Abstract
The aim of this study was to determine whether there are racial/ethnic differences in initiation and timing of adjuvant endocrine therapy (AET) after Medicare Part D drug coverage. We conducted a retrospective cohort study using data from the Surveillance, Epidemiology, and End Results-Medicare-linked data to assess ethnic, socio-demographic, and tumor characteristic variations in the initiation of AET among patients ≥65 with hormone receptor-positive breast cancer in 2007–2009 enrolled in Medicare Part D through 2010. Logistic regression models were performed to assess the association between race/ethnicity and the initiation of tamoxifen, aromatase inhibitors (AIs), and overall AET (tamoxifen or AIs) within the first 12 months of diagnosis. Of the 12,198 women with hormone receptor-positive breast cancer, 74.8 % received AET within 12 months of diagnosis, of which 17.3 % received tamoxifen and 82.8 % received AIs. After controlling for all variables, only Asian women were found to have a greater odds of initiation of overall AET compared to non-Hispanic white women (odds ratio (OR): 1.28, 95 % CI: 1.03–1.58). Hispanic Mexicans and non-Hispanic black patients had a significantly lower odds of tamoxifen initiation (0.70, 0.54–0.91; 0.25, 0.10–0.62). For AI initiation, Hispanic Mexicans and Asians had a higher odds compared to non-Hispanic white women (2.06, 1.34–3.10; 1.33, 1.11–1.61). A suboptimal proportion of women (25.2 %) did not initiate AET within 12 months of diagnosis and therefore did not receive the full benefits of treatment to reduce the risk of breast cancer recurrence and mortality. Racial/ethnic differences in the initiation of tamoxifen and AIs have important implications that require further investigation.
Keywords: Breast cancer, Adjuvant endocrine therapy, Initiation, Hormone receptor-positive
Introduction
Black and Hispanic women experience an increased risk of breast cancer death compared to non-Hispanic white women [1–4]. These ethnic disparities in mortality have been reported to be attributed to late stage at diagnosis [2, 3], socioeconomic status [4], tumor subtypes [5, 6], and the initiation and timing of effective recommended treatment for breast cancer [2, 4]. Nearly two-thirds of all breast cancer cases in the USA are hormone receptor-positive (estrogen or progesterone) and these women are eligible for adjuvant endocrine treatment (AET) [7, 8]. AET is recommended for five years for women with localized- or regional-stage hormone receptor-positive breast cancer [9]. AET treatment includes two classes of drugs, tamoxifen and the aromatase inhibitors (AIs include exemestane, letrozole, and anastrozole). The treatment recommendations are based upon an assessment of menopausal status where premenopausal women are generally indicated to take tamoxifen [9], while recommendations for postmenopausal women can include either tamoxifen, AIs, or a combination of one drug following the other [9].
Given the well-documented efficacy of AET treatment to reduce breast cancer recurrence and mortality [10–13], the timely initiation of AET following a breast cancer diagnosis is potentially important and amenable. However, a number of studies found that a substantial proportion of women with breast cancer indicated for treatment did not take AET altogether [14] or did not initiate the treatment in a timely manner [15–17], especially among ethnic minorities [16–18]. One study found that in a cohort of women enrolled in a large HMO health plan, Hispanic women were less likely to initiate AET compared to non-Hispanic white women [16], but the use of AET was not significantly associated with race/ethnicity in another study of an ethnically diverse national cohort of women [14]. However, this study used a self-report of hormonal therapy use which might have had differential recall or reporting bias [14].
What remains unclear is whether the initiation and timing of AET, including tamoxifen and AIs, is different for an ethnically diverse cohort of older women. Since 2006, the Medicare Part D program started to cover AET for breast cancer for the first time, making it possible to address the above research questions. Although we recently reported an internal validity of Medicare Part D data for hormone therapy and its geographic and racial variation for breast cancer [17], the study did not examine the initiation and timing of hormone therapy. To add new information to the existing literature, we determined whether there were racial/ethnic differences in initiation and timing of AET among a large cohort of older women diagnosed with hormone receptor-positive breast cancer in 2007–2009 with Part D drug claims through 2010 in the SEER areas, accounting for approximately 30 % of the US population. To the best of our knowledge, this is the first study to examine the ethnic disparities in initiation and timing of AET for breast cancer in these women following Medicare Part D drug coverage in SEER areas.
Methods
Data source
This study utilized the Surveillance, Epidemiology, and End Results (SEER)-Medicare-linked data with Part D plan claims from 2007 to 2010. The National Cancer Institute's SEER Program contracts with population-based cancer registries to provide data on all incident cancer cases (with the exception of non-melanoma). The population covered by SEER is comparable to the general US population with regard to measures of poverty [19]. Data collected include patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up vital statistics. The population data such as poverty status and education at the census tract level were from the Census Bureau.
Study design and population
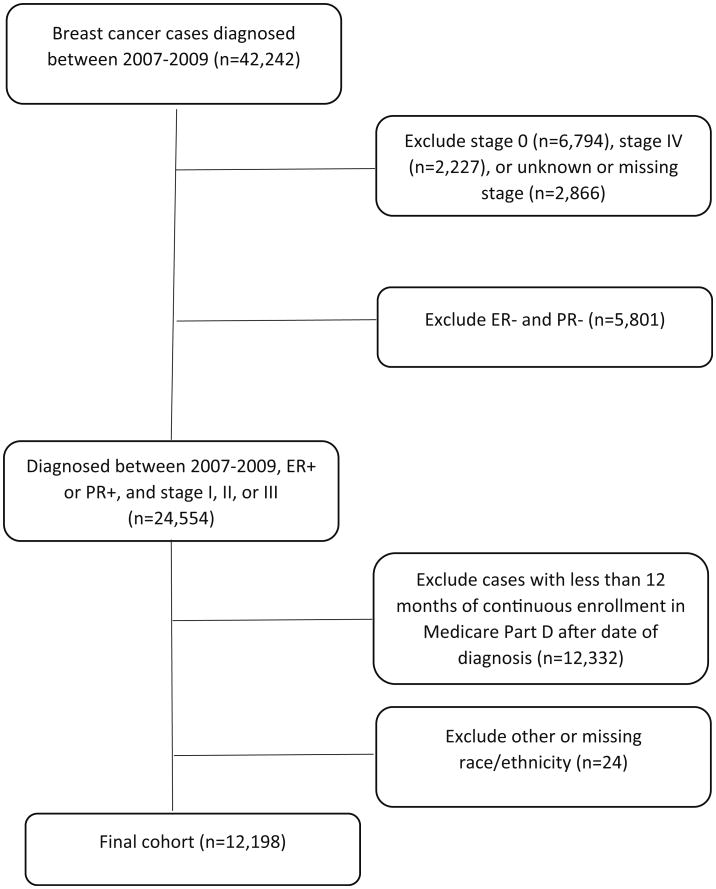
This was a retrospective cohort study. Because AET is recommended to women diagnosed with early-stage (AJCC stages I–III) breast cancer as an adjuvant therapy following cancer-directed surgery with or without chemotherapy [9], we restricted our study cohort to include women with stages I–III and hormone receptor-positive breast cancer at age ≥65 who were enrolled in Medicare Part D for at least 12 months after the date of diagnosis (Fig. 1). Women with tumors of unknown hormone receptor status were excluded. Patients were also excluded if they were not enrolled in Part D plan, had lack of both Medicare Part A and Part B, and were enrolled with a health maintenance organization from the year of diagnosis to the last follow-up. Our final sample included 12,198 women who remained continuously enrolled in Medicare Part D for at least 1 year after breast cancer diagnosis.
Fig. 1.
Diagram for identifying study cohort of women with breast cancer in 2007–2009. Note ER and PR denote estrogen and progesterone receptor status
Dependent variable
Medicare Part D pharmacy claims data contain information on detailed person-specific information for drug utilization such as date of service, product generic drug name identifier, quantity dispensed, days' supply, and fill number. Initiation of AET was defined as a single prescription fill for a tamoxifen or an aromatase inhibitor (AIs) based on their generic drug name in Medicare Part D pharmacy claims data up to 1 year after the date of breast cancer diagnosis. AIs were defined as anastrozole, exemestane, or letrozole. We created a binary variable for initiation if eligible women filled a prescription for any AET medication (yes versus no).
Main exposure variable
We identified women who belonged to six categories of race/ethnicity: non-Hispanic white, black/African American, Hispanic Mexican, Hispanic South or Central American, other Hispanic, or Asian. Race was identified using the SEER race recode variable which is not mutually exclusive for whites, blacks, Asian/Pacific Islanders, and American Indians/Alaska Natives. This variable was combined with the Hispanic origin variable which is derived from the NAACCR Hispanic Identification Algorithm (NHIA) that uses a combination of variables to directly or indirectly classify cases of Hispanic for analytic purposes [20]. If race/ethnicity data were missing or unknown in the SEER data, we used Medicare data to identify the patient's race/ethnicity. Women with other racial/ethnic groups were excluded from this analysis due to small numbers (Fig. 1).
Other study variables
We examined patient socio-demographic, tumor, and clinical characteristics. Demographic information included age at diagnosis and marital status obtained from the SEER data. Socio-demographic information included the percent of residents living below the poverty level at the census tract level and whether the patient lived in a metropolitan region. Tumor characteristics included AJCC tumor stage, tumor size, tumor grade, and lymph node status. Chemotherapy use was identified through Medicare claims within 6 months of diagnosis using procedure codes, and information on radiotherapy and surgery was obtained from both SEER and Medicare data as documented before [21]. The number of comorbid conditions was ascertained from Medicare claims data between 1 year prior to and 1 month after the diagnosis of breast cancer [21–23]. We also included year of diagnosis and SEER geographic area categorized as Northeast, South, Midwest, and West.
Statistical analysis
Differences in the distribution of socio-demographic and tumor characteristics were first examined across racial/ethnic groups. Chi-square tests were used to assess significant differences between groups with respect to categorical variables, and t tests were used to assess differences with respect to continuous variables. Three multivariate logistic regression models were performed to assess the association of race/ethnicity and initiation of AET, tamoxifen, and AI's. Collinearity of all independent variables was tested using multiple collinearity tests, and no variables were removed because no variables had a value greater than 0.7 and the variance inflation factor was >10. We considered a priori significance level at p < 0.05. Analyses were performed using SAS 9.4.
Results
Of the 12,198 women diagnosed with stages I–III hormone receptor-positive breast cancer in 2007–2009 who were continuously enrolled in Medicare Part D, 83 % were non-Hispanic white, 6.5 % were black, 1.2 % were Hispanic Mexican, 0.7 % were Hispanic South or Central American, 4.0 % were other Hispanic, and 4.8 % were Asian. Table 1 presents the distribution of socio-demographic and tumor characteristics by race/ethnicity. Hispanic women of Mexican, South or Central American, or Other Hispanic were younger (median age 73–74) than non-Hispanic white, black, and Asian patients who had a median age of 75 years. Almost all Hispanic women (96.6 %) lived in Metropolitan areas compared to non-Hispanic white women (79.7 %). Compared to non-Hispanic white women (41.5 %), a smaller proportion of South or Central American (<35 %), other Hispanic (34.7 %), and black patients (18 %) were married. A larger proportion of black (71.9 %), Hispanic (Mexican: 66.2 %, South or Central American: 53.4 %, other Hispanic: 58.7 %), and Asian (34.1 %) patients lived in census tract regions where greater than 11.8 % of the population were living below the federal poverty level compared to non-Hispanic white patients (28.5 %). A greater proportion of black (14.4 %) and Hispanic (Mexican: 17.2 %, other Hispanic: 14.1 %) patients were diagnosed with stage III breast cancer compared to non-Hispanic white patients (8.4 %). A greater proportion of black (26.0 %), Hispanic (23.9–30.9 %), and Asian (22.6 %) patients received chemotherapy compared to non-Hispanic white patients (19.3 %). However, a greater proportion of Hispanics (58.3–65.9 %) received radiation therapy compared to non-Hispanic white patients (57.6 %), while a smaller percentage of black patients (47.5 %) received radiation therapy.
Table 1. Characteristics of women diagnosed with stages I–III hormone receptor-positive breast cancer in 2007–2009, by race/ethnicity.
| Non-Hispanic white, n (%) | Black, n (%) | Hispanic Mexican, n (%) | Hispanic South or Central American, n (%) | Other Hispanic, n (%) | Asian, n (%) | |
|---|---|---|---|---|---|---|
| Age, median (range) | 75 (65–115) | 75 (65–101) | 73 (65–93) | 73 (65–93) | 74 (65–95) | 75 (65–97) |
| Age (years) | ||||||
| 65–69 | 2370 (23.4) | 173 (22.3) | 48 (33.1) | 22 (25.0) | 135 (28.0) | 129 (23.9) |
| 70–74 | 2404 (23.7) | 210 (27.0) | 36 (24.8) | 26 (29.6) | 130 (27.0) | 134 (23.1) |
| 75–79 | 2099 (20.7) | 171 (22.0) | 30 (20.7) | 16 (18.2) | 100 (20.8) | 157 (27.0) |
| 80+ | 3252 (32.1) | 223 (28.7) | 31 (21.4) | 24 (27.3) | 117 (24.3) | 151 (26.0) |
| Marital status | ||||||
| Married | 4203 (41.5) | 140 (18.0) | <40 %a | <35 %a | 167 (34.7) | <50 %a |
| Unmarried | 5560 (54.9) | 601 (77.4) | 88 (60.7) | 59 (67.1) | 294 (61.0) | 287 (49.4) |
| Unknown | 362 (3.6) | 36 (4.6) | <8 %a | <13 %a | 21 (4.4) | <5 %a |
| SES (% living below poverty) | ||||||
| First tertile (<5.4 %) | 3655 (36.1) | 74 (9.5) | 14 (9.7) | 20 (22.7) | 82 (17.0) | 180 (31.0) |
| Second tertile (5.4–11.8 %) | 3584 (35.4) | 144 (18.5) | 35 (24.1) | 21 (23.9) | 117 (24.3) | 203 (34.9) |
| Third tertile (>11.8 %) | 2886 (28.5) | 559 (71.9) | 96 (66.2) | 47 (53.4) | 283 (58.7) | 198 (34.1) |
| SEER Cancer Registry region | ||||||
| Northeast | 2189 (21.6) | 157 (20.2) | 0 (0) | <25 %a | 98 (20.3) | 43 (7.4) |
| South | 2574 (25.4) | 377 (48.5) | <8 %a | <13 %a | 18 (3.7) | <5 %a |
| Midwest | 1338 (13.2) | 83 (10.7) | 0 (0) | 0 (0) | <5 %a | <5 %a |
| West | 4024 (39.7) | 160 (20.6) | <98 %a | 62 (70.5) | <75 %a | 510 (87.8) |
| Metropolitan area (yes) | 8069 (79.7) | 656 (84.4) | 140 (96.6) | 85 (96.6) | 425 (88.2) | 554 (95.4) |
| Comorbidity scores | ||||||
| 0 | 5843 (57.7) | 305 (39.3) | 75 (51.7) | 49 (55.7) | 237 (49.2) | 295 (50.8) |
| 1 | 2543 (25.1) | 220 (28.3) | 34 (23.5) | 25 (28.4) | 150 (31.1) | 192 (33.1) |
| 2 | 1001 (9.9) | 119 (15.3) | 22 (15.2) | <13 %a | 53 (11.0) | 58 (10.0) |
| 3+ | 738 (7.3) | 133 (17.1) | 14 (9.7) | <13 %a | 42 (8.7) | 36 (6.2) |
| Year of diagnosis | ||||||
| 2007 | 3235 (32.0) | 249 (32.1) | 43 (29.7) | 28 (31.8) | 151 (31.3) | 176 (30.3) |
| 2008 | 3380 (33.4) | 267 (34.4) | 65 (44.8) | 22 (25.0) | 162 (33.6) | 199 (34.3) |
| 2009 | 3510 (34.7) | 261 (33.6) | 37 (25.5) | 38 (43.2) | 169 (35.1) | 206 (35.5) |
| AJCC tumor stage | ||||||
| Stage I | 6071 (60.0) | 353 (45.4) | 70 (48.3) | 45 (51.1) | 244 (50.6) | 330 (56.8) |
| Stage II | 3206 (31.7) | 312 (40.2) | 50 (34.5) | <40 %a | 170 (35.3) | <35 %a |
| Stage III | 848 (8.4) | 112 (14.4) | 25 (17.2) | <13 %a | 68 (14.1) | <5 %a |
| Tumor size (cm) | ||||||
| <1.0 | 5993 (59.2) | 347 (44.7) | 70 (48.3) | 45 (51.1) | 229 (47.5) | 319 (54.9) |
| >=1.0 | 3593 (35.5) | 396 (51.0) | <50 %a | <45 %a | 231 (47.9) | 227 (39.1) |
| Unknown size | 529 (5.3) | 34 (4.4) | <8 %a | <13 %a | 22 (4.6) | 35 (6.0) |
| Lymph node positivity | ||||||
| 0 (node negative) | 6657 (65.8) | 415 (53.4) | 87 (60.0) | 52 (59.1) | 289 (60.0) | 388 (66.8) |
| 1+ | 1249 (22.2) | 224 (28.8) | 38 (26.2) | <35 %a | 142 (29.5) | <25 %a |
| Unknown | 1219 (12.0) | 138 (17.8) | 20 (13.8) | <13 %a | 51 (10.6) | <5 %a |
| Tumor grade | ||||||
| Well differentiated | 3012 (29.8) | 157 (20.2) | 39 (26.9) | 22 (25.0) | 108 (224) | 163 (28.1) |
| Moderately differentiated | 4890 (48.3) | 353 (45.4) | 73 (50.3) | 43 (48.9) | 237 (49.2) | 276 (47.5) |
| Poorly differentiated | 1792 (17.7) | 214 (27.5) | <20 %a | <25 %a | 115 (23.9) | 122 (21.0) |
| Unknown | 431 (4.3) | 53 (6.8) | <8 %a | <13 %a | 22 (4.6) | 20 (3.4) |
| Surgery treatment | ||||||
| No surgery | 222 (2.2) | 55 (7.1) | <8 %a | <13 %a | 13 (2.7) | 11 (1.9) |
| BCS | 6238 (61.6) | 379 (48.8) | 79 (54.5) | 59 (67.1) | 276 (57.3) | 319 (54.9) |
| Mastectomy | 3665 (36.2) | 343 (44.1) | <45 %a | <30 %a | 193 (40.0) | 251 (43.2) |
| Chemotherapy (yes) | 1954 (19.3) | 202 (26.0) | 39 (26.9) | 21 (23.9) | 149 (30.9) | 131 (22.6) |
| Radiation therapy (yes) | 5830 (57.6) | 369 (47.5) | 90 (62.1) | 58 (65.9) | 281 (58.3) | 314 (54.0) |
| Total | 10,125 (83.0) | 777 (6.4) | 145 (1.2) | 88 (0.7) | 482 (4.0) | 581 (4.8) |
Actual percentages were not reported to avoid N < 11 reporting as required by the data-user agreement
Table 2 presents the percentage of women who received AET, tamoxifen, and AIs by race/ethnicity, socio-demographic, and tumor characteristics. The overall proportion of patients receiving AET was 74.8 %, of which 17.3 % and 82.8 % received tamoxifen and AIs, respectively. A smaller proportion of Hispanic Mexicans (<10.0 %), South/Central Hispanics (<17.0 %), other Hispanics (14.9 %), Asians (13.7 %), and non-Hispanic black patients (13.0 %) received tamoxifen compared to non-Hispanic white patients (18.2 %), whereas a greater percentage of minorities received AIs compared to non-Hispanic white patients. A greater proportion of younger women compared to older women initiated AET (81.7 % for age 65–69 versus 64.9 % for age 80+). A greater proportion of patients without comorbidities initiated AET compared to women with comorbidity score of ≥3. Patients who received chemotherapy or radiotherapy had a higher percentage of receiving AET than those who did not.
Table 2. Percent of patients initiating adjuvant endocrine therapy treatment in those with stages I–III hormone receptor-positive breast cancer, by AET type.
| Percentage (%) of patients receiving AET | Percentage (%) of patients receiving tamoxifen | Percentage (%) of patients receiving AIs | |
|---|---|---|---|
| Race/ethnicity | |||
| Non-Hispanic white | 74.5 | 18.2 | 81.9 |
| Non-Hispanic black | 73.4 | 13.0 | 87.0 |
| Hispanic (Mexican) | 80.7 | <10.0a | <97a |
| Hispanic (South/Central) | 75.0 | <17.0a | <89a |
| Hispanic (other/unknown) | 79.5 | 14.9 | 85.1 |
| Asian | 77.8 | 13.7 | 86.3 |
| Age (years) | |||
| 65–69 | 81.7 | 14.2 | 85.8 |
| 70–74 | 80.6 | 15.7 | 84.3 |
| 75–79 | 75.1 | 17.5 | 82.5 |
| 80+ | 64.9 | 21.5 | 78.5 |
| Marital status | |||
| Married | 77.8 | 16.7 | 83.3 |
| Unmarried | 72.6 | 18.0 | 82.0 |
| Unknown | 76.7 | 12.9 | 87.1 |
| SES (% living below poverty) | |||
| First tertile (<5.4 %) | 75.1 | 14.6 | 85.4 |
| Second tertile (5.4–11.8 %) | 73.8 | 18.1 | 81.9 |
| Third tertile (>11.8 %) | 75.6 | 19.0 | 81.0 |
| SEER Cancer Registry region | |||
| Northeast | 77.3 | 11.7 | 88.3 |
| South | 75.7 | 18.2 | 81.8 |
| Midwest | 71.6 | 27.3 | 72.7 |
| West | 74.0 | 16.8 | 83.2 |
| Metropolitan area (yes) | 75.1 | 15.5 | 84.5 |
| Comorbidity scores | |||
| 0 | 76.5 | 17.7 | 82.3 |
| 1 | 74.5 | 16.9 | 83.1 |
| 2 | 70.6 | 16.1 | 83.9 |
| 3+ | 69.8 | 16.5 | 83.5 |
| Year of diagnosis | |||
| 2007 | 75.4 | 17.5 | 82.5 |
| 2008 | 74.0 | 16.8 | 83.2 |
| 2009 | 75.1 | 17.5 | 82.5 |
| AJCC tumor stage | |||
| Stage I | 71.6 | 20.1 | 79.9 |
| Stage II | 79.5 | 14.4 | 85.6 |
| Stage III | 79.1 | 10.7 | 89.3 |
| Tumor size (cm) | |||
| <1.0 | 74.0 | 18.4 | 81.6 |
| >=1.0 | 77.9 | 14.9 | 85.1 |
| Unknown size | 61.9 | 23.9 | 76.1 |
| Number of positive nodes | |||
| 0 (node negative) | 74.8 | 18.7 | 81.3 |
| 1+ | 82.2 | 12.2 | 87.9 |
| Unknown | 61.1 | 20.5 | 79.5 |
| Tumor grade | |||
| Well differentiated | 71.2 | 19.6 | 80.4 |
| Moderately differentiated | 76.9 | 16.8 | 83.2 |
| Poorly differentiated | 75.3 | 14.8 | 85.2 |
| Unknown | 73.3 | 17.8 | 82.2 |
| Surgery treatment | |||
| No surgery | 68.6 | 11.7 | 88.3 |
| BCS | 74.7 | 17.7 | 82.3 |
| Mastectomy | 75.4 | 16.9 | 83.1 |
| Chemotherapy | |||
| Yes | 78.7 | 10.5 | 89.5 |
| No | 73.8 | 19.1 | 80.9 |
| Radiation therapy | |||
| Yes | 79.3 | 15.3 | 84.7 |
| No | 68.9 | 20.2 | 79.8 |
| Total | 74.8 | 17.3 | 82.8 |
Actual percentages were not reported to avoid N < 11 reporting as required by the data-user agreement
Table 3 presents the percentage of women who initiated AET by month of enrollment and by race/ethnicity, socio-demographic, and tumor characteristics. Among patients who initiated AET, 38.5 % initiated therapy within 0–3 months, 36.7 % initiated therapy within 3–6 months, 16.5 % initiated within 6–9 months, and 8.3 % initiated within 9–12 months of breast cancer diagnosis. A greater proportion of women initiated at earlier months (75.2 % initiated AET within the first 6 months, while 18.8 % initiated during months 6–12). A greater proportion of non-Hispanic white patients (76.2 %) initiated AET during months 0–6 compared to black (70.5 %), Hispanic Mexican (68.3 %), Hispanic South/Central (68.2 %), and other Hispanic patients (67.3 %). Among women who received chemotherapy, a smaller proportion (34.6 %) initiated AET at months 0–6. In women who received radiation treatment, a greater proportion (69.6 %) initiated at earlier months (0–6 months).
Table 3. Time to adjuvant endocrine therapy treatment initiation among those with stages I–III hormone receptor-positive breast cancer receiving endocrine therapy, by month of initiation.
| 0–3 Months (%) | 3–6 Months (%) | 6–9 Months (%) | 9–12 Months (%) | |
|---|---|---|---|---|
| Race/ethnicity | ||||
| Non-Hispanic white | 38.9 | 37.3 | 15.8 | 8.0 |
| Non-Hispanic black | 37.9 | 32.6 | 20.5 | 9.0 |
| Hispanic (Mexican) | 33.3 | 35.0 | 24.8 | <10.0a |
| Hispanic (South/Central) | 39.4 | 31.8 | 19.7 | <17.0 %a |
| Hispanic (other/unknown) | 31.3 | 36.0 | 19.3 | 13.3 |
| Asian | 40.5 | 34.5 | 16.4 | 8.6 |
| Age (years) | ||||
| 65–69 | 27.9 | 37.5 | 21.9 | 12.8 |
| 70–74 | 32.7 | 36.8 | 20.3 | 10.1 |
| 75–79 | 40.1 | 38.7 | 14.3 | 6.9 |
| 80+ | 37.2 | 25.3 | 15.1 | 11.3 |
| Marital status | ||||
| Married | 34.3 | 38.8 | 17.7 | 9.2 |
| Unmarried | 41.6 | 35.4 | 15.3 | 7.8 |
| Unknown | 40.5 | 33.6 | 19.5 | 6.3 |
| SES (% living below poverty) | ||||
| First tertile (<5.4 %) | 38.1 | 37.8 | 16.2 | 7.8 |
| Second tertile (5.4–11.8 %) | 36.5 | 39.1 | 16.0 | 8.5 |
| Third tertile (>11.8 %) | 40.9 | 33.3 | 17.1 | 8.7 |
| SEER Cancer Registry region | ||||
| Northeast | 41.1 | 36.1 | 15.6 | 7.2 |
| South | 46.6 | 31.7 | 14.6 | 7.1 |
| Midwest | 39.6 | 35.4 | 16.1 | 8.9 |
| West | 32.2 | 40.3 | 18.0 | 9.5 |
| Metropolitan area (yes) | 37.4 | 37.5 | 16.8 | 8.3 |
| Comorbidity scores | ||||
| 0 | 36.2 | 38.4 | 17.1 | 8.3 |
| 1 | 39.2 | 35.2 | 16.7 | 8.8 |
| 2 | 41.8 | 35.2 | 14.4 | 8.6 |
| 3+ | 49.3 | 31.0 | 13.4 | 6.2 |
| Year of diagnosis | ||||
| 2007 | 39.6 | 36.2 | 17.2 | 7.0 |
| 2008 | 37.1 | 37.6 | 16.7 | 8.6 |
| 2009 | 38.9 | 36.3 | 15.5 | 9.3 |
| AJCC tumor stage | ||||
| Stage I | 41.4 | 41.2 | 13.2 | 4.2 |
| Stage II | 35.4 | 33.7 | 19.8 | 11.1 |
| Stage III | 32.9 | 21.9 | 23.0 | 22.3 |
| Tumor size (cm) | ||||
| <1.0 | 39.0 | 40.1 | 14.8 | 6.1 |
| >=1.0 | 37.3 | 31.7 | 19.1 | 11.9 |
| Unknown size | 43.0 | 37.7 | 14.1 | 5.3 |
| Number of positive nodes | ||||
| 0 (node negative) | 39.0 | 41.5 | 14.3 | 5.2 |
| 1+ | 29.8 | 28.7 | 23.9 | 17.6 |
| Unknown | 57.2 | 26.4 | 11.5 | 4.9 |
| Tumor grade | ||||
| Well differentiated | 40.0 | 40.5 | 14.0 | 5.4 |
| Moderately differentiated | 38.4 | 37.4 | 16.2 | 8.1 |
| Poorly differentiated | 34.6 | 30.8 | 21.1 | 13.5 |
| Unknown | 46.8 | 31.0 | 14.7 | 7.4 |
| Surgery treatment | ||||
| No surgery | 67.8 | 15.4 | 12.2 | 4.7 |
| BCS | 34.5 | 41.3 | 16.7 | 7.5 |
| Mastectomy | 43.1 | 30.7 | 16.4 | 9.8 |
| Chemotherapy (yes) | ||||
| Yes | 12.2 | 22.4 | 37.1 | 28.3 |
| No | 45.7 | 40.7 | 10.8 | 2.9 |
| Radiation therapy (yes) | ||||
| Yes | 28.7 | 40.9 | 19.8 | 10.6 |
| No | 53.4 | 30.3 | 11.3 | 4.9 |
| Total | 38.5 | 36.7 | 16.5 | 8.3 |
Actual percentages were not reported to avoid N < 11 reporting as required by the data-user agreement
Table 4 presents the adjusted odds ratio (OR) of receiving AET, tamoxifen, and AIs by race/ethnicity, socio-demographic, and tumor characteristics. There were no significant differences in the initiation of AET between any of the Hispanic subgroups compared to non-Hispanic white patients after controlling for all socio-demographic and tumor characteristics. Women of Asian race/ethnicity were associated with a greater odds of initiating AET compared to non-Hispanic white patients (adjusted OR: 1.28, 95 % CI: 1.03–1.58). Black (OR: 0.70, 95 % CI: 0.54–0.91) and Hispanic Mexican patients (0.25, 0.10–0.62) had a significantly lower odds of receiving tamoxifen compared to non-Hispanic white women. Hispanic Mexican patients (2.06, 1.34–3.10) and Asians (1.33, 1.11–1.61) had a significantly higher odds of receiving AIs compared to non-Hispanic white women. Other significant predictors of receiving AET included age, marital status, SES, SEER Cancer Registry region, comorbidity scores, stage at diagnosis, lymph node status, tumor grade, surgical treatment, chemotherapy, and radiation therapy. Significant predictors of initiating tamoxifen were SES, SEER Cancer Registry region, comorbidity score, stage at diagnosis, receipt of surgery, and chemotherapy. Significant predictors of AI initiation were age, marital status, SEER Cancer Registry region, tumor stage, tumor size, lymph node status, tumor grade, receipt of surgery, and radiation treatment.
Table 4. Multivariable logistic regression for the initiation of adjuvant endocrine therapy among women diagnosed with stages I–III hormone receptor-positive breast cancer.
| Initiate any AET | Initiate tamoxifen | Initiate AI | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| AOR | 95 % CIa | AOR | 95 % CIa | AOR | 95 % CIa | |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 | 1 | 1 | |||
| Non-Hispanic black | 0.87 | (0.72–1.04) | 0.70 | (0.54–0.91) | 1.05 | (0.89–1.23) |
| Hispanic (Mexican) | 1.38 | (0.90–2.13) | 0.25 | (0.10–0.62) | 2.06 | (1.34–3.10) |
| Hispanic (South/Central) | 0.91 | (0.55–1.50) | 0.71 | (0.34–1.49) | 1.07 | (0.68–1.70) |
| Hispanic (other/unknown) | 1.23 | (0.97–1.57) | 0.97 | (0.72–1.29) | 1.20 | (0.98–1.48) |
| Asian | 1.28 | (1.03–1.58) | 0.83 | (0.63–1.09) | 1.33 | (1.11–1.61) |
| Age (years) | ||||||
| 65–69 | 1 | 1 | 1 | |||
| 70–74 | 0.93 | (0.81–1.06) | 1.06 | (0.91–1.25) | 0.92 | (0.82–1.03) |
| 75–79 | 0.69 | (0.60–0.79) | 1.10 | (0.93–1.30) | 0.71 | (0.63–0.80) |
| 80+ | 0.47 | (0.41–0.53) | 1.13 | (0.96–1.33) | 0.50 | (0.45–0.57) |
| Marital status | ||||||
| Married | 1 | 1 | 1 | |||
| Unmarried | 0.89 | (0.81–0.98) | 1.01 | (0.90–1.14) | 0.91 | (0.84–0.99) |
| Unknown | 1.04 | (0.82–1.33) | 0.78 | (0.55–1.07) | 1.18 | (0.95–1.47) |
| SES (% living below poverty) | ||||||
| First tertile (<5.4 %) | 1 | 1 | 1 | |||
| Second tertile (5.4–11.8 %) | 1.02 | (0.91–1.13) | 1.10 | (0.96–1.27) | 0.96 | (0.87–1.06) |
| Third tertile (>11.8 %) | 1.15 | (1.02–1.30) | 1.28 | (1.10–1.49) | 0.99 | (0.89–1.11) |
| SEER Cancer Registry Region | ||||||
| Northeast | 1 | 1 | 1 | |||
| South | 0.84 | (0.73–0.97) | 1.35 | (1.11–1.63) | 0.77 | (0.68–0.87) |
| Midwest | 0.70 | (0.59–0.82) | 2.09 | (1.70–2.56) | 0.51 | (0.44–0.59) |
| West | 0.69 | (0.61–0.78) | 1.37 | (1.15–1.62) | 0.65 | (0.58–0.72) |
| Metropolitan area (yes vs no) | 1.08 | (0.96–1.23) | 0.74 | (0.64–0.85) | 1.27 | (1.14–1.42) |
| Comorbidity scores | ||||||
| 0 | 1 | 1 | 1 | |||
| 1 | 0.95 | (0.86–1.05) | 0.92 | (0.81–1.05) | 1.00 | (0.91–1.10) |
| 2 | 0.81 | (0.71–0.94) | 0.81 | (0.67–0.98) | 0.94 | (0.82–1.06) |
| 3+ | 0.81 | (0.69–0.95) | 0.82 | (0.66–1.02) | 0.92 | (0.78–1.07) |
| Year of diagnosis | ||||||
| 2007 | 1 | 1 | 1 | |||
| 2008 | 0.92 | (0.83–1.03) | 0.94 | (0.82–1.08) | 0.96 | (0.88–1.06) |
| 2009 | 0.98 | (0.89–1.09) | 1.00 | (0.88–1.14) | 0.98 | (0.90–1.08) |
| AJCC tumor stage | ||||||
| Stage I | 1 | 1 | 1 | |||
| Stage II | 1.41 | (1.19–1.67) | 0.78 | (0.62–0.96) | 1.50 | (1.29–1.75) |
| Stage III | 1.17 | (0.90–1.51) | 0.66 | (0.47–0.94) | 1.38 | (1.09–1.73) |
| Tumor size (cm) | ||||||
| <1.0 | 1 | 1 | 1 | |||
| >=1.0 | 1.04 | (0.90–1.21) | 1.15 | (0.96–1.38) | 0.97 | (0.85–1.10) |
| Unknown size | 0.56 | (0.47–0.67) | 1.13 | (0.89–1.42) | 0.57 | (0.48–0.68) |
| Number of positive nodes | ||||||
| 0 (node negative) | 1 | 1 | 1 | |||
| 1+ | 1.30 | (1.10–1.53) | 0.99 | (0.81–1.22) | 1.22 | (1.05–1.40) |
| Unknown | 0.74 | (0.64–0.85) | 0.96 | (0.80–1.17) | 0.77 | (0.67–0.87) |
| Tumor grade | ||||||
| Well differentiated | 1 | 1 | 1 | |||
| Moderately differentiated | 1.25 | (1.13–1.38) | 1.03 | (0.91–1.16) | 1.18 | (1.08–1.29) |
| Poorly differentiated | 1.07 | (0.94–1.22) | 0.99 | (0.83–1.17) | 1.07 | (0.95–1.20) |
| Unknown | 1.13 | (0.91–1.40) | 1.11 | (0.84–1.47) | 1.05 | (0.86–1.28) |
| Surgery treatment | ||||||
| No surgery | 1 | 1 | 1 | |||
| BCS | 0.69 | (0.53–0.91) | 1.69 | (1.08–2.63) | 0.58 | (0.45–0.75) |
| Mastectomy | 0.85 | (0.64–1.12) | 1.53 | (0.98–2.40) | 0.73 | (0.56–0.95) |
| Chemotherapy (yes vs no) | 0.74 | (0.65–0.84) | 0.65 | (0.54–0.77) | 0.95 | (0.85–1.06) |
| Radiation therapy (yes vs no) | 1.65 | (1.48–1.85) | 0.86 | (0.74–1.00) | 1.65 | (1.48–1.83) |
Adjusted odds ratio (AOR) controlled for all other socio-demographic and tumor characteristics listed in Table 1
Table 5 presents the adjusted OR of initiating AET, tamoxifen, and AIs by the number of months from diagnosis by race/ethnicity. Non-Hispanic black patients had a significantly lower odds of initiating any AET 0–3 months after the date of diagnosis compared to non-Hispanic white women (adjusted OR: 0.78, 95 % CI: 0.65–0.94), while Asian patients had a higher odds of initiation (1.44, 1.18–1.75). The same pattern was observed for women who initiated AIs during the same time period. Non-Hispanic black women (0.85, 0.72–1.00) compared to non-Hispanic white women who initiated any AET 0–6 months after the date of diagnosis had a lower odds of receiving any AET, while Asian patients had a higher odds (1.23, 1.02–1.48) after controlling for other covariates. Non-Hispanic black and Hispanic Mexican women had a significantly lower odds of initiating tamoxifen therapy 0–6 months after the date of diagnosis compared to non-Hispanic white women (0.67, 0.50–0.90 and 0.27, 0.10–0.74, respectively). Hispanic Mexican women and Asians had a significantly higher odds of initiating AI therapy 0–6 months (1.57, 1.11–2.23 and 1.29, 1.08–1.54, respectively, and of receiving any AET 0–9 months after the date of diagnosis compared to non-Hispanic white women (1.56, 1.05–2.32 and 1.25, 1.03–1.52, respectively). Non-Hispanic black and Hispanic women had a significantly lower odds of initiating tamoxifen 0–9 months after the date of diagnosis compared to non-Hispanic white women (0.72, 0.55–0.93 and 0.28, 0.12–0.70, respectively), while Hispanic Mexicans and Asians had a significantly higher odds of initiation AIs within 0–9 months of diagnosis compared to non-Hispanic white women (2.11, 1.45–3.07 and 1.29, 1.08–1.55, respectively).
Table 5. Multivariable logistic regression by time to initiation of adjuvant endocrine therapy among women diagnosed with stages I–III hormone receptor-positive breast cancer.
| 0–3 Months | 0–6 Months | 0–9 Months | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| AOR | 95 % CIa | AOR | 95 % CIa | AOR | 95 % CIa | |
| Initiate any hormonal therapya | ||||||
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 | 1 | 1 | |||
| Non-Hispanic black | 0.78 | (0.65–0.94) | 0.85 | (0.72–1.00) | 0.92 | (0.78–1.10) |
| Hispanic (Mexican) | 1.24 | (0.84–1.84) | 1.18 | (0.82–1.69) | 1.56 | (1.05–2.32) |
| Hispanic (South/Central) | 1.28 | (0.79–2.07) | 0.97 | (0.62–1.51) | 0.97 | (0.61–1.54) |
| Hispanic (other/unknown) | 1.04 | (0.83–1.30) | 1.10 | (0.99–1.34) | 1.10 | (0.89–1.36) |
| Asian | 1.44 | (1.18–1.75) | 1.23 | (1.02–1.48) | 1.25 | (1.03–1.52) |
| Initiate tamoxifen therapya | ||||||
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 | 1 | 1 | |||
| Non-Hispanic black | 0.77 | (0.55–1.08) | 0.67 | (0.50–0.90) | 0.72 | (0.55–0.93) |
| Hispanic (Mexican) | 0.59 | (0.21–1.62) | 0.27 | (0.10–0.74) | 0.28 | (0.12–0.70) |
| Hispanic (South/Central) | 0.70 | (0.22–2.23) | 0.68 | (0.29–1.57) | 0.79 | (0.38–1.65) |
| Hispanic (other/unknown) | 0.71 | (0.43–1.18) | 0.97 | (0.70–1.35) | 0.98 | (0.72–1.32) |
| Asian | 1.02 | (0.69–1.50) | 0.86 | (0.64–1.17) | 0.86 | (0.64–1.14) |
| Initiate AI therapya | ||||||
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 | 1 | 1 | |||
| Non-Hispanic black | 0.83 | (0.68–1.00) | 0.98 | (0.84–1.15) | 1.07 | (0.91–1.25) |
| Hispanic (Mexican) | 1.43 | (0.95–2.14) | 1.57 | (1.11–2.23) | 2.11 | (1.45–3.07) |
| Hispanic (South/Central) | 1.43 | (0.87–2.36) | 1.10 | (0.71–1.70) | 1.07 | (0.69–1.66) |
| Hispanic (other/unknown) | 1.14 | (0.90–1.45) | 1.11 | (0.91–1.35) | 1.11 | (0.91–1.35) |
| Asian | 1.50 | (1.22–1.84) | 1.29 | (1.08–1.54) | 1.29 | (1.08–1.55) |
Adjusted odds ratio (AOR) controlled for all other socio-demographic and tumor characteristics listed in Table 1
Discussion
This study described the association between race/ethnicity and the initiation of AET (tamoxifen and AIs) in women with early-stage hormone receptor-positive breast cancer with Medicare Part D drug coverage. Overall, close to three quarters (74.8 %) of early-stage hormone receptor-positive breast cancer patients initiated treatment, but still 25 % did not initiate therapy within the first 12 months of diagnosis. There also were substantial racial/ethnic differences in the initiation and timing of AET where Hispanic Mexicans and non-Hispanic black patients had a significantly lower odds of tamoxifen initiation (0.70, 0.54–0.91; 0.25, 0.10–0.62) and black patients had a significantly lower odds of early initiation within the first 6 months of diagnosis compared to non-Hispanic white patients (0.85, 0.72–1.00).
The percentage of women who initiated AET within 12 months was slightly higher than that of previous studies which found between 68 and 71 % of women initiated AET [15–17]. However, those studies included younger commercially insured women [15, 16], or included women with stage 0 breast cancer [17], which may have explained some of the observed differences in proportion of women that initiated AET.
In our study, we found racial/ethnic differences among women who initiated AET where higher levels of initiation were found for Asian women. One study by Livaudais et al. found that Chinese women compared to non-Hispanic white women had a lower odds of AET initiation in a cohort of younger, commercially insured women [16]. We were not able to stratify Asian race/ethnicity into smaller subgroups due to limitations in sample size. Our finding regarding no association between other race/ethnicities and initiation of AET is consistent with previous studies [14, 15]. However, contradictory to these findings, studies showed that the use of AET is independently associated with lower proportion of initiation among Hispanic [16, 18] and black patients [17, 18] compared to white women. These studies were conducted among different study populations and used self-report of AET use, included stage 0 or stage IV breast cancer, and/or studied a younger cohort of women.
This is the first study to find that the proportion of female Medicare beneficiaries with breast cancer who initiated tamoxifen and AIs varied significantly by race/ethnicity even after controlling for other covariates in SEER areas following Medicare Part D coverage, whereas Hispanic Mexican and black patients were less likely to receive tamoxifen and Hispanic Mexican and Asian patients were more likely to receive AIs compared to non-Hispanic white women. A recent study by Wang & Du did find lower levels of initiation by AET type among black women, but it did not examine ethnic differences in the initiation and timing of AET [17]. Other studies combined tamoxifen and AIs into one category of AET [15, 16, 18]. Current national guidelines recommend that women with hormone receptor-positive breast cancer take AIs as part of adjuvant treatment either up-front or following tamoxifen [24], but the guidelines do not provide a clear indication as to which drug is superior at initiation. Despite this, we did find that the initiation of tamoxifen and AIs varied significantly by race/ethnicity. This is an important finding since the type of hormonal therapy use may be associated with adherence to the medication regimen [25, 26], cost of the drugs [27, 28], and potential short- and long-term side effects [29, 30]. These factors may all influence breast cancer recurrence [11, 31] and mortality [31]. While we did control for geographic region, racial/ethnic differences in treatment with tamoxifen versus AIs may be partially attributed to geographic-specific treatment practices [17], especially since racial/ethnic minorities represented in the sample are clustered into regions such that the majority of Hispanic Mexican patients were from the Western region of the USA.
Our study found significant differences in the early initiation of AET by race/ethnicity where black patients are less likely to initiate AET within 6 months from diagnosis and black and Hispanic Mexican women less likely to initiate tamoxifen therapy within 6 months from diagnosis compared to non-Hispanic white patients. However, there is no clear guideline on the timing for which women should initiate hormonal therapy in relationship to her diagnosis, surgical treatment, chemotherapy, and/or radiation therapy date. A previous study examining the cumulative rates of initiation of AET among non-elderly Medicaid insured women found that a small proportion of women continued to initiate AET more than 12 months after breast cancer diagnosis [15]. Similar to other studies, significant predictors of initiating AET included comorbidity status, tumor stage, age, socioeconomic status, geographic region, tumor stage, chemotherapy, and radiation therapy [15–17].
Our study had several strengths. First, our study was able to utilize a large, nationally represented sample of elderly breast cancer patients including subgroups of Hispanic ethnicity. Second, this study applied the innovative use of the nationwide, population-based computerized Medicare claims and SEER Cancer Registry data on cancer therapies which have been validated for internal and external validity [17, 32, 33]. Furthermore, population-based cancer registries alone tend to underestimate the use of adjuvant treatment such as AET [33] and most studies on use of AET have relied solely on medical claims and pharmacy data which do not contain tumor characteristic information that allows to identify which women are indicated for AET treatment [25, 27, 34]. Hence, the SEER-Medicare-linked data used in this study offered the unique opportunity to examine the initiation and timing of AET among those who are indicated for such therapy.
Our study also had limitations. First, our study population included only women aged 65 and older enrolled in Medicare Part D plan and the results may not be generalized to younger patients or those not enrolled in Part D. Second, there could be unmeasured confounding such as patient psychosocial factors that may influence women's initiation of AET which cannot be captured in this study. For example, patients' strong belief in the necessity of a treatment and familial support may improve initiation of therapy [35]. Treatment recommendations made by physicians are also influential, and this information was not available in the current dataset; therefore, we were unable to determine whether treatment with tamoxifen or AIs was influenced by physician recommendation [36]. Third, there may have been misclassification of race/ethnicity. However, this bias may be minimal since the SEER Cancer Registry data use incidence data for Hispanics based on the NAACCR Hispanic/Latino Identification Algorithm (NHIA) which have been previously validated [37]. Also, we used race/ethnicity data from the Medicare dataset which was also well validated for accuracy of race/ethnicity to augment the information on missing or unknown ethnicity in SEER [20].
Conclusions
In conclusion, while a large proportion of women with hormone receptor-positive breast cancer initiated AET, a substantial proportion of women (25.2 %) still did not initiate AET within 12 months of diagnosis. According to the clinical guidelines, all women in our study cohort are eligible and should have initiated AET based on breast cancer tumor characteristics alone in order to maximize the full benefits of effective treatment to reduce the risk of breast cancer recurrence and mortality, with the exception for patients with a history of blood clots, stroke, uterine cancer or osteoporosis [9]. Furthermore, we observed differences in the use of tamoxifen and AIs by race/ethnicity even after controlling for other socio-demographic and tumor characteristics. The underlying factors and consequences associated with prescription of tamoxifen and AIs by race/ethnicity need to be investigated further.
Acknowledgments
We acknowledge the efforts of the National Cancer Institute; Center for Medicare and Medicaid Services; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results Program tumor registries in the creation of this database. The interpretation and reporting of these data are the sole responsibilities of the authors. Albert J. Farias is supported by a postdoctoral fellowship from the University of Texas Health Science Center at Houston (UTHealth) School of Public Health Cancer Education and Career Development Program funded by a grant from the National Cancer Institute (R25-CA57712). This study was also supported in part by a grant from the Agency for Healthcare Research and Quality (R01-HS018956) and in part by a grant from the Cancer Prevention and Research Institute of Texas (RP130051).
Footnotes
Conflict of interest The authors declare that there are no conflicts of interest.
Compliance with ethical standards: Ethical standards The authors declare compliance with ethical standards of the current laws in the USA where the study was performed.
References
- 1.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127:729–38. doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, Wang M, Niknam BA, Ludwig JM, Wang W, Even-Shoshan O, Fox KR. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310:389–97. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 3.Banegas MP, Li CI. Breast cancer characteristics and outcomes among hispanic black and hispanic white women. Breast Cancer Res Treat. 2012;134:1297–304. doi: 10.1007/s10549-012-2142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du XL, Fang S, Meyer TE. Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Am J Clin Oncol. 2008;31:125–32. doi: 10.1097/COC.0b013e3181587890. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC. Intrinsic breast tumor subtypes, race, and long-term survival in the carolina breast cancer study. Clin Cancer Res. 2010;16:6100–10. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society. Breast cancer facts and figures 2013–2014. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 8.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. Nccn clinical practice guidelines in oncology (nccn guidelines®), breast cancer. 2014:2015. doi: 10.6004/jnccn.2004.0021. [DOI] [PubMed] [Google Scholar]
- 10.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R EBCTCG (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haque R, Ahmed SA, Fisher A, Avila CC, Shi J, Guo A, Craig Cheetham T, Schottinger JE. Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer. Cancer Med. 2012;1:318–27. doi: 10.1002/cam4.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lønning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C Study IE. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–92. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 13.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS Group AT. Results of the atac (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–2. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 14.Livaudais JC, Lacroix A, Chlebowski RT, Li CI, Habel LA, Simon MS, Thompson B, Erwin DO, Hubbell FA, Coronado GD. Racial/ethnic differences in use and duration of adjuvant hormonal therapy for breast cancer in the women's health initiative. Cancer Epidemiol Biomark Prev. 2013;22:365–73. doi: 10.1158/1055-9965.EPI-12-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yung RL, Hassett MJ, Chen K, Gesten FC, Roohan PJ, Boscoe FP, Sinclair AH, Schymura MJ, Schrag D. Initiation of adjuvant hormone therapy by medicaid insured women with nonmetastatic breast cancer. J Natl Cancer Inst. 2012;104:1102–5. doi: 10.1093/jnci/djs273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI, Neugut AI, Fehrenbacher L, Thompson B, Coronado GD. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131:607–17. doi: 10.1007/s10549-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Du XL. Socio-demographic and geographic variations in the utilization of hormone therapy in older women with breast cancer after medicare Part-D coverage. Med Oncol. 2015;32:599. doi: 10.1007/s12032-015-0599-6. [DOI] [PubMed] [Google Scholar]
- 18.Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, Keating NL. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117:180–9. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 19.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM, Henley SJ, Eheman CR, Anderson RN, Penberthy L. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NAACCR Race and Ethnicity Work Group: Naaccr guideline for enhancing hispanic/latino identification: Revised naaccr hispanic/latino identification algorithm [nhia v2.2.1] Springfield IL: North American Association of Central Cancer Registries; 2011. [Google Scholar]
- 21.Hu CY, Delclos GL, Chan W, Du XL. Assessing the initiation and completion of adjuvant chemotherapy in a large nationwide and population-based cohort of elderly patients with stage-III colon cancer. Med Oncol. 2011;28:1062–74. doi: 10.1007/s12032-010-9644-7. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with icd-9-cm administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–9. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 24.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ Oncology ASoC. American society of clinical oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 26.Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;73:156–66. doi: 10.1016/j.critrevonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Neugut AI, Subar M, Wilde ET, Stratton S, Brouse CH, Hillyer GC, Grann VR, Hershman DL. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–42. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hershman DL, Tsui J, Meyer J, Glied S, Hillyer GC, Wright JD, Neugut AI. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst. 2014;106(11):1–9. doi: 10.1093/jnci/dju319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299–309. doi: 10.1093/jnci/djr242. [DOI] [PubMed] [Google Scholar]
- 30.Carlson RW, Hudis CA, Pritchard KI. Adjuvant endocrine therapy in hormone receptor-positive postmenopausal breast cancer: evolution of nccn, asco, and st gallen recommendations. J Natl Compr Canc Netw. 2006;4:971–9. doi: 10.6004/jnccn.2006.0082. [DOI] [PubMed] [Google Scholar]
- 31.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–37. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du XL, Key CR, Dickie L, Darling R, Geraci JM, Zhang D. External validation of medicare claims for breast cancer chemotherapy compared with medical chart reviews. Med Care. 2006;44:124–31. doi: 10.1097/01.mlr.0000196978.34283.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du XL, Key CR, Dickie L, Darling R, Delclos GL, Waller K, Zhang D. Information on chemotherapy and hormone therapy from tumor registry had moderate agreement with chart reviews. J Clin Epidemiol. 2006;59:53–60. doi: 10.1016/j.jclinepi.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–62. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 35.Maly RC, Umezawa Y, Ratliff CT, Leake B. Racial/ethnic group differences in treatment decision-making and treatment received among older breast carcinoma patients. Cancer. 2006;106:957–65. doi: 10.1002/cncr.21680. [DOI] [PubMed] [Google Scholar]
- 36.Goff SL, Mazor KM, Meterko V, Dodd K, Sabin J. Patients' beliefs and preferences regarding doctors' medication recommendations. J Gen Intern Med. 2008;23:236–41. doi: 10.1007/s11606-007-0470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonito A, Bann C, Eicheldinger C, Carpenter L. Final report. Rockville, MD: RTI International; 2008. Creation of new race/ethnicity codes and socioeconomic status (ses) indicators for medicare beneficiaries. Sub-task 2. [Google Scholar]