Abstract
This study analyzes the relationships between maternal risk factors present at the time of daughters’ births—namely, young mother, high parity, and short preceding birth interval—and their subsequent adult developmental, reproductive, and socioeconomic outcomes. Pseudo-cohorts are constructed using female respondent data from 189 cross-sectional rounds of Demographic and Health Surveys conducted in 50 developing countries between 1986 and 2013. Generalized linear models are estimated to test the relationships and calculate cohort-level outcome proportions with the systematic elimination of the three maternal risk factors. The simulation exercise for the full sample of 2,546 pseudo-cohorts shows that the combined elimination of risk exposures is associated with lower mean proportions of adult daughters experiencing child mortality, having a small infant at birth, and having a low body mass index. Among sub-Saharan African cohorts, the estimated changes are larger, particularly for years of schooling. The pseudo-cohort approach can enable longitudinal testing of life course hypotheses using large-scale, standardized, repeated cross-sectional data and with considerable resource efficiency.
Keywords: Fertility, Life course, Reproductive health, Cohort analysis, Developing countries
Introduction
Evidence is growing of the effects of maternal factors on fetal programming and consequences for perinatal, neonatal, and infant outcomes and health and well-being in adulthood. The fetal origins of disease hypothesis (Barker 1994) suggests that chronic health disease is linked to fetal development deficits. Fetal programming research has shown that nutrient intake in pregnancy can affect gene expression during the development of the brain structure and other organs and tissues (Barker 1994; Barker et al. 2000; Georgieff 2007). Protein and caloric intake during pregnancy influences infant birth weight, and both low and high birth weight have been linked to adult incidence of noncommunicable diseases (NCDs) (Barker et al. 2000; Shetty and Schmidhuber 2011). From the demographic perspective, the quality of life that contextualizes conditions at birth also enlivens the interpretation and understanding of factors behind variation in life expectancies seen in populations along the poverty-to-wealth continuum.
A woman’s reproductive history influences the survival prospects of her fetus and newborn (Rutstein 2005). Intrauterine conditions have been linked to antecedent factors, such as poor maternal nutritional status, dietary intake, or substance abuse behaviors (Abu-Saad and Fraser 2010). Demographic attention to risky childbearing patterns in developing countries has focused largely on maternal indicators, such as birthing at young or old ages, having closely spaced births, and having many births (e.g., Conde-Agudelo and Belizán 2000; Conde-Agudelo et al. 2012; DaVanzo et al. 2007, 2008; Zimicki 1989). Many of these studies have examined the relationship between these maternal factors and the incidence of infant and child mortality, as well as low birth weight (LBW), preterm births, and births that are small for gestational age. Early childbearing places disproportionate demands on a girl’s physiology to support her own growth and that of her fetus, and can introduce complications during delivery (Gibbs et al. 2012). Older pregnant mothers carry higher risks for congenital anomalies and delivery and postpartum complications (Jolly et al. 2000). Hypertension, diabetes, and kidney or cardiovascular problems are also more common in older pregnant women and can retard fetal growth (Hansen 1986).
Birth complications, such as preeclampsia, prolonged or obstructed labor, uterine rupture, and hemorrhage, are more likely to occur to nulliparous women or grand multiparas and threaten their survival and that of their infants (Shechter et al. 2010). Maternal depletion syndrome—defined as cumulative nutritional deficiencies or negative energy balance—experienced by mothers with too frequent reproductive cycling has been linked to higher infant mortality and child undernutrition (e.g., Christian 2010a; Nenko and Jasienska 2009; Winikoff 1988).
The duration of time between pregnancies defines the interpregnancy interval. In settings where the estimation of gestational ages may not be accurate, researchers have accepted the interval between reported dates of live births as an alternative measure. Within the interbirth interval, events (such as fetal loss, infections, and partner violence) and behaviors (such as sexual abstinence and physical activity) can affect maternal and newborn health. Thus, although a key demographic indicator, the birth interval is actually a fairly crude measure of the probability of a healthy conception.
Short birth intervals, usually defined as fewer than 18 months between live births, are indicative of conceptions occurring within nine or fewer months since the preceding birth, assuming a normal gestation length of nine months. Short birth intervals are associated with LBW and can reflect premature weaning of the preceding child, raising its risk of morbidity and mortality given that the new sibling will be given priority for breast-feeding (Adam et al. 2009). At the other end of the distribution, long intervals, usually defined to be in excess of 60 months, have also been associated with increased infant mortality risk, possibly resulting from macrosomia or prolonged inactivity (physiological regression) of the reproductive system (Conde-Agudelo et al. 2012). The birth interval for the first birth, typically defined as the interval between marriage and birth, is excluded in this study because it incurs a different risk than the interval between later births (Mahy 2003; Nenko and Jasienska 2013).
For a given woman, the three risk factors of maternal age, parity, and birth intervals are intercorrelated. A high-parity birth frequently means an older maternal age and shorter intervals between births. Efforts to model and isolate the individual effects of these three maternal factors are complicated by their overlapping distributions. An in-depth understanding of the underlying physiological and behavioral mechanisms and processes that affect these outcomes remains needed.
Fertility research has shown that a healthy pregnancy is a strong determinant of maternal and newborn survival and health across the life span: optimal early-life conditions, such as healthy fetal growth and development, nutrient intake, and sufficient time for maternal repletion through birth spacing, are considered protective of newborn health, particularly for females. Daughters born under optimal birth conditions are more likely to have strong immune systems and physically develop to withstand the demands of single and repeated pregnancies and breast-feeding in adulthood. Also, girls who are adequately nourished through their teen years experience improved fecundity and are less susceptible to infection, anemia, and adverse reproductive outcomes (Chan, Tsoulis and Sloboda 2015). Epigenetic studies have charted human responses to variations in diet, stress, or toxins in pregnancy that are linked to glucose metabolism and later incidence of adult obesity and diabetes (Wang et al. 2013).
The early childhood development literature has drawn attention to linkages between healthy fetal growth and the pace of cognitive development (Shonkoff and Phillips 2000). In the arc of human development over the life span, it is increasingly important to recognize the effects of healthy fetal origins on adult functioning and productivity. Cognitive abilities nurtured in childhood but often compromised by conditions of poverty (Mani et al. 2013; Vohs 2013) are linked to school performance and educational attainment levels. The latter, in turn, confer economic and social advantages in adulthood and to subsequent generations.
The underlying physiological and cognitive changes during pregnancy, infancy, and child growth and development are not explicitly measured in population survey data. However, birth histories collected in population surveys, particularly in low-income countries, can be used to study relationships between demographic markers, such as maternal factors at birth, and reproductive, health, and socioeconomic outcomes. The evidence base for national populations in low-income countries has been limited, though, with heavy reliance on the cross-sectional surveys, such as those sponsored through the Demographic and Health Surveys (DHS) and UNICEF Multiple Indicator Cluster Survey (MICS) programs. This article seeks to address the absence of a longitudinal perspective on health and development across the life span in low-resource settings by pursuing a pseudo-cohort construction approach to link experiences at birth for females to those of their cohort counterparts in adulthood. Although not as satisfying as following a nationally representative sample of individuals, studying birth cohorts linked over time can offer a deeper appreciation of important early-life exposures and subsequent adult well-being, especially in low-income settings.
Study Objective
This study assesses the relationship between three key maternal factors present at the time of daughters’ births and their reproductive, health, and socioeconomic outcomes in adulthood, using constructed cohorts as units of analysis. The three maternal factors of interest are the daughter’s (1) birth within a short interval (fewer than 18 months since the preceding sibling), (2) birth to a young mother (under age 18), and (3) birth at parity 4 or higher. The influence of older maternal age (above 35 years) has also been considered but subsequently excluded due to multicollinearity with high parity and young maternal age.1 The respondent sample providing birth histories for cohort construction is women with at least one birth in a 15-year period before the survey.
Adult developmental outcomes are measured at the time of the survey for female respondents aged 15 to 49; of interest are (1) short stature or height (<145cm) and (2) poor nutritional status based on the woman’s body mass index (BMI <18.5). Short stature generally reflects acute stunting and chronic nutritional deficiencies or low intake in childhood (Tzioumis and Adair 2014). Short stature and acute underweight status have been found to predict poor pregnancy outcomes (Christian 2010b; WHO 1997) and LBW (Witter and Luke 1991). The reproductive outcomes of interest are (1) the adult female’s experience with child mortality and (2) her self-reported delivery of a “very small” or “small” infant, taken as a proxy measure of LBW. The significance of these outcomes is reinforced by studies such as that by Nordtveit et al. (2009), which examined intergenerational associations in birth weight for mothers and daughters by parity order, and by Chadio and Kotsampasi (2014), which reviewed possible early-life nutritional deficits that influence programming of adult reproductive function.
Also of interest are two socioeconomic outcomes for adult daughters: (1) achieved years of schooling and (2) current paid work status. These outcomes indirectly reflect cognitive development and the ability to work productively for remuneration tied to having a healthy start in childhood and capitalize on the limited socioeconomic data collected in the DHS over time. Our hypotheses are that cohorts of daughters whose mothers have higher levels of the three maternal risk factors will have proportionally worse adult developmental, reproductive, and socioeconomic outcomes than cohorts of daughters whose mothers have lower risk proportions. The lifespan connections between early exposure to potentially impairing events and later adult health and well-being have been investigated in high-income settings but much less frequently in low-income settings because of the limited availability of longitudinal data covering large populations.
Source of Data and Measurement
Data from all DHS surveys with at least two survey rounds in the period 1985–2013 (189 rounds total) are included in the analysis, resulting in a sample of 50 developing countries: 9 in Central, South, and Southeast Asia; 9 in Latin America and the Caribbean; 5 in North Africa/West Asia/Eastern Europe; and 27 in sub-Saharan Africa (SSA).
Longitudinal Insights from Cross-Sectional National Surveys
For many developing countries, DHS surveys are one of the few reliable sources of population-level information about maternal and child health. Because the DHS data are cross-sectional, analyses of these data have been of limited utility for making causal inferences about relationships between early event exposures and later life course conditions. Our understanding of the influences of these risk factors at the population level has been confined primarily to cross-sectional associations with individual-level data and some longitudinal analyses of subnational data with limited generalizability. At the same time, the volume of analytic work on cross-national surveys (such as the DHS, the MICS, or the World Bank Living Standard Measurement Surveys) continues to grow (Fabic et al. 2012), while, with few exceptions (Hallett et al. 2010; Yount et al. 2014), the frequency of exploiting multiple rounds of survey data for longitudinal insights remains low. With the number of countries having a long time series of survey rounds increasing each year, a panel investigation into the effects of maternal covariates on reproductive, child, or adult health and social outcomes becomes possible and promising of new insights.
Constructing and Linking Birth Cohorts
Unlike traditional panel data in which individuals are followed and repeatedly measured, we construct birth cohorts for members born in the same year and follow them across time. The 15-year period before the first available DHS survey in a given country defines the birth years for the cohorts.2 These 15 single-year birth cohorts are then linked to their 15 counterpart cohorts in the subsequent survey round for the same country.
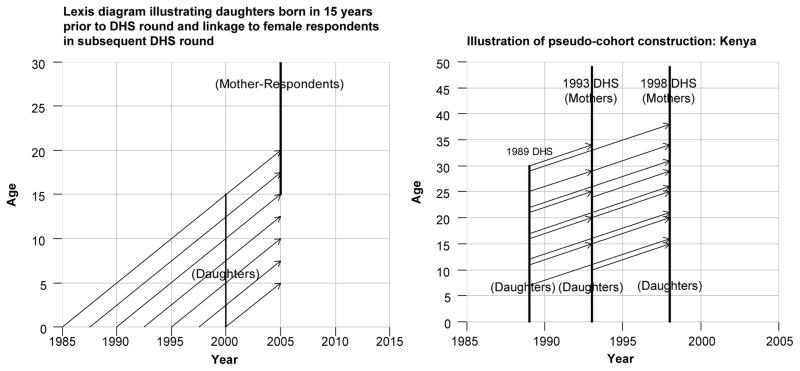
Panel a of Fig. 1 shows that daughters born between 1985 and 2000 are 0–15 years of age in an illustrative 2000 survey round (diagonal lines up to the lower vertical line). The sample of those aged 10–15 years relates age-wise to the sample of female respondents aged 15–20 in the 2005 survey round (upper vertical line). Panel b of Fig. 1 illustrates the cohort linkage with three DHS rounds conducted in Kenya in 1989, 1993, and 1998. The daughters aged 10–15 reported by mothers interviewed in 1989 are linked to their cohort counterparts aged 14–19 in the 1993 survey. Maternal risk and other individual covariates are measured at the time of their birth (1984 to 1989), while their outcomes are those subsequently reported by female survey respondents aged 14–19 in the 1993 survey round.
Fig. 1.
Illustration of birth cohort construction and linkage across DHS rounds
This cohort approach—sometimes called a pseudo-cohort approach—has been used in labor economics and educational achievement studies (e.g., Deaton 1985; McIntosh 2005) but has been used infrequently in public health investigations. Researchers (e.g., Moffitt 1993; Verbeek and Nijman 1992) have suggested that cross-sectional data constructed as cohorts can be treated as genuine panel data, with some limitations. If these limitations can be addressed, analyses with constructed cohorts can expand our understanding of intergenerational change, such as the effects of family environments on child development, educational achievement, and employment prospects that otherwise are unavailable in low- and middle-income countries (LMICs) given the paucity of large-scale, longitudinal population data.
Potential Sources of Bias
Cohorts are then the units of analysis, and cohort-level means or averages are calculated from data on individual members of a cohort defined, in our case, by the year of birth. Cohorts constructed from survey samples of individuals, however, are subject to measurement error. Whereas covariates and outcomes for a panel of individuals followed longitudinally are observed repeatedly over time, with constructed cohorts, one must rely on cohort means or averages. The accuracy of the summary statistics for aggregate units depends on the underlying coverage and size of the survey sample. The obtained cohort averages, however, are consistent, unbiased estimates of the true population cohort means, with the difference resulting from sampling error. Studies have shown that such measurement error is negligible, however, when the cohort size is sufficiently large (Verbeek and Nijman 1992). In this analysis, we use means based on only those cohorts with 100 or more members, as recommended by Verbeek and Nijman (1992). We conducted sensitivity tests using cohort member sizes of 80 and 120, and observed nominal differences.
Another potential limitation of pseudo-cohorts is truncation bias—in our case, the upper age limit of 49 years for female survey respondents. To track a cohort of daughters from birth through their reproductive lifespans requires that countries have a long series of DHS rounds to allow for sufficient exposure time to observe cohort reproductive behavior. Ten countries in our sample allow for observation of adult outcomes to ages 35–38 years for daughters born in the 15 years before the base survey. The average interval across the 189 surveys is 10 years, permitting observation largely over cohorts’ prime childbearing ages of 15–25 years. To address potential truncation bias, our model specifications include the cohort’s mean age of daughters at the time of the measured outcome. This approach enables us to assess the proportional impact of the represented ages on the outcomes, even though, relatively speaking, older women may be excluded. We also have constrained cohort members to be aged 15 or older at the time of the assessed adult outcome. We further tested for truncation bias by restricting the mean ages of the cohorts to 20–35 years and comparing the model coefficients from the two sets. The results were similar, suggesting that the effects of truncation were minimal.
A third possible limitation is that the births for whom exposure to maternal risk factors are based do not perfectly represent their birth cohort in terms of maternal ages. For example, children born in 1985 to mothers older than 45 years will not be reported in a 1990 survey that interviews women aged 15–49. Because older mothers contribute relatively fewer births, our model estimates will not be biased given that they include maternal age, which completely determines the birth’s eligibility to be reported. As noted earlier, our analyses are restricted to cohorts aged 15 or younger at the time of assessing risk factors at birth, which empirically alleviates the representativeness problem.
Other limitations with cohorts constructed from national population surveys include differential mortality and international out-migration. Females who are acutely compromised in infancy may not survive to be represented by their age peers in subsequent surveys. If they are observed, these females may be severely undernourished and more likely to experience adverse pregnancy outcomes. The bias from unobserved factors associated with those who would have died, though, can be addressed by weighting the data using a survival probability distribution (Deaton 1985).
On the other hand, the life course experiences of females who permanently move out of country for work or other reasons will not be captured by their cohort peers present in subsequent survey rounds. The potential bias from out-migration for most countries is likely to be small. Because of the lack of high-quality data on migration, we cannot adjust for this type of bias.
Last, recall bias will be present in some measures, irrespective of the pseudo-cohort approach. Female respondents’ retrospective recall of their reproductive events used to measure maternal risks may not be accurate or complete. Of the three maternal risk factors, maternal age and parity are expected to suffer less recall bias compared with dates of live births, which may be less accurate, especially for births occurring more than 10 years earlier. We assume in this study that recall bias is random and somewhat mitigated with the use of cohort averages.
Cohort Measures
The construction of linked cohort measures requires first characterizing individual records, aggregating them at the cohort level by birth year, and linking birth cohorts across survey rounds. To begin, risk factors at a daughter’s birth reference the conditions reported by the mother in her birth history recorded in her base survey interview. The mother’s other characteristics, such as her education level and place of residence, are similarly captured. Adult nutritional status and reproductive outcomes of interest are obtained from the female respondents in subsequent surveys. For example, a 39-year-old female respondent in a 1998 survey may report her fifth child to be a daughter born in 1984, when the mother was aged 25 and 30 months after her prior birth. In 1998, the index daughter was 14 years old, and we classify her as at risk as a high-parity birth but not at risk in terms of young maternal age or short birth interval. The cohort data for the daughter’s birth year of 1984 is then aligned with same birth-year cohort of female respondents in the 2003 DHS (who are age 19) and associated with the latter’s adult outcomes. That is, the measured height and weight, birth history, LBW and child mortality experience, educational level, and paid work status of those 19-year-old adult females are aggregated to calculate cohort means and are linked to the maternal risk conditions for the cohort of daughters born in 1984.
After limiting the size of pseudo-cohorts to a minimum of 100 females to increase accuracy of the cohort aggregate measures (as discussed in a later section), we arrive at an analysis sample of 2,546 single-year birth cohorts across 189 DHS surveys for 50 countries. The analysis is based on information from a total of 2,422,937 female respondents.
Analytic Framework
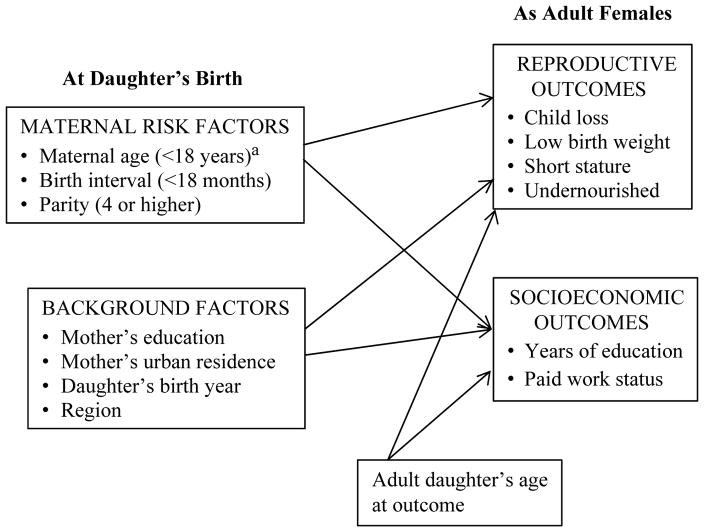
The study’s analytic framework is shown in Fig. 2, wherein the early exposure conditions at birth, along with maternal background characteristics, are hypothesized to influence later adult reproductive and socioeconomic outcomes, and are tested with cohorts as units of analysis. The adult daughter’s age at the time of the measured outcome is included as a control variable. In our effort to focus on daughters’ early-life conditions, we defer from including more measures of adult daughters’ characteristics to establish the utility of the pseudo-cohort approach and its substantive relevance. We are also constrained by the number of continuously measured variables across DHS rounds; for example, maternal anthropometry and household wealth are recently added measures.
Fig. 2.
Analytic framework, with birth cohorts as units of analysis. aMaternal age >34 years not specified due to multicollinearity with other risk factors
Analytic Approach
Of the eight outcomes investigated in the present study, adult years of schooling are continuously measured; the other seven are cohort proportions. A linear model estimated with ordinary least squares (OLS) method is used for years of schooling. Accordingly, its coefficient is simply the expected change in years of schooling for a one-unit change in the covariate while holding the other covariates fixed. The linear model, however, is inappropriate for cohort proportions given their bounded nature because the model can generate predictions below 0 or above 1. A typical way to handle the bounded nature of the outcome is to use the logit transformation, under the assumption that the outcome values are strictly within the unit interval. That assumption does not always hold given that 0 and 1 are plausible outcome values. For example, if all female respondents in a particular cohort are taller than 145cm, the proportion of short-statured women will be 0. In this study, we applied the modeling strategy of Papke and Wooldridge (1996). Their functional form is based on both the logit link function in the generalized linear regression and the binomial distribution. In the model’s equation (Eq. (1)), y is the cohort proportion; Xn and βn are the vectors of hypothesized covariates and coefficients to be estimated.
| (1) |
The model is estimated using the Bernoulli quasi-maximum likelihood estimation method. The estimator is consistent, asymptotically normal, and efficient. All variance estimates for the regression coefficients are obtained by robust variance estimation to adjust for correlated observations (e.g., multiple observations of the same birth cohort) within countries. All models are estimated using Stata 13 (StataCorp 2013).
To interpret the coefficients in models for cohort proportions, we assess change in ln(E(y|x)/1–E(y|x)) given a change in xi:
| (2) |
Thus, βi is the expected change in the natural log of the odds of E(y|x) for a one-unit change in xi while other covariates are held fixed.
Equation (2) shows that the impact of a one-unit change in a model covariate x on the outcome y will vary with their values. To facilitate interpretation of the generalized linear model (GLM) coefficients for the cohort proportions, we calculate and present the average marginal effects (AMEs), which estimate the mean marginal effect for a population given a one-unit change in the covariate x. The standard errors are computed using the delta method (Long 1997).
We first estimate the outcomes models for the full sample of cohorts, including the four regions as dummy variables, and then separately for the SSA cohorts. The six reproductive and two socioeconomic outcomes are defined in Table 1. Child mortality before age 5 is measured both as the cohort proportion of recent births (in the past three years) to mothers and as a proportion of all mothers in the cohort ever experiencing loss of a child under age 5. Low birth weight is based on self-reports by mothers that their infants were born very small or small and is similarly measured as a cohort proportion of all recent births and of mothers. The cohort sample with birth size information is 2,101 for all countries and 1,169 for SSA. Studies comparing maternal reports of infant birth weight with those recorded during health facility deliveries suggest the former are biased upward, which would lead to lower prevalence of LBW infants (Blanc and Wardlaw 2005; Robles and Goldman 1999). If this bias exists across the 50 developing countries, our models will underestimate the association between maternal risk factors at daughters’ birth and their LBW experiences and will serve as a more conservative test than in the absence of recall bias.
Table 1.
Definitions of cohort measures of reproductive and socioeconomic outcomes
| Cohort-Level Measure | Definition |
|---|---|
| Reproductive/Developmental Outcome | |
| Children who died before age 5 | Proportion of births to women in cohort reported to have died before age 5 |
| Mothers ever losing a child before age 5 | Proportion of mothers in cohort who ever report losing a child before age 5 |
| Children reported to be born small | Proportion of births in the three years preceding the survey that are reported by mothers to have been born very small or small |
| Mothers ever reporting a baby born small | Proportion of mothers reporting a recent birth (in the three years before the survey) born very small or small |
| Adult daughters with BMI <18.5 | Proportion of adult female respondents with measured to have a BMI of 18.5 or lower (indicating undernutrition) |
| Adult daughters with height <145cm | Proportion of adult female respondents with height measured to be under 145cm (indicating stunting) |
| Socioeconomic Outcome | |
| Adult daughter’s average years of education | Average years of education of adult female respondents |
| Adult daughters with paid work | Proportion of adult female respondents who currently have paid employment (in cash or kind) |
| Maternal Risk Factors | |
| Maternal age <18 at daughter’s birth | Proportion of daughters born to mothers under age 18 |
| Daughter born parity 4 or higher | Proportion of daughters born at parity 4 or higher |
| Daughter born within 18 months of preceding birth | Proportion of daughters born within 18 months of a preceding birth |
| Covariates | |
| Maternal education | Proportion of daughters born to mothers with any schooling |
| Maternal residence in urban area | Proportion of daughters born to mothers living in urban areas at the time of the survey |
| Birth year of daughter’s cohort | Year of birth for daughter |
| Daughter’s age at outcome survey round | Average age of daughters at the time (survey year) when reproductive/socioeconomic outcome is measured |
Because anthropometry-based outcomes, such as short stature and low BMI, are not available in the earliest surveys, the analytic samples for these models are smaller: 1,985 for all countries, and 1,153 for those in SSA. Widely used cutoff values for short maternal height are below 145–150cm (Christian 2010b; WHO 1997; Witter and Luke 1991) and for undernutrition a BMI <18.5 (Black et al. 2013). For the current paid work outcome, the cohort sample is slightly reduced because of the late introduction of DHS data collection on payment for work: 2,043 total and 1,093 SSA cohorts. We estimated the models for the samples of cohorts as described and for a sample of cohorts with no missing measures. The signs and values of the coefficients for the latter do not change appreciably, but sample composition is heavily weighted to those from the SSA region because of the anthropometry measures included later in the DHS program. To ensure a broad range of development influences, we present the model results based on cohort samples with available measures.
To facilitate interpretation of the models’ results, we conduct post-estimation simulations to calculate the cohort proportions for each outcome that results from alternately eliminating each of the three maternal risk factors. We estimate the model-based outcome proportions if no daughters were born to mothers at young maternal ages (<18 years), at high parity (≥4), or within 18 months of the preceding birth. We then estimate the outcome proportions if all three risk factors are eliminated. The assumption of their independence is more difficult to defend if the analysis is conducted at the individual level; at the cohort level, the mean values for these factors are not highly correlated.
We last compare the estimated risk-eliminated proportions by cohort birth year and by age at outcome survey separately against the observed values. These differences between cohort- and age-specific proportions or averages with the observed levels reveal trends in the benefits for the eight health and socioeconomic outcomes that potentially can be gained with the elimination of the maternal risk factors.
Results
The values of the means, standard deviations and ranges for the maternal risk factors, other covariates, and outcome variables of interest are provided in Table 2. The mean proportion of children dying before age 5 is 79 per 1,000 live births (0.079) across all cohorts and 100 per 1,000 live births for SSA cohorts. The estimates are plausible: the 2013 child mortality rate was 50 per 1,000 births in less-developed countries and 93 per 1,000 births for SSA (UNICEF 2014). Based on proportions of mothers, the experience of ever losing a child under age 5 averages 12.5 % across all cohorts of mothers and 16.6 % for SSA cohorts. The measure based on infants reported to be born small has a cohort average of 17.0 % for the full sample and 15.7 % for the SSA cohorts. The average percentage of mothers reporting a small infant at birth is 22.9 % for all and 22.3 % for SSA cohorts, respectively. For adult health outcomes, mean percentages for undernourished and short-stature/stunted daughters for all cohorts are 11.3 % and 4.2 %, respectively; SSA averages are slightly higher at 12.1 % undernourished and lower at 2.2 % for short stature. Average cohort years of schooling among adult daughters are 6.42 for the total sample and 5.21 for SSA. The percentage having paid work in SSA is 31.7 %, higher than the total of 28.6 %.
Table 2.
Means, standard deviations (SD), and minimum/maximum values of cohort proportions on reproductive health and socioeconomic outcomes for all regions and the sub-Saharan African (SSA) region
| Cohort Measure | All Regions (n = 2,546)
|
SSA (n = 1,386)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min. | Max. | Mean | SD | Min. | Max. | |
| Reproductive/Developmental Outcome | ||||||||
| Proportion of children who died before age 5 | .079 | .046 | .000 | .300 | .100 | .047 | .000 | .300 |
| Proportion of mothers who report ever losing a child by age 5 | .125 | .093 | .000 | .597 | .166 | .103 | .000 | .597 |
| Proportion of children reported born small | .170 | .089 | .000 | .750 | .157 | .081 | .016 | .500 |
| Proportion of mothers ever having a small baby | .229 | .086 | .000 | .750 | .223 | .078 | .050 | .556 |
| Proportion of adult daughters with BMI <18.5 | .113 | .092 | .000 | .571 | .121 | .069 | .000 | .472 |
| Proportion of adult daughters with height <145cm | .042 | .054 | .000 | .464 | .022 | .023 | .000 | .212 |
| Socioeconomic Outcome | ||||||||
| Adult daughter’s average years of education | 6.419 | 2.506 | 0.712 | 12.590 | 5.205 | 2.177 | 0.712 | 9.813 |
| Proportion of adult daughters with paid work | .286 | .160 | .004 | .839 | .317 | .156 | .013 | .839 |
| Maternal Risk Factors | ||||||||
| Proportion of daughters born to mothers under age 18 | .097 | .044 | .000 | .256 | .108 | .039 | .000 | .235 |
| Proportion of daughters born at parity 4 or higher | .425 | .076 | .064 | .619 | .454 | .054 | .271 | .584 |
| Proportion of daughters born within 18 months of preceding birth | .156 | .064 | .018 | .431 | .125 | .042 | .018 | .293 |
| Covariates | ||||||||
| Proportion of daughters born to mothers with any schooling | .555 | .263 | .065 | 1.000 | .465 | .242 | .065 | .983 |
| Proportion of daughters born to mothers living in urban areas at time of survey | .349 | .165 | .074 | .849 | .278 | .107 | .086 | .679 |
| Year of birth for daughter | 1983.7 | 5.4 | 1971.0 | 1996.0 | 1984.0 | 5.3 | 1971.0 | 1995.0 |
| Average age of daughter at time of outcome measurement | 21.76 | 4.66 | 16.00 | 39.00 | 21.85 | 4.77 | 16.00 | 38.00 |
| Proportion of cohorts in region | ||||||||
| North Africa/West Asia/Eastern Europe (235) | .092 | |||||||
| Latin America and Caribbean (ref.) (487) | .191 | |||||||
| Central, South, and Southeast Asia (438) | .172 | |||||||
| Sub-Saharan Africa (1,386) | .544 | |||||||
The mean cohort percentage of daughters born to mothers under 18 years of age averages 9.7 % overall and 10.8 % for the SSA region. The average cohort percentage of daughters born at parity 4 or higher is 42.5 % for all cohorts and 45.4 % for SSA cohorts. The percentage of daughters born within 18 months of their preceding siblings averages 15.6 % and 12.5 % across all and SSA cohorts, respectively.
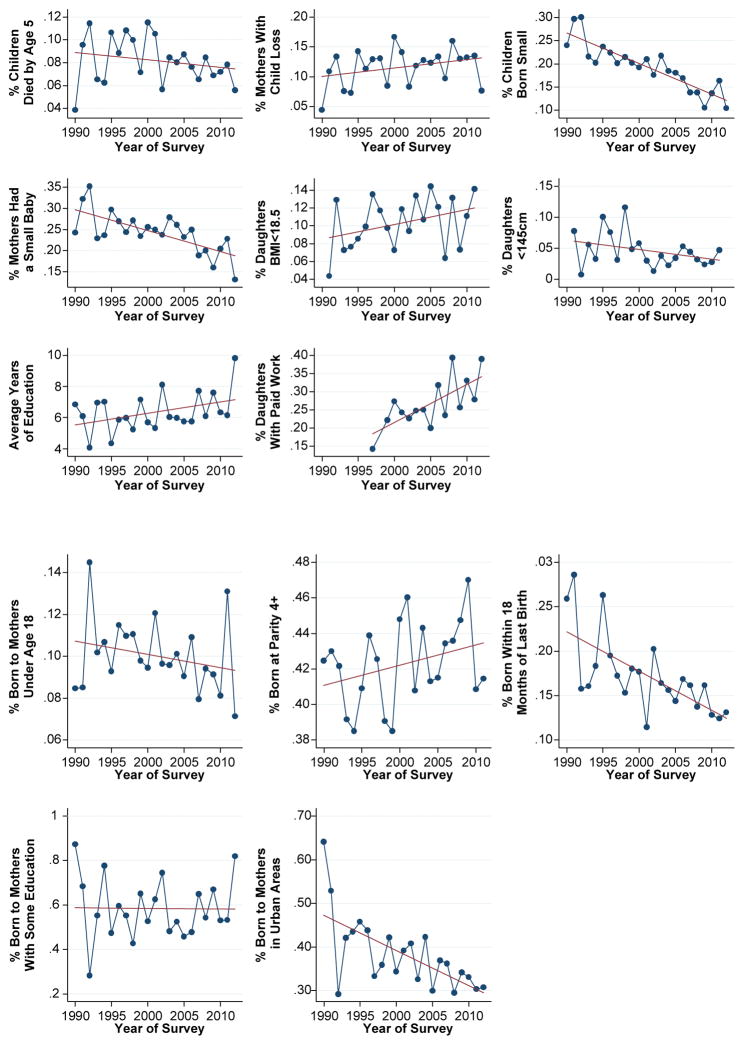
Figure 3 helps visualize the trends in the cohort proportions for the eight health and socioeconomic outcomes, the three reproductive risk factors, and the two control variables by year of the survey capturing the measurement. Trend lines suggest improvements in risk factor exposures and the proportions and averages of outcomes. However, these values are conditioned by the composition of cohorts in the sample. Those with unexpected trends (e.g., cohort proportion of mothers experiencing child loss and low BMI, and proportions of births born at high parity) likely reflect the increasing share of the cohort sample originating from DHS surveys conducted in SSA countries.
Fig. 3.
Means and average proportions of constructed cohorts (n = 2,546) for health and socioeconomic outcomes and maternal risk factors, by year of survey
Exploratory data analysis has not raised concerns about distributions of the outcomes or covariates or possible violation of the assumptions for OLS and GLM estimation. The linear and GLM regression results are provided for all cohorts in Table 3 and for SSA cohorts in Table 4. We observe varying support of the hypothesized relationships between cohort proportions of early risk exposures with cohort experiences of adverse health outcomes (columns 1–6 in each table) and socioeconomic outcomes in adulthood (columns 7 and 8).
Table 3.
Results of generalized linear model estimation of cohort proportions for reproductive and socioeconomic outcomes in 50 developing countries regressed on maternal risk factors, maternal attributes, and region
| Covariate | Proportion of Children Died Before Age 5 | Proportion of Mothers Who Report Ever Losing a Child Before Age 5 | Proportion of Births Reported Born Small | Proportion of Mothers Reporting Ever Having a Small Baby | Proportion of Adult Daughters With BMI < 18.5 | Proportion of Adult Daughters With Height < 145cm | Average Years of Education for Adult Daughters | Proportion of Adult Daughters Currently With Paid Work |
|---|---|---|---|---|---|---|---|---|
| Maternal Age <18 | 2.022** (0.707) | 3.215*** (0.792) | −1.004 (0.687) | −0.121 (0.926) | 2.303† (1.239) | 2.108 (1.280) | −2.194 (2.596) | −2.536† (1.466) |
| Born in Parity ≥4 | 0.938† (0.486) | 1.212* (0.538) | 0.528 (0.419) | 0.656 (0.432) | 0.145 (0.883) | 0.188 (1.685) | 1.585 (1.777) | 0.072 (0.731) |
| Birth Interval <18 months | 0.697 (0.714) | 1.365 (0.836) | 0.900† (0.501) | 1.473* (0.604) | 0.646 (1.291) | 0.411 (2.032) | −3.630 (2.649) | 0.495 (1.114) |
| Maternal Education | −0.698** (0.249) | −0.819** (0.301) | −0.600** (0.177) | −0.769** (0.222) | −0.822* (0.347) | 0.549 (0.604) | 7.426*** (0.677) | 0.189 (0.263) |
| Maternal Residence in Urban Area | −0.037 (0.330) | −0.053 (0.401) | 0.181 (0.286) | 0.303 (0.354) | −0.091 (0.556) | −2.290 (1.459) | −0.337 (1.174) | 0.676 (0.408) |
| Birth Year of the Cohort | −0.026*** (0.005) | −0.023*** (0.006) | −0.002 (0.006) | −0.001 (0.006) | 0.013 (0.010) | −0.041† (0.024) | 0.044† (0.026) | 0.003 (0.015) |
| Age of Cohort at Outcome Survey | −0.021*** (0.005) | 0.055*** (0.007) | −0.125** (0.005) | −0.048*** (0.007) | −0.059*** (0.011) | −0.054** (0.017) | 0.085** (0.026) | 0.107*** (0.013) |
| Region (ref. = Latin America and Caribbean) | ||||||||
| North Africa/West Asia/Europe | −0.377* (0.175) | −0.499* (0.193) | −0.412* (0.173) | −0.518** (0.171) | −0.969† (0.541) | −2.192*** (0.379) | 1.070† (0.565) | − 1.781*** (0.243) |
| Central Asia/South and Southeast Asia | 0.258 (0.175) | 0.086 (0.183) | −0.230† (0.123) | −0.324* (0.141) | 1.313** (0.431) | −0.029 (0.841) | −0.214 (0.469) | −0.278† (0.141) |
| Sub-Saharan Africa | 0.552** (0.179) | 0.625*** (0.195) | −0.388** (0.127) | −0.320* (0.129) | 0.543 (0.385) | −1.701* (0.658) | −1.459** (0.443) | 0.241† (0.141) |
| Constant | 48.833*** (10.043) | 41.731*** (11.821) | 4.575 (11.674) | 2.864 (12.691) | −26.502 (20.441) | 80.174 (48.203) | −86.436 (51.859) | −9.338 (30.746) |
| Cohort Observations | 2,542 | 2,542 | 2,101 | 2,101 | 1,985 | 1,985 | 2,546 | 2,043 |
Note: Regression coefficients are shown, with standard errors in parentheses.
p < .10;
p < .05;
p < .01;
p < .001
Table 4.
Results of generalized linear model estimation of cohort proportions for reproductive and socioeconomic outcomes in 27 sub-Saharan African countries regressed on maternal risk factors and attributes
| Covariate | Proportion of Children Died Before Age 5 | Proportion of Mothers Who Report Ever Losing a Child Before Age 5 | Proportion of Births Reported Born Small | Proportion of Mothers Reporting Ever Having a Small Baby | Proportion of Adult Daughters With BMI <18.5 | Proportion of Adult Daughters With Height <145cm | Average Years of Education for Adult Daughters | Proportion of Adult Daughters Currently With Paid Work |
|---|---|---|---|---|---|---|---|---|
| Maternal Age <18 | 2.760** (0.787) | 3.842** (1.089) | 0.025 (0.884) | 1.482 (1.134) | 0.581 (2.109) | −1.752 (2.405) | −0.167 (4.286) | 0.136 (2.272) |
| Born in Parity ≥4 | 1.233* (0.512) | 1.904** (0.681) | 0.709† (0.348) | 1.324** (0.440) | 0.813 (0.834) | 0.138 (0.989) | −2.184 (1.731) | 0.594 (1.363) |
| Birth Interval <18 months | 1.509 (0.914) | 2.202† (1.100) | 1.275 (0.807) | 1.711† (0.907) | 1.570 (1.796) | 4.569 (3.261) | −7.436† (4.010) | 0.912 (1.640) |
| Maternal Education | −0.480 (0.292) | −0.517 (0.348) | −0.462† (0.234) | −0.583* (0.283) | −0.859* (0.379) | 0.955 (0.669) | 7.401*** (0.767) | 0.111 (0.326) |
| Maternal Residence in Urban Area | 0.393 (0.285) | 0.483 (0.402) | −0.013 (0.346) | 0.070 (0.401) | −0.127 (0.628) | −0.894 (1.051) | −2.109 (1.295) | 0.570 (0.520) |
| Birth Year of the Cohort | −0.027*** (0.006) | −0.023** (0.007) | −0.003 (0.008) | −0.001 (0.008) | 0.008 (0.010) | 0.009 (0.017) | −0.026 (0.020) | −0.004 (0.020) |
| Age of Cohort at Outcome Survey | −0.017* (0.007) | 0.068*** (0.008) | −0.120*** (0.007) | −0.031*** (0.008) | −0.054*** (0.013) | −0.052** (0.019) | −0.009 (0.019) | 0.095*** (0.019) |
| Constant | 51.763*** (11.408) | 41.544** (14.039) | 5.631 (15.837) | 0.547 (16.906) | −17.291 (20.079) | −20.999 (33.527) | 55.083 −40.041 |
3.494 (39.618) |
| Cohort Observations | 1,386 | 1,386 | 1,169 | 1,169 | 1,153 | 1,153 | 1,386 | 1,093 |
Note: Regression coefficients are shown, with standard errors in parentheses.
p < .10;
p < .05;
p < .01;
p < .001
The average marginal effects (AMEs), based on converting the coefficients in Tables 3 and 4, are presented in Table 5. The p values of the GLM coefficients and those of the AMEs are not necessarily the same given that the two are conceptually different measures and calculated under different assumptions (Greene 2011; Long 1997). The interpretation of the AMEs is similar to those for coefficients in a linear regression model and assumes that other covariates are fixed. For example, the AME for cohort levels of being born to a young mother on their adult proportions of children dying before age 5 is a 0.145 percentage point increase in the proportion of their births dying before age 5. A 10 percentage point reduction in proportion of being born to a young mother implies a 1.45 percentage point decrease, or a 14.5 point decrease, in the under-5 mortality rate (e.g., rising from 79 to 94 deaths per 1,000 births). This approximates the child mortality experience for Southern Asia between 1995 and 2005 estimated by the United Nations (2015).
Table 5.
Average marginal effects from generalized linear model estimation of cohort proportions for reproductive and socioeconomic outcomes in 50 developing countries regressed on maternal risk factors and education
| Covariates | Proportion of Children Died Before Age 5 | Proportion of Mothers Who Report Ever Losing a Child Before Age 5 | Proportion of Births Reported Born Small | Proportion of Mothers Reporting Ever Having a Small Baby | Proportion of Adult Daughters With BMI <18.5 | Proportion of Adult Daughters With Height <145cm | Proportion of Adult Daughters Currently With Paid Work |
|---|---|---|---|---|---|---|---|
| All Regions | |||||||
| Maternal age <18 | 0.145** (0.050) | 0.334*** (0.083) | −0.136 (0.093) | −0.021 (0.161) | 0.219† (0.116) | 0.081 (0.050) | −0.476† (0.275) |
| Born in parity ≥4 | 0.067† (0.036) | 0.126* (0.058) | 0.072 (0.057) | 0.114 (0.076) | 0.014 (0.084) | 0.007 (0.064) | 0.014 (0.137) |
| Birth interval <18 months | 0.050 (0.050) | 0.142† (0.085) | 0.122† (0.068) | 0.256* (0.106) | 0.061 (0.123) | 0.016 (0.078) | 0.093 (0.209) |
| Maternal education | −0.050** (0.019) | −0.085** (0.033) | −0.081** (0.025) | −0.134** (0.039) | −0.078* (0.034) | 0.021 (0.023) | 0.035 (0.049) |
| SSA Only | |||||||
| Maternal age <18 | 0.248*** (0.069) | 0.507*** (0.145) | 0.003 (0.114) | 0.255 (0.200) | 0.061 (0.221) | −0.038 (0.051) | 0.028 (0.468) |
| Born in parity ≥4 | 0.111* (0.049) | 0.251** (0.095) | 0.091* (0.046) | 0.228** (0.081) | 0.085 (0.088) | 0.003 (0.021) | 0.122 (0.280) |
| Birth interval <18 months | 0.135† (0.080) | 0.291* (0.142) | 0.164 (0.102) | 0.294 (0.153) | 0.165 (0.194) | 0.098 (0.075) | 0.188 (0.336) |
| Maternal education | −0.043 (0.028) | −0.068 (0.047) | −0.059† (0.031) | −0.100* (0.050) | −0.090* (0.041) | 0.021 (0.016) | 0.023 (0.067) |
Note: Average marginal effects are shown, with standard errors in parentheses.
p < .10;
p < .05;
p < .01;
p < .001
We discuss the model results primarily using the AMEs rather than coefficients, except for the outcome of years of schooling, which is directly interpretable. The influences of the three maternal risk factors in the full sample of cohorts are seen in the first three rows of Table 5, where each is statistically significant with one or more of the reproductive outcomes at p < .05 or better. The AMEs for young maternal age are sizable, positive, and significant for child loss (0.145 as a percentage of births and 0.334 as a percentage of mothers) and low BMI (0.219) at p < .10 or better. Young maternal age is also substantially and negatively associated with a proportion of daughters with paid work, the AME being −0.476 (p < .10).
Although positively related with all cohort proportions for poor reproductive outcomes, the AMEs for high parity are statistically significant (p < .10 or better) only for the experience of child death among births (0.067) and among mothers (0.126). The AMEs for short birth intervals are statistically significant for maternal child loss (0.142) and LBW experience (0.122 for births and 0.256 for mothers, p < .05). Cohorts with high proportions of daughters born to young mothers and at high parity appear to have significantly higher proportions of adult daughters experiencing child loss, and cohorts with high proportions of daughters born shortly after their siblings appear to have higher proportions delivering LBW infants in adulthood.3
The statistical significance of the associations of maternal risk factors with adult socioeconomic outcomes is limited. Cohorts with high proportions of daughters born to a young mother and within an interval of 18 months from the previous birth are associated with fewer years of schooling, on average, for adult daughters (shown in Table 3), but this difference is not statistically significant. Perhaps the strength of the association from maternal education preempts the influence of maternal risk factors. The AMEs for high parity and short birth interval with cohort proportions having paid work are also not statistically significant, although marginal statistical significance is observed for the negative association between cohorts with high proportions born to young mothers and having paid work (p < .10).
Among the control variables, maternal education—measured as the average cohort proportion of daughters born to mothers with any schooling—exhibits the most consistent and statistically significant associations with the reproductive health outcomes, as shown in the fourth row of coefficients in Table 5. With the exception of short stature, an increase in the proportion of daughters born to educated mothers is significantly associated with lower proportions having adverse outcomes. For example, this covariate’s AMEs are statistically significant with the cohort proportions experiencing child mortality (AMEs of −0.05 for births and −0.085 for mothers), being born small (–0.081 for births and −0.134 for mothers), and undernutrition status (–0.078 among adult daughters). The strong relationship of cohort proportions born to educated mothers and average levels of schooling in adulthood (shown in Tables 3 and 4) is suggestive of intergenerational transfer of schooling privilege; the regression coefficient is +7.426 years of schooling overall and 7.401 years for SSA (both significant at p < .001).
For the remaining control variables (shown in Table 3) the cohort proportions of adult females’ age at the time of survey are statistically significant and negatively associated with the reproductive outcomes, suggesting that age at survey is an appropriate control for exposure or length of adult life span and that it captures secular improvements in well-being. For example, in Table 3, the regression coefficients for cohort age at survey with schooling (0.085; p < .001) and with paid work (0.107; p < .001) are both positive and statistically significant. The proportions being born to mothers residing in urban areas at the time of the base survey do not show any consistent pattern of associations with the outcome variables. The control for the country’s region indicates that the levels of adverse reproductive outcomes, relative to Latin America and the Caribbean, are significantly lower for female birth cohorts from the North Africa/West Asia and Eastern Europe and significantly higher for those from SSA. Cohort levels for the two socioeconomic outcomes of years of schooling and paid work for these two regions are likewise significantly different from those in Latin America and the Caribbean.
The AMEs from the GLM results for the SSA cohort subsample are shown in the lower panel of Table 5. In general, the SSA pattern follows that for the full sample of cohorts, although the magnitude of the coefficients (Table 4) and AMEs for the maternal risk factors are often larger. For example, the AMEs of young maternal age on the two child mortality cohort measures are 0.145 for the total sample and 0.334 and for the SSA cohort sample are 0.248 and 0.507; the regression coefficients and AMEs are both statistically significant. For SSA, the AMEs for all three maternal risk factors are consistently positive and significantly related to the proportions of cohorts experiencing child loss. Cohorts with higher proportions of girls born at high parity are significantly associated with cohort proportions of infants born LBW or adult daughters having LBW infants. The AMEs in SSA for maternity risk factors are not significantly related with cohort proportions of adult daughters’ paid work. SSA coefficient sizes for maternal education are smaller than those in the full sample. Regional homogeneity likely constrains variation in the cohort measures and lessens the likelihood of associations being statistically significant. The three risk factors’ AMEs are also not statistically significant for cohort proportions being short in adulthood, a possible consequence of the low prevalence of stunting (the average SSA cohort percentage being 2.2 %).
Table 6 presents post-estimation proportions, where we illustrate the influences of the elimination of each maternal risk factor (young maternal age, high parity birth, and birth interval under 18 months) on the proportion for each outcome for the cohorts, using the GLM and linear regression results, and compare this with the observed level. The simulations are conducted at the cohort level. For example, the AME of being born at high parity on the proportion of mothers ever reporting a child dying before age 5 is 0.334. For a cohort with the population average values for this risk factor and outcome (0.097 and 0.125, respectively), eliminating high-parity births results in a lower proportion of mothers reporting an under-5 death of 0.093 (= 0.125 – 0.097 × 0.334), which is the same as the simulated value shown in Table 6. Note that the AME calculation to obtain the expected change will not always replicate the simulated value given that the latter is computed for each cohort and is then averaged across them.
Table 6.
Observed cohort proportions and simulated proportions with maternal risk factor eliminated: All regions and sub-Saharan Africa (SSA) only
| Outcome | Expected Proportion With Elimination of Risk Factors | All Regions
|
SSA
|
||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Proportion of Children Who Died Before Age 5 | Observed | .079 | .046 | .100 | .047 |
| Maternal age <18 | .065 | .024 | .076 | .015 | |
| Born in parity ≥4 | .054 | .021 | .060 | .016 | |
| Birth interval <18 months | .072 | .030 | .084 | .019 | |
| All three risk factors | .040 | .015 | .037 | .007 | |
| Proportion of Mothers Who Report Ever Losing a Child by Age 5 | Observed | .125 | .093 | .166 | .103 |
| Maternal age <18 | .093 | .053 | .116 | .054 | |
| Born in parity ≥4 | .079 | .051 | .081 | .048 | |
| Birth interval <18 months | .105 | .066 | .131 | .064 | |
| All three risk factors | .048 | .029 | .041 | .021 | |
| Proportion of Children Reported Born Small | Observed | .170 | .089 | .157 | .081 |
| Maternal age < 8 | .182 | .075 | .156 | .063 | |
| Born in parity ≥4 | .141 | .062 | .119 | .049 | |
| Birth interval <18 months | .151 | .065 | .137 | .057 | |
| All three risk factors | .136 | .059 | .104 | .044 | |
| Proportion of Mothers Reporting Ever Having a Small Baby | Observed | .229 | .086 | .223 | .078 |
| Maternal age <18 | .232 | .051 | .199 | .035 | |
| Born in parity ≥4 | .185 | .043 | .137 | .024 | |
| Birth interval <18 months | .192 | .044 | .190 | .033 | |
| All three risk factors | .154 | .036 | .099 | .017 | |
| Proportion of Adult Daughters With BMI <18.5 | Observed | .113 | .092 | .121 | .069 |
| Maternal age <18 | .095 | .056 | .116 | .033 | |
| Born in parity ≥4 | .111 | .067 | .088 | .024 | |
| Birth interval <18 months | .108 | .067 | .103 | .030 | |
| All three risk factors | .083 | .051 | .069 | .020 | |
| Proportion of Adult Daughters With Height <145cm | Observed | .042 | .054 | .022 | .023 |
| Maternal age <18 | .038 | .033 | .026 | .010 | |
| Born in parity 4 | .043 | .038 | .020 | .009 | |
| Birth interval <18 months | .043 | .038 | .012 | .005 | |
| All three risk factors | .033 | .029 | .014 | .005 | |
| Adult Daughter’s Average Years of Education | Observed | 6.419 | 2.506 | 5.205 | 2.177 |
| Maternal age <18 | 6.632 | 2.189 | 5.223 | 1.887 | |
| Born in parity ≥4 | 5.746 | 2.276 | 6.197 | 1.867 | |
| Birth interval <18 months | 6.985 | 2.312 | 6.135 | 1.845 | |
| All three risk factors | 6.525 | 2.323 | 7.144 | 1.822 | |
| Proportion of Adult Daughters With Paid Work | Observed | .286 | .160 | .317 | .156 |
| Maternal age <18 | .326 | .139 | .310 | .102 | |
| Born in parity ≥4 | .272 | .124 | .261 | .096 | |
| Birth interval <18 months | .264 | .121 | .290 | .098 | |
| All three risk factors | .305 | .134 | .238 | .089 | |
Note: Maternal childbearing risk factors are first eliminated individually and then all together.
If we take the proportion of infants reported to be born small as an example, in the third panel of Table 6, the post-estimation simulation shows that eliminating the risk of births at parity 4 or higher results in a cohort proportion of 0.141, lower than the observed cohort proportion of 0.170. If exposure to all three risk factors is eliminated, the estimated proportion is 0.136. (Given that the AME for high parity is 0.072, 0.141 ≈ 0.170 – 0.072 × 0.425.) The observed proportion of mothers ever reporting a small infant at birth of 0.229 may be lowered to an estimated 0.185 if the risk of high-parity births is eliminated and to 0.154 if exposure to all three maternal risk factors is eliminated.
A comparison of the observed outcome levels and the risk-eliminated proportions shows change in the expected directions for all cohorts and those in the SSA region, with the exception of paid work. While mindful that not all maternal risk factors’ associations with reproductive outcomes are statistically significant, we find the post-estimation exercise for combined risk elimination to be informative in illustrating the potential relative magnitudes of life course influences. The elimination of birth risk exposures is proportionally the largest for the estimated proportion of mothers experiencing child loss (declining from 0.125 to 0.048, or by 62 %, overall; and from 0.166 to 0.041, or by 75 %, for SSA) and the smallest for stunting (declining from 0.042 to 0.033, or by 21 % overall). The simulated proportion from improved maternity conditions on daughters’ mean years of schooling is substantial for SSA, increasing from 5.21 to 7.14 years, or by almost two years. Overall, health-related gains may be the greatest for child mortality, followed by birth weight and nutritional status. Socioeconomic gains are the largest for schooling among SSA cohorts.
Age and Cohort Patterns
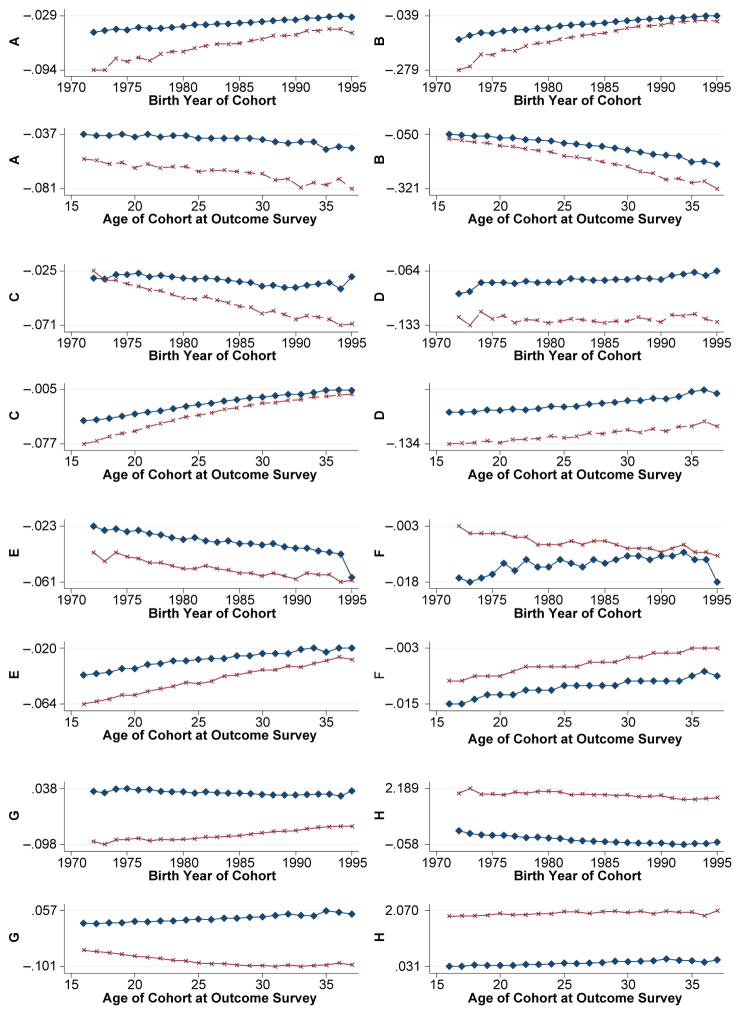
In addition to variation by level of risk factors and regional origin, the cohort sample’s proportions are often significantly influenced by year of birth and age in the year of outcome measurement. To observe patterns by cohort birth year and age at outcome, we illustrate in Fig. 4 the trends in the difference between all risk-eliminated and observed proportions for the eight outcomes (labeled A–H) for these two factors (top and bottom of the pair of graphs) over time (horizontal axis). The lines within the graph relate to the proportions simulated for the full and SSA cohort samples. Moving from a small to a larger difference over time signifies a benefit or loss depending on the outcome, and the steepness of the trend comparing birth year or age graphs is indicative of their relative influences.
Fig. 4.
Observed minus expected change in estimated cohort proportions or averages after eliminating all maternity risk factors by cohort birth year or age at time of outcome survey. Diamond symbols are for all regions; x symbols are for SSA region only. Vertical axes are as follows: A = Change in proportion of children who died before age 5; B = Change in proportion of mothers who report ever losing a child by age 5; C = Change in proportion of children reported born small; D = Change in proportion of mothers ever having a small baby; E = Change in proportion of adult daughters with BMI <18.5; F = Change in proportion of adult daughters with height <145cm; G = Change in proportion of adult daughters with paid work; H = Change in adult daughter’s average years of education
We observe that the estimated benefit of non-exposure to maternal risk factors for averting child loss (panels A and B) is greater for later birth cohorts and younger women and across all cohorts rather than just the SSA cohorts. The estimated benefits of non-exposure to the studied risks for infants born small are largest for earlier cohorts and older daughters (panels C and D). The gains are smaller when based on maternal loss experience than when based on infant survival, and regional gaps persist over time. The benefits of eliminating maternal risks for female nutritional status (panel E) and height (panel F) suggest accrual to earlier birth cohorts and older women and more for SSA cohorts than the full sample.
Although the early exposure to maternal risks was not significantly related to the socioeconomic outcome of paid work (panel G), the benefits for the full and SSA cohort samples exhibit different trends. Later birth cohorts and older women are estimated to derive larger benefits for the SSA than for the full sample. In contrast, the trajectory of benefits for years of education is fairly constant by birth year or age (panel H).
Discussion
Our analysis of national population survey data collected between 1985 and 2013 from 50 LMICs has aimed to assess the relationships between maternal risk factors at daughters’ births associated with eight selected adult developmental, reproductive, and socioeconomic outcomes. Pseudo-cohorts were constructed using birth history and respondent reports from 189 cross-sectional rounds of DHS surveys. To our knowledge, this is the first such application of the pseudo-cohort approach to standardized survey data collected in LMICs to gain longitudinal insights into the life course consequences of adverse exposures at birth. An additional methodological contribution from this study is overcoming the limitation of bounded values of 0 and 1 for cohort averages used in logit models by combining the modeling strategies of Deaton (1985) and Papke and Wooldridge (1996).
The selected outcomes represent important markers for female development over the life course that research suggests may originate from early birth conditions and can be measured through the time series of DHS surveys. We observed significant associations between high cohort proportions of daughters born to young mothers and high levels of child mortality among their births or as mothers themselves. Cohorts with high proportions of young mothers are also associated with high proportions being undernourished or unpaid workers as adults. Similarly, cohorts with high proportions of daughters born at high parity or soon after a preceding sibling are linked to cohorts with high proportions of children being LBW. The associations tend to be larger in magnitude in the SSA cohort sample. Cohorts with a high proportion of daughters born to educated mothers are significantly more likely to be linked to cohorts in which adult daughters have more years of schooling, on average, indicating an intergenerational relationship between mothers’ and daughters’ schooling. This finding may also reflect physical health advantages for better cognitive performance as a result of healthy childbearing.
There is likely intracohort heterogeneity in the reproductive and socioeconomic experiences of the cohort members both at birth and in adulthood. This can constrain the covariance in cohort proportions and result in larger standard errors and less statistical significance. The linked cohort relationships, which are observed here to be statistically significant, robust in magnitude, and in the expected direction, offer some assurance that unhealthy childbearing has life course implications and that the constructed cohort approach merits further pursuit. The simulated cohort proportions from the combined elimination of exposure to all three maternal risks are lower than their observed proportions, and the cohort averages for adult years of schooling are higher, both in the full and SSA regional samples. The one exception is the cohort proportion experiencing paid work, which increases for the elimination of only one risk factor—young maternal age—from 0.286 to 0.326 and only in the full sample. This may reflect variation in the proportions of females having wage or paid work in the SSA region. Folbre (2014) has suggested that women’s time burden of work is greater in African economies but the proportion compensated is smaller.
This analysis has its limitations, some of which are related to the application of the pseudo-cohort approach: for example, truncation bias, differential mortality, and respondent loss resulting from international out-migration. Truncation bias can be mitigated over time with the growing number of DHS surveys, particularly extending the time series in SSA countries. In addition to testing the membership size of cohorts, we also tested restricting the age range for cohort members and found little difference in the regression results. Selective mortality, particularly that attributable to unobservable factors, can be addressed by using the survival probability to weight the cohorts. However, we estimated both weighted and unweighted regression models and obtained similar results, indicating that cohort averages largely mitigated the possibility of survival bias. (We presented the unweighted results.)
Last, linked cohort data offer at least two distinct advantages over individual-level panel data. First, the cost of repeated cross-sectional surveys with standardized content that allow construction of cohort panels is much lower than for longitudinal surveys of comparable sample size. Second, cohort panels are not subject to bias from sample attrition the same way that longitudinal surveys are; therefore, they can cover a longer period. As long as the cross-sectional data are based on a representative sample of the population of interest and dramatic shifts in mortality have not occurred, constructed cohorts can provide consistent estimates of the parameters of interest.
An in-depth understanding of the dynamics underlying reproductive and biological pathways connecting gestational and newborn health with growth and development over the life course is much needed. Ideally, such an understanding will be informed by multidisciplinary research linking micro-level structures and processes—both biological and behavioral—to macro-level ones. Research on fetal development, stress reactivity, reproductive capacity and function, adolescent growth and development, and adult health, lifestyles, and performance increasingly inform how these are shaped by changing nutritional, social, economic, and physical environments. As Shetty and Schmidhuber (2011) suggested, these environmental shifts will inform those occurring for life expectancies and aging experiences. For the health of female populations in LMICs, research evidence on mitigating the effects of risks at birth that can compromise their adult well-being is limited and is needed to help assess gender disparities observed in earlier studies (e.g., Merchant and Martorell 1988; Nenko and Jasienska 2013; Yount et al. 2014). Findings from replicating this study’s analysis with sons born to mothers with the same risk conditions using DHS male samples can be enlightening about any gender disparities in adverse consequences experienced in adulthood. These demographically oriented analyses, applying the pseudo-cohort approach, can give population-scale credence to epigenetic and biobehavioral frameworks that have linked early-life exposures for individuals to their later health outcomes (e.g., Barker 1994; Wang et al. 2013). These studies have underscored the social inheritance conferred by maternal education and the economic costs of poverty on child cognitive ability and educational achievement in adulthood (e.g., Mani et al. 2013).
In sum, we observed a number of significant associations between selected demographic indicators of healthy childbearing and specific adult developmental, health, and socioeconomic outcomes in low-income populations, using pseudo-cohorts as units of analysis. Not all three maternal risk factors are statistically significantly associated with each of the eight measured outcomes. Taken as a whole and when viewed for age and cohort patterns, however, life course linkages are distinguishable. Noteworthy also is that these risks at birth—that is, short birth spacing, young maternal age, and high parity—are preventable and can be addressed through improved access to pre-conception, maternal, newborn, and child health care, as well as expanding social opportunity through female education. Direct and indirect interventions in LMICs are available to reduce health-compromising factors at birth, such as prolonged breast-feeding, improved girl and maternal nutrition, effective contraceptive practice, and extended schooling to delay early childbearing. These paths are known to prevent adverse birth outcomes over the long run and in an enduring manner (Kavanaugh and Anderson 2013). Beyond the substantive findings, this study’s methodological contribution has been to expand the set of statistical tools available to test hypotheses using large-scale, standardized, repeated cross-sectional survey data in a longitudinal manner. The pseudo-cohort approach can confer considerable resource efficiency to future panel analyses.
Acknowledgments
This research received partial support from the Bill & Melinda Gates Foundation through grants to the Johns Hopkins Bloomberg School of Public Health, for which the authors are grateful. Comments from the anonymous reviewers were especially helpful, as were advice and suggestions from other members of the first author’s dissertation committee, Drs. Stan Becker, David Bishai, Robert Moffitt, and Mei-Cheng Wang.
Footnotes
Low cohort-level prevalence will also limit the accuracy of the estimates. Model sensitivity to the inclusion of older maternal age is discussed in a later section.
We first used a 10-year period for births occurring before a DHS round because this is the same base of births used for infant mortality rates. We also constructed cohorts for a 20-year period. To balance sample power and optimize on the range of adult ages, we elected to use births in the 15-year period prior to the DHS for cohort construction.
We tested the inclusion of the cohort proportion of births to mothers older than 34 and observed marginal change. The coefficients for high parity were slightly attenuated with some outcomes but increased modestly for others. The nominal change in coefficients and statistical significance reinforced the decision to avoid multicollinearity and not include older maternal age in the models.
Contributor Information
Qingfeng Li, Email: qli28@jhu.edu.
Amy O. Tsui, Email: atsui@jhu.edu.
References
- Abu-Saad K, Fraser D. Maternal Nutrition and Birth Outcomes. Epidemiologic Reviews. 2010;32:5–25. doi: 10.1093/epirev/mxq001. [DOI] [PubMed] [Google Scholar]
- Adam I, Ismail MH, Nasr AM, Prins MH, Smits LJ. Low birth weight, preterm birth and short interpregnancy interval in Sudan. Journal of Maternal-Fetal and Neonatal Medicine. 2009;22:1068–1071. doi: 10.3109/14767050903009222. [DOI] [PubMed] [Google Scholar]
- Barker DJP. Mothers, babies, and disease in later life. London, UK: Churchill Livingstone; 1994. [Google Scholar]
- Barker DJP, Sheill AW, Barker ME, Law CM. Growth in utero and blood pressure levels in the next generation. Journal of Hypertension. 2000;18:843–846. doi: 10.1097/00004872-200018070-00004. [DOI] [PubMed] [Google Scholar]
- Black RE, Victora C, Walker SP, Bhutta ZA, Christian P, de Onis M … Maternal and Child Nutrition Study Group. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:417–425. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- Blanc A, Wardlaw T. Monitoring low birth weight: An evaluation of international estimates and an updated estimation procedure. Bulletin of the World Health Organization. 2005;83:178–185. [PMC free article] [PubMed] [Google Scholar]
- Chadio S, Kotsampasi B. The role of early life nutrition in programming of reproductive function. Journal of Developmental Origins of Health and Disease. 2014;5:2–15. doi: 10.1017/S204017441300038X. [DOI] [PubMed] [Google Scholar]
- Chan KA, Tsoulis MW, Sloboda DM. Early-life nutritional effects on the female reproductive system. Journal of Endocrinology. 2015;224:R45–62. doi: 10.1530/JOE-14-0469. [DOI] [PubMed] [Google Scholar]
- Christian P. Micronutrients, birth weight and survival. Annual Review of Nutrition. 2010a;30:83–104. doi: 10.1146/annurev.nutr.012809.104813. [DOI] [PubMed] [Google Scholar]
- Christian P. Maternal height and risk of child mortality and undernutrition. Journal of the American Medical Association. 2010b;303:1539–1540. doi: 10.1001/jama.2010.469. [DOI] [PubMed] [Google Scholar]
- Conde-Agudelo A, Belizán JM. Maternal morbidity and mortality associated with interpregnancy interval: Cross sectional study. British Medical Journal. 2000;321:1255–1259. doi: 10.1136/bmj.321.7271.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Agudelo A, Rosas-Bermudez A, Castaño F, Norton M. Effects of birth spacing on maternal, perinatal, infant and child health: A systematic review of causal mechanisms. Studies in Family Planning. 2012;43:93–114. doi: 10.1111/j.1728-4465.2012.00308.x. [DOI] [PubMed] [Google Scholar]
- DaVanzo J, Hale L, Razzaque A, Rahman M. Effects of interpregnancy interval and outcome of the preceding pregnancy on pregnancy outcomes in Matlab, Bangladesh. British Journal of Obstetrics & Gynecology. 2007;114:1079–1087. doi: 10.1111/j.1471-0528.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaVanzo J, Hale L, Razzaque A, Rahman M. The effects of pregnancy spacing on infant and child mortality in Matlab, Bangladesh: How they vary by the type of pregnancy outcome that began the interval. Population Studies. 2008;62:131–154. doi: 10.1080/00324720802022089. [DOI] [PubMed] [Google Scholar]
- Deaton A. Panel data from time series of cross-sections. Journal of Econometrics. 1985;30:109–126. [Google Scholar]
- Fabic M, Choi Y, Bird S. A systematic review of Demographic and Health Surveys: Data availability and utilization for research. Bulletin of the WHO. 2012;90:604–612. doi: 10.2471/BLT.11.095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folbre N. The care economy in Africa: Subsistence production and unpaid care. Journal of African Economies. 2014;23(Suppl 1):i28–i56. [Google Scholar]
- Georgieff MK. Nutrition and the developing brain: Nutrient priorities and measurement. American Journal of Clinical Nutrition. 2007;85:614S–620S. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- Gibbs CM, Wendt A, Peters S, Hogue CJ. The Impact of Early Age at First Childbirth on Maternal and Infant Health. Paediatric and perinatal epidemiology. 2012;26:259–284. doi: 10.1111/j.1365-3016.2012.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene WH. Econometric analysis. Upper Saddle River, NJ: Prentice Hall; 2011. [Google Scholar]
- Hallett T, Stover J, Mishra V, Ghys P, Gregson S, Boerma T. Estimates of HIV incidence from household-based prevalence surveys. AIDS. 2010;24:147–152. doi: 10.1097/QAD.0b013e32833062dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JP. Older maternal age and pregnancy outcome: a review of the literature. Obstetrical & Gynecological Survey. 1986;41:726–742. doi: 10.1097/00006254-198611000-00024. [DOI] [PubMed] [Google Scholar]
- Jolly M, Sebire N, Harris J, Robinson S, Regan L. The risks associated with pregnancy in women aged 35 years or older. Human Reproduction. 2000;15:2433–2437. doi: 10.1093/humrep/15.11.2433. [DOI] [PubMed] [Google Scholar]
- Kavanaugh ML, Anderson RM. Contraception and beyond: the health benefits of services provided at family planning centers. New York: Guttmacher Institute; 2013. [Google Scholar]
- Long S. Regression models for categorical and limited dependent variables: Advanced quantitative techniques in the social sciences. Vol. 7. Thousand Oaks, CA: Sage Publications; 1997. [Google Scholar]
- Mahy M. Childhood mortality in the developing world. Calverton, MD: Demographic and Health Surveys, ORC Macro; 2003. (Comparative Reports No. 4) [Google Scholar]
- Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341:976–980. doi: 10.1126/science.1238041. [DOI] [PubMed] [Google Scholar]
- McIntosh S. Using pseudo cohorts to track changes in the qualifications of national populations. London, UK: Center for Economic Performance, London School of Economics, Department for Education and Skills; 2005. (Research Report RR621) [Google Scholar]
- Merchant K, Martorell R. Frequent reproductive cycling: does it lead to nutritional depletion of mothers? Progress in Food & Nutrition Science. 1988;12:339–369. [PubMed] [Google Scholar]
- Moffitt R. Identification and estimation of dynamic models with a time series of repeated cross-sections. Journal of Econometrics. 1993;59:99–123. [Google Scholar]
- Nenko I, Jasienska G. Fertility, body size and shape: An empirical test of the covert maternal depletion hypothesis. American Journal of Human Biology. 2009;21:520–523. doi: 10.1002/ajhb.20938. [DOI] [PubMed] [Google Scholar]
- Nenko I, Jasienska G. First birth interval, an indicator of energetic status, is a predictor of lifetime reproductive strategy. American Journal of Human Biology. 2013;25:78–82. doi: 10.1002/ajhb.22344. [DOI] [PubMed] [Google Scholar]
- Nordtveit TI, Melve KS, Skjaerven R. Intergenerational birth weight associations by mother’s birth order—The mechanisms behind the paradox: A population-based cohort study. Early Human Development. 2009;85:577–581. doi: 10.1016/j.earlhumdev.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Papke LE, Wooldridge JM. Econometric methods for fractional response variables with an application to 401(k) plan participation rates. Journal of Applied Econometrics. 1996;11:619–632. [Google Scholar]
- Robles A, Goldman N. Can accurate data on birthweight be obtained from health interview surveys? International Journal of Epidemiology. 1999;28:925–931. doi: 10.1093/ije/28.5.925. [DOI] [PubMed] [Google Scholar]
- Rutstein S. Effects of preceding birth intervals on neonatal, infant and under-five years mortality and nutritional status in developing countries: Evidence from the Demographic and Health Surveys. International Journal of Gynecology and Obstetrics. 2005;89(Suppl 1):S7–S24. doi: 10.1016/j.ijgo.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Shechter Y, Levy A, Wiznitzer A, Zlotnik A, Sheiner E. Obstetric complications in grand and great grand multiparous women. Journal of Maternal-Fetal and Neonatal Medicine. 2010;23:1211–1217. doi: 10.3109/14767051003615459. [DOI] [PubMed] [Google Scholar]
- Shetty PS, Schmidhuber J. Nutrition, lifestyle, obesity and chronic disease. New York, NY: United Nations, Department of Economic and Social Affairs; 2011. (Population Division, Expert Paper No. 2011/3) [Google Scholar]
- Shonkoff J, Phillips D. From neurons to neighborhoods: The science of early childhood development. Washington, DC: National Academies Press; 2000. [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- Tzioumis E, Adair LS. Childhood dual burden of under- and over-nutrition in low- and middle-income countries: a critical review. Food and nutrition bulletin. 2014;35:230–243. doi: 10.1177/156482651403500210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF. Levels and trends in child mortality. New York, NY: UNICEF, World Health Organization, World Bank, & United Nations; 2014. (Report 2014) [Google Scholar]
- United Nations. World Population Prospects. 2015 (2015 Revision). Retrieved from http://esa.un.org/unpd/wpp/unpp/panel_population.htm.
- Verbeek M, Nijman T. Can cohort data be treated as genuine panel data? Empirical Economics. 1992;17:9–23. [Google Scholar]
- Vohs K. The poor’s poor mental power. Science. 2013;341:969–970. doi: 10.1126/science.1244172. [DOI] [PubMed] [Google Scholar]
- Wang G, Walker S, Hong X, Bartell T, Wang X. Epigenetics and early life origins of chronic noncommunicable diseases. Journal of Adolescent Health. 2013;52(Suppl 2):S14–S21. doi: 10.1016/j.jadohealth.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Winikoff B. The maternal depletion syndrome: Clinical diagnosis or eco-demographic condition? Biological Sciences. 1988;5:163–170. [Google Scholar]
- Witter F, Luke B. The effect of maternal height on birth weight and birth length. Early Human Development. 1991;25:181–186. doi: 10.1016/0378-3782(91)90114-i. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) A WHO collaborative study of maternal anthropometry and pregnancy outcomes. International Journal of Gynecology and Obstetrics. 1997;57:1–15. [PubMed] [Google Scholar]
- Yount K, Zureick-Brown S, Halim N, LaVilla K. Fertility decline, girls’ wellbeing, and gender gaps in children’s wellbeing in poor countries. Demography. 2014;51:535–561. doi: 10.1007/s13524-014-0282-0. [DOI] [PubMed] [Google Scholar]
- Zimicki S. The relationship between fertility and maternal mortality. In: Parnell AM, editor. Contraceptive use and controlled fertility: Health issues for women and children. Washington, DC: National Academies Press; 1989. pp. 1–47. [PubMed] [Google Scholar]