Abstract
Neutrophils have a crucial role in tumor development and metastatic progression. The contribution of neutrophils in tumor development is multifaceted and contradictory. On the one hand, neutrophils prompt tumor inception, promote tumor development by mediating the initial angiogenic switch and facilitate colonization of circulating tumor cells, and on the other hand, have cytotoxic and anti-metastatic capabilities.
Our understanding of the role of neutrophils in tumor development has greatly depended on different experimental animal models of cancer. In this review we cover important findings that have been made about neutrophils in experimental animal models of cancer, point to their advantages and limitations, and discuss novel techniques that can be used to expand our knowledge of how neutrophils influence tumor progression.
Keywords: neutrophil, tumor development, animal models
1. Introduction
There is growing evidence indicating that neutrophils have an important role in tumor development – from the inception of tumor formation and throughout the malignant progression [1-3]. Neutrophils both promote and prevent tumor progression (reviewed in [1]). The contradictory role of neutrophils in tumor development might be explained be the fact that neutrophils can have a pro- or anti-inflammatory phenotype [4-6]. Depending on their phenotype neutrophils appear to be either antitumoral (N1) or protumoral (N2) [4]. The occurrence of N1/N2 neutrophils has only been shown in murine tumor models and will have to be confirmed in other experimental animal models and more importantly in humans. However, neutrophils with an activated phenotype, producing pro-inflammatory factors and stimulating T cell proliferation has been described in early human lung cancer, while immunosuppressive neutrophils has been reported in human colorectal cancer [5, 6].
Tumor-associated neutrophils (TANs) with a N1 phenotype have a pro-inflammatory cytokine profile, producing nitrate oxide and hydrogen peroxide (H2O2), and are cytotoxic towards tumor cells [4]. N2 TANs, on the other hand, are characterized by high levels of arginase 1 (ARG1) and by their ability to inhibit effector T-cell functions [4, 6].
The tumor microenvironment, evolving with tumor progression, can influence the polarization state of TANs [7]. Transforming growth factor-β (TGFβ) can differentiate neutrophils toward a protumoral phenotype [4]. Intriguingly, neutrophils initially recruited during the early stages of tumor development have an antitumoral N1 phenotype. However, with tumor progression the tumor-infiltrating neutrophils become more protumoral and acquire a N2 phenotype. In line with the polarization paradigm only depletion of neutrophils at a later stage of tumor development reduces tumor growth, further pointing to the fact that neutrophils can have an anti- or protumoral phenotype [7]. Transcriptional profiling of TANs from different mouse tumor models furthermore has revealed that TANs can have strikingly different protumorigenic transcriptional profiles [8]. Thus, even though neutrophils can be polarized into either N1 or N2, they presumably make up a continuum of different phenotypes.
Our understanding of the polarization of neutrophils and how neutrophils contribute to tumor development has largely been elucidated from experimental mouse models. However, alternative models, for example, the zebrafish model, have provided key insights into how neutrophils influence tumor development. In this review, we cover important findings that have been made about neutrophils in experimental animal models of cancer and point to the advantages and limitations of different models. We point to how different experimental animal models and novel techniques can be used to expand our knowledge of how neutrophils influence tumor progression.
1.1 From men to mice
Experimental mouse tumor models have contributed to our understanding of tumor biology and the intricate role of neutrophils in tumor development. There are many advantages with mice; they are small in size, breed in captivity and have biological similarities with humans. However there are important differences between humans and mice [9, 10]. While neutrophils in humans make up the majority (50-70%) of leukocytes in the peripheral blood, they only account for 15-20% of the leukocytes in the peripheral blood of mice [11]. Furthermore, humans live longer, have a lower metabolic rate and undergo more cell divisions. One might therefore anticipate that cancer would be more common among humans, but, interestingly, the lifetime risk of developing cancer is comparable between humans and mice. Mice, however, more often get tumors of mesenchymal origin such as sarcomas and lymphomas, while tumors in humans most often originate from epithelial cells giving rise to carcinomas in, for example, colon, breast and lungs [12].
Different mouse tumor models provide powerful experimental tools to study various cancers that normally develop in humans [13-18]. There are three major experimental mouse tumor models: 1) genetically engineered, 2) transplantable, and 3) humanized tumor models. Each model has different advantages and limitations for elucidating the role of neutrophils in tumor development.
1.1.1 Genetically engineered tumor models
Genetic engineering has allowed for tissue-specific and time-specific induction of various oncogenes or dominant-negative tumor-suppressor genes triggering spontaneous tumor development in mice (Table 1) [13-15]. Spontaneous tumor growth in genetically engineered mouse models faithfully depicts the multistage tumor development seen in humans and, more importantly, the evolving interplay between the tumor microenvironment with neutrophils and initially premalignant tumor cells that with time will become malignant and evolve to full-blown cancer.
Table 1.
Examples of experimental mouse models of cancer
| Genetically engineered models | |
|---|---|
| Genetic model | Cancer type |
| RIP-Tag2 | Pancreatic cancer |
| KrasGD12 | Pancreatic cancer |
| K14-HPV16 | Papilloma |
| MMTV-PyMT | Breast cancer |
| MMTV-Neu | Breast cancer |
| Transplantable models | |
| Transplantable mouse cancer cell lines | Cancer type |
| Lewis lung carcinoma | Lung cancer |
| B16 | Melanoma |
| CT26 | Colon cancer |
| 4T1 | Breast cancer |
| Transplantable human cancer cell lines | Cancer type |
| PC-3 | Prostate cancer |
| MDA-MB-231 | Breast cancer |
Genetically engineered mice can have an intact immune system, hence making the models suitable for studying the interaction, not only between tumor cells and immune cells, but also the intricate interplay between innate and adaptive immune cells during tumor development [19].
Genetic engineering can target different genes expressed by neutrophils and thereby depict how genes of interest contribute to the function of neutrophils in tumor development. By taking advantage of the Tie2:Cre deleter, Finisguerra et al. recently revealed that deletion of Met (coding for MET; the receptor for hepatocyte growth factor (HGF)) in neutrophils attenuated the recruitment of antitumoral, but not protumoral, neutrophils [20, 21]. MET is a prerequisite for the cytotoxic ability of antitumoral neutrophils and reduces tumor growth and metastasis [21].
1.1.2 Transplantable tumor models
While tumor growth in genetically engineered mice can take from a few months up to over a year, transplantable tumor models have the advantage that they can develop full-blown tumors after only a few weeks. A wide range of different cancer cell lines has been extensively used in transplantable tumor models (Table 1). These cancer cell lines have either originated spontaneously or from carcinogen-, transgene- or gene knockout-induced tumors [18].
Transplantation with cancer cell lines into syngeneic mice has the advantage that the mice have an intact immune system. However, the fact that the mice are of the same inbred mouse strain means that the model will not reflect the genetic heterogeneity found among human patients. Also, since the injected cells already are malignant and are put into a naïve microenvironment, the growth of the tumor will not reveal multistage tumor development and the evolving interaction between tumor cells and the tumor microenvironment.
Different strains of immunodeficient mice have made transplantation of human tumor cells, i.e., xenograft transplantation, feasible (Table 1) [22]. A major drawback of the xenograft transplantation model is that, to enable growth of human tissue and prevent rejection, the mice have to be devoid of functional immune system. Hence, even though the xenograft models have greatly contributed to our knowledge of how human cancer cells behave during tumor progression, they are not able to fully depict the functional contribution of tumor cells, innate- and adaptive immune cells and their bidirectional communicate.
1.1.3 Humanized mice model
‘Humanized mice’ are immunodeficient mice that have gained a functional human immune system through the engraftment of human primary hematopoietic cells or tissues. Humanized mice are derived from various mouse strains of immunodeficient mice, which have in common that they have mutations in the interleukin-2 (IL-2) receptor common γ-chain locus (Il2rg). Targeted mutation of Il2rg gives major impairments in the development and function of T, B and natural killer (NK) cells [23]. Currently, immunodeficient mice strains can be humanized with human adaptive immune cells and various innate immune cells including NK cells, monocytes and macrophages, however successful establishment of human neutrophils is a challenge yet to be conquered [24-27].
1.2 Neutrophils and Gr-1+ immune cells
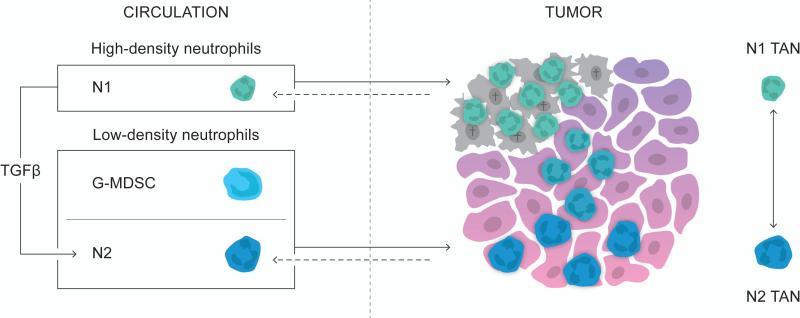
Neutrophils in mice are recognized as CD11b+Ly6G+ cells [28, 29]. However, until recently Gr-1 was broadly used as a marker for bone marrow-derived cells including immune cells of the neutrophil lineage. Gr-1 recognizes the two antigens, Ly6C on monocytes and Ly6G on neutrophils. Even though neutrophils are Gr-1high (while monocytes are Gr-1int), Gr-1 is not able to differentiate between monocytes and neutrophils efficiently [2, 28]. Moreover, Gr-1 has been used to classify immature myeloid derived suppressor cells (MDSCs) as immune cells that primarily exert their protumoral effect through inhibition of antitumoral T-cells [30, 31]. Two subpopulations of MDSCs have been characterized; monocytic-MDSCs (Mo-MDSCs), which are CD11b+Ly6C+, and granulocytic-MDSCs (G-MDSCs), which are CD11b+Ly6G+ [32, 33]. In addition to being classified by the same surface markers, G-MDSCs are morphologically similar to immature neutrophils and have functional overlap with N2 neutrophils (both having immunosuppressive capacity) [32, 34]. The immunosuppressive function of G-MDSCs is however contradictory and accumulating evidence indicate that only Mo-MDSCs and not G-MDSCs mediate T-cell suppression [35-37]. Hence an important question to be unraveled is the exact role of G-MDSCs in tumor development and whether or not neutrophils and GMDSCs are two distinct immune cell populations or if they in fact are the same immune population but with different phenotypes. Due to the lack of definite markers for each population the relationship between neutrophils and G-MDSCs is still largely undefined. However, work by Youn et al. showed that tumor-derived neutrophils (CD11b+Ly6ClowLy6G+) have more functional characteristics in common with splenic G-MDSCs (CD11b+Ly6ClowLy6G+) from tumor-bearing mice than with neutrophils from non-tumor bearing mice, which could imply that G-MDSCs migrate to the tumor site and that TANs acquire a N2 phenotype prior to arriving at the tumor site [38]. On the contrary, transcriptomic analysis, comparing TANs (CD11b+Ly6G+), splenic GMDSCs (CD11b+Ly6G+) from tumor-bearing mice and naïve bone marrow neutrophils, revealed that TANs make up a unique neutrophil population quite distinct from splenic G-MDSCs, which were more closely related to naïve bone marrow-derived neutrophils [39]. The lack of apparent transcriptomic similarity between TANs and splenic G-MDSCs could imply that factors, e.g., TGFβ, in the local tumor microenvironment, indeed, dictate the fate of neutrophils and that TANs are not derived from G-MDSCs but from a peripheral neutrophil population. Indeed, different subpopulations of neutrophils exist in the circulation. Circulating high-density neutrophils are mature and predominant during early tumor development, while low-density neutrophils are immunosuppressive and accumulate with tumor progression [40]. Taken together the exact relationship between neutrophils and G-MDSCs is still largely unclear and more work is needed to determine how they relate. Similarities and differences between neutrophils and G-MDSCs have been reviewed in detail by Pillay et al [41]. Since there currently are no definite markers to distinguish neutrophils from G-MDSCs we cover both in this review and refer to them as neutrophils.
2. Inflammation and tumor inception
Chronic inflammation, i.e., unresolved inflammation with infiltration of immune cells, tissue remodeling and angiogenesis, can prompt the genetic mutation and proliferation rate in mutated cells or directly initiate genomic instability through e.g. DNA damage or intruding on DNA repair pathways. Neutrophils, accompanying chronic inflammation, are involved in creating a mutagenic environment capable of initiating and promoting tumor development [42-45].
The Mutatect tumor model, which allows for the detection of mutagenic incidents in murine fibrosarcoma cells, has been used to study how neutrophils contribute to genetic instability [46]. The genetic damage is detected in a particular target gene, namely Hprt (hypoxanthine phosphoribosyltransferase). Mutatect cells that become mutated, i.e., gain inactivation of Hprt, have acquired a resistance to the cytotoxic drug 6-thioguanine (6-TG) and can be identified with a clonogenic assay. Initially it was revealed that the mutation frequency in this model was elevated in vivo compared to in vitro, implying that factors in the microenvironment contributed to the genetic instability [47]. Indeed, when various Mutatect cell lines are subcutaneously injected into immunocompetent syngeneic C57BL/6 mice, neutrophils are the predominate leukocyte population infiltrating the tumors and the mutation frequency strongly correlates with the number of neutrophils [46].
The significance of neutrophils in tumor inception related to chronic inflammation has further been shown in a model of ulcerative colitis (UC). UC is a form of inflammatory bowel disease that gives an elevated risk of colon cancer [48]. The administrating of azoxymethane (AOM) and dextran sulfate sodium (DSS) to mice recapitulates the chronic inflammation seen in the colon of UC patients [49, 50]. One of the main inflammatory mediators to recruit neutrophils in this model is CXCL2 [51, 52]. CXCL2 attracts CXCR2+ neutrophils, and the infiltration of neutrophils precedes the development of multiple tumors in the colon [51]. Moreover deletion of Cxcr2 in genetically engineered Cxcr2−/− mice impairs AOM/DSS induced colonic chronic inflammation, reduces tumor burden and inhibits malignant progression [52, 53].
Yet another model that has linked neutrophils to tumor inception in chronic inflammation is a model of skin inflammation where mice are topically treated with 7,12-dimethylbenz [a] anthracene (DMBA), followed by repeated application of 12-O-tetradecanoylphorbol-13-acetate (TPA) [53]. The combined treatment induces skin inflammation with persistent recruitment of neutrophil and papillomas eventually develop in the site of tissue perturbation [54]. Importantly, inhibition of CXCR2+ neutrophils prevents the inflammation driven tumor development in this model [53]. Common for both the UC and skin model is that CXCR2+ neutrophils promote early tumor development through the inhibition of antitumoral T-cell immunity.
2.1 Neutrophils and adaptive immune cells
Adaptive immune cells are part of cancer immunosurveillance and can, once they are activated, efficiently eradicate premalignant tumor cells [55-58]. Hence, for a tumor to thrive it must gain capabilities that enable it to evade antitumoral adaptive immune control. Tumors can, for example, recruit protumoral neutrophils that will inhibit the generation and function of cytotoxic T-cells [52, 53]. Since neutrophils are key producers of ARG1, an enzyme that is involved in the impairment of antitumoral T-cells, they can modulate T-cell immunity and thereby control tumor progression. However, adaptive immune cells can exert an effect on neutrophils, emphasizing the two-directional communication between innate and adaptive immune system [2, 59, 60].
Richards et al. showed, in a model of subcutaneous injection of the mouse melanoma cell line B16FasL, that skin-resident regulatory T-cells (Treg) are rapidly mobilized to the site of injection where they limit early recruitment and survival of neutrophils [61]. Interestingly, neutrophils significantly contribute to early tumor rejection in this model, in line with previous data showing that neutrophils arriving during early tumor development are antitumoral [7, 61-63].
2.2 Acute inflammation – what can be learned from zebrafish
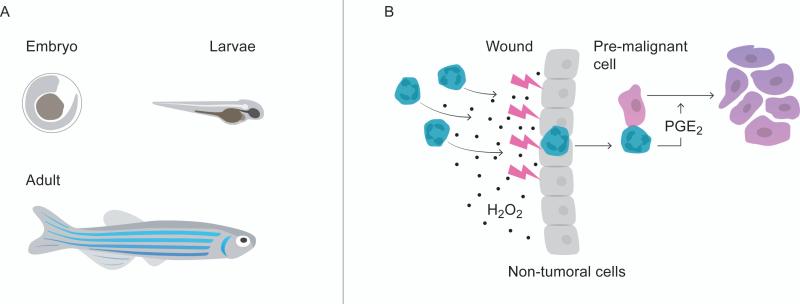
It is now generally appreciated that chronic inflammation can accelerate and induce development of cancer [64]. However, recent evidence from a zebrafish model indicates that even acute inflammation can be detrimental for tumor development (Figure 1) [65]. The zebrafish has emerged as an attractive animal model to study tumor biology and the role of neutrophils in tumor development [66]. An important advantage with the zebrafish model, besides being suitable for genetic manipulation, is the fact that their embryos and larvae are transparent. The transparency of the model allow for efficient live imaging revealing how tumor cells and infiltrating immune cells interact [67-69]. Compared to the mouse model, where the animal number is a limitation both physically and financially, large numbers of zebrafish can by included in each experiment, making the data from each experiment more powerful. Contrary to what one first might think, zebrafish tumor biology has important similarities with human cancers at both histological and genetic levels. Zebrafish neutrophils are an abundant leukocyte population in the circulation similar to humans [70, 71]. Moreover, zebrafish have both an innate and an adaptive arm of the immune system. During the embryonic and larvae stage they will, however, only have innate immune cells, making it an attractive model to study the specific role of neutrophils in tumor inception and early tumor development [72].
Figure 1.
The zebrafish model. A) The transparency of the zebrafish embryo and larvae makes the model suitable for live imaging of neutrophil- and tumor interactions. B) Neutrophils are attracted from the site of acute injury toward pre-malignant cells where the neutrophils promote tumor proliferation through PGE2 production.
Using a zebrafish model where the melanocyte-specific promoter mitfa (microphtalmia-associated transcription factor a) drives oncogenic human HRasG12V expression in melanocytes, Antonio et al. revealed that neutrophils are attracted from the site of acute injury toward premalignant cells where the neutrophils promote tumor progression through PGE2 production (Figure 1) [65, 73, 74]. H2O2, derived from transformed cells but also from non-tumoral neighbors, is the key neutrophil-attractant and inhibiting the generation of H2O2 attenuates infiltration of neutrophils and the proliferation of transformed cells [75]. The finding that neutrophils, not only in chronic, but also in acute inflammation promote tumor development will have to be confirmed in other experimental animal models of cancer and could, if holding true, have important clinical implications.
3. Tumor progression
Tumor development starts in a single or a few cells that have acquired genetic and epigenetic alterations. The abnormal activation of oncogenes or the loss of tumor suppressor genes initiate stable blockade of cell proliferation, i.e., senescence. For a pre-malignant tumor to become malignant it will have to evade senescence [76]. In a model of prostatic tumor development inactivation of the tumor suppressor gene Pten in mouse prostate epithelium induces the formation of benign tumors. Benign Pten−/− tumors are characterized by strong senescence, but over time the tumors grow and become invasive [77, 78]. Intriguingly CD11b+Gr-1+ cells counteract the tumor senescence and promote the development of cancer. CD11b+Gr-1+ cells infiltrate the PTEN null tumors at the onset of senescence and secrete IL-1RA, which antagonizes senescence. Moreover, inhibition of CXCR2 and adoptive transfer of Il1ra knockout myeloid cells to Pten−/− mice enhances senescence of PTEN null tumors [79].
3.1 Neutrophil recruitment in spontaneous tumor models
Human cancer development is a multistage malady that can be depicted in different genetically engineered spontaneous tumor models (Table 1). An important advantage with spontaneous tumor models is that one can elucidate at what stage of tumor development neutrophils are being recruited.
The KrasG12D pancreatic model faithfully recapitulates the development of human pancreatic ductal adenocarcinoma (PDA), which starts with noninvasive pancreatic intraepithelial neoplasia (PanIN) that progresses to invasive cancer [80]. Conditional expression of the mutated allele of Kras in pancreatic islets, specifically drives the progression from early pre-malignancy toward malignancy [81]. The mutational activation of Kras in pancreatic islets induces production of granulocyte-macrophage colony-stimulating factor (GM-CSF) recruiting protumoral CD11b+Gr-1+ cells that inhibit antitumoral T-cell immunity and thereby promote tumor progression. Inhibition of tumor-derived GM-CSF abrogates the recruitment of CD11b+Gr-1+ cells, which inhibits tumor development in an antitumoral T-cell dependent manner, once again emphasizing the important interplay between innate and adaptive immune cells [82, 83].
The MMTV-PyMT is another genetically engineered mouse model that depicts spontaneous tumor development in humans. The mouse mammary tumor virus long terminal repeat (MMTV-LTR) drives mammary gland-specific expression of the oncogenic polyoma virus middle T antigen (PyMT) and, at the age of 6 weeks, MMTV-PyMT mice have developed hyperplasia in their mammary glands, that progresses to adenoma two weeks later, followed by early carcinoma (week 10); by week 11-15 the mice have late-stage carcinoma with metastatic burden, primarily in the lungs [84, 85]. Even though breast cancer is not preceded by chronic inflammation it recruits inflammatory cells. Recently, our group revealed that neutrophils start to expand systemically at the time of malignant transformation from adenoma to early carcinoma. The only hematopoietic growth factor elevated in MMTV-PyMT mice at this time point is granulocyte-colony stimulating factor (G-CSF). G-CSF expands multi-potent progenitors in the bone marrow that give rise to Rblow T-cell immunosuppressive CD11b+Ly6G+ neutrophils [84].
The MMTV promoter has also been used to drive the transforming rat oncogene cerbB-2 (HER2/neu) [86]. The main attractant for neutrophils in this model is the major proangiogenic factor vascular endothelial growth factor (VEGF) [87].
3.2 Angiogenic switch
For a tumor to thrive and grow beyond 1-2 mm2 in size it must acquire an angiogenic phenotype [88, 89]. Neutrophils have a prominent role in tumor angiogenesis both directly and indirectly (reviewed in [90]). Neutrophils have a large intracellular pool of VEGF that can become available upon degranulation. Interestingly, neutrophils in mice deficient in Hck and Fgr (hck−/−fgr−/−), two Src family protein tyrosine kinases expressed by granulocytes and monocytes, have impaired VEGF degranulation [91].
Using the transgenic multistage pancreatic tumor RIP-Tag2 model, where the rat insulin promoter (RIP) induces production of simian virus 40 (SV40) large T-antigen (Tag) oncoproteins in pancreatic islets, Nozawa et al. revealed that the initial angiogenic switch is mediated by MMP-9 derived from infiltrating neutrophils [92, 93]. Also in transplantable tumor models of murine melanoma (B16F10), fibrosarcoma (L929), Lewis lung carcinoma (LLC) and human prostate cancer cells (PC-3), infiltrating neutrophils are the major source of MMP-9 [94]. Importantly unlike other immune cells that also produce MMP-9, neutrophils supply the tumor with a TIMP-free form of MMP-9 that more efficiently induces angiogenesis [94-97]. Activate MMP9 remodels the extracellular matrix and thereby enhances the bioavailability of VEGF and fibroblast growth factor (FGF-2), another angiogenic factor, that otherwise are captured in the extracellular matrix [98, 99]. Intriguingly, neutrophil-derived MMP-9 is also a potent, direct and VEGF-independent angiogenic factor [100]. Transplantable tumor models and the RIP-Tag2 model have furthermore revealed that prokineticin-2 is expressed by CD11b+Gr-1+ cells and promotes not only CD11b+Gr-1+ cell mobilization but also angiogenesis [101, 102].
In contrast, interferon-β (IFNβ) negatively regulates neutrophil-induced angiogenesis [103]. In a tumor mouse model where melanoma B16F10 cells and MCA205 fibrosarcoma cells are transplanted in Ifnb1−/− or Ifnar1−/− syngeneic C57BL76 mice, the tumors grow faster compared to syngeneic control mice. The acceleration in tumor growth in IFNβ deficient mice is due to an increase of proangiogenic neutrophils recruited by CXCR2 ligands [103, 104]. The researchers concluded that adaptive immune cells are not involved in the IFNβ-dependent inhibition of tumor angiogenesis by repeating the experiments in Ifnb1−/−Rag2−/− and Rag2−/− mice that lack T and B-cells [103, 105]. Moreover, in the Ifnb1−/− mice model deficiency of IFNβ delays the apoptosis of pro-angiogenic neutrophils infiltrating the tumor [106].
4. Neutrophils in metastatic progression
Metastatic progression begins with tumor cells in the primary tumor gaining invasive and migratory capabilities that enable them to intravasate local blood and lymphatic vessels and thereby reach distant organs with the circulation. Neutrophils play a crucial role during metastatic progression – beginning in the primary tumor where they favor tumor invasion.
Murine breast cancer cells with Tgfbr2 deletion produce the CXCR2 ligand, CXCL5, and their tumors have enhanced infiltration of CD11b+Gr-1+ cells, which promote tumor invasion through MMP production [107]. Similar results have been obtained in the RETAAD model, a model of uveal melanoma where mice are transgenic for the activated oncogene RET [108]. In the RETAAD model CXCR2+ neutrophils infiltrate the primary tumors where they favor melanoma cell migration and induce an invasive phenotype of melanoma cells [109]. In yet another model of invasive and metastatic melanoma (due to transgenic overexpression of HGF and oncogenic mutation of CDK4(R24C) giving an impaired cell cycle control) ultraviolet radiation induces inflammation. The inflammation is mediated by neutrophils recruited by high mobility group box 1 (HGMB1) produced by UV-damaged epidermal keratinocytes. The infiltration of neutrophils promotes metastatic progression to the lungs through enhanced growth of melanoma cells along the abluminal side of endothelial cells with occasional perivascular invasion [110].
In addition to promoting metastatic progression in the primary tumor neutrophils expand systemically in tumor-bearing mice [33, 84, 111, 112]. Intriguingly, the recruitment of neutrophils begins before circulating tumor cells actually reach the metastatic sites. Hence, neutrophils are part of the pre-metastatic niche [113-115]. Tumor-derived G-CSF is an important attractant for neutrophils in many experimental models of cancer and recruits neutrophils to the lung pre-metastatic niche, while tumor-derived COX2 and PGE2 accumulate CD11b+Gr-1+ cells in the pre-metastatic niche of the brain [116, 117]. Recent work by Wculek and Malachani has further revealed that neutrophils accumulate in the lungs prior to tumor cell arrival; at the metastatic site they produce leukotrienes, which facilitate the colonization of a particular population of tumor cells that have high tumorigenic capacity [113]. The metastatic colonization of circulating tumor cells can further be facilitated by β2-integrin and Mac-1 on neutrophils, which bind ICAM on circulating tumor cells and thereby anchor the circulating tumor cells at the metastatic site [118, 119]. Moreover, neutrophils promote metastatic colonization with neutrophil extracellular traps (NETs) [120]. NETs are extracellular neutrophil-derived DNA structures used by neutrophils to capture circulating tumor cells at the metastatic site. Intriguingly, neutrophils in tumor-bearing mice are more prone to form NETs [113-115].
Although mounting evidence points to the fact that neutrophils promote metastatic progression, there are contradicting data. In a model of renal cell carcinoma deletion of important chemoattractants for neutrophils attenuates recruitment of neutrophils and increases metastatic tumor burden revealing an anti-metastatic role of neutrophils [121]. Furthermore, Granot et al. showed in a model of breast cancer that CCL2 derived from the primary tumor induces an anti-metastatic phenotype of neutrophils, which significantly inhibits the formation of lung metastases [122].
5. Future perspectives and conclusions
It is becoming evident that neutrophils have a prominent role, both preventing and promoting tumor development and metastatic progression. Their dual role indicates that neutrophils are not a homogenous population but make up a continuum of different phenotypes. Accordingly, there are different populations of circulating neutrophils and depending on the stage of tumor development, tumors appear to be infiltrated by either anti- (N1) or protumoral (N2) neutrophils [4, 7, 40]. Intriguingly, protumoral neutrophils can have strikingly different transcriptional profiles implying that there are far more distinct populations of neutrophils [8]. Single-cell analysis could provide a powerful tool to uncover the complex heterogeneity of neutrophils, not only in experimental animal models of cancer, but more importantly in human specimens, and give key insights into the plasticity of neutrophils [123, 124].
The differentiation of neutrophils is dictated by the tumor context. Tumor-derived TGFβ, for example, promotes the differentiation of N2 TANs and high-density neutrophils are able to differentiate into immunosuppressive low-density neutrophils in a TGFβ-dependent manner [4, 40]. Presumably, there is a plethora of other factors, acting autonomously or in combinations that can determine the fate of neutrophils. To develop therapeutics that can tune neutrophils into becoming antitumoral it will be vital to identify the factors that can induce a N1 phenotype. Importantly, N1 and N2 neutrophils have only been explored in murine tumor models; hence the classification of N1 and N2 neutrophils and the factors dictating the fate of neutrophils will have to be further confirmed in human cancers.
Tumor-derived factors will not only differentiate neutrophils but also cause their expansion and recruitment. It is however becoming evident that non-tumoral cells in the tumor microenvironment also attract neutrophils. UV-damaged keratinocytes recruit neutrophils that promote metastatic progression of melanoma cells; H2O2 derived from transformed melanocytes but also from their wounded non-tumoral neighbors attracts neutrophils in a melanoma zebrafish model and in a model of metastatic breast cancer, tumor cells produce IL-1β, activating γδ T-cells, which causes neutrophil expansion [74, 110, 125, 126]. Hence, not only tumor cells but also non-tumoral cells recruit neutrophils and have to be taken into account when elucidating how neutrophils are being recruited during tumor progression.
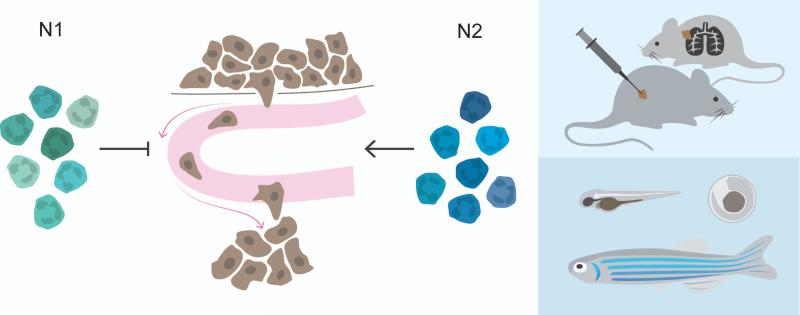
The exact origin of recruited neutrophils is still unknown. One possibility could be that N1 TANs originate from high-density circulating neutrophils, which are more predominant during early tumor development, while N2 TANs emanate from circulating low-density neutrophils, which are immunosuppressive and accumulate with tumor progression[40]. On the contrary, TANs might be the origin of different circulating neutrophil populations (Figure 2). Indeed, retrograde migration of neutrophils, from the site of tissue perturbation, back to the circulation has been reported in the context of inflammation but still has to be proven in experimental animal models of cancer [127-129]. Recently, a new genetically engineered mouse model exhibited successful intravital imaging of neutrophils giving it an important advantage when, e.g., attempting to visualize neutrophil-retrograde migration [130].
Figure 2.
Neutrophils make up a continuum of phenotypes. The tumor microenvironment can influence the polarization state of TANs. TGFβ can differentiate neutrophils toward a protumoral N2 phenotype. The exact origin of recruited neutrophils is still unknown. N1 TANs might originate from high-density circulating neutrophils, while N2 TANs emanate from immunosuppressive low-density neutrophils. Another alternative could be that the local tumor microenvironment dictates the fate of recruited neutrophils. On the contrary, TANs might be the origin of different circulating neutrophil populations.
Taken together neutrophils have an important role in tumor progression, which cannot be overlooked, independent of whether it is anti- or protumoral. The contradictory role of neutrophils in tumor development and metastatic progression can be attributed to the heterogeneity within the neutrophil population. The identification and importance of different neutrophil populations will be uncovered with single-cell technology and functionally attributed with recent development of cluster regularly interspersed short palindromic repeats (CRISPR)-Cas9 [131, 132]. The use of experimental animal models has been and will hence continue to be important in our effort to expand our knowledge about how neutrophils influence tumor development and metastatic progression.
Highlights.
Neutrophils have a dual role in tumor development and metastatic progression
Neutrophils are not a homogenous population but make up a continuum of phenotypes
Mouse and zebrafish models provide key insights into how neutrophils influence tumor development
Acknowledgements
This work was supported by funds from the National Cancer Institute (R01 CA057621), the Wenner-Gren Foundations and the Sweden-America foundation. The authors thank Carl Hagerling for help with illustrating the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Sionov RV, Fridlender ZG, Granot Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer microenvironment : official journal of the International Cancer Microenviron. 2014 doi: 10.1007/s12307-014-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swierczak A, Mouchemore KA, Hamilton JA, Anderson RL. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015;34:735–751. doi: 10.1007/s10555-015-9594-9. [DOI] [PubMed] [Google Scholar]
- 3.Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol Res. 2016;4:83–91. doi: 10.1158/2326-6066.CIR-15-0313. [DOI] [PubMed] [Google Scholar]
- 4.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Wu D, Ni C, Ye J, Chen W, Hu G, et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother. : CII. 2013;62:1745–1756. doi: 10.1007/s00262-013-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elpek KG, Cremasco V, Shen H, Harvey CJ, Wucherpfennig KW, Goldstein DR, et al. The tumor microenvironment shapes lineage, transcriptional, and functional diversity of infiltrating myeloid cells. Cancer Immunol Res. 2014;2:655–667. doi: 10.1158/2326-6066.CIR-13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 10.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 11.Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188:49–71. doi: 10.1016/s0300-483x(03)00043-x. [DOI] [PubMed] [Google Scholar]
- 12.Rangarajan A, Weinberg RA. Opinion: Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2003;3:952–959. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- 13.Jonkers J, Berns A. Conditional mouse models of sporadic cancer. Nat Rev Cancer. 2002;2:251–265. doi: 10.1038/nrc777. [DOI] [PubMed] [Google Scholar]
- 14.Maddison K, Clarke AR. New approaches for modelling cancer mechanisms in the mouse. J Path. 2005;205:181–193. doi: 10.1002/path.1698. [DOI] [PubMed] [Google Scholar]
- 15.Politi K, Pao W. How genetically engineered mouse tumor models provide insights into human cancers. Journal of clinical oncology : official journal of the J Clin Oncol. 2011;29:2273–2281. doi: 10.1200/JCO.2010.30.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fantozzi A, Christofori G. Mouse models of breast cancer metastasis. Breast Cancer Res. : BCR. 2006;8:212. doi: 10.1186/bcr1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eklund L, Bry M, Alitalo K. Mouse models for studying angiogenesis and lymphangiogenesis in cancer. Mol Oncology. 2013;7:259–282. doi: 10.1016/j.molonc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxena M, Christofori G. Rebuilding cancer metastasis in the mouse. Mol Oncology. 2013;7:283–296. doi: 10.1016/j.molonc.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dranoff G. Experimental mouse tumour models: what can be learnt about human cancer immunology? Nat Rev Immunol. 2012;12:61–66. doi: 10.1038/nri3129. [DOI] [PubMed] [Google Scholar]
- 20.Takeda Y, Costa S, Delamarre E, Roncal C, Leite de Oliveira R, Squadrito ML, et al. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature. 2011;479:122–126. doi: 10.1038/nature10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522:349–353. doi: 10.1038/nature14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito R, Takahashi T, Katano I, Ito M. Current advances in humanized mouse models. Cell Mol Immunol. 2012;9:208–214. doi: 10.1038/cmi.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt MR, Appel MC, Giassi LJ, Greiner DL, Shultz LD, Woodland RT. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PloS One. 2008;3:e3192. doi: 10.1371/journal.pone.0003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danner R, Chaudhari SN, Rosenberger J, Surls J, Richie TL, Brumeanu TD, et al. Expression of HLA class II molecules in humanized NOD.Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PloS One. 2011;6:e19826. doi: 10.1371/journal.pone.0019826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lockridge JL, Chen X, Zhou Y, Rajesh D, Roenneburg DA, Hegde S, et al. Analysis of the CD1 antigen presenting system in humanized SCID mice. PloS One. 2011;6:e21701. doi: 10.1371/journal.pone.0021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotech. 2014;32:364–372. doi: 10.1038/nbt.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukocyte Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 29.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 30.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 33.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandau S, Moses K, Lang S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins? Seminars Cancer Biol. 2013;23:171–182. doi: 10.1016/j.semcancer.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Haverkamp JM, Smith AM, Weinlich R, Dillon CP, Qualls JE, Neale G, et al. Myeloid-derived suppressor activity is mediated by monocytic lineages maintained by continuous inhibition of extrinsic and intrinsic death pathways. Immunity. 2014;41:947–959. doi: 10.1016/j.immuni.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 37.Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865–2876. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 38.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, et al. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PloS one. 2012;7:e31524. doi: 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 41.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. : CMLS. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 43.Haqqani AS, Sandhu JK, Birnboim HC. Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia. 2000;2:561–568. doi: 10.1038/sj.neo.7900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomez-Mejiba SE, Zhai Z, Gimenez MS, Ashby MT, Chilakapati J, Kitchin K, et al. Myeloperoxidase-induced genomic DNA-centered radicals. J Biol Chem. 2010;285:20062–20071. doi: 10.1074/jbc.M109.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gungor N, Knaapen AM, Munnia A, Peluso M, Haenen GR, Chiu RK, et al. Genotoxic effects of neutrophils and hypochlorous acid. Mutagenesis. 2010;25:149–154. doi: 10.1093/mutage/gep053. [DOI] [PubMed] [Google Scholar]
- 46.Sandhu JK, Privora HF, Wenckebach G, Birnboim HC. Neutrophils, nitric oxide synthase, and mutations in the mutatect murine tumor model. Am J Path. 2000;156:509–518. doi: 10.1016/S0002-9440(10)64755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkinson D, Sandhu JK, Breneman JW, Tucker JD, Birnboim HC. Hprt mutants in a transplantable murine tumour arise more frequently in vivo than in vitro. Brit J Cancer. 1995;72:1234–1240. doi: 10.1038/bjc.1995.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14:3937–3947. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shang K, Bai YP, Wang C, Wang Z, Gu HY, Du X, et al. Crucial involvement of tumor-associated neutrophils in the regulation of chronic colitis-associated carcinogenesis in mice. PloS One. 2012;7:e51848. doi: 10.1371/journal.pone.0051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, et al. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller MM. Inflammation in epithelial skin tumours: old stories and new ideas. EurJ Cancer. 2006;42:735–744. doi: 10.1016/j.ejca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 56.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 57.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 58.Aruga A, Aruga E, Cameron MJ, Chang AE. Different cytokine profiles released by CD4+ and CD8+ tumor-draining lymph node cells involved in mediating tumor regression. J Leukocyte Biol. 1997;61:507–516. doi: 10.1002/jlb.61.4.507. [DOI] [PubMed] [Google Scholar]
- 59.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 60.Jaillon S, Galdiero MR, Del Prete D, Cassatella MA, Garlanda C, Mantovani A. Neutrophils in innate and adaptive immunity. Sem Immunopath. 2013;35:377–394. doi: 10.1007/s00281-013-0374-8. [DOI] [PubMed] [Google Scholar]
- 61.Richards H, Williams A, Jones E, Hindley J, Godkin A, Simon AK, et al. Novel role of regulatory T cells in limiting early neutrophil responses in skin. Immunology. 2010;131:583–592. doi: 10.1111/j.1365-2567.2010.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neville ME, Pezzella KM, Schmidt K, Galbraith W, Ackerman N. In vivo inhibition of tumor growth of B16 melanoma by recombinant interleukin 1 beta. II. Mechanism of inhibition: the role of polymorphonuclear leukocytes. Cytokine. 1990;2:456–463. doi: 10.1016/1043-4666(90)90055-x. [DOI] [PubMed] [Google Scholar]
- 63.Souto JC, Vila L, Bru A. Polymorphonuclear neutrophils and cancer: intense and sustained neutrophilia as a treatment against solid tumors. Medicinal Res Rev. 2011;31:311–363. doi: 10.1002/med.20185. [DOI] [PubMed] [Google Scholar]
- 64.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Gen Dev. 2010;20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antonio N, Bonnelykke-Behrndtz ML, Ward LC, Collin J, Christensen IJ, Steiniche T, et al. The wound inflammatory response exacerbates growth of preneoplastic cells and progression to cancer. EMBO J. 2015;34:2219–2236. doi: 10.15252/embj.201490147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White R, Rose K, Zon L. Zebrafish cancer: the state of the art and the path forward. Nat Rev Cancer. 2013;13:624–636. doi: 10.1038/nrc3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harvie EA, Huttenlocher A. Neutrophils in host defense: new insights from zebrafish. J Leukocyte Biol. 2015;98:523–537. doi: 10.1189/jlb.4MR1114-524R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shelef MA, Tauzin S, Huttenlocher A. Neutrophil migration: moving from zebrafish models to human autoimmunity. Immunol Rev. 2013;256:269–281. doi: 10.1111/imr.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feng Y, Martin P. Imaging innate immune responses at tumour initiation: new insights from fish and flies. Nat Rev Cancer. 2015;15:556–562. doi: 10.1038/nrc3979. [DOI] [PubMed] [Google Scholar]
- 70.Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE. Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood. 2001;98:3087–3096. doi: 10.1182/blood.v98.10.3087. [DOI] [PubMed] [Google Scholar]
- 71.Le Guyader D, Redd MJ, Colucci-Guyon E, Murayama E, Kissa K, Briolat V, et al. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- 72.Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev Compar Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 73.Michailidou C, Jones M, Walker P, Kamarashev J, Kelly A, Hurlstone AF. Dissecting the roles of Raf- and PI3K-signalling pathways in melanoma formation and progression in a zebrafish model. Disease Mod Mech. 2009;2:399–411. doi: 10.1242/dmm.001149. [DOI] [PubMed] [Google Scholar]
- 74.Feng Y, Renshaw S, Martin P. Live imaging of tumor initiation in zebrafish larvae reveals a trophic role for leukocyte-derived PGE(2). Curr Biol. 2012;22:1253–1259. doi: 10.1016/j.cub.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feng Y, Santoriello C, Mione M, Hurlstone A, Martin P. Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 2010;8:e1000562. doi: 10.1371/journal.pbio.1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, et al. Tumourinfiltrating Gr-1+ myeloid cells antagonize senescence in cancer. Nature. 2014;515:134–137. doi: 10.1038/nature13638. [DOI] [PubMed] [Google Scholar]
- 80.Distler M, Aust D, Weitz J, Pilarsky C, Grutzmann R. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. BioMed Res Internat. 2014;2014:474905. doi: 10.1155/2014/474905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 82.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Casbon AJ, Reynaud D, Park C, Khuc E, Gan DD, Schepers K, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A. 2015;112:E566–575. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 88.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncology. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 89.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 90.Tazzyman S, Niaz H, Murdoch C. Neutrophil-mediated tumour angiogenesis: subversion of immune responses to promote tumour growth. Semin Cancer Biol. 2013;23:149–158. doi: 10.1016/j.semcancer.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Scapini P, Morini M, Tecchio C, Minghelli S, Di Carlo E, Tanghetti E, et al. CXCL1/macrophage inflammatory protein-2-induced angiogenesis in vivo is mediated by neutrophil-derived vascular endothelial growth factor-A. J Immunol i. 2004;172:5034–5040. doi: 10.4049/jimmunol.172.8.5034. [DOI] [PubMed] [Google Scholar]
- 92.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A . 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 94.Deryugina EI, Zajac E, Juncker-Jensen A, Kupriyanova TA, Welter L, Quigley JP. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia. 2014;16:771–788. doi: 10.1016/j.neo.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12:233. doi: 10.1186/gb-2011-12-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J. Biol Chem. 2009;284:25854–25866. doi: 10.1074/jbc.M109.033472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bekes EM, Schweighofer B, Kupriyanova TA, Zajac E, Ardi VC, Quigley JP, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Path. 2011;179:1455–1470. doi: 10.1016/j.ajpath.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bausch D, Pausch T, Krauss T, Hopt UT, Fernandez-del-Castillo C, Warshaw AL, et al. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis. 2011;14:235–243. doi: 10.1007/s10456-011-9207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 102.Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci U S A. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jablonska J, Wu CF, Andzinski L, Leschner S, Weiss S. CXCR2-mediated tumor-associated neutrophil recruitment is regulated by IFN-beta. Internat J Cancer. 2014;134:1346–1358. doi: 10.1002/ijc.28551. [DOI] [PubMed] [Google Scholar]
- 105.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 106.Andzinski L, Wu CF, Lienenklaus S, Kroger A, Weiss S, Jablonska J. Delayed apoptosis of tumor associated neutrophils in the absence of endogenous IFN-beta. Internat J Cancer. 2015;136:572–583. doi: 10.1002/ijc.28957. [DOI] [PubMed] [Google Scholar]
- 107.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kato M, Takahashi M, Akhand AA, Liu W, Dai Y, Shimizu S, et al. Transgenic mouse model for skin malignant melanoma. Oncogene. 1998;17:1885–1888. doi: 10.1038/sj.onc.1202077. [DOI] [PubMed] [Google Scholar]
- 109.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507:109–113. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 111.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A, et al. Neutrophil Extracellular Traps Accumulate in Peripheral Blood Vessels and Compromise Organ Function in Tumor-Bearing Animals. Cancer Res. 2015;75:2653–2662. doi: 10.1158/0008-5472.CAN-14-3299. [DOI] [PubMed] [Google Scholar]
- 113.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev. 2013;32:449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- 115.Sceneay J, Chow MT, Chen A, Halse HM, Wong CS, Andrews DM, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 116.Liu Y, Kosaka A, Ikeura M, Kohanbash G, Fellows-Mayle W, Snyder LA, et al. Premetastatic soil and prevention of breast cancer brain metastasis. Neurooncology. 2013;15:891–903. doi: 10.1093/neuonc/not031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kowanetz M, Wu X, Lee J, Tan M, Hagenbeek T, Qu X, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70:6071–6082. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012;72:3919–3927. doi: 10.1158/0008-5472.CAN-11-2393. [DOI] [PubMed] [Google Scholar]
- 120.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013 doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lopez-Lago MA, Posner S, Thodima VJ, Molina AM, Motzer RJ, Chaganti RS. Neutrophil chemokines secreted by tumor cells mount a lung antimetastatic response during renal cell carcinoma progression. Oncogene. 2013;32:1752–1760. doi: 10.1038/onc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chattopadhyay PK, Gierahn TM, Roederer M, Love JC. Single-cell technologies for monitoring immune systems. Nat Immunol. 2014;15:128–135. doi: 10.1038/ni.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shapiro E, Biezuner T, Linnarsson S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat Rev Genetics. 2013;14:618–630. doi: 10.1038/nrg3542. [DOI] [PubMed] [Google Scholar]
- 125.Derksen PW, Liu X, Saridin F, van der Gulden H, Zevenhoven J, Evers B, et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell. 2006;10:437–449. doi: 10.1016/j.ccr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 126.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mathias JR, Perrin BJ, Liu TX, Kanki J, Look AT, Huttenlocher A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukocyte Biol. 2006;80:1281–1288. doi: 10.1189/jlb.0506346. [DOI] [PubMed] [Google Scholar]
- 128.Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, Schmutz C, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukocyte Biol. 2006;79:303–311. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- 129.Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, Diapouli FM, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hasenberg A, Hasenberg M, Mann L, Neumann F, Borkenstein L, Stecher M, et al. Catchup: a mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat Methods. 2015;12:445–452. doi: 10.1038/nmeth.3322. [DOI] [PubMed] [Google Scholar]
- 131.Sanchez-Rivera FJ, Jacks T. Applications of the CRISPR-Cas9 system in cancer biology. Nat Rev Cancer. 2015;15:387–395. doi: 10.1038/nrc3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mou H, Kennedy Z, Anderson DG, Yin H, Xue W. Precision cancer mouse models through genome editing with CRISPR-Cas9. Genome Med. 2015;7:53. doi: 10.1186/s13073-015-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]