Abstract
Plasmodium parasites are exposed to endogenous and exogenous oxidative stress during their complex life cycle. To minimize oxidative damage, the parasites use glutathione (GSH) and thioredoxin (Trx) as primary antioxidants. We previously showed that disruption of the Plasmodium berghei gamma-glutamylcysteine synthetase (pbggcs-ko) or the glutathione reductase (pbgr-ko) genes resulted in a significant reduction of GSH in intraerythrocytic stages, and a defect in growth in the pbggcs-ko parasites. In this report, time course experiments of parasite intraerythrocytic development and morphological studies showed a growth delay during the ring to schizont progression. Morphological analysis shows a significant reduction in size (diameter) of trophozoites and schizonts with increased number of cytoplasmic vacuoles in the pbggcs-ko parasites in comparison to the wild type (WT). Furthermore, the pbggcs-ko mutants exhibited an impaired response to oxidative stress and increased levels of nuclear DNA (nDNA) damage. Reduced GSH levels did not result in mitochondrial DNA (mtDNA) damage or protein carbonylations in neither pbggcs-ko nor pbgr-ko parasites. In addition, the pbggcs-ko mutant parasites showed an increase in mRNA expression of genes involved in oxidative stress detoxification and DNA synthesis, suggesting a potential compensatory mechanism to allow for parasite proliferation. These results reveal that low GSH levels affect parasite development through the impairment of oxidative stress reduction systems and damage to the nDNA. Our studies provide new insights into the role of the GSH antioxidant system in the intraerythrocytic development of Plasmodium parasites, with potential translation into novel pharmacological interventions.
Keywords: Plasmodium berghei, malaria, glutathione, oxidative stress, growth delay, DNA damage, protein carbonylations
Introduction
Malaria remains the most deadly parasitic disease resulting in approximately 584,000 deaths from 198 million clinical cases reported in 2013 and one third of the world population at risk of infection [1]. This disease is caused by the apicomplexan parasites of the genus Plasmodium, which are transmitted to humans by the bite of infected Anopheles mosquitoes.
Plasmodium parasites are exposed to multiple sources of oxidative stress throughout their complex life cycle. During the intraerythrocytic development, endogenous oxidative stress results from reactive oxygen species (ROS) produced during hemoglobin (Hb) digestion in the parasite’s food vacuole [2, 3]. In addition, the parasite is exposed to exogenous oxidative stress when merozoites egress from the red blood cells (RBC), inducing the production of nitric oxide and ROS by the host’s immune system [2, 4]. Plasmodium parasites depend on two major NADPH-dependent redox networks, the GSH and the Trx antioxidant systems, to detoxify ROS and prevent oxidative damage [5].
Plasmodium synthesizes GSH by the sequential action of the rate limiting enzyme γ-glutamylcysteine synthetase (γ-GCS), and the glutathione synthetase [6]. The reduced GSH is oxidized to GSH disulfide (GSSG) by the reduction of glutaredoxin or glutathione-S-transferase (GST) [3, 6, 7]. The oxidized GSSG is reduced back to GSH by the glutathione reductase (GR), maintaining the GSH/GSSG ratio in the cell [8]. Glutathione plays an important role in a wide range of cellular processes including the detoxification of xenobiotics and protection against ROS [7, 9, 10]. In Plasmodium parasites, GSH is also involved in the degradation of the toxic ferritoprotoporphyrin IX (FP IX), escaped from hemozoin formation [11]. Additionally, GSH functions as an electron donor for the enzyme ribonucleotide reductase (RNR), [12, 13], crucial for DNA synthesis and cellular proliferation [2, 3, 10].
Previous reports from our laboratory demonstrated that disruption of the pbggcs gene in Plasmodium berghei resulted in mutant parasites displaying reduced GSH levels and growth impairment during intraerythrocytic development [14]. Moreover, disruption of the pbggcs gene inhibited oocyst development and the production of sporozoites in the mosquito, indicating that GSH biosynthesis is critical to complete parasite transmission [14]. Similarly, P. berghei parasites with a disrupted glutathione reductase (pbgr-ko) gene displayed significantly reduced GSH levels and interruption of parasite development in the mosquito, with parasites arrested at the oocyst stage [15]. Although the relevance of GSH for parasite development has been shown, the underlying mechanisms of the delayed parasite development as a consequence of reduced GSH levels as well as the oxidative status of the mutant parasites deserves further investigation.
In this study, we further characterized the effects of reduced GSH levels in P. berghei and demonstrate that parasites with significantly low levels of GSH show a growth delay during the ring to schizont transition, have significantly smaller trophozoites and schizonts and a vacuolated cytoplasm. These changes occur concomitantly with an impairment capacity to handle oxidative stress and increased levels of nDNA damage. These results show for the first time a causal interplay between low GSH levels, impaired response to oxidative stress, oxidative DNA damage, and delayed parasite growth during the ring to schizont transition. The findings presented herein provide a better understanding of the need for maintaining GSH homeostasis during P. berghei development. Because the redox balance plays a vital role for parasite survival, our results have important applications for antimalarial treatments. Strategies aiming to promote oxidative stress and/or inhibiting the parasite antioxidant system will lead to a redox imbalance affecting parasite growth, and therefore a potential improvement in treatment response.
Materials and Methods
Mice and P. berghei lines
Random-bred Swiss albino CD-1 female mice (Charles River Laboratories, Wilmington, MA, USA), 6–8 weeks old, weighing 20–35 g were used for the study. All mice procedures were conducted at the AAALAC accredited University of Puerto Rico Medical Sciences Campus (UPR-MSC) Animal Resources Center and approved by the Institutional Animal Care and Use Committee (IACUC). All work was done in strict accordance with the “Guide for the Care and Use of Laboratory Animals” (National-Research-Council, Current Edition) and regulations of the PHS Policy on Humane Care and Use of Laboratory Animals. Mice were maintained and housed according to NIH and AAALAC regulations and guidelines. Mice were allowed to acclimatize for 1 week prior to the beginning of the studies. The P. berghei ANKA WT, reference line (507cl1), which expresses green fluorescent protein under the control of the constitutive eukaryotic elongation factor 1A promoter, was used as a control in all experiments [16]. The following P. berghei lines (mutants) were included in the study: pbggcs-ko1 and pbggcs-ko2 [14], and pbgr-ko1 and pbgr-ko2 [15].
Intraerythrocytic growth assay
To investigate the delayed parasitemia of pbggcs-ko parasites in mice [14], WT and pbggcs-ko parasites were synchronized in in vitro cultures as described by Janse and Waters [17]. Briefly, P. berghei infected blood was harvested from mice with 5–15% parasitemia, diluted in complete medium [RPMI 1640 (Gibco ), 25% heat inactivated fetal bovine serum (Gibco ), 50 IU/ml of neomycin (Sigma)] and cultured for 24–26 h at 37°C in an atmosphere of 10% O2, 5% CO2 and 85% N2 gas mixture. The schizonts were purified by 55% Nycodenz gradient [17] and injected intravenously into mice [18]. The synchronized ring infected blood was collected from mice 4–6 h post infection and cultured in vitro for a total of 28 h. The parasite intraerythrocytic development was analyzed by light microscopy of Diff-Quick stained thin smears in samples collected every 2 h, beginning after the initial 16 h of culture. Parasite intraerythrocytic developmental stages (rings, trophozoites and schizonts) and morphology were determined by counting at least 100 parasites per slide. Images were acquired with a microscope (BX51, Olympus) at 100X magnification using a digital camera (DP72, Olympus). The size of trophozoites and schizonts stages was assessed at 16, 24 and 28 h and vacuoles at 16 h in WT, pbggcs-ko1 and pbggcs-ko2 parasites using a calibrated ocular micrometer at 100X magnification (n=20).
Determination of intraparasitic response to oxidative stress levels by measuring the oxidation of 2′, 7′-dichlorodihydrofluorescein (DCFH)
To determine the levels of intraparasitic response to oxidative stress, the fluorescent intensity of dichlorofluorescein (DCF), the green fluorescent oxidation product of DFCH, was determined in WT and pbggcs-ko parasites cultured in vitro. The DCF fluorescent intensity was measured in the basal (untreated) or hydrogen peroxide (H2O2)-treated cultures as described by Trivedi et al. [19]. Briefly, P. berghei infected blood was cultured, as previously described, in the presence or absence of 2 mM H2O2 for 1 h. The cultures were then incubated with 10 μM of 2,7-dichlorofluorescein diacetate for 30 min at 37°C, washed with 0.01M, potassium phosphate buffer (PBS) (pH 7.4), centrifuged and subsequently lysed with saponin (0.15%) for 10 minutes at 4°C. Parasites were isolated by centrifugation at 1300 xg for 5 min at 10°C and lysed by sonication (30 s pulse, bath-type sonicator) at 4°C. Fluorescent intensities were recorded from the lysates in a spectrofluorometer (SpectraMax M3, Molecular Devices ) at 502 nm and 523 nm excitation and emission wavelengths, respectively. Protein concentrations were determined in each well using the Pierce™ ModifiedLowry Protein Assay Kit (Thermo Scientific) and data was expressed as fluorescence intensity/milligram of parasite lysate protein.
DNA Isolation and Quantification
High molecular weight genomic DNA was isolated from WT, pbggcs-ko and pbgr-ko parasites using a Genomic DNA extraction Kit (Qiagen ) following the manufacturer’s instructions. Quantification of DNA was performed using the PicoGreen® dsDNA Quantitation Reagent (Molecular Probes®) with modifications as previously described [20, 21]. To determine the DNA concentration of the samples, a DNA standard curve was constructed using lambda DNA. The PicoGreen® excitation and emission fluorescence intensities were measured at 485 nm and 530 nm, respectively, using a fluorescence microplate reader (Wallac 1420 Victor2™, PerkinElmer). The integrity of the genomic DNA was examined by analyzing the samples in a 0.8% ethidium bromide-containing agarose gel. The DNA was visualized using UV light (Gel Doc XR System, Bio-Rad).
Analysis of nuclear and mitochondrial DNA damage using the quantitative polymerase chain reaction (QPCR) assay
A QPCR assay was modified to determine the levels of DNA damage to the parasite’s nuclear and mitochondrial genomes as we previously described [22]. The underlying principle of the QPCR assay is that certain types of DNA lesions will prevent the DNA polymerase to effectively replicate the DNA fragment; thereby resulting in a decrease in the amplification yield. To ensure that the QPCR assay was performed within the linear range of amplification, optimal conditions for amplification of the nDNA and mtDNA fragments were determined by performing cycle and template tests. The initial DNA template concentration used for the amplification of nDNA and mtDNA was 5 ng and 7.5 ng, respectively. As a control to ensure quantitative conditions of amplification, a DNA sample with two fold the initial quantity of DNA concentration (10 ng and 15 ng of nDNA and mtDNA template, respectively) was amplified in duplicate. A 40–60% increase in amplification of the control target sequence was considered within the linear range of amplification and adequate for data analysis. To detect nDNA lesions a 6.1 kb fragment from the seryl-tRNA synthetase nuclear gene was amplified using the MasterAmp™ Extra-long PCR Reagents (Epicentre®). An initial denaturation was carried out for 45 s at 94°C, 24 cycles of denaturation for 15 s at 94°C, annealing-extension at 68°C for 12 min, and a final extension at 72°C for 10 min using the following primers: 5′-CACTATCAACATATTCATCTCTTGTTTC-3′ (forward) and 5′-GCTCTATGGACACCAATAGTTCCAAGGG-3′ (reverse).
To detect mtDNA lesions a fragment of 5.7 kb of the mitochondrial genome was amplified using 5′-GAATAGTGGTATAGTCATATCTCCATGAAC-3′ (forward) and 5′-TGGTATCTCGTAATGTAGAACAATAATAGG-3′ (reverse) as primers. The amplification conditions consisted on an initial denaturation for 45 s at 94°C, 21 cycles of denaturation for 15 s at 94°C and annealing-extension at 68°C for 12 min, and a final extension at 72°C for 10 min. To confirm that a single product of the expected size was obtained, the 6.1 kb and 5.7 kb PCR products were resolved on 1% ethidium bromide-stained agarose gels and visualized using UV-light (Gel Doc XR System, Bio-Rad). Nuclear and mitochondrial PCR products were quantified using PicoGreen. The background fluorescence was obtained from a sample without DNA template and subtracted from each experimental sample [20]. Background values were determined in duplicate. The results of P. berghei mutant parasites are expressed as DNA amplification levels relative to the levels of DNA amplification from WT parasites.
Quantification of mtDNA abundance by quantitative polymerase chain reaction (QPCR) assay
Because the number of mtDNA molecules vary between cells, it was critical to make certain that the amplification of the 5.7 kb mtDNA fragment was not affected by possible changes in the abundance of mtDNA molecules. A small mtDNA fragment of 186 bp was amplified to provide a precise measure of mtDNA molecules/abundance because small DNA fragments are unlikely to be damaged. Cycle and template tests were performed to establish optimal conditions of amplification. To assure quantitative PCR conditions, a DNA sample with 50% of the initial quantity of DNA concentration (2.5 ng) was amplified in duplicate. The PCR profile consisted on an initial denaturation for 45 s at 94°C, 25 cycles of denaturation for 15 s at 94°C, annealing for 45 s at 60°C and extension for 45 s at 72°C, and a final extension at 72°C for 10 min. The primer nucleotide sequences for the amplification of the 186 bp mtDNA fragment are 5′-ATTAACAACTATAGCATTATCTGGATGAGA-3′ (forward) and 5′-TTCTTTTTACATTTACATGGTAGCACTAAT-3′ (reverse). PCR products were quantified using PicoGreen after subtracting the background fluorescence. To confirm that a single product of 186 bp was amplified, PCR products were resolved on ethidium bromide-stained 2% polyacrylamide gels and visualized under UV-light (Gel Doc XR System, Bio-Rad). The abundance of mtDNA molecules of P. berghei mutant parasites is expressed relative to the mtDNA abundance in WT parasites.
Determination of DNA lesion frequencies
Because DNA lesions occur as independent events and, thus, are randomly distributed across the genome, the Poisson equation was used to calculate the frequency of DNA lesions per 10 kb/strand as previously described [22]. Using the Poisson distribution, a decrease in amplification is converted into lesion frequency considering that amplification is directly proportional to the portion of undamaged DNA templates (zero class molecules; x=0). Then, the average lesion frequency per DNA strand can be calculated as, λ = −lnAD/AO where AD denotes the amount of amplification product of the damaged DNA template and AO is the amplification product from undamaged DNA. The results of P. berghei mutant parasites are expressed as a relative amplification ratio (AD/AO) and as lesion frequency per 10 kb per strand relative to WT.
Quantification of protein carbonylations by the FTC fluorometric assay
Blood containing P. berghei parasites were harvested from infected mice with 5–15% parasitemia. White blood cells were removed using a Plasmodipur filter (Euro-Diagnostica), washed with 0.01 M PBS (pH 7.4), and the RBC pellet resuspended in 0.01 M PBS (pH 7.4), 1mM DTT, 0.5 mM PMSF and 1 mM EGTA followed by RBC lysis with 0.15% saponin. Samples were centrifuged at 1077 xg for 8 min and the parasite pellet was resuspended in protein isolation buffer (1% DOC and 10% SDS) containing a cocktail of protease inhibitors (0.01mg of leupeptin A, 0.001 mg of pepstatin A, 0.35 mg of PMSF). The supernatant was collected, and DTT was added to a final concentration of 75 mM, to avoid spontaneous protein oxidation, and stored at −80°C until further use. The protein concentration was determined using the Bicinchoninic acid (BCA) assay (Bio-Rad DC™ Protein Assay) or the RC DC™ Protein Assay (Bio-Rad). Infected blood was cultured for 24–28 h in complete RPMI medium in a low oxygen (10%) atmosphere as described above. Schizont cultures were harvested, diluted with 0.01 M PBS (pH 7.4), centrifuged, and subsequently lysed to obtain the parasite proteins as described above. Carbonyl content of proteins was assessed in parasites from mice blood (rings, trophozoites and schizonts) and in schizonts from cultures using a modified fluorometric assay [23]. Briefly, proteins (60 μg) were incubated with 50 μl of 0.2 mM fluorescein 5-thiosemicarbazide (FTC) overnight in the dark, precipitated with acetone, air dried, and solubilized with 50 μl of 6M guanidine hydrochloride. Protein precipitates were sonicated (30 s pulse, bath-type sonicator) for 10 min, vortexed, resuspended in 450 μl of 0.1 M PBS, vortexed, centrifuged (10,000 xg for 10 min at 4°C) and the supernatant aliquoted (150 μl/well) in triplicate into a black 96-well microplate (Thermo Scientific™). The fluorescence intensity was determined in a spectrofluorometer (Wallac 1420 VICTOR F) with excitation at 485 nm and emission at 535 nm. Protein concentrations were determined in each well using the Micro BCA™ Protein Assay Kit (Pierce). The average of the fluorescence readings and the protein concentrations were measured in triplicate from 2–4 independent experiments. Nanomoles (nmol) of FTC-reacted carbonyls were calculated from the readings of FTC standard curve. Carbonyl content was expressed as nmol/milligram of parasite lysate protein.
Quantification of mRNA expression
Total RNA from parasite intraerythrocytic stages (5–15% parasitemia) was extracted using Tri-Reagent (Molecular Research Center, Inc.) following the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized using the SuperScript® VILO kit (Life Technologies). The P. berghei sequences of target genes were retrieved from PlasmoDB at plasmodb.org (Supplemental Table 1). Analysis of gene expression was done by RT-qPCR using the StepOnePlus™ System (Applied Biosystems) with the Fast SYBRR® Green Master Mix and 50 ng/μL of cDNA per sample. The samples were assayed in triplicate from three independent experiments. Gene expression data was analyzed using the StepOne™ Software v2.2 (Applied Biosystems) and normalized against the expression of 18S rRNA (Supplemental Table 1) [24] according to the 2−Δ ΔCt method [25, 26]. Melt curve analysis was used to confirm the specificity of amplified PCR products.
Modeling of Parasite Growth
A non-linear curve fit using the Weibull model for the growth curve of the parasite schizonts stages was applied Y = YM − YM − Y0 e−(kx)g. Parameters of this function were used to determine the delay in growth of the WT and mutant parasites. Specifically, YM corresponds to the maximum in the growth curve, Y0 to the initial value of the growth curve, k to the time constant, g corresponds to a paremeter showing the inflection point of the growth, and t represents time. The g values from the fitted function were used to calculate the growth delay based on the parasitemia curves (Table 1).
Table 1.
Non-linear fit Weibull Growth Model
| Best-fit values | WT | pbggcs-ko1 | pbggcs-ko2 |
|---|---|---|---|
| YM | 72.22 | 41.24 | 12.20 |
| Y0 | 1.366 | −0.4789 | −0.3949 |
| k | 0.04333 | 0.04197 | 0.04640 |
| g | 15.14 | 11.65 | 11.15 |
Statistical Analysis
Statistical analyses were performed using GraphPad Prism v 5.0 (GraphPad Software Inc.). A p value <0.05 was considered statistically significant. Comparisons of the parasite stage percentages during parasitemia, and measurements of intraparasitic response to oxidative stress in the presence or absence of external H2O2, were made by Two-Way Analysis of Variance (Two-Way ANOVA) with Repeated Measures with post-hoc testing using a t-test with Bonferroni correction to account for multiple comparisons.
Diameter of parasites, DNA damage and protein carbonylation assays, were analyzed using One-Way ANOVA. A Two-Way ANOVA was used for the RT-qPCR data analysis. Both analyses were done using multiple comparisons with post-hoc testing using a t-test with Bonferroni corrections. A correlation matrix analysis was undertaken to find if there were associations between the biological variables measured and the parameters derived from the Weibull growth model used to analyze the parasitemia curves. The Pearson correlation coefficient was used as a measure of association. A p value of 0.05 was used to determine if a correlation in the matrix was significant (Table 2).
Table 2.
Correlation Matrix Analysis and p Values
| Parameter g | Parameter k | DNA damage | GSH levels | |
|---|---|---|---|---|
| Parameter g | 0.786 | 0.504 | 0.013 | |
| Parameter k | −0.330 | 0.282 | 0.798 | |
| DNA damage | 0.703 | −0.904 | 0.517 | |
| GSH levels | 1.000 | −0.311 | 0.688 |
Results
Depleted GSH levels induce developmental delay and morphological changes during P. berghei intraerythrocytic growth
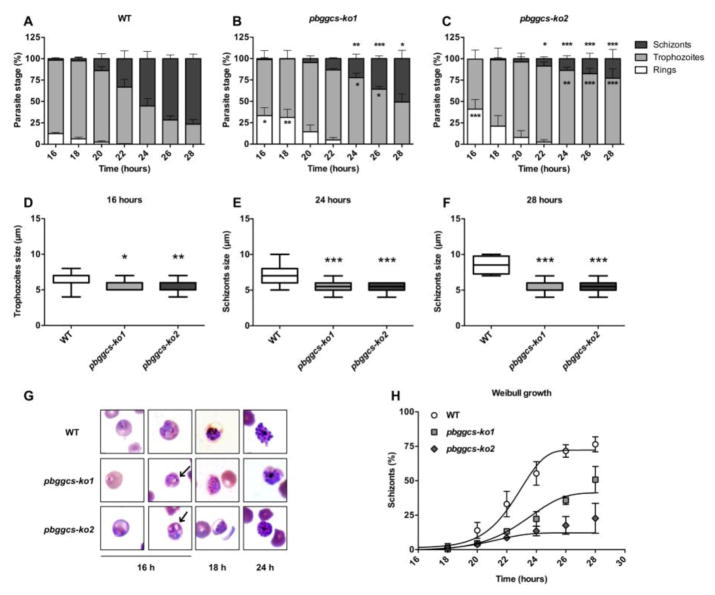
To assess the effects of GSH depletion on intraerythrocytic growth, the ring to schizont transition was monitored in synchronized cultures of the pbggcs-ko1 and pbggcs-ko2 parasites and compared to WT parasites. Parasite clones from two independent transfections with a disrupted ggcs gene (pbggcs-ko1 and pbggcs-ko2) showed growth delay during the ring to schizont progression (Fig. 1). As depicted in Fig. 1, panels A, B, and C demonstrate an approximately two-hour delay during the ring to trophozoite transition, and an approximately 4 to 6-hour delay in the trophozoite to schizont transition. After 16 h, WT parasite cultures contained 12.4% rings, 85.9% trophozoites and 1.7% schizonts (Fig. 1A), while the mutant pbggcs-ko1 and pbggcs-ko2 parasites exhibit 33.3% and 41.1% rings (p<0.05; p<0.001) and 65.6% and 58.4% of trophozoites, respectively (Fig. 1B–C). Note that at 16 h, the pbggcs-ko1 culture had only 1.1% of schizonts and no schizonts were detected in the pbggcs-ko2 parasites (Fig. 1B–C). At 28 h, WT parasite cultures displayed 23.5% trophozoites and 76.5% schizonts, while the pbggcs-ko1 and the pbggcs-ko2 mutants presented 49.2% and 77.2% (p<0.001) trophozoites, and 50.8% (p<0.05) and 22.8% schizonts (p<0.001), respectively (Fig. 1B–C). The growth curves were analyzed separately for schizonts using a Weibull growth model. The results of the model fitting are shown in Table 1 and Fig. 1H. The non-linear fit of the schizont growth shows a decrease in the maximum level of growth of approximately 40% to 85% during the culture time period (Table 1), and also a change of time of the inflection point of the curve representing a two-hour growth delay (Fig. 1H).
Figure 1. In vitro development of WT and pbggcs-ko parasites.
(A–C) Percentage of intraerythrocytic stages of WT (A), pbggcs-ko1 (B), and pbggcs-ko2 (C) parasites throughout a 28 h time course. Three morphological stages were distinguished by microscopy: rings (white bars), trophozoites (light grey bars), and schizonts (dark grey bars). (D–F) The size of trophozoites and schizonts stages from WT (white box), pbggcs-ko1 (light grey box) and pbggcs-ko2 (dark grey box) parasites are shown at 16 (D), 24 (E) and 28 h (F) (n=20). (G) Representative images of intraerythrocytic stages of WT, pbggcs-ko1 and pbggcs-ko2 parasites at 16, 24 and 28 h of in vitro culture. Cytoplasmic vacuoles are indicated by arrows. (H) Weibull growth model for the percentage of schizonts for WT (circle), pbggcs-ko1 (square) and pbggcs-ko2 (diamond) parasites. Significant differences were observed between the WT parasites and the pbggcs-ko1 and pbggcs-ko2 mutant parasites (*p<0.05, **p<0.01, ***p<0.001) analyzed by Two-way repeated measures ANOVA with Bonferroni multiple comparison test. Bars represent the SEM from 3 independent experiments.
The correlation matrix analysis showed that the inflection point (growth delay) correlated with the GSH levels present in each parasite clone (WT, 7.4 nmoles/109 parasites; pbggcs-ko1, 1.0 nmoles/109 parasites; pbggcs-ko2, 0.2 nmoles/109 parasites [14]) (r2=1.0, p = 0.013). The complete correlation matrix is shown in Table 2. The upper triangular matrix shows the p values, while the lower triangular matrix (shaded in gray) shows the correlation coefficients. The only correlation that meets the statistical significance criterion is the association detected between the Weibull model parameter g and the levels of GSH. Due to sparsity of the data, more work is required to better understand this potentially causal correlation.
Morphological changes in the GSH deficient mutants, pbggcs-ko1 and pbggcs-ko2, were assessed during the erythrocytic cycle (rings, trophozoites, schizonts) (Fig. 1D–G). Analysis of Diff-Quick stained cultured parasites revealed a significant size reduction and increased vacuolated cytoplasm in the mutants (Fig. 1G). A significant reduction in size was observed in both pbggcs-ko1 and the pbggcs-ko2 trophozoites at 16 h of culture (p<0.05 and p<0.01, respectively) and in schizonts at 24 and 28 h of culture (p<0.001 and p<0.001) (Fig. 1E–F). To further analyze the morphology, the number of parasites with vacuolated cytoplasm was determined. A remarkable number of the mutant parasites displayed vacuoles in their cytoplasm (60%, pbggcs-ko1 and 45%, pbggcs-ko2), (Fig. 1G and Fig. S1–S3). These results suggest that low GSH levels result in morphological changes and a significant delay of the intraerythrocytic development in P. berghei.
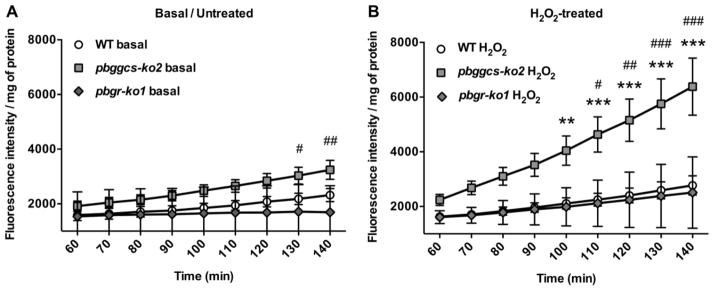
P. berghei ggcs deficient parasites display augmented intracellular oxidation of DCFH and an impaired response to oxidative stress
In order to determine whether depleted GSH is associated with an increase in oxidative stress, the green fluorescent intensity of DCF, the oxidation product of the nonfluorescent probe DCFH, was measured in WT and mutant parasites. The pbggcs-ko2 (GSH levels: 0.2 nmoles/109 parasites [14]), show higher levels of DCF fluorescence under basal conditions when compared to WT parasites as depicted in Fig 2A (130–140 min; p<0.05, p<0.01). Similarly, DCF fluorescence under basal conditions is higher in pbggcs-ko2 (1.9-fold) as compared to pbgr-ko1 (GSH levels: 5.0 nmoles/109 parasites [15]) (Fig. 2A). There were no basal differences in intracellular oxidation, as determined by DCF fluorescence, between the pbgr-ko1 and the WT parasites.
Figure 2. P. berghei ggcs-ko parasites show increased levels of intracellular oxidative stress.
DCF fluorescence intensity was determined at various time points in basal/untreated and H2O2-treated in vitro cultures of WT, pbggcs-ko2 and pbgr-ko1 parasites. Cultures were treated with 2 mM H2O2 for 1 h. (A) Basal levels of DCF fluorescence in WT, pbggcs-ko2 and pbgr-ko1 parasites. (B) H2O2-induced DCF fluorescence intensity in WT, pbggcs-ko2 and pbgr-ko1 parasites. Significant differences were observed between the H2O2-treated WT and pbggcs-ko2 (**p<0.01, ***p<0.001) and pbggcs-ko2 and pbgr-ko1 mutant parasites (#p<0.05, ##p<0.01, ###p<0.001) at different time points analyzed by Two-way repeated measures ANOVA with Bonferroni multiple comparison test. Bars represent the SD from 2 (pbgr-ko1) and SEM from 4 (pbggcs-ko2) independent experiments.
To test the response to exogenous oxidative stress the parasites were exposed to H2O2. Treatment with 2 mM H2O2 had no effect on DCF fluorescent intensity in the WT or pbgr-ko1 parasites (Fig. 2B). In contrast, incubation with H2O2 significantly increased (2.3 fold) the oxidation of DCFH in pbggcs-ko2 parasites as compared to WT as depicted in Fig. 2B (100–140 min; p<0.01, p<0.001). Similarly, incubation of pbggcs-ko2 with H2O2 significantly increased (2.5 fold) the oxidation of DCFH as compared to pbgr-ko1 (110–140min; p<0.05, p<0.01, p<0.001) (Fig. 2B). These results suggest that the pbggcs-ko2 parasites with low GSH levels exhibit higher basal oxidative stress and an impaired response to exogenous oxidative stress than the WT or the pbgr-ko1 parasites.
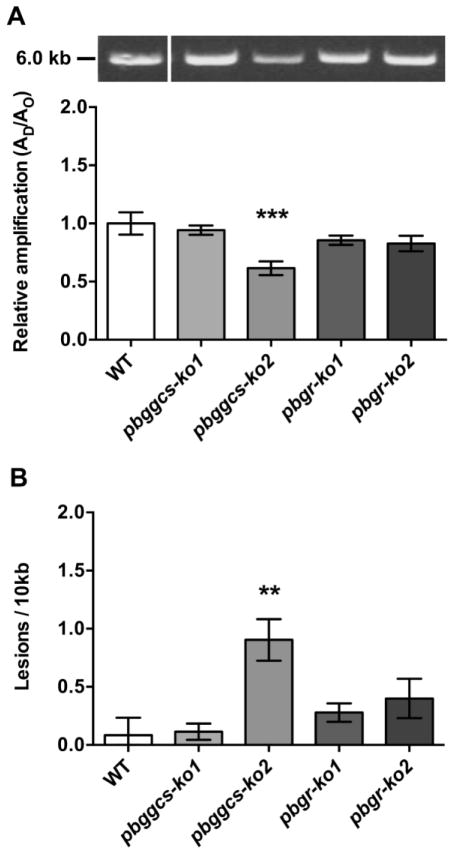
pbggcs-ko parasites show increased levels of nuclear DNA damage
To investigate whether reduced GSH levels cause damage to the parasite’s DNA, a QPCR assay was used to quantify the levels of nuclear (nDNA) and mitochondrial DNA (mtDNA) damage [22]. To measure nDNA damage in the WT, pbggcs-ko and pbgr-ko parasites, a 6.1 kb nDNA fragment of the P. berghei seryl t-RNA synthetase gene was amplified. The pbggcs-ko2 parasites show a significant (p<0.001) 38.5% reduction in the amplification of the 6.1 kb nDNA fragment (Fig. 3A), indicating an increase in levels of nDNA damage (Fig. 3B), compared to WT, whereas the amplification levels of the 6.1 kb nDNA fragment from the pbggcs-ko1, pbgr-ko1 and pbgr-ko2 were similar to those of the WT parasites (Fig. 3A). The frequency of nDNA lesions was 10.6-fold higher for the pbggcs-ko2 parasites compared to WT parasites (p<0.01) (Fig. 3B). However, no statistically significant differences in the number of nDNA lesions between the WT, the pbggcs-ko1, or pbgr-ko mutant parasites were detected.
Figure 3. P. berghei ggcs-ko2 mutant shows increased levels of nDNA damage.
(A) Upper panel, representative gel showing the amplification of a 6.1 kb nDNA fragment of the seryl t-RNA synthetase gene from WT, pbggcs-ko and pbgr-ko parasites. Lower panel, relative amplification levels of the 6.1 kb nDNA fragment from WT and mutant parasites. (B) Frequency of nDNA lesions per 10 kb per strand was calculated as described in Materials and Methods. Representative results of the 6.1 kb amplified fragment are shown in the upper panel. The 6 kb molecular weight marker is indicated in the left. Significant differences were observed between WT and pbggcs-ko2 parasites (**p<0.01, ***p<0.001) analyzed by One-way ANOVA with Bonferroni multiple comparison test. Bars represent the SEM from 3 independent experiments and 10 QPCR analyses.
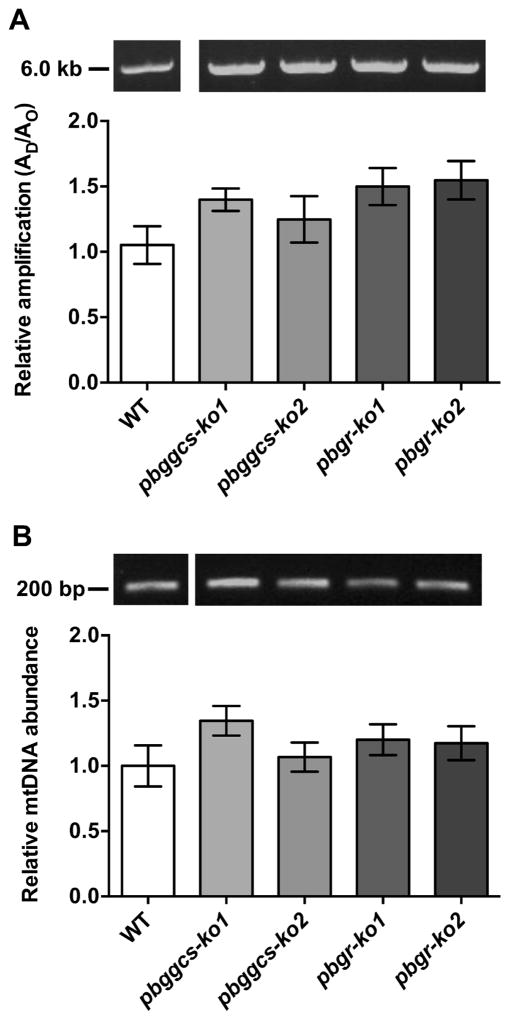
The levels of mtDNA lesions in the WT and the mutant parasites were also determined by amplifying a 5.7 kb mtDNA fragment which represents 95% of the mitochondrial genome [27]. The analysis revealed no significant differences in the relative amplification of the mtDNA fragment between the pbggcs-ko, the pbgr-ko or the WT parasites, demonstrating no increase in mtDNA damage (Fig. 4A). Because high oxidative stress levels can affect the replication rates of the mtDNA, the steady-state levels of mtDNA molecules were assessed in WT, pbggcs-ko, and pbgr-ko. Steady-state levels of mtDNA molecules were similar in the mutant and the WT parasites (Fig. 4B).
Figure 4. Mutant parasites show no changes in the levels of mtDNA damage or mtDNA abundance.
(A) Upper panel, representative gel showing the amplification of a 5.7 kb mtDNA fragment from WT, pbggcs-ko and pbgr-ko parasites. Lower panel, relative amplification of a 5.7 kb mtDNA fragment that was normalized for changes in mtDNA abundance. (B) Upper panel, representative gel showing the amplification of the 186 bp mtDNA fragment. Lower panel, relative amplification of a 186 bp mtDNA fragment representative of mitochondrial abundance. No significant differences in mtDNA damage or mtDNA abundance were observed between WT and mutant parasites. Bars represent the SEM from 3 independent experiments, and 14 and 12 QPCR analyses for mtDNA damage or mtDNA abundance, respectively.
Collectively, these results suggest that parasites with reduced GSH levels show an increase in nDNA damage but no increase in damage to the mitochondrial genome.
Reduced GSH levels have no effect on protein carbonyl content in parasite intraerythrocytic stages
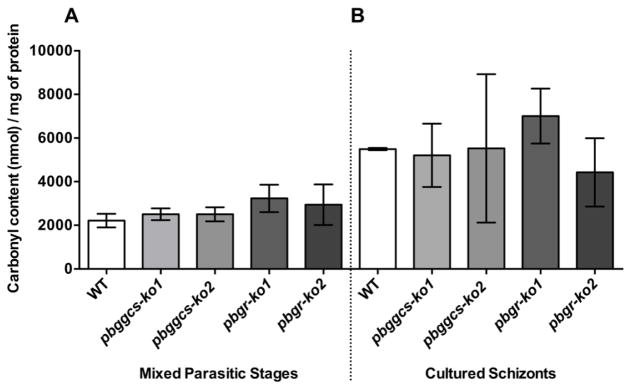
Protein carbonylation is one of the most common protein modifications caused by oxidative stress [28]. The extent of protein carbonylation was examined in total protein extracts from WT, pbggcs-ko and pbgr-ko mixed asynchronous blood stages and from synchronized schizonts cultures using the FTC fluorometric assay. The amount of carbonylated proteins from the intraerythrocytic blood stages of pbggcs-ko and pbgr-ko mutants was similar to those detected in the WT parasites (Fig. 5A). Similar results were obtained when protein carbonyls were measured using the dinitrophenylhydrazine (DNPH) method (Fig. S4). Cultured schizonts from mutant and WT parasites exhibited a 1.5–2.5 fold increase in carbonyl content as compared to the intraerythrocytic parasites (Fig. 5B; Table 3). However, no significant differences (One-way ANOVA and unpaired t-test) were observed between the mixed blood stages and cultured schizonts.
Figure 5. Levels of protein carbonylation in intraerythrocytic stages from WT and mutant parasites.
Levels of protein carbonylation were assessed in WT, pbggcs-ko, and pbgr-ko parasites using the FTC fluorescent assay. Panel A: protein carbonyl content in intraerythrocytic asynchronous blood stages. Panel B: protein carbonyl content in cultured schizonts. No significant differences in the levels of protein carbonylations were observed between WT and mutant intraerythrocytic asynchronous parasites and cultured schizonts. Bars represent the SEM of 4 independent experiments from intraerythrocytic stages and SD of 2 independent experiments from cultured schizonts.
Table 3.
Protein Carbonylation Content in Wild Type versus Mutant Parasites
| WT | pbggcs-ko1 | pbggcs-ko2 | pbgr-ko1 | pbgr-ko2 | |
|---|---|---|---|---|---|
| Mixed Asexual Stages (nmol/mg ± SEM) | 2216 ± 312 | 2506 ± 272 | 2502 ± 320 | 3234 ± 630 | 2945 ± 931 |
| Schizonts Stages (nmol/mg ± SD) | 5496 ± 55 | 5207 ± 1452 | 5526 ± 3401 | 7006 ± 1257 | 4428 ± 1568 |
| Fold | 2.48 | 2.08 | 2.21 | 2.17 | 1.50 |
GSH depletion induces upregulation of genes involved in detoxification and DNA synthesis
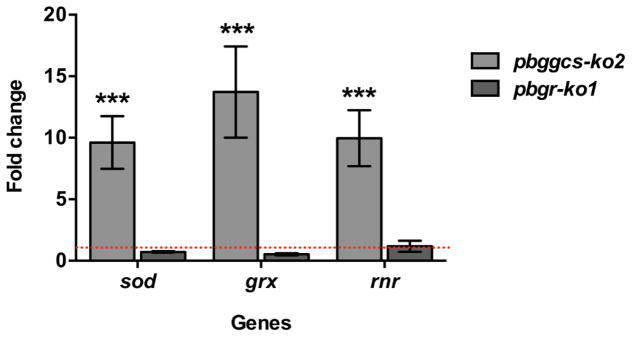
To establish whether or not reduced GSH levels affects the expression of genes involved in oxidative detoxification and DNA synthesis, the mRNA levels of the superoxide dismutase (sod), glutaredoxin (grx) and ribonucleotide reductase (rnr) genes were determined in the pbggcs-ko2, the pbgr-ko1 and the WT parasites. Increased levels of sod, grx and rnr mRNA were detected in the pbggcs-ko2 parasites as compared to the WT, while no differences in gene expression were observed between the pbgr-ko1 and the WT parasites (Fig. 6). The P. berghei SOD, an enzyme responsible for the dismutation of the superoxide anion (O2• −), was upregulated (9.6-fold induction) in the pbggcs-ko2 parasites (p<0.01). The glutaredoxin (Grx) enzyme serves as a hydrogen-donor for RNR in the first step of DNA synthesis. Both grx and rnr genes showed a significant increase in expression in the pbggcs-ko2 parasites, with 13.7 and 10-fold upregulation, respectively (p<0.001). These results suggest that P. berghei parasites exhibiting GSH depletion display increased expression of genes involved in ROS detoxification and DNA synthesis.
Figure 6. P. berghei ggcs-ko parasites show upregulated levels of sod, grx and rnr mRNA expression.
The relative gene expression was determined by RT-qPCR and expressed as fold change for the pbggcs-ko2 and pbgr-ko2 as compared to WT. The data was normalized against the expression of 18S rRNA. The sod (superoxide dismutase), grx (glutaredoxin) and rnr (ribonucleotide reductase) genes showed increased gene expression in the pbggcs-ko2 mutant parasites (***p<0.001) analyzed by Two-way ANOVA with Bonferroni multiple comparison test. Bars represent the SEM from 3 independent experiments. Red dotted line represents the expression level in WT parasites.
Discussion
Previous work from our laboratory showed that the Plasmodium GSH metabolism is dispensable for parasite development during the intraerythrocytic cycle [2, 3]. However, low GSH levels delayed progression of the infection in mice, presumably by extending the parasite’s cellular cycle in order to compensate for the absence of the GSH antioxidant [2]. In this study we show that pbggcs-ko parasites with low GSH levels exhibit a delay in the ring to schizont progression, an impaired response to increased oxidative stress, and an increase in nDNA damage. In addition, we report an increased in mRNA expression of genes involved in oxidative stress detoxification and DNA synthesis. These results suggest that optimal GSH levels are needed in order to protect the P. berghei nuclear genome from oxidative stress and for proper development. Furthermore, due to the impaired response to external oxidative stress, parasites will face a major hurdle to grow under conditions where environmental oxidative stress is high, such as the infected erythrocytes [3] and particularly the internal compartments of the mosquito host [14, 15].
In previous work, we reported that pbggcs-ko mutant parasites exhibit a delayed development in mice [14]. In this work we observed a two-hour delay in the transition from rings to trophozoites, and a 4 to 6-hour delay in the transition from trophozoites to schizonts when comparing the pbggcs-ko1 and pbgccs-ko2 mutants to the WT parasites. Our non-linear Weibull model of the schizont growth reveals a two-hour delay in the growth between the mutants and the WT parasites. Results from the present study also show a highly significant correlation between GSH levels and the delayed transition from ring to schizonts (r2=1.0, p = 0.013), supporting the notion that GSH plays an important role on Plasmodium growth development. The observed delay and smaller size of the pbggcs-ko parasites suggest a slow-down in the cell cycle presumably to repair the oxidative damage observed in the nuclear genome. This is consistent with previous observations where a Candida albicans and a Saccharomyces cerevisiae strain harboring a deletion in the ggcs homologue, showed decreased growth rates and prolonged cell cycle [29, 30]. Additional evidence supporting the hypothesis that low GSH levels affect cell growth is provided by studies of human 3T3 fibroblasts treated with diethyl maleate, an agent that decreases nuclear GSH levels, resulted in slower cell cycle [31]. Similarly, we speculate that a longer cell cycle during P. berghei intraerythrocytic stages could provide the parasite with enough time to repair the oxidative damage to the nDNA occurring as a consequence of low GSH levels.
It should be pointed out that in contrast to P. berghei, deletion of the γGCS or glutathione synthetase genes was lethal for P. falciparum, suggesting that GSH levels are critical for P. falciparum development [32]. It is possible that P. falciparum is incapable of uptaking sufficient amounts of GSH from the mature RBC to allow parasite survival during the erythrocytic cycle [32, 33]. Furthermore, P. falciparum infected RBC display increased GSH efflux [34], which may result in a decrease in GSH available for parasite uptake. P. berghei prefers to invade young erythrocytes (reticulocytes), which are metabolically different from mature RBC, supporting the hypothesis that host environment have an impact in parasite growth and survival [34, 35]. Differences between both species should be taken into consideration and the GSH uptake in P. berghei be further evaluated, since the pbggcs-ko parasites have reduced but detectable GSH levels.
Plasmodium parasites depend on the digestion of hemoglobin as a major amino acid source. Heme/FP IX (Fe2+/Fe3+), generated as a by-product of Hb digestion, is toxic for the parasite and is detoxified via a biomineralization process into hemozoin, a chemically inert crystal that confers the distinctive pigment of Plasmodium parasites [36, 37, 38, 39]. However, some heme/FP IX (Fe2+/Fe3+) escapes the biomineralization process and reaches the cytosol [40,41] resulting in the generation of the superoxide (O2• −) [4, 42]. The superoxide (O2• −) can be dismutated by the cytosolic superoxide dismutase (SOD) to H2O2 and O2 and can react with H2O2 to form the highly reactive hydroxyl radical (•OH) [3]. In addition, free iron released from heme/FP IX (Fe2+/Fe3+) acts as a pro-oxidant mediating the generation of hydroxyl (•OH) via the Fenton reaction [3]. To maintain low levels of oxidants the parasites uses the GSH antioxidant system. The pbggcs-ko parasites, with low GSH levels are unable to detoxify the non-crystalized heme/FP IX and thus, will potentially have higher levels of free iron (Fe2+/Fe3+) and consequently higher levels of oxidative stress. It is plausible that in a low GSH environment like the one present in the pbggcs-ko, the parasites are unable to prevent the harmful effects of the FP IX; resulting in enhanced oxidative stress, leading to parasite growth delay and changes in the morphology during development. The H2O2 treatment significantly increased the oxidation of DCFH in the pbggcs-ko2 parasites when compared to the WT and pbgr-ko1 parasites, further supporting that low GSH levels are associated with increased oxidative stress. It is possible that the heme/FP IX (Fe2+/Fe3+) produced from Hb digestion may have promoted H2O2-induced DCFH oxidation. This idea is consistent with evidence showing that the H2O2-induced intracellular DCFH oxidation in bovine aortic endothelial cells is facilitated by uptake of iron mediated by the transferrin receptor [43]. Hence, we conclude that the mutant parasites have an impaired system to handle oxidative stress.
Our observations are consistent with studies where conditions leading to increased oxidative stress in P. falciparum cultures resulted in parasite growth delay [19, 44, 45]. Treatments that promote oxidative stress such as clotrimazole [19], pro-oxidant concentrations of curcumin [44] or bilirubin [45] result in increased accumulation of ROS and inhibition of parasite growth [19]. In addition, treatment with the antimalarial drug artesunate leads to increased oxidative stress and reduced GSH levels, causing parasite death [46]. The association between inhibition of parasite growth and increased oxidative stress was further supported by the addition of antioxidants that restored parasite growth [46]. Interestingly, our results show that the pbgr-ko1 parasites with intermediate levels of GSH did not present growth delay (5.0 nmoles/109 parasites) [15] nor increased oxidation of DCFH. These data suggest that the increase in oxidative stress and the parasite growth delay are a consequence of reduced GSH levels as is the case for the pbggcs-ko parasites. We hypothesize that there is a threshold in the GSH levels required for normal P. berghei intraerythrocytic development.
Interestingly, an increase of nDNA damage was detected in pbggcs-ko2 parasites with low GSH levels (0.2 nmoles/109 parasites [14]). This observation supports the notion that the increased oxidative stress associated with GSH depletion leads to the induction of oxidative damage in the P. berghei nuclear genome. Our findings are in agreement with a recent study in P. falciparum demonstrating that nDNA damage is associated with GSH depletion and increased oxidative stress after artesunate treatment [46]. It should be noted that neither the pbgccs-ko1 (GSH levels of 1.0 nmoles/109 parasites [14]) nor the pbgr-ko mutants (GSH levels of 5.0 nmoles/109 parasites [15]) show a statistically significant increase in nDNA damage compared to WT (GSH levels of 7.4 nmoles/109 parasites [14]), demonstrating that only a drastic reduction in GSH levels (i.e. the 97% reduction in GSH levels detected in the pbggcs-ko2 when compared to WT) are associated with increased oxidation of DCFH and nDNA damage. Surprisingly, increased mtDNA damage or changes in the abundance of mtDNA molecules in the pbggcs-ko parasites were not detected, suggesting that under physiological conditions, damage to the nDNA and not the mtDNA may lead to P. berghei growth delay. In contrast, P. falciparum parasites treated with high pro-oxidant concentrations of curcumin resulted in increased damage to both the nuclear and mitochondrial genomes [44]. Mitochondria are the main ROS producers in the cell, mainly as a byproduct of oxidative phosphorylation [47]. Although Plasmodium parasites are fully capable of mitochondrial oxidative phosphorylation during the intraerythrocytic stages [48], they rely on anaerobic glycolysis as the main source of energy [49, 50] using the electron transport chain mainly for de novo pyrimidine biosynthesis [51, 52]. Our results showed that the absence of mtDNA damage under depleted GSH levels is in agreement with Plasmodium mitochondria not being the main source of ROS during the intraerythrocytic development. These results suggest that GSH plays a role in protecting the nuclear genome from oxidative stress.
Surprisingly, no significant changes in the amounts of protein carbonyl content in the pbggcs-ko or pbgr-ko parasites were detected in our analysis, suggesting that reduced GSH levels did not increase oxidative damage to proteins. Radfar et al. [53] reported that in P. falciparum cultures, chloroquine treatment led to an increase in protein carbonylation mainly in the mature schizont. Chloroquine is hypothesized to prevent hemozoin formation allowing the release of ferroprotoporphyrin IX (FP IX), which in turns induces oxidative stress (lethal to the parasite). Although a similar environment of increased oxidative stress is present in the pbggcs-ko mutants due to the significantly reduced GSH levels, our results show that the GSH deficiency did not further increase the levels of oxidized proteins neither in the intraerythrocytic stages nor in the synchronized schizont cultures.
The mRNA expression of sod, an enzyme responsible for the dismutation of O2− into H2O2 [54] was significantly upregulated in the pbggcs-ko2 deficient parasites but not in pbgr-ko2 parasites. The upregulation of cytosolic sod mRNA could be a protective response to the increased oxidative stress levels observed in the GSH depleted pbggcs-ko2 mutants. The SOD enzyme represents an important antioxidant defense of Plasmodium parasites [55]. Two Plasmodium SODs have been identified, PfSOD1 and PfSOD2 [56], but the iron-dependent cytosolic PfSOD1 has been shown to be highly expressed in the intraerythrocytic stages [57]. Our results suggest that the extreme reduction of GSH levels in the pbggcs-ko2 mutants induces an increase in the expression of the antioxidant genes presumably as a response to oxidative stress.
Optimal levels of dNTPs are required for DNA replication and DNA repair, a reaction catalyzed by RNR, the rate-limiting enzyme during DNA synthesis. The Grx enzyme can reduce RNR and exert redox regulation, cell growth and proliferation activities. Analysis of the P. berghei mutant parasites revealed that the grx and rnr genes displayed a significant increase in mRNA expression in the pbggcs-ko2 parasites, while no differences were detected in the pbgr-ko2 parasites. These results suggest that depletion of GSH is associated with a significant induction in the grx and rnr expression, probably in response to the oxidative damage observed in nDNA of the mutant parasites. These results are in agreement with findings showing induction of the RNR enzyme by DNA damage [58]. It is plausible that the pbggcs-ko parasites increased synthesis of dNTPs for DNA replication to compensate for deficiencies in proliferation or to repair the oxidative damage observed in the nDNA. However, these changes in gene expression are not detected in the pbgr-ko2 parasites, which show normal growth during the intraerythrocytic cycle [15] and no lesions in nDNA, suggesting that intermediate GSH levels are sufficient for normal parasite growth.
The reduced GSH levels present in the pbggcs-ko parasites have implications for antimalarial treatments [59]. We recently reported that the pbggcs-ko parasites were more sensitive to clearance after sub lethal doses of artemisinin (ART) treatment in a recrudescence assay [59]. These results suggest that GSH contributes to the ART response, making the parasites more tolerant to the drug. In addition, RRx-001, a new agent that inhibits the pentose phosphate pathway (PPP) of the parasite, was effective against P. berghei cerebral malaria and enhanced the activity of artemether when used in combination [60]. The PPP provides the only source of NADPH used by the parasite’s GSH and the Trx systems during protection against the harmful effects of oxidative stress. This drug also binds to Hb and GSH, increasing the ROS levels and oxidative damage caused by artemether [61]. These reports together with our results encourage research to explore drugs that inhibit the GSH biosynthesis of the parasite as a potential combination drug for ART treatment.
This study provides evidence supporting that GSH deficient parasites exhibit a growth delay and damage to the nuclear genome during development of the intraerythrocytic stages. The reduced GSH levels did not increase damage to the parasite’s mitochondrial genome nor protein carbonylation of the parasites. This report highlights a potential key role of GSH to support parasite development and to protect the nuclear genome from oxidative damage (Fig. 7). A threshold level of GSH appears to be necessary to observe the effects of oxidative stress in the parasite. Further work will be needed to clearly establish the existence of a GSH threshold level. Understanding the mechanisms that result in the inhibition of parasite development represents a promising approach for the development of new antimalarial drugs.
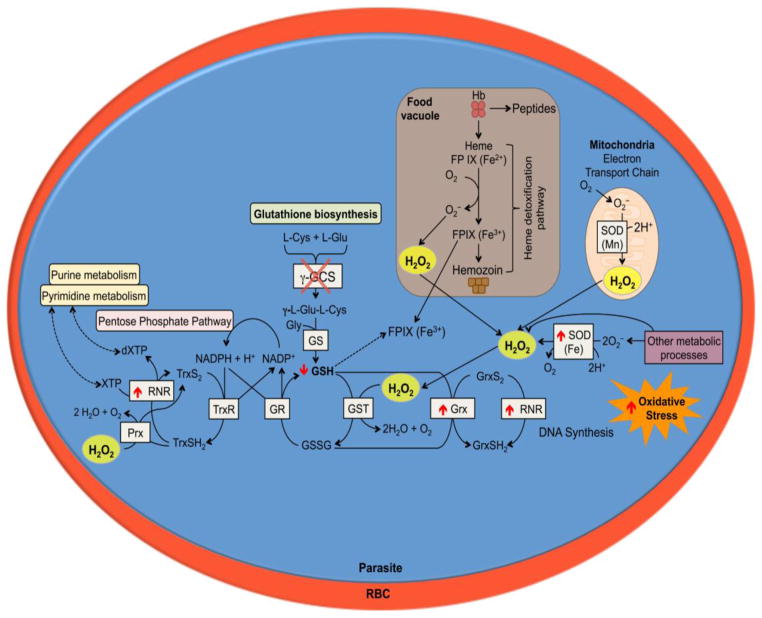
Figure 7. Proposed effects of GSH deficiency in P. berghei during asexual blood stages.
Representation of the P. berghei intracellular blood stage with the biochemical processes involved in the antioxidant defense. The red circle represents the red blood cell and the blue circle represents the parasite and the biochemical antioxidant pathways. The pbggcs-ko mutant parasites with significantly low GSH levels exhibit growth delay during intraerythrocytic development probably due to an impaired response to oxidative stress and nDNA damage. As a result of increased oxidative stress, the pbggcs-ko mutant parasites increase expression of sod for cytosolic dismutation of the superoxide, and grx and rnr to support dNTP synthesis. These events may be necessary to sustain development of P. berghei parasite in the absence of GSH.
Supplementary Material
Figure S1. Intraerythrocytic development of P. berghei WT, pbggcs-ko1, and pbggcs-ko2 parasites at 16 h culture. Light microscopy images of trophozoites from WT, pbggcs-ko1, and pbggcs-ko2 parasites during intraerythrocytic development at 16 h culture. Note the presence of vacuoles in the mutant parasites. Parasites are indicated by black arrows and cytoplasmic vacuoles by red arrows. Microscopy images were adjusted for brightness and contrast to improve image clarity.
Figure S2. Intraerythrocytic development of P. berghei WT, pbggcs-ko1, and pbggcs-ko2 parasites at 24 h culture. Light microscopy images of WT, pbggcs-ko1, and pbggcs-ko2 parasites during intraerythrocytic development at 24 h culture. Note mature schizonts in the WT and late trophozoites/immature schizonts in the mutants. Parasites are indicated by black arrows and cytoplasmic vacuoles by red arrows. Microscopy images were adjusted for brightness and contrast to improve image clarity.
Figure S3. Intraerythrocytic development of P. berghei WT, pbggcs-ko1, and pbggcs-ko2 parasites at 28 h culture. Light microscopy images of WT, pbggcs-ko1, and pbggcs-ko2 parasites during intraerythrocytic development at 28 h culture. Mature schizonts from WT parasites are beginning to burst from the RBC. Note smaller and disorganized immature schizonts in the mutants. Parasites are indicated by black arrows. Microscopy images were adjusted for brightness and contrast to improve image clarity.
Figure S4. Protein carbonylation in P. berghei ggcs-ko and gr-ko parasites by the dinitrophenylhydrazine (DNPH) method. The carbonyl content of proteins from WT, pbggcs-ko, and pbgr-ko parasites was determined by the dinitrophenylhydrazine (DNPH) method using the OxyBlot Protein Oxidation Detection Kit (Millipore) as we previously described [62]. Parasite samples (30 μg) were incubated with 2,4 DNPH to form 2,4 dinitrophenyl hydrazone derivates (DNP-hydrazone) and non-derivatized proteins were used as negative controls. Proteins were resolved in an SDS-polyacrylamide gel (12%) and blotted onto a PVDF membrane. Binding of DNP to carbonylated proteins was detected with a rabbit anti-DNP antibody (1:150) (Millipore) followed by detection with a goat anti-rabbit IgG antibody conjugated to horseradish peroxidase (1:300) (Millipore). Carbonylated proteins were visualized using Super Signal West Pico Substrate (Pierce Biotechnology). Band intensities were quantified using the Bio-Rad VersaDoc™ Imaging System and the Quantity One computer software. Carbonylated protein levels were normalized to the signal obtained with an antibody against the 75kDa heat shock protein-70 (HSP-70) co-chaperone protein (1:100) of P. berghei (kindly provided by Dr. Fidel Zavala). Levels of carbonylations were expressed as intensity proportions after correcting for variations in protein loading based on the expression of the co-chaperone. Intensity proportions of carbonyl content were determined by densitometry. (A) Intensity for the pbggcs-ko and pbgr-ko parasites is expressed relative to the WT. A 75 kDa HSP-70 co-chaperone of P. berghei was used as a loading control. (B) Representative blot showing the immunodetection of carbonylated proteins in WT and mutant parasites (D=Derivatized; ND= Non Derivatized negative control). No significant differences in protein carbonylations were observed between WT and pbggcs-ko or pbgr-ko parasites. Bars represent the SD from 2 independent experiments.
Table S1. Primer sequences used for RT-qPCR.
Highlights.
Decreased GSH levels correlate with a delay of the ring to schizont transition.
Damage to the nDNA but not the mtDNA is higher in GSH-deficient parasites.
Optimal GSH levels are required for parasite normal growth and to avoid nDNA damage
Acknowledgments
The authors want to thank Keila Crespo, Jesús F. Muñiz and Natalia Vega for technical support, Wieslaw Kozek for advice on microscopic analysis of parasite size and cytoplasmic vacuoles. This investigation was partially supported by the National Institute of General Medical Sciences, Research Centers in Minority Institutions Award G12RR03051, Minority Biomedical Research Support Grant 3S06-GM-008224 and Minority Biomedical Research-RISE Program Award R25-GM-061838. RGM was partially supported by National Institutes of Health Minority Access to Research Careers (MARC) grant: T36-GM-095335.
Abbreviations
- ART
artemisinin
- DCF
dichlorofluorescein
- DCFH
2′, 7′-dichlorodihydrofluorescein
- DHOD
dihydroorotate dehydrogenase
- DNPH
dinitrophenylhydrazine
- FP IX
ferritoprotoporphyrin IX
- FTC
fluorescein 5-thiosemicarbazide
- GR
glutathione reductase
- Grx
Glutaredoxin
- GSH
glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- Hb
hemoglobin
- mtDNA
mitochondrial DNA
- nDNA
nuclear DNA
- pbggcs-ko
P. berghei gamma-glutamylcysteine synthetase knockout
- pbgr-ko
P. berghei glutathione reductase knockout
- PBS
phosphate-buffered saline
- PPP
Pentose phosphate pathway
- QPCR
quantitative polymerase chain reaction
- RBC
red blood cells
- RNR
Ribonucleotide Reductase
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- Trx
thioredoxin
- WT
wild type
- γGCS
gamma glutamylcysteine synthetase
Footnotes
Competing interests
The authors have no competing interests to declare.
Authors’ contribution
Conceived and designed the experiments: JVR EECL RGM SAP AES
Performed the experiments: VPI EECL MRC
Analyzed the data: VPI EECL RGM SAP AES
Contributed reagents/materials/analysis tools: AES
Wrote the paper: VPI EECL SAP AES
Revised the article critically: JVR RGM
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization (WHO) [accessed 11.19.14];World Malaria Report 2014. 2014 http://www.who.int/malaria/publications/world_malaria_report_2014/en/index.html.
- 2.Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int J Parasitol. 2004;34:163–189. doi: 10.1016/j.ijpara.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Müller S. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Mol Microbiol. 2004;53(5):1291–305. doi: 10.1111/j.1365-2958.2004.04257.x. [DOI] [PubMed] [Google Scholar]
- 4.Tilley L, Loria P, Foley M, editors. Antimalarial Chemotherapy. Vol. 2001. Humana Press; 2001. Chloroquine and other quinoline antimalarials; pp. 87–121. [Google Scholar]
- 5.Krauth-Siegel RL, Bauer H, Schirmer RH. Dithiol proteins as guardians of the intracellular redox milieu in parasites: old and new drug targets in trypanosomes and malaria-causing plasmodia. Angew Chem Int Ed Engl. 2005;44:690–715. doi: 10.1002/anie.200300639. [DOI] [PubMed] [Google Scholar]
- 6.Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 7.Müller S, Gilberger TW, Krnajski Z, Lüersen K, Meierjohann S, Walter RD. Thioredoxin and glutathione system of malaria parasite Plasmodium falciparum. Protoplasma. 2001;217:43–49. doi: 10.1007/BF01289412. [DOI] [PubMed] [Google Scholar]
- 8.Atamna H, Ginsburg H. The malaria parasite supplies glutathione to its host cell: investigation of glutathione transport and metabolism in human erythrocytes infected with Plasmodium falciparum. Eur J Biochem. 1997;250:670–679. doi: 10.1111/j.1432-1033.1997.00670.x. [DOI] [PubMed] [Google Scholar]
- 9.Meister A, Anderson ME. Glutathione. Annual Reviews Biochemistry. 1983;52:711–60. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 10.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 11.Ginsburg H, Ward SA, Bray PG. An integrated model of chloroquine action. Parasitol Today. 1999;15(9):357–60. doi: 10.1016/s0169-4758(99)01502-1. [DOI] [PubMed] [Google Scholar]
- 12.Schirmer RH, Becker K, Matuschewski K. Depletion of Plasmodium berghei plasmoredoxin reveals a non-essential role for life cycle progression of the malaria parasite. PLoS One. 2008;3(6):e2474. doi: 10.1371/journal.pone.0002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchholz K, Putrianti ED, Rahlfs S, Schirmer RH, Becker K, Matuschewski K. Molecular genetics evidence for the in vivo roles of the two major NADPH-dependent disulfide reductases in the malaria parasite. J Biol Chem. 2010;285(48):37388–95. doi: 10.1074/jbc.M110.123323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vega-Rodríguez J, Dinglasan RR, Franke-Fayard B, Janse CJ, Pastrana-Mena R, Coppens I, Waters AP, Jacobs-Lorena M, Rodríguez-Orengo J, Serrano AE. The glutathione biosynthetic pathway is essential for mosquito transmission. PLoS Pathog. 2009;5(2):e1000302. doi: 10.1371/journal.ppat.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastrana-Mena R, Dinglasan RR, Franke-Fayard B, Vega-Rodriguez J, Fuentes-Caraballo M, Baerga-Ortiz A, Coppens I, Jacobs-Lorena M, Janse CJ, Serrano AE. Glutathione reductase-malaria parasites have normal blood stage growth but arrest during oocyst development in the mosquito. J Biol Chem. 2010;285(35):27045–56. doi: 10.1074/jbc.M110.122275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, van Gemert GJ, Sauerwein RW, Waters AP. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol. 2006;145(1):60–70. doi: 10.1016/j.molbiopara.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Janse CJ, Water AP. Plasmodium berghei: the application of cultivation and purification techniques to molecular studies of malaria parasites. Parasitol Today. 1995;11:138–43. doi: 10.1016/0169-4758(95)80133-2. [DOI] [PubMed] [Google Scholar]
- 18.Waters AP, Thomas AW, van Dijk MR, Janse CJ. Transfection of malaria parasites. Methods. 1997;13(2):134–47. doi: 10.1006/meth.1997.0506. [DOI] [PubMed] [Google Scholar]
- 19.Trivedi V, Chand P, Srivastava K, Puri SK, Maulik PR, Bandyopadhyay U. Clotrimazole inhibits hemoperoxidase of Plasmodium falciparum and induces oxidative stress. Proposed antimalarial mechanism of clotrimazole. J Biol Chem. 2005;280(50):41129–36. doi: 10.1074/jbc.M501563200. [DOI] [PubMed] [Google Scholar]
- 20.Acevedo-Torres K, Berríos L, Rosario N, Dufault V, Skatchkov S, Eaton MJ, Torres-Ramos CA, Ayala-Torres S. Mitochondrial DNA damage is a hallmark of chemically induced and the R6/2 transgenic model of Huntington’s disease. DNA Repair. 2009;8(1):126–36. doi: 10.1016/j.dnarep.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqui A, Rivera-Sánchez S, del Castro MR, Acevedo-Torres K, Rane A, Torres-Ramos CA, Nicholls DG, Andersen JK, Ayala-Torres S. Mitochondrial DNA damage is associated with reduced mitochondrial bioenergetics in Huntington’s disease. Free Radic Biol Med. 2012;53(7):1478–88. doi: 10.1016/j.freeradbiomed.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayala-Torres S, Chen Y, Svoboda T, Rosenblatt J, Van Houten B. Analysis of gene-specific DNA damage and repair using quantitative polymerase chain reaction. Methods. 2000;22:135–147. doi: 10.1006/meth.2000.1054. [DOI] [PubMed] [Google Scholar]
- 23.Mohanty JG, Bhamidipaty S, Evans MK, Rifkind JM. A fluorimetric semi-microplate format assay of protein carbonyls in blood plasma. Anal Biochem. 2010;400(2):289–94. doi: 10.1016/j.ab.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yano K, Komaki-Yasuda K, Kobayashi T, Takemae H, Kita K, Kano S, Kawazu S. Expression of mRNAs and proteins for peroxiredoxins in Plasmodium falciparum erythrocytic stage. Parasitol Int. 2005;54(1):35–41. doi: 10.1016/j.parint.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative Ct method. Nature Protocols. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaidya AB, Akella R, Suplick K. Sequences similar to genes for two mitochondrial proteins and portions of ribosomal RNA in tandemly arrayed 6-kilobase-pair DNA of a malarial parasite. Mol Biochem Parasitol. 1989;35(2):97–107. doi: 10.1016/0166-6851(89)90112-6. [DOI] [PubMed] [Google Scholar]
- 28.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–18. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 29.Yadav AK, Desai PR, Rai MN, Kaur R, Ganesan K, Bachhawat AK. Glutathione biosynthesis in the yeast pathogens Candida glabrata and Candida albicans: essential in C. glabrata, and essential for virulence in C. albicans. Microbiology. 2011;157(2):484–95. doi: 10.1099/mic.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 30.Sharma KG, Sharma V, Bourbouloux A, Delrot S, Bachhawat AK. Glutathione depletion leads to delayed growth stasis in Saccharomyces cerevisiae: evidence of a partially overlapping role for thioredoxin. Curr Genet. 2000;38:71–77. doi: 10.1007/s002940000137. [DOI] [PubMed] [Google Scholar]
- 31.Markovic J, Mora NJ, Broseta AM, Gimeno A, de-la-Concepción N, Viña J, Pallardó FV. The depletion of nuclear glutathione impairs cell proliferation in 3t3 fibroblasts. PLoS One. 2009;4(7):e6413. doi: 10.1371/journal.pone.0006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patzewitz EM, Wong EH, Müller S. Dissecting the role of glutathione biosynthesis in Plasmodium falciparum. Mol Microbiol. 2012;83(2):304–18. doi: 10.1111/j.1365-2958.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller S. Role and regulation of glutathione metabolism in Plasmodium falciparum. Molecules. 2015;20(6):10511–34. doi: 10.3390/molecules200610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrand MA, Winterberg M, Ng F, Nguyen M, Kirk K, Hladky SB. Glutathione export from human erythrocytes and Plasmodium falciparum malaria parasites. Biochem J. 2012;448(3):389–400. doi: 10.1042/BJ20121050. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava A, Creek DJ, Evans KJ, De Souza D, Schofield L, Müller S, Barrett MP, McConville MJ, Waters AP. Host reticulocytes provide metabolic reservoirs that can be exploited by malaria parasites. PLoS Pathog. 2015;11(6):e1004882. doi: 10.1371/journal.ppat.1004882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitch CD, Kanjananggulpan P. The state of ferriprotoporphyrin IX in malaria pigment. J Biol Chem. 1987;262(32):15552–5. [PubMed] [Google Scholar]
- 37.Pagola S, Stephens PW, Bohle DS, Kosar AD, Madsen SK. The structure of malaria pigment beta-haematin. Nature. 2000;404(6775):307–10. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- 38.Egan TJ, Combrinck JM, Egan J, Hearne GR, Marques HM, Ntenteni S, Sewell BT, Smith PJ, Taylor D, van Schalkwyk DA, Walden JC. Fate of haem iron in the malaria parasite Plasmodium falciparum. Biochem J. 2002;365:343–347. doi: 10.1042/BJ20020793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hempelmann E. Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors. Parasitol Res. 2007;100(4):671–6. doi: 10.1007/s00436-006-0313-x. [DOI] [PubMed] [Google Scholar]
- 40.de Almeida Ribeiro MC, Augusto O, da Costa Ferreira AM. Influence of quinoline-containing antimalarials in the catalase activity of ferriprotoporphyrin IX. J Inorg Biochem. 1997;65:15–23. [Google Scholar]
- 41.Loria P, Miller S, Foley M, Tilley L. Inhibition of the peroxidative degradation of haem as the basis of action of chloroquine and other quinoline antimalarials. Biochem J. 1999;339:363–370. [PMC free article] [PubMed] [Google Scholar]
- 42.Liochev SI, Fridovich I. Superoxide and iron: partners in crime. IUBMB Life. 1999;48(2):157–61. doi: 10.1080/713803492. [DOI] [PubMed] [Google Scholar]
- 43.Tampo Y, Kotamraju S, Chitambar CR, Kalivendi SV, Keszler A, Joseph J, Kalyanaraman B. Oxidative stress-induced iron signaling is responsible for peroxide-dependent oxidation of dichlorodihydrofluorescein in endothelial cells: role of transferrin receptor-dependent iron uptake in apoptosis. Circ Res. 2003;92(1):56–63. doi: 10.1161/01.res.0000048195.15637.ac. [DOI] [PubMed] [Google Scholar]
- 44.Cui L, Miao J, Cui L. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob Agents Chemother. 2007;51(2):488–94. doi: 10.1128/AAC.01238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S, Guha M, Choubey V, Maity P, Srivastava K, Puri SK, Bandyopadhyay U. Bilirubin inhibits Plasmodium falciparum growth through the generation of reactive oxygen species. Free Radic Biol Med. 2008;44(4):602–13. doi: 10.1016/j.freeradbiomed.2007.10.057. [DOI] [PubMed] [Google Scholar]
- 46.Gopalakrishnan AM, Kumar N. Antimalarial action of artesunate involves DNA damage mediated by reactive oxygen species. Antimicrob Agents Chemother. 2015;59(1):317–25. doi: 10.1128/AAC.03663-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saraste M. Oxidative phosphorylation at the fin de siècle. Science. 1999;283(5407):1488–93. doi: 10.1126/science.283.5407.1488. [DOI] [PubMed] [Google Scholar]
- 48.Kawahara K, Mogi T, Tanaka TQ, Hata M, Miyoshi H, Kita K. Mitochondrial dehydrogenases in the aerobic respiratory chain of the rodent malaria parasite Plasmodium yoelii yoelii. J Biochem. 2009;145(2):229–37. doi: 10.1093/jb/mvn161. [DOI] [PubMed] [Google Scholar]
- 49.Bryant C, Voller A, Smith MJ. The incorporation of radioactivity from (14C) glucose into the soluble metabolic intermediates of malaria parasites. Am J Trop Med Hyg. 1964;13:515–19. doi: 10.4269/ajtmh.1964.13.515. [DOI] [PubMed] [Google Scholar]
- 50.Scheibel LW, Pflaum WK. Cytochrome oxidase activity in platelet-free preparations of Plasmodium falciparum. J Parasitol. 1970;56(6):1054. [PubMed] [Google Scholar]
- 51.Gutteridge WE, Dave D, Richards WH. Conversion to dihydroorotate to orotate in parasitic protozoa. Biochem Biophys Acta. 1979;582(3):390–401. doi: 10.1016/0304-4165(79)90131-4. [DOI] [PubMed] [Google Scholar]
- 52.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446(7131):88–91. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 53.Radfar A, Diez A, Bautista JM. Chloroquine mediates specific proteome oxidative damage across the erythrocytic cycle of resistant Plasmodium falciparum. Free Radic Biol Med. 2008;44:2034–42. doi: 10.1016/j.freeradbiomed.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 54.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–55. [PubMed] [Google Scholar]
- 55.Bécuwe P, Gratepanche S, Fourmaux MN, Van Beeumen J, Samyn B, Mercereau-Puijalon O, Touzel JP, Slomianny C, Camus D, Dive D. Characterization of iron-dependent endogenous superoxide dismutase of Plasmodium falciparum. Mol Biochem Parasitol. 1996;76(1–2):125–34. doi: 10.1016/0166-6851(95)02552-9. [DOI] [PubMed] [Google Scholar]
- 56.Sienkiewicz N, Daher W, Dive D, Wrenger C, Viscogliosi E, Wintjens R, Jouin H, Capron M, Müller S, Khalife J. Identification of a mitochondrial superoxide dismutase with an unusual targeting sequence in Plasmodium falciparum. Mol Biochem Parasitol. 2004;137(1):121–32. doi: 10.1016/j.molbiopara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 57.Bozdech Z, Ginsburg H. Antioxidant defense in Plasmodium falciparum--data mining of the transcriptome. Malar J. 2004;3:23. doi: 10.1186/1475-2875-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elledge SJ, Davis RW. DNA damage induction of ribonucleotide reductase. Mol Cell Biol. 1989;9(11):4932–4940. doi: 10.1128/mcb.9.11.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vega-Rodríguez J, Pastrana-Mena R, Crespo-Lladó KN, Ortiz JG, Ferrer-Rodríguez I, Serrano AE. Implications of glutathione levels in the Plasmodium berghei response to chloroquine and artemisinin. PLoS One. 2015;10(5):e0128212. doi: 10.1371/journal.pone.0128212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yalcin O, Oronsky B, Carvalho LJ, Kuypers FA, Scicinski J, Cabrales P. From METS to malaria: RRx-001, a multi-faceted anticancer agent with activity in cerebral malaria. Malar J. 2015;14:218. doi: 10.1186/s12936-015-0720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scicinski J, Oronsky B, Taylor M, Luo G, Musick T, Marini J, Adams CM, Fitch WL, Elledge SJ, Davis RW. DNA damage induction of ribonucleotide reductase. Mol Cell Biol. 1989;9(11):4932–4940. doi: 10.1128/mcb.9.11.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.del Castro MR, Suarez E, Kraiselburd E, Isidro A, Paz J, Ferder L, Ayala-Torres S. Aging increases mitochondrial DNA damage and oxidative stress in liver of rhesus monkeys. Exp Gerontol. 2012;47(1):29–37. doi: 10.1016/j.exger.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Intraerythrocytic development of P. berghei WT, pbggcs-ko1, and pbggcs-ko2 parasites at 16 h culture. Light microscopy images of trophozoites from WT, pbggcs-ko1, and pbggcs-ko2 parasites during intraerythrocytic development at 16 h culture. Note the presence of vacuoles in the mutant parasites. Parasites are indicated by black arrows and cytoplasmic vacuoles by red arrows. Microscopy images were adjusted for brightness and contrast to improve image clarity.
Figure S2. Intraerythrocytic development of P. berghei WT, pbggcs-ko1, and pbggcs-ko2 parasites at 24 h culture. Light microscopy images of WT, pbggcs-ko1, and pbggcs-ko2 parasites during intraerythrocytic development at 24 h culture. Note mature schizonts in the WT and late trophozoites/immature schizonts in the mutants. Parasites are indicated by black arrows and cytoplasmic vacuoles by red arrows. Microscopy images were adjusted for brightness and contrast to improve image clarity.
Figure S3. Intraerythrocytic development of P. berghei WT, pbggcs-ko1, and pbggcs-ko2 parasites at 28 h culture. Light microscopy images of WT, pbggcs-ko1, and pbggcs-ko2 parasites during intraerythrocytic development at 28 h culture. Mature schizonts from WT parasites are beginning to burst from the RBC. Note smaller and disorganized immature schizonts in the mutants. Parasites are indicated by black arrows. Microscopy images were adjusted for brightness and contrast to improve image clarity.
Figure S4. Protein carbonylation in P. berghei ggcs-ko and gr-ko parasites by the dinitrophenylhydrazine (DNPH) method. The carbonyl content of proteins from WT, pbggcs-ko, and pbgr-ko parasites was determined by the dinitrophenylhydrazine (DNPH) method using the OxyBlot Protein Oxidation Detection Kit (Millipore) as we previously described [62]. Parasite samples (30 μg) were incubated with 2,4 DNPH to form 2,4 dinitrophenyl hydrazone derivates (DNP-hydrazone) and non-derivatized proteins were used as negative controls. Proteins were resolved in an SDS-polyacrylamide gel (12%) and blotted onto a PVDF membrane. Binding of DNP to carbonylated proteins was detected with a rabbit anti-DNP antibody (1:150) (Millipore) followed by detection with a goat anti-rabbit IgG antibody conjugated to horseradish peroxidase (1:300) (Millipore). Carbonylated proteins were visualized using Super Signal West Pico Substrate (Pierce Biotechnology). Band intensities were quantified using the Bio-Rad VersaDoc™ Imaging System and the Quantity One computer software. Carbonylated protein levels were normalized to the signal obtained with an antibody against the 75kDa heat shock protein-70 (HSP-70) co-chaperone protein (1:100) of P. berghei (kindly provided by Dr. Fidel Zavala). Levels of carbonylations were expressed as intensity proportions after correcting for variations in protein loading based on the expression of the co-chaperone. Intensity proportions of carbonyl content were determined by densitometry. (A) Intensity for the pbggcs-ko and pbgr-ko parasites is expressed relative to the WT. A 75 kDa HSP-70 co-chaperone of P. berghei was used as a loading control. (B) Representative blot showing the immunodetection of carbonylated proteins in WT and mutant parasites (D=Derivatized; ND= Non Derivatized negative control). No significant differences in protein carbonylations were observed between WT and pbggcs-ko or pbgr-ko parasites. Bars represent the SD from 2 independent experiments.
Table S1. Primer sequences used for RT-qPCR.