Abstract
The biosynthesis of iron sulfur (Fe-S) clusters in Bacillus subtilis is mediated by a SUF-type gene cluster, consisting of the cysteine desulfurase SufS, the scaffold protein SufU, and the putative chaperone complex SufB/SufC/SufD. Here, we present the high-resolution crystal structure of the SufS homodimer in its product-bound state (i.e., in complex with pyrodoxal-5ʹ-phosphate, alanine, Cys361-persulfide). By performing hydrogen/deuterium exchange (H/DX) experiments, we characterized the interaction of SufS with SufU and demonstrate that SufU induces an opening of the active site pocket of SufS. Recent data indicate that frataxin could be involved in Fe-S cluster biosynthesis by facilitating iron incorporation. H/DX experiments show that frataxin indeed interacts with the SufS/SufU complex at the active site. Our findings deepen the current understanding of Fe-S cluster biosynthesis, a complex yet essential process, in the model organism B. subtilis.
Introduction
Iron sulfur (Fe-S) clusters are amongst the most versatile enzyme cofactors in Nature, as they are involved in cellular respiration, carbohydrate metabolism, DNA repair and various other vital functions throughout all kingdoms of life [1–4]. The biosynthesis of Fe-S clusters must be tightly regulated because of the toxicity of free sulfur and iron. Therefore, the systems for Fe-S cluster biogenesis are mainly conserved from bacteria to human, although elaborate transport systems have diverged through evolution [5–7]. Three distinct systems have been described for prokaryotic cells: i.) the NIF system (nitrogen fixation, [8]), ii.) the ISC system (iron-sulfur cluster, [9]) and iii.) the SUF system (sulfur mobilization, [10]). While the NIF system is specific for nitrogenase maturation in azototrophic bacteria, the ISC and/or SUF systems function in housekeeping in most–if not all–bacteria. In some bacterial species (e.g., Escherichia coli), both systems are found, while others (e.g., B. subtilis) rely on a single type of Fe-S machinery [10]. In eukaryotic cells, Fe-S biosynthesis takes place in the mitochondria in an ISC-like system [6] and in plastids in a SUF-like system [11]. In all of these systems, the Fe-S cluster is formed on a scaffold protein before it is transferred to a target apoprotein [12]. A cysteine desulfurase acquires sulfur from cysteine in a pyrodoxal-5ʹ-phosphate (PLP)-dependent reaction and then transfers it as persulfide to the scaffold protein [13,14]. In the E. coli SUF and eukaryotic systems, auxiliary proteins (SufS/SufE and Nfs1/Isd11, respectively) were found to enhance the activity of the cysteine desulfurase and aid in persulfide transfer [15,16]. The assembly also relies on electron transport, most likely for the reduction of sulfane (S0) to sulfide (S2-). For this purpose, it was shown that the E. coli ISC system utilizes ferredoxin [17], while the E. coli SUF system relies on a scaffold protein (SufB) associated with FAD [15] (in the latter case, however, in vitro experiments suggested a role in reduction of ferric iron rather sulfur) [18]. The mechanism of iron insertion remains elusive. It has been suggested that the highly conserved protein frataxin might act as the iron donor, although this role is still under debate. Structural analysis showed that frataxin assumes an α/β fold in which the N-terminal α-helix consists of several acidic residues, commonly referred to as the ‘acidic ridge’ [19–23]. Deletion of frataxin in Saccharomyces cerevisiae results in accumulation of iron in the mitochondria and drastically decreases biosynthesis of Fe-S clusters [24]. Frataxin can bind iron and interact with the cysteine desulfurase as well as the Fe-S scaffold protein. These observations led to the suggestion that frataxin could act as an iron chaperone [25]. The latter hypothesis was challenged by the finding that deletion of the E. coli gene encoding frataxin does not lead to iron accumulation or a decrease in Fe-S clusters as observed in S. cerevisiae, even though E. coli frataxin also forms a complex with SufS/SufU homologs IscS/IscU [26,27].
In Bacillus subtilis (Bs), a frataxin homolog Fra (formerly YdhG) shares only little sequence identity with other frataxins. However, structural analysis of BsFra showed a conserved α/β sandwich fold with a cluster of acidic residues on the N-terminal α1 and α2 helices, forming an “acidic ridge” [28]. BsFra was found to bind two equivalents of iron with a moderate Kd and to interact with the Thermotoga maritima (Tm) iron-sulfur cluster scaffold protein TmIsu [28]. Based on deletion mutants, it was suggested that BsFra is a global iron regulator involved in the distribution of iron in B. subtilis [29]. Previously, the cysteine desulfurase BsSufS and putative scaffold protein BsSufU were characterized in vivo and in vitro, and it was shown that BsFra can be utilized as an iron source [28–31]. We recently demonstrated that BsFra interacts with the ferrochelatase HemH and is crucial for the incorporation of iron into protoporphyrin [32]. However, the role of frataxin in delivery of iron to SufS/SufU is still poorly understood; in particular, in Fe-S biosynthesis, no interaction between BsFra and the BsSufS/BsSufU complex has been observed so far. Herein, we demonstrate that BsFra can indeed interact with BsSufS and BsSufU, as characterized by hydrogen/deuterium exchange (H/DX) experiments. Furthermore, we present the crystal structure of BsSufS and suggest a model for the protein complex consisting of BsSufS/BsSufU/BsFra.
Material and Methods
Protein expression and purification
Plasmids for heterologous expression were previously prepared [30,33]. The heterologous expression of fra [29,32], sufU [30] and sufS [31] was carried out in E. coli BL21(DE) cells for 20 h at 22°C in LB (lysogeny broth) medium with 50 μg/mL kanamycin and 0.2 mM IPTG. Cells were collected and washed in HEPES buffer A (50 mM HEPES, pH 8, 300 mM NaCl, 5 mM imidazole), treated with DNaseI, and lysed with a French press. The crude extract was cleared by centrifugation (17000 rpm, 4°C, 30 min) and the supernatant was filtered (0.25 μm). The target protein was purified by Ni-affinity chromatography on an FPLC system (NGC Quest, Biorad) using a gradient of 5–100% HEPES buffer B (50 mM HEPES, pH 8, 300 mM NaCl, 250 mM imidazole) over 20 min. The elution fractions were concentrated and further purified by size exclusion chromatography (HiLoad 26/60 Superdex 200, GE Healthcare Life Sciences) in HEPES buffer C (50 mM HEPES, pH 8, 300 mM NaCl). Fractions containing the target protein were concentrated, flash frozen in liquid nitrogen and stored at -80°C in HEPES buffer C supplemented with 10% glycerol.
Crystallization, data collection, and structure determination
Crystallization was performed by the sitting-drop method at 20°C in 0.6-μl drops consisting of equal parts protein and crystallization solutions. BsSufS crystallized at 20 mg/ml within one week in 0.1 M HEPES, pH 7.5, 50% (v/v) PEG 400. Prior to data collection, crystals were cryoprotected in a solution consisting of the well solution supplemented with 20% glycerol and then flash-frozen in liquid nitrogen. Data were collected under cryogenic conditions at the European Synchrotron Radiation Facility at Beamline ID29. Data were processed with XDS [34] and scaled with CCP4-implemented SCALA [35]. The structure was determined by molecular replacement with PHASER [36], manually built in COOT [37], and refined with PHENIX [38]. The SufS homolog from Brucella suis (PDB ID 4W91) was employed as a search model. Figures were prepared with Pymol (www.pymol.org). Coordinates and structure factors were deposited in the Protein Data Bank with the accession code 5J8Q.
Hydrogen/deuterium exchange experiments
H/DX mass spectrometric analysis of the samples was performed using an automated H/DX setup (Waters) including a two-arm robotic autosampler (LEAP Technologies), an ACQUITY UPLC M-Class System and HDX Manager (Waters). For the exchange reaction, BsFra, BsSufU, BsSufS, BsFra/BsSufU, BsFra/BsSufS, BsFra/BsSufU/BsSufS, and BsSufU/BsSufS (60 μM final concentration of each component) were individually prepared in H2O buffer (25 mM Tris-Cl, pH 7.5, 100 mM NaCl) [32] and pre-cooled to 1°C. For each LC-MS run, 7.5 μL of protein solution was pipetted into a fresh vial on an exchange plate at 25°C and diluted with 61.8 μL of either H2O buffer (t0 runs) or D2O buffer (exchange runs). After incubation for 15, 30, 60, or 600 s, 55 μL of the reaction solution was transferred to a fresh vial containing 55 μL of quenching solution (400 mM H3PO4/KH2PO4, pH 2.2, pre-dispensed and pre-cooled to 1°C for 10 min before the first run). After quenching, 95 μL of the resulting solution was immediately injected into the HDX Manager.
Digestion was done online using an Enzymate BEH Pepsin Column (Waters) at 20°C with water/0.1% formic acid at a flow rate of 100 μL/min. Subsequently peptic peptides were trapped at 0.5°C using a C18 trap column. Separation of peptides was achieved at 0.5°C utilizing a 1 x 100 mm ACQUITY UPLC BEH C18 1.7 μm column (Waters) at a flow rate of 30 μL/min with the following gradient of solvents A (water/0.1% formic acid) and B (acetonitrile/0.1% formic acid): Linear increase from 5–35% B within 7 min, followed by a ramp to 85% B within 1 min and isocratic 85% B for additional 2 min. Finally, the column was washed with 95% B for 1 min and re-equilibrated with 5% B for 5 min.
During separation of peptides using the chromatographic column, the pepsin column was washed by injecting 3 × 80 μL 4% acetonitrile and 0.5 M guanidinium chloride. HDMSe was used for t0 peptide detection and HDMS for exchanged peptides. Lock mass spectra were measured every 45 s using Glu-fibrinopeptide B as a standard ([M+H]2+ = 785.8427 m/z). t0 peptide identification was performed using ProteinLynx Global SERVER (Waters) with custom databases and the setting “no enzyme”. Final assignment of deuterium incorporation was done with DynamX 3.0 (Waters).
Microscale thermophoresis
The determination of binding constants was done by microscale thermophoreis using a Monolith NT.115 instrument (NanoTemper). 100 μM BsFra was labeled at 8°C overnight using the Monolith NT.115 Protein Labeling Kit RED MALEIMID (cysteine-reactive; NanoTemper). Labeled BsFra was buffer-exchanged into a binding buffer (25 mM Tris-Cl, 100 mM NaCl, 10 mM 2-mercaptoethanol, pH 7.5) and the concentration was adjusted to 0.25 μM. A serial dilution series of BsSufS, BsSufU, and BsSufS/BsSufU was prepared in the same buffer in a range from 560 μM to 0.030 μM and then mixed 1:1 with labeled BsFra. The titration was transferred into NT.115 MST Premium Coated capillaries (NanoTemper). Measurements were performed at 25°C, 20% LED power and 20% MST power with a heating time of 30 s and cooling time of 5 s. The binding constant for each interaction was calculated from the average of three measurements using the NT Analysis software (NanoTemper).
Cysteine Desulfurase Activity Assay
The activity of BsSufS was measured by the amount of sulfide released during conversion of cysteine to alanine. Free sulfide was quantified using N,N-dimethyl-p-phenylenediamine sulfate (DMPD) and FeCl3 as described previously [30,31,39]. We incubated 0.5 μM BsSufS with 10 μM BsSufU and 50 μM BsFra in 25 mM Tris-Cl, pH 7.5, 100 mM NaCl, 5 mM dithiothreitol (DTT) for 5 min at room temperature. The 200 μL reaction was started by addition of l-cysteine (2 mM) and quenched after 10 min by addition of 25 μL of DMPD (20 mM in 7.2 M HCl) and 25 μL FeCl3 (30 mM in 1.2 M HCl). After 30 min of incubation in the dark, the absorbance was measured at 670 nm. All reactions were carried out in triplicate.
Fe-S Biosynthesis Assays
The BsSufS-dependent biosynthesis of Fe-S clusters on BsSufU was assayed in an anaerobic chamber (Coy Laboratories) with forming gas (2% H2/98% N2) as previously described [30,33]. Briefly, 0.5 μM BsSufS was incubated with 10 μM BsSufU in 25 mM Tris-Cl, pH 7.5, 100 mM NaCl, 5 mM DTT for 5 min at 15°C. Next, 100 μM ammonium iron(II) sulfate was added to the reaction mixture with or without 50 μM BsFra. The reaction was started by addition of 2 mM cysteine, and Fe-S cluster formation on BsSufU was monitored by UV-Vis at 465 nm (ε456 = 5.8 mM-1cm-1) [40].
Results
Crystal structure of BsSufS in the product-bound state
As no structure of B. subtilis SufS was available at the start of our study, we sought to fill this gap. BsSufS was thus crystallized and its structure was determined to 1.7 Å resolution by molecular replacement using the B. suis homolog (PDB ID 4W91) [41] as a search model (Table 1). One molecule of SufS was found within the asymmetric unit (AU) and could be built to completeness. The crystal structure of BsSufS is highly similar to that of E. coli SufS (PBD ID 1I29) with a root mean square deviation (r.m.s.d.) of 1.28 Å over 407 Cα atoms (S1 Fig). The BsSufS monomer forms a tightly intertwined homodimer with another monomer across the crystallographic symmetry axis (Fig 1A). The interface and architecture of the BsSufS homodimer closely resemble those of E. coli SufS [42].
Table 1. Data collection and refinement statistics for BsSufS.
| Data collection | ||
|---|---|---|
| Space group | P 31 2 1 | |
| Unit cell | ||
| a, b, c (Å) | 93.2, 93.2, 130.5 | |
| α, β, γ (°) | 90, 90, 120 | |
| Wavelength (Å) | 0.9790 | |
| Resolution (Å) | 46.60–1.70 (1.76–1.70)* | |
| No. of unique reflections | 72121 (6911) | |
| Multiplicity | 9.7 (9.4) | |
| Completeness (%) | 1.00 (0.97) | |
| Mean I/sigma(I) | 18.5 (2.7) | |
| Rmerge | 0.071 (0.753) | |
| Refinement | ||
| No. of unique reflections | 72119 (6911) | |
| Rwork/Rfree | 0.134/0.159 (0.175/0.212) | |
| No. of atoms | 3562 | |
| Protein | 3254 | |
| Ligand | 15 | |
| Water | 293 | |
| R.m.s. deviations | ||
| Angles (°) | 1.58 | |
| Bonds (Å) | 0.018 | |
| Ramachandran allowed (%) | 2.9 | |
| Ramachandran outliers (%) | 0.2 | |
| Average B-factor (Å2) | 30.2 | |
| Protein | 29.0 | |
| Ligand | 26.1 | |
| Water | 43.8 |
*Statistics for the highest-resolution shell are shown in parentheses.
Data were collected from a single crystal.
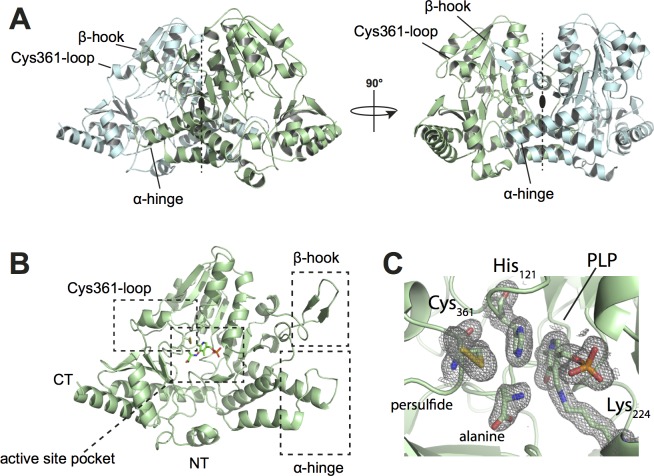
Fig 1. Crystal Structure of the B. subtilis cysteine desulfurase SufS at 1.7 Å.
(A) Overall structure of the BsSufS homodimer. The dimer forms a two-fold symmetry axis which covers the active site pocket. One monomer is colored light green and the other, cyan. (B) Monomeric BsSufS consists of two domains: The larger, N-terminal domain harbors the PLP binding pocket and the smaller, C-terminal domain contains the flexible Cys361-loop (on which the catalytic Cys361 resides). (C) Magnified view of the active site pocket of BsSufS, showing the products alanine and Cys361-persulfide. Pyrodoxal-5ʹ-phosphate is bound to Lys224 as an aldimine. A 2Fo-Fc map density (grey mesh) is shown, contoured at 1.0 r.m.s.d. N-terminus (NT), C-terminus (CT).
BsSufS assumes a type I fold of the aminotransferase class V family, consisting of an overall α/β fold that is highly similar to that of E. coli SufS, IscS, NifS, and CsdA [41,43–46]. The enzyme consists of two domains: A large, N-terminal domain (residues 1–294) and a smaller, C-terminal domain (residues 295–406). In the N-terminal domain, a 7-stranded, parallel β-sheet is sandwiched between several α-helices to form a tightly packed core. This domain harbors the active site pocket, where the cofactor PLP is bound to Lys224 as an aldimine. Furthermore, residues 253–265 (herein referred to as the ‘β-hook’) form a hairpin-like structure that latches on to the other monomer. The small, C-terminal domain consists of a 4-stranded, parallel β-sheet and four α-helices containing the flexible ‘Cys361-loop’ with the nucleophile Cys361 (Fig 1B). The dimer interface covers much of the active site pocket and protects PLP and Cys361. We found BsSufS in the product-bound state, with alanine bound near Cys361-persulfide, suggesting that one reaction cycle had occurred.
BsSufS forms a homodimer in solution
We performed time-resolved H/DX experiments in order to understand the dynamics of BsSufS in solution. We identified 176 peptides between 5 and 20 amino acids in length, resulting in 98.6% sequence coverage and a redundancy of 4.4 (Fig 2). BsSufS was incubated in D2O buffer for 15, 30, 60, and 600 s, and the exchange of backbone amide hydrogen to deuterium was measured for each peptide. In good agreement with the expectations from our crystallographic analysis, the solvent-exposed N- and C-termini of BsSufS exchanged readily, whereas most of the core structure was strongly protected (compare to Fig 1). Strong protection was observed for BsSufS regions involved in formation of the homodimer interface, validating the existence of the enzyme as a homodimer in solution [47] (Fig 2B).
Fig 2. H/DX heat map of BsSufS.
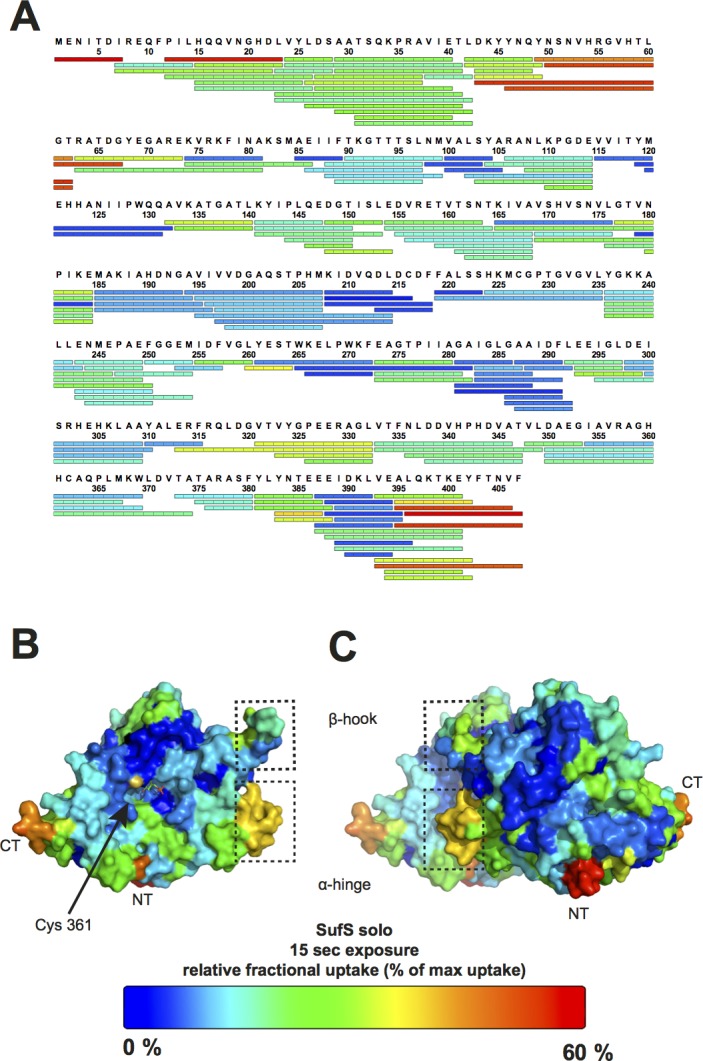
The dynamics of BsSufS were analyzed in solution by incubation in deuterated buffer. The relative amount of deuterium incorporated after 15 s, indicated by a color code ranging from blue (low; stable region) to red (high; flexible region), is mapped on the detected peptic peptides of (A) BsSufS or mapped onto the surface of the BsSufS (B) monomer and (C) homodimer. N-terminus (NT), C-terminus (CT).
BsSufU binds to the BsSufS homodimer
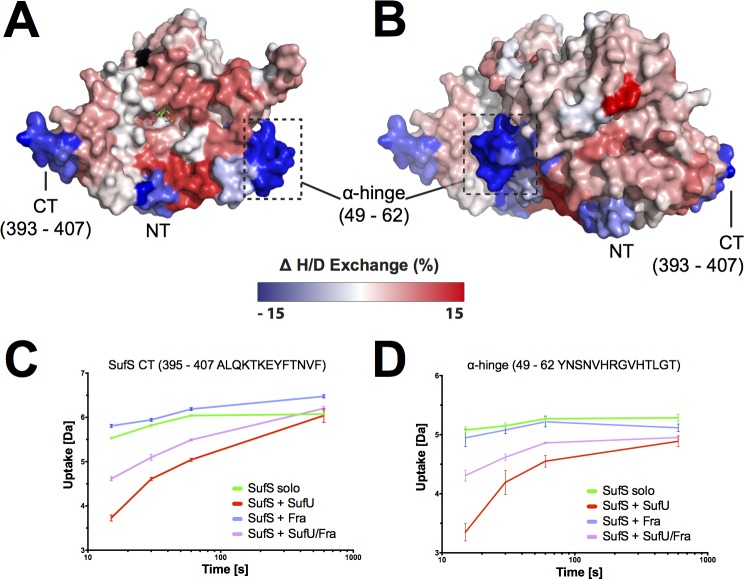
Next, we sought to investigate the interaction interface and conformational dynamics of SufS and SufU. Therefore, SufS was incubated with SufU in deuterated buffer. Upon completion of the H/DX reaction and peptic digest, we observed that several peptides of SufS showed a change in deuterium uptake when compared to their counterparts from H/DX with SufS alone (see above). Most notably, the C-terminal α-helix of SufS showed a high degree of protection in the presence of SufU (Fig 3). This observation agrees with recent findings that the C-terminus of E. coli IscS is required for interaction with IscU [48,49]. In addition, residues 50–63 of SufS (herein referred to as the ‘α-hinge’) were protected against deuterium incorporation, suggesting the presence of a second SufU interaction site at the SufS homodimer interface.
Fig 3. BsSufU alters the H/D exchange of BsSufS upon binding.
Changes in relative fractional deuterium uptake of BsSufS after incubation with BsSufU for 15 s in D2O buffer compared to BsSufS alone were mapped onto the surface of the BsSufS (A) monomer and (B) homodimer. A decrease (blue) in deuterium uptake signals protection (i.e., a binding event), whereas an increase (red) signals a structural rearrangement. Black regions were not detected. Binding of BsSufU to (C) the C-terminus and (D) the α-hinge of BsSufS as a function of deuterium uptake over time. Color code: BsSufS alone (green), BsSufS + BsSufU (red), BsSufS + BsFra (blue), and BsSufS + BsSufU/BsFra (violet). N-terminus (NT) and C-terminus (CT).
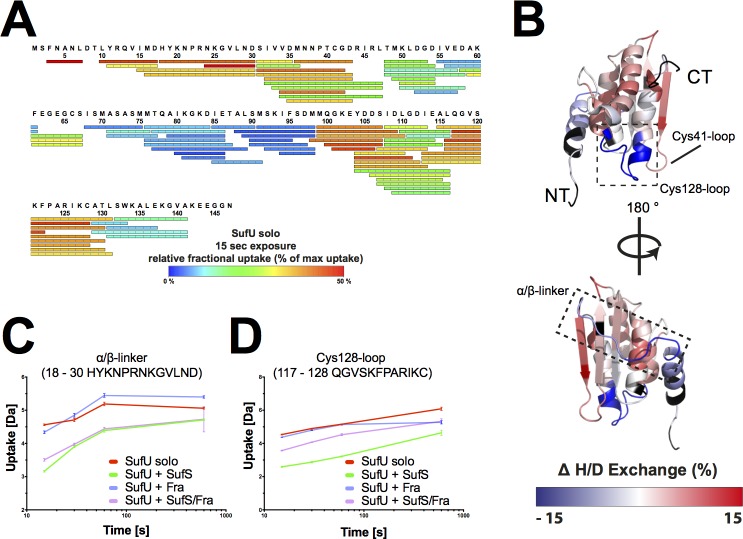
We then utilized our H/DX approach to analyze changes in SufU upon binding SufS. We identified 87 peptides, which covered 93% of the sequence with a redundancy of 6.0 (Fig 4). The NMR solution structure of apo-SufU (PDB ID 2AZH) reveals a compact, α/β fold. Our H/DX analysis supports this structure in solution, as medium to low H/D exchange was observed for the core of SufU, whereas high H/D exchange was observed for an extended loop region between the N-terminal helix α1 and strand β1 (the ‘α/β linker’) as expected for a solvent-exposed, unstructured region. In the presence of SufS, the ‘α/β-linker’ of SufU is strongly protected from deuterium uptake, which indicates that SufS interacts with SufU at this location (Fig 4C). Residues 117–130, which form a loop carrying Cys128 (the ‘Cys128-loop’), are also protected from deuterium uptake, suggesting a second SufS binding site. Therefore, we conclude that the ‘α/β-linker’ and ‘Cys128-loop’ of SufU bind to the C-terminus and ‘α-hinge’ of SufS, respectively, bringing the active site of SufS and SufU into close proximity. A similar scenario has been proposed for the interaction of IscU and IscS in E. coli [49].
Fig 4. BsSufS alters the H/D exchange of BsSufU upon binding.
(A) Detected peptic peptides of BsSufU with the relative fractional uptake after 15 s of incubation in deuterated buffer. (B) Changes in the relative fractional deuterium uptake of BsSufU after incubation with BsSufS for 15 s in D2O buffer compared to BsSufU alone were mapped onto the surface of BsSufU (PDB ID 2AZH). The heat map represents the differences in deuterium uptake compared to BsSufU alone. A decrease (blue) in deuterium uptake signals protection (i.e., a binding event), whereas an increase (red) signals a structural rearrangement. Black regions were not detected. Binding of BsSufU to (C) the α/β-linker and (D) the Cys128-loop of BsSufS as a function of deuterium uptake over time. Color code: BsSufU alone (red), BsSufU + BsSufS (green), BsSufU + BsFra (blue), and BsSufU + BsSufS/BsFra (violet). N-terminus (NT) and C-terminus (CT).
Binding of BsSufU to BsSufS induces conformational changes in both proteins
Next, we analyzed the H/DX data for changes in the dynamic behavior of SufS in the presence of SufU. Significantly higher H/D exchange rates were observed for the active site pocket of SufS when SufU was present, particularly at the ‘Cys361-loop’ and ‘β-hook’ of SufS (Fig 3). These observations strongly suggest that the SufS homodimer opens in the presence of SufU, allowing the ‘Cys361-loop’ to move freely. This is in agreement with findings in E. coli, where the ‘Cys361-loop’ of IscS undergoes a major, 14-Å movement during transfer of sulfur to IscU [48,49].
Our data also show that, in the presence of SufS, SufU undergoes significant structural rearrangements at the ‘α-helical bundle’ (i.e., helices α2, α3, and α5) and ‘β-sheet surface’ (i.e., residues 32–47). The active site of SufU contains four residues (i.e., Cys41, Asp43, Cys66, and Cys128) that coordinate a structurally important zinc ion [30,50]. In particular, the ‘Cys41-loop’ showed a significantly increased deuterium uptake. To summarize, the interaction of BsSufS with BsSufU induces structural rearrangements near the active sites of both proteins, potentially facilitating persulfide transfer. This notion supports the 40-fold increase in BsSufS desulfurase activity previously observed upon interaction with BsSufU [30,31,47,50].
BsFra binds to BsSufU and BsSufS
After demonstrating the dynamic behavior and interaction of the BsSufS/BsSufU complex, we sought to integrate B. subtilis frataxin into the picture. We conducted microscale thermophoresis (MST) experiments between fluorophore-labelled BsFra and BsSufU, BsSufS, and BsSufS/BsSufU, and found that while BsFra binds its partners fairly weakly: BsSufU (Kd = 57.4 ± 13.8 μM), BsSufS (Kd = 50.6 ± 17.4 μM) and the BsSufS/BsSufU complex (Kd = 32.5 ± 3.6 μM) (Fig 5). In contrast, the interaction of BsSufS with BsSufU (Kd = 2.63 μM) [31] and BsFra with BsHemH (Kd = 1.63 μM) [32] were found to be significantly tighter. Nevertheless, our affinity measurement for BsFra with BsSufS/BsSufU is of the same order of magnitude as that for the homologous E. coli system comprised of CyaY and IscS (Kd = 18.5 μM) [51].
Fig 5. Characterization of the affinity of BsFra for BsSufS, BsSufU, and BsSufS/BsSufU using microscale thermophoresis.
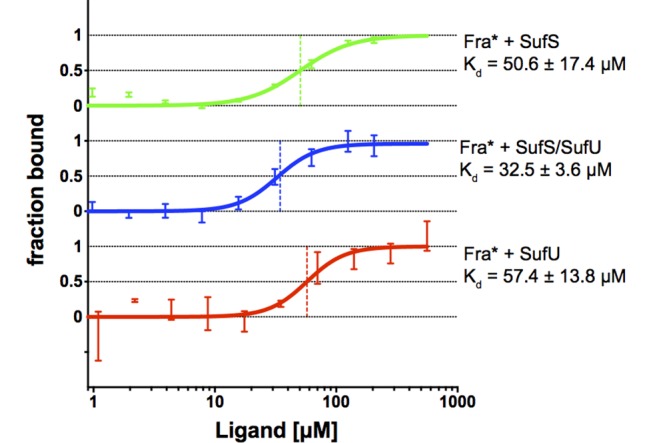
MST binding curve from the interaction of fluorophore-labeled BsFra with (A) BsSufS, (B) BsSufS/BsSufU, and (C) BsSufU. A Hill model was applied for Kd determination. Fra* indicates fluorophore-tagged frataxin.
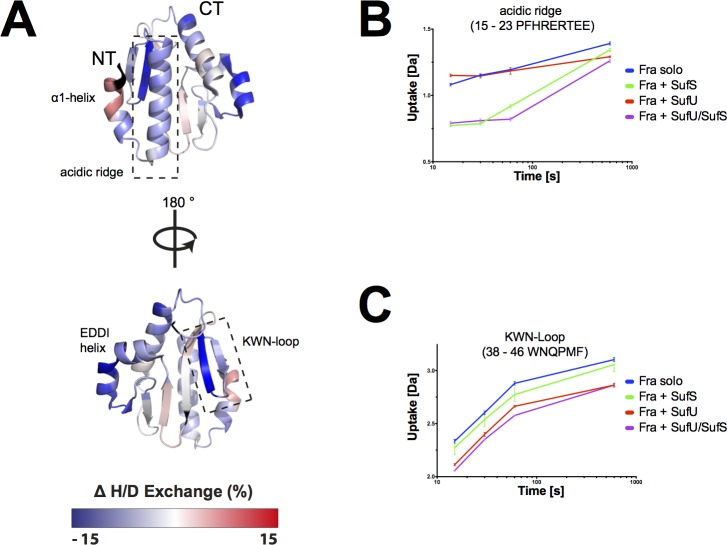
To further characterize the interaction of BsFra with its partners we applied H/DX experiments. Analysis of BsFra alone yielded 61 unique peptides with 98% sequence coverage and a redundancy of 4.7 (Fig 6). Human, S. cerevisiae, and E. coli frataxin (PDB ID 3S4M, 2GA5, and 2EFF, respectively) assume an α/β fold in which two α-helices are stacked against a 6-stranded β-sheet, with the N-terminal α-helix harboring several acidic residues (the ‘acidic ridge’) [21]. BsFra (PDB ID 2OC6) has an additional short, N-terminal helix (herein referred to as ‘α1-helix’) followed by the helix carrying the ‘acidic ridge’ (compare S2 Fig). The ‘α1-helix’ shows medium to high deuterium uptake in BsFra, suggesting that in solution it can move freely. Furthermore, a helix connecting β4 to β5 (the ‘EDDI-helix’) is present in BsFra but not homologous frataxin structures [19–21]. A high rate of H/D exchange was observed for this ‘EDDI-helix’, especially for residues E75, D76, D77, and I78.
Fig 6. Peptic peptides of BsFra detected from H/DX measurements.
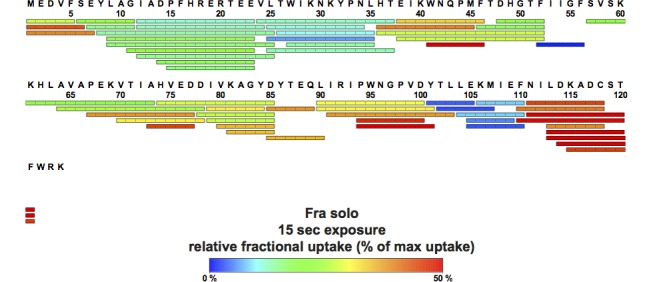
The relative amount of deuterium incorporated after 15 s, indicated by a color code ranging from blue (low; stable region) to red (high; flexible region), is mapped on the detected peptic peptides of BsFra.
We repeated the H/DX experiments of BsFra in the presence of BsSufU (S3 Fig), BsSufS (S4 Fig), and the BsSufS/BsSufU complex (Fig 7). These measurements were compared with those of BsFra alone for differences in deuterium uptake. In the presence of BsSufU, we observed a decrease of H/D exchange in β-strands 1 and 2 of BsFra (residues 37–46, herein referred to as the ‘KWN-loop’), which harbor residues K40, W41, and N42 (Fig 7C). As we found that the same residues were protected in the reaction of BsFra and BsSufU, we conclude that BsFra binds to BsSufU at the ‘KWN-loop’ area. The involvement of the ‘KWN-loop’ in the interaction with BsSufU is in agreement with previous studies in which mutations of in this region disrupted the interaction [52–55]. In the presence of both BsSufU and BsSufS, BsFra was additionally to the KWN loop protected from H/D uptake at the ‘acidic ridge’ (residues 9–25) (Fig 7and S4 Fig), suggesting an interaction with BsSufS. This is in accordance with previously published work on E. coli IscS/CyaY [51,56]. Furthermore, BsSufS induces an increased rate of deuterium uptake at the N-terminal ‘α1-helix’ of BsFra (Fig 7). We propose that, upon binding of BsFra to BsSufS, the ‘α1-helix’ undergoes a structural rearrangement. Interestingly, BsFra was protected from deuterium uptake at the C-terminus and ‘EDDI helix’ (residues 74–85) compared to the protein alone. However, this was found in the BsFra/BsSufS and BsFra/BsSufU reactions (Fig 7, S3 Fig and S4 Fig), suggesting that the either protein can stabilize these highly dynamic areas (compare Fig 6). Taken together, the results indicate that BsFra binds to BsSufS and BsSufU as well as to the BsSufS/BsSufU complex. A similar binding of frataxin to a cysteine desulfurase/scaffold protein complex was described in E. coli [51,56].
Fig 7. H/DX analysis of BsFra upon binding BsSufU/BsSufS.
(A) Changes in the relative fractional deuterium uptake of BsFra after incubation with BsSufS/BsSufU for 15 s in D2O buffer compared to BsFra alone were mapped onto the surface of BsFra (PDB ID 2OC6). The heat map represents the differences in deuterium uptake compared to BsFra alone. A decrease (blue) in deuterium uptake signals protection (i.e., a binding event), whereas an increase (red) signals a structural rearrangement. Black regions were not detected. Binding of BsSufS/BsSufS to (B) the ‘acidic ridge’ and (C) the KWN-loop of BsFra as a function of deuterium uptake over time. Color code: BsFra alone (blue), BsFra + BsSufS (green), BsFra + BsSufU (red), and BsFra + BsSufU/BsSufS (violet). N-terminus (NT) and C-terminus (CT).
Binding of BsFra to BsSufU/BsSufS induces conformational changes in all three proteins
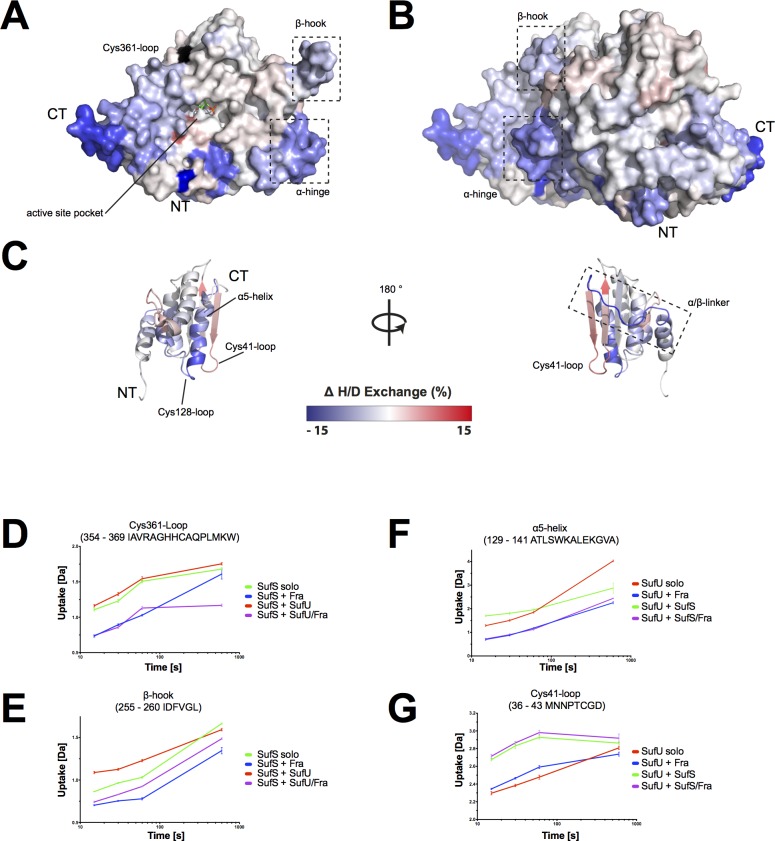
Next, we tested whether the binding of BsFra would alter the interaction of BsSufS with BsSufU. We analyzed the H/DX data of the BsFra/BsSufU/BsSufS interaction and compared it to that of the BsSufS/BsSufU complex and proteins alone (see above). The scaffold protein BsSufU showed decreased deuterium uptake at the C-terminal helix (‘α5-helix’), indicating binding to BsFra (Fig 8 and S3 Fig). The cysteine desulfurase BsSufS showed protection from H/D exchange at the ‘Cys361-loop’ and the ‘β-hook’ when BsFra was present (Fig 8), also indicating a binding event. Several positively charged residues are located in this region (e.g., R356, H359, H360, and K367) and may bind the negatively charged residues of the ‘acidic ridge’ of BsFra (see above). In contrast to the reaction of BsSufS/BsSufU with BsFra, no increase in deuterium uptake was observed at the active site pocket of BsSufS when BsFra was present (compare Fig 8to Fig 3). The BsSufU binding site on BsSufS (see above) was still observable, supporting the idea of simultaneous binding.
Fig 8. BsFra alters the H/D exchange of BsSufS and BsSufU upon binding the BsSufS/BsSufU complex.
Changes in the relative fractional deuterium uptake of BsSufS/BsSufU after incubation with BsFra for 15 s in D2O buffer compared to BsSufS and BsSufU alone were mapped onto the surface of the BsSufS (A) monomer and (B) homodimer as well as (C) BsSufU (PBD ID 2AZH). The heat map represents the differences in deuterium uptake compared to the solo incubation. A decrease (blue) in the uptake signals protection (i.e., a binding event), whereas an increase (red) signals a structural rearrangement. Black regions were not detected. Changes in (D) the SufS Cys361-loop and (E) the β-hook as a function of deuterium uptake over time. Color code: BsSufS alone (green), BsSufS + BsFra (blue), BsSufS + BsSufU (red), and BsSufS + BsSufU/BsFra (violet). Changes in (F) the SufU α5-helix and (G) the Cys41-loop as a function of deuterium uptake over time. Color code: BsSufU alone (red), BsSufU + BsFra (blue), BsSufU + BsSufS (green), and BsSufU + BsSufS/BsFra (violet). N-terminus (NT) and C-terminus (CT).
We propose that BsFra binds via the ‘acidic ridge’ to the positively charged ‘Cys361-loop’ and ‘β-hook’ of BsSufS. BsFra may act as a clamp at the BsSufS homodimer interface, locking the ‘Cys361-loop’ and ‘β-hook’ and thus preventing the opening of the BsSufS active site pocket. Furthermore, BsFra binds via its ‘KWN-loop’ to the ‘β-sheet surface’ of BsSufU. A similar binding site was suggested for E. coli IscS/CyaY [51,56].
To test whether the binding of BsFra to the BsSufS/BsSufU complex alters the activity of BsSufS we conducted cysteine desulfurase activity assays in vitro. We found that BsSufU greatly enhances the activity of BsSufS, in agreement with previous reports [30,33]. The introduction of BsFra, however, does not appear to affect the activation of BsSufS by BsSufU (Fig 9). This is in agreement with the E. coli IscS/CyaY interaction, where conversion of cysteine to alanine by IscS is not affected by CyaY [56]. We next analyzed whether frataxin has an effect on the formation of Fe-S clusters in vitro. In an assay described by Albrecht and colleagues [30], we incubated BsSufS and BsSufU in the presence of ferrous iron and cysteine and monitored the formation of Fe-S clusters by UV-Vis spectroscopy. When BsFra was present in the reaction mixture the initial rate of formation was unaffected, although we observed a very minor increase in the yield of Fe-S clusters formed on BsSufU. Such a subtle change could indicate that either BsFra does not efficiently donate iron to BsSufU under assay conditions or that its presence is redundant under the supplied concentration of iron. Additionally, other, unidentified components may be required for efficient transfer.
Fig 9. Sulfur transfer and Fe-S cluster biosynthesis assays of BsSufS and BsSufU in the presence of BsFra.
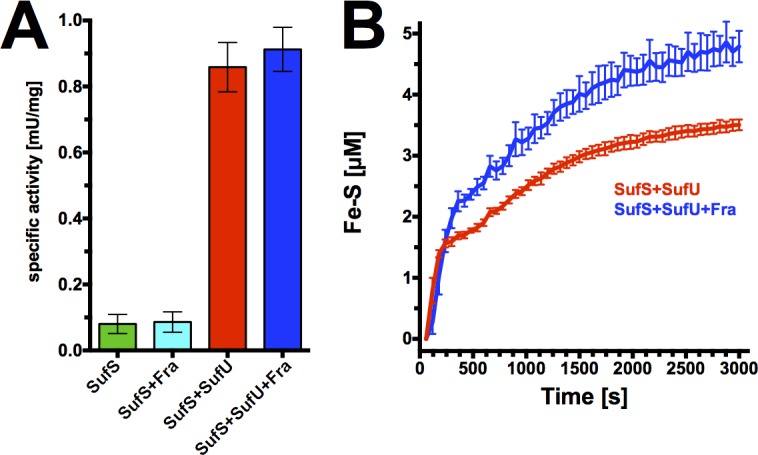
(A) The specific activity of BsSufS was measured in vitro by the release of sulfide from cysteine. The activity of BsSufS (green) is unaffected by BsFra (cyan), but is greatly increased by the presence of BsSufU (red). The addition of BsFra to BsSufS/BsSufU does not affect the activity of BsSufS. (B) In vitro biogenesis of Fe-S clusters by BsSufS/BsSufU over time (red). Addition of BsFra (blue) results in a very slight increase in cluster yield.
Discussion
Bacillus subtilis has five paralogous cysteine desulfurase genes, namely nifS [57], yrvO [58], nifZ [59] and ycbU [60]. However, only the product of the sufS gene is involved in biosynthesis of Fe-S clusters. Until now, no structural information was available on the B. subtilis cysteine desulfurase. We were able to determine the crystal structure of the BsSufS dimer to 1.7 Å resolution, revealing high structural homology to its E. coli counterpart. To better understand the conformational dynamics of SufS and its interaction with SufU and Fra, we conducted H/DX experiments with proteins from the Gram-positive model organism B. subtilis. Many insights into the SUF system were previously obtained from studies on E. coli, in which the SUF system only acts as a backup under stress conditions [7]. The ISC system mediates the major “housekeeping” functions for Fe-S cluster biosynthesis in E. coli [9]. In B. subtilis, we find similarities to both E. coli ISC and SUF systems [30,31,33]. We applied hydrogen/deuterium exchange experiments on BsSufS in order to investigate structural dynamics upon its interaction with the putative iron delivery protein BsFra and the scaffold protein BsSufU. H/DX detects the exchange of hydrogen on the backbone amides with deuterium in the solvent, where the exchange rate of highly dynamic or surface-exposed areas is rapid compared to residues that are buried in the protein core or otherwise protected. Investigation of BsSufS confirmed the homodimer interface in solution and suggested that the enzyme is tightly packed. Nevertheless, the strength of H/DX lies in its ability to detect changes in residues following a binding event. We showed that the C-terminal region and ‘α-hinge’ of BsSufS interact with the long ‘α/β-linker’ and ‘Cys128-loop’ of BsSufU (Fig 10). This interaction is accompanied by an opening of the BsSufS dimer interface and rearrangement of the ‘Cys361-loop’. BsSufU, on the other hand, showed H/D exchange in the loop carrying Cys41 and Asp43. BsSufU fits on the BsSufS interaction site, which brings the ‘Cys361-loop’ of BsSufS and the ‘Cys41-loop’ of BsSufU into close proximity for persulfide transfer.
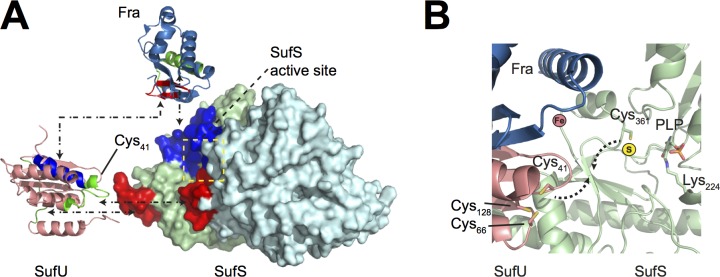
Fig 10. Proposed model for the interaction of BsFra/BsSufU/BsSufS.
(A) Based on the H/DX results, we identified the binding sites of BsSufU (PDB ID 2AZH, shown in salmon), BsFra (PDB ID 2OC6, shown in sky blue) and the BsSufS homodimer (light green and cyan) in the BsFra/BsSufS/BsSufU complex. The binding epitopes are highlighted according to the color of the respective interaction partner (i.e., residues protected by BsFra are shown in blue, those by BsSufS in green, and those by BsSufU in red). (B) Proposed binding of BsFra and BsSufU to BsSufS. The binding of BsSufU to BsSufS brings the SufS residue Cys361 and the SufU residue Cys41 into close proximity for persulfide transfer. While binding of BsFra to BsSufS does not affect the transfer of persulfide from BsSufS to BsSufU, it brings the putative iron binding site into close proximity to the SufS/SufU active site.
The E. coli SufS/SufE interaction was previously characterized by H/DX [61] and the crystal structure of the homologous E. coli CsdA/CsdE enzyme complex was solved recently [41]. In both cases, SufE/CsdE binds near the active site of SufS/CsdA to accept the sulfur as persulfide, which is then passed on to the scaffold protein complex SufBC2D of the E. coli SUF system. E. coli SufB has been shown to act as a scaffold and carries an FAD binding site that is possibly important for the reduction of Fe3+ to Fe2+ in the generation of Fe-S clusters [18]. In B. subtilis, the roles of SufB, SufC, and SufD are still unclear. BsSufB, though conserved, lacks the Fe-S cluster and FAD binding sites, suggesting an important role which differs from that of its E. coli homolog. We hypothesize that the BsSufBCD enzyme complex is involved in later steps of Fe-S cluster maturation, similar to the E. coli HscA/HscB proteins of the ISC system [62–64]. Taken together, our data suggest that binding of BsSufU to BsSufS activates the latter for transfer of sulfur from persulfide to the active site of BsSufU.
In the present study, we further established an interaction between BsSufS/BsSufU and the B. subtilis frataxin homolog BsFra. Previous biochemical characterization showed that BsFra can bind ferric and ferrous iron, and plays a role in Fe-S cluster biosynthesis and transfer to a target protein [28,29]. The frataxin family has been extensively studied in E. coli, S. cerevisiae, and H. sapiens. Generally, this family consists of a conserved α/β sandwich fold and harbors several acidic residues at the N-terminal α-helix and the first β-sheet (S2 Fig). In vitro enzyme assays measuring the rate at which Fe-S clusters form on the scaffold protein in the presence of a cysteine desulfurase and frataxin highlights a discrepancy: While frataxin inhibits cluster formation in the E. coli system [51,56], it enhances the rate of cluster formation in the yeast system [65]. It was shown that the cysteine desulfurase determines whether frataxin acts as an inhibitor or activator [66]. It was further shown that a single point mutation on the scaffold protein renders E. coli as frataxin-dependent and S. cerevisiae as frataxin-independent [67–69]. We conducted analogous assays with BsFra and found that, in contrast to E. coli and S. cerevisiae homologs, it did not appear to alter the initial rate of cluster formation, but resulted in a very minor increase in cluster yield. The cluster formed on BsSufU is highly labile and most likely degrades to an uncharacterized species upon isolation of holo-BsSufU [30,50]. That we were unable to isolate holo-BsSufU may suggest that our current model for Fe-S biosynthesis includes only the minimal number of participants.
We performed H/DX experiments to analyze the interaction of BsSufS and BsSufU with BsFra. We observed binding of BsFra to BsSufU and BsSufS, which did not change in the presence of BsSufU/BsSufS and therefore indicates formation of a BsFra/BsSufU/BsSufS complex in solution. The binding epitope of BsFra associates with the ‘Cys361-loop’ and ‘β-hook’ motifs of BsSufS (Fig 10). The interaction points between BsFra and BsSufU are the ‘KWN-loop’ and ‘β-sheet surface’, respectively. The short ‘α1-helix’ of BsFra appears to rearrange upon binding BsSufS, forming an extended α1-α2 helix hybrid, which then fits in the groove of BsSufS/BsSufU. This interaction brings the putative iron binding site of BsFra into close proximity to the active site of BsSufU, and it is reasonable to propose that iron incorporation is mediated by the ‘acidic ridge’ of BsFra. Previous SAXS measurements of E. coli IscS/IscU/CyaY revealed a similar binding mode [51], and characterization of the interaction of frataxin with the ferrochelatase established that BsFra binds with its acidic residues in a similar fashion to the iron acceptor BsHemH [32]. In B. subtilis, it is well established that frataxin is involved in incorporating iron into the nascent Fe-S cluster in vivo [33], but whether frataxin also serves as an intracellular iron carrier remains elusive.
We determined that BsSufU binds BsSufS and further showed how BsFra binds to the BsSufU/BsSufS complex as well as the individual proteins. In accordance with what is known about E. coli IscS/IscU/CyaY [51,56], the interaction of BsSufU/BsSufS is tight compared to that of BsFra with the complex. We assume that the interaction of BsFra to the complex is transient and that BsFra competes with additional participants of the Fe-S biogenesis pathway, as is known for E. coli IscS/Fdx [17]. The results presented here represent a single snapshot in a highly dynamic assembly process whose parts have not been fully identified.
The biogenesis of Fe-S clusters is a multistep process consisting of sulfur abstraction from cysteine, persulfide transfer, iron delivery and incorporation, and reductive generation of the Fe-S cluster, followed by transfer onto a target protein. Further studies will be necessary to identify any additional participants and determine how the biosynthetic steps are organized. In particular, the identity of the electron donor for Fe-S cluster biogenesis in vivo is unknown, and the role of SufB/SufC/SufD is yet to be determined.
The authors declare no financial conflict of interest.
Supporting Information
The structure of a B. subtilis SufS monomer (green) is superimposed with: (A) E. coli CsdA monomer (cyan; PDB ID 4LW2) with an r.m.s.d. of 3.30 Å over 401 Cα atoms; (B) E. coli SufS monomer (yellow; PDB ID 1I29) with an r.m.s.d. of 1.28 Å over 407 Cα atoms; and (C) E. coli IscS monomer (magenta; PDB ID 3LVL) with an r.m.s.d. of 5.55 Å over 389 Cα atoms
(TIFF)
Frataxin usually consists of two α-helices and one 6-stranded β-sheet. Two additional helices appear in BsFra.
(TIFF)
Differences in H/D uptake of the interaction complex compared to each individual protein are mapped onto the structures of (A) BsFra (PDB ID 2OC6) and (B) BsSufU (PDB ID 2AZH). The relative amount of deuterium incorporated is indicated by a color code ranging from blue (low; stable region) to red (high; flexible region). Black regions were not detected. N-terminal (NT) and C-terminal (CT).
(TIFF)
Differences in H/D uptake of the interaction complex compared to each protein alone are mapped onto the structures of (A) BsFra (PDB ID 2OC6), (B) the BsSufS monomer, and (C) the BsSufS homodimer. The relative amount of deuterium incorporated is indicated by a color code ranging from blue (low; stable region) to red (high; flexible region). Black regions were not detected. N-terminal (NT) and C-terminal (CT).
(TIFF)
(XLS)
Acknowledgments
This work was supported by SFB987 Project B1 (M.A.M.) and the LOEWE excellence initiative of the state of Hesse, Germany, (M.A.M. and G.B.) and the Scientific Instrumentation Grant 160/621-1 FUGG (G.B. and U.L.). We also acknowledge excellent support by the European Synchrotron Radiation Facility (ESRF), Grenoble, France.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) by Sonderforschungsbereich 987 (SFB 987) Project B1(http://www.sfb987.de) to MAM, LOEWE excellence initiative of the state of Hesse, Germany (http://www.synmikro.com/en/) to GB and MAM and the Deutsche Forschungsgemeinschaft (DFG) Scientific Instrumentation Grant 160/621-1 FUGG (http://www.dfg.de/en/research_funding/programmes/infrastructure/scientific_instrumentation/equipment_programmes/index.html) to GB and UL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Beinert H. Iron-Sulfur Clusters: Nature's Modular, Multipurpose Structures. Science. 1997;277: 653–659. 10.1126/science.277.5326.653 [DOI] [PubMed] [Google Scholar]
- 2.Beinert H, Kiley PJ. Fe-S proteins in sensing and regulatory functions. Current Opinion in Chemical Biology. 1999;3: 152–157. 10.1016/S1367-5931(99)80027-1 [DOI] [PubMed] [Google Scholar]
- 3.Beinert H. Iron-sulfur proteins: ancient structures, still full of surprises. J Biol Inorg Chem. 2000;5: 2–15. [DOI] [PubMed] [Google Scholar]
- 4.Kiley PJ, Beinert H. The role of Fe-S proteins in sensing and regulation in bacteria. Current Opinion in Microbiology. 2003;6: 181–185. [DOI] [PubMed] [Google Scholar]
- 5.Bandyopadhyay S, Chandramouli K, Johnson MK. Iron-Sulphur Cluster Biosynthesis. Biochem Soc Trans. NIH Public Access; 2008;36: 1112–1119. 10.1042/BST0361112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lill R, Mühlenhoff U. Maturation of Iron-Sulfur Proteins in Eukaryotes: Mechanisms, Connected Processes, and Diseases. Annu Rev Biochem. Annual Reviews; 2008. 10.1146/annurev.biochem.76.052705.162653 [DOI] [PubMed] [Google Scholar]
- 7.Fontecave M, de Choudens SO, Py B, Barras F. Mechanisms of iron–sulfur cluster assembly: the SUF machinery. J Biol Inorg Chem. Springer-Verlag; 2005;10: 713–721. 10.1007/s00775-005-0025-1 [DOI] [PubMed] [Google Scholar]
- 8.Dean DR, Bolin JT, Zheng L. Nitrogenase metalloclusters: structures, organization, and synthesis. Journal of Bacteriology. American Society for Microbiology (ASM); 1993;175: 6737–6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng L, Cash VL, Flint DH, Dean DR. Assembly of iron-sulfur clusters. Identification of an iscSUA-hscBA-fdx gene cluster from Azotobacter vinelandii. J Biol Chem. 1998;273: 13264–13272. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y, Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J Biol Chem. 2002;277: 28380–28383. 10.1074/jbc.C200365200 [DOI] [PubMed] [Google Scholar]
- 11.Balk J, Lobréaux S. Biogenesis of iron–sulfur proteins in plants. Trends in Plant Science. 2005;10: 324–331. 10.1016/j.tplants.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 12.Fontecave M, Ollagnier de Choudens S. Iron-sulfur cluster biosynthesis in bacteria: Mechanisms of cluster assembly and transfer. Archives of Biochemistry and Biophysics. 2008;474: 226–237. 10.1016/j.abb.2007.12.014 [DOI] [PubMed] [Google Scholar]
- 13.Flint DH. Escherichia coli contains a protein that is homologous in function and N-terminal sequence to the protein encoded by the nifS gene of Azotobacter vinelandii and that can participate in the synthesis of the Fe-S cluster of dihydroxy-acid dehydratase. J Biol Chem. 1996;271: 16068–16074. [PubMed] [Google Scholar]
- 14.Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR. IscU as a scaffold for iron-sulfur cluster biosynthesis: sequential assembly of [2Fe-2S] and [4Fe-4S] clusters in IscU. Biochemistry. 2000. [DOI] [PubMed] [Google Scholar]
- 15.Outten FW. The SufE protein and the SufBCD complex enhance SufS cysteine desulfurase activity as part of a sulfur transfer pathway for Fe-S cluster assembly in Escherichia coli. J Biol Chem. American Society for Biochemistry and Molecular Biology; 2003;278: 45713–45719. 10.1074/jbc.M308004200 [DOI] [PubMed] [Google Scholar]
- 16.Adam AC, Bornhövd C, Prokisch H, Neupert W, Hell K. The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. EMBO J. EMBO Press; 2006;25: 174–183. 10.1038/sj.emboj.7600905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Frederick RO, Reinen NM, Troupis AT, Markley JL. [2Fe-2S]-ferredoxin binds directly to cysteine desulfurase and supplies an electron for iron-sulfur cluster assembly but is displaced by the scaffold protein or bacterial frataxin. J Am Chem Soc. 2013;135: 8117–8120. 10.1021/ja401950a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wollers S, Layer G, Garcia-Serres R, Signor L, Clemancey M, Latour JM, et al. Iron-Sulfur (Fe-S) Cluster Assembly: THE SufBCD COMPLEX IS A NEW TYPE OF Fe-S SCAFFOLD WITH A FLAVIN REDOX COFACTOR. Journal of Biological Chemistry. 2010;285: 23331–23341. 10.1074/jbc.M110.127449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho S-J, Lee MG, Yang JK, Lee JY, Song HK, Suh SW. Crystal structure of Escherichia coli CyaY protein reveals a previously unidentified fold for the evolutionarily conserved frataxin family. Proc Natl Acad Sci USA. National Acad Sciences; 2000;97: 8932–8937. 10.1073/pnas.160270897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhe-Paganon S, Shigeta R, Chi YI, Ristow M, Shoelson SE. Crystal structure of human frataxin. J Biol Chem. 2000;275: 30753–30756. 10.1074/jbc.C000407200 [DOI] [PubMed] [Google Scholar]
- 21.Musco G, Stier G, Kolmerer B, Adinolfi S, Martin S, Frenkiel T, et al. Towards a structural understanding of Friedreich's ataxia: the solution structure of frataxin. Structure. 2000;8: 695–707. [DOI] [PubMed] [Google Scholar]
- 22.Adinolfi S, Trifuoggi M, Politou AS, Martin S, Pastore A. A structural approach to understanding the iron-binding properties of phylogenetically different frataxins. Human Molecular Genetics. Oxford University Press; 2002;11: 1865–1877. 10.1093/hmg/11.16.1865 [DOI] [PubMed] [Google Scholar]
- 23.Pastore C, Adinolfi S, Huynen MA, Rybin V, Martin S, Mayer M, et al. YfhJ, a molecular adaptor in iron-sulfur cluster formation or a frataxin-like protein? Structure. 2006;14: 857–867. 10.1016/j.str.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 24.Mühlenhoff U, Richhardt N, Ristow M, Kispal G, Lill R. The yeast frataxin homolog Yfh1p plays a specific role in the maturation of cellular Fe/S proteins. Human Molecular Genetics. Oxford University Press; 2002;11: 2025–2036. 10.1093/hmg/11.17.2025 [DOI] [PubMed] [Google Scholar]
- 25.Yoon T, Cowan JA. Iron-sulfur cluster biosynthesis. Characterization of frataxin as an iron donor for assembly of [2Fe-2S] clusters in ISU-type proteins. J Am Chem Soc. 2003;125: 6078–6084. 10.1021/ja027967i [DOI] [PubMed] [Google Scholar]
- 26.Li DS, Ohshima K, Jiralerspong S, Bojanowski MW, Pandolfo M. Knock-out of the cyaY gene in Escherichia coli does not affect cellular iron content and sensitivity to oxidants. FEBS Letters. 1999;456: 13–16. [DOI] [PubMed] [Google Scholar]
- 27.Pohl T, Walter J, Stolpe S, Soufo JHD, Grauman PL, Friedrich T. Effects of the deletion of the Escherichia coli frataxin homologue CyaY on the respiratory NADH:ubiquinone oxidoreductase. BMC Biochem. BioMed Central Ltd; 2007;8: 13 10.1186/1471-2091-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi W, Cowan JA. A structural and functional homolog supports a general role for frataxin in cellular iron chemistry. Chem Commun. 2010;46: 719–721. 10.1039/b911975b [DOI] [PubMed] [Google Scholar]
- 29.Albrecht AG, Landmann H, Nette D, Burghaus O, Peuckert F, Seubert A, et al. The frataxin homologue Fra plays a key role in intracellular iron channeling in Bacillus subtilis. ChemBioChem. 2011;12: 2052–2061. 10.1002/cbic.201100190 [DOI] [PubMed] [Google Scholar]
- 30.Albrecht AG, Netz DJA, Miethke M, Pierik AJ, Burghaus O, Peuckert F, et al. SufU is an essential iron-sulfur cluster scaffold protein in Bacillus subtilis. Journal of Bacteriology. 2010;192: 1643–1651. 10.1128/JB.01536-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albrecht AG, Peuckert F, Landmann H, Miethke M, Seubert A, Marahiel MA. Mechanistic characterization of sulfur transfer from cysteine desulfurase SufS to the iron-sulfur scaffold SufU in Bacillus subtilis. FEBS Letters. 2011;585: 465–470. 10.1016/j.febslet.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 32.Mielcarek A, Blauenburg, Miethke M, Marahiel MA. Molecular insights into frataxin-mediated iron supply for heme biosynthesis in Bacillus subtilis. PLoS ONE. 2015;10: e0122538 10.1371/journal.pone.0122538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albrecht AG, Landmann H, Nette D, Burghaus O, Peuckert F, Seubert A, et al. The frataxin homologue Fra plays a key role in intracellular iron channeling in Bacillus subtilis. ChemBioChem. WILEY‐VCH Verlag; 2011;12: 2052–2061. 10.1002/cbic.201100190 [DOI] [PubMed] [Google Scholar]
- 34.Kabsch W, IUCr. XDS. Acta Crystallogr D Biol Crystallogr. International Union of Crystallography; 2010;66: 125–132. 10.1107/S0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. International Union of Crystallography; 2011;67: 235–242. 10.1107/S0907444910045749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ, et al. Phaser crystallographic software. J Appl Crystallogr. International Union of Crystallography; 2007;40: 658–674. 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. International Union of Crystallography; 2004;60: 2126–2132. 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 38.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. International Union of Crystallography; 2010;66: 213–221. 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SIEGEL LM. A DIRECT MICRODETERMINATION FOR SULFIDE. Analytical Biochemistry. 1965;11: 126–132. [DOI] [PubMed] [Google Scholar]
- 40.Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR. IscU as a Scaffold for Iron−Sulfur Cluster Biosynthesis: Sequential Assembly of [2Fe-2S] and [4Fe-4S] Clusters in IscU†. Biochemistry. American Chemical Society; 2000. 10.1021/bi000931n [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Park S. Structural changes during cysteine desulfurase CsdA and sulfur acceptor CsdE interactions provide insight into the trans-persulfuration. Journal of Biological Chemistry. 2013;288: 27172–27180. 10.1074/jbc.M113.480277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mihara H, Fujii T, Kato S-I, Kurihara T, Hata Y, Esaki N. Structure of external aldimine of Escherichia coli CsdB, an IscS/NifS homolog: implications for its specificity toward selenocysteine. J Biochem. 2002;131: 679–685. [DOI] [PubMed] [Google Scholar]
- 43.Fujii T, Maeda M, Mihara H, Kurihara T, Esaki N, Hata Y. Structure of a NifS homologue: X-ray structure analysis of CsdB, an Escherichia coli counterpart of mammalian selenocysteine lyase. Biochemistry. 2000;39: 1263–1273. 10.1021/bi991732a [DOI] [PubMed] [Google Scholar]
- 44.Lima CD. Analysis of the E. coli NifS CsdB protein at 2.0 A reveals the structural basis for perselenide and persulfide intermediate formation. Journal of Molecular Biology. 2002;315: 1199–1208. 10.1006/jmbi.2001.5308 [DOI] [PubMed] [Google Scholar]
- 45.Cupp-Vickery JR, Urbina H, Vickery LE. Crystal structure of IscS, a cysteine desulfurase from Escherichia coli. Journal of Molecular Biology. 2003;330: 1049–1059. [DOI] [PubMed] [Google Scholar]
- 46.Clausen T, Kaiser JT, Steegborn C, Huber R, Kessler D. Crystal structure of the cystine C-S lyase from Synechocystis: stabilization of cysteine persulfide for FeS cluster biosynthesis. Proc Natl Acad Sci USA. National Academy of Sciences; 2000;97: 3856–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selbach B, Earles E, Santos Dos PC. Kinetic analysis of the bisubstrate cysteine desulfurase SufS from Bacillus subtilis. Biochemistry. 2010;49: 8794–8802. 10.1021/bi101358k [DOI] [PubMed] [Google Scholar]
- 48.Marinoni EN, de Oliveira JS, Nicolet Y, Raulfs EC, Amara P, Dean DR, et al. (IscS-IscU)2 complex structures provide insights into Fe2S2 biogenesis and transfer. Angew Chem Int Ed Engl. 2012;51: 5439–5442. 10.1002/anie.201201708 [DOI] [PubMed] [Google Scholar]
- 49.Shi R, Proteau A, Villarroya M, Moukadiri I, Zhang L, Trempe J-F, et al. Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol. 2010;8: e1000354 10.1371/journal.pbio.1000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selbach BP, Chung AH, Scott AD, George SJ, Cramer SP, Santos Dos PC. Fe-S cluster biogenesis in Gram-positive bacteria: SufU is a zinc-dependent sulfur transfer protein. Biochemistry. 2014;53: 152–160. 10.1021/bi4011978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prischi F, Konarev PV, Iannuzzi C, Pastore C, Adinolfi S, Martin SR, et al. Structural bases for the interaction of frataxin with the central components of iron-sulphur cluster assembly. Nature Communications. 2010;1: 95 10.1038/ncomms1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, Craig EA. Binding of yeast frataxin to the scaffold for Fe-S cluster biogenesis, Isu. J Biol Chem. American Society for Biochemistry and Molecular Biology; 2008;283: 12674–12679. 10.1074/jbc.M800399200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Correia AR, Wang T, Craig EA, Gomes CM. Iron-binding activity in yeast frataxin entails a trade off with stability in the alpha1/beta1 acidic ridge region. Biochem J. 2010;426: 197–203. 10.1042/BJ20091612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cook JD, Kondapalli KC, Rawat S, Childs WC, Murugesan Y, Dancis A, et al. Molecular details of the yeast frataxin-Isu1 interaction during mitochondrial Fe-S cluster assembly. Biochemistry. American Chemical Society; 2010;49: 8756–8765. 10.1021/bi1008613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leidgens S, De Smet S, Foury F. Frataxin interacts with Isu1 through a conserved tryptophan in its beta-sheet. Human Molecular Genetics. 2010;19: 276–286. 10.1093/hmg/ddp495 [DOI] [PubMed] [Google Scholar]
- 56.Adinolfi S, Iannuzzi C, Prischi F, Pastore C, Iametti S, Martin SR, et al. Bacterial frataxin CyaY is the gatekeeper of iron-sulfur cluster formation catalyzed by IscS. Nature Structural & Molecular Biology. Nature Publishing Group; 2009;16: 390–396. 10.1038/nsmb.1579 [DOI] [PubMed] [Google Scholar]
- 57.Sun D, Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis nadB gene and a nifS-like gene, both of which are essential for NAD biosynthesis. Journal of Bacteriology. American Society for Microbiology (ASM); 1993;175: 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Black KA, Santos Dos PC. Abbreviated Pathway for Biosynthesis of 2-Thiouridine in Bacillus subtilis. de Boer P, editor. Journal of Bacteriology. 2015;197: 1952–1962. 10.1128/JB.02625-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jurgenson CT, Begley TP, Ealick SE. The Structural and Biochemical Foundations of Thiamin Biosynthesis. Annu Rev Biochem. 2009;78: 569–603. 10.1146/annurev.biochem.78.072407.102340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barbe V, Cruveiller S, Kunst F, Lenoble P, Meurice G, Sekowska A, et al. From a consortium sequence to a unified sequence: the Bacillus subtilis 168 reference genome a decade later. Microbiology. 2009;155: 1758–1775. 10.1099/mic.0.027839-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh H, Dai Y, Outten FW, Busenlehner LS. Escherichia coli SufE sulfur transfer protein modulates the SufS cysteine desulfurase through allosteric conformational dynamics. Journal of Biological Chemistry. 2013;288: 36189–36200. 10.1074/jbc.M113.525709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoff KG, Silberg JJ, Vickery LE. Interaction of the iron-sulfur cluster assembly protein IscU with the Hsc66/Hsc20 molecular chaperone system of Escherichia coli. Proc Natl Acad Sci USA. National Acad Sciences; 2000;97: 7790–7795. 10.1073/pnas.130201997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silberg JJ, Hoff KG, Tapley TL, Vickery LE. The Fe/S assembly protein IscU behaves as a substrate for the molecular chaperone Hsc66 from Escherichia coli. J Biol Chem. American Society for Biochemistry and Molecular Biology; 2001;276: 1696–1700. 10.1074/jbc.M009542200 [DOI] [PubMed] [Google Scholar]
- 64.Tokumoto U, Takahashi Y. Genetic analysis of the isc operon in Escherichia coli involved in the biogenesis of cellular iron-sulfur proteins. J Biochem. Oxford University Press; 2001;130: 63–71. [DOI] [PubMed] [Google Scholar]
- 65.Tsai C-L, Barondeau DP. Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry. 2010;49: 9132–9139. 10.1021/bi1013062 [DOI] [PubMed] [Google Scholar]
- 66.Bridwell-Rabb J, Iannuzzi C, Pastore A, Barondeau DP. Effector role reversal during evolution: the case of frataxin in Fe-S cluster biosynthesis. Biochemistry. 2012;51: 2506–2514. 10.1021/bi201628j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoon H, Golla R, Lesuisse E, Pain J, Donald JE, Lyver ER, et al. Mutation in the Fe-S scaffold protein Isu bypasses frataxin deletion. Biochem J. Portland Press Limited; 2012;441: 473–480. 10.1042/BJ20111637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoon H, Knight SAB, Pandey A, Pain J, Turkarslan S, Pain D, et al. Turning Saccharomyces cerevisiae into a Frataxin-Independent Organism. PLoS Genet. Public Library of Science; 2015;11: e1005135–28. 10.1371/journal.pgen.1005135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roche B, Agrebi R, Huguenot A, Ollagnier de Choudens S, Barras F, Py B. Turning Escherichia coli into a Frataxin-Dependent Organism. Casadesús J, editor. PLoS Genet. Public Library of Science; 2015;11: e1005134 10.1371/journal.pgen.1005134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The structure of a B. subtilis SufS monomer (green) is superimposed with: (A) E. coli CsdA monomer (cyan; PDB ID 4LW2) with an r.m.s.d. of 3.30 Å over 401 Cα atoms; (B) E. coli SufS monomer (yellow; PDB ID 1I29) with an r.m.s.d. of 1.28 Å over 407 Cα atoms; and (C) E. coli IscS monomer (magenta; PDB ID 3LVL) with an r.m.s.d. of 5.55 Å over 389 Cα atoms
(TIFF)
Frataxin usually consists of two α-helices and one 6-stranded β-sheet. Two additional helices appear in BsFra.
(TIFF)
Differences in H/D uptake of the interaction complex compared to each individual protein are mapped onto the structures of (A) BsFra (PDB ID 2OC6) and (B) BsSufU (PDB ID 2AZH). The relative amount of deuterium incorporated is indicated by a color code ranging from blue (low; stable region) to red (high; flexible region). Black regions were not detected. N-terminal (NT) and C-terminal (CT).
(TIFF)
Differences in H/D uptake of the interaction complex compared to each protein alone are mapped onto the structures of (A) BsFra (PDB ID 2OC6), (B) the BsSufS monomer, and (C) the BsSufS homodimer. The relative amount of deuterium incorporated is indicated by a color code ranging from blue (low; stable region) to red (high; flexible region). Black regions were not detected. N-terminal (NT) and C-terminal (CT).
(TIFF)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.