Abstract
Background
Women with venous thromboembolism (VTE), thrombophilias or mechanical heart valves may require anticoagulation during pregnancy and postpartum. The incidence of postpartum hemorrhage (PPH) in the literature is 2.9–6%, but the rate while on anticoagulation is not well documented.
Aims
To determine the incidence of haemorrhagic complications associated with the use of peripartum anticoagulation, and the types and risk factors for haemorrhagic complications.
Methods
A retrospective chart review was conducted on women who delivered at an academic teaching hospital and received peripartum anticoagulation between January 2000 and August 2009. Women with known bleeding disorders were excluded.
Results
In total, 195 cases were identified with mean age 31.3 years and gestational age of 37.7 weeks. Of these, 49% had a history of VTE, 21% had active VTE in the index pregnancy, and 63% had vaginal delivery. Types of anticoagulation used antepartum were unfractionated heparin (UFH) (43%) and low molecular weight heparin (LMWH) (36%), with 26% receiving therapeutic doses. The rate of haemorrhagic complications was 12.8%, with majority being PPH (80%). Sixty percent of the PPH occurred before reintroduction of anticoagulation postpartum. Use of therapeutic UFH antepartum was associated with increased risk of haemorrhagic complications compared to LMWH (OR 3.08, 95% CI 0.663 – 15.03, p = 0.183).
Conclusion
The rate of haemorrhagic complications is higher in women on peripartum anticoagulation compared with published incidence in unselected obstetric populations; however, this rate is similar to our institution’s reported rates. Our findings inform clinicians about competing risks of thrombotic and haemorrhagic complications in this population.
Keywords: Anticoagulation, pregnancy, peripartum, haemorrhagic complications, chart review, retrospective
Introduction
Venous thromboembolism (VTE) is a leading cause of maternal death in Canada and other developed countries.1,2 To prevent thrombotic complications, anticoagulation may be warranted in pregnant women at higher risk of thrombosis, such as those with prior or current VTE, heritable or acquired thrombophilias, and mechanical heart valves.3,4 Despite the increased risk of thrombosis in these women, the peripartum period also poses a significant bleeding risk, and haemorrhage remains the leading cause of maternal death in other countries worldwide.2
The overall incidence of postpartum haemorrhage (PPH) reported in the literature is 2.9–6%, but the incidence in those on anticoagulation in pregnancy is not well documented.5–10 Previously, the rate of haemorrhagic complications while on peripartum anticoagulation has been estimated to be 2–11%, but most of the evidence in the literature focuses on low molecular weight heparins (LMWH) and prophylactic doses of anticoagulation.11–17 In addition, thrombotic complications are often the main outcome, and studies have been underpowered to detect differences in rates of haemorrhagic complications.11,14–17
We sought to determine the incidence and types of haemorrhagic complication associated with peripartum anticoagulation use at a tertiary obstetrics referral center as compared to that described in the literature. In addition, we wanted to understand the risk factors for haemorrhagic complications in women on peripartum anticoagulation in our tertiary referral center.
Methods
A retrospective, cross-sectional chart review of women who received peripartum anticoagulation and delivered at an academic teaching hospital in Edmonton, Canada between 1 January 2000 and 31 August 2009 was conducted. The study was approved by the University of Alberta Health Research Ethics Board.
Women were included in the study if they received prophylactic or therapeutic doses of anticoagulation (unfractionated heparin (UFH), LMWH or warfarin) peripartum (before and/or after delivery). Cases were selected based on ICD-10 codes for indication for anticoagulation; these cases were then linked to ICD-10 codes for delivery to capture the population of interest (see Appendix 1). Patient charts identified from ICD-10 codes were reviewed and accepted as cases if they met the inclusion criteria. Women with more than one pregnancy in the study period were also included and each pregnancy was considered separately. Women with documented bleeding disorders or if they had a haemorrhagic complication before receiving anticoagulation were also excluded.
A standardized form was used to extract data directly from the patients’ charts. Information collected included patient demographics, mode of delivery (vaginal or cesarean), indication for anticoagulation, type and dose of anticoagulation, timing of re-initiation of anticoagulation, haemorrhagic complications postpartum, concomitant risk factors for PPH, and sequelae of haemorrhagic complications. Anti-Xa levels, International Normalized Ratios, and the lowest hemoglobin level postpartum were collected on patients with haemorrhagic complications. The type of anticoagulation was documented as the anticoagulant the patient was receiving closest to the antepartum and/or postpartum period. Prophylactic anticoagulation was defined as prophylactic or intermediate dosing as per Bates et al.3 and therapeutic anticoagulation was any dose greater than prophylactic. Re-initiation of anticoagulation was analyzed according to time, dose of anticoagulation, and mode of delivery. Concomitant risk factors for PPH were extracted from the chart as history of postpartum haemorrhage, fetal macrosomia (>4000 g), multiple gestations, polyhydramnios, placenta previa, labor induction, precipitous labor (<3 h in duration), prolonged third stage of labor (>30 min), episiotomy, genital tract laceration, shoulder dystocia, presence of retained placenta, and use of non-steroidal anti-inflammatory drugs postpartum.
The primary outcome was to determine the rate of obstetric haemorrhagic complications in this obstetric population on peripartum anticoagulation. For the purpose of this study, peripartum was defined as three days before and seven days after delivery. Obstetric haemorrhagic complications were defined as PPH, haematomas in any location, and any other complication that may be relevant to bleeding. PPH was determined by the documentation of “postpartum hemorrhage” in patient charts and/or “excessive bleeding” of >500 mL as indicated on the delivery record by the obstetrician.18 Early and late PPH were captured, with early defined as ≤24 h after delivery and late as >24 h after delivery. Secondary outcomes included determining the types of haemorrhagic complications, timing of re-initiation of anticoagulation postpartum, and concomitant risk factors for PPH on the types and frequency of obstetric haemorrhagic complications.
For statistical analyses, univariate analysis (Fisher’s exact or chi-squared test for categorical variables and Student’s t-test for continuous variables) was used to examine associations between the indication, type, and dose of anticoagulation, timing of re-initiation of anticoagulation postpartum, mode of delivery, and concomitant risk factors for PPH on the type and frequency of haemorrhagic complications. Step-wise forward selection multivariate analysis using logistic regression was conducted to identify risk factors for haemorrhagic complications with the dependent variable being the number of cases with haemorrhagic complications and the independent variables being the risk factors for haemorrhagic complications with a p < 0.1 identified in the univariate analysis. For the purposes of the logistic regression model, the time of reinitiating anticoagulation was categorized as follows: <6 h, 6–12 h, and >12 h postpartum. Statistical significance was defined as p < 0.05 and all data analyses were completed using the statistical software package PASW Version 18.0 (IBM Corporation, Somers, NY, USA).
Results
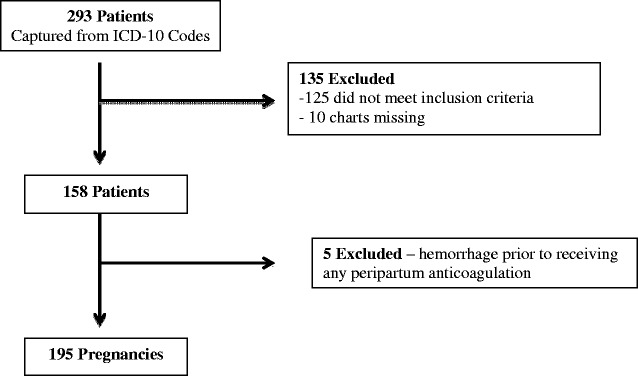
Of the 293 women identified from ICD-10 codes, 195 pregnancies from 158 women met the inclusion criteria (Figure 1). A summary of patient characteristics is shown in Table 1. The mean age was 31.3 ± 5.2 years with median gravidity and parity of 3 and 1, respectively. Medical history revealed 12% with gestational diabetes and 11% with hypertension. Mean gestational age was 37.7 weeks with 63% having vaginal delivery and 37% with cesarean delivery.
Figure 1.
Patient flow chart.
Table 1.
Patient characteristics.
| Characteristic | n = 195 |
|---|---|
| Demographics | |
| Mean age ± SD in years (range) | 31.3 ± 5.2 (18–43) |
| Median gravity/parity (range) | 3/1 (1–13/0–8) |
| History of medical conditions, n (%) | 74 (37.9) |
| Gestational diabetes | 24 (12) |
| Hypertension | 22 (11) |
| Hypothyroidism | 12 (6) |
| Asthma | 12 (6) |
| Antepartum ASA use, n (%) | 38 (19) |
| Anticoagulation | |
| Antepartum only | 6 (3) |
| Antepartum and postpartum | 156 (80) |
| Postpartum only | 33 (17) |
| Delivery Information | |
| Mean gestational age ± SD in weeks (range) | 37.7 ± 3.4 (23.1–41.7) |
| Mode of delivery, n (%) | |
| Vaginal | 122 (63) |
| Cesarean | 73 (37) |
| Labor induction, n (%) | 83 (43) |
| Neuraxial anesthesia, n (%) | 113 (58) |
| Oxytocic agent used, n (%) | 188 (96) |
| Mean duration of stages I–III (h:mm ± SD) (range) | |
| Stage I | 6:09 ± 3:47 (0:20–18:45) |
| Stage II | 0:53 ± 1:01 (0:01–5:15) |
| Stage III | 0:05 ± 0:08 (0:00–1:42) |
SD: standard deviation; ASA: acetylsalicylic acid; h: hours; mm: minutes.
The most common indication for peripartum anticoagulation was a history of VTE (49%); 35% had history of deep vein thrombosis (DVT) and 13% had history of pulmonary embolism (PE) (Table 2). Moreover, 21% were being treated for active VTE, and 59% had history of thrombophilia, the most common being Factor V Leiden (23%). Thirty-one percent of cases had more than one indication for anticoagulation. Eighty-three percent of cases were on antepartum anticoagulation, and 97% were on postpartum anticoagulation. UFH was the most common type of anticoagulation used antepartum (43%) and postpartum (49%). The LMWH used were enoxaparin, dalteparin, nadroparin, and tinzaparin, with dalteparin the most common. More women were on prophylactic versus therapeutic dosing of anticoagulation antepartum (54% vs. 26%) and postpartum (69% vs. 28%). Of the 162 cases on antepartum anticoagulation, 53 (33%) were on a different anticoagulant earlier in pregnancy compared to the peripartum period. Of these patients, 87% were switched to UFH, 6% were switched to a different LMWH, and 8% discontinued anticoagulation prior to delivery. No patient was on warfarin antepartum, while two patients were on it postpartum. The mean time from the last dose of anticoagulation antepartum to delivery was 9:28 ± 6:25 h (median 8:37; range 0:11–23:36 h) and time of delivery to re-initiation of anticoagulation postpartum was 8:41 ± 5:47 h (median 7:12; range 0:24–23:32 h).
Table 2.
Indication for anticoagulation and types of anticoagulation used.
| Variables | n = 195 | |
|---|---|---|
| Indications for anticoagulation, n (%) | ||
| History of VTE | 95 (49) | |
| History of DVT | 69 (35) | |
| History of PE | 26 (13) | |
| Active VTE | 41 (21) | |
| Active DVT | 19 (10) | |
| Active PE | 18 (9) | |
| Thrombophilia | 115 (59) | |
| Thrombophilia only | 71 (36) | |
| Thrombophilia + history of VTE | 37 (19) | |
| Thrombophilia + active VTE | 6 (3) | |
| Other | 13 (7) | |
| Anticoagulation | Antepartuma | Postpartumb |
| On anticoagulation, n (%) | 162 (83) | 189 (97) |
| Therapeutic dose, n (%) | 50 (26) | 54 (28) |
| UFH | 23 (12) | 25 (13) |
| LMWH | 27 (14) | 29 (15) |
| Prophylactic dose, n (%) | 105 (54) | 135 (69) |
| UFH | 61 (31) | 71 (36) |
| LMWH | 44 (23) | 64 (33) |
| Unknown dose | 7 (4) | 0 (0) |
Active: in index pregnancy; LMWH: low molecular weight heparin; UFH: unfractionated heparin; VTE: venous thromboembolism (includes deep vein thrombosis and/or pulmonary embolism); DVT: deep vein thrombosis; PE: pulmonary embolism.
Antepartum anticoagulation refers to the type and dose of anticoagulation the patient was on immediately prior to delivery.
Postpartum anticoagulation refers to the type and dose of anticoagulation the patient was on immediately postpartum.
The rate of all haemorrhagic complications was 25/195 deliveries (12.8%) (Table 3). Two types of haemorrhagic complications occurred: PPH (20/25) and hematomas (5/25), which equates to an overall rate of PPH of 10% and hematomas of 3%. The majority of haemorrhagic complications were in women who received both antepartum and postpartum anticoagulation (23/25); however, 60% occurred before anticoagulation was re-initiated, with most occurring <24 h postpartum (median = 0 days; range = 0–23 days postpartum). Sequelae occurred in 76% of those with haemorrhagic complications such as requiring blood transfusion (n = 14), surgery (n = 8), administration of uterotonic agents (i.e. systemic prostaglandins, ergotamine, or misoprostol) (n = 9), or admission to ICU (n = 1). In addition to being on peripartum anticoagulation, 150 cases (77%) had at least one other concomitant risk factor for PPH with 39% having two or more risk factors. There were no cases of maternal death due to haemorrhagic complications.
Table 3.
Incidence and types of haemorrhagic complications.
| Variable | Total n = 195 | Antepartuma and postpartum n = 156 | Postpartum only n = 33 |
|---|---|---|---|
| Number of haemorrhagic complications, n (%) | 25 (13) | 23 (15) | 2 (6) |
| Types of haemorrhagic complications | |||
| Post-partum hemorrhage | 20 (10) | 18 (12) | 2 (6) |
| Hematoma | 5 (3) | 5 (3) | 0 |
| Timing of haemorrhagic complication | |||
| Before re-initiation of anticoagulation postpartum | 15 (8) | 15 (10) | – |
| After re-initiation of anticoagulation postpartum | 10 (5) | 8 (5) | 2 (6) |
| Sequelaeb in patients with haemorrhagic complications, n (%) | 19 (10) | 17 (11) | 2 (6) |
There were no haemorrhagic complications in those who were on antepartum anticoagulation alone (n = 6).
Sequelae = blood transfusion, surgery, administration of uterotonic agents or admission to ICU.
A comparison of women with and without haemorrhagic complications is found in Table 4. Haemorrhagic complications occurred more frequently in women with active VTE (p = 0.011) and those with retained placenta (p < 0.001). Women who had haemorrhagic complications were more likely to be on therapeutic doses of anticoagulation both antepartum (p = 0.012) and postpartum (p = 0.032). Additionally, these women were more likely to be on therapeutic doses of UFH both antepartum (32% vs. 9%, p = 0.003) and postpartum (36% vs. 11%, p = 0.002) compared to women who did not have a haemorrhagic complication. In contrast, women on LMWH (either antepartum or postpartum) had no increase in haemorrhagic complication rates.
Table 4.
Characteristics of women with and without haemorrhagic complications.
| No haemorrhagic complications (n = 170) | Haemorrhagic complications (n = 25) | p value | |
|---|---|---|---|
| Indication for anticoagulation, n (%) | |||
| Active VTE | 27 (16) | 10 (40) | 0.011 |
| History of VTE | 82 (48) | 14 (56) | 0.525 |
| Antiphospholipid antibodies | 27 (16) | 7 (28) | 0.158 |
| Antithrombin deficiency | 3 (2) | 0 (0) | 1 |
| Protein C deficiency | 2 (1) | 0 (0) | 1 |
| Protein S deficiency | 22 (13) | 0 (0) | 0.084 |
| Factor V Leiden | 40 (24) | 3 (12) | 0.3 |
| Prothrombin gene mutation | 18 (11) | 1 (4) | 0.477 |
| Concomitant risk factors for PPH, n (%) | |||
| Polyhydramnios | 4 (2) | 1 (4) | 0.5 |
| Episiotomy | 15 (9) | 5 (20) | 0.147 |
| Placenta previa | 1 (0.6) | 1 (4) | 0.241 |
| Retained placenta | 2 (1) | 5 (20) | <0.001 |
| NSAID postpartum | 33 (19) | 3 (12) | 0.58 |
| Anticoagulation, n (%) | |||
| Antepartum only | 6 (4) | 0 (0) | 1 |
| Antepartum and postpartum | 133 (78) | 23 (92) | 0.177 |
| Postpartum only | 31 (18) | 2 (8) | 0.263 |
| On Antepartum Anticoagulation, n (%) | 139 a (82) | 23 (92) | 0.263 |
| Therapeutic | 38 (22) | 12 (48) | 0.012 |
| UFH | 15 (9) | 8 (32) | 0.003 |
| LMWH | 23 (14) | 4 (16) | 0.757 |
| Prophylactic | 94 (55) | 11 (44) | 0.391 |
| UFH | 54 (32) | 7 (28) | 0.819 |
| LMWH | 40 (23) | 4 (16) | 0.608 |
| On Postpartum Anticoagulation | 164 (97) | 25 (100) | 1 |
| Therapeutic dose n (%) | 44 (26) | 12 (48) | 0.032 |
| UFH | 18 (11) | 9 (36) | 0.002 |
| LMWH | 26 (15) | 3 (12) | 1 |
| Prophylactic dose, n (%) | 120 (71) | 13 (52) | 0.07 |
| UFH | 63 (37) | 6 (24) | 0.264 |
| LMWH | 57 (34) | 7 (28) | 0.654 |
LMWH: low molecular weight heparin; NSAID: non-steroidal anti-inflammatory drug; PPH: postpartum hemorrhage; UFH: unfractionated heparin; VTE: venous thromboembolism (includes deep vein thrombosis and pulmonary embolism).
Unknown dose in 7 patients who had no haemorrhagic complications.
Haemorrhagic complications based on type of antepartum anticoagulation are further broken down in Table 5. When analyzing haemorrhagic complications according to dose and type of anticoagulation used antepartum, therapeutic doses of UFH was associated with more haemorrhagic complications compared to therapeutic LMWH; however, this finding did not reach statistical significance [OR 3.08, 95% CI 0.663 – 15.03, p = 0.183). Most women on therapeutic UFH were on intravenous (IV) UFH (19/23) with few receiving subcutaneous (SC) UFH (4/23), adjusted with a mid-interval activated partial thromboplastin time (aPTT). Of the eight women on therapeutic UFH antepartum who had a haemorrhagic complication, seven were receiving IV UFH and one was receiving SC UFH. In those with haemorrhagic complications, the mean duration from the end of the IV UFH infusion to delivery was 7:25 + 0:13 h (range 2:45–10:27 h). The one woman on SC UFH antepartum who bled received the dose 18 min prior to delivery.
Table 5.
Breakdown of haemorrhagic complication based on type of antepartum anticoagulation and dose.a
| Variable | Totala n = 162 | >Therapeutic dose | >Prophylactic dose | ||||
|---|---|---|---|---|---|---|---|
| Total n = 50 | UFHb n = 23 | LMWH n = 27 | Total n = 105 | UFH n = 61 | LMWH n = 44 | ||
| Number of haemorrhagic complications n (%) | 23 (14.2) | 12 (24) | 8 (34.7) | 4 (14.8) | 11 (10.5) | 7 (11.5) | 4 (9) |
| Types of haemorrhagic complications, n (%) | |||||||
| Post-partum hemorrhage | 18 (11.1) | 12 (24) | 8 (26.7) | 4 (14.8) | 6 (5.7) | 5 (8.1) | 1 (2.3) |
| Hematoma | 5 (3.1) | 0 | 0 | 0 | 5 (4.8) | 2 (3.3) | 3 (6.8) |
Patients on antepartum anticoagulation.
Odds ratio for haemorrhagic complications in therapeutic dose UFH compared to therapeutic dose LMWH = OR 3.08, 95% CI 0.663 – 15.03, p = 0.183.
No associations were found between the type of anticoagulation or timing of re-initiation of anticoagulation postpartum and the rate or type of haemorrhagic complications in the univariate analysis. However, when analyzed according to the mode of delivery and type of haemorrhagic complication, anticoagulation was re-initiated earlier postpartum in women with vaginal deliveries compared to cesarean delivery (7:58 ± 5:18 vs. 9:53 ± 6:21 h, p = 0.028) and in women with haematomas compared to PPH (6:17 ± 2:44 vs. 10.49 ± 6:07 h, p = 0.138). Since most of the haemorrhagic complications occurred before the re-initiation of anticoagulation postpartum, a post hoc analysis of timing of last dose of anticoagulation antepartum to delivery in those with and without haemorrhagic complications was conducted. It revealed a trend toward later discontinuation of anticoagulation in those with haemorrhagic complications compared to those without (7:11 ± 4:44 vs. 9:58 ± 6:38 h before delivery, p = 0.057). In the multivariate logistic regression model, adjusted for age, gravidity, parity, body mass index (BMI), presence of medical conditions, or concomitant risk factors for PPH, the only independent risk factor for haemorrhagic complications was retained placenta (p < 0.001). This variable retained statistical significance even when repeat cases were removed from the analysis (p < 0.001).
Discussion
Our study found a 12.8% rate of obstetric haemorrhagic complications in women receiving peripartum anticoagulation, with the rate of PPH 10% and hematomas 3%. Retained placenta was the only independent risk factor associated with these complications. Our findings for the rate of PPH in patients on anticoagulation are higher than the 2.9–6% reported incidence of PPH in unselected obstetric populations.5–10 However, our institution, a tertiary obstetrics referral center, may serve a different obstetrics population and capture PPH differently compared to published reports. The PPH rates in our study are similar to our institution rates for PPH as obtained from our institution’s Health Records (reported to be 12.9%) during the same time period as our study (Janes-Kelley, 2013, personal communication). However, it may be difficult to compare these numbers because our study excluded documented bleeding disorders and the institution rates are captured only through ICD-10 codes without considering similar exclusions.
Our incidence for all haemorrhagic complications is also higher than previous literature reports of women receiving peripartum anticoagulation (range 2–11%).11–17 There are some differences in our study population, compared with previous studies, which could account for our rates and may limit the generalizability of our study results. Our study had a high number of women receiving therapeutic doses of anticoagulation (∼27%), and we found that haemorrhagic complications occurred more frequently in women receiving therapeutic anticoagulation. Most reports in the literature have studied prophylactic anticoagulation, primarily with LMWH with few studies differentiating between prophylactic or therapeutic dosing.11–17 A systematic review of LWMH use for both thromboprophylaxis and treatment of VTE found that only 6.3% of patients were on therapeutic anticoagulation and the overall rate of haemorrhagic complications was 1.98%.11 In contrast, a case-control study looking at bleeding complications with enoxaparin found similar results to our study: 23.6% of the cases had active VTE and the overall PPH rate was higher at 11%.12 Our study also affirms these findings as 8/71 (11%) of women who were on antepartum LMWH had a haemorrhagic complication, which is also comparable to the overall rate in our institution.
In addition, we noted an increased use of UFH compared to previously published studies. This study was performed at a time when there was often a transition from LWMH to UFH near term to facilitate the possibility of neuraxial anesthesia. The rate of bleeding complications with prophylactic UFH did not appear to differ much from our institution rate. In contrast, use of therapeutic dose UFH antepartum was associated with a three-fold higher rate of haemorrhagic complications compared to LMWH; even though it did not reach statistical significance, it is still a clinically important finding. At the time of the review, aPTT was used to monitor the anticoagulation effect of UFH as anti-Xa levels were not readily available. However, changes in coagulation factors in pregnancy may result in lower aPTT levels for equivalent UFH plasma concentrations compared to non-pregnant women.19 Therefore, it is possible that a higher than necessary dose of UFH was used in these patients, and may have contributed to the increased trend in bleeding complications.
The finding that most of the postpartum bleeding complications occurred prior to re-initiating anticoagulation was somewhat surprising. There was a trend to later discontinuation of antepartum anticoagulation in these patients, suggesting that we may need to pay closer attention to this factor. Only 43% of our patients had labor induction and this trend could suggest that planning more of these deliveries may decrease the risk of PPH.
Our rate of haemorrhagic complications could also be partly due to factors unrelated to anticoagulation. Most women had at least one concomitant risk factor for PPH in addition to anticoagulation and 39% had more than two risk factors. Since the occurrence of haemorrhagic complications occurred prior to re-initiation of anticoagulation, these risk factors and other variables that were difficult to capture in our study, such as differences in the management of the third stage of labor, may have contributed more than the use of anticoagulation.
Clinicians have the difficult task of balancing the competing risks of bleeding versus thrombosis in these complex patients. It is not surprising that peripartum anticoagulation poses a bleeding risk postpartum and that more aggressive dosing of anticoagulation (therapeutic versus prophylactic) confers a higher risk. There are also no evidence-based guidelines to direct the clinician as to how to best to manage peripartum anticoagulation to balance the risk of thrombosis and bleeding. The 2012 American College of Chest Physicians (ACCP) guidelines on antithrombotic therapy suggest discontinuing adjusted-dose SC UFH or LMWH 24 h prior to a planned delivery in order to avoid an unwanted anticoagulant effect, especially with concomitant neuraxial anesthesia.20 However, spontaneous labor can occur prior to a planned delivery, or women may not desire induction of labor or planned cesarean section. The guidelines suggest that women at very high risk of thrombosis may be best managed with IV UFH, which is stopped 4–6 h prior to planned delivery. Given the pharmacokinetics of UFH, this time interval should be sufficient to normalize coagulation parameters. However in our study, it was surprising that more of the bleeding complications associated with the therapeutic UFH were with intravenous UFH (7/19). The mean duration from end of the IV UFH infusion to delivery in this group was 7:25 h, which is longer than the recommended 4–6 h. It is unclear if this issue is related to monitoring of UFH, duration of stopping of UFH or to factors unrelated to anticoagulation. It is possible the current availability of anti-Xa levels in practice may improve haemorrhagic rates, though our study did not assess this impact. This is an area that deserves further research.
Our study has several limitations. First, being a chart review, the accuracy of the information is limited to the documentation available in the chart, other potential confounders for haemorrhagic complications may not have been captured and there may be a selection bias. As this information was collected retrospectively, we are relying on the clinician’s assessment and documentation of outcomes such as PPH. Unfortunately, the diagnosis of PPH is often subjective at the time of delivery and clinicians often underestimate actual blood loss.18,21 In addition, we were not able to capture data on planned versus unplanned deliveries which may have provided further insight into our findings. Second, as patient selection criteria used ICD-10 codes, we may not have captured all of the women in the population of interest, which could lead to either under or overestimating our incidence of haemorrhagic complications. Furthermore, a wide range of potential errors in coding of ICD-10 codes has been well identified in the literature.22 Third, our sample size is small and as the study lacks a control group, we are unable to measure the obstetric haemorrhagic complications in women not receiving anticoagulation. Our study captured a time span of nine years. Changing clinical practices in obstetrics during this time may have influenced the rate of haemorrhagic complications. We also did not capture re-admissions of women with late haemorrhagic complications to different hospitals in the region, which may have resulted in underestimation of the primary outcome. However, since early PPH is more common than late PPH and most women remained in hospital >24 h after delivery, we likely captured the majority of the haemorrhagic complications in these women.
Conclusion
The clinical dilemma in women requiring anticoagulation in pregnancy is balancing the risks of bleeding and thrombosis. Previous studies have shown that women on LMWH in pregnancy have low recurrence rates of VTE, which favors the use of anticoagulation.11 Our study showed that peripartum anticoagulation poses a bleeding risk of 12.8%, which is higher than currently reported rates in the literature, but which was comparable to our institution rate. This study adds to the existing literature, especially since we had more women requiring therapeutic anticoagulation (in particular UFH) compared to other studies. Further highlights include a trend toward higher risk of haemorrhagic complications with therapeutic dose UFH antepartum (as compared to LMWH) and with shorter time frames between final antepartum dose and delivery. Clinicians need to carefully assess and balance individual risk factors in women receiving anticoagulation peripartum.
Acknowledgements
The abstract of the results was published and previously presented at the International Society of Obstetric Medicine 2010 Congress, Melbourne, Australia, 1–3 October 2010 (presented on 2 October 2010). The authors would like to acknowledge the members of EW's residency panel: Dr Sheri Koshman, Dr Tammy Bungard, and Dr Deon Druteika, for providing feedback on the study design and methodology.
Appendix1. ICD-10 Codes for indication of anticoagulation and delivery mode
| ICD-10 code | Indication for anticoagulation |
|---|---|
| D68.8 | Other specified coagulation defects |
| D68.9 | Coagulation defect, unspecified |
| I26 | Pulmonary embolism |
| I74 | Arterial embolism and thrombosis |
| O22.2 | Superficial thrombophlebitis in pregnancy |
| O22.3 | Deep phlebothrombosis in pregnancy |
| O22.5 | Cerebral venous thrombosis in pregnancy |
| O22.8 | Other venous complications in pregnancy |
| O22.9 | Venous complication in pregnancy, unspecified |
| O28.0 | Abnormal haematological finding on antenatal screening of mother |
| O88 | Obstetric embolism |
| Z86.7 | Personal history of diseases of the circulatory system |
| Z95.2 | Presence of prosthetic heart valve |
| ICD-10 code | Delivery mode |
| O80 | Single spontaneous delivery |
| O81 | Single delivery by forceps and vacuum extractor |
| O82 | Single delivery by cesarean section |
| O83 | Other assisted single delivery |
| O84 | Multiple delivery |
Conflict of Interest
None declared
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethics approval
The study was approved by the University of Alberta Health Research Ethics Board (Health Panel) on December 8, 2009.
Guarantor
NY
Contributorship
All authors were involved in study concept, design and protocol development. EW coordinated study, performed the chart reviews and completed data analysis. EW, NY and CM drafted initial manuscript. EW, CM, RK, WS and NY reviewed and revised drafts of manuscript.
References
- 1.Health Canada. Special report on maternal mortality and severe morbidity in Canada: enhanced surveillance. The path to prevention. Ottawa, 2004.
- 2.World Health Organization. The World Health Report 2005 – make every mother and child count. The World Health Report 2005, Geneva: World Health Organization, 2005. [Google Scholar]
- 3.Bates SM, Greer IA, Pabinger I, et al. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133: 844S–886S. [DOI] [PubMed] [Google Scholar]
- 4.Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol 2002; 101: 6–14. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994–2006. Am J Obstet Gynecol 2010; 202: 353.e1–353.e6. [DOI] [PubMed] [Google Scholar]
- 6.Bateman BT, Berman MF, Riley LE, et al. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg 2010; 110: 1368–1373. [DOI] [PubMed] [Google Scholar]
- 7.Joseph KS, Rouleau J, Kramer MS, et al. Investigation of an increase in postpartum haemorrhage in Canada. BJOG 2007; 114: 751–759. [DOI] [PubMed] [Google Scholar]
- 8.Dupont C, Touzet S, Colin C, et al. Incidence and management of postpartum haemorrhage following the dissemination of guidelines in a network of 16 maternity units in France. Int J Obstet Anesth 2009; 18: 320–327. [DOI] [PubMed] [Google Scholar]
- 9.Ford JB, Roberts CL, Simpson JM, et al. Increased postpartum hemorrhage rates in Australia. Int J Gynaecol Obstet 2007; 98: 237–243. [DOI] [PubMed] [Google Scholar]
- 10.Carroli G, Cuesta C, Abalos E, et al. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol 2008; 22: 999–1012. [DOI] [PubMed] [Google Scholar]
- 11.Greer I, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005; 106: 401–407. [DOI] [PubMed] [Google Scholar]
- 12.Kominiarek M, Angelopoulos SM, Shapiro NL, et al. Low-molecular-weight heparin in pregnancy: peripartum bleeding complications. J Perinatol 2007; 27: 329–334. [DOI] [PubMed] [Google Scholar]
- 13.Freedman R, Bauer K, Neuberg DS, et al. Timing of postpartum enoxaparin administration and severe postpartum hemorrhage. Blood Coagul Fibrinolysis 2008; 19: 55–59. [DOI] [PubMed] [Google Scholar]
- 14.Pettilä V, Kaaja R, Leinonen P, et al. Thromboprophylaxis with low molecular weight heparin (dalteparin) in pregnancy. Thromb Res 1999; 96: 275–282. [DOI] [PubMed] [Google Scholar]
- 15.Burrows RF, Gan ET, Gallus AS, et al. A randomised double-blind placebo controlled trial of low molecular weight heparin as prophylaxis in preventing venous thrombolic events after caesarean section: a pilot study. BJOG 2001; 108: 835–839. [DOI] [PubMed] [Google Scholar]
- 16.Rodie VA, Thomson AJ, Stewart FM, et al. Low molecular weight heparin for the treatment of venous thromboembolism in pregnancy: a case series. BJOG 2002; 109: 1020–1024. [DOI] [PubMed] [Google Scholar]
- 17.Casele H, Haney EI, James A, et al. Bone density changes in women who receive thromboprophylaxis in pregnancy. Am J Obstet Gynecol 2006; 195: 1109–1113. [DOI] [PubMed] [Google Scholar]
- 18.Schuurmans N, MacKinnon C, Lane C, et al. SOGC Clinical Practice Guidelines. Prevention and management of postpartum haemorrhage. J Soc Obstet Gynaecol Can 2000; 22: 271–281. [Google Scholar]
- 19.Whitfield LR, Leie AS, Levy G. Effect on pregnancy on the relationship between concentration and anticoagulant action of heparin. Clin Pharmacol Ther 1983; 14: 23–28. [DOI] [PubMed] [Google Scholar]
- 20.Bates SM, Greer IA, Middeldorp S, et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e691S–e736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rath WH. Postpartum hemorrhage – update on problems of definitions and diagnosis. Acta Obstet Gynecol Scand 2011; 90: 421–428. [DOI] [PubMed] [Google Scholar]
- 22.O'Malley KJ, Cook KF, Price MD, et al. Measuring diagnoses: ICD code accuracy. Health Serv Res 2005; 40: 1620–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]