Abstract
Diagnosis and treatment of renal stones during pregnancy is a complex problem. Risks to the fetus from ionising radiation and interventional procedures need to be balanced with optimising clinical care for the mother. Management of such patients requires a clear understanding of available options, with a multidisciplinary team approach. In this review, we discuss the role of different diagnostic tests including ultrasound, magnetic resonance urography, and computerized tomography. We also provide an update on recent developments in the treatment of renal stones during pregnancy. Expectant management remains first-line treatment. Where definitive treatment of the stone is required, new evidence suggests that ureteroscopic stone removal may be equally safe, and possibly better than traditional temporising procedures.
Keywords: Renal stones, urolithiasis, nephrolithiasis, pregnancy
Introduction
Renal stones are relatively rare during pregnancy. However, they are a common cause of non-obstetric abdominal pain in pregnant women. Management of renal stones in pregnancy is challenging. It can be difficult to differentiate between physiological and pathological changes, and diagnostic tests and treatment options are limited. Clinical care of the woman needs to be optimised while balancing potential risks to the fetus and pregnancy.
Renal tract changes in pregnancy
Significant dilatation of the pelvicalyceal system and ureters occurs during pregnancy. Progesterone increases smooth muscle relaxation and reduces peristalsis in the ureter.1 The enlarging gravid uterus compresses the ureter, especially later in pregnancy.2 Physiologic hydronephrosis occurs in 90% of pregnant women,3 and pelvicalyceal dilatation up to 2 cm can be regarded as normal in pregnancy.4 The right side is generally more dilated than the left, possibly as a result of dextro-rotation of the uterus, and the protective effect of the sigmoid colon over the left ureter.2,5 Dilatation starts as early as 6 weeks gestation and resolves by 6 weeks postpartum.
Renal plasma flow and the glomerular filtration rate both increase by over 50% during pregnancy.6 This leads to increased urinary excretion of calcium, uric acid, sodium, and oxalate, all of which are lithogenic.7,8 Calcium tubular reabsorption is also reduced due to suppression of parathyroid hormone. These changes, and urinary stasis secondary to hydronephrosis, promote stone formation in pregnancy. However, the overall risk of stone formation is similar in pregnant and non-pregnant women.7,9,10 It is thought that this may be due to increased urinary excretion of inhibitors of stone formation, such as citrate, magnesium, and the glycoprotein nephrocalcin, and due to the alkalinity of urine in pregnancy.8,10
Calcium stones account for over 80% of stones in the general population (Table 1). They are also the most common stones found in pregnancy.10,11 However, up to 74% of pregnant patients with kidney stones have calcium phosphate stones, in contrast to the general population, where calcium oxalate is more common. This is thought to be secondary to the renal excretory changes and higher pH of urine in pregnancy.11,12
Table 1.
Stone composition (table modified from Moe13).
| Stone composition | Frequency | Comment |
|---|---|---|
| Calcium | 80% | Radio-opaque |
| Struvite stones | 5–15% | Associated with infection |
| Uric acid | 5–10% | Radiolucent |
| Cystine | 1–2.5% | |
| Ammonium urate | 0.5–1% | |
| Others: xanthine, protein matrix 2,8-dihydroxyadenine drugs, e.g. indinavir, triamterene | Rare |
Epidemiology
Renal stones affect 5–15% of the world's population, with recurrence rates of around 50%.13,14 Incidence is thought to be increasing due to lifestyle factors that lead to obesity and metabolic syndrome, particularly in women. However, nephrolithiasis is relatively more common in men.15 Risk factors include a positive family history, dietary factors such as low intake of water or increased intake of animal protein and sodium, environment factors such as hot climate, and underlying medical conditions such as hyperparathyroidism.15,16
The incidence of renal stones in pregnancy is quoted to be 1 in 1500, which is similar to non-pregnant women.9,17,18 However there is a wide variation in reported incidence in individual case series, ranging from 1/188 to 1/4600.17,19 Lower incidences are quoted more frequently in studies of un-referred populations. Stones appear to be more common in multiparous women, with 80–90% stones occurring in the second and third trimester.19–22 Ureteral stones are encountered twice as often as renal calculi, and both the right and left side appear to be equally affected, despite greater dilatation of the right renal tract.20 Patients are more likely to be Caucasian and have a history of renal disease and hypertension. A quarter have a history of previous stone disease.20,23,24
Clinical presentation
Clinical presentation is typically with ‘renal colic’, or severe flank pain, radiating to the groin.25 Nausea and vomiting may also occur. Dysuria and frequency are common, particularly when the stone has moved to the lower urinary tract or there is infection. Microscopic or gross haematuria is usually present and renal angle tenderness may be elicited on examination.
During pregnancy, flank pain is the most common presentation, affecting 89–100% of women, and haematuria is seen in 75–95% of cases.17,19,20 Renal stones may also present as preterm labour or uterine contractions.17,19 In one series, 28% of patients were incorrectly diagnosed as appendicitis, diverticulitis, or placental abruption.20
Impact of renal stones on pregnancy
Presence of renal stones in pregnant women has been associated with a significant increase in the risk of recurrent miscarriage, mild pre-eclampsia, chronic hypertension, gestational diabetes mellitus, and caesarean deliveries.26,27 It has also been associated with premature rupture of membranes in one study.23 Preterm delivery rates ranging between 2.5 and 40% have been reported.17,18,20,23,27 However, these findings are not seen consistently among different studies. Although an association with low birthweight has been reported in one study,27 most studies do not report increased risks for the baby, including risk of congenital malformations, low birthweight, low Apgar scores, and perinatal death.23,26,28 As data are conflicting among the different studies, the true risks of renal stones on pregnancy outcomes are difficult to ascertain.
Diagnosis
Laboratory assessment
Dipstick analysis of a midstream specimen of urine should be performed to assess for underlying infection. An alkaline pH > 7 may suggest infection with a urea-splitting organism; pH < 5 may be associated with a uric acid stone. If the dipstick is positive for nitrites, a urine culture and sensitivity should be sent to confirm infection and the microorganism involved. Blood should be sent to check for anaemia, kidney function, and any derangement in electrolytes including calcium. Increased serum calcium should prompt investigations for hyperparathyroidism.
Radiological diagnosis
In the non-pregnant population, non-contrast computerized tomography (CT) scan has become the modality of choice for diagnosing renal stones. Sensitivities and specificities approach 100%, with detection of all types of stones, except rare stones such as indinavir.29 CT is now considered superior to the previous ‘gold standard’, intravenous urography (IVU).30 However, IVU may be used to provide anatomic and functional information and verify site and grade of obstruction prior to planning surgery. Plain Kidney Ureter Bladder (KUB) radiographs can only identify radio-opaque stones, but are cheaper than CT and give less radiation exposure. For this reason, some advocate using plain KUB x-rays for follow up of radio-opaque stones once diagnosis has been established with a CT.31 Magnetic resonance imaging (MRI) and magnetic resonance urography (MRU) do not visualise stones, but detect ‘signal voids’, making it harder to identify small stones, so they are generally not used. Ultrasound (US) is limited by its poor sensitivity for stone detection.
Risks from ionising radiation for investigative procedures during pregnancy are dependent on gestational age of the fetus and radiation dose. Effects that increase in severity with increasing dose include fetal death, congenital malformations, growth restriction, and neurodevelopment problems. Risks where the probability of effect increases with dose, but there is no known threshold dose include childhood cancer and inheritable genetic mutations.32 The risk of abnormality is considered to be negligible at 50 mGy or less, and the risk of malformations is significantly increased above control levels only at doses above 150 mGy.33 The mean and maximum radiation exposure from common procedures is listed in Table 2. The National Radiological Protection Board has concluded that radiation doses resulting from most diagnostic procedures in an individual pregnancy present no substantial risk of causing fetal death or malformation or impairment of mental development.32 However, as there is no known threshold dose for carcinogenic effects, the principle of ALARA (As Low As Reasonably Achievable) should be adhered to. Radiological exposure carries a risk of less than about 1 in 5000 for fatal childhood cancers (1 in 33,000 per mGy) and less than 1 in 10,000 for induced inheritable diseases (1 in 40,000 per mGy).32 Fetal radiation exposure may be reduced further by imaging only the involved side where possible, shielding the maternal pelvis, and keeping the exposure time or number of radiographs to a minimum.
Table 2.
Radiation dose for common procedures (modified from NRPB32).
| Diagnostic procedure | Mean radiation exposure (mGy) | Maximum radiation exposure (mGy) |
|---|---|---|
| X-ray abdomen | 1.4 | 4.2 |
| X-ray pelvis | 1.1 | 4.0 |
| X-ray chest | <0.01 | <0.01 |
| IVU | 1.7 | 10 |
| CT abdomen | 8 | 49 |
| CT pelvis | 25 | 79 |
Iodinated and gadolinium-based contrast agents also cross the placenta, so may affect the fetus. Although no mutagenic or teratogenic effects have been demonstrated, iodinated contrast exposure in later pregnancy may suppress fetal thyroid function, so neonates should be screened for hypothyroidism within the first week of life.34
Ultrasonography
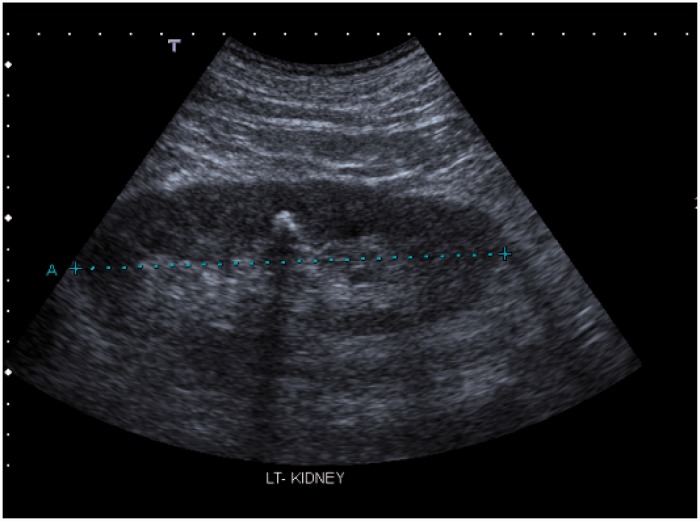
Given the established risks to the fetus from radiation exposure, ultrasound is the first line investigation used in pregnant women (Figure 1). However, sensitivities for stone detection vary considerably, ranging from 29 to 69%.19,20,35–37 Although US provides additional information on hydronephrosis and hydroureter, it may be difficult to differentiate between pathologic and physiologic hydronephrosis. Around 20% of patients with complete obstruction may be missed because they are thought to have ‘physiological hydronephrosis’ of pregnancy.38 In physiological hydronephrosis, it is important to note that ureteral dilatation does not usually extend below the pelvic brim, beyond the iliac artery.39 In a study that compared the value of US and renography in pregnant women with hydronephrosis, a renal pelvic diameter of less than 17 mm in asymptomatic patients effectively excluded the diagnosis of ureteral calculi.40
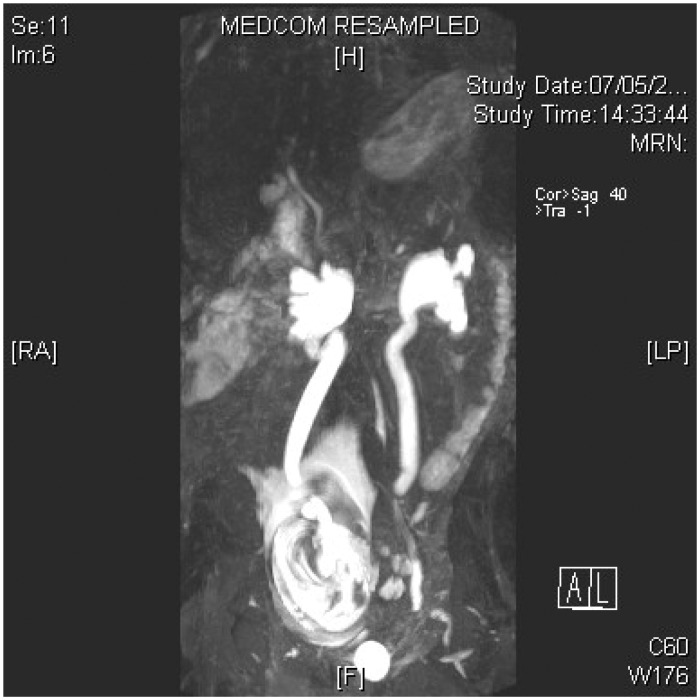
Figure 1.
MRU demonstrating ureteric stone as a ‘signal void’.
Where transabdominal scan is inconclusive, transvaginal US may be used to improve detection rates. Laing et al. reported detection of 13 distal ureteral stones, where transabdominal scan had detected only two of the 13 stones.41
Sensitivity for stone detection may also be improved in pregnancy by using Doppler US to measure renal vascular resistance. Measurement of the resistive index (RI) of intrarenal blood flow has shown that using a threshold of 0.70 for RI and a change in RI of 0.06 is useful in the diagnosis of acute unilateral ureteral obstruction, if done within 6–48 h of presentation (sensitivity 45%, specificity 91% and sensitivity 95%, specificity 100%, respectively).42,43 However, detection rates may be reduced if done outside this time window, in patients with renal disease and with use of non-steroidal anti-inflammatory drugs. Moreover, it requires skilled sonographers to perform such procedures.
Colour Doppler can also be used to detect ureteral jets, or passage of urine, at the uretero-vesical junction. Absence of a ureteral jet on the symptomatic side suggests complete obstruction with a stone. However, false positives may occur due to compression of the ureter by the gravid uterus. It has been suggested that absence of jets should be confirmed in contralateral decubitus patient position.44,45
Intravenous urography
Where ultrasound has failed to diagnose stones, and symptoms such as fever, vomiting, and pain are persistent, or there is deteriorating renal function, alternative diagnostic procedures may be considered. However, there is no clear agreement on which is preferred. Limited IVU has been proposed as one option. Several different protocols of limited IVU have been suggested,19,20,22,46,47 with sensitivities ranging from 60 to 94%. Stothers and Lee reported that IVU demonstrated calculi in 16 of 17 patients with a three film study (using a scout film, a film at 30 seconds and one at 20 minutes).20 Irving and Burgess used a two film series (one plain film and one at 20 minutes) and reported improved detection of stones in three of 15 patients47 and Butler et al. have reported better detection rates than renal ultrasound with a ‘single-shot’ IVP film, taken 30 min after contrast (60% versus 93%).19
Magnetic resonance urography
MRI uses electromagnetic radio waves rather than ionising radiation. No harmful effects to the fetus have been reported, however it should be avoided in the first trimester due to limited data on safety during fetal organogenesis.48 MRU is the visualisation of the urinary tract using MRI. Studies have used the unenhanced, heavily T2 weighted pulsed signals to obtain images in which static fluid exhibits higher signal intensity relative to background (static MRU). MRU may also be performed with contrast material (gadolinium) to provide information on excretory function of the kidneys (excretory MRU).49
Although MRI does not visualize ureteral calculi, certain features may suggest the presence of obstructing calculi. Stones appear as signal voids overlying the high signal of urine within a dilated ureter (Figure 2). Obstructing distal ureteral calculi may be seen as a standing column of urine below the level of the pelvic brim, along with proximal ureteral dilation, the ‘double kink’ sign.50 Other MRI features that suggest pathologic rather than physiologic hydronephrosis include an abrupt ending of the ureter, rather than a smooth taper at the level of the pelvic brim, and the presence of perinephric or periureteral edema.50 A number of studies have demonstrated that MRI was able to distinguish physiological from pathological obstruction of the ureter, and with a high level of accuracy in pregnant women,50,51 and is better than ultrasound Doppler studies for detection of stones.52 In a case series of 220 pregnant women with loin pain, 13 underwent second-line imaging with MRU for persistent symptoms.53 A stone was confirmed in one patient who then underwent a nephrostomy, while 12 of the 13 patients were able to avoid intervention. In another study of 15 pregnant women, fast imaging sequence rapid acquisition with relaxation enhancement (RARE-MRU) was able to detect urinary tract dilatation and localise level of obstruction in all cases. It was possible to differentiate between intrinsic and extrinsic obstruction but the exact nature of the intrinsic obstruction could not be specified.54 MRI may also be able to provide information on other underlying pathology. However, MRI does have limited availability, is time consuming and expensive, and visualisation of small stones can be difficult.
Figure 2.
Echogenic area with posterior acoustic shadow, suggestive of a renal stone.
CT scanning
While unenhanced CT is generally not recommended in pregnancy because of higher ionising radiation exposure, some have advocated the use of low-dose CT where US has been inconclusive.55 A retrospective study in 20 pregnant women with suspected urolithiasis was conducted where the women underwent renal US followed by low-dose CT scan. The study found that CT was more sensitive in locating urinary calculi than renal US. The dose of radiation used varied from 2 to 13.7 mGy, in contrast to the standard mean 25 mGy for CT pelvis. They concluded that patient care may be improved with judicious use of low-dose CT, where appropriate.
Management
The natural course of renal tract stones depends on the size and location of the stone. In the general population, 68% of stones <5 mm may be passed spontaneously within 4 weeks, whereas only 47% of stones 5–10 mm will pass spontaneously.31,56,57 Gettman and Segura reported that 79% of stones were passed if they were located in the vesico-ureteric junction (VUJ) compared to 43% in the proximal ureter, regardless of stone size.56 In pregnancy, 64–84% stones have been reported to be passed spontaneously with conservative therapy, and 50% of those that are not passed during pregnancy may be passed after delivery.20–22,37
Expectant management
As the rate of spontaneous passage of stones is high, expectant management is first-line treatment in both the general population and during pregnancy. It includes analgesia, hydration, and antibiotics if infection is suspected. Hydration promotes passage of the stone by increasing urinary flow and output. Opioids are generally prescribed to treat acute renal colic and may be used safely in pregnancy. Non-steroidal anti-inflammatory drugs (NSAIDS) may also be used for pain management in the non-pregnant population where renal function is not compromised, but they are generally avoided in pregnancy due to risk of adverse effects on the fetal kidney, oligohydramnios, and premature closure of ductus arteriosus.58 They may be used in short courses before 30 weeks gestation in individual cases where benefits outweigh risks, as effects on the ductus are reversible before 32 weeks.
Medical management
Medical management with expulsive therapy using the alpha adrenergic blocker tamsulosin and calcium channel blocker nifedipine, with or without steroids, is effective in facilitating the spontaneous passage of stones in the general population.59 This may be secondary to inhibitory effects on ureteral peristaltic activity. In a systematic review of 47 randomised trials, alpha-blockers had a 45% higher and nifedipine a 49% higher and faster stone expulsion rate than controls (relative risk (RR): 1.45; 95% confidence interval (CI) 1.34–1.57 and RR 1.49; 95% CI 1.33–1.66, respectively). Additionally, lower analgesic requirements, fewer colic episodes, and fewer hospitalisations were observed within treatment groups.
No data are available on medical expulsive therapy use in pregnancy. However, nifedipine has been used safely in pregnancy to control hypertension, and for tocolysis, in doses of 20 mg, repeated if necessary, with maximum of 160 mg per day. The proposed dose of nifedipine used in expulsive therapy (20–30 mg) is not significantly different from that used for tocolysis.
Active intervention
Indications for active intervention include uncontrolled pain, persistent vomiting, sepsis, obstruction of a solitary or transplanted kidney, bilateral obstruction, impending renal failure, stones > 1 cm, or, in pregnant women, obstetric complications such as premature onset of labour.31,60,61 An estimated 25–40% of pregnant women require active intervention.17,19–21,23 When active intervention is needed during pregnancy, it is imperative to use a multidisciplinary team approach, with involvement of urologists, obstetricians, anaesthetists, radiologists, and neonatologists.
Active management of renal stones includes (1) temporising measures to relieve obstruction through insertion of a ureteral stent or percutaneous nephrostomy (PCN) tube, or (2) definitive treatment of the stone by lithotripsy, percutaneous nepthrolithotomy, or ureteroscopic stone removal (URS).
Temporising treatments: Ureteral stents and Percutaneous nephrostomy (PCN)
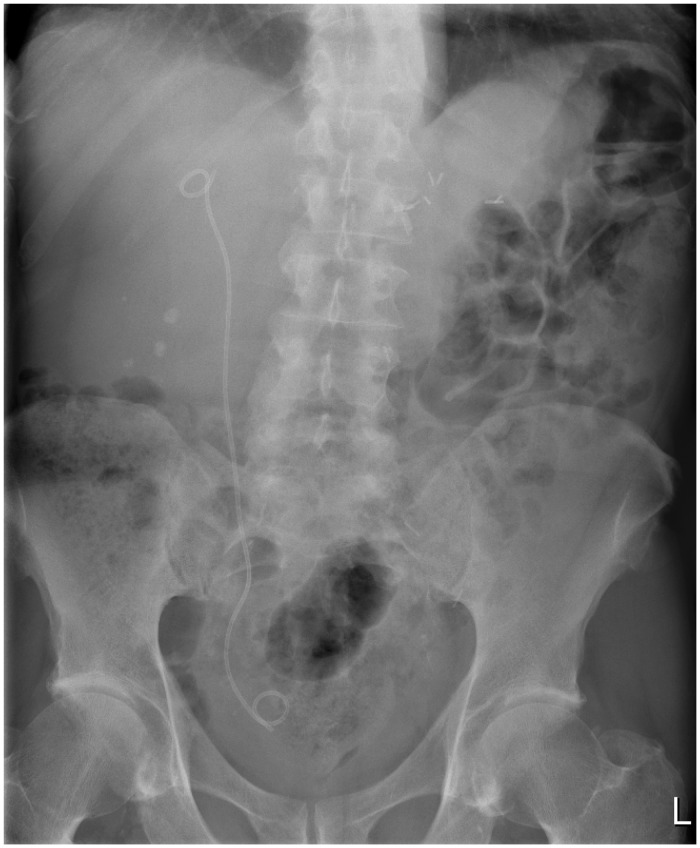
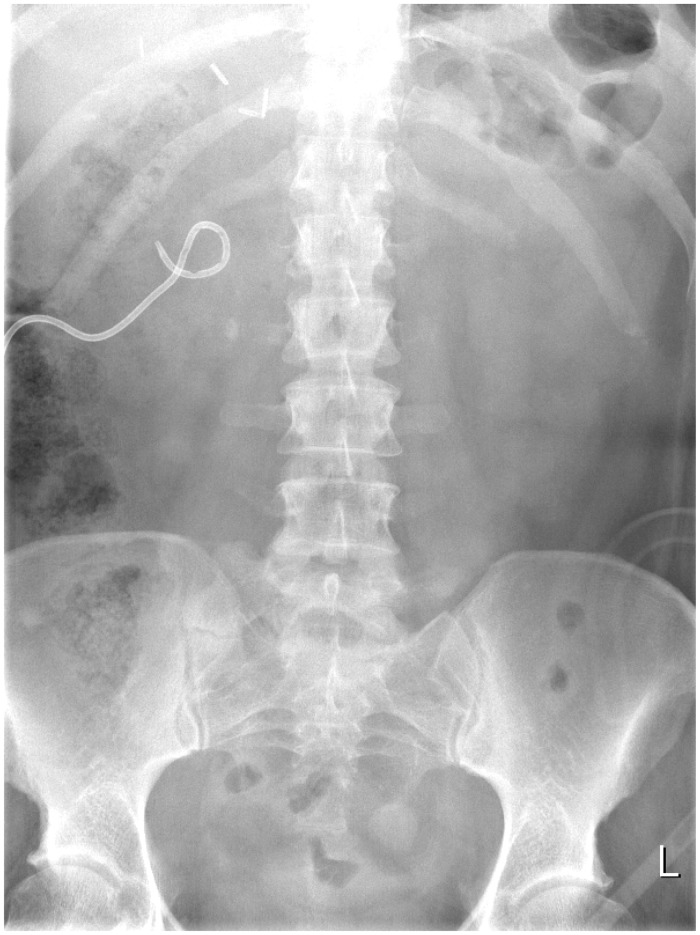
Insertion of an indwelling ureteral Double-J stent allows rapid decompression of the ureter. It may be done retrograde via cystoscopy or antegrade after percutaneous renal puncture, under ultrasound guidance (Figure 3). PCN creates a temporary diversion of urine through the PCN tube, which is inserted under ultrasound guidance via a percutaneous renal puncture (Figure 4). These procedures are generally done under local anaesthesia.
Figure 3.
Ureteric stent with lower pole renal stones.
Figure 4.
Percutaneous nephrostomy with proximal ureteric stone.
Temporary drainage is preferred to definitive stone removal in the presence of infection, a large stone burden, altered anatomy or transplanted kidney, bilateral stone disease, obstetric complications, presentation in the first trimester or near full term, and patient or surgeon preference.61 The advantage of temporising treatment is that it is quicker and can be done with minimal anaesthesia, without radiation exposure. However, temporising measures are less well tolerated by the patients, and the tubes are prone to dislodgement or migration, bacterial colonisation and infection, and tubal encrustation with recurrent blockage. To minimize these risks, it is advisable to change the tube at 6- to 8-week intervals but this requires multiple procedures.62,63 Moreover, a definitive procedure is needed at a later date.
A randomised controlled trial comparing ureteral catheters, ureteral stents, and PCNs found that all are equally effective for decompressing the urinary tract.61 PCN is usually preferred in the presence of sepsis, when excessive ureteral manipulation is best avoided. However, it does require the patient to deal with an external apparatus, which may be troublesome.64,65 In comparison to PCN, non-pregnant patients with indwelling ureteral stents are more likely to complain of irritative lower urinary tract symptoms, stent-related pain, and decreased quality of life.66 They are also at greater risk of ascending urinary tract infections.
Until recently, temporising treatments were preferred during pregnancy as they are minimally invasive. However, complications such as tube encrustation are seen more often in pregnancy secondary to hypercalciuria and hyperuricosuria. This results in recurrence of obstruction and more frequent need for drain exchange.67 Khoo et al. presented a case series of eight women between 16 and 30 weeks who underwent PCN.62 There were no technical problems with catheter insertion, and PCN resulted in clinical improvement in all cases. However, one developed sepsis, three had tubal blockage, and five required stent insertion. They also reviewed 10 published reports of PCN in pregnant women (n = 1 to 7 patients, total 29 women). Nearly half (45%) of the women required tube replacement or flushing due to dislodgement or obstruction. They concluded that early ureteroscopic inspection and stone extraction, with or without ureteral stent implantation, is preferred to long-term nephrostomy during pregnancy. If a nephrostomy catheter is left in situ until delivery, regular flushing is advised.
Definitive methods of stone removal
Ureteroscopic stone removal (URS)
URS involves retrograde visualisation of the collecting system using an endoscope. Stones are fragmented using the holmium:YAG laser, pulsed-dye laser, or ballistic or ultrasonic lithotripter, and removed by basket retrieval, or forceps. Significant advances have been made in endourology over the last decade. Better fibreoptics and reduction in the size of ureteroscopes (from 11 to 4.5 Fr) have improved the views and access to renal passages. Ureteral dilatation is generally not needed, making the procedure less traumatic.
URS is used increasingly as an alternative to extra-corporeal shock wave lithotripsy (ESWL) for treating renal calculi, both in the proximal and distal ureter. It is particularly useful where lithotripsy is contraindicated or would be less effective, such as in coagulopathic, morbidly obese, or pregnant patients or where the stone is >1 cm or is in the lower pole.31,68,69 Ureteroscopy appears to be more effective than ESWL for treating stones in lower ureter, with success rates of 93–100%. It is also more effective (73% versus 67%) and cost effective than ESWL for treating stones >1 cm in the upper ureter.56,57
During pregnancy, URS is now being advocated increasingly as the procedure of choice where definitive intervention is needed, and if adequate expertise and resources are available, unless there are clear indications for temporising measures, as listed earlier.61,62 Laing et al. reported a systematic review of 15 published retrospective case series between 1990 and 2011, with 116 procedures, where ureteroscopy was used in pregnant women.70 Complete stone clearance was achieved in 86%. There were two major complications (one ureteral perforation and one case of premature uterine contraction) and seven minor complications (five urinary tract infections and two cases of post-operative pain). Semins et al. published a systematic review of 108 pregnancies and found no significant difference in the risk of urinary tract infections or ureteral injury with URS in pregnancy compared to non-pregnant women.71 Both authors concluded that URS is a relatively safe option in pregnancy with a high success rate. URS may be performed under a general or spinal anaesthesia or even sedation. The majority of procedures can be performed without ionising radiation under US guidance, with fluoroscopy used only in the event of failure to advance the guidewire.70,71 It is recommended that a temporary stent is inserted after the procedure to prevent obstruction and pain secondary to oedema or stone fragments. This can cause some discomfort to the patient. The stent is usually removed in 72 h but may need to be kept in longer if there are complications such as trauma or bleeding.72
Although a variety of methods have been used safely and successfully for stone fragmentation, holmium:YAG laser is advocated by some as the safest option for intracorporeal lithotripsy during pregnancy. It has little periureteral thermal effect and does not result in energy transmission to the fetus. It also has lower sound intensities compared to US and electrohydraulic probes, thus reducing any potential risk of damage to fetal hearing.72–75
There is a theoretical risk of cyanide formation by the action of laser on uric acid stones, but this has not been reported to be a problem in clinical practice, as any cyanide produced is likely to be removed by the irrigation fluid.7
Extra-corporeal shock wave lithotripsy (ESWL)
ESWL, available since the 1980s, is the only non-invasive method for fragmentation of renal stones. It uses high intensity ultrasonic pulses to cause stone fragmentation. It is the most common treatment for renal stones in the non-pregnant population, and the preferred treatment for simple stones less than 1 cm, in the kidney and upper ureter.57 Although ESWL is well tolerated in the short term, it carries a potential risk of trauma to renal vessels, haematoma formation, and acute kidney injury.76 CT and MRI images have shown renal injury in 63–85% of patients treated with ESWL.77,78,79
ESWL is contraindicated in pregnancy because of fetal damage and death observed in animal studies, particularly with exposure later in pregnancy.80–83 However, there are case reports of successful delivery of healthy babies despite inadvertent exposure to ESWL during pregnancy, and therefore, some have advocated further research in this area.84–86
Percutaneous nephrolithotomy (PCNL)
PCNL involves creating an access tract into the renal collecting system through which a nephroscope can be introduced (Figure 3). The nephroscope has a working channel through which intracorporeal lithotripsy may be performed using lithotrite or laser. Stone fragments are then removed using suction, graspers, or a basket devise. Although PCNL is thought to be more invasive than other treatments, a large meta-analysis has demonstrated its safety and efficacy, particularly when stones are large, multiple, or complex.87 PCNL is the preferred treatment for staghorn calculi, complex large stones (≥2 cm), lower pole calculi >1 cm, and impacted proximal ureteral stones.31,88
Two cases of PCNL in early pregnancy have been reported with good outcome.89,90 However, PCNL is not advised during pregnancy because it requires general anaesthesia, prolonged fluoroscopy time, and prone position of the patient.
Conclusion
Renal stones during pregnancy can lead to significant morbidity for the woman and may also increase the risk of obstetric complications. Management of renal stones in pregnant women is challenging because the optimum diagnostic tests and treatments are also associated with increased risks for the fetus. A high level of suspicion for stones is needed when dealing with pregnant women who present with abdominal pain where obstetric causes have been ruled out. Ultrasound should be the first line of investigation, and this could also include a transvaginal approach, which is better at detecting ureteral stones that are more commonly found in pregnancy. Where ultrasound is inconclusive and symptoms are persistent, an MRU, limited IVU or low-dose CT scan may be considered. First-line treatment should be expectant management, but a third of the women may need active intervention. Where expertise and resources are available, URS may be considered as first-line treatment unless there are clear indications for a temporising treatment with a ureteral stent or PCN. Such women should be cared for by a multidisciplinary team that should include obstetricians, urologists, radiologists, anaesthetists, and neonatologists.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Guarantor
Shireen Meher is the guarantor.
Contributorship
Shireen Meher and Ranan DasGupta drafted the manuscript. Norma Gibbons commented on the final draft of the manuscript.
References
- 1.Marchant DJ. Effects of pregnancy and progestational agents on the urinary tract. Am J Obstet Gynecol 1972; 112: 487–501. [DOI] [PubMed] [Google Scholar]
- 2.Rasmussen PE, Neilson FR. Hydronephrosis during pregnancy: A literature survey. Eur J Obstet Gynecology Reprod Biol 1988; 27: 249–259. [DOI] [PubMed] [Google Scholar]
- 3.Peake SL, Roxburgh HB, Langlois SL. Ultrasonic assessment of hydronephrosis of pregnancy. Radiology 1983; 146: 167–170. [DOI] [PubMed] [Google Scholar]
- 4.Nelson-Piercy Handbook of obstetric medicine, 4th ed London: Informa healthcare, 2010, pp. 176. [Google Scholar]
- 5.Roberts JA. Hydronephrosis of pregnancy. Urology 1976; 8: 1–4. [DOI] [PubMed] [Google Scholar]
- 6.Conrad KP, Lindheimer MD. Renal and cardiovascular alterations. In: Lindheimer MD, Roberts JM, Cunningham FG. (eds). Chesley's hypertensive disorders in pregnancy, 2nd ed Stamford, CT: Appleton and Lange, 1999, pp. 263–326. [Google Scholar]
- 7.Smith CL, Kristensen C, Davis M, et al. An evaluation of the physicochemical risk for renal stone disease in pregnancy. Clin Nephrol 2001; 55: 205–211. [PubMed] [Google Scholar]
- 8.Maikranz P, Parks JH, Holley JH, et al. Gestational hypercalciuria causes pathological urine calcium oxalate supersaturations. Kidney Int 1989; 36: 108–113. [DOI] [PubMed] [Google Scholar]
- 9.Coe FL, Parks JH, Lindheimer MD. Nephrolithiasis during pregnancy. N Engl J Med 1978; 298: 324–326. [DOI] [PubMed] [Google Scholar]
- 10.Maikranz P. Nephrolithiasis in pregnancy. Baillieres Clin Obstet Gynaecol 1994; 375: 375–386. . [DOI] [PubMed] [Google Scholar]
- 11.Ross AE, Handa S, Lingeman JE, et al. Kidney stones during pregnancy: An investigation into stone composition. Urol Res 2008; 36: 99–102. [DOI] [PubMed] [Google Scholar]
- 12.Meria P, Hadjadj H, Jungers P, et al. Stone formation and pregnancy: Pathophysiological insights gained from morphoconstitutional stone analysis. J Urol 2010; 183: 1412–1418. [DOI] [PubMed] [Google Scholar]
- 13.Moe OW. Kidney stones: Pathophysiology and medical management. Lancet 2006; 367: 333–344. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland JW, Parks JH, Coe FL. Recurrence after a single renal stone in a community practice. Miner Electrolyte Metab 1985; 11: 267–269. [PubMed] [Google Scholar]
- 15.Trinchieri A. Epidemiology of urolithiasis: An update. Clin Cases Miner Bone Metab 2008; 5: 101–106. [PMC free article] [PubMed] [Google Scholar]
- 16.Soucie JM, Thun MJ, Coates RJ, et al. Demographic and geographic variability of kidney stones in the United States. Kidney Int 1994; 46: 893–899. [DOI] [PubMed] [Google Scholar]
- 17.Drago JR, Rohner TJ, Chez RA. Management of urinary calculi in pregnancy. Urology 1982; 20: 578–581. [DOI] [PubMed] [Google Scholar]
- 18.Hendricks SK, Ross SO, Krieger JN. An algorithm for diagnosis and therapy of management and complications of urolithiasis during pregnancy. Surg Gynecol Obstet 1991; 172: 49–54. [PubMed] [Google Scholar]
- 19.Butler E, Cox SM, Eberts EG, et al. Symptomatic nephrolithiasis complicating pregnancy. Obstet Gynecol 2000; 96: 753–756. [DOI] [PubMed] [Google Scholar]
- 20.Stothers L, Lee L. Renal colic in pregnancy. J Urol 1992; 148: 1383–1387. [DOI] [PubMed] [Google Scholar]
- 21.Parulkar BG, Hopkins TB, Wollin MR, et al. Renal colic during pregnancy: A case for conservative treatment. J Urol 1998; 159: 365–368. [DOI] [PubMed] [Google Scholar]
- 22.Lewis DF, Robichaux AG, 3rd, Jaekle RK, et al. Urolithiasis in pregnancy, Diagnosis, management, pregnancy outcome. J Reprod Med 2003; 48: 28–32. [PubMed] [Google Scholar]
- 23.Swartz MA, Lydon-Rochelle MT, Simon D, et al. Admission for nephrolithiasis in pregnancy, risk of adverse birth outcomes. Obstet Gynecol 2007; 109: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 24.Jones WA, Correa RJ, Jr, Ansell JS. Urolithiasis associated with pregnancy. J Urol 1979; 122: 333–335. [DOI] [PubMed] [Google Scholar]
- 25.Eskelinen M, Ikonen J, Lipponen P. Usefulness of history-taking, physical examination and diagnostic scoring in acute renal colic. Eur Urol 1998; 34: 467–473. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg E, Sergienko R, Abu-Ghanem S, et al. Nephrolithiasis during pregnancy: Characteristics, complications, and pregnancy outcome. World J Urol 2011; 29: 743–747. [DOI] [PubMed] [Google Scholar]
- 27.Chung SD, Chen YH, Keller JJ, et al. Urinary calculi increase the risk for adverse pregnancy outcomes: A nationwide study. Acta Obstet Gynecol Scand 2013; 92: 69–74. [DOI] [PubMed] [Google Scholar]
- 28.Bánhidy F, Acs N, Puhó EH, et al. Maternal kidney stones during pregnancy and adverse birth outcomes, particularly congenital abnormalities in the offspring. Arch Gynecol Obstet 2007; 275: 481–487. [DOI] [PubMed] [Google Scholar]
- 29.Vieweg J, Teh C, Freed K, et al. Unenhanced helical computerized tomography for the evaluation of patients with acute flank pain. J Urol 1998; 160: 679–684. [DOI] [PubMed] [Google Scholar]
- 30.Miller OF, Rineer SK, Reichard SR, et al. Prospective comparison of unenhanced spiral computed tomography and intravenous urogram in the evaluation of acute flank pain. Urology 1998; 52: 982–987. [DOI] [PubMed] [Google Scholar]
- 31.Miller NL, Lingeman JE. Management of kidney stones. BMJ 2007; 334: 468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.NRPB. Diagnostic medical exposure – Advice on exposure to ionising radiation during pregnancy, National Radiological Protection Board: Chilton, 1998. [Google Scholar]
- 33.American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Guidelines for diagnostic imaging during pregnancy. ACOG Committee opinion no 158, Washington, DC: ACOG, 1995. [Google Scholar]
- 34.Webb JA, Thomsen HS, Morcos SK. Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol 2005; 15: 1234–1240. [DOI] [PubMed] [Google Scholar]
- 35.Lifshitz DA, Lingeman JE. Ureteroscopy as a first line intervention for ureteral calculi in pregnancy. J Endourol 2002; 16: 19. [DOI] [PubMed] [Google Scholar]
- 36.Denstedt JD, Razvi H. Management of urinary calculi during pregnancy. J Urol 1992; 148: 1072–1075. [DOI] [PubMed] [Google Scholar]
- 37.Isen K, Hatipoglu NK, Dedeoglu S, et al. Experience with the diagnosis and management of symptomatic ureteric stones during pregnancy. Urology 2012; 79: 508–512. [DOI] [PubMed] [Google Scholar]
- 38.Laing FC, Jeffrey RB, Jr, Wing VW. Ultrasound versus excretory urography in evaluating acute flank pain. Radiology 1985; 154: 613–616. [DOI] [PubMed] [Google Scholar]
- 39.MacNeily AE, Goldenberg SL, Allen GJ, et al. Sonographic visualization of the ureter in pregnancy. J Urol 1991; 146: 298–301. [DOI] [PubMed] [Google Scholar]
- 40.Müller-Suur R, Tyden O. Evaluation of hydronephrosis in pregnancy using ultrasound and renography. Scand J Urol Nephrol 1985; 19: 267–273. [DOI] [PubMed] [Google Scholar]
- 41.Laing FC, Benson CB, DiSalvo DN, et al. Distal ureteral calculi: Detection with vaginal US. Radiology 1994; 192: 545–548. [DOI] [PubMed] [Google Scholar]
- 42.Shokeir AA, Mahran MR, Abdulmaaboud M. Renal colic in pregnant women: Role of renal resistive index. Urology 2000; 55: 344–347. [DOI] [PubMed] [Google Scholar]
- 43.Shokeir AA, Abdulmaaboud M. Resistive index in renal colic: A prospective study. BJU Int 1999; 83: 378–382. [DOI] [PubMed] [Google Scholar]
- 44.Asrat T, Roossin MC, Miller EI. Ultrasonographic detection of ureteral jets in normal pregnancy. Am J Obstet Gynecol 1998; 178: 1194–1198. [DOI] [PubMed] [Google Scholar]
- 45.Karabulut N, Karabulut A. Colour Doppler evaluation of ureteral jets in normal second and third trimester pregnancy: Effect of patient position. Br J Radiol 2002; 75: 351–355. [DOI] [PubMed] [Google Scholar]
- 46.Horowitz E, Schmidt JD. Renal calculi in pregnancy. Clin Obstet Gynecol 1985; 28: 324–338. [PubMed] [Google Scholar]
- 47.Irving S, Burgess N. Managing severe loin pain in pregnancy. Int J Obstet Gynecol 2002; 109: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 48.Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices. Am J Roentgenol 2007; 188: 1447–1474. [DOI] [PubMed] [Google Scholar]
- 49.A-valtuille G, A-valtuille AIG, Abascal G, et al. Magnetic resonance urography: A pictorial overview. Brit J Radiol 2006; 79: 614–626. [DOI] [PubMed] [Google Scholar]
- 50.Spencer JA, Chahal R, Kelly A, et al. Evaluation of painful hydronephrosis in pregnancy: Magnetic resonance urographic patterns in physiological dilatation versus calculous obstruction. J Urol 2004; 171: 256–260. [DOI] [PubMed] [Google Scholar]
- 51.Mullins JK, Semins MJ, Hyams ES, et al. Half Fourier single-shot turbo spin-echo magnetic resonance urography for the evaluation of suspected renal colic in pregnancy. Urology 2012; 79: 1252–1255. . [DOI] [PubMed] [Google Scholar]
- 52.Spencer JA, Tomlinson AJ, Weston MJ, et al. Early report: Comparison of breath-hold MR excretory urography, Doppler ultrasound and isotope renography in evaluation of symptomatic hydronephrosis in pregnancy. Clin Radiol 2000; 55: 446–453. [DOI] [PubMed] [Google Scholar]
- 53.Au E, DasGupta R, Sandhu C, et al. Optimising the diagnosis of renal colic during pregnancy: What is the role of MRU? J Endourology 2008; 22: A1–A310. [Google Scholar]
- 54.Roy C, Saussine C, Jahn C, et al. Fast imaging MR assessment of ureterohydronephrosis during pregnancy. Magn Reson Imaging 1995; 13: 767–772. [DOI] [PubMed] [Google Scholar]
- 55.White WM, Zite NB, Gash J, et al. Low-dose computed tomography for the evaluation of flank pain in the pregnant population. J Endourol 2007; 21: 1255–1260. [DOI] [PubMed] [Google Scholar]
- 56.Gettman MT, Segura JW. Management of ureteric stones: issues and controversies. Brit J Urol Int 2005; 95: 85–93. [DOI] [PubMed] [Google Scholar]
- 57.Preminger GM, Tiselius HG, Assimos DG, et al. Guideline for the management of ureteral calculi. Eur Urol 2007; 52: 1610–1631. [DOI] [PubMed] [Google Scholar]
- 58.Moise KJ, Huhta JC, Sharif DS, et al. Indomethacin in treatment of premature labor: Effects on the ductus arteriosis. N Engl J Med 1988; 319: 327–331. [DOI] [PubMed] [Google Scholar]
- 59.Seitz C, Liatsikos E, Porpiglia F, et al. Medical therapy to facilitate the passage of stones: What is the evidence? Eur Urol 2009; 56: 455–471. [DOI] [PubMed] [Google Scholar]
- 60.Biyani CS, Joyce AD. Urolithiasis in pregnancy. II: Management. BJU Int 2002; 89: 819–823. [DOI] [PubMed] [Google Scholar]
- 61.Semins MJ, Matlaga BR. Management of urolithiasis in pregnancy. Int J Womens Health 2013; 5: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khoo L, Anson K, Patel U. Success and short-term complication rates of percutaneous nephrostomy during pregnancy. J Vasc Interv Radiol 2004; 15: 1469–1473. [DOI] [PubMed] [Google Scholar]
- 63.Kavoussi LR, Albala DM, Basler JW, et al. Percutaneous management of urolitihiasis during pregnancy. J Urol 1992; 148: 1069. [DOI] [PubMed] [Google Scholar]
- 64.Pearle MS, Pierce HL, Miller GL, et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol 1998; 160: 1260–1264. [PubMed] [Google Scholar]
- 65.Stables DP. Percutaneous nephrostomy: Techniques, indications, and results. Urol Clin North Am 1982; 9: 15–29. [PubMed] [Google Scholar]
- 66.Mokhmalji H, Braun PM, Martinez Portillo FJ, et al. Percutaneous nephrostomy versus ureteral stents for diversion of hydronephrosis caused by stones: A prospective, randomized clinical trial. J Urol 2001; 165: 1088–1092. . [PubMed] [Google Scholar]
- 67.Loughlin KR, Bailey RB., Jr Internal ureteral stents for conservative management of ureteral calculi during pregnancy. N Engl J Med 1986; 315: 1647–1649. [DOI] [PubMed] [Google Scholar]
- 68.Grasso M, Beaghler M, Loisides P. The case for primary endoscopic management of upper urinary tract calculi. II. Cost and outcome assessment of 112 primary ureteral calculi. Urology 1995; 45: 372–376. [DOI] [PubMed] [Google Scholar]
- 69.Pearle MS, Lingeman JE, Leveillee R, et al. Prospective, randomized trial comparing shock wave lithotripsy and ureteroscopy for lower pole caliceal calculi 1 cm or less. J Urol 2005; 173: 2005–2009. [DOI] [PubMed] [Google Scholar]
- 70.Laing KA, Lam TB, McClinton S, et al. Outcomes of ureteroscopy for stone disease in pregnancy: Results from a systematic review of the literature. Urol Int 2012; 89: 380–386. [DOI] [PubMed] [Google Scholar]
- 71.Semins MJ, Trock BJ, Matlaga BR. The safety of ureteroscopy during pregnancy: A systematic review and meta-analysis. J Urol 2009; 181: 139–143. [DOI] [PubMed] [Google Scholar]
- 72.Akpinar H, Tüfek I, Alici B, et al. Ureteroscopyandholmium laser lithotripsy in pregnancy: Stents must be used postoperatively. J Endourol 2006; 20: 107–110. [DOI] [PubMed] [Google Scholar]
- 73.Karlsen SJ, Bull-Njaa T, Krokstad A. Measurement of sound emission by endoscopic lithotriptors: An in vitro study and theoretical estimation of risk of hearing loss in a fetus. J Endourol 2001; 15: 821–826. [DOI] [PubMed] [Google Scholar]
- 74.Watterson JD, Girvan AE, Beiko DT, et al. Ureteroscopy and holmium:YAG laser lithotripsy: An emerging definitive management strategy for symptomatic ureteral calculi in pregnancy. Urology 2002; 60: 383–387. [DOI] [PubMed] [Google Scholar]
- 75.Srirangam SJ, Hickerton B, Van Cleynenbreugel B. Management of urinary calculi in pregnancy: a review. J Endourol 2008; 22: 867–875. [DOI] [PubMed] [Google Scholar]
- 76.Shock Wave Lithotripsy Task Force. Current perspective on adverse effects in shock wave lithotripsy. Clinical guidelines, Linthicum, MD: American Urological Association, 2009. http://www.auanet.org/education/guidelines/shock-wave-lithotripsy.cfm (accessed 27 September 2013). [Google Scholar]
- 77.Willis LR, Evan AP, Connors BA, et al. Shockwave lithotripsy: Dose-related effects on renal structure, hemodynamics, and tubular function. J Endourol 2005; 19: 90–101. [DOI] [PubMed] [Google Scholar]
- 78.Rubin JI, Arger PH, Pollack HM, et al. Kidney changes after extracorporeal shock wave lithotripsy: CT evaluation. Radiology 1987; 162: 21–24. [DOI] [PubMed] [Google Scholar]
- 79.Krambeck AE, Gettman MT, Rohlinger AL, et al. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of follow up. J Urol 2006; 175: 1742–1747. . [DOI] [PubMed] [Google Scholar]
- 80.Streem SB. Contemporary clinical practice of shockwave lithotripsy: A re-evaluation of contraindications. J Urol 1997; 157: 1197–1203. [PubMed] [Google Scholar]
- 81.Smith DP, Graham JB, Prystowsky JB, et al. The effects of ultrasound guided shock waves during early pregnancy in Sprague-Dawley rats. J Urol 1992; 147: 231–234. [DOI] [PubMed] [Google Scholar]
- 82.Yalcin O, Tahmaz L, Yumbul Z, et al. Effects of shock waves on a rat foetus. Scand J Urol Nephrol 1998; 32: 167–170. [DOI] [PubMed] [Google Scholar]
- 83.Ohmori K, Matsuda T, Horii Y, et al. Effects of shock waves on the mouse fetus. J Urol 1994; 151: 255–258. [DOI] [PubMed] [Google Scholar]
- 84.Asgari MA, Safarinejad MR, Hosseini SJ, et al. Extracorporeal shock wave lithotripsy of renal calculi during early pregnancy. BJU Int 1999; 84: 615–617. [DOI] [PubMed] [Google Scholar]
- 85.Deliveliotis CH, Argyropoulus B, Chrisofos M, et al. Shockwave lithotripsy in unrecognized pregnancy: Interruption or continuation? J Endourol 2001; 15: 787–788. [DOI] [PubMed] [Google Scholar]
- 86.Frankenschmidt A, Sommerkamp H. Shockwave lithotripsy during pregnancy: A successful clinical experience. J Urol 1998; 159: 501–502. [DOI] [PubMed] [Google Scholar]
- 87.Preminger GM, Assimos DG, Lingeman JE, et al. Chapter 1: AUA guideline on management of staghorn calculi: Diagnosis and treatment recommendations. J Urol 2005; 173: 1991–2000. [DOI] [PubMed] [Google Scholar]
- 88.Albala DM, Assimos DG, Clayman RV, et al. Lower pole I: A prospective randomized trial of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy for lower pole nephrolithiasis—initial results. J Urol 2001; 166: 2072–2080. [DOI] [PubMed] [Google Scholar]
- 89.Shah A, Chandak P, Tiptaft R, et al. Percutaneous nephrolithotomy in early pregnancy. Int J Clin Pract 2004; 58: 809–810. [DOI] [PubMed] [Google Scholar]
- 90.Tóth C, Tóth G, Varga A, et al. Percutaneous nephrolithotomy in early pregnancy. Int Urol Nephrol 2005; 37: 1–3. [DOI] [PubMed] [Google Scholar]