Abstract
Over the previous 50 years survival of patients with cystic fibrosis has progressively increased. As a result of improvements in health care, increasing numbers of patients with cystic fibrosis are now considering starting families of their own. For the health care professionals who look after these patients, the assessment of the potential risks, and the process of guiding prospective parents through pregnancy and beyond can be both challenging and rewarding. To facilitate appropriate discussions about pregnancy, health care workers must have a detailed understanding of the various important issues that will ultimately need to be considered for any patient with cystic fibrosis considering parenthood. This review will address these issues. In particular, it will outline pregnancy outcomes for mothers with cystic fibrosis, issues that need to be taken into account when planning a pregnancy and the management of pregnancy for mothers with cystic fibrosis or mothers who have undergone organ transplantation as a result of cystic fibrosis.
Keywords: Cystic fibrosis, pregnancy, management, outcomes
Background
Cystic fibrosis (CF) is most common in people of northern European descent. Birth prevalence varies from region to region but occurs in approximately one in 3000 births. Ethnic background has significant impact on birth prevalence.1 Improved treatment has resulted in longer survival, and in a few decades the disease has changed from a predominantly paediatric disease to one which has its major impact in adult life. Median survival in most developed nations now approaches or even exceeds 40 years,2–6 and in some countries numbers of adults with CF outstrip the numbers of children.3,5 As survival has improved, the number of people with CF becoming parents has increased. Of the 13,095 adults with CF in the USA, 211 had a pregnancy in 2011 which represented a live birth rate of 1.6 per 100 US women with CF aged from 14 to 45 years.2 In Australia, approximately one in seven adults have had children.4
As patients live longer, age- and treatment-related complications have broadened, and the complexity of care has increased. Common extra-pulmonary manifestations of the disease include pancreatic exocrine insufficiency, CF-related diabetes (CFRD), reduced bone density, recurrent distal intestinal obstruction and gastrooesophageal reflux disease. In addition, cumulative antibiotic exposure is associated with multi-resistant bacterial infections, and treatment limiting antibiotic allergy and toxicity may emerge (Table 1). These complications can have significant implications for the management of pregnancy.
Table 1.
Common clinical manifestations of cystic fibrosis in adults.
| Sinopulmonary | Gastrointestinal | Endocrine | Renal | Psychiatric | Other |
|---|---|---|---|---|---|
| Chronic suppurative lung disease with airflow obstruction and bronchiectasis Allergic bronchopulmonary aspergillosis Pneumothorax Haemoptysis Respiratory failure with cor pulmonale and pulmonary hypertension Chronic sinusitis Nasal polyposis | Distal intestinal obstruction syndrome Constipation Intussusception Liver cirrhosis with portal hypertension Bowel cancer Pancreatic exocrine insufficiency Recurrent pancreatitis Gastrooesophageal reflux disease Fat soluble vitamin deficiency | CF related diabetes Delayed puberty Metabolic bone disease Oligomenorrhea | Nephrolithiasis Hyponatraemic hypochloraemic metabolic alkalosis Chronic kidney disease (CF diabetes, aminoglycoside exposure) Oxalate nephropathy | Depression Anxiety | Difficult vascular access Drug allergy CF arthropathy/ hypertrophic pulmonary osteoarthropathy |
Despite improvements in care, the majority of patients with CF will eventually develop respiratory failure and many are considered for lung transplantation. Most transplants performed are bilateral lung transplants. In a minority of patients, combined transplants (e.g. heart–lung–liver transplants), isolated liver transplants and a small number of renal and pancreas transplants have been performed. Since 1995, the International Heart Lung Transplant Registry reports >6200 patients with CF have received lung transplants, accounting for 16.6% of the total number of patients undergoing lung transplantation.7 As the number of CF patients that have received transplants increases, so too are there are increasing reports of pregnancy in patients with CF after lung transplantation.2,8
This review will address considerations for the planning of a CF pregnancy, the impact of pregnancy on the fetus and mother and the outcomes for pregnancy in women post-transplantation for CF.
Outcomes of pregnancies in CF
Historical perspective
The first successful delivery of a baby in a woman with CF was reported in 1960,9 followed by a case series of 13 pregnancies in 10 mothers.10 Median age at pregnancy was 21 years. There were eight healthy deliveries at term, three pre-term deliveries and two who were still-born or died shortly after delivery. This was an era when median survival for CF was less than 5 years,11 and until the 1980s pregnancy for mothers with CF was generally discouraged.12–14 However as CF care and outcomes have improved there is increasing recognition that pregnancies can result in favourable fetal and maternal outcomes.15–23
Physiological impact
Cardiac output, blood volume and resting ventilation all increase during pregnancy.24 Static lung volumes including total lung capacity and residual volume are reduced as a result of upward displacement of the diaphragm. For healthy women, spirometry (a key measure of lung function used routinely in the CF clinic) is generally preserved throughout pregnancy, although some series have noted reductions in both forced expiratory volume in 1 s (FEV1) and forced vital capacity as pregnancy progresses.25,26 The extent of change in spirometry during pregnancy in women with CF is variable. Elevations in pulmonary artery pressure are common in CF (37% of patients were found to have a peak pulmonary artery pressure >30 mmHg as estimated by echocardiographic criteria, with FEV1% and PaO2 independent predictors of transtricuspid pressure gradient27) and haemodynamics may be significantly worsened in those with advanced lung disease and severe pulmonary hypertension. Therefore, patients with severe airflow limitation and/or markedly impaired gas exchange are at risk of respiratory decompensation as pregnancy progresses.
Impact of CF on short-term fetal outcomes
In recent reports of pregnancy outcomes in CF, the most frequently encountered adverse fetal outcome was pre-term delivery, with between 10 and 25% babies born before 37 weeks gestation.18,22,23,28,29 Increased rates of low birth weight (<2.5 kg) have been reported in some studies but not all.18,22,23,28 For most infants, Apgar scores will be normal,29 rates of congenital malformations do not appear to be increased and perinatal death is rare.12,18,22,23,28,29 Short-term neurological and cognitive development appears to be normal, although subtle sequelae of CF pregnancy on the child have not been comprehensively studied to date.22
Impact of CF on maternal outcomes
Impact of CF on delivery outcomes
In some reports caesarean section was more common in women with an FEV1 less than 60% predicted. Caesarean section was performed for obstetric indications (e.g. breech presentation, narrow birth canal)18 and due to maternal respiratory decompensation.22 In other reports caesarean section rates have not been increased.29 Many women with CF will be able to deliver successfully by vaginal delivery, although induction of labour after 37 weeks gestation is often required for respiratory deterioration.29 Under these circumstances early administration of epidural anaesthesia to reduce the physiological stress of labour and assisted second stage may be required.
Impact of CF on long-term maternal outcomes
Whilst there has been debate about the impact of pregnancy on long-term prognosis for mothers with CF, recent literature does not indicate that pregnancy has adverse effects on lung function decline or mortality. A study from the 1990s demonstrated a general pattern of increased loss of lung function during pregnancy (i.e. more than 10% of predicted loss). However, individual patterns of changes in spirometry were variable, and many patients returned to baseline levels after delivery. Nonetheless, those with an FEV1<60% had a higher rate of death within the preschool lifetime of their children (mean 3.2 years after delivery). Whilst mortality may have reflected the natural progression of CF-related bronchiectasis, it is possible that pregnancy also contributed to decline.30 More recently, Goss et al. published a large cohort study from the US CF Patient Registry examining the impact of pregnancy on 680 women matched with 3327 non-pregnant controls and observed for 12 years.31 At inception of this cohort, the pregnant CF women were generally healthier, with a higher FEV1 and body mass index (BMI). The 10-year survival of CF women who became pregnant was no worse compared to those who had not. Similar results have been demonstrated in other recent studies, with women who became pregnant experiencing similar respiratory and health trends as those who did not, although in some reports their utilization of health resources was greater.32–34
Whether or not pregnancy impacts on the trajectory of progression of lung disease for patients with CF, there are physical and emotional demands of child rearing that have the potential to reduce any individual parent’s ability to adhere to maintenance therapies themselves. This may lead to a deterioration in their own health. These issues should be discussed in detail with any prospective CF parents.
Fertility, contraception and family planning for patients with CF
People with CF are as likely as peers to consider starting a family,35,36 however, significant knowledge deficits in sexual and reproductive health in people with CF have been identified.37–39 Thus, pregnancy planning should include a detailed discussion between the patient, the multi-disciplinary CF team and the multi-disciplinary obstetrics team.40 Opportunity should be provided for the patient and partner, and where relevant other support persons, to meet with and discuss the various aspects of obstetric and post-natal care with the CF team and the obstetric team.41
Fertility and contraception
For CF patients, there are gender-specific issues that need to be considered in planning for pregnancy. Congenital absence of the vas deferens and obstructive azoospermia is present in 95% of males with CF. Therefore, most are unable to father their own children without specific intervention such as microepididymal sperm aspiration or testicular sperm aspiration followed by intracytoplasmic sperm injection.42 There is little work describing the impact of fatherhood on CF, although the difficulties of child rearing will have the potential to distract any individual from an arduous CF regimen and negatively impact on health as a consequence. As for females, difficult ethical dilemmas are raised for male patients with CF who have more severe lung disease and limited life expectancy who are considering assisted reproductive techniques in order to become a father.
For females with CF, local and systemic factors can impair fertility. Tenacious cervical mucus and fallopian tube obstruction can present physical barriers to conception. Poor nutrition may adversely impact on ovulation and may lead to oligomenorrhoea though, for most women with normal nutritional status, menses is not perturbed and pregnancy is possible. Interventions such as follicular stimulation (e.g. clomiphene) and in vitro fertilization (IVF) have been successfully used to assist women with CF to achieve pregnancy, depending on the likely mechanism of infertility.43 Despite this, studies of knowledge of reproductive potential demonstrate that many young women with CF may not be aware (or fully aware) of the risk of pregnancy following unprotected intercourse.44
A key role of the CF team is to ensure all young women with CF are aware that pregnancy can and does occur.39,41,45–47 There is limited research to inform the optimal form of contraception in women with CF. Whilst there are theoretical risks of oral contraceptive failure when antibiotics are administered, studies have cast some doubt on how much of an effect is evident.48 The effects of azithromycin and other oral antibiotics are unclear, though they are likely to have the greatest interaction with the oral contraceptive pill when they have recently been initiated. Similarly, the effect of intravenous antibiotics is unclear. Therefore in the case of recent commencement or cessation of new antibiotics it is wise to recommend a second effective form of contraception for at least one cycle (e.g. barrier method). Limited pharmacokinetic studies in CF support the use of standard dose of the oral contraceptive pill,49 though similar to the general population they should not be prescribed in patients with a prior history of venous thrombosis, significant CF-related liver disease or pulmonary hypertension.
Other forms of contraception that can be considered include barrier techniques, injectable medroxyprogesterone and long-term implantable progesterone-based devices, and intrauterine devices (IUDs).50 Indefinite use of systemic progestogens is unwise in patients with CF as it may aggravate pre-existing low bone mineral density. The choice between each of these options should be considered on an individual basis and in consultation with the patient, the CF team and the general practitioner or a specialist obstetrician. The latter should be consulted if an IUD is considered appropriate in the nulliparous woman with CF. As with all younger women (and men) advice about safe sexual practices is important, including access to appropriate vaccinations such as human papilloma virus vaccination.
Genetic counselling
CF is an autosomal recessively inherited condition resulting from mutations in the CF transmembrane conductance regulator (CFTR) gene. The number of identified CFTR mutations now exceeds 1900.51 Children of a mother with CF are obligate carriers. There is consensus that screening of partners for carriage of a CFTR mutation should be offered.40 Patients with CF are generally supportive of carrier screening,52 however this is not universal35,53 and discussions should be handled with sensitivity.
The risk that the fetus will have CF will depend on the risk the partner carries a CFTR mutation. Carrier screening of the unaffected partner provides a more accurate estimate of risk. Screening panels need to include mutations common in people of the partner’s ethnic background. A negative result reduces the likelihood that the baby will have CF; however, CF remains possible as screening is limited.
When a partner is found to be a carrier of a CFTR gene, the risk of this couple having a child with CF is 50%. This requires careful discussion between the couple, the CF physician and a medical geneticist, as the implications for decision making are complex. Recognition of milder CF phenotypes and improved outcomes for people with CF generally may lead to a couple to consider proceeding to or continuing with a pregnancy. In some health care systems, IVF is available and may support selective implantation of an embryo unaffected by CF by pre-implantation genetic diagnostic techniques.
Predicting outcomes of a CF pregnancy
Anticipating outcomes can be difficult, and a number of variables have been proposed as potential predictors. Pre-pregnancy pulmonary function, microbiological status, nutritional status and CFRD are particularly important considerations.
Pulmonary function and microbiology
The most useful predictor appears to be pre-pregnancy FEV1.15,31,32,40,54 Overall, CF mothers with mild to moderate pre-pregnancy airflow limitation (FEV1>60%) tolerate pregnancy well. Conversely, a pre-pregnancy FEV1<60% is associated with a higher rate of maternal complications such as pulmonary exacerbations, pneumothorax and preeclampsia.40,55 However, significant bronchiectasis is possible even with relatively preserved spirometry, and if present is often associated with chronic antibiotic-resistant gram negative infections (Figures 1 and 2). Pre-existing cor pulmonale and severe pulmonary hypertension are contraindications to pregnancy.56,57 Data from case series of patients with primary pulmonary arterial hypertension demonstrate a high maternal mortality (20–30%), even in the modern era.58 These results are generally extrapolated to patients with pulmonary hypertension secondary to CF lung disease. Ventilatory failure as evidenced by daytime and/or nocturnal hypercapnoea portends a poor prognosis. The presence of chronic, exertional or nocturnal hypoxia is also an indicator of severe lung disease and is therefore likely to result in suboptimal outcomes. Although systematic data addressing these particular clinical parameters are not available, they should also be considered relative contraindications.40 Similarly, Burkholderia cenocepacia pulmonary infection has been associated with rapid decline in lung function, and patients with this infection should be counselled about the excessive risk of pregnancy.18,59–61 At present, there are insufficient data to advice on likely pregnancy outcomes in patients infected with other Burkholderia species. However, these are often more benign and therefore may run a similar course as with infection with other gram negative bacteria such as Pseudomonas.
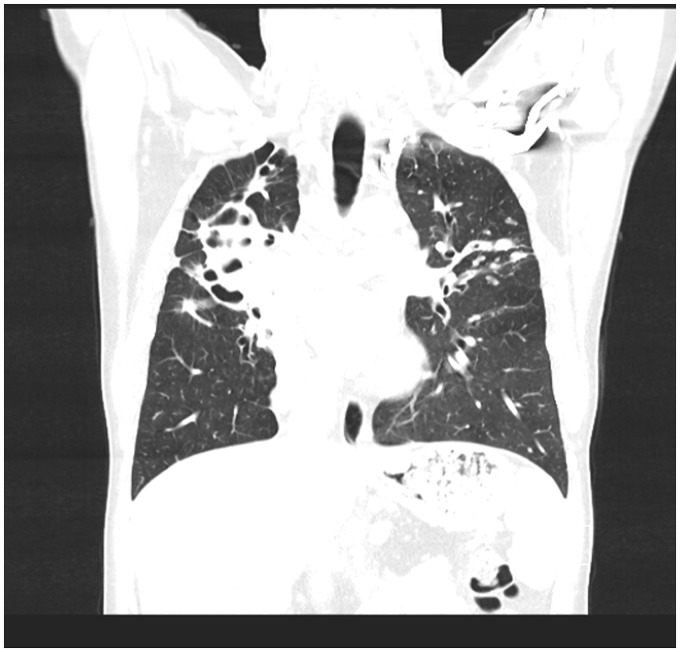
Figure 1.
Coronal CT chest performed on the patient described in Figure 1 demonstrating gross cylindrical bronchiectasis with collapse of the right upper lobe, and mucus filled airways in the left upper lobe and associated inflammatory nodules.
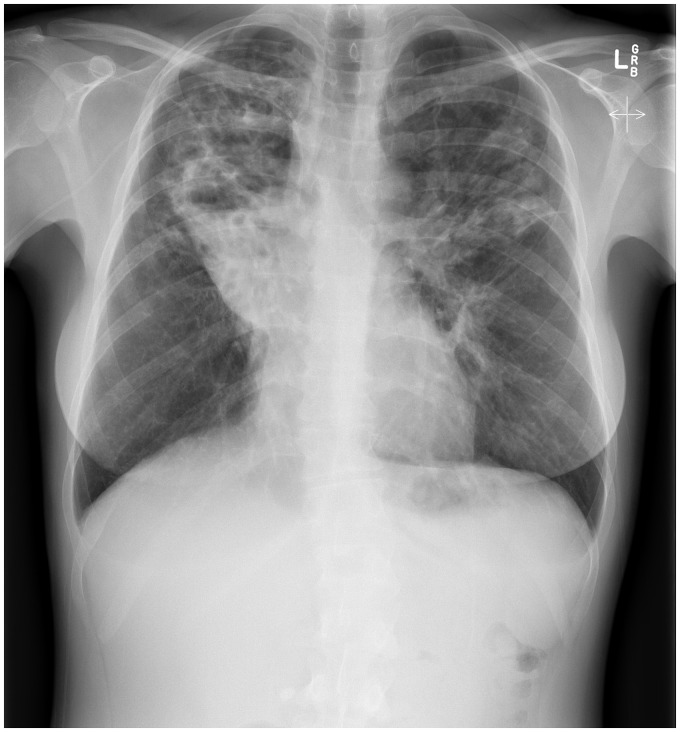
Figure 2.
CXR of a 36-year-old G3P2 mother with CF demonstrating severe bilateral upper lobe bronchiectasis and chronic collapse of the right upper lobe.
Nutrition
Nutrition is an important consideration when attempting to predict outcomes. Impaired pre-pregnancy nutrition is a significant risk factor for suboptimal maternal and fetal outcomes.15,62 A higher preconception body weight has been associated with favourable pregnancy outcomes, including a reduced rate of caesarean section.23,30,31 In general, it is recommended a pre-pregnancy BMI of 22 kg/m2 be targeted40 and pregnancy be approached with caution if BMI is ≤18 kg/m2.24,62
Diabetes
The prevalence of CFRD is more than 25% in young adults and increases throughout adult life.63 It is predominantly an insulin deficient state, though insulin resistance can further aggravate impaired glucose tolerance during acute respiratory infection and during pregnancy.64 Women with CF but without diabetes also have an increased risk of developing gestational diabetes compared with the healthy population due to inability to increase insulin secretion to compensate for the physiological insulin resistant state that develops late in pregnancy.64,65 Data from patients with non-CFRD demonstrate an increased risk of fetal malformations, macrosomia and shoulder dystocia in patients with inadequately controlled diabetes,66–69 and for the mother the presence of diabetes confers greater obstetric risks.65 There are no data addressing the relative impact of CFRD on outcomes of CF pregnancies.
Management of pregnancy in a woman with CF
In addition to routine pre-pregnancy care (including cervical smears, prior viral infection screen, dietary folic acid supplementation, immunizations and lifestyle advice) it is imperative in the CF pregnancy that careful attention is given to:
Maintenance of pulmonary health;
Medications during pregnancy;
Maintenance of nutrition;
Surveillance and treatment of diabetes;
Inter-disciplinary teamwork and communication.
Pulmonary health
Prior to pregnancy it is important to optimize all aspects of maintenance therapies. During pregnancy, regular review by the CF specialist and multi-disciplinary team, and close monitoring of lung function and sputum microbiology are mandatory. Due to the potential adverse impact on the developing fetus in the event of severe respiratory sepsis, aggressive and early treatment of pulmonary exacerbations is encouraged. However, selection of antibiotics required for exacerbations of lung disease requires careful consideration. Airway clearance therapy should be even more intensive than was required prior to pregnancy. Musculoskeletal pain is common during pregnancy secondary to hormone-induced relaxation of ligaments, which can in turn adversely impact on airway clearance. Exercise is important for maintaining optimal lung health in people with CF, although there are no systematic data assessing its role in pregnancy.
Medications in pregnancy
Excess fetal malformations have not been demonstrated in CF pregnancies, despite requirements for extensive drug therapy.12 Although the majority of modern maintenance therapies for people with CF have excluded pregnant patients in clinical trials, reassuring data to date suggest that most commonly administered therapies are unlikely to contribute significantly to fetal malformations.40 However, some commonly prescribed drugs in CF (e.g. ciprofloxacin) should be used with caution.70,71 Whilst there are a paucity of adequate, well-controlled studies of ciprofloxacin in pregnant women, and one study has not demonstrated an increase in major malformations or significant musculoskeletal dysfunctions after fluoroquinolone exposure during embryogenesis,70 animal studies have demonstrated an increased incidence of abortion, intrauterine deaths, fetal retardation and arthropathy with ciprofloxacin exposure.71
Careful thought should also be given to intravenous antibiotics. Consensus guidelines outside pregnancy recommend that treatment of severe CF exacerbations in patients chronically infected with Pseudomonas aeruginosa include a parenteral beta-lactam antibiotic (e.g. ceftazidime) in combination with an aminoglycoside (e.g. tobramycin).72 However, aminoglycosides cross the placenta, have demonstrated selective uptake in fetal kidneys and have been associated with 8th cranial nerve damage after in utero exposure.71 Therefore, the use of parenteral aminoglycosides is generally avoided. Alternatives include beta-lactam monotherapy73 or nebulized tobramycin which is associated with lower serum levels.74 Nevertheless, in the deteriorating pregnant woman with CF consideration should be given to the use of drug therapies which normally would be avoided during pregnancy, as securing a well mother and a thriving infant are key at the end of the pregnancy.
Nutrition
Pregnancy increases nutritional demands for the mother, by approximately ∼300 kcal/day.31 Many women with CF (especially those with pancreatic insufficiency) may struggle to meet such increased requirements.62 Dietetic counselling with focus on optimizing energy and nutrient intake is important before conception. During pregnancy, the recommendation for CF mothers is to eat a diet with 120–150% of the recommended energy intake for non-CF age- and sex-matched patients, and to aim for weight gain of >11 kg.24 Options for nutritional support (including during pregnancy) include aggressive oral supplementation and in some cases enteral feeding (nasogastric or gastrostomy).15,32 Nutritional supplementation via enteral feeding can aggravate gastrooesophageal reflux, which is common in CF, is frequently aggravated by the gravid uterus, and which may exacerbate airway inflammation.75–77 Of the various gastric acid suppressive options, H2-blocking therapy (e.g. ranitidine) has the most robust safety profile, but proton pump inhibitors are also likely to be safe.75,76,78 Pro-kinetic agents such as domperidone can also be used in more severe cases of gastrooesophageal reflux.
Nutritional review also relies on the assessment of micronutrient status, which should ideally be performed prior to conception. This requires an appraisal of dietary intake and blood levels.62 Most patients with CF have pancreatic insufficiency leading to malabsorption of fat soluble vitamins A, D, E and K and require supplementation. Generally, vitamin A supplementation is contraindicated during pregnancy due to concerns of teratogenicity.79 However, Vitamin A supplementation in pregnant women who are deficient reduces rates of maternal anaemia and may be associated with reduced rates of maternal infection.80,81 If vitamin A supplementation is necessary to maintain adequate levels, a daily intake of less than 10,000 units is recommended.40 Vitamin D deficiency is associated with an increased risk of gestational diabetes, pre-eclampsia and small for gestational age infants, and should be supplemented when required.82 Vitamin E supplementation should be adjusted according to blood levels, and vitamin K supplementation should continue as for the patient’s requirements before pregnancy. Folic acid supplementation is advised to continue for at least 12 weeks, and regular follow up with a CF specialist dietician is recommended.40 Finally adequate iron stores are important for pregnant women; however, there is emerging evidence that parenteral replacement of iron may aggravate respiratory infection. Therefore, the judicious prescription of iron replacement in people with CF is recommended.83,84
Nutrition is also an important consideration post-partum, and discussions around infant feeding should ideally occur before birth. Breast feeding is a vexed issue in the woman with CF. For some mothers with CF breast feeding can be successfully undertaken.18,85 However, it is not possible for many women, due to poor milk flows and to the further nutritional demands it places on the mother. Close monitoring of nutritional status and fatigue should be undertaken, and consideration for weaning may be desirable for the health of the mother. Careful assessment of each medication and its ability to be concentrated in breast milk is vital.
Surveillance for gestational diabetes in CF
A glucose tolerance test is recommended during each trimester in those women with CF who do not have a diagnosis of CFRD. Insulin is the treatment of choice for CFRD86 and is often required in woman who develop CF-related gestational diabetes. Data demonstrating improved fetal and maternal outcomes with aggressive treatment of diabetes in the general population are extrapolated to patients with CF, and tight control of gestational diabetes is strongly advocated.87,88 Importantly, there are no CF-specific data assessing interventions aimed at tight glycaemic control, and the nutritional approach for women with CF-related gestational diabetes has not been well studied. A high-fat high-energy diet is central to maintenance of nutrition in CF, has been linked to improved long-term outcomes89 and is standard advice for most patients with CF. Conversely, pregnant women in the general population with diabetes are encouraged to eat a low glycaemic-index diet and possibly to reduce total calorific intake.90 Therefore, a particular challenge when diabetes complicates pregnancy in CF is how to sustain the nutritional requirements of the mother and fetus at the same time as maintaining glycaemic control. This again highlights the vital role of an expert CF dietician in assessing the best form and timing of enhanced calorie intake.
Models of care and inter-disciplinary team work
Ideally every CF pregnancy should be carefully planned, and the issues outlined earlier should be discussed between the patient and their CF and obstetric multi-disciplinary teams (Tables 2 and 3). Due to the unpredictable effect of pregnancy on an individual, every pregnancy in CF should be regarded as a potentially high risk for both mother and fetus. Antenatal care of pregnant patients with CF should include at least monthly CF outpatient follow up, increasing to 2 weekly in the last trimester, and more frequently as indicated. Each review should include specific physiotherapy, dietetic, nursing and medical review. As a specific example, urinary incontinence is common in women with CF91 and is likely to be exacerbated during pregnancy. Therefore, it is reasonable that similar recommendations for the general population with regards to pelvic floor strengthening should be extrapolated to pregnant women with CF.92
Table 2.
Members of an adult CF specialist team.
| Members of an Adult CF Teama |
|---|
| CF medical specialists |
| CF nurse specialists |
| Physiotherapists |
| Dieticians |
| Social workers |
| Psychologist |
| Pharmacist |
| Junior medical staff |
| Administration support and data entry staff |
Table 3.
Suggested members of a high risk obstetric team caring for CF women.
| Suggested members of a high risk obstetric team caring for CF women |
|---|
| Obstetrician |
| Obstetric physician, or maternal fetal medicine specialist with an interest in high risk pregnancy |
| Anaesthetist |
| Neonatologist |
| Social worker |
| Midwives |
| Diabetes educators, dieticians |
Patient reviews should support multi-disciplinary team management through a high-risk pregnancy clinic, lead by obstetric and maternal–fetal medicine specialists.40
As delivery approaches, regular communication and planning between the CF and obstetric teams is vital, particularly in those women whose lung and or nutritional health are sub-optimal approaching the third trimester. Given the increased rates of pre-term delivery, ready access to specialist neonatal services including neonatal intensive care is important.
Many patients with CF will have limited vascular access due to frequent prolonged courses of intravenous antibiotics. Such problems will require careful planning, particularly when considering potential peri-partum haemorrhage. Many patients will have totally implantable vascular access devices. It is imperative that all nursing staff accessing such devices are familiar with their use.
Finally, spontaneous abortions may occur or therapeutic terminations may be required. Again, close collaboration between CF and specialist obstetric multi-disciplinary teams is required to manage the complex medical, obstetric and psychological issues placed on the woman with CF facing these procedures.
Comprehensive details of all aspects of pregnancy in CF are available in the European CF Society Pregnancy in CF Guidelines.40
CF pregnancy post-lung transplantation
Despite improved outcomes for patients with CF over the previous three decades, the majority of patients will ultimately develop respiratory failure. In many cases lung transplantation can be performed and can prolong life. Therefore for health care professionals counselling prospective CF parents, particularly those with moderate to severe lung disease, there will necessarily be conversations about transplantation. Survival rates following lung transplantation for patients with CF have progressively improved. In the recently published International Society of Heart and Lung Transplant Report (2012), the median survival rate for CF was 7.5 years and 40% of patients survived 10 years or more after lung transplantation.96
Patients with CF often enquire about the possibility of children after transplantation. It is certainly possible for CF women who have undergone lung transplantation to become pregnant, and the experience of organ transplant recipients achieving pregnancy is expanding.2,6 There are now a number of case reports and series that have highlighted both favourable and adverse effects of pregnancy on lung graft function.97–99 However to date the long-term effect of pregnancy and child rearing on allograft function has not been systematically documented. Where appropriate, this should be explored during assessment for or following recovery from lung transplantation.
The US National Transplantation Pregnancy Registry has recently reported results which included 21 female lung recipients who had 30 pregnancies (including one triple pregnancy).100 Ten of the 21 transplant recipients had CF, and these women had 12 pregnancy outcomes reported, including seven live births, two spontaneous abortions and three therapeutic abortions. Across the entire group, mean gestational age was reduced at 33.9 weeks. At the time of publication, 13 of the 21 transplant recipients had adequate allograft function, five had died (including two with CF) and one had a poorly functioning graft.
Planning a CF pregnancy post-transplant
Many of the principles relevant to pregnancy planning and management in a patient with CF and native lungs apply to CF patients who have undergone a transplant. However, there are additional considerations which must also be taken into account. Respiratory insufficiency occurring as a consequence of obliterative bronchiolitis, monitoring for allograft rejection (bronchoscopy), the need for immunosuppressive therapy and potential impact on extra-pulmonary manifestations of CF, especially diabetes, are important considerations in the woman undergoing pregnancy following lung transplantation. Therefore, planning and preparation is particularly important so that decisions about changes to therapies that may be necessary (especially changes to immunosuppressive drugs) can be considered and implemented. This will require detailed discussion between the obstetric team, the transplant team and where required a pharmacologist with an interest in pregnancy.
Looking to the future and areas of uncertainty
The rapid evolution of CF care over the previous three decades has meant that parenthood has become reality for an increasing number of patients with this disease. For patients born with CF in the 21st century, a median life expectancy of more than 50 years has been projected.101 This is likely to increase as novel therapies that correct the underlying genetic defect become available in clinical practice.102–106 In pregnancy, the advent of new CFTR specific treatments will raise ethical and clinical dilemmas. For all CF drugs in development, under clinical trial or recently licensed for use, the effect on fetal development will be unknown. For women who may derive profound clinical benefit from these medications and who wish to become pregnant, difficult decisions may be required, including balancing the risks of continuing a therapy with an unknown safety profile against the risk of disease progression if therapy is ceased.
To enhance understanding of the impact of pregnancy and starting a family in CF, future areas of research should include:
Long-term outcomes of children born to patients with CF.
The optimal management of CF-related gestational diabetes including nutritional management.
The prognostic implications of CF-related gestational diabetes including subsequent development of overt CFRD.
The effect of historically highly aggressive organisms such as B. cenocepacia on CF pregnancy in the current era.
The most appropriate management of contraception for patients with CF.
The psychological impact of both pregnancy and conversely the inability to conceive for patients with CF.
The impact of fatherhood on long-term outcomes for men with CF.
The impact of new CF therapies such as ivacaftor on long-term maternal outcomes.
Conclusions
The number of CF women commencing and successfully completing pregnancy continues to rise. Historically, fetal and maternal outcomes were poor but have improved dramatically. For the woman with CF who has well-preserved lung function, stable and adequate nutritional status and, if present, well controlled diabetes, long-term maternal outcomes appear to equal those who have not been pregnant. Where possible, planning including genetic counselling should be undertaken with the woman with CF and their partner and include the obstetric and clinical genetics services. Very close monitoring of pulmonary status, weight gain and for the presence of gestational diabetes is vital and requires close team work between the CF specialist team and the high-risk obstetric service, the latter having a sound working knowledge of the complications and treatment of people with CF. Similarly, close inter-disciplinary team work is required in the planning and management of women with CF planning or having a pregnancy after transplantation, where the potential risks appear to be even greater.
Declaration of conflicting interests
None declared.
Funding
SCB is the recipient of a Queensland Health Health Research Fellowship. This review received no other specific grant from any funding agency in the public, commercial, or not for profit sector.
Guarantor
JG.
Contributorship
JG, GT, and SCB wrote the manuscript. SCB and LC reviewed and edited the manuscript.
References
- 1.O’Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009; 373: 1891–1904. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation Patient Registry 2011 Annual Data Report. Bethesda: The CF Foundation, 2011.
- 3.Canadian Cystic Fibrosis Registry 2011 Annual Report. Toronto: Cystic Fibrosis Canada, 2011.
- 4.15th Annual Report from the Australian Cystic Fibrosis Data Registry. Sydney: Cystic Fibrosis Australia, 2012.
- 5.ECFS Patient Registry Annual Data Report 2008–2009 data. Denmark: European Cystic Fibrosis Society, 2012.
- 6.UK Cystic Fibrosis Registry Annual Data Report 2012. Bromley: Cystic Fibrosis Trust, 2013.
- 7.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth adult lung and heart-lung transplant report—2013; focus theme: Age. J Heart Lung Transplant 2013; 32: 965–978. [DOI] [PubMed] [Google Scholar]
- 8.14th Annual Report from the Australian Cystic Fibrosis Data Registry. Sydney: Cystic Fibrosis Australia, 2011.
- 9.Siegel B, Siegel S. Pregnancy and delivery in a patient with cystic fibrosis of the pancreas. Obstet Gynecol 1960; 16: 438–440. [Google Scholar]
- 10.Grand RJ, Talamo RC, Di Sant' Agnese PA, et al. Pregnancy in cystic fibrosis of the pancreas. JAMA 1966; 195: 117–124. [PubMed] [Google Scholar]
- 11.Pugh RJ, Pickup JD. Cystic fibrosis in the Leeds region: incidence and life expectancy. Arch Dis Child 1967; 42: 544–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen LF, di Sant’Agnese PA, Friedlander J. Cystic fibrosis and pregnancy. A national survey. Lancet 1980; 2: 842–844. [DOI] [PubMed] [Google Scholar]
- 13.Matson JA, Capen CV. Pregnancy in the cystic fibrosis patients. An update. J Reprod Med 1982; 27: 373–375. [PubMed] [Google Scholar]
- 14.Geddes DM. Cystic fibrosis and pregnancy. J R Soc Med 1992; 85: 36–37. [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng EY, Goss CH, McKone EF, et al. Aggressive prenatal care results in successful fetal outcomes in CF women. J Cyst Fibros 2006; 5: 85–91. [DOI] [PubMed] [Google Scholar]
- 16.Pittard WB, 3rd, Sorensen RU, Schnatz PT. Pregnancy outcome in mothers with cystic fibrosis: Normal neonatal immune responses. South Med J 1987; 80: 344–346. [DOI] [PubMed] [Google Scholar]
- 17.Edenborough FP, Mackenzie WE, Stableforth DE. The outcome of 72 pregnancies in 55 women with cystic fibrosis in the United Kingdom 1977–1996. BJOG 2000; 107: 254–261. [DOI] [PubMed] [Google Scholar]
- 18.Gilljam M, Antoniou M, Shin J, et al. Pregnancy in cystic fibrosis. Fetal and maternal outcome. Chest 2000; 118: 85–91. [DOI] [PubMed] [Google Scholar]
- 19.Boyd JM, Mehta A, Murphy DJ. Fertility and pregnancy outcomes in men and women with cystic fibrosis in the United Kingdom. Hum Reprod 2004; 19: 2238–2243. [DOI] [PubMed] [Google Scholar]
- 20.Gillet D, de Braekeleer M, Bellis G, et al. Cystic fibrosis and pregnancy. Report from French data (1980–1999). BJOG 2002; 109: 912–918. [DOI] [PubMed] [Google Scholar]
- 21.Barak A, Dulitzki M, Efrati O, et al. Pregnancies and outcome in women with cystic fibrosis. Isr Med Assoc J 2005; 7: 95–98. [PubMed] [Google Scholar]
- 22.Odegaard I, Stray-Pedersen B, Hallberg K, et al. Maternal and fetal morbidity in pregnancies of Norwegian and Swedish women with cystic fibrosis. Acta Obstet Gynecol Scand 2002; 81: 698–705. [PubMed] [Google Scholar]
- 23.Lau EM, Barnes DJ, Moriarty C, et al. Pregnancy outcomes in the current era of cystic fibrosis care: A 15-year experience. Aust N Z J Obstet Gynaecol 2011; 51: 220–224. [DOI] [PubMed] [Google Scholar]
- 24.Whitty JE. Cystic fibrosis in pregnancy. Clin Obstet Gynecol 2010; 53: 369–376. [DOI] [PubMed] [Google Scholar]
- 25.Neeraj, Sodhi C, Pramod J, Singh J, et al. Effect of advanced uncomplicated pregnancy on pulmonary function parameters of North Indian subjects. Indian J Physiol Pharmacol 2010; 54: 69–72. [PubMed] [Google Scholar]
- 26.Hirnle L, Lysenko L, Gerber H, et al. Respiratory function in pregnant women. Adv Exp Med Biol 2013; 788: 153–160. [DOI] [PubMed] [Google Scholar]
- 27.Bright-Thomas RJ, Ray SG, Webb AK. Pulmonary artery pressure in cystic fibrosis adults: Characteristics, clinical correlates and long-term follow up. J Cyst Fibros 2012; 11: 532–538. [DOI] [PubMed] [Google Scholar]
- 28.Thorpe-Beeston JG, Madge S, Gyi K, et al. The outcome of pregnancies in women with cystic fibrosis—Single centre experience 1998–2011. BJOG 2013; 120: 354–361. [DOI] [PubMed] [Google Scholar]
- 29.Burden C, Ion R, Chung Y, et al. Current pregnancy outcomes in women with cystic fibrosis. Eur J Obstet Gynecol Reprod Biol 2012; 164: 142–145. [DOI] [PubMed] [Google Scholar]
- 30.Edenborough FP, Stableforth DE, Webb AK, et al. Outcome of pregnancy in women with cystic fibrosis. Thorax 1995; 50: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goss CH, Rubenfeld GD, Otto K, et al. The effect of pregnancy on survival in women with cystic fibrosis. Chest 2003; 124: 1460–1468. [DOI] [PubMed] [Google Scholar]
- 32.McMullen AH, Pasta DJ, Frederick PD, et al. Impact of pregnancy on women with cystic fibrosis. Chest 2006; 129: 706–711. [DOI] [PubMed] [Google Scholar]
- 33.Schechter MS, Quittner AL, Konstan MW, et al. Long-term effects of pregnancy and motherhood on disease outcomes of women with cystic fibrosis. Ann Am Thorac Soc 2013; 10: 213–219. [DOI] [PubMed] [Google Scholar]
- 34.Ahluwalia M, Hoag JB, Hadeh A, et al. Cystic fibrosis and pregnancy in the modern era: A case control study. J Cyst Fibros 2014; 13: 69–73. [DOI] [PubMed]
- 35.Simcox AM, Hewison J, Duff AJ, et al. Decision-making about pregnancy for women with cystic fibrosis. Brit J Health Psychol 2009; 14: 323–342. [DOI] [PubMed] [Google Scholar]
- 36.Sueblinvong V, Whittaker LA. Fertility and pregnancy: Common concerns of the aging cystic fibrosis population. Clin Chest Med 2007; 28: 433–443. [DOI] [PubMed] [Google Scholar]
- 37.Gage LA. What deficits in sexual and reproductive health knowledge exist among women with cystic fibrosis? A systematic review. Health Soc Work 2012; 37: 29–36. [DOI] [PubMed] [Google Scholar]
- 38.Tuchman LK, Kalogiros ID, Forke CM, et al. Reproductive knowledge and preferences of adolescents and adults with cystic fibrosis: A web-based assessment. Int J Sex Health 2010; 22: 72–83. [Google Scholar]
- 39.Korzeniewska A, Grzelewski T, Jerzynska J, et al. Sexual and reproductive health knowledge in cystic fibrosis female patients and their parents. J Sex Med 2009; 6: 770–776. [DOI] [PubMed] [Google Scholar]
- 40.Edenborough FP, Borgo G, Knoop C, et al. Guidelines for the management of pregnancy in women with cystic fibrosis. J Cyst Fibros 2008; 7: S2–32. [DOI] [PubMed] [Google Scholar]
- 41.Fair A, Griffiths K, Osman L. Attitudes to fertility issues among adults with cystic fibrosis in Scotland. Thorax 2000; 55: 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCallum TJ, Milunsky JM, Cunningham DL, et al. Fertility in men with cystic fibrosis: An update on current surgical practices and outcomes. Chest 2000; 118: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 43.Rodgers HC, Knox AJ, Toplis PJ, et al. Successful pregnancy and birth after IVF in a woman with cystic fibrosis. Hum Reprod 2000; 15: 2152–2153. [DOI] [PubMed] [Google Scholar]
- 44.Sawyer SM, Phelan PD, Bowes G. Reproductive health in young women with cystic fibrosis: Knowledge, behavior and attitudes. J Adolesc Health 1995; 17: 46–50. [DOI] [PubMed] [Google Scholar]
- 45.Plant BJ, Goss CH, Tonelli MR, et al. Contraceptive practices in women with cystic fibrosis. J Cyst Fibros 2008; 7: 412–414. [DOI] [PubMed] [Google Scholar]
- 46.McEwan FA, Hodson ME, Simmonds NJ. The prevalence of “risky behaviour” in adults with cystic fibrosis. J Cyst Fibros 2012; 11: 56–58. [DOI] [PubMed] [Google Scholar]
- 47.Gatiss S, Mansour D, Doe S, et al. Provision of contraception services and advice for women with cystic fibrosis. J Fam Plann Reprod Health Care 2009; 35: 157–160. [DOI] [PubMed] [Google Scholar]
- 48.Orme M, Back DJ. Oral contraceptive steroids—pharmacological issues of interest to the prescribing physician. Adv Contracept 1991; 7: 325–331. [DOI] [PubMed] [Google Scholar]
- 49.Stead RJ, Grimmer SF, Rogers SM, et al. Pharmacokinetics of contraceptive steroids in patients with cystic fibrosis. Thorax 1987; 42: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thorpe-Beeston JG. Contraception and pregnancy in cystic fibrosis. J R Soc Med 2009; 102 Suppl): S3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The Clinical and Functional Translation of CFTR (CFTR2). CF Foundation, 2013.
- 52.Maxwell SJ, Kyne G, Molster C, et al. Perceptions of population cystic fibrosis prenatal and preconception carrier screening among individuals with cystic fibrosis and their family members. Genet Test Mol Biomarkers 2011; 15: 159–164. [DOI] [PubMed] [Google Scholar]
- 53.Ioannou L, Massie J, Lewis S, et al. ‘No thanks’—reasons why pregnant women declined an offer of cystic fibrosis carrier screening. J Commun Genet 2014; 5: 109–117. [DOI] [PMC free article] [PubMed]
- 54.Tonelli MR, Aitken ML. Pregnancy in cystic fibrosis. Curr Opin Pulm Med 2007; 13: 537–540. [DOI] [PubMed] [Google Scholar]
- 55.Hilman BC, Aitken ML, Constantinescu M. Pregnancy in patients with cystic fibrosis. Clin Obstet Gynecol 1996; 39: 70–86. [DOI] [PubMed] [Google Scholar]
- 56.Larsen JW., Jr Cystic fibrosis and pregnancy. Obstet Gynecol 1972; 39: 880–883. [PubMed] [Google Scholar]
- 57.Kotloff RM, FitzSimmons SC, Fiel SB. Fertility and pregnancy in patients with cystic fibrosis. Clin Chest Med 1992; 13: 623–635. [PubMed] [Google Scholar]
- 58.Rosengarten D, Blieden LC, Kramer MR. Pregnancy outcomes in pulmonary arterial hypertension in the modern management era. Eur Respir J 2012; 40: 1304–1305. [DOI] [PubMed] [Google Scholar]
- 59.Bose D, Yentis SM, Fauvel NJ. Caesarean section in a parturient with respiratory failure caused by cystic fibrosis. Anaesthesia 1997; 52: 578–582. [DOI] [PubMed] [Google Scholar]
- 60.Frangolias DD, Nakielna EM, Wilcox PG. Pregnancy and cystic fibrosis: A case-controlled study. Chest 1997; 111: 963–969. [DOI] [PubMed] [Google Scholar]
- 61.Tanser SJ, Hodson ME, Geddes DM. Case reports of death during pregnancy in patients with cystic fibrosis—Three out of four patients were colonized with Burkholderia cepacia. Respir Med 2000; 94: 1004–1006. [DOI] [PubMed] [Google Scholar]
- 62.Michel SH, Mueller DH. Nutrition for pregnant women who have cystic fibrosis. J Acad Nutr Diet 2012; 112: 1943–1948. [DOI] [PubMed] [Google Scholar]
- 63.Plant BJ, Goss CH, Plant WD, et al. Management of comorbidities in older patients with cystic fibrosis. Lancet Respir Med 2013; 1: 164–174. [DOI] [PubMed] [Google Scholar]
- 64.Hardin DS, Rice J, Cohen RC, et al. The metabolic effects of pregnancy in cystic fibrosis. [Erratum appears in Obstet Gynecol 2005; 106: 869]. Obstet Gynecol 2005; 106: 367–375. [DOI] [PubMed]
- 65.Cousins L. Etiology and prevention of congenital anomalies among infants of overt diabetic women. Clin Obstet Gynecol 1991; 34: 481–493. [PubMed] [Google Scholar]
- 66.Catalano PM, McIntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012; 35: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stuebe AM, Landon MB, Lai Y, et al. Maternal BMI, glucose tolerance, and adverse pregnancy outcomes. Am J Obstet Gynecol 2012; 207: 62 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 69.Suhonen L, Hiilesmaa V, Teramo K. Glycaemic control during early pregnancy and fetal malformations in women with type I diabetes mellitus. Diabetologia 2000; 43: 79–82. [DOI] [PubMed] [Google Scholar]
- 70.Loebstein R, Addis A, Ho E, et al. Pregnancy outcome following gestational exposure to fluoroquinolones: A multicenter prospective controlled Study. Antimicrob Agents Chemother 1998; 42: 1336–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.MIMS Online [Internet]. St Leonards: UBM Medica, 2012, p.1.
- 72.Döring G, Flume P, Heijerman H, et al. Treatment of lung infection in patients with cystic fibrosis: Current and future strategies. J Cyst Fibros 2012; 11: 461–479. [DOI] [PubMed] [Google Scholar]
- 73.Prickett M, Jain M. Efficacy of β-lactams as single agents for treating severe pulmonary exacerbations during pregnancy in cystic fibrosis. Am J Respir Crit Care Med 2012; 186: 1310–1311. [DOI] [PubMed] [Google Scholar]
- 74.Geller DE, Pitlick WH, Nardella PA, et al. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest 2002; 122: 219–226. [DOI] [PubMed] [Google Scholar]
- 75.Sheikh SI, Ryan-Wenger NA, McCoy KS. Outcomes of surgical management of severe GERD in patients with cystic fibrosis. Pediatr Pulmonol 2013; 48: 556–562. [DOI] [PubMed] [Google Scholar]
- 76.Palm K, Sawicki G, Rosen R. The impact of reflux burden on Pseudomonas positivity in children with cystic fibrosis. Pediatr Pulmonol 2012; 47: 582–587. [DOI] [PubMed] [Google Scholar]
- 77.Pauwels A, Decraene A, Blondeau K, et al. Bile acids in sputum and increased airway inflammation in patients with cystic fibrosis. Chest 2012; 141: 1568–1574. [DOI] [PubMed] [Google Scholar]
- 78.Gill SK, O’Brien L, Einarson TR, et al. The safety of proton pump inhibitors (PPIs) in pregnancy: A meta-analysis. Am J Gastroenterol 2009; 104: 1541–1545; quiz 0, 6. [DOI] [PubMed] [Google Scholar]
- 79.Ackermans MM, Zhou H, Carels CE, et al. Vitamin A and clefting: Putative biological mechanisms. Nutr Rev 2011; 69: 613–624. [DOI] [PubMed] [Google Scholar]
- 80.van den Broek N, Dou L, Othman M, et al. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev 2010; 10: CD008666–CD008666. [DOI] [PubMed] [Google Scholar]
- 81.Thorne-Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr Perinat Epidemiol 2012; 26: 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aghajafari F, Nagulesapillai T, Ronksley PE, et al. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: Systematic review and meta-analysis of observational studies. BMJ 2013; 346: f1169–f1169. [DOI] [PubMed] [Google Scholar]
- 83.Smith DJ, Anderson GJ, Lamont IL, et al. Accurate assessment of systemic iron status in cystic fibrosis will avoid the hazards of inappropriate iron supplementation. J Cyst Fibros 2013; 12: 303–304. [DOI] [PubMed] [Google Scholar]
- 84.Hoo ZH, Wildman MJ. Intravenous iron among cystic fibrosis patients. J Cyst Fibros 2012; 11: 560–562. [DOI] [PubMed] [Google Scholar]
- 85.Michel SH, Mueller DH. Impact of lactation on women with cystic fibrosis and their infants: A review of five cases. J Am Diet Assoc 1994; 94: 159–165. [DOI] [PubMed] [Google Scholar]
- 86.Moran A, Brunzell C, Cohen RC, et al. Clinical care guidelines for cystic fibrosis-related diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010; 33: 2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009; 361: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005; 352: 2477–2486. [DOI] [PubMed] [Google Scholar]
- 89.Corey M, McLaughlin FJ, Williams M, et al. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol 1988; 41: 583–591. [DOI] [PubMed] [Google Scholar]
- 90.IDF Clinical Guidelines Task Force. Global guideline on pregnancy and diabetes. Brussels: International Diabetes Federation, 2009.
- 91.Cornacchia M, Zenorini A, Perobelli S, et al. Prevalence of urinary incontinence in women with cystic fibrosis. BJU Int 2001; 88: 44–48. [DOI] [PubMed]
- 92.Boyle R, Hay-Smith EJ, Cody JD, et al. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev 2012; 10: CD007471. [DOI] [PubMed]
- 93.Bell SC and Robinson PJ. Introduction – facilities, staffing and services. In: Fitzgerald DA (ed.) Cystic fibrosis standards of care. Australia, Sydney: Cystic Fibrosis Australia, 2008.
- 94.Cystic Fibrosis Trust. Standards for the Clinical Care of Children and Adults with Cystic Fibrosis in the UK. 2nd ed. London: Cystic Fibrosis Trust, 2011.
- 95.Kerem E, Conway S, Elborn S, et al. Standards of care for patients with cystic fibrosis: A European consensus. J Cyst Fibros 2005; 4: 7–26. [DOI] [PubMed]
- 96.Yusen RD, Christie JD, Edwards LB, et al. The registry of the international society for heart and lung transplantation: Thirtieth adult lung and heart-lung transplant report-2013; focus theme: Age. J Heart Lung Transplant 2013; 32: 965–978. [DOI] [PubMed] [Google Scholar]
- 97.Gyi KM, Hodson ME, Yacoub MY. Pregnancy in cystic fibrosis lung transplant recipients: Case series and review. J Cyst Fibros 2006; 5: 171–175. [DOI] [PubMed] [Google Scholar]
- 98.Armenti VT, Radomski JS, Moritz MJ, et al. Report from the National Transplantation Pregnancy Registry (NTPR): Outcomes of pregnancy after transplantation. Clin Transpl 2004, pp. 103–114. [PubMed] [Google Scholar]
- 99.Zurbano F, Lopez F, Fornet I, et al. Maternity and lung transplantation: Cases in Spain. Arch Bronconeumol 2012; 48: 379–381. [DOI] [PubMed] [Google Scholar]
- 100.Shaner J, Coscia LA, Constantinescu S, et al. Pregnancy after lung transplant. Prog Transplant 2012; 22: 134–140. [DOI] [PubMed] [Google Scholar]
- 101.Dodge JA, Lewis PA, Stanton M, et al. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J 2007; 29: 522–526. [DOI] [PubMed] [Google Scholar]
- 102.Sermet-Gaudelus I, de Blic J, LeBourgeois M, et al. Potentiating and correcting mutant CFTR in patients with cystic fibrosis. In: Mall MA, Elborn JS. (eds). ERS cystic fibrosis monograph, Sheffield: European Respiratory Society, 2014, pp. 129–149. [Google Scholar]
- 103.Caci E, Galietta JV. Correcting the basic ion transport defects in cystic fibrosis. In: Mall MA, Elborn JS. (eds). ERS cystic fibrosis monograph, Sheffield: European Respiratory Society, 2014, pp. 116–128. [Google Scholar]
- 104.Griesenbach U, Featherstone RF, Alton EWFW. New horizons for cystic fibrosis gene and cell therapy. In: Mall MA, Elborn JS. (eds). ERS cystic fibrosis monograph, Sheffield: European Respiratory Society, 2014, pp. 150–168. [Google Scholar]
- 105.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bell SC, De Boeck K and Amaral MD. New pharmacological approaches for cystic fibrosis: Promises, progress, pitfalls. Pharmacol Ther. Epub ahead of print 14 June 2014. DOI: 10.1016/j.pharmthera.2014.06.005. [DOI] [PubMed]