Abstract
Due to advances in paediatric congenital heart surgery, there are a growing number of women with congenital heart disease (CHD) reaching childbearing age. Pregnancy, however, is associated with haemodynamic stresses which can result in cardiac decompensation in women with CHD. Many women with CHD are aware of their cardiac condition prior to pregnancy, and preconception counselling is an important aspect of their care. Preconception counselling allows women to make informed pregnancy decisions, provides an opportunity for modifications of teratogenic medications and, when necessary, repair of cardiac lesions prior to pregnancy. Less commonly, the haemodynamic changes of pregnancy unmask a previously unrecognised heart lesion. In general, pregnancy outcomes are favourable for women with CHD, but there are some cardiac lesions that carry high risk for both the mother and the baby, and this group of women require care by an experienced multidisciplinary team. This review discusses preconception counselling including contraception, an approach to risk stratification and management recommendations in women with some common CHDs.
Keywords: Pregnancy, congenital heart disease, preconception counselling, heart disease
Preconception considerations in women with congenital heart disease
Cardiovascular changes during pregnancy
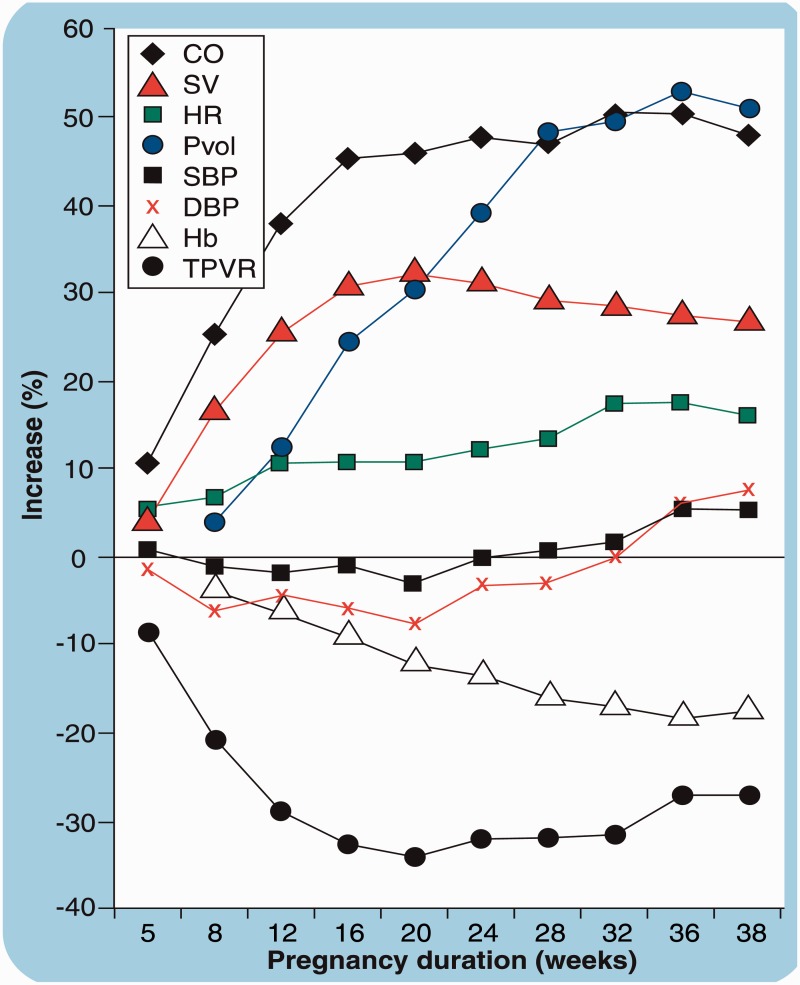
Pregnancy results in significant maternal cardiovascular changes (Figure 1)1 most of which begin early in the first trimester. There is approximately a 40% increase in blood volume, a 30% decrease in peripheral vascular resistance and, later in pregnancy, a 10–20% increase in heart rate. These changes contribute to the 25–40% increase in cardiac output (CO) that occurs over the course of pregnancy. CO increases by a further 50% at the time of labour secondary to the catecholaminergic response to pain and anxiety and auto-transfusion from uterine contractions. Following delivery, there is a rapid (within hours) and significant fall in CO, although full resolution of all haemodynamic changes can take as long as six months. These changes in plasma volume and CO during and after pregnancy contribute to the increased risk of cardiac complications in women with pre-existing heart disease. In addition to haemodynamic changes, there is an increase in thrombotic tendencies during pregnancy, which can contribute to thromboembolic complications (TEC) in women with congenital heart disease (CHD).
Figure 1.
Haemodynamic changes during pregnancy (from Karamermer et al.1).
CO: cardiac output; SV: stroke volume; HR: heart rate; Pvol: plasma volume; SBP: systolic blood pressure; DBP: diastolic blood pressure; Hb: haemoglobin; TPVR: total peripheral vascular resistance.
Preconception counselling
Preconception counselling is an important aspect of cardiac care for women with CHD and should begin early, ideally in adolescence, by cardiologists and maternal–fetal medicine specialists with experience in pregnancy and CHD. Despite surgical repair, many women with CHD will have residua and sequelae which can have important implications for pregnancy. Women who have not had regular cardiac care prior to pregnancy should be re-assessed by a cardiologist, in early pregnancy.
Counselling should include general recommendations for a healthy pregnancy such as weight control, cessation of smoking and periconceptional folic acid supplementation. Specific cardiac recommendations include education on the maternal and fetal risks, contraception advice, pre-pregnancy optimisation of blood pressure, modification of fetotoxic medications, interventions to optimise pregnancy outcomes and, when appropriate, the potential late effect of pregnancy on the heart as well as maternal life expectancy.2–5 Counselling should also include a detailed obstetric history, including an assessment of the risk of complications such as pre-eclampsia that might impact on cardiovascular function. Pre-eclampsia may cause an increase in afterload and potential deterioration in cardiac function.6 There may be persistent cardiovascular abnormalities following pregnancy.7,8 These changes can be detrimental, specifically in the setting of structural heart disease.
Assessment of pregnancy risks
Assessment of pregnancy risk should include a detailed cardiac, surgical and obstetric history, a physical examination, documentation of oxygen saturations, a 12-lead electrocardiogram and a transthoracic echocardiogram. Maternal functional status is an important determinant of outcome and should be documented. Exercise stress testing provides an objective measure of functional capacity and can assess blood pressure response to exercise in women with aortic stenosis (AS). Chronotrophic incompetence during stress testing predicts pregnancy complications.9 Stress echocardiography is helpful to assess ischaemia in women with coronary anomalies/disease and to assess ventricular reserve in women with cardiomyopathies or valve lesions. Magnetic resonance imaging (MRI) is useful to define complex congenital anatomy, assess right ventricular size and function and visualise the aorta.
There are a number of risk assessment tools to predict maternal cardiac complications during pregnancy including: the WHO classification10 (Table 1), the CARPREG risk score11 (Table 2) and the ZAHARA risk score12 (Table 3). The CARPREG risk score assigns one point to each of four predictors of adverse maternal cardiac events. The risk of a cardiac event in pregnancy increases from 5% for a woman with a score of 0 to 27% with a score of 1 and 75% when the score is ≥2.11 The ZAHARA risk score incorporates eight weighted clinical predictors to quantitate pregnancy risk on a scale of 0–13.12 The risk of cardiac complications increases from 2.9% with a score of 0–0.50 to 70% with a score > 3.51. In addition to these general risk scores, it is also important to consider lesion-specific risks as discussed below.
Table 1.
Maternal cardiovascular risk based on World Health Organization (WHO) classifications (from Thorne et al.10).
| Conditions in which pregnancy risk is WHO class I: risk not significantly higher than the general population • Uncomplicated small or mild pulmonary stenosis, patent ductus arteriosus or mitral valve prolapse • Successfully repaired simple lesions (atrial or ventricular septal defect, patent ductus arteriosus, anomalous pulmonary venous connection) • Isolated atrial and ventricular ectopic beats |
| Conditions in which pregnancy risk is WHO class II (low to moderate risk compared with the general population) – III (significantly higher risk compared with the general population) depending on the individual • Mild left ventricular impairment • Hypertrophic cardiomyopathy • Native or tissue valvular heart disease not considered WHO class I or WHO class IV • Marfan syndrome without aortic dilatation (aortic size < 40 mm) • Aorta < 45 mm in association with bicuspid aortic valve disease • Repaired coarctation of the aorta |
| Conditions in which pregnancy risk is WHO class III: significantly increased risk of maternal morbidity and mortality compared with the general population • Mechanical heart valve • Systemic right ventricle • Fontan circulation • Unrepaired cyanotic heart disease • Other complex congenital heart disease • Aortic dilatation 40–45 mm in Marfan syndrome • Aortic dilatation 45–50 mm in bicuspid aortic valve disease |
| Condititions in which pregnancy risk is WHO class IV: extremely high risk of maternal morbidity and mortality; patients should be counselled against pregnancy • Pulmonary arterial hypertension from any cause • Severe systemic ventricular dysfunction from any cause (ejection fraction < 30%, New York Heart Association class II–IV symptoms) • Previous peripartum cardiomyopathy with any residual impariment of systemic ventricular function • Severe mitral stenosis • Severe symptomatic aortic stenosis • Marfan syndrome with dilated aorta >45 mm • Bisucspid aortic valve disease with dilated aorta >50 mm • Native severe coarctation of the aorta |
Table 2.
The CARPREG risk-predictor score.11
| Predictors | Points |
|---|---|
| • Previous cardiac event: heart failure, transient ischaemic attack, cerebrovascular accident or arrhythmia | 1 |
| • New York Heart Association class > II or cyanosis | 1 |
| • Left heart obstruction: Mitral valve area < 2 cm2 or aortic valve area < 1.5 cm2 or peak left ventricular outflow tract obstruction >30 mmHg on echocardiogram | 1 |
| • Reduced left ventricular function (ejection fraction < 40%) | 1 |
| Risk score | Frequency of maternal cardiac complications |
|---|---|
| 0 | 5% |
| 1 | 27% |
| ≥2 | 75% |
Table 3.
The ZAHARA risk score.12
| Predictors | Points |
|---|---|
| 1. History of arrhythmias | 1.50 |
| 2. Cardiac medication before pregnancy | 1.50 |
| 3. New York Heart Association class prior to pregnancy ≥ II | 0.75 |
| 4. Left heart obstruction (peak gradient > 50 mmHg or aortic valve area <1.0 cm2) | 2.50 |
| 5. Systemic atrioventricular valve regurgitation (moderate/severe) | 0.75 |
| 6. Pulmonary atrioventricular valve regurgitation (moderate/severe) | 0.75 |
| 7. Mechanical valve prosthesis | 4.25 |
| 8. Cyanotic heart disease (corrected/ uncorrected) | 1.00 |
| Total number of points: 0–13 points | |
| Risk score |
Frequency of maternal cardiac complication (%) |
| 0–0.50 | 2.9 |
| 0.51–1.50 | 7.5 |
| 1.51–2.50 | 17.5 |
| 2.51–3.50 | 43.1 |
| >3.51 | 70.0 |
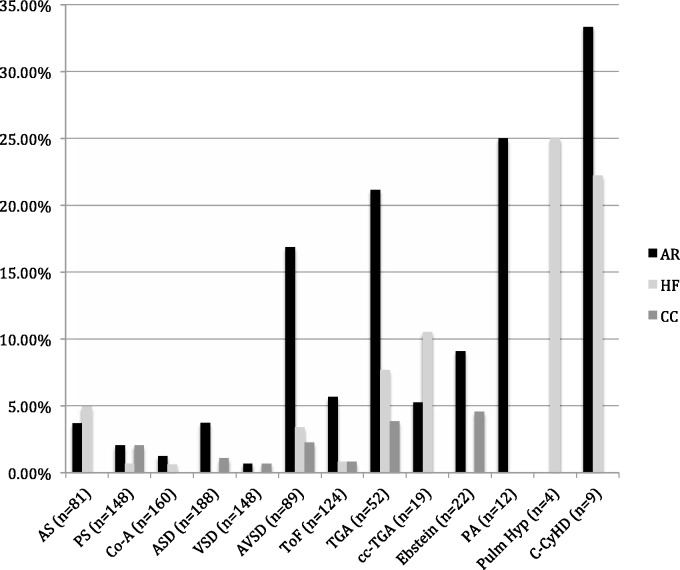
Maternal health is an important determinant of fetal and neonatal health. Fetal mortality (1.7%) and perinatal mortality (2.3%) are still rare, but increased above the baseline rate of 1%.13 Fetal and perinatal morbidity is more common, ranging between 16% and 18% and primarily includes low-birth-weight babies (8%), prematurity (16%) and the complications of prematurity.11,13 The incidence of fetal and neonatal complications varies depending on the cardiac lesion (Figure 2). Risk of transmission of CHD to offspring is variable and dependent on the maternal or paternal cardiac condition. For CHD not associated with a genetic syndrome, transmission to offspring is in the range of 3–5% with higher rates in left-sided outflow track lesions such as AS (10%) as shown in Table 4.14 There is also a risk of congenital anomalies in women with diabetes.
Figure 2.
Lesion-specific risk of adverse cardiac events (created from data presented in Drenthen et al.12)
AS: aortic stenosis; PS: pulmonary stenosis; Co-A: aortic coarctation; ASD: atrial septal defect; VSD: ventricular septal defect; AVSD: atrioventricular septal defect; TOF: tetralogy of Fallot; TGA: transposition of the great arteries; cc-TGA: congenitally corrected transposition of great arteries; Ebstein: Ebstein anomaly; PA: pulmonary atresia; Pulm Hyp: pulmonary hypertension or Eisenmenger syndrome; C-CyHD: complex cyanotic heart disease; AR: arrhythmia; HF: heart failure; CC: other cardiac complications.
Table 4.
Lesion-specific risk for eight CHD in offspring given one affected parent (%).14
| Defect | Father affected | Mother affected |
|---|---|---|
| Atrial septal defect | 1.5 | 4–4.5 |
| Ventricular septal defect | 2 | 6–10 |
| Patent ductus arteriosus | 2.5 | 3.5–4 |
| Atrioventricular canal | 1 | 14 |
| Pulmonary stenosis | 2 | 4–6.5 |
| Aortic stenosis | 3 | 13–18 |
| Coarctation of the aorta | 2 | 4 |
| Tetralogy of Fallot | 1.5 | 2.5 |
CHD: congenital heart disease.
Lesion-specific risks
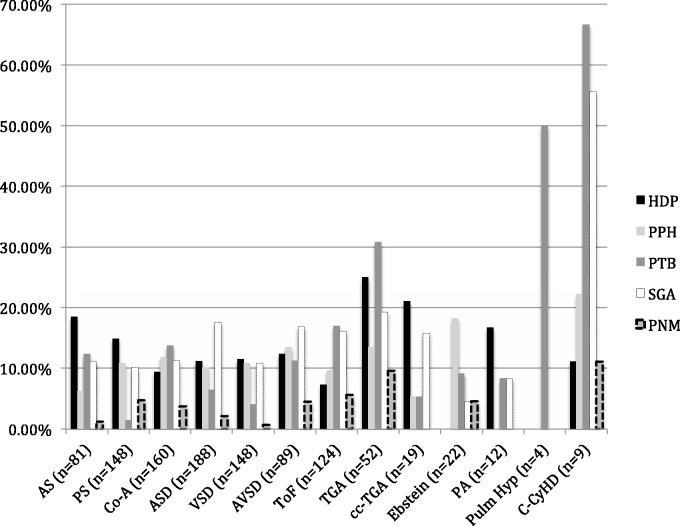
In addition to general pregnancy risks discussed above, lesion-specific risks need to be considered. Figures 2 and 3 show the rates of adverse cardiac and perinatal outcomes in women with CHD.12
Figure 3.
Lesion-specific risks of adverse pregnancy and perinatal events (created from data presented in Drenthen et al.12).
AS: aortic stenosis; PS: pulmonary stenosis; Co-A: aortic coarctation; ASD: atrial septal defect; VSD: ventricular septal defect; AVSD: atrioventricular septal defect; TOF: tetralogy of Fallot; TGA: transposition of the great arteries; cc-TGA: congenitally corrected transposition of great arteries; Ebstein: Ebstein anomaly; PA: pulmonary atresia; Pulm Hyp: pulmonary hypertension or Eisenmenger syndrome; C-CyHD: complex cyanotic heart disease; HDP: hypertensive disorders of pregnancy (including pregnancy-induced hypertension, pre-eclampsia, eclampsia and HELLP syndrome); PPH: postpartum haemorrhage; PTB: preterm birth (under 37 weeks); SGA: small for gestational age infants; PNM: perinatal (fetal and neonatal) mortality.
Valvular heart disease
Congenital AS
Most congenital AS is secondary to bicuspid aortic valve disease. In general, mild or moderate AS is well tolerated during pregnancy. Women with severe AS who are symptomatic should have an intervention prior to pregnancy. Asymptomatic women with severe AS may become symptomatic during pregnancy and require careful preconception risk stratification. Complications such as heart failure and arrhythmias occur in approximately 10% of pregnancies in women with AS. The risk of complications is dependent on the functional status of the woman, the severity of obstruction and the systolic function of the left ventricle.12 Maternal mortality is rare (<1%). If women become symptomatic during pregnancy and medical management fails, percutaneous balloon valvuloplasty can be performed in women with suitable valve anatomy.
Aortic disease
Coarctation of the aorta
Coarctation of the aorta typically occurs distal to the origin of the left subclavian artery. It can be associated with other congenital defects, the most common of which is bicuspid aortic valve. Most pregnant women with coarctation have undergone a surgical repair. There are a number of possible surgical repairs including the end-to-end anastomosis, subclavian flap repair, interpositional grafts and stenting. Assessment should focus on the identification of residual lesions that can impact pregnancy risk including persistent hypertension, re-coarctation, aneurysms at the site of the repair, aortopathy at other sites and aortic valve disease. Women with anatomically good repairs and no residual hypertension generally do well. The most common pregnancy complication is hypertension which is seen in approximately 30% of pregnancies; aortic dissection is rare.15
Hereditary aortopathies
These conditions are discussed elsewhere.16
Intracardiac shunts
Secundum atrial septal defects
The most common type of atrial septal defect (ASD) is the secundum ASD. Secundum ASD can be associated with right ventricular dilation, right ventricular dysfunction and rarely, pulmonary hypertension. Isolated secundum ASD, repaired or unrepaired, are well tolerated during pregnancy unless pulmonary hypertension is present. These are considered low-risk lesions with low rates of cardiovascular complications (1.3%).12 Atrioventricular septal defects are more complex lesions with a higher pregnancy risk.12,13
Ventricular septal defects
The most common types of ventricular septal defects (VSD) are perimembranous and muscular VSD. Significant VSD are associated with left ventricular volume overload, left ventricular dysfunction and pulmonary hypertension. Small restrictive VSD are considered low-risk lesions in the absence of pulmonary hypertension. Large non-restrictive VSD are associated with pulmonary hypertension and significant risk during pregnancy. VSD associated with Eisenmenger syndrome is discussed separately. Cardiovascular events are rare in women with simple VSD (1.2%) (Figures 2).12,13
Complex CHD
Tetralogy of Fallot
Tetralogy of Fallot (TOF) is a complex lesion consisting of a VSD, an overriding aorta, subpulmonary stenosis and right ventricular hypertrophy. Almost all pregnant women with TOF will have undergone an intracardiac repair in childhood. Although pulmonary regurgitation and right ventricular volume overload are common after TOF repair, in general, this group of women does well during pregnancy.17,18 Pregnancy outcomes relate to the severity of pulmonary regurgitation and the size and systolic function of ventricles. By comparison, women with unrepaired or palliated TOF have chronic cyanotic heart disease, with much higher rates of maternal cardiovascular complications (32%), although maternal mortality is low (1%).19 Recurrent miscarriages are common in women with cyanotic heart disease. Fetal complications are related to the maternal oxygen saturations. The chance of carrying a fetus to term is lowest if oxygen saturations are below 85%.20
Transposition of the great arteries
All women with complete transposition of the great arteries (TGA) will have undergone some form of surgical correction. Possible surgical repairs included Mustard/Senning operations, Jatene operations or Rastelli operations. The type of surgical correction is a major determinant of the associated late sequelae and hence the pregnancy risk. Women with Mustard/Senning repairs often have residual subaortic ventricular dysfunction and/or tricuspid valve regurgitation. These women are also at risk for developing arrhythmias. Preconception risk stratification is important in this group of women. Pregnancy may result in worsening of subaortic ventricular function or tricuspid valve regurgitation.21 Pregnancies in women with Jatene operations are becoming increasingly more common as this cohort of women reach childbearing age.22
Congenitally corrected TGA
ccTGA is defined by discordant atrioventricular and ventricular arterial connections. Congenitally corrected TGA (cc-TGA) can occur in isolation or in conjunction with other cardiac lesions such as VSD or pulmonary stenosis. Women with VSD and/or pulmonary stenosis will often have undergone surgical repair in childhood. Women with cc-TGA have a morphologic right subaortic ventricle. This is associated with variable degrees of subaortic ventricular dysfunction and atrioventricular valve regurgitation. In general, women with isolated or repaired cc-TGA do well if they have preserved subaortic ventricular function and no significant regurgitation or stenosis.23
Ebstein anomaly
Ebstein anomaly of the tricuspid valve is typically associated with tricuspid regurgitation, an abnormal ‘functional right ventricle’ and a dilated right atrium. Patients may also have multiple accessory pathways. Women of childbearing age with Ebstein anomaly may or may not have undergone a tricuspid valve repair. Women with unrepaired Ebstein anomaly will have variable degrees of tricuspid regurgitation and abnormalities of the ‘functional right ventricle’. These factors are important determinants of pregnancy outcome. Pregnancy is well tolerated provided that there is reasonable right ventricular size and systolic function, the absence of significant arrhythmias or cyanosis. The most common complications are atrial arrhythmias.23
Single ventricle physiology and the Fontan circulation
Single ventricle physiology refers to a variety of very complex congenital cardiac malformations where one dominant ventricle supports both the systemic and pulmonary circulations. The Fontan operation refers to a procedure for single ventricle physiology, where systemic venous blood is diverted into the lung, bypassing the subpulmonary ventricle. The operation has undergone a number of revisions since it was first described. Preconception risk stratification is very important in this group of women because some will be at very high risk for pregnancy complications because of the cardiac (heart failure, refractory arrhythmias) and non-cardiac (liver disease and protein losing enteropathy) sequelae of the Fontan physiology. The most common problems encountered during pregnancy are arrhythmias (25%) and systolic and diastolic heart failure (5%).24,25 Fetal complications are common and include premature birth (32%), intrauterine growth restriction (IUGR) (12%) and fetal death (4%).2,13
Eisenmenger syndrome
Eisenmenger syndrome refers to cardiac defects associated with high pulmonary vascular resistance, reversal or bi-directional shunt flow at the great vessel, ventricular or atrial level and cyanosis. Pregnancy in women with Eisenmenger syndrome is associated with a high maternal mortality; reported ranges have been between 28% and 50%.26,27 Small contemporary series have suggested that outcomes may be more optimistic;29 however, because of the high risk nature of this condition, pregnancy is discouraged.2 For women who elect to continue pregnancy, it is important that they have care by an experienced multidisciplinary team that includes a pulmonary hypertension specialist. There is growing experience using pulmonary vasodilators during pregnancy.27,28 The risks of Bosentan in pregnancy are undetermined and for this reason it is best avoided when possible. Eisenmenger syndrome is associated with significant risk to the fetus and neonate including recurrent miscarriage, IUGR, prematurity and death.
Contraception
All women should be educated about contraception options. Lesion-specific contraception is discussed elsewhere.10,29,30 Unfortunately, many women with CHD do not have adequate knowledge of safe contraception options.10,29–31 In brief, oestrogen-containing preparations are associated with thromboembolic (TE) risks and are contraindicated in women with cardiac conditions who are high risk for TE complications. This includes women with mechanical valves, Eisenmenger syndrome and Fontan circulation. Progesterone-only pills are safer for women with these conditions but have high failure rates and are therefore not suggested when prevention of pregnancy is crucial. Non-oral progesterone-only contraception such as progesterone implants and depot injections have very low failure rates and can be used in most conditions. Intrauterine devices are good choices for many women with CHD, but can be associated with vagal responses at the time of insertion, which is a potentially serious complication for women with pulmonary vascular disease or a Fontan circulation. Barrier methods have high failure rates and are not advised when pregnancy risk is high and prevention of pregnancy is important. Tubal sterilisation is a permanent, surgical method of contraception. The procedure carries a risk for some women with complex CHD such as those with Eisenmenger syndrome. Hysteroscopic intratubal stenting can be a safe alternative. Vasectomy may be an option for some couples, but it is important to consider the possibility that maternal life span may be shortened and that males may outlive their partners and consider families later.
Infertility and assisted reproductive technologies
If pregnancy is not advised on medical grounds, alternative options such as surrogacy or adoption may be considered. For women in whom pregnancy is considered safe, it is important to ensure that fertility medications and techniques are safe for the patient with CHD. Maternal and fetal risks are increased in women with CHD receiving assisted reproductive therapies.32 For women at risk for TEC, gonadotropins should be used with caution as they increase oestrogen levels, thereby potentially increasing this risk further. Adequate analgesia should be offered at the time of egg collection and cervical dilatation. For some women with high-risk cardiac conditions such as a Fontan circulation, complications of fertility therapy such as ovarian hyperstimulation syndrome could result in serious cardiac compromise and modifications in protocols may be required to prevent this complication. Multiple embryo transfers may not be appropriate for women with high-risk lesions.
Management of pregnant woman with CHD
The multidisciplinary team
While cardiovascular deaths overall are now the most common indirect cause of maternal mortality in developed countries, deaths secondary to CHD have decreased.33 The UK confidential enquiries into maternal deaths highlighted multidisciplinary team management with good communication as the key to reducing complications in women with CHD.33 The team should involve maternal–fetal medicine specialists, cardiologists with expertise in CHD and pregnancy, obstetric anaesthetists, haematologists, trained nursing staff and social workers. Antenatal, intrapartum and postpartum care plans should be individualised, clearly outlined and communicated promptly to all caregivers.
Antenatal care
Referral to a specialist centre for initial assessment is recommended, specifically in moderate to high-risk cases. Even those deemed as ‘low-risk’ can benefit from an assessment by an expert in pregnancy and CHD. This can help to relieve anxiety and prevent unnecessary treatment during pregnancy. Women at low risk for complications may continue routine antenatal care and delivery at a local hospital, but women with moderate or high-risk lesions should be followed in a centre with expertise in pregnancy and CHD. Follow-up is individualised based on the diagnosis, baseline cardiac function and development of complications.2
Cardiac investigations in pregnancy
Transthoracic echocardiography is routinely performed before and during pregnancy to assess cardiac structure and function. When necessary, transoesophageal echocardiography is also safe in pregnancy. Investigations that involve radiation exposure should not be withheld if indicated, following a discussion with the patient on risks and benefits of the test. In all cases, the radiation dose and duration of exposure should be minimised and the gravid uterus should be shielded. Cardiac MRI is only required when the diagnosis of complex heart disease or aortic pathology cannot be determined echocardiographically. MRI is considered safe during pregnancy. While interpreting blood tests, normal pregnancy reference values must be used. Troponin is not normally increased in pregnancy and is the diagnostic test of choice to detect myocardial injury. A normal B-type natriuretic peptide level can be helpful to exclude cardiac decompensation.34
Cardiac medications
The physiological changes of pregnancy affect drug absorption, action and metabolism. Fetal adverse effects are dependent on gestational age and whether the drug crosses the placenta. A summary of commonly used cardiac drugs and their potential fetal and neonatal effects can be found in the European Cardiology Society guidelines on the management of cardiovascular diseases during pregnancy.2 Cardiac medications that are often withheld during pregnancy include Angiotensin-converting-enzyme (ACE) inhibitors, angiotensin receptor blockers and statins. Use of anticoagulants, especially in women with mechanical valves, is complex and guidelines are available from the European Society of Cardiology and the American Heart Association/American College of Cardiology.2,35
Cardiac complications in pregnancy
The most common cardiac complications that occur in women with CHD during pregnancy are arrhythmias (atrial or ventricular tachyarrhythmias) and heart failure. Less common complications include infective endocarditis, aortic dissection and cardiac arrest. Management of these conditions should be individualised. In rare instances, when medical management fails, percutaneous therapy or cardiac surgery may be required during pregnancy. Cardiac surgery carries significant fetal and neonatal risks including a 20–33% risk of fetal loss.36
Intrapartum and postpartum care
With few exceptions, vaginal delivery is preferred over Caesarean delivery as it carries lower risks for both mother and fetus due to smaller shifts in blood volume, less haemorrhage, fewer clotting complications and fewer infections.2,37 Modifications that may help to diminish the cardiovascular stress at the time of labour include good pain management with early epidural analgesia and labouring in the left lateral position. For some women, such as those with a Fontan circulation, avoidance of excessive maternal expulsive effort by assisting the active second stage of labour may be helpful.38 Non-invasive monitoring such as oximetry and telemetry can be used to monitor women at risk for decompensation during labour and delivery. Invasive cardiac monitoring is rarely required. Oxytocin for the management of the third stage of labour is best given as a continuous slow IV infusion to avoid the risks of peripheral vasodilation, tachycardia and fluid retention that could be potentially life threatening in patients with fixed COs or left-sided obstructive lesions.39
Conclusions
Pregnancy is associated with varying degrees of risk in women with CHD. Women with simple lesions generally do very well, whereas other more complex lesions are associated with high maternal and fetal morbidity and mortality. The key to successful management of these pregnancies is comprehensive pre-pregnancy counselling, the appropriate use of contraception, optimisation of cardiac status prior to pregnancy, multidisciplinary team involvement, meticulous antenatal, intrapartum and postpartum planning and prompt attention to adverse events.
Acknowledgements
None
Declaration of conflicting interests
The authors have no conflict of interest.
Funding statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
Patient consent and ethical approval were not obtained, as this was a review paper.
Guarantor
The guaranteeing author is Dr Rohan D'Souza.
Contributorship
All authors contributed to the manuscript development and approved the final version of the manuscript for submission.
References
- 1.Karamermer Y, Roos-Hesselink JW. Pregnancy and adult congenital heart disease. Expert Rev Cardiovasc Ther 2007; 5: 859–869. [DOI] [PubMed] [Google Scholar]
- 2.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011; 32: 3147–3197. [DOI] [PubMed] [Google Scholar]
- 3.Silversides CK, Kiess M, Beauchesne L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan's syndrome. Can J Cardiol 2010; 26: e80–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balint OH, Siu SC, Mason J, et al. Cardiac outcomes after pregnancy in women with congenital heart disease. Heart 2010; 96: 1656–1661. [DOI] [PubMed] [Google Scholar]
- 5.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52: e143–e263. [DOI] [PubMed] [Google Scholar]
- 6.Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation 2014; 130: 703–714. [DOI] [PubMed] [Google Scholar]
- 7.Edlow AG, Srinivas SK, Elovitz MA. Investigating the risk of hypertension shortly after pregnancies complicated by preeclampsia. Am J Obstet Gynecol 2009; 200: e60–e62. [DOI] [PubMed] [Google Scholar]
- 8.Melchiorre K, Sutherland GR, Liberati M, et al. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 2011; 58: 709–715. [DOI] [PubMed] [Google Scholar]
- 9.Lui GK, Silversides CK, Khairy P, et al. Heart rate response during exercise and pregnancy outcome in women with congenital heart disease. Circulation 2011; 123: 242–248. [DOI] [PubMed] [Google Scholar]
- 10.Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart 2006; 92: 1520–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siu SC, Colman JM, Sorensen S, et al. Adverse neonatal and cardiac outcomes are more common in pregnant women with cardiac disease. Circulation 2002; 105: 2179–2184. [DOI] [PubMed] [Google Scholar]
- 12.Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J 2010; 31: 2124–2132. [DOI] [PubMed] [Google Scholar]
- 13.Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol 2007; 49: 2303–2311. [DOI] [PubMed] [Google Scholar]
- 14.Nora JJ, Nora AH. Maternal transmission of congenital heart diseases: new recurrence risk figures and the questions of cytoplasmic inheritance and vulnerability to teratogens. Am J Cardiol 1987; 59: 459–463. [DOI] [PubMed] [Google Scholar]
- 15.Beauchesne LM, Connolly HM, Ammash NM, et al. Coarctation of the aorta: outcome of pregnancy. J Am Coll Cardiol 2001; 38: 1728–1733. [DOI] [PubMed] [Google Scholar]
- 16.Stewart F. Marfan's syndrome and other aortopathies in pregnancy. Obstet Med 2013; 6: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldtman GR, Connolly HM, Grogan M, et al. Outcomes of pregnancy in women with tetralogy of Fallot. J Am Coll Cardiol 2004; 44: 174–180. [DOI] [PubMed] [Google Scholar]
- 18.Greutmann M, Von Klemperer K, Brooks R, et al. Pregnancy outcome in women with congenital heart disease and residual haemodynamic lesions of the right ventricular outflow tract. Eur Heart J 2010; 31: 1764–1770. [DOI] [PubMed] [Google Scholar]
- 19.Presbitero P, Somerville J, Stone S, et al. Pregnancy in cyanotic congenital heart disease. Outcome of mother and fetus. Circulation 1994; 89: 2673–2676. [DOI] [PubMed] [Google Scholar]
- 20.Thorne SA. Pregnancy in heart disease. Heart 2004; 90: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guedes A, Mercier LA, Leduc L, et al. Impact of pregnancy on the systemic right ventricle after a Mustard operation for transposition of the great arteries. J Am Coll Cardiol 2004; 44: 433–437. [DOI] [PubMed] [Google Scholar]
- 22.Tobler D, Fernandes SM, Wald RM, et al. Pregnancy outcomes in women with transposition of the great arteries and arterial switch operation. Am J Cardiol 2010; 106: 417–420. [DOI] [PubMed] [Google Scholar]
- 23.Connolly HM, Grogan M, Warnes CA. Pregnancy among women with congenitally corrected transposition of great arteries. J Am Coll Cardiol 1999; 33: 1692–1695. [DOI] [PubMed] [Google Scholar]
- 24.Canobbio MM, Mair DD, van der Velde M, et al. Pregnancy outcomes after the Fontan repair. J Am Coll Cardiol 1996; 28: 763–767. [DOI] [PubMed] [Google Scholar]
- 25.Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Pregnancy and delivery in women after Fontan palliation. Heart 2006; 92: 1290–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss BM, Zemp L, Seifert B, et al. Outcome of pulmonary vascular disease in pregnancy: a systematic overview from 1978 through 1996. J Am Coll Cardiol 1998; 31: 1650–1657. [DOI] [PubMed] [Google Scholar]
- 27.Bedard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur Heart J 2009; 30: 256–265. [DOI] [PubMed] [Google Scholar]
- 28.Kiely DG, Condliffe R, Webster V, et al. Improved survival in pregnancy and pulmonary hypertension using a multiprofessional approach. BJOG 2010; 117: 565–574. [DOI] [PubMed] [Google Scholar]
- 29.Silversides CK, Sermer M, Siu SC. Choosing the best contraceptive method for the adult with congenital heart disease. Curr Cardiol Rep 2009; 11: 298–305. [DOI] [PubMed] [Google Scholar]
- 30.Vigl M, Kaemmerer M, Seifert-Klauss V, et al. Contraception in women with congenital heart disease. Am J Cardiol 2010; 106: 1317–1321. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs AH, Harrison JL, Colman JM, et al. Pregnancy and contraception in congenital heart disease: what women are not told. J Am Coll Cardiol 2008; 52: 577–578. [DOI] [PubMed] [Google Scholar]
- 32.Dayan N, Laskin CA, Spitzer K, et al. Pregnancy complications in women with heart disease conceiving with fertility therapy. J Am Coll Cardiol 2014; 64: 1862–1863. [DOI] [PubMed] [Google Scholar]
- 33.CMACE. Saving Mothers' Lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. London, UK: Centre for Maternal and Child Enquiries, 2011. [DOI] [PubMed]
- 34.Tanous D, Siu SC, Mason J, et al. B-type natriuretic peptide in pregnant women with heart disease. J Am Coll Cardiol 2010; 56: 1247–1253. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: 2438–2488. [DOI] [PubMed] [Google Scholar]
- 36.John AS, Gurley F, Schaff HV, et al. Cardiopulmonary bypass during pregnancy. Ann Thorac Surg 2011; 91: 1191–1196. [DOI] [PubMed] [Google Scholar]
- 37.Steer PJ. Pregnancy and contraception. In: Gatzoulis MA, Swan L, Therrien J, Pantley GA. (eds). Adult congenital heart disease: a practical guide, Oxford: BMJ publishing, Blackwell publishing, 2005, pp. 16–35. . [Google Scholar]
- 38.Robertson JE, Silversides CK, Mah ML, et al. A contemporary approach to the obstetric management of women with heart disease. J Obstet Gynaecol Can 2012; 34: 812–819. [DOI] [PubMed] [Google Scholar]
- 39.Tamhane P, O'Sullivan G, Reynolds F. Oxytocin in parturients with cardiac disease. Int J Obstet Anesth 2006; 15: 332–333. author reply 3. [DOI] [PubMed] [Google Scholar]