Abstract
Almost 30% of pregnant women attending public health clinics in South Africa are HIV positive; which represents approximately 280,000 women each year. South Africa has the largest antiretroviral therapy programme in the world, with over 2.7 million people on treatment in 2013. Since its belated and controversial beginning, the Prevention of Mother-to-Child Transmission programme has achieved a substantial reduction in vertical transmission. South Africa is justifiably proud of this success. However, the history of Prevention of Mother-to-Child Transmission (PMTCT) and antiretroviral therapy programmes in South Africa has been fraught with delays and political intervention. South Africa could have started both PMTCT and antiretroviral therapy programmes in 2000. Instead, the AIDS denialist views of the government allowed the HIV epidemic to spiral out of control. Roll-out of a national PMTCT programme began in 2002, but only after the government was forced to do so by a Constitutional Court ruling. Now, a decade later, HIV treatment and prevention programmes have been completely transformed. This article will discuss the evolution of the HIV epidemic in South Africa, and give a historical overview of the struggle to establish a national PMTCT, and the impact of delaying PMTCT and treatment programmes on infant and maternal health.
Keywords: HIV, AIDS, maternal child transmission, complications, drugs (medication), maternal-fetal medicine, infectious diseases, maternal mortality
Introduction
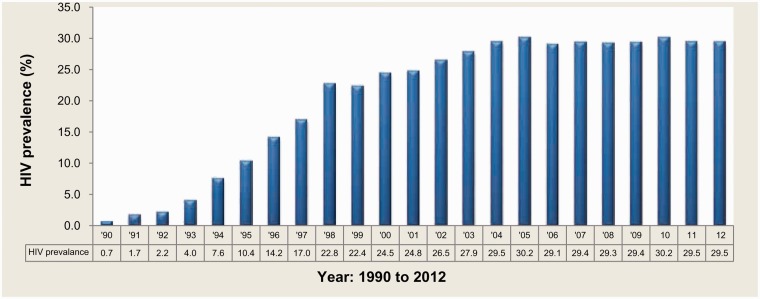
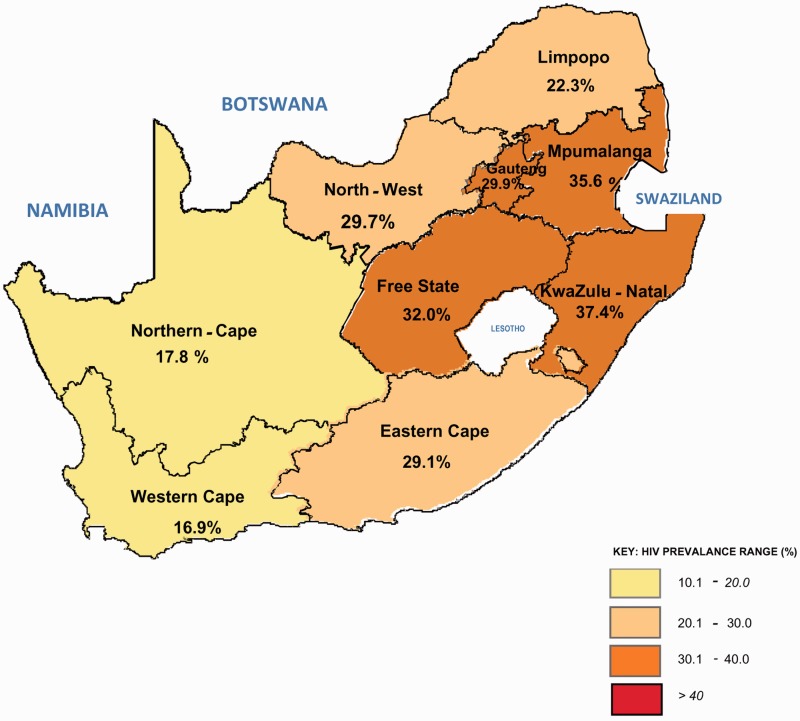
Almost 30% of pregnant women attending public health clinics in South Africa are HIV positive; which represents approximately 280,000 women each year (Figures 1 and 2).1 South Africa has the largest antiretroviral therapy (ART) programme in the world, with over 2.7 million people on treatment in 2013.2 Since its belated and controversial beginning, the Prevention of Mother-to-Child Transmission (PMTCT) programme has achieved a substantial reduction in vertical transmission. In 2011, the latest year for which data are available, 2.7% of HIV-exposed infants attending baby follow-up services were HIV-positive at 4–8 weeks of age, compared to an expected 30% transmission rate in the absence of any PMTCT measures.3 South Africa is justifiably proud of this success. However, the history of PMTCT and ART programmes in South Africa has been fraught with delays and political intervention, with many thousands of preventable deaths. South Africa could have started both PMTCT and ART programmes in 2000.4 Instead, the AIDS denialist views and resulting disastrous health policy of the government at that time delayed implementation, and allowed the HIV epidemic to spiral out of control.5 Roll-out of a national PMTCT programme began in 2002, but only after the government was forced to do so by a Constitutional Court ruling.6
Figure 1.
HIV prevalence trends among antenatal women, South Africa 1990 to 2012.1
Figure 2.
HIV prevalence distribution by province, South Africa, 2012.1
The Constitutional Court case involved a single issue – the provision of single-dose nevirapine (sdNVP) to HIV-positive pregnant women. At the time, there was no national ART programme, and therefore no treatment to keep HIV-positive women alive. A generation of AIDS orphans and child-headed households resulted.7 The national ART programme finally began in 2004.8 However, implementation was slow with uneven access to treatment across the country. The AIDS denialist health minister remained in office, and continued to undermine public confidence in ART. Avoidable transmission of HIV and deaths from AIDS continued.9
Now, a decade later, HIV treatment and prevention programmes have been completely transformed (Table 1). South Africa is implementing the latest WHO Guidelines, which have expanded access to treatment for both pregnant women and all other adults.10 From January 2015, all pregnant and breastfeeding women will be eligible for lifelong highly active antiretroviral therapy (HAART), and the CD4 threshold for initiation of HAART will increase to 500 cells/mm3.11
Table 1.
Timeline of national PMTCT and ART guidelines, South Africa.
| PMTCT Guidelines | Year | ART Guidelines |
|---|---|---|
| National PMTCT programme begins after Constitutional Court judgement against the Government: • Single dose nevirapine to the mother in labour, and to the infant within 72 hours of birth | 2002 | |
| • AZT to mother from 28 weeks gestation; single dose nevirapine to mother in labour and to infant within 72 hours of birth | 2004 | National roll-out of Antiretroviral programme. • Eligibility: CD4 < 200 cells/mm3, or WHO stage 4 disease |
| • AZT to mother from 28 weeks gestation; single dose nevirapine to mother in labour and to infant within 72 hours of birth | 2008 | |
| • AZT from 14 weeks gestation: single dose nevirapine plus tenofovir/3TC in labour; infant prophylaxis with nevirapine for 6 weeks if mother on HAART or formula feeding, or until the end of all breastfeeding if mother not eligible for HAART. | 2010 | • Eligibility for HAART includes CD4 < 350 cells/mm3, and all people with TB irrespective of CD4 count |
| • All pregnant women eligible for HAART, irrespective of CD4 count. Infant prophylaxis with nevirapine for 6 weeks. Women initiating HAART with CD4 < 350 and no other indication for HAART, to stop treatment after all breastfeeding has ceased. | 2013 | |
| • All pregnant and breastfeeding women eligible for lifelong HAART | 2015 | • Eligibility for HAART includes CD4 < 500 cells/mm3 |
ART: antiretroviral therapy; AZTL: zidovudine; HAART: highly active antiretroviral therapy; PMTCT: Prevention of Mother-to-Child Transmission.
For health care professionals working in HIV care, it would have been unbelievable in 2004 to imagine that ART provision in South Africa would progress so rapidly. This article will briefly discuss the evolution of the HIV epidemic in South Africa, and give a historical overview of the struggle to establish a national PMTCT programme, and the impact of delaying PMTCT and treatment programmes on infant and maternal health. It will then look at the more recent success of the PMTCT and ART programmes in improving outcomes for pregnant women living with HIV and their children, and future issues and challenges.
AIDS in South Africa in the 1990s
HIV appeared in South Africa during the last years of apartheid.9 The first national antenatal HIV prevalence survey, in 1990, found 0.8% of pregnant women were HIV-positive.12 Annual surveys followed; these monitored the escalating HIV prevalence, but no plan was made to prevent vertical transmission.
The 1990s were a time of major political change in South Africa. In the 1994 election, for the first time ever, all citizens had a right to vote. The newly elected government, with Nelson Mandela as president, showed a commitment to improving maternal and child health. However, with many priorities for change, HIV did not get the attention it deserved. Social and economic inequalities and poor access to health care continued to be a significant challenge, particularly for the black population, who have been hardest hit by the HIV epidemic.5,13
The response to the growing HIV pandemic was inadequate and lacked leadership.5 The Treatment Action Campaign (TAC), a civil society response of people living with HIV and activists, was founded in 1998, and supported by concerned health care professionals.6 By this time, antenatal prevalence had risen to 22% and the upward trend continued; 1500 people were newly infected with HIV each day.12 TAC played a defining role in pressurising the government to provide PMTCT and a national ART treatment programme.14
In 1999, President Thabo Mbeki became convinced by the writings of AIDS denialists that AIDS in Africa was caused by poverty rather than a transmissible virus.15 He promoted the view that AIDS was a creation of western pharmaceutical companies, aiming to generate a large market (and large profits) in Africa for antiretroviral drugs. He maintained that ART was toxic, and that drugs, rather than HIV, were the cause of death of people diagnosed with AIDS. The Health Minister, Manto Tshabalala-Msimang, loyally supported these ideas, and enthusiastically promoted them. It is difficult to know how many senior politicians shared the dissident views of the president, but the majority were complicit by their silence.
During this era, HIV prevalence continued to rise, and mother-to-child transmission continued unchecked. Doctors in the public sector were unable to provide lifesaving medication to their patients. Doctors who had access to ART provided by non-government organisations and provided them to pregnant women and women who had been raped were intimidated and suspended.16 By 2000, national antenatal prevalence had increased to 26.5%.17
However, pressure from health care workers and civil society for the government to provide ART was increasing. PMTCT became a major focus, with published evidence to show its benefit and cost effectiveness in low-resource settings. In 1994, the AIDS Clinical Trials Group 076 trial showed that zidovudine (AZT) monotherapy from 14 weeks gestation, in labour and to the infant for 6 weeks postpartum reduced vertical transmission by 67%.18 For developing countries, cost and lack of infrastructure were major barriers in implementing this regimen. However, in 1999, two large studies in developing countries demonstrated the benefits of short ART regimens to prevent vertical transmission in resource-poor countries. The ‘Thai Trial’ randomised women to AZT monotherapy or placebo from 36 weeks gestation; all infants were formula fed. Vertical transmission was reduced by almost 50% in women receiving AZT.19 The HIVNET 012 trial in Uganda compared sdNVP to the mother in labour and to the infant post delivery to AZT 3 hourly in labour and for 7 days to the infant. Intrapartum and postnatal sdNVP also significantly reduced the risk of vertical transmission.20
Despite the evidence that short courses of ART could significantly reduce vertical transmission in resource-poor countries, the government resisted providing AZT for PMTCT. Thabo Mbeki publicly declared AZT to be toxic and despite studies showing its efficacy and safety.21 Manto Tshabalala-Msimang asked the Medicines Control Council of South Africa to review the safety of AZT prior to its use for PMTCT; when it was approved in February 2000, she rejected its findings.
In addition to unfounded concerns about toxicity, the government maintained that a national PMTCT programme was unaffordable, and there was a lack of capacity within the health service. An editorial in the Lancet in 2002 noted that these were ‘just government excuses’ to rationalise their AIDS denialist stance.22 In fact the Health Minister had turned down an offer from Boehringer Ingelheim to provide nevirapine free for 5 years, and blocked grants from the Global Fund and The President's Emergency Plan for AIDS Relief (PEPFAR). That there was a lack of capacity in the health service to provide PMTCT would be disproven by three provinces which set up their own PMTCT programmes, in defiance of the government.
Reluctantly, the government implemented an sdNVP regimen in May 2001 at two ‘pilot sites’ in each of the nine provinces, once nevirapine had been reviewed and registered (after long delays) by the Medicines Control Council.6 It was planned that the pilot sites would run for 2 years, and then a decision made to roll out the programme nationally. With only 18 sites providing PMTCT across the entire country, the majority of pregnant women in South Africa were excluded from PMTCT care.
The Constitutional Court case
In mid-2001, TAC filed a constitutional claim against the government, actively supported by many health professionals.6,14 It requested a ruling that the lack of provision of PMTCT was a violation of the right to health care and therefore unconstitutional; and further that the government be ordered to make treatment available at all public health facilities for pregnant women.
The court ruled in favour of TAC in December 2001, stating that there had been a serious breach by the government of its human rights obligations and duties. The government immediately appealed.
The Constitutional Court dismissed the appeal in August 2002. This was almost a year and an estimated 100,000 infant infections after TAC had initiated legal proceedings. The national PMTCT programme was therefore implemented by a judgement of a court of law, rather than being determined by international best practice and evidence-based medicine.6
While the national Department of Health rigidly opposed PMTCT implementation, three of South Africa's nine provinces broke ranks with government policy, and initiated their own PMTCT programmes. The Western Cape programme started in 1999, at two sites in Khayelitsha, a township on the outskirts of Cape Town with the highest antenatal prevalence in the province.23 HIV counselling and testing were offered to all pregnant women; those testing positive were given AZT from 36 weeks gestation. Médicins Sans Frontières greatly contributed to the implementation and expansion of PMTCT services in Khayelitsha, and the programme was subsequently rolled out to the rest of the province. The provinces of Gauteng and KwaZulu Natal also implemented PMTCT programmes, in 2001 and 2002, respectively, without awaiting the court ruling.14 All three provinces were pressurised by the government not to provide PMTCT services, indicating the extent to which PMTCT had become a political issue, rather than about providing access to lifesaving treatment. These provinces demonstrated that government insistence that there was no capacity to introduce PMTCT services in the public sector was not true. The remaining six provinces complied rigidly with the government position, to the extent of closing down non-government organisations that were providing single-dose nevirapine to pregnant women.6
Implementation of national PMTCT and ART programmes
The Constitutional Court case in 2002 was a landmark victory. In 2003 the South African Cabinet sidelined the views of the president and health minister, and for the first time publicly stated that ‘HIV causes AIDS’. Plans for a national ART treatment programme were drawn up, involving the Department of Health, medical experts, and TAC, to begin in early 2004.14
This should have been the end of AIDS denialism, and the beginning of rapid rollout of life-saving ART. However, implementation of both PMTCT and ART programmes was slow, geographically uneven, and characterised by a lack of political will.24 Manto Tshabalala-Msimang remained health minister, and openly continued to fuel public mistrust in ART and stress its toxicity. She actively promoted unproven ‘alternative’ nutritional treatments for HIV, particularly beetroot, lemon, garlic, and African potato, purporting that they boosted the immune system in HIV-positive people and prevented progression to AIDS.25 She presented these ‘nutritional treatments’ as traditional African medicine, and therefore an African alternative to western medicine. Traditional medicine is commonly used in South Africa, with estimates that at least 70% of South Africans consult traditional healers.26 After apartheid, there were government plans for research, development, and regulation of traditional medicine, to reclaim African cultural heritage. The health minister used her support for traditional medicine as a means of undermining confidence in ART. She actively endorsed untested and unregulated medicines promoted by international AIDS dissidents and others from within South Africa as viable alternatives to ART.
The health minister's unquestioning acceptance of untested medication was in contrast to her ongoing criticism of nevirapine. Concerns about drug resistance were increasingly being reported in the medical literature. At the 2004 International AIDS Conference in Bangkok, she responded to a paper showing that resistance could occur after a single dose by stating that South Africa would immediately halt the PMTCT programme. No alternative treatment to nevirapine plan was proposed in place of nevirapine, provoking international condemnation. An editorial in Nature responded that the latest evidence indicated ‘doctors should provide more treatment for pregnant women with HIV – not less’.27 The PMTCT programme did continue, but the opportunity was not taken to resolve concerns about resistance by changing to AZT to prevent vertical transmission.
Undeterred by international disdain, the health minister again caused worldwide condemnation at the next International AIDS Conference in Toronto, in 2006. Beetroot, lemon, and garlic were prominently displayed on the South African government stand, and received widespread derision. The UN special envoy for AIDS in Africa, Stephen Lewis, in his closing speech denounced the South African government response to the HIV epidemic saying ‘it is the only country in Africa … whose government is still obtuse, dilatory and negligent about rolling out treatment. This is the only country in Africa whose government continues to propound theories more worthy of a lunatic fringe than of a concerned and compassionate state’.28 After the conference, 65 of the world's leading HIV/AIDS experts signed a letter to President Mbeki asking that he dismiss Manto Tshabalala-Msimang. However, this did not happen. She was finally dismissed from her post as health minister in 2008, after Mbeki was forced by the ANC to resign as president.
The interim health minister, Barbara Hogan, announced that ‘the era of AIDS denialism is over completely in South Africa’, to widespread acclaim from health professionals and civil society.14 She immediately prioritised rapid implementation of PMTCT and ART services. Following the 2009 election, Dr Aaron Motsoaledi was appointed Minister of Health. Under his leadership HIV testing, PMTCT, and ART programmes significantly expanded. In 2010 eligibility for HAART increased from a CD4 threshold of 200 to 350 cells/mm3. The national PMTCT guideline had changed in early 2008 from single-dose nevirapine alone to AZT monotherapy from 28 weeks gestation.29 In 2010, pregnant women ineligible for HAART initiated AZT earlier in pregnancy, from 14 weeks gestation.30 A national HIV counselling and testing campaign was initiated, and 13.3 million HIV tests were performed in the following 18 months.31 Over 2 million people tested positive, and over 400,000 were initiated on HAART. At last there was optimism that the HIV epidemic in South Africa would be controlled, and that people would live with HIV, rather than dying from it.
The lost benefits of ART, 2000–2005
Harvard researchers calculated the lost benefits of ART, in terms of a lack of PMTCT and treatment programmes in South Africa from 2000 to 2005.4 They considered it would have been feasible to start rolling out PMTCT and ART programmes in 2000, and that this should have achieved at least 50% coverage by 2005, as drug prices fell and international support increased. They compared what would have been possible in South Africa with what happened in Botswana and Namibia, neighbouring countries with similar HIV epidemics.
Botswana started its PMTCT programme in 1999, and Namibia in 2001. By 2005, 70% of pregnant women in both countries received PMTCT. In contrast, in South Africa, they calculated that 23% of women received PMTCT in 2005, with less than 10% in 2004, and less than 3% in preceding years. They estimated a minimum of 35,000 preventable infant infections occurred during 2000–2005, based on the assumption that single-dose nevirapine had been available to 50% of pregnant women by 2005. This figure is likely an underestimate of actual deaths. They calculated a conservative estimate of 60,000 infant infections per year. However as the authors note, government statistics suggest 105,000 infant infections a year. Using this higher figure gives 62,000 preventable infant infections.32 A similar model was applied to adult mortality from HIV infection: assuming limited availability of ART treatment programmes, they estimated there were between 330,000 and 500,0000 preventable adults deaths from HIV in the years 2000–2005.4
Saving mothers lives: the impact of HIV on maternal deaths
PMTCT services initially had a single goal – to prevent vertical transmission. Despite the victory that the court case represented, there was no sense of concern about keeping mothers alive. Women who tested HIV positive in pregnancy knew they were unlikely to stay alive to see their children grow up. AIDS orphans and child-headed households became common. Even today, it is estimated that there are 2.4 million AIDS orphans in South Africa.33
The impact of HIV infection on maternal mortality in South Africa has been overwhelming. The National Committee for Confidential Enquiries into Maternal Mortality in South Africa (NCCEMD) was established in the 1990s, with the goal of documenting substandard care, to enable improvements in health care delivery to be identified. It first reported in 1998, and issued triennial reports therafter.34 The 1998 report highlighted the contribution of HIV to maternal mortality. Non-pregnancy-related infections caused 23% of all maternal deaths, and were the second most common cause. The majority of deaths in this category were due to HIV; and as only women who had been tested for HIV were included, this was undoubtedly an underestimate.
This report highlighted how little knowledge existed regarding the optimal management of sick HIV-positive pregnant women: stating that ‘the management of women with AIDS during pregnancy is unclear. The disease is relatively new and few doctors have experience in managing such cases’. The report noted that although few examples of substandard care were identified in women dying of HIV ‘this reflects the uncertainty as to the appropriate management of these women, rather than good obstetric practice’ and commented on the absence of accepted, practical guidelines. What was not mentioned was that treating opportunistic infections in HIV-positive women would not give good outcomes without access to ART. In 1999, the National ART roll out was still 5 years away, AIDS denialism was taking root and ART to reduce maternal mortality was not being considered.
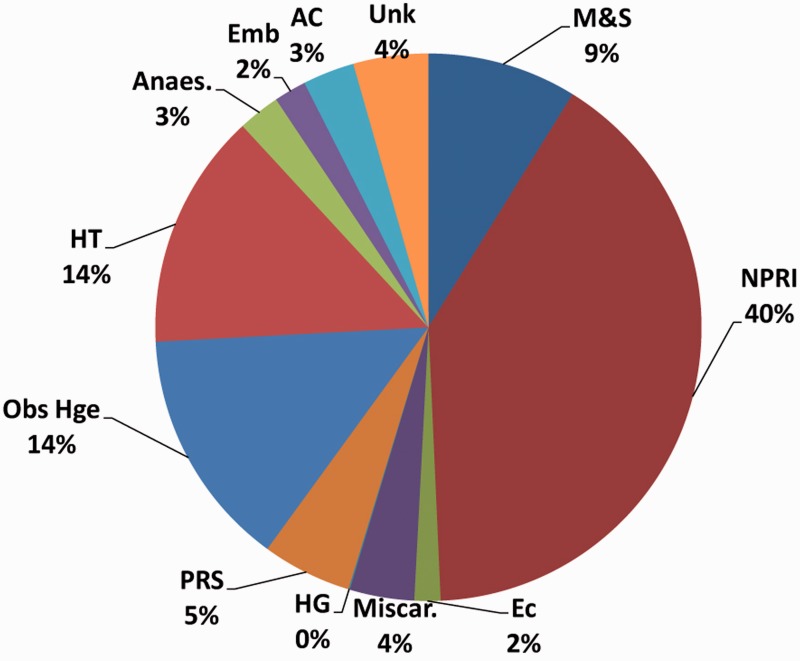
Subsequent triennial reports illustrated the overwhelming impact of HIV on the rising maternal mortality rate, with deaths from non-pregnancy-related infections increasing with each report.35–38 The percentage of deaths for which HIV status was recorded also progressively increased. In 1998, HIV status was known in only 24% of maternal deaths.34 In the 2008–2010 report, this had risen to 79%.38 In this report, 40.5% of all maternal deaths were attributed to non-pregnancy-related infections, with the majority being HIV-related (Figure 3). Tuberculosis was the single most common opportunistic infection. Institutional maternal mortality was the highest ever recorded in South Africa, at 176 per 100,000 live births overall, and 430 per 100,000 live births amongst HIV-positive women. In contrast, institutional maternal mortality in the UK is 10 per 100,000 live births.39
Figure 3.
Distribution of underlying causes of maternal deaths in South Africa, 2008–2010.38 NPRI: non-pregnancy-related infections; EC: ectopic pregnancy; Miscar: miscarriage; HG: hyperemesis gravidarum; PRS: pregnancy-related sepsis; Obs Hge: obstetric haemorrhage; HT: hypertension; Anaes: anaesthetic complications; Emb: embolism; AC: acute collapse, cause unknown; Unk: unknown; M&S: medical and surgical conditions (previously pre-existing medical diseases).
The 2008–2010 South African report was the first to document whether HIV-positive women were on HAART by the time of death, and found that over half of eligible women had not initiated treatment. The root cause of missed opportunities included delayed appointments, inaccessible clinics, attending multiple clinics, and long and inflexible pre-ART preparation. The report recommended that every maternity facility must be able to initiate and monitor HAART, rather than referring women to separate ART clinics. It also stated that HAART should be initiated on the same day that a woman was determined eligible. At this time, policy for initiation of ART required three counselling sessions, spread over 2–3 weeks, some clinics required a treatment supporter to also attend sessions. Same day initiation was a radical approach in this context.
However, there is now optimism that maternal mortality due to HIV may at last be falling. An interim report on maternal deaths for 2011 has shown a significant reduction in maternal mortality.40 Overall, there has been a 13% reduction, to 153 per 100,000 live births, with a 17.7% reduction to 354 per 100,000 live births amongst HIV-positive women. Deaths from non-pregnancy-related infections remain the highest single category, but showed a remarkable 28% reduction in mortality amongst HIV-positive women. The scale-up of HAART, which included pregnant women with CD4 counts < 350 cells/mm3 in 2010, and reducing barriers to pregnant women starting treatment are likely responsible for this change.
This is great cause for optimism. However, had South Africa started a national ART programme earlier, maternal mortality would not have risen so high, would have fallen earlier, and many HIV-related maternal deaths would have been avoided.
HAART for pregnant women: nevirapine or efavirenz?
The 2008–2010 NCCEMD report highlighted an issue that may otherwise have taken significantly longer to be revealed. Maternal deaths were occurring due to adverse effects of ART; deaths due to liver failure or Steven Johnson's syndrome were reported in pregnant women taking nevirapine containing HAART regimens.38 Deaths increased each year, with 14 in 2008, 17 in 2009, and 42 in 2010. The 2011 interim report showed a further increase, with 64 deaths in that year alone.40
Nevirapine was recommended as part of first-line HAART in South Africa, in line with WHO guidelines.8,41 The increase in deaths correlated with increasing numbers of pregnant women taking nevirapine-based HAART. This was due both to increased access to ART, and the increased CD4 threshold for treatment of 350 cells/mm3. The risk of nevirapine hypersensitivity is higher in women with nadir CD4 counts > 250 cells/mm3; below this threshold, the risk is lower, but still exists.42–44 The NCCEMD report does not document how many maternal deaths occurred in women starting nevirapine with CD4 counts between 250 and 350 cells/ mm3, however there is no CD4 count cut-off below which nevirapine is safe. Deaths have also been reported amongst non-pregnant women changing to nevirapine from efavirenz because they were planning a pregnancy.44 It should be noted that nevirapine hypersensitivity relates to ongoing use as part of HAART regimens, and does not occur after a single dose.
Nevirapine was included in first-line HAART for women of reproductive age and pregnant women because of concerns that efavirenz, the alternative non-nucleoside reverse transcriptase inhibitor, was a teratogen. In 2005, the FDA reclassified efavirenz from category C (that risk of fetal harm cannot be ruled out) to category D (that there was positive evidence of fetal risk).45 Six case reports had been published of neural tube defects in women who had used efavirenz in the first trimester. Similar malformations had been found in animal studies, with 3 of 20 fetuses of cynomolgus monkeys given efavirenz having neural tube defects.46,47 WHO and South African guidelines stated that women of reproductive age should take efavirenz only if using reliable contraception; if not, nevirapine-based regimens should be used.8,47 Pregnant women taking efavirenz in the first trimester were to be switched to nevirapine. Neural tube closure occurs around 28 days post-conception, and it is rare for women in low-resource settings to have booked by this stage. For the majority of women, switching in the first trimester would not have avoided any potential neural tube defects. In practice, many women were switched from efavirenz at later gestations.
A meta-analysis of worldwide data published in 2011 included 1437 live births with efavirenz exposure in the first trimester of pregnancy.48 No overall increase in birth defects was found, and only one neural tube defect identified. This gave a prevalence of 0.07%, less than the baseline in any population worldwide. However, the small sample size is a limitation of the meta-analysis, and sufficient only to rule out a 10-fold increase in risk. More than 3000 conceptions would be needed to detect a doubling of the risk. This number has no doubt been far exceeded globally, however the data have not been documented. A recent update includes 2026 live births with first trimester exposure; there is still only a single neural tube defect reported in this meta-analysis.49
The finding that nevirapine was causing a significant and increasing number of maternal deaths and the lack of evidence that efavirenz was teratogenic precipitated a rapid change in guidelines on HAART regimens for women of reproductive age. In 2012, efavirenz rather than nevirapine was recommended as first-line treatment for everyone, including women of reproductive age and all pregnant women.50–52 This ended an ongoing controversy about efavirenz use in pregnant women.
Vertical transmission and infant feeding
The issue of infant feeding has been complex and controversial in South Africa, given that vertical transmission can occur through breastfeeding. In the early years of the PMTCT programme, South Africa followed guidelines of resource-rich countries, and advocated formula feeding for HIV-positive women.8,41 Free formula milk was provided until the infant was 6 months of age. However, South Africa has a developing country profile, with many women living in areas without access to safe drinking water, making formula feeding inappropriate and unsafe. Many were also unwilling to formula feed, concerned that this might reveal their HIV status to family and neighbours.53 A study in Durban reported in 2001 that amongst women who self-selected to exclusively breast feed for 3–6 months there was no difference in the rate of HIV transmission compared to those who formula fed. However, women who breast fed, but who also provided additional liquids or solid food (mixed feeding) to their infants, had a considerably higher rate of transmission.54
Several randomised studies in other Sub-Saharan African countries had shown that formula feeding did not increase HIV-free survival compared to breastfeeding with the mother on HAART or the infant given extended prophylaxis.55 Formula feeding led to increased mortality from respiratory and gastrointestinal disease irrespective of infant HIV status. These findings resulted in WHO releasing new recommendations in 2010 supporting breastfeeding in HIV-positive mothers living in low-resource settings, with mothers or their infants taking ART throughout the period of breastfeeding.56 By following evidence-based regimens, infants would benefit from breastfeeding while reducing the risk of mother-to-child HIV transmission.
On this basis, in 2011 South Africa changed to a policy of promoting only exclusive breastfeeding to HIV-positive mothers, with a high-profile announcement by the health minister.57 At the time, South Africa was one of the 12 countries where child mortality was increasing. Rates of exclusive breastfeeding were extremely low. A 2008 study found that less than 25.7% of infants under 6 months of age were exclusively breast fed, and 24% formula fed; over half were given mixed feeds.58 Provision of free formula milk in South Africa was discontinued, with strengthened support for exclusive breastfeeding.
Moving forward
In line with WHO recommendations, in 2013 South African PMTCT guidelines changed with all pregnant women eligible to initiate HAART, irrespective of CD4 count (WHO option B).52 A fixed-dose combination (FDC) of tenofovir, emtricitabine, and efavirenz is now available; the Department of Health negotiated the lowest price in the world, making provision of HAART cost-effective and simple.59 All HIV-positive pregnant women not already on HAART start the FDC at their first antenatal visit unless there are specific contraindications. Midwives and nurses are being trained to prescribe and monitor the FDC in antenatal clinics, with a Nurse-Initiated Management of ART (NIMART) programme being rolled out across the country.60
The Western Cape provincial guidelines in 2013 stated that all pregnant and breastfeeding women would be eligible to continue lifelong HAART (WHO Option B+).61 All other provinces followed the national guidelines that women with CD4 counts > 350 cells/mm3 would discontinue after breastfeeding had ceased. The Western Cape decision was seen as controversial by many. Several concerns were raised.62 First was that this would lead to poor adherence and resistance amongst women with high CD4 counts who might not be motivated to continue. Second was that providing HAART to a population that otherwise would not qualify for treatment would lead to spiralling costs and stock outs of drugs. And finally that prioritising pregnant women was unfair to men and to non-pregnant women. However despite these concerns, the national guidelines are about to change again. In January 2015, all pregnant and breastfeeding women will be eligible to continue lifelong HAART irrespective of CD4 count and the CD4 threshold for initiating all HIV-positive adults will increase to 500.11
Conclusion
HIV took only a few years to have a major impact on the lives of many South Africans. Hundreds of thousands of preventable deaths occurred, and millions of children were orphaned. However in just 10 years, the treatment of HIV has been radically transformed. It no longer means inevitable death, a lost generation of working age adults, and grandparents looking after children who survived HIV exposure. As in resource-rich countries, HIV is now becoming a chronic disease, with people on lifelong treatment. However, HIV still dominates health care at all levels; from community clinics to tertiary care. HIV is a major focus of maternity care and the routine work of midwives in South Africa in areas of high HIV prevalence is challenging. PMTCT programmes require HIV counselling and repeated testing of HIV-negative women, starting at booking, again at 32 weeks, in labour and every 3 months during breastfeeding. Midwives are prescribing and monitoring the FDC. South Africa must be credited for rapidly implementing comprehensive international best practices for prevention of mother-to-child transmission and addressing the difficult historical legacy of AIDS denialism in the recent past.
Declaration of conflict of interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Guarantor
RB
Contributorship
The authors are the sole contributors to this review.
References
- 1.National Department of Health. The 2012 National Antenatal Sentinel HIV & Herpes Simplex Type-2 Prevalence Survey in South Africa. Report. Pretoria, 2014.
- 2.UNAIDS. Getting to zero: HIV in Eastern and Southern Africa. Report. Johannesburg, 2013.
- 3.Barron P, Pillay Y, Doherty T. Eliminating mother-to-child HIV transmission in South Africa. Bull World Health Organ 2013; 91: 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chigwedere P, Seage GR, III, Gruskin S, et al. Estimating the lost benefits of antiretroviral drug use in South Africa. JAIDS 2008; 49: 410–415. [DOI] [PubMed] [Google Scholar]
- 5.Mayosi BM, Lawn JE, van Niekerk A, et al. Health in South Africa: changes and challenges since 2009. Lancet 2012; 380: 2029–2043. [DOI] [PubMed] [Google Scholar]
- 6.Heywood M. Preventing mother-to-child HIV transmission in South Africa: background, strategies and outcomes of the Treatment Action Campaign case against the Minister of Health: current developments. South Afr J Human Rights 2003; 19: 278–315. [Google Scholar]
- 7.Cullinan K, Thom A. Introduction. In: Cullinan K, Thom A. (eds). The virus, vitamins and vegetables: the South African HIV/AIDS mystery, Auckland Park: Jacana Media, 2009, pp. ix–xvi. [Google Scholar]
- 8.National Department of Health. Antiretroviral Treatment (ART) in adults. Pretoria, 2004.
- 9.Karim SSA, Churchyard GJ, Karim QA, et al. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet 2009; 374: 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. Geneva, 2013. [PubMed]
- 11.Cullinan K. Lifelong ARVs for pregnant women says Health Minister. Health-e news 24 July 2014, http://www.health-e.org.za/2014/07/24/lifelong-arvs-pregnant-women-says-health minister (accessed 04 October 2014).
- 12.National Department of Health. National HIV and Syphilis antenatal sero-prevalence survey in South Africa 1998. Report. Pretoria, 1999.
- 13.Chopra M, Daviaud E, Pattinson R, et al. Saving the lives of South Africa's mothers, babies, and children: can the health system deliver? Lancet 2009; 374: 835–846. [DOI] [PubMed] [Google Scholar]
- 14.Geffen N. Debunking delusions: the inside story of the Treatment Action Campaign, Auckland Park: Jacana Media, 2010. [Google Scholar]
- 15.Nattrass N. Mortal combat – AIDS denialism and the struggle for antiretrovirals in South Africa, Scottsville: University of KwaZulu-Natal Press, 2007. [Google Scholar]
- 16.Von Mollendorff T. Daring to care: a doctor's persecution in Mpumalanga. In: Cullinan K, Thom A. (eds). The virus, vitamins and vegetables: the South African HIV/AIDS mystery, Auckland Park: Jacana Media, 2009, pp. 77–90. [Google Scholar]
- 17.National Department of Health. National HIV and syphilis sero-prevalence survey in South Africa, 2000. Report. Pretoria, 2001.
- 18.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994; 331: 1173–1180. [DOI] [PubMed] [Google Scholar]
- 19.Shaffer N, Chuachwoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group 1999; 353: 773–780. Lancet. [DOI] [PubMed] [Google Scholar]
- 20.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET012 randomised trial. Lancet 1999; 354: 795–802. [DOI] [PubMed] [Google Scholar]
- 21.Baleta A. South Africa's AIDS care thrown into confusion. Lancet 1999; 354: 1711. [DOI] [PubMed] [Google Scholar]
- 22.Editorial Enough time wasted in South Africa. Lancet Infect Dis 2000; 2: 197. [DOI] [PubMed] [Google Scholar]
- 23.Stinson K, Giddy J, Cox V, et al. Reflections on a decade of delivering PMTCT in Khayelitsha, South Africa. SAJHIVMED 2014; 15: 30–32. [Google Scholar]
- 24.Nattrass N. South Africa's “rollout” of highly active antiretroviral therapy. A critical assessment. J Acquir Immune Def Syndr 2006; 43: 618–623. [DOI] [PubMed] [Google Scholar]
- 25.McGregor L. Garlic, olive oil, lemon and beetroot. In: Cullinan K, Thom A. (eds). The virus, vitamins and vegetables: the South African HIV/AIDS mystery, Auckland Park: Jacana Media, 2009, pp. 130–142. [Google Scholar]
- 26.Ndaki K. Traditional alternatives? In: Cullinan K, Thom A. (eds). The virus, vitamins and vegetables: the South African HIV/AIDS mystery, Auckland Park: Jacana Media, 2009, pp. 143–156. [Google Scholar]
- 27.Check E. Health minister ignites row over drugs for HIV mothers. Nature 2004; 430: 389. [DOI] [PubMed] [Google Scholar]
- 28.Blandy F. South Africa under fire at AIDS conference. Mail and Guardian 19 Aug 2006, http://mg.co.za/article/2006-08-19-sa-govt-under-fire-at-aids-conference (accessed 15 September 2014).
- 29.National Department of Health. Policy and guidelines for the implementation of the PMTCT programme. Pretoria, 2008.
- 30.National Department of Health. The South African antiretroviral treatment guidelines. Pretoria, 2010.
- 31.Pillay Y, White C, McCormick N. How times have changed – HIV and AIDS in South Africa in 2011. SAMJ 2012; 102: 77–78. [DOI] [PubMed] [Google Scholar]
- 32.National Department of Health. National HIV and Syphilis antenatal sero-prevalence survey in South Africa 2005. Report. Pretoria, 2006.
- 33.UNAIDS. South Africa, http://www.unaids.org/en/regionscountries/countries/South Africa (accessed 04 October 2014).
- 34.Department of Health. Saving Mothers: report on Confidential Enquiries into Maternal Deaths in South Africa, 1998. Pretoria, 1999.
- 35.Department of Health. Saving Mothers: report on Confidential Enquiries into Maternal Deaths in South Africa, 1999-2001. Pretoria, 2002.
- 36.Department of Health. Saving Mothers: report on Confidential Enquiries into Maternal Deaths in South Africa, 2002-2004. Pretoria, 2005.
- 37.National Committee on Confidential Enquiries into Maternal Deaths. Saving mothers 2005-2007: Fourth report on confidential enquiry into maternal deaths. Pretoria, 2008.
- 38.National Committee on Confidential Enquiries into Maternal Deaths. Saving mothers 2008-2010: Fifth report on confidential enquiry into maternal deaths. Pretoria, 2012.
- 39.Knight M, Kenyon S, Brocklehurst P, et al. Saving Lives, Improving Mothers' Care – Lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–12, Oxford: National Perinatal Epidemiology Unit, University of Oxford, 2014. on behalf of MBRRACE-UK. [Google Scholar]
- 40.National Committee on Confidential Enquiries into Maternal Deaths. Ninth interim report on the confidential enquiry into maternal deaths. 2011. Pretoria, 2012.
- 41.World Health Organisation. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva, 2004.
- 42.Stern JO, Robinson PA, Love J, et al. A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients. J Acquir Immune Defic Syndr 2003; 34(Suppl 1): S21–S33. [DOI] [PubMed] [Google Scholar]
- 43.Hitti J, Frenkel LM, Stek AM, et al. PACTG 1022 Study Team. Maternal toxicity with continuous nevirapine in pregnancy: results from PACTG 1022. J Acquir Immune Defic Syndr 2004; 36: 772–776. [DOI] [PubMed] [Google Scholar]
- 44.Smart T and Alcorn K. Switching from efavirenz to nevirapine in women with higher CD4 counts. HATIP 2009; 136: 1-9, http://www.aidsmap.com/pdf/HATIP-136-7th-May-2009/page/1321567/ (accessed 05 October 2014).
- 45.World Health Organisation. Technical update on treatment optimisation. Use of efavirenz during pregnancy: a public health perspective. Geneva, 2012.
- 46.Chersich MF, Urban MF, Venter FWD, et al. Efavirenz use during pregnancy and for women of childbearing potential. AIDS Res Ther 2006; 3: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford N, Mofenson L, Kranzer K, et al. Safety of efavirenz in first-trimester of pregnancy: a systematic review and meta-analysis of outcomes from observational cohorts. AIDS 2010; 24: 1461–1470. [DOI] [PubMed] [Google Scholar]
- 48.Ford N, Calmy A and Mofenson L. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS 2011; 25: 2301–2304. [DOI] [PubMed] [Google Scholar]
- 49.Ford N, Mofenson L, Shubber Z, et al. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS 2014; 28(Suppl 2): S123–S131. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organisation. Programmatic update. Use of antiretroviral drugs for treating pregnant women and preventing HIV infections in infants. Geneva, 2012.
- 51.W Cape Department of Health. Circular H3/2013. Update to antiretroviral treatment, December 2012. Cape Town, 2013.
- 52.National Department of Health. The South African antiretroviral treatment guidelines, 2013. Pretoria, 2013.
- 53.Doherty T, Chopra M, Nkonki L, et al. Effect of the HIV epidemic on infant feeding in South Africa: “When they see me coming with the tins they laugh at me'. Bull World Health Organ 2006; 84: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coutsoudis A, Pillay K, Kuhn L, et al. South African Vitamin A Study Group. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban. AIDS 2001; 15: 379–387. [DOI] [PubMed] [Google Scholar]
- 55.Kuhn L, Aldrovandi G. Survival and health benefits of breastfeeding versus artificial feeding in infants of HIV-infected women: developing versus developed world. Clin Perinatol 2010; 37: 843–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organisation. Guidelines on HIV and infant feeding. Geneva, 2010.
- 57.National Department of Health. The Tshwane declaration of support for breastfeeding in South Africa. S Afr J Clin Nutr 2011; 24: 214. [Google Scholar]
- 58.Media statement by the Minister of Health Dr Aaron Motsoaledi: Government adopts breastfeeding-only infant feeding strategy. 23 August 2011, http://www.gov.za/speeches/view.php?sid=20973 (accessed 05 October 2014).
- 59.Southern African HIV Clinicians Society. Fixed-dose combination for adults accessing antiretroviral therapy. SAJHIV Med 2013; 14: 41–43. [Google Scholar]
- 60.Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet 2012; 380: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.W Cape Department of Health. PMTCT clinical guidelines update. Cape Town, 2013.
- 62.Coutsoudis A, Goga A, Desmond C, et al. Is option B + the best choice? Lancet 2013; 381: 269–271. [DOI] [PubMed] [Google Scholar]