Abstract
Background
Gestational diabetes mellitus can be defined as ‘glucose intolerance or hyperglycaemia with onset or first recognition during pregnancy.’
Objective
The objective of our systematic review was to see if there was any intervention that could be used for primary prevention of gestational diabetes mellitus in women with risk factors for gestational diabetes mellitus.
Search strategy
Major databases were searched from 1966 to Aug 2012 without language restriction.
Selection criteria
Randomised trials comparing intervention with standard care in women with risk factors for gestational diabetes were included. Meta-analysis was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement. The primary outcome assessed was the incidence of gestational diabetes.
Data collection and analysis
Data from included trials were extracted independently by two authors and analysed using Rev-Man 5.
Main results
A total of 2422 women from 14 randomised trials were included; which compared diet (four randomised trials), exercise (three randomised trials), lifestyle changes (five randomised trials) and metformin (two randomised trials) with standard care in women with risk factors for gestational diabetes mellitus. Dietary intervention was associated with a statistically significantly lower incidence of gestational diabetes (Odds ratio 0.33, 95% CI 0.14 to 0.76) and gestational hypertension (Odds ratio 0.28, 95% CI 0.09, 0.86) compared to standard care. There was no statistically significant difference in the incidence of gestational diabetes mellitus or in the secondary outcomes with exercise, lifestyle changes or metformin use compared to standard care.
Conclusions
The use of dietary intervention has shown a statistically significantly lower incidence of gestational diabetes mellitus and gestational hypertension compared to standard care in women with risk factors for gestational diabetes mellitus.
Keywords: Gestational diabetes, prevention, diet, exercise, lifestyle and pharmacological intervention
Introduction
Gestational diabetes mellitus (GDM) can be defined as ‘glucose intolerance or hyperglycaemia with onset or first recognition during pregnancy.’1–4 Worldwide GDM incidence varies depending on risk factors within communities but its estimated incidence is between 2 and 8% of all pregnancies.4–8 Maternal hyperglycaemia results in fetal hyperglycaemia and macrosomia leading to shoulder dystocia, birth trauma, perinatal death, neonatal hypoglycaemia and long-term risk of obesity and diabetes in the child. The maternal risks include increased incidence of caesarean section, increased rates of induction of labour and its associated sequelae and subsequent development of type 2 diabetes mellitus.
The NICE guideline detailed a screening programme targeting biochemical screening to women with the following risk factors4 BMI ≥ 30 kg/m2; previous macrosomia ≥4500 g; previous GDM, first-degree relative with diabetes; family origin group with high risk of diabetes.4 Data on recent trends in maternal age at birth and on the prevalence of overweight and obesity indicate that women are older and heavier when having children, which will increase the prevalence of GDM.6–13 The IADPSG consensus14 recommends a one-step 75 g OGTT for all women not already known to be diabetic at 24 to 28 weeks of gestation and diabetes is diagnosed where one or more threshold values is exceeded (fasting ≥ 5.1 mmol/L, 1 h ≥ 10.0 mmol/L, 2 h ≥ 8.5 mmol/L). Application of these criteria is predicted to result in per pregnancy incidence of GDM of over 16% from the current level of 3.5%.14 The potential benefits of recognising and treating GDM include reductions in ill health in the woman and/or the baby during or immediately after pregnancy, as well as the benefits of reducing the risk of progression to type 2 diabetes in the longer term.4,5,15–17
Treatment of GDM is within a multidisciplinary environment which includes lifestyle interventions, such as diet, exercise, self-monitoring of blood glucose and hypoglycaemic therapy.16,17 It has been estimated that an average additional cost per mother with GDM is £1733 and cost per newborn to mother with GDM is £110.18 When taking into account the short- and long-term risks, for both mother and neonate, and the interventions required for women diagnosed with GDM, primary prevention would be beneficial to both the individual and would reduce the health cost burden of diagnosis.
The objective of our systematic review was to see if there was any intervention which could be used for primary prevention of GDM in women with risk factors for GDM.
Methods
A prospective peer-reviewed protocol was prepared a priori. Meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.19 All published randomised or quasi-randomised controlled trials comparing intervention with standard care in women with risk factors for GDM were included. Risk factors included raised BMI, previous GDM, previous infant with birth weight greater than 4500 g, family history of diabetes, high-risk ethnic groups and polycystic ovarian syndrome (PCOS). The interventions were sub-grouped into diet versus standard management; exercise versus standard management; lifestyle (diet, exercise, weight check at each visit) intervention versus standard management and pharmacological intervention versus standard management.
Trials comparing interventions in pregnant women with no risk factors for GDM and one intervention versus another were excluded. There were no exclusion criteria based on language or publication status. Studies were identified through Medline, Embase, Cochrane specialised trials register, ClinicalTrials.gov, conference abstract databases from 1966 to August 2012. A literature search was performed independently in August 2012 by two authors (PM and GG) using search terms Pregnancy/Gestational Diabetes/Prevention/Obesity/Previous gestational diabetes/Dietary intervention/Lifestyle intervention/Exercise/Pharmacological intervention/Metformin. All titles were screened and studies excluded if obviously irrelevant. If there was any doubt concerning the eligibility of the study, abstracts were examined and if necessary, the full text. Data were extracted independently by two authors (PM and GG). If any disagreements arose the complete paper was reviewed and disagreements resolved by discussion between the two authors (GG and PM). If we had not been able to agree we would have contacted a third author. Extracted data were put into a spreadsheet that had been developed prior to conducting the study. Authors were contacted if supplementary information was required.
The primary outcome assessed was the incidence of GDM. The secondary outcomes were caesarean section rate, fetal macrosomia (>4000 g), large for gestational age (LGA) (≥90th percentile for gestational age), small for gestational age (SGA) (≤10th percentile for gestational age), mean birth weight, pre-eclampsia (blood pressure ≥140/90 on two separate occasions with 1+ proteinuria arising after 20 weeks of gestation), gestational hypertension (blood pressure ≥140/90 on two separate occasions arising after 20 weeks of gestation), induction of labour, preterm birth (Birth before 37 weeks), shoulder dystocia, neonatal hypoglycaemia (<2.0 mmol/L), perinatal mortality and health cost analysis.
Data analysis
Data were analysed using Review Manager 5 (Cochrane Collaboration, Oxford, UK); Odds ratios (ORs) and weighted mean difference (WMD) were used as summary measures. Methodological heterogeneity was assessed during the selection, and statistical heterogeneity was measured using the chi-square test and I2 scores. A random effect model20 was used throughout to reduce the effect of statistical heterogeneity. Risk of bias across studies was assessed using risk of bias tables generated through Review Manager. Sensitivity analysis was performed by excluding studies with unclear quality. Funnel plots were not used to measure publication bias because of the small number of studies and their similar sizes.
Results
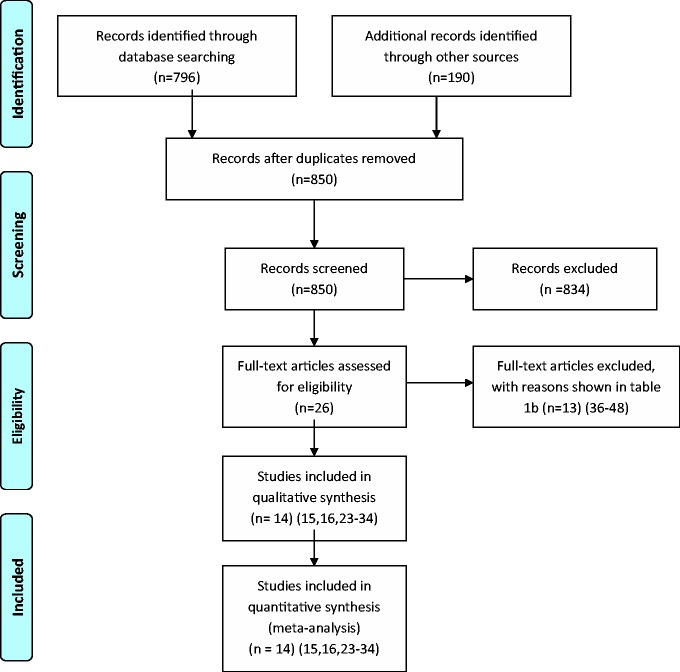
Figure 1 describes the literature search outcome. In all, 14 randomised controlled trials (RCTs) were included with a total of 2422 women (Table 1) comparing: diet (three RCTs; n = 455), exercise (three RCTs; n = 183), lifestyle changes (six RCTs; n = 1470) and drugs (two RCTs; n = 314) with standard care in women with risk factors for GDM. Twelve studies were excluded; reasons for exclusion are listed in Table 2. A total of 302 (12.5%) women were lost to follow-up. The mean age was 28.8 years and mean BMI was 31.6 kg/m2.
Figure 1.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram.
Table 1.
Characteristics of included studies.
| Study | Design | Participants | Intervention | Definition of GDM | Outcomes assessed |
|---|---|---|---|---|---|
| Diet | |||||
| Quinlivan et al.21 | Randomised Single centre Australia | Participants 124 Intervention: n = 61 Control: n = 63 Inclusion: Pregnant with fetus with no known anomalies, spoke English, did not intend to relinquish their infant, were able to attend the hospital for AN care and were overweight (BMI > 25–29.9) or obese (BMI > 29.9) | Four steps; 1. Continuity of care by a single maternity care provider. 2. Assessing weight gain at each antenatal visit. 3. A brief dietary intervention (5 min) by a food technologist at every antenatal visit. 4. Psychological assessment and intervention if indicated. If difficulties were identified an individualised solution focused treatment plan was implemented. | 75 g OGTT and WHO criteria for diagnosis. 2-h plasma glucose > 6.6 mmol/L for gestational glucose tolerance and > 7.7 mmol/L for GDM | Gestational glucose tolerance, GDM, weight gain in pregnancy, birth weight. |
| Thornton et al.22 | Randomised 3 tertiary centres USA | 257 participants Intervention group: n = 124 Control care group: n = 133 Inclusion: singleton pregnancy 12–28/40 with BMI ≥ 30. Exclusion: Pre-existing hypertension, diabetes or chronic renal disease. | Registered dietician prescribed a balanced nutritional regimen (18–24 kcal/kg consisting of 40% carbohydrate, 30% protein and 30% fat, no patients received less than 2000 calories daily) based on their weight at entry to the study. Asked to record all food and beverages consumed each day. Records reviewed and participants weighed at each visit and 6/52 postpartum. Both groups encouraged to participate in 30 min daily walking and seen at least once by a registered dietician. | Diagnostic criteria not stated | Last weight before delivery, 6/52 postpartum weight, mean loss difference from last weight before delivery to 6/52 postpartum, GDM, Pre-eclampsia, Ketonuria, Gestational hypertension, Haemorrhage/infection postpartum, Preterm delivery < 37 weeks, Post-term delivery > 41 weeks, labour induction, caesarean delivery, macrosomia (>4.5 kg), shoulder dystocia, Apgar score (<7 at 5 min), adherence to prescribed nutritional regime (non-adherence described as not bringing logbook to clinic for review or not recording food intake for greater than 1 week) |
| Wolff et al.23 | Randomised Dual centre Denmark | 66 Participants Intervention: n = 28 Control: n = 38 Inclusion: Non-diabetic Caucasian obese (BMI ≥ 30) pregnant women 12–18 weeks. Exclusion: smoking, <18years or >45years, multiple pregnancy and medical complications known to affect fetal growth or contraindicate limitation of weight gain. | 10 consultations of 1 h with a trained dietician during the pregnancy. Instructed to eat a healthy diet according to official Danish dietary recommendations (fat intake: max 30 energy percent, protein 15–20%, carbohydrate 50–55%). The energy intake was restricted based on individually estimated energy requirements and estimated energetic cost of fetal growth. Seven day weighed food records obtained at inclusion, 27 and 36 weeks. | 50 g OGTT diagnostic criteria not stated | Gestational weight gain, serum insulin, serum leptin, blood glucose at inclusion, 27 and 36 weeks, birth weight, infant length, gestational age, placental weight, Apgar score, Head Circumference and Abdominal Circumference, GDM, PIH, Preeclampsia, prolonged pregnancy, caesarean delivery. |
| Exercise | |||||
| Callaway et al.24 | Randomised Single centre Australia | Participants 50 Intervention: n = 25 Control: n = 25 Inclusion: Obese pregnant women recruited at 12 weeks gestation Exclusion: unclear | An individualised exercise programme with an energy expenditure of 900 kcal/week. Data collection at 12, 20, 28 & 36 weeks. Scheduled for 6 face to face visits, on average attended 4. | 2-h 75 g OGTT and Australasian Diabetes in Pregnancy Society criteria was used for the diagnosis of GDM. | Energy expenditure expressed as a weekly metabolic equivalent hours (MET hours), kilocalories per week, insulin resistance(HOMA-IR), fasting insulin, fasting glucose, GDM |
| Oostdam et al.15 | Randomised Multicentre Amsterdam | Participants 121 Intervention: n = 62 Control: n = 59 Inclusion: Pregnant women at increased risk of GDM if they were obese (BMI > 30) or overweight (BMI > 25) and had one of following: history of macrosomia (offspring >97th centile for gestational age); history of GDM, first grade relative with DM2. Exclusion: recruitment after 20 weeks, age <18 y, inadequate knowledge of Dutch language, GDM diagnosis prior to randomisation, HTN, alcohol abuse, drug abuse, use of medication that affects insulin secretion or insulin sensitivity, serious pulmonary, cardiac, hepatic or renal impairment, malignant disease, serious mental or physical impairment. | Exercise programme on 2 days of the week for 60 min for the remainder of pregnancy. The exercise sessions consisted of aerobic and strength exercises under supervision of trained physiotherapist. | Not stated | Maternal: Fasting blood glucose, fasting insulin, HbA1c, body weight, weight gain, BMI, daily activity, LSCS, GDM. Neonatal: birth weight, LGA (>97th centile for gestation) and fetal growth. |
| Ong et al.25 | Randomised Single centre Australia | 12 Participants Intervention: n = 6 Control: n = 6 Inclusion: sedentary obese women, singleton pregnancy, normal 18 week anatomy USS, no evidence of cardiovascular or pre-existent diabetes. Exclusion: not stated | 10 weeks of home-based supervised exercise (three sessions per week) on an upright stationary cycle. Each session involved a 10-min warm-up followed by 1 or 2 15-min bouts of cycling at an intensity of 50–60% HRmax. As the weeks progressed the exercise intensity was increased to 60–70% HRmax, while the duration was increased 40–45 min. Sessions ended with a 10-min cool down of easy pedalling. | 75 g OGTT Diagnostic criteria not stated. | Fasted serum glucose, serum glucose at 1 and 2 h after OGTT, fasted serum insulin levels, serum insulin levels at 1 and 2 h after OGTT Date collected at baseline (18 weeks) and post-intervention at 28 weeks |
| Lifestyle changes | |||||
| Guelincks et al.26 | Randomised, Single centre, Belgium | 130 randomised. Intervention group : n = 65 Standard care group: n = 65 Inclusion: Obese (BMI > 29) white women attending prenatal clinic before 15 weeks gestation Exclusion: Pre-existing diabetes or developing GDM, recruitment after 15 weeks gestation, premature labour, primary need for nutritional advice in case of metabolic disorder, Crohn’s, allergic conditions and inadequate knowledge of Dutch language. | Brochure which provided advice on nutrition, PA and tips to limit pregnancy related weight gain. Counselled by a trained nutritionist in 3 group sessions(5 women, 1 h)at 15,20 and 32 weeks. The sessions provided subjects with recommendations on a balanced, healthy diet based on official National Dietary recommendation. | Carpenter Coustan criteria by using ≥2 abnormal plasma glucose values (fasting > 95 mg/dL, 1-h >180 mg/dL, 2-h >155 mg/dL and 3-h >140 mg/dL) | GDM, pregnancy induced hypertension, induction of labour, mode of delivery, birth weight, and height, macrosomia (birth weight >4000 gm), Apgar score after 1 and 5 min and admission to neonatal intensive care. |
| Vinter et al.27 | Randomised 2 university hospitals Denmark | 360 Participants Intervention: n = 180 Control: n = 180 Inclusion: 18–40 y, 10–14 weeks gestation, BMI 30-45 kg/m2. Exclusion: Prior serious obstetric complications, chronic diseases, positive OGTT in early pregnancy, alcohol or drug abuse, non-Danish speaking, multiple pregnancy. | Dietary counselling by trained dieticians on 4 separate occasions 15/20/28/35 weeks. Dietary advice was based on official Danish recommendations. Energy requirements were individually estimated according to weight and level of activity Encouraged to be moderately physically active and equipped with a pedometerto motivate and improve daily activity. Women had free full time membership to a fitness centre for 6 months where they had closed training classes with physiotherapists for 1 h each week. After training women were grouped 4–6 times with the physiotherapist using coaching inspired methods for improving participants integration of physical activities in pregnancy. | GDM diagnosed if the 2 h capillary blood glucose was ≥ 9 mmol/L | Gestational weight gain, preeclampsia, pregnancy-induced hypertension, GDM, caesarean section, macrosomia (≥4000 g), LGA (≥90th centile) and admission to neonatal intensive care unit. |
| Korpi-Hyovalti et al.28 | Randomised Multicentre Finland | 54 participants Intervention: n = 27 Control: n = 27 Inclusion: Women 8–12 weeks gestation with one risk factor for GDM (BMI > 25 kg/m2, previous history of GDM, previous infant >4.5 kg at delivery, >40 years, FH of diabetes) and 12-h fasting glucose of 4.8–5.5 mmol/L and 2-h OGTT glucose of <7.8 mmol/L Exclusion: GDM at baseline. | Diet counselling: Goals of the diet, carbohydrate 50–55% diet, fibre 15 g/1000 kcal, fat 30%, sat fat <10% and protein 15–20%. Recommendations for energy intake was 30 kcal/kg/day if normal weight and 25 kcal/kg/day of overweight. Average of 13 appointments with the nurse and 6 with a nutritionist who gave individually tailored dietary advice. Exercise counselling: Encouraged moderate-intensity physical exercise. Six appointments with the physiotherapist who motivated women to either start or continue exercising during their pregnancy and gave written instructions. Goal was 30 min of daily physical activity if previously exercised <2.5 h weekly or 45 min if previously >2.5 h weekly. Women were offered both aerobic and aquafit classes | 2 h 75 g OGTT. Modified from WHO. Fasting plasma glucose 5.6 mmol/ L or 2 h 7.8 mmol/L. | Maternal outcomes: total weight gain, weight at the end of pregnancy, fasting glucose, OGTT 1- and 2-h glucose, gestational diabetes, insulin therapy, pre-eclampsia, IOL, lacerations, LSCS Neonatal outcomes: birthweight, macrosomia, gestational age, admissions to NICU, jaundice requiring phototherapy or respiratory distress. |
| Polley et al.29 | Randomised Single centre USA | Participants: 120 Intervention: n = 61 Control: n = 59 Inclusion: Pregnant women below 20 weeks gestation. Includes both normal weight and obese women. Exclusion: Underweight (BMI < 19.8), women < 18 y, first AN visit >12 weeks, high-risk pregnancy. Outcomes for obese women reported separately which was utilised for the analysis. | Intervention delivered at regular clinics by masters and doctoral level staff with training in nutrition or clinical psychology. Given written and oral information in a) appropriate weight gain during pregnancy b)exercise during pregnancy c)healthful eating during pregnancy Newsletters prompting healthy eating and exercise were mailed biweekly. After each clinic visit women were sent a personalised graph of their weight gain. Women who gained correct amount were encouraged, too little were advised to seek guidance from physician outside of the study. Women who exceeded recommended weight gain were given additional individualised nutrition and behavioural counselling. | Not stated | Weight gain during pregnancy, those who exceed IOM weight recommendation during pregnancy, weight gain within IOM recommendations, weight gain below IOM recommendations, total weight gain, postpartum weight loss, net weight retention, mean birthweight, low birthweight <2500 g, macrosomia >4000 g, weeks at delivery, preterm delivery <36 weeks, LSCS, Pre-eclampsia, maternal hypertension, GDM |
| Luoto et al.30 | Randomised Multicentre Finland | Participants: 442 Intervention: 196 Control: 246 Inclusion: 8–12 weeks gestation, 1 risk factor for GDM (BMI > 25, GDM or any signs of glucose intolerance or macrosomia > 4500 g in a previous pregnancy, first- or second-degree relative with diabetes, age > 40 Exclusion: abnormal baseline OGTT (fasting > 5.3 mmol/L, 1 h > 10 mmol/L, 2 h > 8.6 mmol/L), pre-pregnancy type 1 or 2 diabetes, unable to speak Finnish, age < 18 y, multiple pregnancy, physical restriction preventing physical activity, substance abuse, treatment or clinical history of psychiatric illness. | Physical activity counselling and recommendations for appropriate weight gain at 8–12/40 and enhanced at next 4 visits, dietary counselling at 16–18 weeks and all enhanced at next 3 visits until OGTT at 26–28/40. | OGTT with either fasting ≥ 5.3 mmol/L, 1 h > 10.0 mmol or 2 h > 8.6 mmol/L | GDM, fasting glucose levels and OGTT 1- and 2-h blood glucose levels, insulin levels, HOMA-IR, pre-eclampsia, birthweight, macrosomia >4000 g and >4500 g, SGA, crown-heel length, ponderal index, HC, gestational weight gain |
| Phelan et al.31 | Randomised Multicentre USA | Participants: 400 Intervention: n = 201 Control: n = 200 Inclusion: Gestational age 10–16 weeks, BMI 19.8–40, non-smoking, > 18 y, fluently English speaking, access to a telephone and a singleton pregnancy. Exclusion: Major health or psychiatric disease, weight loss during pregnancy or a history of > 3 miscarriages. Outcomes for obese women reported separately which was utilised for the analysis. | One face-to-face visit at the onset of treatment who discussed appropriate weight gains during pregnancy, physical activity (walking 30 min most days) and calorie goals (20 kcal/kg). Body weight scales, food records and pedometers were provided to promote adherence to daily self monitoring. Automated postcards that prompted healthy eating and exercise habits were mailed weekly. Personalised graphs of weight gain with feedback. Three supportive phone calls from dietician. If over or under weight gain then additional calls (2 per month) to provide structured meal plans and specific goals until weight gain returned to appropriate amount. | Not stated | Weight gain to delivery, weight 6 months post delivery, low birthweight (<2500 g), birthweight, macrosomia >4000 g), weeks gestation at delivery, preterm delivery (<36 weeks), LSCS, preeclampsia, maternal hypertension, GDM. |
| Metformin | |||||
| Metformin | |||||
| Vanky et al.32 | Randomised Double blinded Single centre Norway | 40 Participants Intervention: n = 18 Placebo: n = 22 Inclusion: diagnosis of PCOS prior to pregnancy, aged 18–40, gestation between 5 and 12 weeks, a singleton viable fetus on USS Exclusion: Liver disease, creatinine >130 mm/L, alcohol abuse, previously known diabetes mellitus, fasting glucose >5.6 mmol/L, treatment with oral glucocorticoids or use of drugs known to interfere with metformin. | Metformin 850 mg od week 1 increased to 850 mg bd. All participants received od multivitamin, folic acid and were given verbal and written dietary and lifestyle counselling at inclusion. | Diagnosed using WHO 1998 criteria 75 g OGTT with 2-h value ≥ 7.8 mmol/L. | Androgen levels, all pregnancy complications (Preterm delivery, PE, LSCS, postpartum endometritis, ARDS), GDM, gestational age at birth, gestational length, HC, birthweight, placental weight, Apgar @5 and 10 min, umbilical PH. |
| Vanky et al.33 | Randomised, double-blind 11 Centres (3 university hospitals, 7 local hospitals and 1 gynaecology specialist practice) Norway | 274 Randomised Intervention: n = 136 Placebo: n = 138 Inclusion: PCOS diagnosed according to the Rotterdam criteria, age 18–45, gestational age 5–12 weeks, single viable fetus on ultrasonography. Exclusion: ALT > 90 IU/L, serum creatinine > 1.7 mg/dL, alcohol abuse, previously diagnosed diabetes or fasting glucose > 126 mg/dL at time of inclusion, treatment with oral glucocorticoids or use of drugs known to interfere with metformin. | Metformin 500 mg bd for first week and then 1000 mg bd for the rest of the study. All participants advised to take metformin and one daily multivitamin. All participants received written and verbal counselling on dietary and lifestyle advice. | 75 g OGTT and diagnosis with fasting glucose > 126 mg/dL or 2-h level > 140 mg/dL. WHO criteria | Primary outcomes: Pre-eclampsia, preterm delivery, new GDM. Secondary outcomes: weight gain, BP, HR, labour onset (spontaneous vs induction), labour length, mode of delivery, blood loss, birthweight, fetal length, ponderal index, HC, placental weight, Apgar @1, 5 and 10 min, umbilical artery pH, sex, weight. |
Table 2.
Studies excluded.
| Study | Intervention | Reason for exclusion |
|---|---|---|
| Asbee et al.34 | Diet, lifestyle vs control | Pregnant women with no risk factors |
| Barakat et al.35 | Exercise vs control | Healthy pregnant women |
| Begum et al.36 | Metformin vs placebo | Observational study |
| Clapp37 | low GI vs high GI diet | One intervention vs another |
| Rhodes et al.38 | Low GI vs low fat diet | One intervention vs another |
| Fraser et al.39 | High fibre vs control | Healthy pregnant women with no risk factors |
| Hopkins et al.40 | Exercise vs control | Healthy pregnant women |
| Hui et al.41 | Exercise and nutrition vs standard management | Pregnant women with no risk factors |
| Jeffries et al.42 | Weight recording vs standard management | Pregnant women with no risk factors |
| Luoto et al.43 | Probiotic vs control | Pregnant women with no risk factors |
| Leitinen et al.44 | Diet vs control | Pregnant women with no risk factors |
| Moses et al.45 | Low GI vs high GI | One intervention vs another |
| Walsh et al.46 | Low GI vs standard care | Included women who previously delivered macrosomic baby weighing >4 kg |
Comparison of diet versus standard care
Three studies compared diet with standard antenatal care.21–23 A total of 455 women were included; 49 were lost to follow-up. Mean age (Diet group 27.7 years vs standard care 29.0 years) and mean BMI ((Diet group 36.1 kg/m2 vs standard care 36.4 kg/m2) were comparable between the groups.
Primary outcome
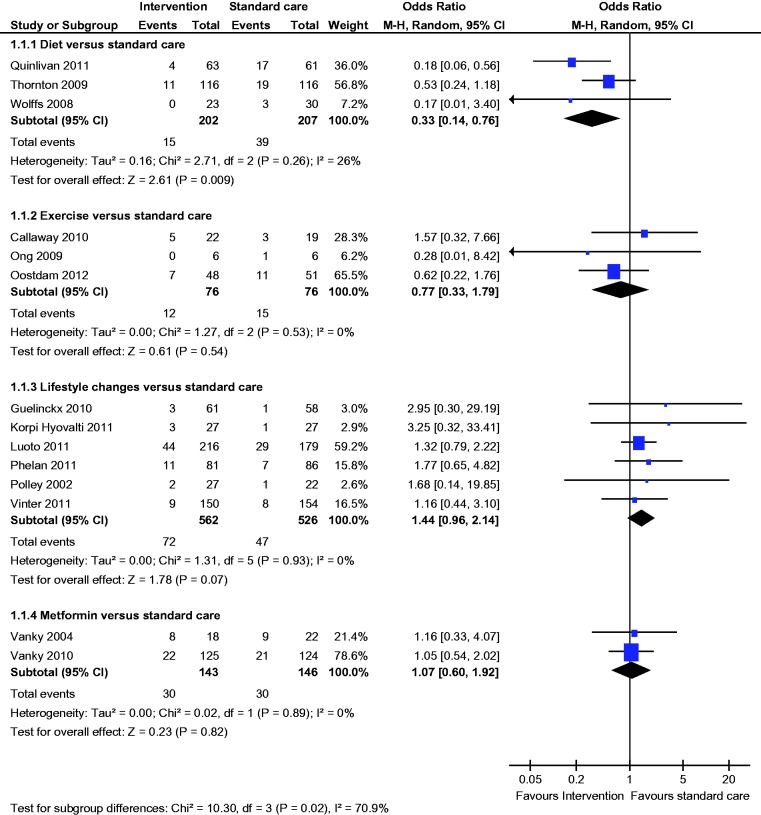
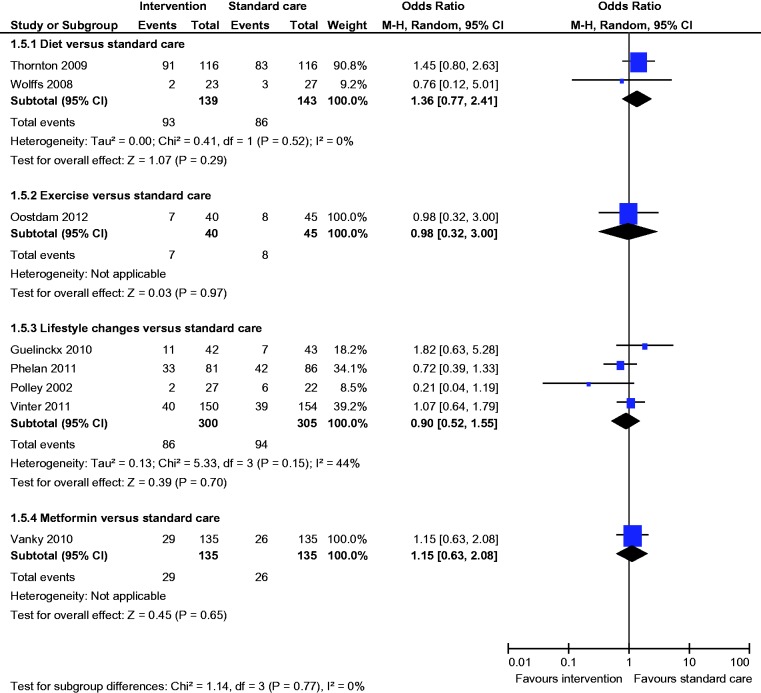
All three studies reported the incidence of GDM; there was a statistically significantly lower incidence of GDM (OR 0.33, 95% CI 0.14 to 0.76) with dietary intervention compared to standard care (diet 7% vs standard care 18%) (Figure 2).
Figure 2.
Gestational diabetes.
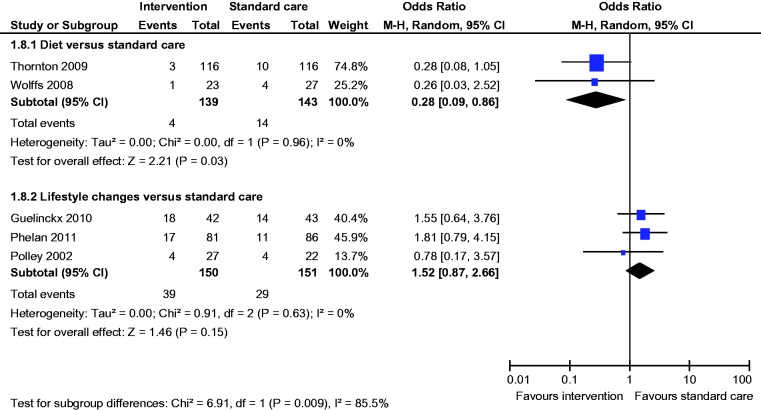
Secondary outcomes
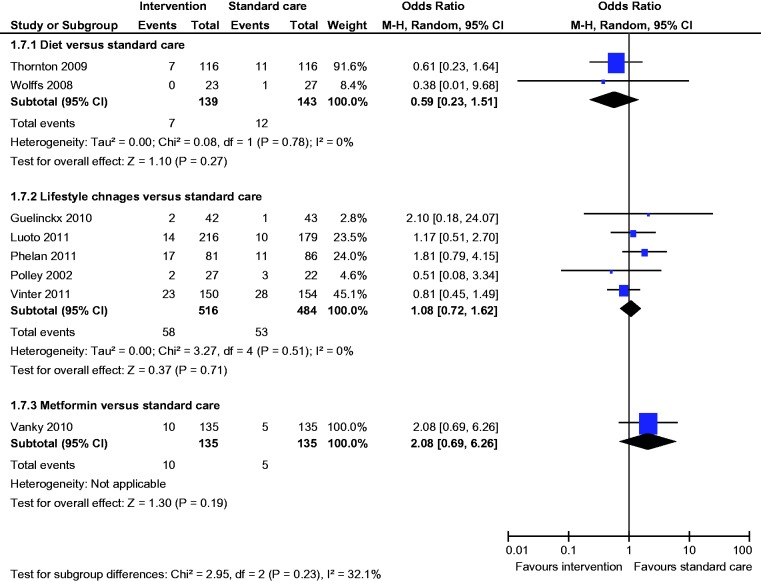
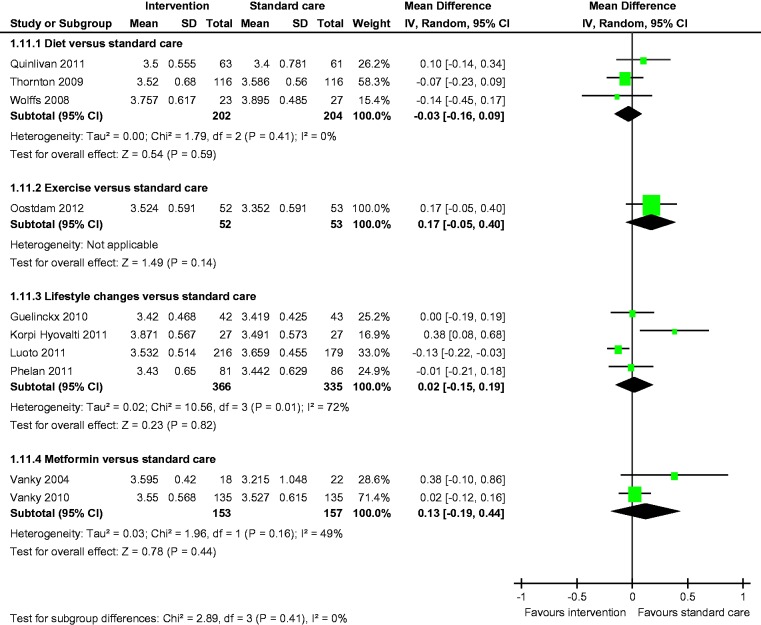
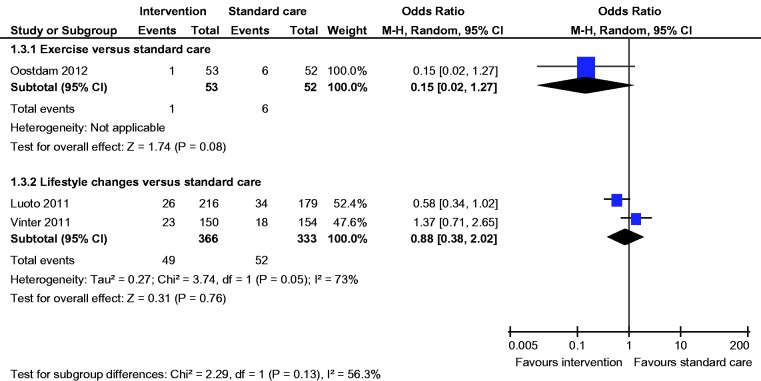
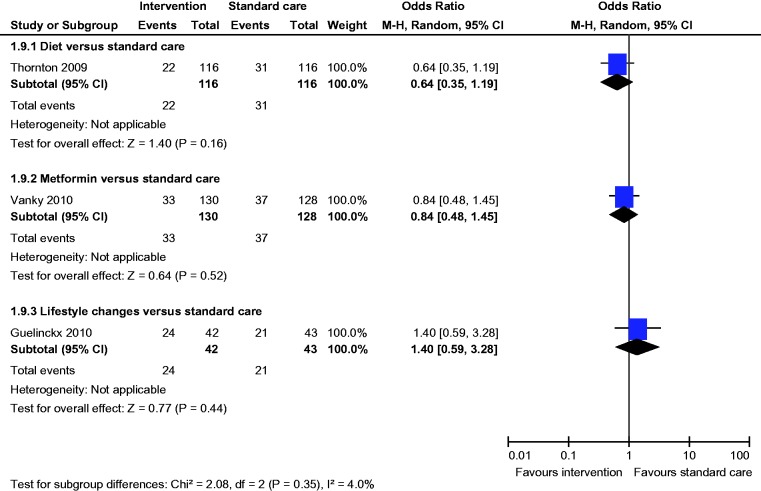
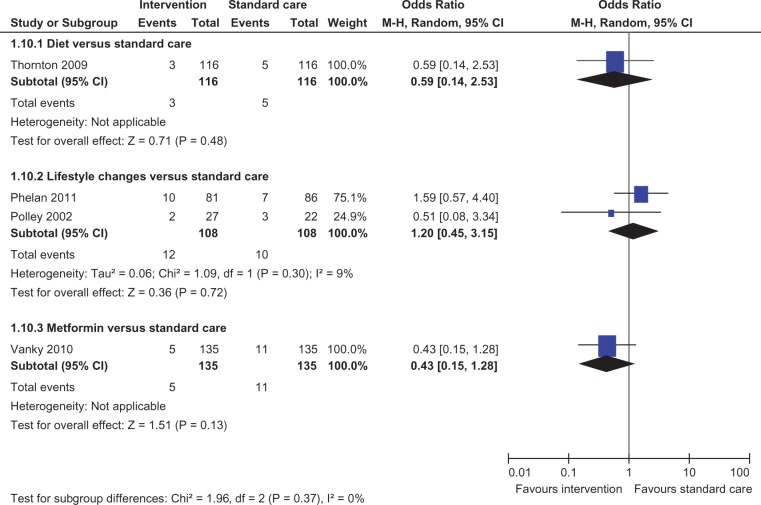
Meta-analysis of two studies22,23 showed a statistically significant lower incidence of gestational hypertension with dietary intervention compared to standard care (OR 0.28, 95% CI 0.09 to 0.86) (Figure 8). There was no statistically significant difference in the rates of macrosomia, caesarean section, pre-eclampsia, induction of labour, preterm birth and mean birth weight (Figures 5, 6, 8–12).
Figure 8.
Pre-eclampsia.
Figure 5.
Macrosomia.
Figure 6.
Caesarean section.
Figure 12.
Mean birth weight.
Comparison of exercise versus standard care
Three studies compared exercise with standard antenatal care.15,24,25 A total of 183 women were included; 50 were lost to follow-up. Mean age (Exercise group 30.4 years vs standard care group 30.5 years) and BMI (Exercise group 34.05 kg/m2 vs standard care group 34.5 kg/m2) were comparable between the groups.
Primary outcome
All three studies reported the incidence of GDM; there was no statistically significant difference in the incidence of GDM (OR 0.77, 95% CI 0.33 to 01.79) between the groups (Figure 2).
Secondary outcomes
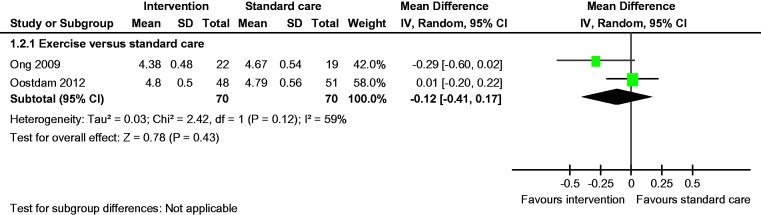
There was no statistically significant difference in the rates of LGA and caesarean section between the groups (Figures 3 and 6). Fasting blood glucose and mean birth weight of both the groups were similar (Figures 3 and 12).
Figure 3.
Fasting blood glucose.
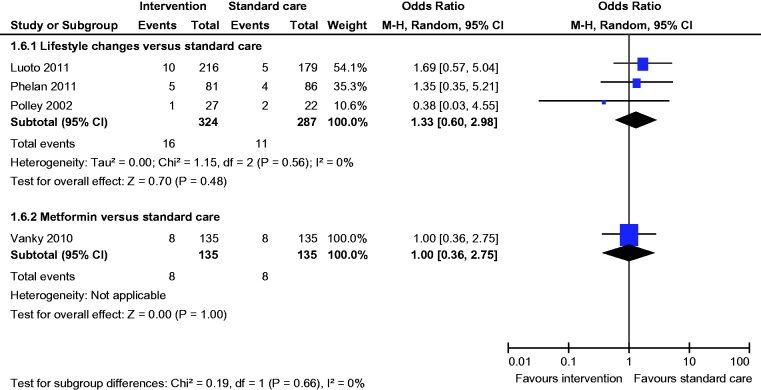
Comparison of lifestyle changes versus standard care
Six studies compared lifestyle changes with standard antenatal care.26–31 A total of 1470 women were included; 189 were lost to follow-up. Mean age (Diet and exercise group 28.3 y vs Standard care group 28.8 years) and BMI (Diet and exercise group 30.3 kg/m2 vs Standard care group 29.4 kg/m2) were comparable between both groups.
Primary outcome
All six studies reported the incidence of GDM; there was no statistically significant difference in the incidence of GDM (OR 1.44, 95% CI 0.96 to 2.14) between the groups (Figure 2).
Secondary outcomes
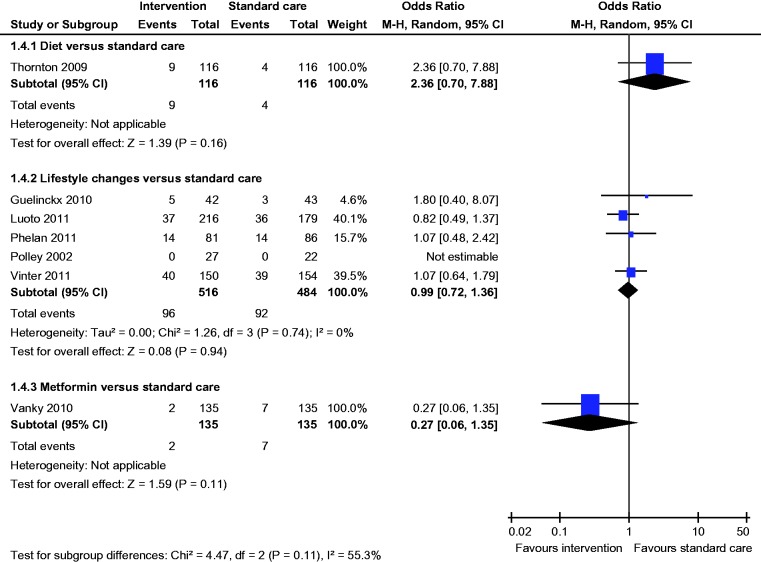
There was no statistically significant difference in the rates of LGA, macrosomia, caesarean section, SGA, pre-eclampsia, gestational hypertension, induction of labour, preterm birth and mean birth weight (Figures 4–12).
Figure 4.
Large for gestational age.
Figure 7.
Small for gestational age.
Figure 9.
Gestational hypertension.
Figure 10.
Induction of labour.
Figure 11.
Preterm birth.
Comparison of metformin versus standard care
Two studies compared metformin with standard antenatal care.32,33 A total of 314 women were included; 17 were lost to follow-up. Mean age (Metformin group 29.3 years vs Standard care group 28.8 years) and BMI (Metformin group 30.8 kg/m2 vs Standard care group 28.9 kg/m2) were comparable between both groups.
Primary outcome
Both studies reported the incidence of GDM; there was no statistically significant difference in the incidence of GDM (OR 1.04, 95% CI 0.60 to 1.92) between the groups (Figure 2).
Secondary outcomes
There was no statistically significant difference in the rates of macrosomia, caesarean section, SGA, pre-eclampsia, induction of labour, preterm birth and mean birth weight (Figures 5–8 and 10–12).
Health economic evaluation
None of the studies assessed the cost to health services.
Heterogeneity
Methodological heterogeneity was assessed before analysis. No studies were excluded on the basis of methodological heterogeneity. There was a low estimate of statistical heterogeneity (I2 ≤ 25%) in GDM (comparison Diet vs Standard care). There was moderate heterogeneity (I2 between 25% and 75%) in Fasting blood glucose (comparison exercise vs standard care), LGA (Comparison diet and exercise vs standard care), caesarean section (comparison Diet and exercise vs standard care) and Mean Birth Weight (Comparison Diet and exercise vs standard care and metformin vs standard care).
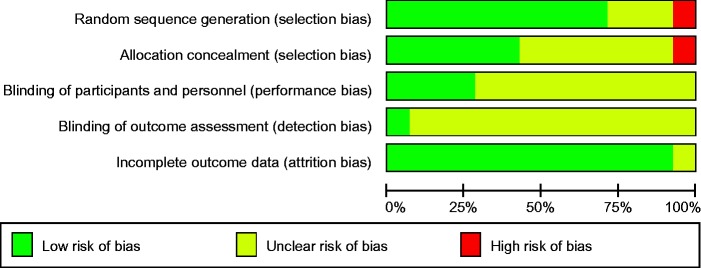
Risk of bias
The risk of bias was assessed using a risk-of-bias graph (Figure 13). Allocation concealment and blinding were poorly reported.
Figure 13.
Risk of bias graph.
Discussion
Main findings
GDM continues to be a challenging obstetric condition and the incidence is increasing. This review has evaluated primary prevention of GDM in women who have risk factors for developing GDM during pregnancy. We have found that dietary interventions have demonstrated a significantly reduced rate of GDM and gestational hypertension in pregnant women with risk factors for GDM but lifestyle, exercise or drug interventions have not. Healthy eating advice by a trained dietician, weighing at each antenatal visit and review of food records were utilised by all three included dietary intervention trials, however the number of follow-up visits and duration of each consultation were variable.
Strengths and limitations
This review has several strengths. The search was thorough and systematic without language restrictions. Two reviewers independently performed the study selection and data extraction to minimise errors. We contacted the authors for unpublished information. We adhered to PRISMA19 statement in reporting our review. We used the random effect model20 throughout the meta-analysis and therefore reduced the impact of statistical heterogeneity. Our inclusion criteria were well defined with only high-risk women, thus targeting a specific cohort of women to whom the interventions could be applied. Obviously, with all interventions there are cost implications and by limiting interventions to a high-risk group, rather than the entire pregnant population, it is more likely that the intervention will be cost-effective to run as the risk/benefit ratio will be greater.
Our review has a number of limitations: methodological heterogeneity, whilst only high-risk women were included, this is still a heterogeneous group with all risk factors (modifiable and non-modifiable) for GDM, different dietary and exercise interventions used by the trialist and also different diagnostic criteria used to diagnose GDM by different trialist. Small numbers of studies were included in the meta-analysis and not all outcomes were reported by the trials included in the review. The allocation concealment and blinding of outcome assessor were not reported in most studies.
Interpretation
Oostdam et al.47 conducted a similar systematic review which included all pregnant women not just high-risk women, therefore a heterogeneous group. They also found dietary intervention significantly reduced the incidence of GDM in pregnant women. The results of Oostdam et al.’s systematic review also suggest that a low glycaemic diet reduced the risk of LGA and exercise programme significantly reduced macrosomia. Our review did not show a significant difference in LGA or macrosomia rates with any of the interventions.
Thangaratinam et al.48 conducted a systematic review to evaluate the effect of dietary and lifestyle interventions in pregnancy on maternal and fetal weight. They concluded that among the interventions, those based on diet are the most effective and are associated with significant reductions in maternal weight gain, pre-eclampsia, GDM, gestational hypertension and preterm labour. However their review included trials on women with any BMI, obese and overweight or only obese women, women with diagnosis of GDM and with pre-existing diabetes, therefore a heterogeneous group unlike our review which included only trials with pregnant women with risk factors for GDM. Hence the application of the results of Thangaratinams et al.’s review to groups of women with risk factors cannot be justified.
A systematic review49 to assess the benefits and harm of antenatal dietary or lifestyle interventions for pregnant women who are overweight or obese did not show any statistically significant difference for LGA infant, mean gestational weight gain, GDM, pre-eclampsia, preterm labour or caesarean section. However this review was published in 2010, since then several trials have been published, hence the difference in the results between this review and ours.
There are two Cochrane reviews5,50 looking at primary prevention of GDM. Tieu et al.50 looked at dietary advice in pregnancy for preventing GDM. Three trials were included in the review. One trial analysed high-fibre diets and two trials assessed low glycaemic index (LGI) versus high glycaemic index diets for pregnant women. Again all three trials included healthy pregnant women without any risk factors for GDM. Whilst they did not find a statistically significant difference in the incidence of GDM, they did find that there was a reduction in fasting blood sugars, LGA incidence, a reduction in Ponderal index and birth centiles with an LGI diet. These trials were excluded from our review as we included only trials performed on women with risk factors.
Han et al.5 also published a Cochrane review looking at exercise and the incidence of GDM. Like our review they found no significant difference in the incidence of GDM and other outcomes. Again, they included all pregnant women not just high-risk women.
Although dietary intervention significantly reduced the incidence of GDM and gestational hypertension, exercise and lifestyle intervention (which included diet, exercise) were not associated with statistically significant differences in any of the outcomes. Compliance with exercise was reported to be poor from second trimester in most of the trials which could be a reason for not finding a statistically significant difference with exercise. This situation mimics real life, thereby making the value of exercise in preventing GDM in pregnancy questionable. Moreover, dietary advice was provided by a qualified dietician, which included healthy eating, energy intake based on energy requirement, prescribed balanced regime (carbohydrate 40%, fat 30% and protein 30%) and review of food diary in all the diet-only trials. Whereas lifestyle intervention trials provided dietary intervention by a trained dietician, which included dietary advice in a group sessions, written information about healthy eating did not include a prescribed regime, intake based on energy requirement or food diary. This difference in the dietary intervention could explain the lower incidence of GDM with diet-only trials whereas there was no difference with the lifestyle intervention trials. Moreover, none of the trials reported per protocol analysis due to small numbers; therefore, the true effect of exercise and lifestyle intervention could not be assessed.
Conclusion
This review has demonstrated that dietary interventions have a statistically significant effect on the primary prevention of GDM and gestational hypertension in women who are at risk of developing GDM. No statistically significant difference was found for interventions involving exercise, lifestyle (dietary and exercise) or drug interventions. This evidence should be interpreted with caution as only a small number of trials were included. Whilst the results for dietary intervention and its role in primary prevention is encouraging, adequately powered larger multicentre RCTs using standardised dietary intervention and standardised outcome measures need to be performed to determine whether these interventions can be applied to women at risk of GDM. The cost of the interventions also needs to be calculated to identify if the interventions used are cost effective.
Acknowledgements
The authors thank Mireille van Poppel and Kym Guelfi for providing unpublished data and Librarian, Sheffield Teaching Hospital, for helping with literature search.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical approval
Not required.
Guarantor
PM is the guarantor.
Contributorship
PM and GG developed the protocol with input from other authors. PM and GG performed the search, study selection and data extraction. PM and GG analysed the results. PM, GG, TF, SS and RB drafted the manuscript. All authors provided input into the development of manuscript.
References
- 1.ACOG Practice Bullitin. Clinical management guidelines for obstetrician-gynaecologists. Obstetr Gynaecol 2001; 98: 525–538. [PubMed] [Google Scholar]
- 2.Hoffman L, Nolan C, Wilson JD, et al. Gestational diabetes mellitus—management guidelines. The Australasian Diabetes in Pregnancy Society. Med J Aust 1998; 169: 93–97. [DOI] [PubMed] [Google Scholar]
- 3.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998; 21: B161–B167. [PubMed] [Google Scholar]
- 4.CG63 Diabetes in Pregnancy – NICE guideline. London: National Institute for Clinical Excellence, http://www.nice.org.uk/nicemedia/live/11946/41320/41320.pdf (2008, accessed January 2014).
- 5.Han S, Middleton P, Crowther CA. Exercise for pregnant women for preventing gestational diabetes mellitus. Cochrane Database Syst Rev 2012; 7: CD009021–CD009021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottalico JN. Recurrent gestational diabetes: risk factors, diagnosis, management, and implications. Semin Perinatol 2007; 31: 176–184. [DOI] [PubMed] [Google Scholar]
- 7.Dabelea D, Snell-Bergeon JK, Hartsfield CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 2005; 28: 579–584. [DOI] [PubMed] [Google Scholar]
- 8.Mulla WR, Henry TQ, Homko CJ. Gestational diabetes screening after HAPO: has anything changed? Curr Diabetes Rep 2010; 10: 224–228. [DOI] [PubMed] [Google Scholar]
- 9.Office for National Statistics. Conception Statistics, England and Wales, www.statistics.gov.uk/downloads/theme_population/Table_2.xls (2009, accessed 4 August 2009).
- 10.ISD Scotland. Births & Babies, www. isdscotland.org/isd/1436.html (2009, accessed 4 August 2009).
- 11.Yogev Y, Metzger BE, Hod M. Establishing diagnosis of gestational diabetes mellitus: impact of the hyperglycemia and adverse pregnancy outcome study. Semin Fetal Neonatal Med 2009; 14: 94–100. [DOI] [PubMed] [Google Scholar]
- 12.The NHS Information Centre. Health survey for England 2007: healthly lifestyles: knowledge, attitudes and behaviour, www.ic.nhs.uk/pubs/hse07healthylifestyles (2008, accessed 4 August 2009).
- 13.Heslehurst N, Ells LJ, Simpson H, et al. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG 2007; 114: 187–194. [DOI] [PubMed] [Google Scholar]
- 14.International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. International Association of Diabetes and Pregnancy Study Groups Consensus Panel*. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luoto R, Kinnunen TI, Aittasalo M, et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: a cluster-randomized controlled trial. PLoS Med 2011; 8: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oostdam N, van Poppel MNM, Wouters MGAJ, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. Br J Obstetr Gynaecol 2012; 119: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 17.Crowther AC, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New Eng J Med 2005; 352: 2477–2486. [DOI] [PubMed] [Google Scholar]
- 18.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009; 361: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waugh N, Royle P, Clar C, et al. Screening for hyperglycaemia in pregnancy: a rapid update for the National Screening Committee. Health Technol Assess 2010; 14: 45–45. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A, Altman DG, Tetjlaf J, et al. The PRISMA statement for reporting systematic reviews and meta-analysis of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: 65–94. [DOI] [PubMed] [Google Scholar]
- 21.Biggerstaff BJ, Tweedie RL. Incorporating variability in estimates of heterogeneity in the random effects model in mata-analysis. Stat Med 1997; 16: 753–768. [DOI] [PubMed] [Google Scholar]
- 22.Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust N Z J Obstetr Gynaecol 2011; 51: 141–146. [DOI] [PubMed] [Google Scholar]
- 23.Thornton YS, Smarkola C, Kopacz SM, et al. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. J National Med Assoc 2009; 101: 569–577. [DOI] [PubMed] [Google Scholar]
- 24.Wolff S, Legarth J, Vangsgaard K, et al. A randomised trial of the effects of dietary councelling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obesity 2008; 32: 495–501. [DOI] [PubMed] [Google Scholar]
- 25.Callaway LK, Colditz PB, Byrne NM, et al. Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care 2010; 33: 1457–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ong MJ, Guelfi KJ, Hunter T, et al. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab 2009; 35: 418–421. [DOI] [PubMed] [Google Scholar]
- 27.Guelinckx I, Devlieger R, Mullie P, et al. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr 2010; 91: 373–80. [DOI] [PubMed] [Google Scholar]
- 28.Vinter CA, Dorte MJ, Ovesen P, et al. The LiP (Lifestyle in Pregnancy) Study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 2011; 34: 2502–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korpi-Hyövälti EAL, Laaksonen DE, Schwab US, et al. Feasibility of a lifestyle intervention in early pregnancy to prevent deterioration of glucose tolerance. BMC Public Health 2011; 11: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obesity 2002; 26: 1494–1502. [DOI] [PubMed] [Google Scholar]
- 31.Phelan S, Phipps MG, Abrams B, et al. Randomized trial of a behavioural intervention to prevent excessive gestational weight gain: the Fit for Delivery Study. Am J Clin Nutr 2011; 93: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanky E, Salvesen KA, Heimstad R, et al. Metformin reduces pregnancy complications without affecting androgen levels in pregnant polycystic ovary syndrome women: results of a randomized study. Hum Reprod 2004; 19: 1734–1740. [DOI] [PubMed] [Google Scholar]
- 33.Vanky E, Solhild S, Hemistad R, et al. Metformin versus placebo from First Trimester to Delivery in Polycystic Ovary Syndrome: a randomized, controlled multicenter study. J Clin Endocrinol Metab 2010; 95: E448–E455. [DOI] [PubMed] [Google Scholar]
- 34.Asbee SM, Jenkins TR, Butler JR, et al. Preventing excessive weight gain during pregnancy through dietary and lifestyle couseling: a randomized controlled trial. Obstetr Gynaecol 2009; 113: 305–312. [DOI] [PubMed] [Google Scholar]
- 35.Barakat R, Cordero Y, Coteron J, et al. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: a randomised controlled trial. Br J Sports Med 2012; 46: 656–661. [DOI] [PubMed] [Google Scholar]
- 36.Begum MR, Khanam NN, Quadir E, et al. Prevention of gestational diabetes mellitus by continuing metformin therapy throughout pregnancy in women with polycystic ovary syndrome. J Obstet Gynaecol Res 2009; 35: 282–286. [DOI] [PubMed] [Google Scholar]
- 37.Clapp JF., 3rd Maternal carbohydrate intake and pregnancy outcome. Proc Nutr Soc 2002; 61: 45–50. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes ET, Pawlak DB, Takoudes TC, et al. Effects of a lowglycemic load diet in overweight and obese pregnant women: a pilot randomized controlled trial. Am J Clin Nutr 2010; 92: 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser RB, Ford FA, Milner RD. A controlled trial of a high dietary fibre intake in pregnancy—Effects on plasma glucose and insulin levels. Diabetologia 1983; 25: 238–241. [DOI] [PubMed] [Google Scholar]
- 40.Hopkins SA, Baldi JC, Cutfield WS, et al. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J Clin Endocrinol Metab 2010; 95: 2080–2088. [DOI] [PubMed] [Google Scholar]
- 41.Hui A, Back L, Ludwig S, et al. Lifestyle intervention on diet and exercise reduced excessive gestational weight gain in pregnant women under a randomised controlled trial. BJOG 2012, pp. 70–77. [DOI] [PubMed] [Google Scholar]
- 42.Jeffries K, Shub A, Walker SP, et al. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Med J Aust 2009; 191: 429–433. [DOI] [PubMed] [Google Scholar]
- 43.Luoto R, Laitinen K, Nermes M, et al. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcomes and prenatal and postnatal growth: a double blind, placebo-controlled study. Br J Nutr 2010; 1--8. [DOI] [PubMed]
- 44.Laitinen K, Poussa T, Isolauri E. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr 2009; 101: 1679–1687. [DOI] [PubMed] [Google Scholar]
- 45.Moses RG, Luebcke M, Davis WS, et al. Effect of a lowglycemic- index diet during pregnancy on obstetric outcomes. Am J Clin Nutr 2006; 84: 807–812. [DOI] [PubMed] [Google Scholar]
- 46.Walsh JM, McGowan CA, Mahony R, et al. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. Br Med J 2012; 345: e5605–e5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oostdam N, van Poppel MNM, Wouters MGAJ, et al. Interventions for preventing gestational diabetes mellitus: a systematic review and meta-analysis. J Women’s Health 2011; 20: 1551–1563. [DOI] [PubMed] [Google Scholar]
- 48.Thangaratinam S, Rogozińska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. Br Med J 2012; 344: e2088–e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dodd JM, Grivell RM, Crowther CA, et al. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG 2010; 117: 1316–26. [DOI] [PubMed] [Google Scholar]
- 50.Tieu J, Crowther CA, Middleton P. Dietary advice in pregnancy for preventing gestational diabetes. Cochrane Database Syst Rev 2008; 2: CD006674–CD006674. [DOI] [PubMed] [Google Scholar]