Abstract
An increasing number of women with rare inherited disorders of metabolism are becoming pregnant. Whilst, in general, outcomes for women and their children are good, there are issues that need to be considered. Due to the rarity of many conditions, there is limited specific guidance available on best management. Prepregnancy counselling with information on inheritance, options for reproduction, teratogenicity risk, potential impact on maternal health and long-term health of children should be offered. With appropriate specialist management, the teratogenic risk of conditions such as maternal phenylketonuria (PKU) can be eliminated, and the risk of metabolic decompensation in other disorders of intoxication or energy metabolism significantly reduced. Newer therapies, such as enzyme replacement therapy, appear to be safe in pregnancy, but specific advice should be sought. Multidisciplinary management, and close liaison between obstetricians and other specialists is required for women in whom there is cardiac, renal, respiratory, joint or other organ involvement.
Keywords: Metabolic, pregnancy, inherited, teratogenicity, decompensation, post-partum
Introduction
Inherited metabolic diseases (IMDs) are clinically heterogeneous, individually rare disorders that can present at any age, and typically, but not always, are associated with abnormal biochemical tests (usually specialist rather than routine laboratory testing). Broadly speaking they can be divided into three groups:
Disorders of intoxication give rise to an acute or progressive intoxication secondary to the accumulation of toxic compounds proximal to a metabolic block, e.g. disorders of amino acid metabolism such as phenylketonuria (PKU, Online Mendelian Inheritance in Man (OMIM) catalogue of human genes and genetic condition number #261600), the organic acidurias and the urea cycle defects such as ornithine transcarbamylase (OTC) deficiency (#311250).
Disorders of energy metabolism result in an energy deficiency in tissues such as liver, muscle, brain or heart, e.g. mitochondrial respiratory chain defects, fatty acid oxidation defects and glycogen storage disorders (GSDs).
Disorders of complex molecules cause disturbance in the synthesis or catabolism of complex molecules. Symptoms tend to be progressive and not dependent on dietary / energy intake, e.g. the lysosomal storage disorders and the peroxisomal disorders.
An increasing number of patients with IMD are surviving to adulthood and wishing to have children of their own.1 Although many women have successful pregnancies, with an excellent outcome, these patients present challenges from the reproductive perspective. Apart from PKU, for which there is substantial evidence for management guidelines during pregnancy, information on pregnancy for most of the other inherited metabolic conditions comes either from isolated case reports or small case series and no single centre is likely to have enough experience with any single condition to provide definitive guidelines for management.
This review highlights how these disorders may impact on pregnancy and the puerperium, with emphasis on both maternal and fetal outcome. Table 1 lists some of the specific issues that need to be considered when a woman with an inherited disorder of metabolism plans a pregnancy. The care of women with an inherited disorder of metabolism in pregnancy should be managed together with a physician and/or dietician with expertise in this field. The British Inherited Metabolic Disease Group (BIMDG: www.bimdg.org.uk) can provide details of centres with the UK. Similarly, the Society for the Study of Inborn Errors of Metabolism - Adult Metabolic Physicians Group (SSIEM-AMG: http://www.ssiem.org/amp/contact.asp) can provide contact details for specialist centres worldwide.
Table 1.
Issues to be considered when a woman with an inherited disorder of metabolism plans a pregnancy.
| Pre-pregnancy |
During pregnancy and labour |
Post-partum |
|||
|---|---|---|---|---|---|
| Issue | Comment / Examples | Issue | Comment / Examples | Issue | Comment |
| Fertility | Conditions associated with premature ovarian insufficiency, e.g. Galactosemia | Metabolic control | Nausea and vomiting (‘morning sickness’) – risk factor for metabolic decompensation in any disorder of energy metabolism, e.g. FAOD, GSD, UCD, MSUD, GA1, disorders of ketone body metabolism | Metabolic control | Puerperial stress, Involution of the uterus, breastfeeding – risk period for decompensation of disorders of protein or energy metabolism |
| Risk of miscarriage | Conditions with poor metabolic control, e.g. PKU, HCU | Effects on fetus | Potential teratogenicity, e.g. maternal PKU syndrome | Contraception | Discuss if required |
| Medications | Consider possible teratogenicity, e.g. statins, ACE inhibitors, some anticonvulsants | Growth retardation (secondary to protein and / or calorie restriction or recurrent hypoglycemia) | Long-term outcome of the child | Consider follow-up for children born to mothers with rare conditions where long-term outcome remains uncertain | |
| Genetic counselling | Advice re reproductive options and options for PGD, antenatal and postnatal diagnosis | Effects on mother | Worsening of underlying condition, e.g. dyslipidemia in LPL deficiency, or secondary co-morbidities (see also Table 2) | ||
| Metabolic control | Optimise treatment, e.g. PKU, urea cycle disorders, HCU, any disorder of energy metabolism | Other maternal issues | Maternal learning difficulties, support network and financial concerns | ||
| Nutritional issues and general health | Optimisation of weight, ensure adequate vitamin and mineral supplementation in those on restricted diets | During labour and delivery | Put a plan in place to consider options for spontaneous labour, planned elective labour or need for emergency intervention. | ||
| Ensure energy requirements are met in any disorder of energy metabolism | |||||
| Muscular involvement, e.g. GSD III, mitochondrial disorders, acid maltase deficiency. Cardiac involvement, e.g. GSD III, mucopolysaccharidoses | |||||
FAOD: fatty acid oxidation disorders; GSD: glycogen storage disorders; UCD: urea cycle disorders; MSUD: maple syrup urine disease; GA1: glutaric aciduria type I; PKU: phenylketonuria; HCU: homocystinuria; LPL: lipoprotein lipase; PGD: preimplantation genetic diagnosis.
The pre-conception period
When women with an inherited disorder of metabolism reach childbearing age they should be counselled with regard to the potential impact of pregnancy on their condition, as well as the impact of their condition on pregnancy and the outcome for their children. As with any woman planning a pregnancy, prepregnancy advice includes starting folic acid supplementation, stopping smoking, limiting caffeine and alcohol intake and optimising weight, diet and general physical health.2 Many women with an inherited disorder of amino acid or energy metabolism will be treated with a modified diet, which, depending on the specific condition, may be low in protein, high in carbohydrate, low in fat, or high in fat. The aim is to optimise metabolic control and nutritional status if possible prior to pregnancy.
Treatment may need to be altered if the patient is prescribed any known teratogenic medications. A decision to stop other specific medications, e.g. sodium benzoate and sodium phenylbutyrate in women with symptomatic urea cycle disorders, enzyme replacement therapy in Gaucher disease (#230800), or biotin in biotinidase deficiency (#253260) is likely to have significant detrimental effects on maternal health. Successful pregnancies have been described with the use of these and other specialist medications (including enzyme replacement therapies for the lysosomal storage disorders, Fabry disease (#301500) and acid maltase deficiency (#232300)).3–8 Many manufacturers will keep a registry of pregnancies occurring on their product and should be contacted directly for advice if no specific information is available in the literature.
Women with cardiac disease, epilepsy, respiratory disease, or other issues such as significant skeletal disease, need specialist prepregnancy advice and may require additional support and monitoring.
Genetic considerations
Many disorders of metabolism are autosomal recessive in inheritance, thus in non-consanguineous families there is a very low risk of having an affected child but potential parents are often anxious regarding the risks to their children, and genetic counselling should be offered. Preimplantation genetic diagnosis is available for a number of (usually) X-linked inherited disorders of metabolism, e.g. OTC deficiency, adrenoleukodystrophy (#300100) and Fabry disease. Antenatal diagnosis is also available and may have a role in detecting autosomal recessive conditions in communities where there is a founder effect or a high rate of consanguinity.
Maternally-inherited mitochondrial disorders affect approximately 1 in 8000 people.9 These conditions can cause variable phenotypes including heart and liver failure, defects in energy metabolism, blindness, deafness, loss of motor skills and premature death. In 2013/2014 the Human Fertilisation and Embryology Authority provided advice to the UK Government regarding the use of enucleated donated oocytes with normal (wild-type) mitochondria to be used as recipients of nuclear DNA from intending mothers to overcome transmission of mitochondrial disorders. At the time of writing, such techniques are not available clinically.
Teratogenicity
PKU is an autosomal recessive condition caused by deficiency of the enzyme phenylalanine hydroxylase, which converts the dietary amino acid phenylalanine to tyrosine. It is one of the most common disorders seen in an adult IMD clinic, with a prevalence of 1 in 10,000 in northern Europe.10 High phenylalanine levels damage the developing brain and, without treatment, the classical presentation is of delayed motor milestones, microcephaly, and progressive severe mental retardation with seizures. In contrast, patients diagnosed on newborn screening, treated appropriately with a low protein diet and phenylalanine-free amino acid, vitamin and mineral supplementation from an early age, with good control of phenylalanine levels, have normal intellectual development. There is no impact of well-treated PKU on fertility and many women with treated PKU will wish to have a pregnancy.
However, in the early 1960s it was recognised that high maternal phenylalanine levels are also teratogenic to the developing fetus in utero.11 The maternal PKU (mPKU) syndrome includes developmental delay (92%), microcephaly (73%), cardiac defects (12%), low birth weight (40%) and dysmorphic features in the children born to mothers with untreated classic PKU.12 Unlike some harmful substances which affect only a single trimester, excess phenylalanine is associated with a significant increased risk of congenital heart disease in weeks 0–8, brain, fetal and postnatal growth retardation, wide nasal bridge and anteverted nares in weeks 8–12 and neurologic deficits throughout all 40 weeks of pregnancy in a dose-dependent manner.13
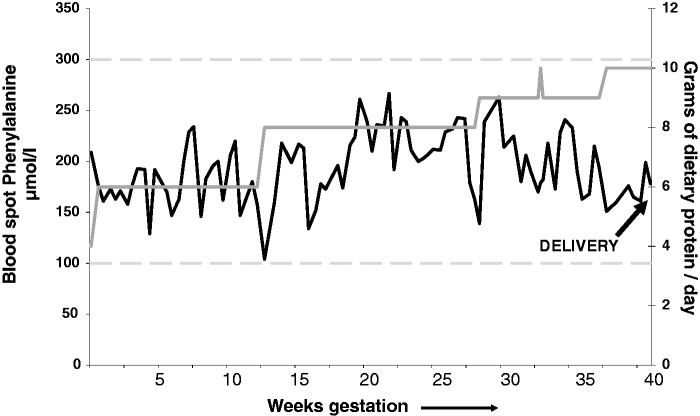
Fortunately, the mPKU syndrome is entirely preventable if women with PKU maintain strict metabolic control of their phenylalanine levels throughout pregnancy. The Maternal PKU Collaborative Study (MPKUCS) established that if mPKU levels are maintained <360 µmol/L, there is no harmful effect on the fetus.14 Target levels of phenylalanine vary slightly from country to country – in the UK the target range during pregnancy is 100–250 µmol/L. As in childhood, lowering and maintenance of maternal phenylalanine levels is achieved by a combination of a diet low in natural protein, supplemented with a phenylalanine-free amino acid mixture, vitamins and minerals. Monitoring is by regular phenylalanine blood spot measurements. Depending on the maternal phenylalanine level, maternal dietary protein restriction may need to be very strict in the first trimester and then, as the fetus grows and protein tolerance increases, protein intake will need to be increased in the second and third trimesters. Figure 1 shows an example of phenylalanine levels and changes in prescribed dietary protein intake throughout pregnancy in a woman with well-controlled PKU.
Figure 1.
Blood spot phenylalanine levels and dietary protein intake in a pregnant woman with well-controlled PKU. Blood spot phenylalanine levels (3 measurements per week during pregnancy) are indicated by the solid black line. Prescribed dietary protein intake (g/day) is indicated by the solid grey line. Target phenylalanine levels (upper and lower limits) are indicated by the dashed grey lines. As the pregnancy progresses protein tolerance increases and dietary protein intake is increased to allow fetal growth. To put the protein intake into context: 1 slice of white bread contains 2.5 g protein; a cup of full-fat milk contains 8 g; and a baked potato contains 2.5 g. A healthy baby was born at 37 + 5 weeks weighing 3090 g.
Girls and women with PKU should therefore be educated and advised to plan their pregnancies, obtain good metabolic control of their phenylalanine levels prior to conception and use appropriate contraception to avoid an unplanned pregnancy if desired.
The potential teratogenicity of other abnormal / elevated metabolites in women with IMD is less clearly defined or unknown. For most conditions and most metabolites there is simply not enough experience available to allow us to draw any firm conclusions about teratogenicity. For example, although the majority of women with homocystinuria (#236200) have successful pregnancies, complications including spontaneous abortion and fetal abnormalities have been described.15–17 Interpreting the significance of these cases is complicated by the fact that many patients with marked hyperhomocysteinemia, may also have secondary B12 deficiency and / or folate deficiency. Advice to women with conditions causing hyperhomocysteinemia therefore is to maximise metabolic control prior to pregnancy and ensure adequate B12 and folate supplementation.
Again, although successful pregnancies have been reported, there is some data to suggest that high maternal tyrosine levels, such as occur in the disorders of tyrosine metabolism, tyrosinemia type I (#276700) and type II (#276600), may have adverse effects on fetal neurodevelopment.18–21 With appropriate treatment, maternal tyrosine levels can be lowered, but there is little consensus as to the target range of tyrosine levels required in pregnancy to protect the fetus.
Although the number of reported pregnancies is small, there is no definitive evidence that the elevated organic acids found in disorders such as methylmalonic acidemia, propionic acidemia (#606054) or glutaric aciduria type I (#231670) are teratogenic, and successful pregnancies have been reported.15,22–25
Given the paucity of data available on the potential teratogenicity of many rare disorders of metabolism, and hence the difficulty in drawing firm conclusions and providing comprehensive pregnancy counselling, publications of case reports of pregnancy remain a useful source of information.
Fertility and miscarriage
In general, fertility is not a major issue in the majority of inherited disorders of metabolism. An exception however is classic galactosemia (#230400), caused by deficient activity of the enzyme galctose-1-phosphate uridyltransferase, this condition has an overall incidence of about 1 in 16,000 to 1 in 60,000.26,27 It typically presents in early infancy with hepatorenal failure, cataracts and sepsis, and is included in the newborn screening programme of a number of countries, although not currently the UK. Prompt recognition of the condition with removal of lactose from the diet results in resolution of liver and kidney involvement but patients remain at risk of long-term complications including movement disorders, speech delay, cognitive problems and premature ovarian insufficiency (POI). POI occurs in up to 90% of women with galactosemia. Fertility is possible however for some women and a small number of successful spontaneous pregnancies, or pregnancies following stimulation with follicular stimulating hormone (FSH) have been reported.28,29
To date, there is no data on the use of techniques such as ovarian tissue cryopreservation, or mature oocyte cryopreservation in women with galactosemia.30 The majority of women who have undergone these procedures worldwide have done so prior to gonadotoxic therapies such as chemotherapy and so have had normal healthy ovaries. The ovaries of many girls with galactosemia are probably already damaged at a young age and so the success rate of these techniques may be lower. Embryo cryopreservation relies on ovarian stimulation, which may be inadequate in many women with galactosemia. For these women, options, such as the use of donor oocytes or adoption should be discussed. Legislation regarding assisted reproductive options may differ between countries and the involvement of an institutional ethics committee, particularly if treatment of a child or use of an experimental technique is proposed, is advisable.
There is an increased risk of miscarriage, usually associated with maternal poor metabolic control, recognized in mPKU, and possibly other conditions including those associated with hypertyrosinemia, hyperhomocystinemia and some disorders of energy metabolism. Regular discussion with women of reproductive age regarding the value of good metabolic control around the time of conception and the early pregnancy period is essential. Prompt treatment of intercurrent illness, significant nausea and / or vomiting and avoidance of known triggers of metabolic decompensation are critical in supporting women with a known disorder of energy metabolism through a pregnancy. Similarly, effective and appropriate means of contraception should be discussed with those women who do not wish to plan a pregnancy.
Other specific issues
Dietary restrictions
Many women with an inherited disorder of metabolism are treated with a modified diet. Vitamin and mineral supplementation should therefore be considered.
Adaptations in maternal metabolism during a normal pregnancy are designed to ensure an adequate supply of nutrients and energy to the fetus.31 A constant and adequate supply of glucose is important, since it is the main energy substrate for fetal metabolism and is essential for normal growth and development.32
Women with defects of energy metabolism may not be able to match glucose production with the increasing demands of pregnancy, e.g. in conditions such as the hepatic GSDs or disorders of ketone body metabolism.15,33 The risk of maternal hypoglycemia is usually greatest in the early morning, after the overnight fast. During prolonged hypoglycemia, the fetal metabolic rate is maintained by oxidation of glucose derived from fetal glycogenolysis and gluconeogenesis. Thus protein breakdown and amino acid oxidation are increased and net gain in terms of protein synthesis is diminished, resulting in intrauterine growth retardation. Prolonged intrauterine exposure to hypoglycemia may also result in long-term/permanent changes in fetal metabolism, which may be responsible for an increased predilection to diseases such as type 2 diabetes in later life.34 Prolonged maternal ketosis may result in a lower IQ in offspring.35 Treatment options include the use of slow release starch, e.g. uncooked cornstarch (UCCS) before bed (with / without another dose during the night) or, in more severe cases, an overnight tube feed.
Protein requirements increase in the second and third trimester.36 Women on a therapeutic restricted protein diet may be at risk of protein-energy malnutrition and micronutrient deficiency at this time resulting in intrauterine growth retardation and children born with low birth weight. In women with urea cycle disorders, who are often protein averse, or with an organic acidemia where appetite is often poor, increasing oral intake appropriately can be a challenge and requires specialist dietetic input. The most common of the urea cycle disorders is X-linked OTC deficiency. Many females with this condition are asymptomatic, but others can have recurrent episodes of hyperammonemia from childhood or present later in adulthood for the first time during a period of metabolic ‘stress’.
Nausea and vomiting in pregnancy
Pregnancy, if complicated by nausea and vomiting, can lead to acute metabolic decompensation in women with inherited disorders of energy metabolism or intoxication due to reduced calorie intake and difficulties taking essential supplements and medications (e.g. the urea cycle defects, the hepatic GSDs, disorders of ketone body metabolism, the organic acidemias and fatty acid oxidation defects). Although rare, if prompt treatment is not given, such decompensation can be sufficiently severe so as to lead to maternal and / or fetal death.15,37 In other conditions, e.g. PKU, reduced oral intake does not lead to acute maternal illness, but will result in maternal protein catabolism leading to higher levels of metabolites such as phenylalanine, thereby increasing the risk of fetal teratogenicity.
Nausea and anorexia must always be taken seriously in such conditions and effective anti-emetic medication and dietary support given early on. The majority of women known to metabolic services will have an oral emergency regimen to start at home in such circumstances. If the oral emergency regimen is not tolerated then women may require hospital admission for intravenous treatment. Oral and emergency guidelines for the management of many inherited disorders of metabolism are available on the BIMDG website (www.BIMDG.org.uk).
Secondary co-morbidities
The potential for impaired organ function is wide and varied. Table 2 lists some of the issues that may need to be considered with specific disease examples. Pregnancy may lead to worsening of an underlying condition, e.g. cardiomyopathy in GSD type III (#232400); growth of hepatic adenoma in GSD type I; dyslipidemia, particularly hypertriglyceridemia, of lipoprotein lipase deficiency (#238600). Alternatively a pre-existing complication may influence factors such as timing and mode of delivery, e.g. skeletal dysplasia in certain lysosomal storage disorders.
Table 2.
Pre-existing organ involvement or other issues that may impact on the management of pregnancy, labour and delivery.
| Issue | Clinical problem | Specific inherited metabolic disease examples |
|---|---|---|
| Liver | Adenoma growth | GSD I |
| Impaired function | Hepatic GSDs, Wilson disease | |
| Cardiac | Valvular disease | Mucopolysaccharidoses / mucolipidoses |
| Impaired ventricular function | Fabry disease, GSD III, mucopolysaccharidoses / mucolipidoses | |
| Dysrhythmia | Fabry disease, carnitinine transporter deficiency, propionic acidemia | |
| Respiratory | Disordered sleep breathing | Acid maltase deficiency, mucopolysaccharidoses / mucolipidoses, muscle GSDs |
| Thoracic deformities | Mucopolysaccharidoses / mucolipidoses | |
| Narrowed airways – difficult intubation | Mucopolysaccharidoses / mucolipidoses | |
| Orthopaedic | Pelvic / hip / knee involvement | Mucopolysaccharidoses / mucolipidoses / x-linked hypophosphatemia |
| Ligamentous laxity | Mucopolysaccharidoses / mucolipidoses | |
| Spinal involvement / fusion | Mucopolysaccharidoses / mucolipidoses | |
| Endocrine | Thyroid dysfunction | Mitochondrial disorders |
| Diabetes | Mitochondrial disorders | |
| Lipid | Hypertriglyceridemia / pancreatitis | Lipoprotein lipase deficiency |
| Renal | Proteinuria | GSD I, Fabry disease |
| Impaired renal function | GSD I, Fabry disease | |
| Muscle | Weakness | Acid maltase deficiency, mitochondrial disorders, some fatty acid oxidation disorders |
| Risk of rhabdomyolysis | Fatty acid oxidation disorders | |
| Coagulation | Increased thrombotic risk | Any condition associated with hyperhomocystinemia, e.g. HCU |
| Post-partum bleeding | GSD I | |
| Intellectual | Ability to adher to treatment recommendations and care for child | Any metabolic condition associated with severe childhood decompensation (e.g. hyperammonemia, hypoglycemia, encephalopathy) leading to fixed intellectual impairment or progressive neurodegeneration |
| Medications | Potential impact if medication stopped | In particular for management of epilepsy, hypertension, dyslipidemia |
A variable degree of learning disability is associated with a number of IMDs, e.g. poorly treated PKU, or following childhood decompensation with hyperammonemia (e.g. urea cycle defects) or hypoglycemia (fatty acid oxidation defects, GSDs). Women with IMD who also have learning difficulties may need additional social and psychological support during pregnancy and following childbirth.
Many obstetricians will be aware of the increased risk of acute fatty liver of pregnancy (AFLP) and haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome in the heterozygous mothers of a fetus with long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD, #609016) deficiency.38–40 No pregnancies have been reported to date in women who have autosomal recessive LCHAD deficiency. A number of these women are now surviving in adulthood and may wish to plan pregnancies of their own. It is not known what their risk of developing similar pregnancy-related complications is, but it is likely to be significantly increased and very close monitoring is advised.
Pregnancy is a prothrombotic period and presentation of hypercoagulable conditions, e.g. homocystinuria during pregnancy and the post-partum period with a thrombus is well described.16,17,41 Homocystinuria is an inherited thrombophilia with a high lifetime risk of thrombosis and guidelines for similar conditions suggest anticoagulation with low weight molecular heparin (LWMH) throughout pregnancy and for seven days to six weeks post-partum.42
Monitoring during pregnancy
The frequency and type of monitoring depend on the particular condition and its severity. For example, at our unit, pregnant women with PKU send in blood spot cards by post three times a week for measurement of phenylalanine levels, whilst women with hepatic GSD III have 24-h metabolic profiling towards the end of the first trimester and fax / email the results of fasting home blood glucose and ketone levels regularly for review thereafter. In addition to their obstetric care, pregnant women are seen routinely approximately once every trimester by the metabolic team. Regular telephone contact with the metabolic dietician is arranged and women are encouraged to email / fax through results of home monitoring. Hospital admission for optimisation of metabolic control is very rarely needed but can be offered if required.
Labour and delivery
Labour and delivery are times of increased energy requirement and women often have poor oral intake for the duration of labour. In women with known defects of energy metabolism, we advise that their babies should be delivered in a hospital setting and our policy is to prescribe additional energy supplementation (usually intravenous 10% dextrose at 2 ml/kg/h, i.e. 120 ml/h for a 60 kg woman) once labour is established. Euglycemia should be maintained throughout the period of delivery. In the absence of other complications, vaginal delivery is usually possible. A planned induction at term may be desirable.
Women with skeletal muscle involvement may find the second stage of labour difficult, necessitating caesarean section. Deterioration of underlying organ damage, e.g. cardiomyopathy in women with GSD type III is also possible. Haemodynamic stress can be reduced by appropriate analgesia and decreasing patients’ anxiety. For any women in whom specific issues can be anticipated, liaison with the obstetrician and obstetric anaesthetist prior to delivery is advised.
Post-partum
Rebound hypoglycemia in the neonate should be monitored if the mother received intravenous dextrose during labour.
Following delivery, there is a well-recognized risk period for acute decompensation of some metabolic conditions, particularly of disorders of urea cycle metabolism such as OTC deficiency.3,43 Classically this decompensation occurs between days 3 and 14 post-partum. Post-partum decompensation is thought to relate to the relative metabolic stress of the changes of the puerperium and an increased protein load for catabolism following involution of the uterus. The behavioural changes of hyperammonemia must not be confused with symptoms of post-partum psychosis or depression. It is worth remembering however, that the majority of women who are heterozygote for an OTC mutation go through pregnancy and labour uneventfully without anyone being aware of the underlying problem (until the diagnosis is made in a more severely affected male relative).
Elevations in post-partum metabolites have also been described in other disorders of protein metabolism such as maple syrup urine disease (#248600) and methylmalonic acidemia.24,44 Recurrent post-partum rhabdomyolysis has been noted following labour in the fatty acid oxidation disorder carnitine palmitoyltransferase type II deficiency (#255110, personal communication, Robin Lachmann). There are no specific guidelines for management of this period in women at risk but treatments used have included; reduction of dietary protein intake, increase in non-protein calorie intake, increase in ammonia-scavenger medications (if appropriate), and use of oral or intravenous emergency regimens.
Breast feeding
The energy requirements for breastfeeding are at least as large as those of pregnancy and women with IMD wishing to breastfeed need to ensure adequate energy intake. Breastfeeding has been reported to cause hypoglycemia in mothers with GSD, but, with specialist dietetic input and providing nutritional requirements can be met, some women have successfully breastfed. However, in symptomatic patients with urea cycle disorders and organic acidemias, the combination of the catabolic state of the puerperium with the nutritional demands of breastfeeding and a poor calorie intake may be a potential trigger for metabolic decompensation and breastfeeding women need to be closely monitored.
Currently there is very little safety information available regarding many of the specific medications used for IMD, and women wishing to breastfeed need to discuss this on an individual basis with their physician.
Conclusions
In general, with appropriate treatment, the outcome of pregnancy for the majority of women with known IMD appears to be good and there are very few contraindications to pregnancy. The need for pre-pregnancy counselling is particularly important as even within our specialist adult metabolic unit at least 40% of all pregnancies annually are unplanned.
It is important not to just carefully monitor the fetus during pregnancy, but also to follow up the babies of mothers with IMDs longer term to identify any subtle neurocognitive or developmental issues. Physicians and obstetricians treating women with IMDs should continue to report pregnancy management and outcomes, both successful and unsuccessful, to add to the information available for consultation when counselling and managing women and their partners.
Acknowledgements
This work was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health's NIHR Biomedical Research Centers’ funding scheme.
Funding
The author has received unrestricted educational grant funding from Genzyme and Shire Pharmaceuticals, honoraria from Genzyme and Vitaflo, and funding for clinical trial work from Genzyme, Shire, Synageva BioPharma and Biomarin Pharmaceuticals.
Ethical approval
No ethical approval was required for this article. Patient consent has been obtained for use of anonymised clinical data.
Guarantor
Dr Elaine Murphy.
Contributorship
Dr Elaine Murphy.
References
- 1.Lee PJ. Pregnancy issues in inherited metabolic disorders. J Inherit Metab Dis 2006; 29(2–3): 311–316. [DOI] [PubMed] [Google Scholar]
- 2.Seshadri S, Oakeshott P, Nelson-Piercy C, et al. Prepregnancy care. BMJ 2012; 344: e3467–e3467. [DOI] [PubMed] [Google Scholar]
- 3.Mendez-Figueroa H, Lamance K, Sutton VR, et al. Management of ornithine transcarbamylase deficiency in pregnancy. Am J Perinatol 2010; 27(10): 775–784. [DOI] [PubMed] [Google Scholar]
- 4.Lamb S, Aye CY, Murphy E, et al. Multidisciplinary management of ornithine transcarbamylase (OTC) deficiency in pregnancy: essential to prevent hyperammonemic complications. BMJ Case Rep 2013; doi:10.1136/bcr-2012-007416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granovsky-Grisaru S, Belmatoug N, vom Dahl S, et al. The management of pregnancy in Gaucher disease. Eur J Obstet Gynecol Reprod Biol 2011; 156(1): 3–8. [DOI] [PubMed] [Google Scholar]
- 6.Hendriksz CJ, Preece MA, Chakrapani A. Successful pregnancy in a treated patient with biotinidase deficiency. J Inherit Metab Dis 2005; 28(5): 791–792. [DOI] [PubMed] [Google Scholar]
- 7.Kalkum G, Macchiella D, Reinke J, et al. Enzyme replacement therapy with agalsidase alfa in pregnant women with Fabry disease. Eur J Obstet GynecolReprod Biol 2009; 144(1): 92–93. [DOI] [PubMed] [Google Scholar]
- 8.de Vries JM, Brugma JD, Ozkan L, et al. First experience with enzyme replacement therapy during pregnancy and lactation in Pompe disease. Mol Genet Metab 2011; 104(4): 552–555. [DOI] [PubMed] [Google Scholar]
- 9.Chinnery PF. Mitochondrial disorders overview. In: Pagon RA, Adam MP, Ardinger HH, et al. (eds). GeneReviews(R), Seattle, WA: University of Washington, 1993. [PubMed] [Google Scholar]
- 10.Mitchell JJ, Trakadis YJ, Scriver CR. Phenylalanine hydroxylase deficiency. Genet Med 2011; 13(8): 697–707. [DOI] [PubMed] [Google Scholar]
- 11.Mabry CC, Denniston JC, Nelson TL, et al. Maternal phenylketonuria. A cause of mental retardation in children without the metabolic defect. N Engl J Med 1963; 269: 1404–1408. [DOI] [PubMed] [Google Scholar]
- 12.Lenke RR, Levy HL. Maternal phenylketonuria and hyperphenylalaninemia. An international survey of the outcome of untreated and treated pregnancies. N Engl J Med 1980; 303(21): 1202–1208. [DOI] [PubMed] [Google Scholar]
- 13.Rouse B, Azen C, Koch R, et al. Maternal Phenylketonuria Collaborative Study (MPKUCS) offspring: facial anomalies, malformations, and early neurological sequelae. Am J Med Genet 1997; 69(1): 89–95. [DOI] [PubMed] [Google Scholar]
- 14.Koch R, Hanley W, Levy H, et al. The Maternal Phenylketonuria International Study: 1984-2002. Pediatrics 2003; 112(6 Pt 2): 1523–1529. [PubMed] [Google Scholar]
- 15.Langendonk JG, Roos JC, Angus L, et al. A series of pregnancies in women with inherited metabolic disease. J Inherit Metab Dis 2012; 35(3): 419–424. [DOI] [PubMed] [Google Scholar]
- 16.Levy HL, Vargas JE, Waisbren SE, et al. Reproductive fitness in maternal homocystinuria due to cystathionine beta-synthase deficiency. J Inherit Metab Dis 2002; 25(4): 299–314. [DOI] [PubMed] [Google Scholar]
- 17.Calvert SM, Rand RJ. A successful pregnancy in a patient with homocystinuria and a previous near-fatal postpartum cavernous sinus thrombosis. Br J Obstet Gynaecol 1995; 102(9): 751–752. [DOI] [PubMed] [Google Scholar]
- 18.Francis DE, Kirby DM, Thompson GN. Maternal tyrosinaemia II: management and successful outcome. Eur J Pediatr 1992; 151(3): 196–199. [DOI] [PubMed] [Google Scholar]
- 19.Cerone R, Fantasia AR, Castellano E, et al. Pregnancy and tyrosinaemia type II. J Inherit Metab Dis 2002; 25(4): 317–318. [DOI] [PubMed] [Google Scholar]
- 20.Vanclooster A, Devlieger R, Meersseman W, et al. Pregnancy during nitisinone treatment for tyrosinaemia type I: first human experience. JIMD Rep 2012; 5: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fois AB, Borgogni P, Cioni M, et al. Presentation of the Data of the Italian Registry for Oculocutaneous Tyrosinaemia. J Inherit Metab Dis 1986; 9(Suppl 2): 2–2. [Google Scholar]
- 22.Deodato F, Rizzo C, Boenzi S, et al. Successful pregnancy in a woman with mut- methylmalonic acidaemia. J Inherit Metab Dis 2002; 25(2): 133–134. [DOI] [PubMed] [Google Scholar]
- 23.Diss E, Iams J, Reed N, et al. Methylmalonic aciduria in pregnancy: a case report. Am J Obstet Gynecol 1995; 172(3): 1057–1059. [DOI] [PubMed] [Google Scholar]
- 24.Wasserstein MP, Gaddipati S, Snyderman SE, et al. Successful pregnancy in severe methylmalonic acidaemia. J Inherit Metab Dis 1999; 22(7): 788–794. [DOI] [PubMed] [Google Scholar]
- 25.Van Calcar SC, Harding CO, Davidson SR, et al. Case reports of successful pregnancy in women with maple syrup urine disease and propionic acidemia. Am J Med Genet 1992; 44(5): 641–646. [DOI] [PubMed] [Google Scholar]
- 26.Coss KP, Doran PP, Owoeye C, et al. Classical galactosaemia in Ireland: incidence, complications and outcomes of treatment. J Inherit Metab Dis 2013; 36(1): 21–27. [DOI] [PubMed] [Google Scholar]
- 27.Berry GT, Elsas LJ. Introduction to the Maastricht workshop: lessons from the past and new directions in galactosemia. J Inherit Metab Dis 2011; 34(2): 249–255. [DOI] [PubMed] [Google Scholar]
- 28.Gubbels CS, Land JA, Rubio-Gozalbo ME. Fertility and impact of pregnancies on the mother and child in classic galactosemia. ObstetGynecol Surv 2008; 63(5): 334–343. [DOI] [PubMed] [Google Scholar]
- 29.Rubio-Gozalbo ME, Gubbels CS, Bakker JA, et al. Gonadal function in male and female patients with classic galactosemia. Hum Reprod Update 2010; 16(2): 177–188. [DOI] [PubMed] [Google Scholar]
- 30.van Erven B, Gubbels CS, van Golde RJ, et al. Fertility preservation in female classic galactosemia patients. Orphanet J Rare Dis 2013; 8: 107–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000; 71(5 Suppl): 1256S–1261S. [DOI] [PubMed] [Google Scholar]
- 32.Morriss FH, Jr, Makowski EL, Meschia G, et al. The glucose/oxygen quotient of the term human fetus. Biol Neonate 1974; 25(1–2): 44–52. [DOI] [PubMed] [Google Scholar]
- 33.Ramachandran R, Wedatilake Y, Coats C, et al. Pregnancy and its management in women with GSD type III - a single centre experience. J Inherit Metab Dis 2012; 35(2): 245–251. [DOI] [PubMed] [Google Scholar]
- 34.Petry CJ, Hales CN. Long-term effects on offspring of intrauterine exposure to deficits in nutrition. Hum Reprod Update 2000; 6(6): 578–586. [DOI] [PubMed] [Google Scholar]
- 35.Rizzo T, Metzger BE, Burns WJ, et al. Correlations between antepartum maternal metabolism and child intelligence. N Engl J Med 1991; 325(13): 911–916. [DOI] [PubMed] [Google Scholar]
- 36.Picciano MF. Pregnancy and lactation: physiological adjustments, nutritional requirements and the role of dietary supplements. J Nutr 2003; 133(6): 1997S–2002S. [DOI] [PubMed] [Google Scholar]
- 37.Schimanski U, Krieger D, Horn M, et al. A novel two-nucleotide deletion in the ornithine transcarbamylase gene causing fatal hyperammonia in early pregnancy. Hepatology 1996; 24(6): 1413–1415. [DOI] [PubMed] [Google Scholar]
- 38.Jebbink J, Wolters A, Fernando F, et al. Molecular genetics of preeclampsia and HELLP syndrome - a review. Biochim Biophys Acta 2012; 1822(12): 1960–1969. [DOI] [PubMed] [Google Scholar]
- 39.Ibdah JA, Bennett MJ, Rinaldo P, et al. A fetal fatty-acid oxidation disorder as a cause of liver disease in pregnant women. N Engl J Med 1999; 340(22): 1723–1731. [DOI] [PubMed] [Google Scholar]
- 40.Strauss AW, Bennett MJ, Rinaldo P, et al. Inherited long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency and a fetal-maternal interaction cause maternal liver disease and other pregnancy complications. Semin Perinatol 1999; 23(2): 100–112. [DOI] [PubMed] [Google Scholar]
- 41.Novy J, Ballhausen D, Bonafe L, et al. Recurrent postpartum cerebral sinus vein thrombosis as a presentation of cystathionine-beta-synthase deficiency. Thrombo Haemost 2010; 103(4): 871–873. [DOI] [PubMed] [Google Scholar]
- 42.Thrombosis and embolism during pregnancy and the puerperium, reducing the risk (Green-top Guideline No. 37a), 2009. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg37a/ (2009, accessed December 2014).
- 43.Arn PH, Hauser ER, Thomas GH, et al. Hyperammonemia in women with a mutation at the ornithine carbamoyltransferase locus. A cause of postpartum coma. N Engl J Med 1990; 322(23): 1652–1655. [DOI] [PubMed] [Google Scholar]
- 44.Grunewald S, Hinrichs F, Wendel U. Pregnancy in a woman with maple syrup urine disease. J Inherit Metab Dis 1998; 21(2): 89–94. [DOI] [PubMed] [Google Scholar]