Abstract
This narrative review discusses gestational weight gain (GWG) and medical outcomes of pregnancy, including metabolic, cardiovascular, respiratory, musculoskeletal and psychiatric systems. Taken as a whole, the available evidence shows that excessive GWG increases the risk of all medical complications of pregnancy, and negatively impacts the long-term health and weight of both mothers and their offspring. Briefly, interventions to encourage appropriate GWG are discussed and readers are directed to resources to facilitate discussion of pregnancy weight.
Keywords: Pregnancy, weight gain, gestational, diabetes, hypertension, preeclampsia, intervention
Introduction
Gestational weight gain (GWG) is a normal and expected component of healthy pregnancy. Separate from pregravid body weight, inappropriate GWG is an independent and modifiable risk factor for adverse medical, obstetric, and neonatal outcomes of pregnancy.1–4 Recently, attention has been directed towards GWG given its independent relationship with adverse medical outcomes of pregnancy.
The majority of research examining guideline-discordant GWG is largely focused on obstetric, neonatal, and child outcomes.1–4 In this narrative review, we aim to define appropriate weight gain in pregnancy, highlight the effects of inappropriate GWG on medical outcomes of pregnancy, discuss the maternal long-term health risks of inappropriate GWG, and offer insight towards intervention strategies and novel frameworks to aid maternity care providers with pregnancy weight management. Although we limit our discussion to appropriate and excessive GWG, the effects of inadequate GWG should not be dismissed. As this article is of a review nature, neither research ethics approval nor patient consent was required for the production of this manuscript, which was produced without funding support.
How much weight gain during pregnancy is considered ideal?
In 2009, the Institute of Medicine established evidence-based recommendations designed to help maternity care providers assist their patients in managing pregnancy-associated weight.5 These guidelines offer specific weekly (kg/wk) and absolute (total kg) weight gain recommendations based on a woman's pregravid body mass index (BMI) (Table 1). The Institute of Medicine (2009) defines and attempts to standardize healthy weight gain targets for pregnant women,5 yet only a small percentage of patients currently adhere to these recommendations.6
Table 1.
Institute of Medicine recommended gestational weight gain, based on Pregravid body mass index.5
| Classification | Target weekly GWG (second and third trimesters) | Target total GWG |
|---|---|---|
| Underweight (BMI < 18.5 kg/m2) | 1 pound per week (0.51 kg) | 28–40 pounds (12.7–18.2 kg) |
| Normal weight (BMI 18.5–24.9 kg/m2) | 1 pound per week (0.42 kg) | 25–35 pounds (11.4–15.9 kg) |
| Overweight (BMI 25.0–29.9 kg/m2) | 0.6 pounds per week (0.28 kg) | 15–25 (6.8–11.4 kg) |
| Obese (BMI ≥ 30 kg/m2) | 0.5 pounds per week (0.22 kg) | 11–20 pounds (5–9.1 kg) |
Weight gain during pregnancy and the risk of abnormal glucose metabolism
While the relationship between BMI and diabetes (type 2 or gestational) is well defined in pregnancy and otherwise, fewer studies have examined the association between GWG and the independent risks of abnormal glucose metabolism, including gestational diabetes mellitus (GDM) and postpartum type 2 diabetes. In their report, the Institute of Medicine5 suggests that more evidence is required to delineate the role that GWG plays in predisposing mothers towards aberrant glycemic control.
There is now sufficient evidence to state that optimal weight gain in early pregnancy is critical in maintaining normoglycemia. In a nested case-control study of 345 women with GDM and 800 women with a negative screening test, a significant increase in GDM (OR 1.74, 95% CI 1.16–2.60) was observed if the rate of weight gain prior to screening was between 0.41 and 0.97 kg/week compared to women who gained <0.27 kg/week (after adjusting for BMI, age, race/ethnicity, parity, and blood pressure).7 This finding was similar to that from Morisset et al.8 who concluded that, after adjusting for traditional GDM risk factors, weight gain in the first trimester was significantly higher in patients who developed GDM compared to normoglycemic controls (3.40 ± 0.42 vs. 1.87 ± 0.16 kg, p ≤ 0.01), and was above the acceptable limits defined in the Institute of Medicine guidelines. This suggests that first trimester weight gain is both a significant and independent predictor of GDM (OR 1.25, 95% CI 1.10–1.42).8
Evidence suggests that there is a positive relationship between GWG and glucose concentration. In a recent prospective cohort study,9 413 women were enrolled prior to 14 weeks gestation. Information was collected on GWG, body fat distribution, and glucose metabolism (50 g, 1 h glucose challenge test). Independent of pregravid BMI, increased early pregnancy GWG was directly related to maternal glucose concentrations at 24–28 weeks. For each additional 0.3 kg/week gained during pregnancy prior to glucose challenge testing, there was a 2.2 mg/dl (95% CI 0.1–4.3) increase in glucose concentration. In addition, for each 8.6 mm increase in biceps skinfold thickness and each 11.7 mm increase in triceps skinfold thickness, there was a 4.3 mg/dl (95% CI 0.2–8.5) increase in maternal glucose.9 For reference, women in the cohort had a mean (SD) biceps skinfold thickness of 14.6 (8.6) mm and triceps skinfold thickness of 25.9 (11.7) mm.
Women entering pregnancy carrying excess weight are more susceptible to abnormal glucose metabolism than their normal weight counterparts; consequently, optimizing GWG is of particular importance for them. It has been reported that both subtle and overt abnormal glucose metabolism (Impaired Glucose Tolerance and GDM) were more common in women who had increased GWG prior to 24 weeks of pregnancy and were either overweight or obese.10 This observation was confirmed in a second study that also noted that elevated GWG prior to glucose screening predicted impaired glucose tolerance (but not GDM) in women of all BMI categories.11 Specifically, each additional kilogram of weight gain in the first trimester increased the risk of GDM by 25%, and each additional 0.1 kg/week before GDM diagnosis increased the risk of GDM by 28%. Controversy does exist; one study found that while women with pre-pregnancy overweight and obesity had significant risk of developing GDM, there was no link between excessive GWG and GDM.12
Excess GWG, however, does increase the risk of type 2 diabetes later in life. Data suggest that there is a positive relationship between excessive GWG and maternal abdominal adiposity, a risk factor strongly linked to increased risk of cardiovascular and metabolic diseases such as type 2 diabetes.13 In a prospective birth cohort of 7223 mother–child pairs, women with excess GWG had a 47% greater risk of presenting with type 2 diabetes within 21 years of delivering compared to women who gained weight within recommendations (age-adjusted OR 1.47, 95% CI 1.11–1.94).14 Likely, much of this relationship can be explained by higher post-partum weight retention in women who gain excessive weight during pregnancy, leading to accelerated weight tracking throughout the life course (i.e. shifting BMI upwards). In a recent study of women who were diagnosed with GDM during pregnancy, both pre-pregnancy BMI and weight change from pre-pregnancy to postpartum were shown to increase the risk of prediabetes and overt diabetes later in life.15
Thus, excessive GWG, particularly in the first and early second trimesters, appears to be related to increased risk of abnormal glucose metabolism during pregnancy (including both Impaired Glucose Tolerance and GDM) and in later life (type 2 diabetes). One possible mechanism for this phenomenon is related to an early increase in insulin resistance resulting from rapid accrual of weight (maternal effect). This insulin resistance leads to reduced capacity of the pancreatic β cells to secrete adequate levels of insulin. As a result, the mother is less able to compensate for further increases in insulin resistance that are encountered later in pregnancy (fetal/placental effect). This effect is known as “B cell exhaustion”.6
Weight gain during pregnancy and the risk of hypertensive disorders
Hypertensive disorders of pregnancy (HDP) are a leading cause of maternal and perinatal morbidity and mortality.16 Since HDP affect up to 10% of pregnancies and currently can only be cured by delivery, there has been intense interest in preventive strategies. Excessive GWG is a significant and modifiable risk factor for HDP. Prospective cohort studies have consistently demonstrated a relationship between GWG and risk of HDP.17 For example, the Avon longitudinal study of parents and children, which prospectively evaluated a cohort of 12,522 women found that excessive GWG was associated with increased risks of gestational hypertension and preeclampsia compared with weight gain within the recommended range (OR 1.51, 95% CI: 1.32–1.73 and OR 2.14, 95% CI: 1.46–3.12, respectively). For every 200 g of GWG per week (up to 18 weeks), the likelihood of HDP and preeclampsia was significantly higher; OR 1.26, 95% CI: 1.16–1.38 and OR 1.31, 95% CI: 1.07–1.62, respectively after adjusting for confounding variables. In normotensive women, early pregnancy GWG was directly related to blood pressure change in mid-pregnancy, and negatively with blood pressure change in late pregnancy. Throughout the entire pregnancy, GWG was positively associated with concurrent blood pressure change.17 Further evidence supporting the Institute of Medicine pregnancy weight gain recommendations comes from a recent prospective evaluation of 56,101 pregnant women included in the Norwegian Mother and Child Cohort Study (MoBa).18 Among women of normal weight in this study, excessive GWG significantly increased the risk of HDP in both nulliparas and multiparas. Similar results were found for nulliparous women who were overweight and experienced excessive GWG. An additional prospective cohort of 6959 women from the Netherlands indicated that GWG was associated with a higher risk of gestational hypertension (OR 2.07, 95% CI: 1.43–2.99), independent of pregravid BMI.19 The strongest effects were demonstrated for first trimester weight gain.
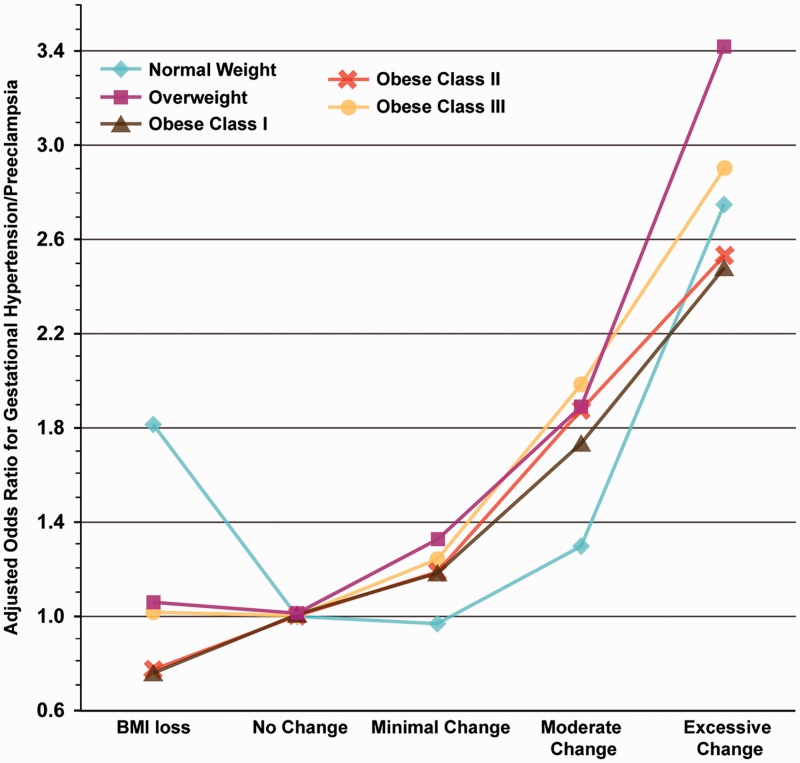
Several retrospective studies also suggest that HDP are more likely to develop in women who have greater GWG. First, a retrospective population cohort of 436,414 Californian women found that, independent of pre-pregnancy BMI, women with excessive GWG had nearly a two-fold increase in the odds of developing HDP (OR 1.94, 95% CI: 1.7–2.2).20 The same study reported on the odds of developing gestational hypertension/preeclampsia by change in BMI in pregnancy, accounting for pre-pregnancy BMI. The increase in the risk of HDP associated with increased GWG was present in women of all pre-pregnancy BMI categories. Importantly, women who started their pregnancies heavier incurred the increase in risk of HDP with lighter weight gain during pregnancy than women who were normal weight (Figure 1). A second study showed that excessive GWG was associated with an increased risk of pregnancy-induced hypertension (OR 1.35, 95% CI: 1.07–1.7, p = 0.03). The risk was nearly 6-fold higher in patients with combined pregravid obesity and excessive GWG compared to women with normal pregravid weight and adequate GWG.12 Finally, in a secondary analysis of a multicentre RCT that evaluated the effects of vitamins C and E to prevent HDP,21 it was found that excess GWG in all pregravid BMI categories increased the risk of HDP after controlling for relevant confounders. Importantly, a total of 73% of 9969 nulliparous women gained in excess of the Institute of Medicine guidelines – excessive GWG is now exceedingly common.
Figure 1.
Adjusted OR for the development of gestational hypertension/preeclampsia as a function of change in BMI during pregnancy, accounting for prepregnancy BMI.20
There is significant heterogeneity among studies examining HDP. It is a challenge to disentangle the unique and independent contribution of excessive GWG from other factors related to the pathogenesis of HDP including, but not limited to, the inflammatory milieu and maternal metabolic syndrome, pregravid obesity, maternal body composition, body fat distribution (waist circumference), hyperlipidemia, insulin resistance, and coagulation abnormalities. These patient characteristics are strongly related to the development of preeclampsia.22,23 Furthermore, although it appears that GWG in excess of the guidelines increases HDP risk, the contribution of fluid retention secondary to oedema is often not considered in simple associations between total GWG and prevalence of HDP.
Other pregnancy complications affected by GWG
Although the effects of GWG on metabolic and cardiovascular outcomes are better studied and arguably more important, excessive pregnancy weight is related to other medical problems as well. It is biologically plausible that respiratory complications, including obstructive sleep apnoea, exacerbation of asthma, and complications of general anaesthesia are more likely with increased GWG24 – this has not yet been well established in the literature. Like increased weight in general, excessive GWG places increased stress on the musculoskeletal system, resulting in increased pain in the back, hips and knees. Carpal tunnel syndrome is also more common in women who gain more weight than recommended. Finally, women who gain excessive weight are prone to depression and anxiety, irrespective of their starting BMI.25 As maternity care providers, we must be sensitive to the emotional needs of patients when we discuss weight-related matters, while still coaching and providing much-needed information.
What can be done to assist with achieving appropriate GWG?
Collectively, the information available to date validates the notion that rate of weight gain and early excessive GWG are independent risk factors that warrant discussion during preconception counselling and in early pregnancy if medical complications are to be avoided or at least attenuated.
Several intervention studies employing dietary counselling and/or physical activity to support healthy GWG have been published. Many prenatal interventions target pregnancy weight with a focus on maternal–fetal and down-stream child outcomes.4 It is beyond the scope of this review to discuss the individual interventions, which are varied and often complex. Encouragingly, several reviews suggest that engagement in healthy behaviours can positively modify GWG.4,26 For instance, Thangaratinam et al.26 completed a meta-analysis of RCTs that aimed to optimize GWG and examined the effects on maternal weight and obstetric outcomes. A total of 44 relevant randomized controlled trials (n = 7278 women) that evaluated three categories of interventions (diet, physical activity, and mixed interventions) were included. Overall, if any intervention was employed, there was a GWG reduction of 1.42 kg (95% CI 0.95–1.89 kg) compared to control. Prenatal intervention showed a beneficial effect on preeclampsia risk (OR 0.74, 95% CI 0.60–0.92), with no statistically significant effect on other critically important outcomes. Dietary interventions resulted in the largest reduction in GWG (3.84 kg, 2.45–5.22 kg) and improved pregnancy outcomes compared with other interventions. Although promising, the evidence rating was low to very low for medical complications of pregnancy including preeclampsia, GDM, gestational hypertension and preterm delivery. Nonetheless, management with dietary and lifestyle interventions in pregnancy can reduce GWG and improve the health and well-being of mother and baby. Although one recent systematic review and meta-analysis did not show a therapeutic effect of lifestyle intervention on GWG, preeclampsia, GDM or induction of labour,27 one cannot discount the health benefits independent of body weight changes (i.e. improved physical fitness, glucose homeostasis, body composition, etc.). Importantly, safety assessments found that engagement in healthy lifestyle did not pose harm to mother or baby. Indeed, a systematic review and meta-analysis by Russo et al.28 reports a 28% reduction in GDM risk (95% CI 9–42%) when physical activity interventions are compared with standard care (RR 0.72, P=.005). Thus, an active and non-sedentary pregnancy appears to offer a slight protective effect against the development of GDM. Care providers should feel comfortable promoting behaviour change given the numerous health benefits that can be accrued independent of body weight changes.
Given that the majority of women now enter pregnancy with excess weight (i.e. overweight or obesity), and that the risk of medical complications of excessive GWG are more pronounced in these women, attention should focus on GWG management for optimal maternal–fetal health.3 Most recent evidence suggests that it is safe for women with obesity (obesity class I, II, II) to gain close to no weight until mid-pregnancy and that the rate of weight increase from this point forward should be inversely proportional to pregravid obesity status (i.e. women with prepregnancy class III obesity should gain at a slower rate, if at all).29 For all women, however, guideline concordant GWG is recommended to limit medical complications of pregnancy and optimize outcomes. In light of the majority who gain in excess, it is thought that discrepancies exist with respect to care provider messaging and patient uptake of behavioural (dietary and physical activity) recommendations30,31 that encourage guideline adherence. In an attempt to improve patient-provider dialog, reconcile these perceived differences and subsequent knowledge transfer concerning GWG, the Canadian Obesity Network has developed a tool that aims to facilitate a discussion about healthy pregnancy weights to harmonize the messages delivered to all women. This evidence-based practitioner guide is called the ‘5 As for healthy pregnancy weight gain’ (http://www.obesitynetwork.ca/pregnancy) and encourages prenatal care providers to initiate a conversation about guideline concordant weight gain. Briefly, the 5 As are: Ask (for permission to discuss weight), Assess (the causes of guideline discordant gain), Advise (on risks and management options), Agree (on a SMART plan to achieve goals), and Assist (women in identifying barriers/facilitators, educate, refer, arrange follow-up). While in its infancy, this framework provides a first step towards a thoughtful, empathetic dialog about a sensitive topic, and aims to encourage healthy behaviours that subsequently limit the adverse effects of excessive pregnancy weight gain. Aside from the Canadian Obesity Network 5 As, additional prenatal coaching/counselling resources can be found within the Institute of Medicine guideline implementation report6 which includes GWG and physical activity prescription pads for prenatal care providers, GWG tracking sheets for the patient (i.e., a self-monitoring tool), and an online module for calculating BMI-specific GWG.
Conclusion
Pregnancy has been referred to as a “crystal ball”, because medical complications encountered during pregnancy frequently recur later in life. Pregnancy unveils the presence of abnormalities in glucose metabolism or underlying cardiovascular health that appear to “normalize” when the pregnancy ends, but become clinically important again in the future. The importance of both timing and rate of weight gain during pregnancy should be a focal point for discussion in the first clinical encounter for prenatal care. Furthermore, public education campaigns should be undertaken, since many women have already gained significant weight before they are seen for the first prenatal appointment. If this is the case, GWG management is still encouraged as it is beneficial to slow the rate of gain and focus on weight stability via the adoption of healthy behaviours.32
Pregnancy is a time of immense excitement for parents-to-be, who almost universally feel a desire to provide their offspring with every advantage to succeed. Over the last several decades, the importance of appropriate GWG has been less emphasized than in generations past, at the same time as both pregravid BMI and GWG have increased due to complex sociocultural–socioeconomic reasons. All individuals who provide care to pregnant women have a responsibility to discuss optimal GWG targets that are individualized to each woman, to monitor GWG, identify women who are gaining outside of the guidelines, and to identify local resources to aid women who exceed recommendations. By doing so, we can help to empower women, minimize lifelong weight trajectories for women and their offspring, and decrease the risk of important short- and long-term medical complications related to excessive pregnancy weight gain.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
ZMF is supported by a Canadian Institutes of Health Research (CIHR) Postdoctoral Fellowship from the Institute of Human Development, Child and Youth Health. KA holds a CIHR New Investigator Award from the Institute of Human Development, Child and Youth Health.
Guarantor
LG
Contributorship
ZMF, FC, AT performed the literature search and reviewed the literature. ZMF wrote the initial draft of the manuscript. ZMF, KBA, and LG edited the manuscript. All authors reviewed and approved the final draft.
References
- 1.El-Chaar D, Finkelstein SA, Tu X, et al. The impact of increasing obesity class on obstetrical outcomes. J Obstet Gynaecol Can 2013; 35: 224–233. [DOI] [PubMed] [Google Scholar]
- 2.Nohr EA, Vaeth M, Baker JL, et al. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 2008; 87: 1750–1759. [DOI] [PubMed] [Google Scholar]
- 3.Ferraro ZM, Barrowman N, Prud'homme D, et al. Excessive gestational weight gain predicts large for gestational age neonates independent of maternal body mass index. J Matern Fetal Neonatal Med 2012; 25: 538–542. [DOI] [PubMed] [Google Scholar]
- 4.Adamo KB, Ferraro ZM, Brett KE. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health 2012; 9: 1263–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weight gain during pregnancy: reexamining the guidelines. In: Rasmussen KM, Yaktine AL. (eds). In: Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines, Washington, DC: National Academies Press, 2009. . [PubMed] [Google Scholar]
- 6.IOM (Institute of Medicine) and NRC (National Research Council). Leveraging action to support dissemination of the pregnancy weight gain guidelines: Workshop summary, Washington, DC: The National Academies Press, 2013. [PubMed] [Google Scholar]
- 7.Heddersson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol 2010; 115: 597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morriset AS, Tchernof A, Dubé MC, et al. Weight gain measures in women with gestational diabetes mellitus. J womens Health (Larchmt) 2011; 20: 375–380. [DOI] [PubMed] [Google Scholar]
- 9.Tomedi LE, Simhan HN, Chang CC, et al. Gestational weight gain, early pregnancy maternal adiposity distribution, and maternal hyperglycemia. Matern Child Health J 2014; 18: 1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson KS, Waters TP, Catalano PM. Maternal weight gain in women who develop gestational diabetes mellitus. Obstet Gynecol 2012; 119: 560–565. [DOI] [PubMed] [Google Scholar]
- 11.Herring SJ, Oken E, Rifas-Shiman SL, et al. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol 2009; 201: e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nan L, Enging L, Jia G, et al. Maternal prepregnancy body mass index and gestational weight gain on pregnancy outcomes. PLoS One 2013; 8: e82310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClure CK, Catov JM, Ness R, et al. Associations between gestational weight gain and BMI, abdominal adiposity, and traditional measures of cardiometabolic risk in mothers 8 y postpartum. Am J Clin Nutr 2013; 98: 1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Mamun A, Mannan M, O'Callaghan M, et al. Association between gestational weight gain and postpartum diabetes: evidence from a community based large cohort study. PLoS One 2013; 8: e75679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Zhang C, Zhang S, et al. Prepregnancy body mass index and weight change on postpartum diabetes risk among gestational diabetes women. Obesity (Silver Spring) 2014; 22: 1560–1567. [DOI] [PubMed] [Google Scholar]
- 16.Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol 2013; 25: 124–132. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald-Wallis, Tilling K, Fraser A, et al. Gestational weight gain as a risk factor for hypertensive disorders of pregnancy. Am J Obstet Gynecol 2013; 209: 327.e1–327.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haugen M, Brantsæter AL, Winkvist A, et al. Associations of pre-pregnancy body mass index and gestational weight gain with pregnancy outcome and postpartum weight retention: a prospective observational cohort study. BMC Pregnancy Childbirth 2014; 14: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaillard R, Durmuş B, Hofman A, et al. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring) 2013; 21: 1046–1055. [DOI] [PubMed] [Google Scholar]
- 20.Swank ML, Caughey AB, Farinelli CK, et al. The impact of change in pregnancy body mass index on the development of gestational hypertensive disorders. J Perinatol 2014; 34: 181–185. [DOI] [PubMed] [Google Scholar]
- 21.Johnson J, Clifton RG, Roberts JM, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine Guidelines. Obstet Gynecol 2013; 121: 969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaiworapongsa T, Chaemsaithong P, Yeo L, et al. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 2014; 10: 466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaiworapongsa T, Chaemsaithong P, Korzeniewski SJ, et al. Pre-eclampsia part 2: prediction, prevention and management. Nat Rev Nephrol 2014; 10: 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies GA, Maxwell C, McLeod L, et al. Obesity in pregnancy. J Obstet Gynaecol Can 2010; 32: 165–173. [DOI] [PubMed] [Google Scholar]
- 25.Hill B, Skouteris H, McCabe M, et al. A conceptual model of psychosocial risk and protective factors for excessive gestational weight gain. Midwifery 2013; 29: 110–114. [DOI] [PubMed] [Google Scholar]
- 26.Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012; 16: 344, e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruifrok AE, van Poppel MN, van Wely M, et al. Association between weight gain during pregnancy and pregnancy outcomes after dietary and lifestyle interventions: a meta-analysis. Am J Perinatol 2014; 31: 353–364. [DOI] [PubMed] [Google Scholar]
- 28.Russo LM, Nobles C, Ertel KA, et al. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol 2015; 125: 576–582. [DOI] [PubMed] [Google Scholar]
- 29.Hutcheon JA, Platt RW, Abrams B, et al. Pregnancy weight gain charts for obese and overweight women. Obesity (Silver Spring) 2015; 23: 532–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferraro ZM, Rutherford J, Keely EJ, et al. An assessment of patient information channels and knowledge of physical activity and nutrition during pregnancy. Obstet Med 2011; 4: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraro ZM, Boehm KS, Gaudet LM, et al. Counseling about gestational weight gain and healthy lifestyle during pregnancy: Canadian maternity care providers' self-evaluation. Int J Womens Health 2013; 5: 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferraro ZM, Patterson S, Chaput JP. Unhealthy weight control practices: culprits and clinical recommendations. Clin Med Insights Endocrinol Diabetes 2015; 8: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]