Abstract
Background
Severe headache during pregnancy is a challenging condition that may rarely imply endocrine disturbances. Rapid recognition of pituitary apoplexy is needed to improve pregnancy outcome.
Objective
To review and compare maternal and fetal outcomes after pituitary apoplexy.
Methods
Four cases of pituitary apoplexy during pregnancy in our centre are reported and literature review covering the past 54 years was performed.
Results
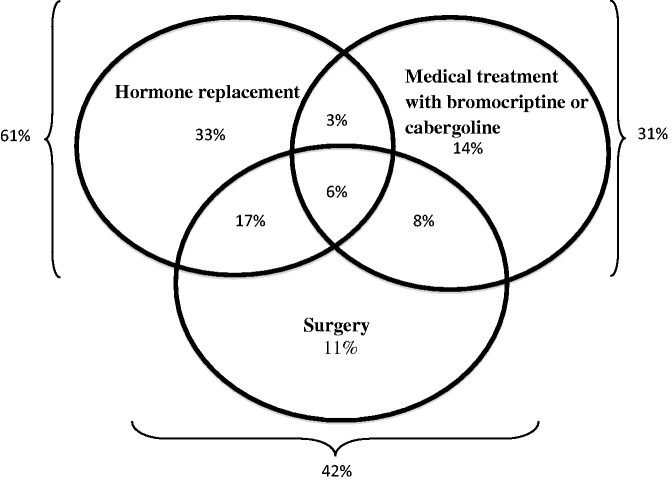
In the four cases presented and the 33 reported in the literature, most women presented with severe headaches and systemic symptoms. Overall, 42% were treated surgically, 31% received bromocriptine or cabergoline and 61% were given hormone replacement. No major obstetrical complication was reported and all babies were healthy.
Conclusion
Pituitary apoplexy is a rare cause of sudden and severe headache during pregnancy. Rapid identification of this condition with potentially associated endocrine disturbances is important to ensure maternal and fetal well-being. A multidisciplinary team approach seems to reduce morbidity and mortality.
Keywords: Pituitary, endocrine insufficiency, pregnancy, apoplexy
Introduction
Sudden and severe headache during pregnancy can be challenging for clinicians. Pituitary apoplexy is a very rare cause of headache and was first described by Baily1 in 1898. It is defined as the abrupt destruction of pituitary tissue resulting from infarction or haemorrhage into the pituitary. It usually involves an underlying pituitary tumour. However, it had been also described in women without any pre-existing pituitary lesion but where the pituitary is physiologically enlarged as a result of pregnancy. The increase in pituitary size during pregnancy is due to hyperplasia and hypertrophy of the lactotroph cells by oestrogen stimulation and through their transformation to prolactin-producing pregnancy cells.2 The pituitary gland reaches its maximum size approximately three days postpartum, measuring from 120 to 136% of its usual size.3
Increased intracapsular pressure in the sella turcica by the enlarged pituitary favours ischemia and thrombosis. Once infarction has occurred, the pituitary or the tumour may become haemorrhagic and swells rapidly. Pituitary hormone deficiencies may develop quickly after infarction but are not always present. The principal symptoms of apoplexy are sudden headache (97%), nausea (80%) and loss of visual fields (71%).4 Pituitary apoplexy is a medical emergency because it can impair pituitary function and it is therefore critical to search for endocrine disturbances and start appropriate hormonal replacement when necessary.
The objective of this study was to report four cases of pituitary apoplexy related to pregnancy, present our experience and recommendations in treating this rare pathology and to compare the clinical presentations, treatment, and maternal/fetal outcomes with cases from the literature.
Subjects and methods
We reviewed four cases of pituitary apoplexy occurring in pregnancy or during the postpartum period which had been treated at the Centre hospitalier de l'Université de Montréal (CHUM) between 1993 and 2014. The prevalence of pituitary apoplexy occurring during gestation and the peripartum period could be estimated to 1 per 10,000 term pregnancies since a total of 49,093 deliveries were registered during this period in our centre. Pituitary apoplexy was defined as a haemorrhage in the pituitary gland identified with imaging (CT scan or MRI), either with or without associated hormonal deficiencies.
Patients were identified from reported cases by our team and through a retrospective search in hospital charts using ICD-9 indicating prolactinoma (227.31), benign pituitary tumour (227.3) and other pituitary anomalies (E23.6) for patients hospitalised on the obstetric ward from 1993 to 2014. Charts were reviewed to collect information on maternal characteristics (age, parity, medical and pregnancy past history), clinical presentation (signs and symptoms, gestational age), diagnostic studies, therapeutic modalities, maternal outcomes (gestational age at delivery, spontaneous delivery, caesarean, obstetrical complications, breastfeeding, follow-up) and fetal outcomes (weight at delivery, newborn well-being and neonatal complications).
We performed a literature search using PubMed, Medline and Embase to identify relevant articles published between 1960 and 2014. Our search was limited to articles in English and French, and we included abstracts only when enough data were available. The search was conducted using the MeSH terms ‘pituitary diseases’, ‘pregnancy’, ‘pituitary apoplexy’ and the non-MeSH term ‘apoplexy’. We searched the reference lists of the primary articles and reviews for relevant articles not already identified in the literature search. This study was approved by the CHUM research ethics committee.
Statistical analysis
Appropriate summary statistics were calculated to describe the characteristics of our patients and those from the literature (medians for non-normally distributed continuous variables and proportions for binary or categorical variables).
Results
Case 1
A 33-year-old woman was admitted to the CHUM obstetrical ward at 39 weeks of gestation (WG) with sudden onset of severe headache. Obstetrical history of this Gravida 6, Para 3, Abortions 2 (G6P3A2) woman revealed that her previous pregnancies had been complicated by gestational hypertension and preeclampsia. However, no previous history of hypertension was noted outside of pregnancy. The present pregnancy had been uneventful until she reported a sudden and severe bilateral headache accompanied by nausea, blurry vision and dizziness 24 h prior to admission. No head trauma was reported, and since blood pressure and preeclampsia investigations were within normal values, the patient was discharged after 10 h of observation.
She returned the next day with residual headache. Repeat preeclampsia investigations were again normal. However, she fainted when leaving the hospital and was immediately readmitted for acute medical stabilisation and further evaluation. A complete neurological examination showed neck stiffness, a sign of meningeal irritation. Visual fields were normal. A lumbar puncture was performed, and both opening pressure and cerebrospinal fluid examination were normal.
A cerebral computed tomography (CT) scan without contrast showed a prominent and slightly hyperdense pituitary gland of 12 mm in contact with the optic chiasm. Pituitary apoplexy was suspected and a magnetic resonance imaging (MRI) performed seven days after onset of her headache confirmed sellar central haemorrhagic infarction and pituitary hyperplasia without underlying lesion, compatible with sub-acute pituitary apoplexy. Labour was induced at 40 WG after administration of intravenous hydrocortisone, a preventive measure for possible maternal relative hypocortisolaemia. She delivered a healthy 3.6 kg boy and began breastfeeding successfully. Initial hormonal work-up (Table 1) and biochemical results including sodium, potassium and blood glucose were normal. At two months postpartum, her endocrine work-up was still normal (Table 1) and a follow-up MRI showed a regression of the pituitary size with a central remnant cyst of 6 mm. One year later, she had a spontaneous pregnancy without any complication. She delivered a healthy baby at term and breastfed normally.
Table 1.
Laboratory results of four cases from the CHUM.
| Case 1 |
Case 2 |
Case 3 |
Case 4 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 39 WG | 2 months PP | 20 WG | 2 months PP | 16 WG | 3 months PP | 4 days PP | 10 months PP | ||
| TSH (mU/L) | 1.64 | 0.56 | 0.22 | 3.69 | 0.75 | 0.47 | 1.42 | 2.42 | |
| FT4 (pmol/L) | 12.3 | 14.2 | 14.7 | 13.5 | 15.2 | 11.3 | – | 13.3 | |
| Prolactin (μg/L) | 391 | 13.5 | 7.2 | 145.8 | 12.1 | 18.3 | 20.2 | 5.2 | |
| Cortisol (nmol/L) | 475 | 310 | 756 | 281 | 347 | 170 | 64 | 485 | |
| ACTH (pmol/L) | 12.5 | – | – | – | – | – | – | – | |
| IGF-1 (μg/L) | 253 | – | – | 190 | 110 | 158 | – | 51 | |
| Urinary free cortisol (nmol/24 h) | – | – | 459 | - | 445 | – | – | 343 (on treatment) | |
Reference range in general population: TSH: thyroid stimulating hormone, 0.35–5.50 mU/L; FT4: free thyroxine, 10.0–23.0 pmol/L; Prolactin, 3.0–29.0 μg/L; Cortisol, 119.0–618.0 nmol/L; ACTH: adrenocorticotropic hormone, 2.0–11.0 pmol/L; IGF-1: insulin growth factor, 95.0–320.0 μg/L; Urinary free cortisol, < 220 nmol/24 h; PP: post-partum; WG: week of gestation; CHUM: Centre hospitalier de l'Université de Montréal.
Case 2
A 30-year-old woman (G1) presented with secondary amenorrhea and infertility. Initial prolactin level was at 218.6 μg/L (normal range 3.0–29.0 μg/L). A pituitary MRI was performed and revealed a macroprolactinoma with an initial size tumour of 13 × 17 × 10 mm. The rest of her pre-pregnancy hormonal work-up was normal. She commenced cabergoline at 0.5 mg/week and seven months later her prolactin level was reduced to 30.5 μg/L, her menses returned and an MRI showed a regression of the tumour size to 5 × 16 × 3 mm. Cabergoline was ceased by the patient and shortly after she became pregnant. At 13 WG, cabergoline was restarted (0.5 mg/week) for residual macroprolactinoma noted on MRI just before pregnancy and because her prolactin had rapidly increased to 338.7 μg/L, which was over the normal expected 10-fold increase during pregnancy (serum prolactin usually reaching levels of 150 to 300 μg/L by term).5 At 20 WG, she fainted and was brought to the CHUM, complaining of non-specific headaches. A cerebral MRI was immediately performed and showed a recurrent pituitary mass of 17 × 22 ×14 mm with a hypointense signal compatible with acute bleeding and pituitary apoplexy. She did not have any pituitary insufficiency at work-up (Table 1) and cabergoline was continued and titrated during the rest of the pregnancy. The dose was increased at 24 WG to 0.5 mg twice a week for persistent incapacitating headaches and was stopped completely at delivery because symptoms were controlled, her prolactin level was acceptable (222.7 μg/mL) and the patient wished to breastfeed. She had a spontaneous vaginal delivery at term and gave birth to a healthy 3.2 kg girl who was breastfed without problem. Two months postpartum, her laboratory results remained normal (Table 1) and an MRI showed a diminished mass of 9 × 9 mm. Because of suckling difficulties, she chose to stop breastfeeding and the cabergoline was therefore restarted at 0.5 mg/week.
She became pregnant 10 months later and the cabergoline was continued at the same dose throughout pregnancy. She had an uneventful second pregnancy and had a spontaneous vaginal delivery at term. The cabergoline was stopped after delivery and she was still breastfeeding normally six months postpartum.
Case 3
A 37-year-old woman was diagnosed with a microprolactinoma two years before her first pregnancy (this case was previously published by Couture et al.6 in 2012). An MRI performed three months before pregnancy showed a microadenoma of 7 × 7 mm that had been stable for the previous six months. She was initially treated with cabergoline 0.5 mg/week, which was stopped at 6 WG. At 16 WG, she came to the obstetrical ward because of headaches, nausea and visual disturbances. Her hormonal work-up was completely normal apart from the prolactin level, which was low for a woman in her first trimester (Table 1). An MRI showed a pituitary measuring 11 × 11 ×18 mm with a central haemorrhage compatible with pituitary apoplexy. The cabergoline was then restarted at 0.5 mg/week and later stopped at 36 WG. Her blood pressure increased to 140/90 at 38 WG and she was induced for suspicion of preeclampsia but eventually had a caesarean for failure of labour progression. She gave birth to a healthy but small 2.2 kg girl and had normal lactation. She also had a pulmonary embolism postpartum that was treated with warfarin for four months.
Two weeks postpartum, an MRI showed resolution of the microadenoma and her biochemical work-up was normal at three months postpartum (Table 1). She became pregnant two years later and received dalteparin and aspirin prophylaxis. At 21 WG she complained of headache and her 24-h urinary free cortisol was low for pregnancy at 78 nmol/d. Hydrocortisone was started at 10 mg in the morning and 5 mg at night for relative secondary hypocortisolism induced by the physiological stress of pregnancy. The remainder of her pregnancy was uneventful and she had an elective caesarean at 38 WG with a baby weighing 3.4 kg. Her postpartum hormonal work-up was normal and the hydrocortisone was stopped.
Case 4
A 40-year-old woman (G4P1A3) with type 1 diabetes and primary hypothyroidism was induced at 36 WG for recurrent hypoglycaemic episodes and suspicion of uteroplacental insufficiency with 50% reduction of insulin requirements in two weeks. She had a vaginal delivery with forceps and gave birth to a healthy 4 kg girl. Six hours postpartum, she complained of a severe headache. A blood patch was performed since post-dural puncture headache was suspected. The headache did not improve and the patient was not able to lactate. A cerebral MRI performed six days after initial symptoms showed a slightly oedematous pituitary gland without any mass or visible bleeding, which was considered normal for a woman in the early postpartum period. She received cortisol supplementation for new-onset adrenal insufficiency that was needed until seven months postpartum (Table 1). She is still followed by the endocrinology team and, at one-year postpartum, injections of growth hormone (somatropin) were started because of persistent confirmed GH deficiency (IGF-1 37 μg /L) and tiredness.
Although the patient presented some risk factors for lymphocytic hypophysitis (Type 1 diabetes and primary hypothyroidism), initial radiologic imaging did not support this diagnosis; no visible mass, no thickened pituitary stalk, no compression or displacement of chiasm. However, hemosiderin visible on T2-weighted images led us to believe that there was some pituitary bleeding missed by the first MRI performed almost one week after presentation. Clear enhancement of the tractus optici also suggested recent chiasm compression by the pituitary on the first MRI. On the last MRI in 2014 (one year later), the pituitary gland is clearly atrophic (4 mm) and no hemosiderosis is visible.
Literature review
We found 33 cases of pituitary apoplexy during pregnancy reported in the literature (Table 2). Four of them were available as abstracts only. The cases of Kannuki7 and Ohtsubo8 (articles published in Japanese) were presented in the literature review of Kita et al.,9 and we obtained information from Kita's Table 1 (cases 6 and 11). The median maternal age was 28.5 years old and 14 women (42%) were known to have a pituitary lesion before pregnancy: eight macroadenomas (including 4 macroprolactinomas) and four microadenomas (3 microprolactinomas). Two other patients had known adenomas but the size was not specified. Two patients were diagnosed with a macroadenoma during pregnancy because of severe headaches.10,11 One was diagnosed at 28 WG and suffered from apoplexy eight weeks later. The other was also diagnosed at 28 WG; however, apoplexy occurred one week later. One patient was known to have a pre-existing Nelson syndrome prior to apoplexy.12
Table 2.
Summary of apoplexy cases during pregnancy and the peripartum period from the literature review and our academic centre.
| Age (years) | Prior lesion | WG at presentation | Symptoms |
Treatment |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Headaches | Nausea | Visual disturbances | Hormonal replacement | Bromocriptine and cabergoline | Surgery | ||||
| Kannuki et al.7 | 28 | PRL | 6 | + | + | ? | ? | ? | |
| Ohtsubo et al.8 | 29 (multi) | AD | 24 | + | + | + | + | ||
| Kita et al.9 | 26 (G1) | – | 26 | + | + | + | |||
| Atmaca et al10 | 33 | MAD | 37 | + | + | + | + | ||
| Luliano et al.11 | 28 | MAD | 29 | + | + | + | + | + | |
| Gheorghiu et al.12 | 33 | AD | 22 | + | + | + | |||
| Perotti et al.13 | 29 | – | 2 h PP | + | + | + | + | + | |
| Schrupp et al.14 | 31 (G1) | – | 1 wk PP | + | + | − | |||
| Jassal et al.15 | 32 (multi) | – | 4 days PP | + | + | + | |||
| Freeman et al.16 | 22 | MAD | 32 | + | + | + | |||
| Tonda et al.17 | 22 | – | 32 | + | + | + | |||
| Krull et al.18 | 28 (G1) | – | 7 | + | + | ||||
| O'Donovan et al.19 | 37 (multi) | MPRL | 8 | + | + | + | + | ||
| Murao et al.20 | 35 | – | 39 | + | + | ||||
| Nagulesparan et al. 21 | 34 | – | 31 | + | + | + | |||
| Gondim et al.22 | 29 | MPRL | 30 | + | + | + | + | + | |
| Zammit-Mangion et al.23 | 28 | – | 16 | + | + | + | |||
| Zammit-Mangion et al.23 | 31 | - | 18 | + | + | + | |||
| Onesti et al.24 | 28 | – | ? | + | + | + | |||
| Hervet et al.25 | 26 | – | 24 | + | + | + | |||
| Parihar et al.26 | 22 | MPRL | 20 | + | + | + | |||
| Witek et al.27 | 25 (G1) | MPRL | 14 | + | + | + | + | ||
| Scherrer et al.28 | ? | MAD | 16 | + | + | + | |||
| Scherrer et al.28 | ? | mAD | 28 | + | + | + | |||
| Lunardi et al.29 | 21 (multi) | – | 24 | + | + | + | |||
| Fujimaki et al.30 | 23 (G1) | – | 24 | + | + | ||||
| Ginath et al.31 | 31 (G1) | PRL | 39 | + | + | + | |||
| Heide et al.32 | 26 (multi) | – | 23 | + | + | + | |||
| Janssen et al.33 | 27 (G1) | – | 10 | + | + | + | + | ||
| Bamfo et al.34 | 31 (G1) | – | 10 | + | + | + | |||
| Lee et al.35 | 26 (G1) | – | 24 | + | + | + | + | ||
| Lamberts et al.36 | 30 (G1) | PRL | 23 | + | + | + | + | ||
| Rosen et al.37 | 32 | – | 22 | + | + | + | |||
| Patients from the CHUM | |||||||||
| Case 1 | 33 (G6) | – | 39 | + | + | − | − | – | − |
| Case 2 | 30 (G1) | MPRL | 20 | − | − | − | − | + | − |
| Case 3 | 37 (G1) | mPRL | 16 | + | + | + | − | + | − |
| Case 4 | 40 (G4) | – | 6 h PP | + | − | − | + | − | − |
| All 37 patients | 29a | 9 MAD (5 PRL) 5 mAD (4 MPRL) | 24 (4 PP) | 35/37 (95%) | 13/37 (35%) | 22/37 (59%) | 21/36 (58)% | 11/36 (31%) | 15/36 (42%) |
Median age.
Note: The treatment used in Kannuki's case6 was not specified, but we found the treatment in Ohtshubo's case7 in an English abstract. In total, treatments were specified for 36 patients. AD: adenoma non-specified; MAD: macroadenoma non-specified; mAD: microadenoma non-specified; PRL: prolactinoma; MPRL: macroprolactinoma; mPRL: microprolactinoma; GA: Gestational age; PP: postpartum; CHUM: Centre Hospitalier de l'Université de Montréal; WG: weeks of gestation.
Including our four cases, the median gestational age at symptom onset was 24 weeks and 4 (11%) cases occurred postpartum.13–15 Headaches were reported in 35/37 (95%) of the cases, 22/37 (59%) had visual disturbances and 13/37 (35%) had nausea. Two patients had polyuria and polydipsia16,17 and three had altered mental status at presentation.13,18,19 As shown in Figure 1, a total of 15/36 (42%) patients were treated surgically, while 11/36 (31%) received bromocriptine or cabergoline. Twenty-two of 36 (61%) were treated with hormone replacement, mostly levothyroxine and hydrocortisone (9/25). Twelve patients (33%) had at least two types of treatment. Two patients (including case 1) received no treatment at all14 and another received postpartum radiotherapy.10 The median gestational age at delivery was 38.5 weeks. Mode of delivery was vaginal birth for 16/25 (64%) of women and caesarean section for 9/25 (36%) of cases examined (data missing). There was only one spontaneous abortion at nine weeks of pregnancy due to apoplexy at 6 WG.18 The progress of this patient was unfortunately marked by persistent symptoms of ischaemic encephalopathy and seizures after the episode. Data were missing for fetal outcomes such as birth weight. All newborns (except for case 15) appeared to be healthy.
Figure 1.
Summary of the management used in the literature and in our cases of pituitary apoplexy.
Discussion
Pituitary apoplexy is a very rare cause of sudden headache in pregnancy and should be considered a medical emergency because of possible hormonal insufficiency. In 20 years, we have had four cases in our academic centre (estimated prevalence of 1 per 10,000 pregnancies) and 33 cases were found over the past 54 years (1960–2014) in the literature.
In his 11-year retrospective study, Randeva et al.4 found that the principal symptoms of pituitary apoplexy in non-pregnant subjects are sudden headache (97%), nausea (80%) and loss of visual fields (71%). All four of our patients had sudden and severe headaches, two had visual disturbances, two presented with nausea and two fainted. The results of our literature review are comparable (headache 94% and visual disturbances 61%) except that fewer patients had nausea (only 33%). The median gestational age at the beginning of symptoms in the literature was 24 weeks, which is unchanged when we include our patients. Including our last patient, 4 women had symptoms of postpartum.
Pituitary apoplexy usually happens in an underlying lesion. Fourteen cases from the literature (42%–47% with our cases) were known to have a pituitary lesion before apoplexy. The second patient in our series was known to have a macroprolactinoma and was treated with cabergoline before pregnancy. The third one was known to have a microprolactinoma and was also treated with cabergoline. Both had their medication stopped in the first trimester but later needed to restart it. Table 3 summarises the principal recommendations related to management and follow-up of pituitary adenoma during pregnancy.
Table 3.
Summary of recommendations for management of pituitary adenoma before and during pregnancy.a
| Pre-pregnancy • Complete pituitary hormonal work-up with visual fields assessment on physical examination to establish proper diagnosis of pituitary lesion – consider evaluation in endocrinology and/or multidisciplinary team. • If diagnosis of micro or macroprolactinoma is confirmed and desire to become pregnant is expressed, treatment with dopaminergic agonist (bromocriptine or cabergoline) to reduce size of the tumour and controlled prolactin level before pregnancy is recommended. • For all macroadenoma, consider treatment for reduction in size to less than 1 cm before pregnancy preferably with effective medication (e.g. dopaminergic therapy for macroprolactinoma) and/or surgery/radiotherapy if indicated, to minimise the risk of apoplexy and pressure on the optic chiasm during pregnancy. |
| During pregnancy • In women with microprolactinoma, discontinue dopamine agonist therapy when pregnancy is confirmed. In women with macroprolactinoma who become pregnant under therapy, it seems reasonable to continue dopamine agonist therapy throughout the pregnancy, especially if the initial tumour was invasive or close to the optic chiasm given the high risk (31%) of tumour growth or apoplexy during pregnancy. • Plan a physical examination and an evaluation of thyroidotroph and corticotroph axis functions at each trimester (with follow-up of T4 level and urinary free cortisol, which is more reliable than plasma cortisol during pregnancy) in near-to and confirmed macroadenomas to avoid unrecognised relative pituitary insufficiency. Routine evaluation of prolactin level during pregnancy is not recommended for asymptomatic patient. • Visual fields should be checked by an ophthalmologist once during pregnancy for women with near-to or established macroadenomas. Repeat visual fields during the third trimester for macroadenoma only. • Inform all patients with micro or macroadenomas about symptoms that could be related to tumour growth or apoplexy (sudden thunderclap headache, visual disturbance) and advise them to come rapidly to the hospital if they become symptomatic for a hormonal and radiologic work-up. • Routine radiologic follow-up of adenomas is not recommended during pregnancy. However, if clinical suspicion of tumour growth or apoplexy (development of neurological or visual symptoms), proceed to urgent pituitary MRI without gadolinium, formal visual fields assessment and hormonal work-up. • If significative growth of a prolactinoma is established and the patient experiences some neurological symptoms, reinitiation or increase of the dose of dopamine agonist therapy is recommended. If dopamine agonist therapy does not decrease tumour size and improved symptoms, consider surgical resection, especially in patient with documented optic chiasm compression and visual fields disturbance. If the fetus is near-term, it may be reasonable to induce delivery before neurosurgical intervention. |
| Postpartum • Close follow-up of apoplexy symptoms with rapid radiologic and hormonal work-up if needed. • Clinical and hormonal follow-up are recommended during subsequent pregnancy. |
This summary have taken into account the recommendations made by the Endocrine Society for diagnosis and treatment of hyperprolactinemia – see Melmed et al..38
Patient 1 had pituitary apoplexy without underlying lesion, which suggested that it probably occurred due to physiological hypertrophy of the pituitary gland during pregnancy. We found only two other cases in the literature similar to our case with pituitary apoplexy during pregnancy without evidence of an underlying lesion.18,20 Pituitary apoplexy should be included in the differential diagnosis of severe and sudden headache in pregnant women, even if they are not known to have any pituitary lesion. As described with our patients 2 and 3, subsequent pregnancies also seem to be safe for these patients with adequate follow-up.
Most of the obstetrical and fetal outcomes were missing from the cases reported in the literature. The available data showed that 13/21 (62%–64% with our cases) women had vaginal delivery and 8/21 (38%–36% with our cases) had caesarean sections. All newborns seen in our centre were healthy. Pituitary apoplexy is considered to be a medical emergency, but even if hormonal insufficiency occurs, it does not appear to have any consequence for the fetus if it is recognised early and treated rapidly.
The retrospective chart review is limited by the coding system of the ICD-9, since pituitary apoplexy is not coded. We feel that we identified these women adequately by using the appropriate coding for pituitary-associated lesions. Because of the limited data found in the literature search on obstetrical and fetal outcomes, it is difficult to draw hard conclusions about these important issues.
Conclusion
Pituitary apoplexy is a rare cause of severe and sudden headache during pregnancy. It can result from an acute haemorrhagic infarction of a pre-existing pituitary lesion or, more exceptionally as with our first case, in a physiologically enlarged gland. It is imperative to search for endocrine disturbances and start appropriate hormonal replacement since apoplexy can impair pituitary function. The diagnosis of pituitary apoplexy needs to be considered by clinicians for pregnant and postpartum women presenting with severe and sudden onset of headache, since rapid treatment of hormonal deficiencies need to be addressed when present, and this also seems to help in delivering a healthy baby. Endocrine follow-up is needed for those women on subsequent pregnancy.
Acknowledgements
The authors thank the study participants and their families, the endocrinology and obstetrics teams at the CHUM, as well as John Davison for the manuscript review. This work was presented as a poster at the International Congress of Endocrinology on 21 June 2014 and at the North American Society of Obstetrical Medicine Annual Meeting on 25 October 2014.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
All patients gave written consent. Our local ethics committee also approved this research study.
Guarantor
AG
Contributorship
SG wrote the manuscript, researched data, and contributed to discussion. FW and MJB researched data and reviewed/edited the manuscript. MM and AG researched data, contributed to discussion and reviewed/edited the manuscript.
References
- 1.Baily P. Pathological report of a case of acromegaly, with special reference to the lesions in the hypophysis cerebri and in the thyroid gland; and a case of haemorrhage into the pituitary. Phila Med J 1898; 1: 789–792. [Google Scholar]
- 2.Karaca Z, Tanriverdi F, Unluhizarci K, et al. Pregnancy and pituitary disorders. Eur J Endocrinol 2010; 162: 453–475. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez JG, Elizondo G, Saldivar D, et al. Pituitary gland growth during normal pregnancy: an in vivo study using magnetic resonance imaging. Am J Med 1988; 85: 217–220. [DOI] [PubMed] [Google Scholar]
- 4.Randeva HS, Schoebel J, Byrne J, et al. Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol 1999; 51: 181. [DOI] [PubMed] [Google Scholar]
- 5.Rigg LA, Lein A, Yen SS. Pattern of increase in circulating prolactin levels during human gestation. Am J Obstet Gynecol 1977; 129: 454–456. [DOI] [PubMed] [Google Scholar]
- 6.Couture N, Aris-Jilwan N, Serri O. Apoplexy of a microprolactinoma during pregnancy: case report and review of literature. Endocr Pract 2012; 18: 147–150. [DOI] [PubMed] [Google Scholar]
- 7.Kannuki S, Bando K, Shirakawa N, et al. [MRI findings and endocrinological dysfunction in hemorrhagic pituitary adenoma]. No Shinkei Geka 1993; 21: 1005–1012. [PubMed] [Google Scholar]
- 8.Ohtsubo T, Asakura T, Kadota K, et al. [A report of a transsphenoidal operation during pregnancy for a pituitary adenoma]. No Shinkei Geka 1991; 19: 867–870. [PubMed] [Google Scholar]
- 9.Kita D, Hayashi Y, Sano H, et al. Postoperative diabetes insipidus associated with pituitary apoplexy during pregnancy. Neuro Endocrinol Lett 2012; 33: 107–112. [PubMed] [Google Scholar]
- 10.Atmaca A, Dagdelen S, Erbas T. Follow-up of pregnancy in acromegalic women: different presentations and outcomes. Exp Clin Endocrinol Diabetes 2006; 114: 135–139. [DOI] [PubMed] [Google Scholar]
- 11.Iuliano S, Laws ER., Jr Management of pituitary tumors in pregnancy. Semin Neurol 2011; 31: 423–438. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiu ML, Chirita C, Coculescu M. Partial remission of Nelson's syndrome after pituitary apoplexy during pregnancy. Endocrin Abstracts 2009; 19: 191. [Google Scholar]
- 13.Perotti V, Dexter M. Post-partum pituitary apoplexy with bilateral third nerve palsy and bilateral carotid occlusion. J Clin Neurosci 2010; 17: 1328–1330. [DOI] [PubMed] [Google Scholar]
- 14.Schrupp Berg HL, Edlow JA. Post-partum pituitary apoplexy: a case report. Intern Emerg Med 2007; 2: 311–314. [DOI] [PubMed] [Google Scholar]
- 15.Jassal DS, McGinn G, Embil JM. Pituitary apoplexy masquerading as meningoencephalitis. Headache 2004; 44: 75–79. [DOI] [PubMed] [Google Scholar]
- 16.Freeman R, Wezenter B, Silverstein M, et al. Pregnancy-associated subacute hemorrhage into a prolactinoma resulting in diabetes insipidus. Fertil Steril 1992; 58: 427–429. [DOI] [PubMed] [Google Scholar]
- 17.Tonda C, Rizvi AA. Headache, pituitary lesion and panhypopituitarism in a pregnant woman: tumor, apoplexy or hypophysitis? Am J Med Sci 2011; 342: 247–249. [DOI] [PubMed] [Google Scholar]
- 18.Krull I, Christ E, Kamm CP, et al. Hyponatremia associated coma due to pituitary apoplexy in early pregnancy: a case report. Gynecol Endocrinol 2010; 26: 197–200. [DOI] [PubMed] [Google Scholar]
- 19.O'Donovan PA, O'Donovan PJ, Ritchie EH, et al. Apoplexy into a prolactin secreting macroadenoma during early pregnancy with successful outcome. Case report. Br J Obstet Gynaecol 1986; 93: 389–391. [PubMed] [Google Scholar]
- 20.Murao K, Imachi H, Muraoka, et al. Hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome with pituitary apoplexy. Fertil Steril 2011; 96: 260–261. [DOI] [PubMed] [Google Scholar]
- 21.Nagulesparan M, Roper J. Hemorrhage into the anterior pituitary during pregnancy after induction of ovulation with clomiphene. Br J Obstet Gynaecol 1978; 85: 153–155. [DOI] [PubMed] [Google Scholar]
- 22.Gondim J, Ramos FJ, Pinheiro I, et al. Minimally invasive pituitary surgery in hemorrhagic necrosis of adenoma during pregnancy. Minim Invasive Neurosurg 2003; 46: 173–176. [DOI] [PubMed] [Google Scholar]
- 23.Zammit-Mangion M, Rogers A, Mackillop L. Pituitary apoplexy in pregnancy: two case reports. Reg Clin Cases. Abstract and poster, pp. 16. Society for Endocrinology, Clinical Cases Meeting, 10 July 2012, Oxford, UK. [Google Scholar]
- 24.Onesti ST, Wisniewski T, Post KD. Clinical versus subclinical pituitary apoplexy: presentation, surgical management, and outcome in 21 patients. Neurosurgery 1990; 26: 980–986. [PubMed] [Google Scholar]
- 25.Hervet E, Barrat J, Pigne A, et al. Prolactin adenoma. Hypophysectomy during pregnancy. Nouv Presse Méd 1975; 4: 2393–2395. [PubMed] [Google Scholar]
- 26.Parihar V, Yadav YR, Sharma D. Pituitary apoplexy in a pregnant woman. Ann Indian Acad Neurol 2009; 12: 54–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witek P, Zieliński G, Maksymowicz M, et al. Transsphenoidal surgery for a life-threatening prolactinoma apoplexy during pregnancy. Neuro Endocrinol Lett 2012; 33: 483–488. [PubMed] [Google Scholar]
- 28.Scherrer H, Turpin G, Darbois Y, et al. [Pregnancy and hyperprolactinemia. Review of therapeutic measures apropos of a series of 35 patients]. Ann Méd Interne 1986; 137: 621–626. [PubMed] [Google Scholar]
- 29.Lunardi P, Rizzo A, Missori P, et al. Pituitary apoplexy in an acromegalic woman operated on during pregnancy by transphenoidal approach. Int J Gynaecol Obstet 1991; 34: 71–74. [DOI] [PubMed] [Google Scholar]
- 30.Fujimaki T, Hotta S, Mochizuki T, et al. Pituitary apoplexy as a consequence of lymphocytic adenohypophysitis in a pregnant woman: a case report. Neurol Res 2005; 27: 399–402. [DOI] [PubMed] [Google Scholar]
- 31.Ginath S, Golan A. Gestational pituitary-tumor apoplexy. N Engl J Med 2010; 363: e10. [DOI] [PubMed] [Google Scholar]
- 32.De Heide LJM, Van Tol KM, Doorenbos B. Pituitary apoplexy presenting during pregnancy. Neth J Med 2004; 62: 393–396. [PubMed] [Google Scholar]
- 33.Janssen NM, Dreyer K, Van der Weiden R. Management of pituitary tumour apoplexy with bromocriptine in pregnancy. J R Soc Med Short Rep 2012; 3: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamfo JE, Sharif S, Donnelly T, et al. A case of pituitary apoplexy masquerading as hyperemesis gravidarum. J Obstet Gynaecol 2011; 31: 662. [DOI] [PubMed] [Google Scholar]
- 35.Lee MS, Pless M. Apoplectic lymphocytic hypophysitis. Case report. J Neurosurg 2003; 98: 183–185. [DOI] [PubMed] [Google Scholar]
- 36.Lamberts SW, Klijn JG, de Lange SA, et al. The incidence of complications during pregnancy after treatment of hyperprolactinemia with bromocriptine in patients with radiologically evident pituitary tumors. Fertil Steril 1979; 31: 614–619. [DOI] [PubMed] [Google Scholar]
- 37.Rosen SG, Kharlip J. Pituitary apoplexy during pregnancy. Supplement, abstract and poster, pp. 1–438, The Endocrine Society's 93th Meeting, 4–7 June 2011, Boston, MA, USA. [Google Scholar]
- 38.Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011; 96: 273–288. [DOI] [PubMed] [Google Scholar]