Abstract
Liver disease in pregnancy can be classified as predating, co-incidental or unique to pregnancy. Medications are often overlooked as a significant cause of liver disease. We present the case of a 39-year-old patient who presented at 20 weeks with jaundice, elevated liver enzymes, and abnormal liver function progressing eventually to fulminant hepatic failure. The patient was on methyldopa and labetalol from 12 weeks’ gestational age. Liver biopsy was consistent with drug-induced liver injury. Both methyldopa and labetalol have been associated with hepatotoxicity including liver failure. This case highlights the importance of including medications as a cause of liver failure in pregnant patients.
Keywords: Hepatitis, liver failure, liver dysfunction, drug induced, methyldopa, labetalol
Introduction
The cause of liver disease in pregnancy can be difficult to diagnose. Making the correct diagnosis is critical as it has implications for both the mother and her fetus. Medications can often be overlooked as a cause of liver failure. We present a case of fulminant hepatic failure in a pregnant woman secondary to methyldopa and labetalol.
Case report
A 39-year-old G2P0 woman with twin gestation conceived through in vitro fertilization was admitted to hospital at 20+0 weeks’ gestational age (GA) with jaundice and elevated liver enzymes. Her medical history was relevant for hypertension, type II diabetes, and obesity. She had one previous first trimester loss.
Medications in the current pregnancy included labetalol, started at 12 weeks’ GA and methyldopa, which was added at 16 weeks’ GA. When asked about allergies, the patient recalled being told that she had atorvastatin (Lipitor)-related pneumonitis in 2002. However, the pathology report obtained later stated she was on labetalol at that time. The biopsy was consistent with a bronchiolitis obliterans organizing pneumonia pattern suggestive of eosinophilic pneumonia possibly secondary to labetalol use.
In this pregnancy, she was well until 14 weeks’ GA when she developed pruritis lasting two weeks and occurring on the chest, back, and soles of the feet. At that time, ALT was 910 U/L and bile acids of 26.4 µmol/L. Subsequent ALT done one week apart were 531 U/L and 452 U/L, respectively. She was feeling well up until the week before admission when she developed jaundice, pale stools, and dark urine. She did not have any autoimmune features and denied symptoms of pre-eclampsia, recent travel, blood transfusion or high-risk behavior. All medications were discontinued on admission.
The patient was normotensive. She had scleral icterus but no asterixis or stigmata of chronic liver disease. The rest of the physical examination was unremarkable. Hematology profile, electrolytes, creatinine, and urine protein to creatinine ratio were normal. Liver enzymes and function were the following: ALT 1406 U/L, AST 2701 U/L, Alkaline Phosphatase 159 U/L, GGT 37 U/L, LDH 807 U/L, bilirubin 304 µmol/L, albumin 28 g/L, INR 3.1, fibrinogen 3.1 g/L, and glucose 8.2 mmol/L. Autoimmune panel was remarkable for positive antinuclear antibody (ANA) with a titre of 1:320 and homogenous pattern and mild elevations of IgG and IgA. The rest of the autoimmune panel was negative. Infectious work-up was negative for hepatitis A, B, C, cytomegalovirus (CMV), Epstein–Barr virus (EBV), and herpes simplex virus (HSV). Toxicology screen was negative as was work-up for hereditary causes. Both the abdominal ultrasound and magnetic resonance cholagiopancreatography (MRCP) showed cholelithiasis but no obstruction or other abnormalities.
On the third day of admission, the patient became acutely encephlopathic and developed acute kidney injury with creatinine rising to 109. She was immediately transferred to a hospital with a liver transplant unit. She was managed supportively with lactulose and observed in a step down unit. Due to her coagulopathy, she underwent a transjugular liver biopsy two days later. Following her biopsy, she spontaneously delivered Twin A. A decision was made to induce delivery of Twin B in the operating room. She had a significant postpartum hemorrhage and required dilatation and curettage for removal of retained products of conception.
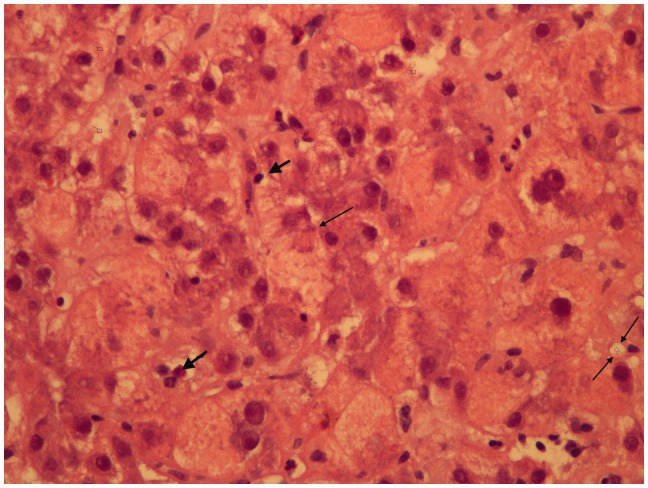
Hepatic core biopsy showed acute liver injury consistent with drug-/toxin-induced liver injury (Figure 1). The patient was managed conservatively and observed for one week as an inpatient. Prior to discharge, synthetic liver function showed improvement and the creatinine normalized. Her synthetic liver function has normalized, but the most recent ultrasound done eight months after the event shows marked cirrhosis. She is currently followed by the liver transplant team and is planning surrogacy.
Figure 1.
The hepatocytes show vacuolization of their cytoplasm and focal cytoplasmic expansion, i.e. ballooning degeneration, (single thin arrow) indicating trans-membrane transport mechanisms are breaking down and scattered necrotic hepatocytes. This appearance is quite distinctive from fatty liver of pregnancy, where the hepatocytes would show a uniform microvescicular and foamy appearance. Intracanalicular cholestasis is present (two thin arrows). There are some neutrophilic leukocytes responding to cell death. There is a light patchy sinusoidal lymphocyte infiltrate (thick arrows)—but the appearance is not typically auto-immune, where a more intense lymphoplasmacytic infiltrate would be expected. This appears to be more of a direct toxicity to the hepatocytes.
Discussion
Liver disease in pregnancy can be classified as predating, co-incidental or unique to pregnancy. It is essential to make the correct diagnosis as it has significant implications for management. Since our patient presented at 20 weeks’ GA, a cause from any of the three categories of liver disease in pregnancy was possible and thus, a broad differential diagnosis and work-up was applied. Pre-eclampsia was felt to be less likely as she was normotensive without medication and did not have maternal symptoms, proteinuria or other biochemical abnormalities. Acute fatty liver of pregnancy was a strong consideration given the dramatic synthetic liver dysfunction. Obstetric cholestasis can occur concurrently with other etiologies of liver disease and may have developed at 14 weeks’ GA as she had pruritis and elevated liver enzymes and bile acids.1 Drug-induced liver injury (DILI) and autoimmune hepatitis were the other two possibilities although after applying the criteria for autoimmune hepatitis, it was less likely on the differential.2 The biopsy was, therefore, critical in making a diagnosis in this patient as the cause was not obvious.
DILI is a broad term applied to any injury to the liver by a prescribed medication, over-the-counter medication, herbs or dietary supplements (Table 1) manifesting as a spectrum from asymptomatic liver enzyme elevations to acute liver failure.3,14 The risk factors for DILI are complex, but female gender and obesity have both been listed as risk factors.3,14 Additionally, it has been suggested that obstetric cholestasis is associated with higher rates of drug sensitivities.11 Our patient developed fulminant hepatic failure approximately eight weeks after starting methyldopa and four weeks after starting labetalol. The exact medication that caused this patient’s acute liver failure is difficult to discern and it is unclear whether the combination of the two hepatotoxic medications contributed to her dramatic presentation. This patient had a previous lung injury likely secondary to labetalol, which also raises the possibility of a genetic predisposition.
Table 1.
Common medications associated with liver failure.
| Drug class | Pattern of drug-induced liver injury (DILI) |
|---|---|
| Antibiotics | |
| Amoxicillin/clavulanate | Cholestasis2 |
| Isoniazid | Hepatocellular2 |
| Nitrofurantoin | Mixed2 |
| Rifampin | Hepatocellular2 |
| Trimethoprim-sulfamethoxazole | Mixed2 |
| Anticonvulsants | |
| Carbamazepine | Mixed2 |
| Valproic acid | Hepatocellular2 |
| Antihypertensives | |
| Labetalol | Hepatocellular4 |
| Methyldopa | Hepatocellular5–10 |
| Analgesics | |
| Acetaminophen | Hepatocellular2 |
| Acetysalicyclic acid, other NSAIDs | Hepatocellular2 |
| Anti-thyroid medications | |
| Amiodarone | Hepatocellular2 |
| Other | |
| Azathioprine | Mixed2 |
| Chemotherapy | – |
| Herbal medications | – |
| Methotrexate | Hepatocellular2 |
The literature shows that the risk of hepatotoxicity from methyldopa in pregnant patients is approximately 1% and can occur between 1 and 20 weeks within starting the drug.12 The clinical spectrum ranges from minor elevation of liver enzymes to fulminant hepatitis.12 The severity of liver injury does not appear to be related to the dose of methyldopa.12 Although no case reports of labetalol-induced hepatotoxicity during pregnancy were identified, there are numerous case reports in non-pregnant adults starting in the early 1980s.12 Previous case reports of labetalol-induced hepatotoxicity described patients who presented with vomiting, jaundice, dark urine, and pruritus; the onset of symptoms varied from 21 to 189 days after starting the therapy (median 60 days).12 In addition, most patients also had an increase in AST and/or ALT of more than seven times the upper limit of normal.12 In the literature, there have been multiple deaths from labetalol-induced hepatotoxicity; however, most cases have reported symptom resolution after stopping the medication.12
In the case of our patient, her liver enzymes dramatically decreased after stopping the medications, while the synthetic function took longer to recover.
The mechanism of methyldopa-induced liver dysfunction is speculated to be related to its activation.12 Methyldopa is a pro-drug that is metabolized to alpha-methylnorepinephrine (its active component) when the transformation of the pro-drug via CYP 450 enzymes is disrupted and an immune reaction to the resulting metabolite occurs.12 Two mechanisms of labetalol related liver injury are hypothesized: one could be idiosyncratic, potentially due to differences in CYP 450 enzymes, and the other mechanism could be allergic as eosinophils have been seen in pathology specimens.4
While these medications are commonly used for the treatment of hypertension in pregnancy, there is no guidance around monitoring liver enzymes especially with methyldopa. A 2009 Cochrane review of methyldopa for primary hypertension in non-pregnant patient cautions that healthcare practitioners should be aware that there are potential serious side effects associated with the use of methyldopa.13 Based on the available literature (Table 2), we advocate for monitoring liver enzymes at baseline and then intermittently throughout pregnancy. Typically, when methyldopa is stopped, the liver enzyme elevation should resolve.
Table 2.
Case repots of methyldopa associated liver failure.
| Case report | Maternal age (years) | GA at which methyldopa initiated (weeks) | GA at symptom onset (weeks) | Symptoms | Outcome |
|---|---|---|---|---|---|
| Picaud et al.5 *Abstract only | 22 | 33 | 3rd trimester | Initial symptoms of nausea, vomiting, and increased temperature; associated with febrile jaundice and coagulopathy in the third stage of labor. Patient passed away 3 days after delivery due to hematemesis, renal failure, and hepatic encephalopathy. | Maternal death 3 days after delivery |
| Smith and Piercy6 | 30 | 10 | 13 | Jaundice, abdominal pain, nausea | Methyldopa discontinued at 14 weeks and re-introduced at 36 weeks’ gestation; C-section for family planning |
| Thomas and Cardwell7 | 37 | 6 | 15 | Jaundice, dark urine | Complete resolution of symptoms at 21 weeks with discontinuation of medication |
| Phadis et al.12 | 40 | 6 | 8 | Jaundice, dark urine, myalgia, fatigue, fever | Switched to labetalol and liver function gradually improved between 10 and24 weeks |
| Ali et al.8 | 33 | 6 | 12 | Jaundice, dark urine | Resolved 2 weeks later with a course of prednisone |
| Ozsvar et al.9 | 35 | 21 | 23 | Transaminitis | Switched to nifedipine; no information on outcome |
| Slim et al.10 | 34 | 13 | 17 | Jaundice, pruritus, dark urine, pale stool | Liver function test results returned to normal levels within 5 weeks of discontinuation |
GA: gestational age.
Conclusion
Methyldopa and labetalol are common antihypertensive agents used in pregnancy. Drug-induced fulminant liver failure is a rare but serious complication of these medications. This case report, along with many others in both pregnant and non-pregnant adults, draws attention to these drugs and consideration of monitoring of liver enzymes while on these medications.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Written consent was obtained from the patient.
Guarantor
TF.
Contributorship
TF conceptualized the manuscript. TF and HR drafted the manuscript. DW provided the image and wrote the histological description.
References
- 1.Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol 2009; 15: 2049–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc 2014; 89: 95–106. [DOI] [PubMed] [Google Scholar]
- 3.Manns MP, Czaja AJ, Gorham JD, et al. Practice guidelines: diagnosis and management of autoimmune liver disease. Hepatology 2010; 51: 1–29.19827164 [Google Scholar]
- 4.Clark JA, Zimmerman HJ, Tanner LA. Labetalol hepatotoxicity. Ann Intern Med 1990; 113: 210–213. [DOI] [PubMed] [Google Scholar]
- 5.Picaud A, Walter P, de Preville G, et al. Fatal toxic hepatitis in pregnancy. A discussion of the role of methyldopa. J Gyencol Obstet Biol Reprod (Paris) 1990; 19: 192–196. [PubMed] [Google Scholar]
- 6.Smith GN, Piercy WN. Methyldopa hepatotoxicity in pregnancy: a case report. Am J Obstet Gynecol 1995; 172: 222–224. [DOI] [PubMed] [Google Scholar]
- 7.Thomas LA, Cardwell MS. Acute reactive hepatitis in pregnancy induced by alpha-methyldopa. Obstet Gynecol 1997; 90: 658–659. [DOI] [PubMed] [Google Scholar]
- 8.Ali T, Srinivasan N, Le V, et al. Alpha-methyldopa hepatotoxicity in pregnancy. J Coll Physicians Surg Pak 2009; 19: 125–126. [PubMed] [Google Scholar]
- 9.Ozsvar Z, Solymossi Z, Monostory K. Methyldopa-induced acute reactive hepatitis in pregnancy, drug metabolizing capacity of the liver. Orv Hetil 2010; 151: 457–461. [DOI] [PubMed] [Google Scholar]
- 10.Slim R, Ben Salem C, Hmouda H, et al. Hepatotoxicity of alpha-methyldopa in pregnancy. J Clin Pharm Ther 2010; 35: 361–363. [DOI] [PubMed] [Google Scholar]
- 11.Johnson P, Samsioe G, Gustafson A. Studies in cholestasis of pregnancy. I. Clinical aspects and liver function tests. Acta Obstet Gynecol Scand 1975; 54: 77–84. [DOI] [PubMed] [Google Scholar]
- 12.Phadis SV, Sangay MR, Sanusi FA. Alpha-methyldopa-induced acute hepatitis in pregnancy. Aust N Z J Obstet Gynaecol 2006; 46: 256–257. [DOI] [PubMed] [Google Scholar]
- 13.Mah GT, Tejani AM and Musini VM. Methyldopa for primary hypertension. Cochrane Database Syst Rev, CD003893, http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003893.pub2/abstract (2009). [DOI] [PMC free article] [PubMed]
- 14.Hayashi PH, Fontana RJ. Clinical features, diagnosis and natural history of drug-induced liver injury. Semin Liver Dis 2014; 34: 134–144. [DOI] [PubMed] [Google Scholar]