Abstract
Severe gestational hypertriglyceridemia is a potentially life threatening and complex condition to manage, requiring attention to a delicate balance between maternal and fetal needs. During pregnancy, significant alterations to lipid homeostasis occur to ensure transfer of nutrients to the fetus. In women with an underlying genetic predisposition or a secondary exacerbating factor, severe gestational hypertriglyceridemia can arise, leading to devastating complications, including acute pancreatitis. Multidisciplinary care, implementation of a low-fat diet with nutritional support, and institution of a hierarchical therapeutic approach are all crucial to reduce maternal and fetal morbidity. To avoid maternal pancreatitis, close surveillance of triglycerides throughout pregnancy with elective hospitalization for refractory cases is recommended. Careful dietary planning is required to prevent neural and retinal complications from fetal essential fatty acid deficiency. Questions remain about the safety of fibrates and plasmapheresis in pregnancy as well as the optimal timing for induction and delivery of these women.
Keywords: High-risk pregnancy, hypertriglyceridemia, maternal–fetal medicine, pancreatitis, maternal morbidity, complications
Severe gestational hypertriglyceridemia is a potentially life-threatening and complex condition to manage, requiring attention to a delicate balance between maternal and fetal needs. To date, there has been a lack of practical guidance for clinicians, particularly with respect to the nuances of planning a dietary prescription to avoid essential fatty acid deficiency. We highlight a prototypical case of severe gestational hypertriglyceridemia, discuss clinical considerations, and propose a real-world approach based on what is known from the literature.
Case
A 27-year-old primigravada woman at 22 weeks’ gestation presented to the emergency room with severe abdominal pain and nausea. Her history was significant for poorly controlled type 2 diabetes (HbA1c 7.9%) as well as hypertriglyceridemia-induced acute pancreatitis three years previously. There was no family history of lipid disorders. She had been lost to follow-up and had not been on dietary fat restriction or lipid-lowering therapy prior to her pregnancy. Her home medications were insulin determir 30 units at breakfast and bedtime, aspart 5 units with correction pre-meals, as well as a prenatal multivitamin. Upon presentation, in addition to an elevated blood glucose (12.8 mmol/L) and lipase (514 U/L), her bloodwork revealed a lipemic sample due to a markedly elevated plasma triglycerides of 99 mmol/L. Total cholesterol was also elevated at 22.3 mmol/L. Liver enzymes were normal. An abdominal ultrasound revealed a prominent pancreas with peripancreatic fluid consistent with acute pancreatitis. Fetal growth by obstetrical ultrasound was appropriate for gestational age and the amniotic fluid index was normal.
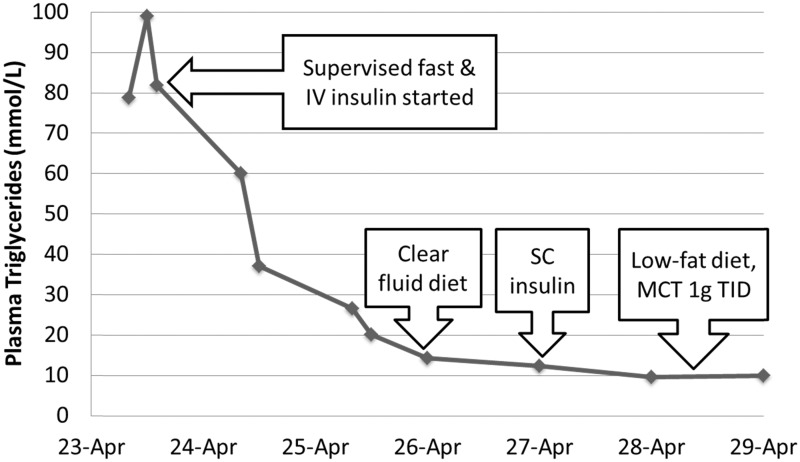
She was admitted for management of severe gestational hypertriglyceridemia-induced pancreatitis. She was followed by a multidisciplinary team consisting of obstetricians, endocrinologists, maternal–fetal medicine physicians, registered dietitians and diabetes nurse specialists. Supervised fasting and intravenous fluid therapy were instituted in conjunction with an intravenous insulin and 5% dextrose infusion. After two days, she was started on a low-fat diet (<20% of total calories from fat/day) and medium-chain triglycerides (Figure 1). Within 72 h of admission, her plasma triglyceride level had reduced by more than two-thirds, and a week later at discharge was 10 mmol/L. She was discharged on a 20% fat-restricted diet with medium chain triglycerides (MCT) 15 g and omega-3-fatty esters 3 g daily. Two weeks following her discharge, her plasma triglycerides were again elevated at 33 mmol/L. She was admitted electively to institute more stringent dietary management and counselling. Subsequently, she presented in preterm labour at 36 weeks and five days’ gestation. Due to fetal compromise and labour dystocia, she required urgent C-section and delivered a healthy female infant weighing 3658 g.
Figure 1.
Changes in our patient’s plasma triglyceride levels as a result of several interventions during her first hospitalization: (a) supervised fast and intravenous (iv) insulin infusion; (b) transitioned to clear fluid diet; (c) iv insulin infusion discontinued and subcutaneous (sc) insulin started; (d) transitioned to low-fat diet (<20 % of total calories from fat/day) with medium chain triglycerides (MCT) 1 g orally three times daily.
What is normal lipoprotein metabolism and how does it differ in pregnancy?
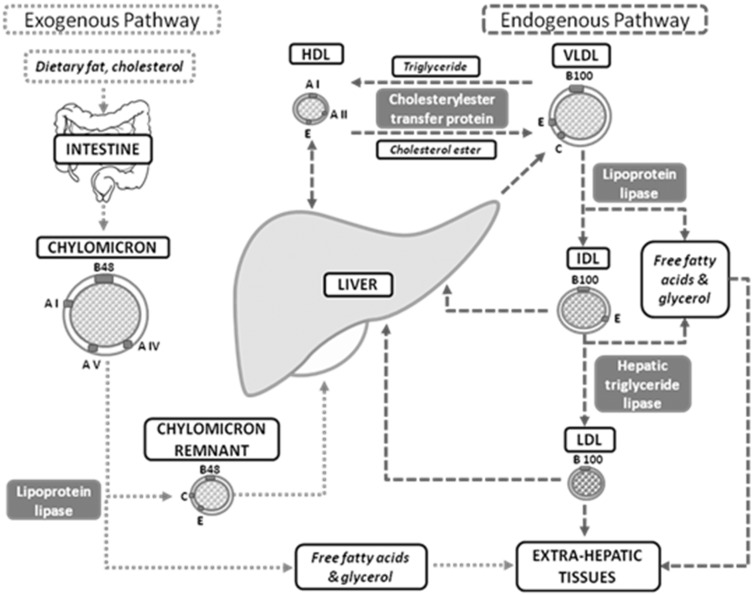
Comprising three fatty acids attached to a glycerol backbone, triglycerides are derived from both dietary intake and endogenous hepatic production, as depicted in Figure 2.
Figure 2.
Endogenous and exogenous triglyceride pathways.
In the small intestine, exogenous dietary triglycerides combine with phospholipids, apolipoproteins and cholesterol esters to form CMs, which are secreted into intestinal lymph and thoracic duct and then the circulation. CMs, which are TRLs, serve as an essential mode of transport for dietary triglycerides to peripheral tissue and the liver. In contrast, endogenous triglycerides are produced from hepatic fatty acids and these triglycerides are incorporated into very low density lipoprotein (VLDL) particles, another TRL particle. They contain apolipoprotein B100 (as opposed to apolipoprotein B48 in CMs). VLDL particles are secreted directly into circulation and allow for the transport of hepatically derived lipids to peripheral tissues. Both CMs and VLDL particles undergo extensive processing in the circulation. Through the hydrolytic action of capillary-anchored LPL in adipose tissue, skeletal muscle and cardiac muscle, these TRLs are metabolized, liberating free fatty acids and glycerol which are now able to diffuse across cellular membranes. Adipocytes and myocytes take up these free fatty acids to be either oxidized or stored. Fatty acid oxidation provides immediate generation of energy, while the remaining free fatty acids, in conjunction with de novo fatty acid synthesis from carbohydrates (lipogenesis), are esterified to form cytosolic fat droplets.
In both pregnant and non-pregnant states, the maintenance of normal triglyceride levels requires a careful equilibrium among several dynamic factors:
production of triglyceride-rich lipoproteins (TRLs), namely chylomicrons (CMs) and very low density lipoprotein (VLDL)
lipolytic processing of circulating TRLs by lipoprotein lipase (LPL) with apolipoprotein C-II (apoC-II) as its activator, and hepatic triglyceride lipase (HTGL), as well as
hepatic clearance of CMs and VLDL remnants from circulation by LDL receptor and LDL receptor-related protein (LRP), mediated by apolipoprotein E (apoE).
During pregnancy, significant alterations to lipid homeostasis occur to ensure transfer of nutrients to the fetus. Hormonally driven changes in the liver and adipose tissue alter levels of circulating triglycerides, fatty acids, cholesterol, and phospholipids. While all plasma lipids increase following the first eight weeks of pregnancy, the most profound change is in plasma triglyceride levels which increase 2–3 fold by third trimester (with mean triglyceride levels of 0.89 mmol/L, 1.71 mmol/L, and 2.77 mmol/L in the first, second and third trimesters, respectively).1,2 Further triglyceride increases are seen at all gestational stages in diabetic pregnancies.1
Maternal triglycerides do not freely cross the placenta but serve as an important source of energy for the mother. Glycerol from triglycerides may be used for glucose synthesis, while free fatty acids may be oxidized to form ketone bodies during maternal fasting. This allows other nutrients such as essential amino acids and glucose to be diverted to the fetus as energy sources and building blocks for fetal development.1
In the early stages of pregnancy (first and second trimesters), rising progesterone levels result in hyperphagia and increased intestinal lipid synthesis.3 The increased intake of dietary fats and cholesterol provides more substrate for CMs formation. This, in combination with increased hepatic lipogenesis, facilitates the accumulation of maternal fat stores and weight gain.
As pregnancy progresses, this anabolic state gives way to a catabolic state as fetal nutritional requirements increase markedly.1 Maternal fat stores, in turn, decrease as free fatty acids and glycerol are used by the liver to synthesize energy-rich compounds such as glucose and ketone bodies for fetal utilization, although it is still controversial whether elevated ketone levels have an adverse impact on fetal development. This is driven predominantly by significant increases in estrogen and human placental lactogen (hPL) in the late-second and third trimesters.4 The high estrogen state further enhances lipogenesis and hepatic VLDL synthesis, but suppresses hepatic lipase (HL) activity, resulting in increased triglyceride-rich LDL and high-density lipoprotein (HD) particles in the maternal circulation.5 Concurrently, insulin resistance secondary to high levels of hPL counteracts the usual targets of insulin action leading to decreased LPL activity and increased adipose tissue lipolysis.6,7 This results in increased levels of circulating free fatty acids, which are available as substrates for hepatic triglyceride synthesis and assembly of VLDL. The lipolytic cascade is further exacerbated by hypoglycemic-induced catecholamine surges of late pregnancy.8
How do we define gestational hypertriglyceridemia?
Although lipometabolism changes are seen in the majority of pregnant women,9 gestational hypertriglyceridemia is defined as fasting plasma triglyceride level above the age-adjusted 95th percentile for the non-pregnant population. Of particular clinical relevance, is severe gestational hypertriglyceridemia, arbitrarily defined as a plasma triglyceride level greater than 11.4 mmol/L as levels above this are associated with an increased risk of pancreatitis. A proportion of these women may have had pre-existing hypertriglyceridemia but many present for the first time in pregnancy.
What contributes to severe gestational hypertriglyceridemia?
The development of severe gestational hypertriglyceridemia typically occurs in the presence of an underlying genetic abnormality in triglyceride metabolism (Table 1). This can be the result of TRLs overproduction (e.g. increased number or size of VLDL as seen in familial combined hyperlipidemia and familial hypertriglyceridemia). It can also be caused by reduced lipolysis of circulating TRLs due to any mechanism resulting in defective lipolytic activity (e.g. homozygous mutations in LPL, apoC-II, LPL chaperone lipase maturation factor 1 or glycosylphosphatidylinositol-anchored HD-binding protein 1). Causative LPL gene mutations10–12 as well as apoE variants10,11,13 have been described in association with severe gestational hypertriglyceridemia. Decreased hepatic clearance of remnants has been seen as a contributing factor, as in familial dysbetalipoproteinemia associated with apoE2/E2 genotype. In some instances, secondary factors exacerbate the hypertriglyceridemic changes of pregnancy. The most commonly seen secondary risk factor in pregnancy by far is poorly controlled diabetes mellitus, which reduces the activity of LPL.2 The other factors tend to be less clinically relevant in gestational hypertriglyceridemia.
Table 1.
Primary and secondary factors contributing to severe gestational hypertriglyceridemia.
| Predisposing factors | Examples |
|---|---|
| Primary (genetic) factors | |
| • Increased production | ▪ Familial combined hyperlipidemia (FCH) |
| ▪ Familial hypertriglyceridemia (FHTG) | |
| • Ineffective lipolysis | ▪ Familial chylomicronemia disorders |
| • Decreased remnant clearance | ▪ Familial dysbetalipoproteinemia |
| Secondary (non-genetic) factors | |
| • States of altered physiology | ▪ Insulin-resistant states (e.g. untreated/ poorly-controlled diabetes mellitus) |
| ▪ Hypothyroidism | |
| ▪ Nephrotic syndrome | |
| • Medications/toxins | ▪ Glucocorticoids |
| ▪ Beta-blockers | |
| ▪ Protease inhibitors | |
| ▪ Alcohol | |
What are the risks of severe gestational hypertriglyceridemia to mother and fetus?
Gestational hypertriglyceridemia can lead to devastating and even life-threatening complications. One of its well-known risks is hypertriglyceridemia-induced acute pancreatitis with over three-quarters of cases occurring in the second and third trimesters.14 To date, its pathophysiological mechanism has not yet been fully elucidated. A favoured theory suggests that markedly TRL-rich environments promote lipolysis by pancreatic lipase. This results in increased liberation of high concentrations of free fatty acids which, in turn, inflict damage to the vascular endothelium and the pancreatic acinar cells.15 In-vitro studies also suggest a role for fatty acid-induced mitochondrial toxicity in the pathogenesis of hypertriglyceridemia-induced pancreatitis.16 The resultant ischemia generates an acidic environment, which amplifies the toxicity of the free fatty acids and promotes further pancreatic injury.15,17,18 Another favoured hypothesis is pancreatic capillary ischemia and acidosis due to chylomicronemia-related hyperviscosity.17
Although it occurs at relatively low incidence (between 3 and 7 in 10,000 cases), acute pancreatitis can be complicated by pseudocysts, pancreatic necrosis, significant electrolyte derangements, acute respiratory distress syndrome, shock and preeclampsia.1,19 Historically, gestational hypertriglyceridemia-induced acute pancreatitis portended significant mortality for both mother and fetus ranging from 7.5 to 21.0% and 19.0 to 20.0%, respectively.20–22 However, with the advent of improved and timely supportive treatments, contemporary mortality rates are lower, highlighting the importance of early diagnosis and intervention.23 Yet, the diagnosis can be challenging. It is a frequent mimicker of other conditions such as perforated peptic ulcer, ruptured ectopic pregnancy, pre-eclampsia, placental abruption and uterine rupture. Furthermore, lipemia can cause a spuriously low plasma amylase level, which can be misinterpreted and lead to diagnostic uncertainty.24 Thus, maintaining a high index of clinical suspicion in the appropriate setting is essential to avoid a delayed or missed diagnosis of gestational hypertriglyceridemia-induced acute pancreatitis.
Other maternal complications of gestational hypertriglyceridemia include hyperviscosity syndrome,25 pre-eclampsia26 and a greater likelihood for development of hyperlipoproteinemia in the future.13 Fetal risks include macrosomnia and the multiple pancreatitis-related complications such as in-utero fetal death, preterm labour, and prematurity.1,27
Who should be screened for severe gestational hypertriglyceridemia?
Early identification of women at risk for severe gestational hypertriglyceridemia is essential. This should ideally happen at preconception. Women with a history of pancreatitis or abdominal pain associated with prior estrogen use and those with a family history of hypertriglyceridemia should be screened. Individuals with known hypertriglyceridemia should have their triglycerides monitored during pregnancy. Signs suggestive of hypertriglyceridemia include eruptive xanthomatous skin lesions, lipemia retinalis and hepatosplenomegaly, although not all patients with severe hypertriglyceridemia have these signs. For some women, the only clue may be a lab report that suggests a lipemic sample.
What should I do if a patient with hypertriglyceridemia tells me she is planning to become pregnant?
Preconceptional counselling for women with hypertriglyceridemia should involve three key components:
-
For women who are taking lipid-lowering therapies, the benefits and risks of continuing such agents should be discussed. Caution is needed as fibrates are known to cross the human blood–placenta barrier.28 While no increase in congenital abnormalities was seen with gemfibrozil in preclinical animal testing,29 and no teratogenicity was noted in several case reports of fibrate use after the first t trimester,28,30,31 the numbers are too small to be confident in their safety. Similarly, although 18 mg daily of niacin is recommended in pregnancy, there are no studies assessing the safety of niacin at the much higher triglyceride-lowering doses of 1500 to 3000 g/day.25
Given that safety is not well established for fibrates and niacin, most clinicians would advocate discontinuing these lipid-lowering therapies while attempting pregnancy. The safety data on statins are conflicting;32 however, since statins do not have a significant triglyceride-lowering effect, they should be discontinued prior to and during pregnancy.
All women with pre-existing hypertriglyceridemia should be counselled about possible exacerbating factors. Non-pharmacologic interventions such as preconception weight loss in obese women and moderate-to-intense aerobic exercise should be instituted. Glycemic control should be optimized in the preconception stage to reduce not only hypertriglyceridemia but also the risks of congenital abnormalities, spontaneous abortion and pre-eclampsia.33,34
Dietary counselling should occur early with the expertise of a registered dietitian. Avoidance of concentrated sugars and a fat-restricted diet are paramount and will be discussed in further detail in the next section. Given the increased risk of pancreatitis with plasma triglyceride levels >11.4 mmol/L, we would advocate tailoring dietary recommendations to a target baseline triglyceride level less than this threshold, and as close to normal as possible.
How should I manage a patient with severe gestational hypertriglyceridemia?
Published guidelines such as those by The Endocrine Society35 advise a combination of dietary fat and simple carbohydrate intake reduction with drug treatment (using fibrates, niacin, or omega-3 fatty acids with or without statins) for management of severe hypertriglyceridemia in the non-pregnant state. In contrast, no formal clinical practice guidelines exist for severe gestational hypertriglyceridemia, which requires a nuanced approach to maintain the delicate balance between maternal and fetal needs. Much of the current recommendations extend largely from observational data in the form of case reports. Based on the available evidence for each treatment modality, we propose a hierarchical management strategy, as summarized in Figure 3 and Table 2. The care of all women with gestational hypertriglyceridemia should include: (1) multidisciplinary team management and (2) a low-fat low-glycemic carbohydrate diet, while avoiding essential fatty acid deficiency, with omega-3-acid ethyl esters and MCT as additional nutritional support as required. If hypertriglyceridemia is refractory to above treatments, consideration should be given towards: (3) elective hospitalization for supervised fast, parenteral nutrition, or intravenous insulin therapy, (4) fibrate therapy after first trimester and (5) plasmapheresis.
Figure 3.
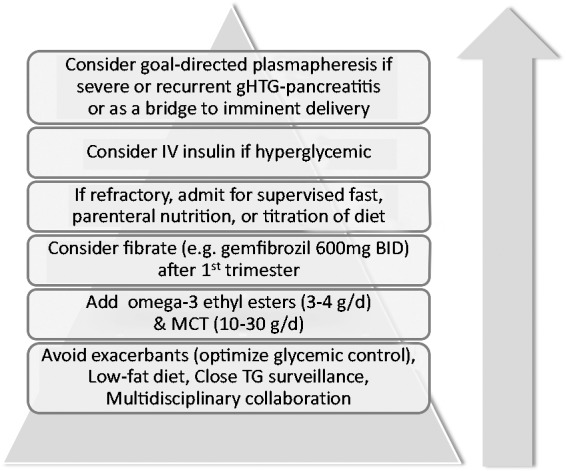
Recommended evidence-based approach to severe gestational hypertriglyceridemia management.
Table 2.
Management of gestational hypertriglyceridemia.
| Mechanism | Benefit | Risk | Key references | |
|---|---|---|---|---|
| Low fat diet < 20% of calories from fat/day | • Reduce substrates for exogenous TG synthesis pathway | • Effective in plasma TG lowering | • Difficult patient adherence • Risk of maternal weight loss and fetal essential fatty acid (EFA) deficiency | Sanderson58 Ma10 Mizushima21 Shenhav (2002)13 Al-Shali51 Tsai28 Abu Musa30 Basaran2 Sivakumaran53 Goldberg25 Basar54 Han11 Gupta56 |
| Omega-3-acid ethyl esters 3 to 4 g/d orally | • Reduce hepatic TG synthesis • Increase fatty acid oxidation in the liver and skeletal muscle • Enhance LPL activity | • Reduce TG by 25–50% via several mechanisms • Helps avoid deficiency of key omega-3 fatty acids including DHA and EPA | • Fishy taste, mild gastrointestinal side effects (e.g. burping) • May not lower TG quickly enough in acute setting | Goldberg25 Basar54 Han11 |
| Medium-chain triglycerides (MCT) 10 to 30 g/d orally Available as supplement but also in coconut oil, palm kernel oil, butter | • Provide nutritional support with rapid small intestine absorption and direct transport of TG via portal vein to liver for oxidation without CM formation • Mitigates the increase in dietary CHO in an isocaloric diet | • Densely caloric (8.3 kcal/g for MCT vs. 3–4 kcal/g for carbohydrate and protein) • Potential positive impact on fetal brain development | • Gastrointestinal side effects (e.g. abdominal discomfort, diarrhea, nausea, intestinal gas) | Mizushima21 Shenhav13 |
| Fibrates e.g. Gemfibrozil 600 mg twice-daily | • Transcription regulation via (+)PPARα • Increase LPL-mediated catabolism of VLDL particles by up-regulation of LPL, apoA-I, and apoA-II • Decrease apoB and VLDL production by down-regulation of apoCIII expression | • Effective gradual reduction in TG in many genetic forms of HTG, although response genotype dependent | • Safety in pregnancy controversial • May not lower TG quickly enough in acute setting | Gemfibrozil: Al-Shali51 Tsai28 Goldberg25 Fenofibrate (with niacin): Abu Musa30 |
| Parenteral nutrition | • Less increase in TG from iv carbohydrate ingestion compared to enteral carbohydrate nutrition | • Provides source of calories • Helps prevent/reverse maternal weight loss | • Typically requires hospitalization | Sanderson58 Shenhav13 Al-Shali51 Goldberg25 |
| Insulin Intravenous most often | • Rapid and potent LPL activator | • Immediate dramatic TG-lowering effect | • No clear role for euglycemic patients (risk of hypoglycemia) | Al-Shali51 Basaran2 Basar54 |
| Plasmapheresis | • Rapid removal of TG-rich lipoproteins • Removal of inflammatory mediators/cytokine levels in acute pancreatitis | • Immediate dramatic TG-lowering effect | • Limited availability • High cost • Risk of infection/thrombosis of plasmapheresis catheter line • Transient effect | Ma10 Sivakumaran53 Basar54 Gupta56 Safi55 |
CHO: carbohydrate; CM: chylomicron; DHA: docosahexaenoic acid; EFA: essential fatty acid; EPA: eicosapentaenoic acid; MCT: medium-chain triglycerides; TG: triglyceride; LPL: lipoprotein lipase; VLDL: very low density lipoprotein.
Multidisciplinary care team collaboration
Successful management of gestational hypertriglyceridemia requires proactive planning with a multidisciplinary health care team. In addition to the primary care provider, a high-risk obstetrician and an endocrinologist, the team often comprised a registered dietitian and diabetes nurse. An overall nutritional evaluation including a thorough assessment of caloric and fat intake by the registered dietitian is crucial as is optimization of glycemic control by the diabetes nurse. In conjunction with primary health care providers, endocrinologists assess, treat and monitor for clinical and biochemical signs of worsening hypertriglyceridemia prior to and throughout pregnancy. Obstetrical concerns for maternal or fetal complications may also dictate a need for change in management or for hospitalization. Close monitoring of fetal development is crucial. While there is currently insufficient evidence for a specific triglyceride threshold to dictate delivery, early induction of labour or preterm C-section should be considered for refractory third trimester hypertriglyceridemia to reduce the risk of acute pancreatitis. Open communication between team members and patient is paramount to provide much needed support and counselling through every stage of pregnancy.
Dietary prescription with low-fat diet with omega-3-acid ethyl esters and MCT as nutritional support
The management of gestational hypertriglyceridemia entails balancing fetal health needs with the needs of the mother. Under the careful guidance of a registered dietitian, women should be counselled specifically on establishing an isocaloric fat-restricted diet that does not incur an increased risk of maternal and fetal essential fatty acid deficiency or maternal pancreatitis from carbohydrate-driven hypertriglyceridemia. An example of a dietary prescription highlighting these characteristics is included in Table 3.
Table 3.
Summary of key management principles in the management of gestational hypertriglyceridemia.
| Prior to attempting to conceive: |
| 1. Holding lipid-lowering agents due to unknown teratogenicity in first trimester. |
| 2. Avoidance of exacerbating factors (secondary causes). |
| 3. Institution of dietary recommendations early, as part of pregnancy planning. |
| Attempting to conceive and during gestation: |
| 1. Multidisciplinary alliance is essential for patient-centred care and successful outcomes. |
| 2. Dietary prescription of low-fat diet and nutritional support (omega-3, MCT) is the cornerstone of therapy, and must balance fetal essential fatty acids requirements. |
| a. Establish ideal caloric intake based on pregnancy state (pre-pregnancy, first, second or third trimester), single vs. multiple gestation, age, and activity level. |
| b. Total daily fat should be at minimum less than 20% of total caloric intake, but may require further restriction (e.g. less than 10%) depending on triglyceride levels. In consultation with a registered dietitian, foods such as non-fat dairy products (e.g. Greek yogurt, cheese, egg whites), skinless chicken or turkey breast, cooked fish, low glycemic-index fibers (e.g. cooked quinoa, whole wheat bread), and vegetables may be selected to design a meal plan that meets the patient’s needs. The portion and frequency of each food item, along with any diet-appropriate dressing/condiment, must be carefully delineated in the meal plan. |
| c. Ensure adequate essential fatty acid intake (LA 2–6% and ALA 0.7%; approximately 4.4–13 g/day and 1.4 g/day, respectively) and include minimum of 300 mg EPA & DHA, ideally targeting 500–650 mg if possible. Although many foods such as fish are good sources of omega-3 fatty acids, their inclusion in the diet may be limited to avoid exceeding the total daily fat threshold. Thus, omega-3-esters by prescription can help increase intake to lower triglycerides. |
| d. Add MCT by prescription to help maintain an isocaloric diet without significantly increasing carbohydrate intake. |
| e. Ensure appropriate intake of other macronutrients, i.e. carbohydrate and protein should generally be 45–65% and 10–35% of total caloric intake, respectively. Emphasize importance of limiting high-glycemic and high-fructose content foods/beverages. |
| f. Maintain sufficient intake for key micronutrients (such as folate, iron, calcium) with diet and supplementation as needed |
| 3. Should dietary prescription be insufficient, other medical interventions may be considered to lower TG levels: |
| a. Insulin useful if hyperglycemic. |
| b. Avoid heparin if not otherwise indicated. |
| c. Fibrates may be considered after first trimester after careful discussion with patient. |
| d. Plasmapheresis can be considered if refractory and severe. |
| 4. Close surveillance of triglyceride levels throughout pregnancy and consideration for elective hospitalization to optimize therapy and avoid gestational hypertriglyceridemia-induced acute pancreatitis. |
| 5. No evidence for specific timing of induction and delivery. This should be determined in conjunction with endocrinologist, obstetrician and anesthetist based on fetal development and maternal risk for acute pancreatitis. However, early delivery by induction or C-section should be strongly considered in women with severe hypertriglyceridemia refractory to treatment in the third trimester. |
| Lactation: |
| 1. Decision to continue/resume niacin and fibrate therapy should be considered on a case-by-case basis, taking into account the importance of the drug to the mother versus the unknown impact to nursing infants. |
CM: chylomicron; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; MCT: medium-chain triglycerides; TG: triglyceride; LA: linoleic acid; AL alpha-linolenic acid.
At the fundamental cornerstone of therapy is the low-fat diet, with total saturated and unsaturated fat restricted to no more than 20% of daily caloric intake. This is true for all non-pregnant and pregnant individuals with severe hypertriglyceridemia as it is very effective in lowering plasma triglyceride levels. In some patients, especially those with LPL or apo C-II deficiency, greater fat restriction to less than 10% may be needed. This frequently amounts to only 20–40 g dietary fat/day, much lower than the average Western diet of 120 g/day. Adherence to such a restrictive diet can be challenging for many women and occasionally hospitalization may be required to initiate or maintain these stringent dietary regimens.
Furthermore, adherence to this diet during pregnancy can also lead to several unintended consequences. Hypocaloric diet can result in maternal weight loss, which has been linked to increased adverse perinatal outcomes, including preterm birth and small for gestational age birth-weight infants.36 Conversely, efforts to reduce fat intake can often result in diets high in carbohydrates, which can result in a paradoxical increase in fasting triglyceride levels. Women with gestational hypertriglyceridemia should be counselled to restrict their consumption of high glycemic index foods, including refined sugars, and high fructose beverages, as they enhance the de novo hepatic synthesis of fatty acids and triglycerides derived predominantly from carbohydrates.37
In addition, restricted fat intake can lead to maternal, but more importantly fetal, deficiency of omega-3 and omega-6 essential fatty acids (EFA) (i.e. linoleic acid (LA) and alpha-linolenic acid (ALA), respectively) as well as key omega-3 long-chain polyunsaturated fatty acids (LCPUFA) (i.e. eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)). Derived from plant sources, ALA is used to make EPA and DHA, which are also found in animal-based foods. Due to inefficient conversion of ALA to EPA and DHA, fetal DHA concentrations are determined primarily by maternal DHA diet.
Recommended dietary intake varies in the literature: while the accepted intake for LA ranges from 2%38 to 6%39, there is general consensus among expert groups38,39 that ALA should consist of at least 0.7% of total energy intake in pregnant women, approximately 1.4 g/day. In addition, given the potential benefits and weighing the absence of toxicity, expert panels such as ISSFAL,38 World Association of Perinatal Medicine Dietary Guidelines Working Group,40 Food and Agriculture Organization of the United Nations (FAO),41 and the Perinatal Lipid Intake Working Group42 have recommended pregnant and lactating women consume an average DHA and EPA total of 300–650 mg/day, of which DHA should be at least 200–300 mg/day. Meeting these requirements (which constitute a minimum of 13 g of EFA and LCPUFA) without exceeding a fat restriction of 20–40 g is difficult and requires careful planning to avoid EFA and LCPUFA deficiency.
Although symptoms of EFA deficiency are relatively mild for the mother (typically skin dryness and desquamation), fetal EFA and LCPUFA deficiency are of much greater significance. Deficiency in DHA, a building block of the central nervous system and retinal tissue, can lead to impaired brain and visual development, especially in low birth-weight infants.28,43 Both topical EFA-rich oil (e.g. sunflower oil) and oral omega-3-acid ethyl esters (purified prescription omega-3-acid concentrates) have been used to avoid LCPUFA deficiency.28 At higher doses (e.g. EPA and DHA ≥2 g/day), omega-3-acid ethyl esters also have the additive benefit of triglyceride lowering, which is thought to occur through several mechanisms, including reduced hepatic triglyceride synthesis, increased fatty acid oxidation in the liver and skeletal muscle and increased TRL removal through enhanced LPL activity. Safety of maternal omega-3 supplementation has been previously confirmed in a well-designed double-blind randomized placebo-controlled trial of 98 healthy pregnant women who received fish oil (DHEA 2.2 g and EPA 1.1 g/day) or olive oil from 20 weeks’ gestation until delivery.44 No adverse effects were seen for the fetus or infant at 12 year follow-up.45
Aside from omega-3-acid ethyl esters, nutritional support can also be derived with the addition of oral MCT, available by prescription. MCTs are an excellent source of calories (8.3 kcal/g for MCT versus 4 kcal/g for carbohydrates and proteins). By supplying MCT, the need to increase carbohydrate intake in order to maintain an isocaloric diet is mitigated to some extent. Unlike conventional fats such as long-chain triglycerides (LCT), MCT do not raise plasma triglyceride levels. In contrast to LCT, they do not require CMs formation for their absorption and do not stimulate CMs release into the general circulation. Instead, they are rapidly absorbed in the small intestine and directly transported via the portal vein to the liver for immediate oxidation as energy fuel. Furthermore, their downstream products, including acetyl coenzyme-A, are thought to be beneficial in fetal brain myelination.46 Although the safety of MCT has not been specifically evaluated in the fetus, a randomized double-blind study in preterm infants by Rayyan et al. demonstrated the safety of 7 - to 14-day exposure to a parenteral lipid emulsion containing a mixture of MCT, soybean oil, olive oil and fish oil compared to soybean emulsion alone. Adverse effects such as infections, hepatobiliary disorders and hyperglycemia were not shown to be increased in those exposed to the MCT-containing emulsion. Similarly, there were no serious adverse effects felt to be related to the MCT-containing emulsion.47 Thus, the risks to the fetus are thought to be extremely low.
Elective hospitalization for supervised fast, parenteral nutrition, or insulin therapy
Close surveillance of plasma triglyceride concentrations throughout pregnancy is essential and should be followed on a monthly basis at minimum. As pregnancy progresses, triglyceride levels may need to be monitored on a q1 to 2 weekly basis. Consideration should be given for elective hospitalization to optimize therapy and reduce the risk of gestational hypertriglyceridemia-induced acute pancreatitis. While some advocate an arbitrarily selected triglyceride level of 28 mmol/L,44 the threshold for admission likely depends on numerous factors aside from the absolute plasma triglyceride level including rapid rate of increase, poor adherence to diet, symptoms or signs of pancreatitis, previous triglyceride thresholds for pancreatitis in each individual (when available) and evidence of pregnancy complications.
Short-term hospitalizations can be used proactively to rapidly reduce triglyceride levels through supervised fast or parenteral nutrition (e.g. either through intravenous dextrose or total parenteral nutrition). It has been hypothesized that intravenous feeding may have an attenuated triglyceride-raising effect compared to enteral carbohydrate intake, based on a study in healthy males by DenBesten and colleagues.48
Another treatment modality often used in the short term to acutely lower triglyceride levels is insulin infusion. Not only does it reverse hyperglycemia and thereby hyperglycemia-induced inhibition of LPL, insulin is also a rapid and potent LPL activator. The benefits of insulin in gestational hypertriglyceridemia have only been seen in hyperglycemic women.49 Insulin therapy is not recommended in euglycemic individuals with gestational hypertriglyceridemia.
Similarly, intravenous heparin has also sometimes been used as part of the acute management of gestational hypertriglyceridemia. Although it is thought to liberate endothelial LPL into the circulation, many case reports have consistently shown transient effects due to LPL stores depletion and resultant LPL deficiency. This can result in a secondary paradoxical increase in plasma triglyceride levels.50 The use of heparin may not enhance LPL activity as capillary-anchored LPL is already enzymatically active. There is also an associated risk of hemorrhage into the pancreas with pre-existing pancreatic necrosis in the context of acute pancreatitis. As such, heparin is not recommended for gestational hypertriglyceridemia management.
Fibrates and niacin
As outlined previously, both fibrates and niacin are used as mainstays in the management of non-pregnant hypertriglyceridemia; however, their use in gestational hypertriglyceridemia is much more controversial. Although there have been case reports of fibrates (gemfibrozil) and niacin use after first trimester with no clear adverse effect documented, 28,30,31,51 it remains controversial whether fibrate and/or niacin can be restarted and used in conjunction with other therapies to maintain triglyceride levels after the first trimester. However, even if initiated in conjunction with dietary recommendations, the onset of their action is gradual and often more rapid triglyceride lowering may be desired in acute settings.
In the postpartum period, decision to resume these medications may depend on the latest values and trend of the serum triglyceride as well as maternal breastfeeding plans. Niacin is known to be excreted in breast milk and may cause serious adverse reactions in nursing infants at the lipid-lowering doses, although this has not been formally evaluated. It is not known if fibrates are excreted in breast milk. In both cases, the decision to continue/resume niacin and fibrate therapy should be considered on a case-by-case basis, taking into account the importance of the drug to the mother versus the unknown impact to nursing infants.
Plasmapheresis
Plasmapheresis therapy can be considered if hypertriglyceridemia is severe and refractory to all other therapies. It is considered a category III indication for hypertriglyceridemic pancreatitis by the American Society of Apheresis52 due to the poor quality of the available evidence. Although the Society does not have a specific recommendation for gestational hypertriglyceridemia, various regimens have been utilized for treatment of gestational hypertriglyceridemia-induced acute pancreatitis. In four of the reported cases,53,54 plasmapheresis was used for prevention of gestational hypertriglyceridemia-induced acute pancreatitis. There was wide variability in timing of the first plasmapheresis session (from 16 to 33 gestational weeks) as well as total sessions required (3 to 27 sessions). In all four of these cases, however, there was successful prevention of pancreatitis and resulted in the delivery of a healthy infant. In addition to removal of TRLs and inflammatory mediators, it has been theorized that the plasma used as replacement fluid can be an additional source of LPL.55 While rapid reduction in triglycerides from apheresis has been described in a few case reports and case series for treatment and prophylaxis of hypertriglyceridemia-induced pancreatitis,55–56 broad employment of this strategy has been limited by its availability, high cost and the risks of catheter-related infections and thromboses. Furthermore, its effect appears to also be transient so its use should be ideally confined as a temporizing measure while other therapies are being employed or as a bridge to imminent delivery.57
Conclusion
Management of gestational hypertriglyceridemia is associated with many clinical challenges. Multidisciplinary care, careful dietary planning and the institution of a hierarchical therapeutic approach are all crucial to optimize maternal and perinatal outcomes. Future studies are required to delineate the safety of fibrate therapy and plasmapheresis in pregnancy as well as the optimal timing for induction and delivery. Strong consideration should be given towards induction once fetal maturity is established (e.g. at 36 weeks), especially in women whom triglyceride levels show a steep upward trend in the third trimester.
Acknowledgements
The authors wish to thank Colleen Gilchrist, R.D., for her invaluable assistance.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Guarantor
BW.
Contributorship
BW reviewed the literature and prepared the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version.
References
- 1.Ghio A, Bertolotto A, Resi V, et al. Triglyceride metabolism in pregnancy. Adv Clin Chem 2011; 55: 133–153. [DOI] [PubMed] [Google Scholar]
- 2.Basaran A. Pregnancy-induced hyperlipoproteinemia: review of the literature. Reprod Sci 2009; 16: 431–437. [DOI] [PubMed] [Google Scholar]
- 3.Murphy SP, Abrams BF. Changes in energy intakes during pregnancy and lactation in a national sample of US women. Am J Public Health 1993; 83: 1161–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000; 71: 1256S–1261S. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez JJ, Montelongo A, Iglesias A, et al. Longitudinal study on lipoprotein profile, high density lipoprotein subclass and postheparin lipases during gestation in women. J Lipid Res 1996; 37: 299–308. [PubMed] [Google Scholar]
- 6.Martin-Hidalgo A, Holm C, Belfrage P, et al. Lipoprotein lipase and hormone-sensitive lipase activity and mRNA in rat adipose tissue during pregnancy. Am J Physiol 1994; 266: E930–E935. [DOI] [PubMed] [Google Scholar]
- 7.Desoye G, Schweditsch MO, Pfeiffer KP, et al. Correlation of hormones with lipid and lipoprotein levels during normal pregnancy and postpartum. J Clin Endocrinol Metab 1987; 64: 704–712. [DOI] [PubMed] [Google Scholar]
- 8.Herrera E, Ortega-Senovilla H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr Pharm Biotechnol 2014; 15: 24–31. [DOI] [PubMed] [Google Scholar]
- 9.Knopp RH, Warth MR, Charles D, et al. Lipoprotein metabolism in pregnancy, fat transport to the fetus, and the effects of diabetes. Biol Neonate 1986; 50: 297–317. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Ooi TC, Liu MS, et al. High frequency of mutations in the human lipoprotein lipase gene in pregnancy-induced chylomicronemia: possible association with apolipoprotein E2 isoform. J Lipid Res 1994; 35: 1066–1075. [PubMed] [Google Scholar]
- 11.Han DH, Moh IH, Kim DM, et al. Gestational hyperlipidemia and acute pancreatitis with underlying partial lipoprotein lipase deficiency and apolipoprotein E3/E2 genotype. Korean J Intern Med 2013; 28: 609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson H, Leisegang F, Hassan F, et al. A novel Glu421Lys substitution in the lipoprotein lipase gene in pregnancy-induced hypertriglyceridemic pancreatitis. Clin Chim Acta 1998; 269: 1–12. [DOI] [PubMed] [Google Scholar]
- 13.Shenhav S, Gemer O, Schneider R, et al. Severe hyperlipidemia-associated pregnancy: prevention in subsequent pregnancy by diet. Acta Obstet Gynecol Scand 2002; 81: 788–790. [DOI] [PubMed] [Google Scholar]
- 14.Ramin KD, Ramin SM, Richey SD, et al. Acute pancreatitis in pregnancy. Am J Obstet Gynecol 1995; 173: 187–191. [DOI] [PubMed] [Google Scholar]
- 15.Havel RJ. Pathogenesis, differentiation and management of hypertriglyceridemia. Adv Intern Med 1969; 15: 117–145. [PubMed] [Google Scholar]
- 16.Navina S, Acharya C, Delany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med 2011; 3: 107ra110–107ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura W, Mossner J. Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats. Int J Pancreatol 1996; 20: 177–184. [DOI] [PubMed] [Google Scholar]
- 18.Saharia P, Margolis S, Zuidema GD, et al. Acute pancreatitis with hyperlipemia: studies with an isolated perfused canine pancreas. Surgery 1977; 82: 60–67. [PubMed] [Google Scholar]
- 19.Scherer J, Singh VP, Pitchumoni CS, et al. Issues in hypertriglyceridemic pancreatitis: an update. J Clin Gastroenterol 2014; 48: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery WH, Miller FC. Pancreatitis and pregnancy. Obstet Gynecol 1970; 35: 658–664. [PubMed] [Google Scholar]
- 21.Mizushima T, Ochi K, Matsumura N, et al. Prevention of hyperlipidemic acute pancreatitis during pregnancy with medium-chain triglyceride nutritional support. Int J Pancreatol 1998; 23: 187–192. [DOI] [PubMed] [Google Scholar]
- 22.Eddy JJ, Gideonsen MD, Song JY, et al. Pancreatitis in pregnancy. Obstet Gynecol 2008; 112: 1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juneja SK, Gupta S, Virk SS, et al. Acute pancreatitis in pregnancy: a treatment paradigm based on our hospital experience. Int J App Basic Med Res 2013; 3: 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson-Piercy C, Crook MA. Severe hypertriglyceridemia complicating pregnancy, management by dietary intervention and ω-3 fatty acid supplementation. Nutrition 2009; 25: 1098–1099. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg AS, Hegele RA. Severe hypertriglyceridemia in pregnancy. J Clin Endocrinol Metab 2012; 97: 2589–2596. [DOI] [PubMed] [Google Scholar]
- 26.Gallos ID, Sivakumar K, Kilby MD, et al. Pre-eclampsia is associated with, and preceded by, hypertriglyceridaemia: a meta-analysis. BJOG 2013; 120: 1321–1332. [DOI] [PubMed] [Google Scholar]
- 27.Tang SJ, Rodriquez-Frias E, Singh S, et al. Acute pancreatitis during pregnancy. Clin Gastroenterol Hepatol 2010; 8: 85–90. [DOI] [PubMed] [Google Scholar]
- 28.Tsai EC, Brown JA, Veldee MY. Potential of essential fatty acid deficiency with extremely low fat diet in lipoprotein lipase deficiency during pregnancy: a case report. BMC Pregnancy Childbirth 2004; 4: 27–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald JE, Petrere JA, de la Iglesia FA. Experimental studies on reproduction with the lipid-regulating agent gemfibrozil. Fundam Appl Toxicol 1987; 8: 454–464. [DOI] [PubMed] [Google Scholar]
- 30.Abu Musa AA, Usta IM, Rechdan JB, et al. Recurrent hypertriglyceridemia-induced pancreatitis in pregnancy: a management dilemma. Pancreas 2006; 32: 227–228. [DOI] [PubMed] [Google Scholar]
- 31.Saadi HF, Kurlander DJ, Erkins JM, et al. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract 1999; 5: 33–36. [DOI] [PubMed] [Google Scholar]
- 32.Zarek J, Koren G. The fetal safety of statins: a systematic review and meta-analysis. J Obstet Gynaecol Can 2014; 36: 506–509. [DOI] [PubMed] [Google Scholar]
- 33.Feig DS, Razzaq A, Sykora K. Trends in deliveries, prenatal care, and obstetrical complications in women with pregestational diabetes. A population-based study in Ontario, Canada, 1996–2001. Diabetes Care 2006; 29: 232–235. [DOI] [PubMed] [Google Scholar]
- 34.MacIntosh MCM, Fleming KM, Bailey JA. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales and Northern Ireland: population based study. BMJ 2006; 333: 177–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berglund L, Brunzell JD, Goldberg AC, et al. Endocrine society. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2012; 97: 2969–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viswanathan M, Siega-Riz AM, Moos MK, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess (Full Rep) 2008; 168: 1–223. [PMC free article] [PubMed] [Google Scholar]
- 37.Fried SK, Rao SP. Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr 2003; 78: 873S–880S. [DOI] [PubMed] [Google Scholar]
- 38.International Society for the Study of Fatty Acids and Lipids. Report of the sub-committee on recommendations for intake of polyunsaturated fatty acids in healthy adults, Brighton: Author, June 2004. [Google Scholar]
- 39.Institute of Medicine (US). Panel on micronutrients. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Panel on macronutrients panel on the definition of dietary fiber, subcommittee on upper reference levels of nutrients, subcommittee on interpretation and uses of dietary reference intakes, and the standing committee on the scientific evaluation of dietary reference intakes, food and nutrition board, Washington, DC: National Academies Press, 2005. [Google Scholar]
- 40.Koletzko B, Lien E, Agostoni C, et al. World Association of Perinatal Medicine Dietary Guidelines Working Group. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med 2008; 36: 5–14. [DOI] [PubMed] [Google Scholar]
- 41.Food and Agriculture Organization of the United Nations (FAO). Fats and fatty acids in human nutrition, report of an expert consultation, www.fao.org/ag/humannutrition/nutrition/en/ (2010, accessed 1 2015 February). [PubMed]
- 42.Koletzko B, Cetin I, Brenna JT. Perinatal Lipid Intake Working Group; Child Health Foundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Society; Society for Clinical Nutrition and Metabolism; European Society for Paediatric Gastroenteriology, Hepatology and Nutrition, Committee on Nutrition; International Federation of Placenta Associations; International Society for the Study of Fatty Acids and Lipids. Dietary fat intakes for pregnant and lactating women. Br J Nutr 2007; 98: 873–877. [DOI] [PubMed] [Google Scholar]
- 43.Uauy R, Mena P, Pojas C. Essential fatty acid metabolism in the micropremie. Clin Perinatol 2000; 27: 71–93. [DOI] [PubMed] [Google Scholar]
- 44.Dunstan JA, Simmer K, Dixon G, et al. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2008; 93: 45–50. [DOI] [PubMed] [Google Scholar]
- 45.Meldrum S, Dunstan JA, Foster JK, et al. Maternal fish oil supplementation in pregnancy: a 12 year follow-up of a randomised controlled trial. Nutrients 2015; 7: 2061–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bach AC, Babyan VK. Medium-chain triglycerides: an update. Am J Clin Nutr 1982; 36: 950–962. [DOI] [PubMed] [Google Scholar]
- 47.Rayyan M, Devlieger H, Jochum F, et al. Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: a randomized double-blind study in preterm infants. J Parenter Enteral Nutr 2012; 36: 81S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DenBesten L, Reyna RH, Connor WE, et al. The different effects on the serum lipids and fecal steroids of high carbohydrate diets given orally or intravenously. J Clin Invest 1973; 52: 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jabbar MA, Zuhri-Yafi MI, Larrea J. Insulin therapy for a non-diabetic patient with severe hypertriglyceridemia. J Am Coll Nutr 1998; 17: 458–461. [DOI] [PubMed] [Google Scholar]
- 50.Whayne TF., Jr Concerns about heparin therapy for hypertriglyceridemia. Arch Intern Med 2010; 170: 108–109. [DOI] [PubMed] [Google Scholar]
- 51.Al-Shali K, Wang J, Fellows F, et al. Successful pregnancy outcome in a patient with severe chylomicronemia due to compound heterozygosity for mutant lipoprotein lipase. Clin Biochem 2002; 35: 125–130. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz J, et al. guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing committee of the American Society for apheresis: the sixth special issue. J Clin Apher 2013; 28: 145–284. [DOI] [PubMed] [Google Scholar]
- 53.Sivakumaran P, Tabak SW, Gregory K. Management of familial hypertriglyceridemia during pregnancy with plasma exchange. J Clin Apher 2009; 24: 42–46. [DOI] [PubMed] [Google Scholar]
- 54.Basar R, Uzum AK, Canbaz B, et al. Therapeutic apheresis for severe hypertriglyceridemia in pregnancy. Arch Gynecol Obstet 2013; 287: 839–843. [DOI] [PubMed] [Google Scholar]
- 55.Safi F, Toumeh A, Abuissa Qadan MA, et al. Management of familial hypertriglyceridemia-induced pancreatitis during pregnancy with therapeutic plasma exchange: a case report and review of literature. Am J Ther 2014; 21: e134–e136. [DOI] [PubMed] [Google Scholar]
- 56.Gupta N, Ahmed S, Shaffer L. Severe hypertriglyceridemia induced pancreatitis in pregnancy. Case Rep Obstet Gynecol 2014; 2014: 485493–485493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuff-Werner P, Sebastian F, Kohlschein P. Role of lipid apheresis in changing times. Clin Res Cardiol Suppl 2012; 7: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanderson SL, Iverius P, Wilson DE. Successful hyperlipidemic pregnancy. JAMA 1991; 265: 1858–1860. [PubMed] [Google Scholar]