Abstract
Purpose
Curcumin (CUR), the main polyphenol in turmeric, is poorly absorbed and rapidly metabolized following oral administration, which severely curtails its bioavailability. Poly-(lactic-co-glycolic acid)-based CUR nanoparticles (CUR-NP) have recently been suggested to improve CUR bioavailability, but this has not been fully verified. Specifically, no data are available about curcumin glucuronide (CURG), the major metabolite of CUR found in the plasma following oral administration of CUR-NP. Herein, we investigated the absorption and metabolism of CUR-NP and evaluated whether CUR-NP improves CUR bioavailability.
Methods
Following oral administration of CUR-NP in rats, we analyzed the plasma and organ distribution of CUR and its metabolites using high-performance liquid chromatography-tandem mass spectrometry. To elucidate the mechanism of increased intestinal absorption of CUR-NP, we prepared mixed micelles comprised of phosphatidylcholine and bile salts and examined the micellar solubility of CUR-NP. Additionally, we investigated the cellular incorporation of the resultant micelles into differentiated Caco-2 human intestinal cells.
Results
Following in vivo administration of CUR-NP, CUR was effectively absorbed and present mainly as CURG in the plasma which contained significant amounts of the metabolite compared with other organs. Thus, CUR-NP increased intestinal absorption of CUR rather than decreasing metabolic degradation and conversion to other metabolites. In vitro, CUR encapsulated in CUR-NP was solubilized in mixed micelles; however, whether the micelles contained CUR or CUR-NP had little influence on cellular uptake efficiency. Therefore, we suggest that the high solubilization capacity of CUR-NP in mixed micelles, rather than cellular uptake efficiency, explains the high intestinal absorption of CUR-NP in vivo.
Conclusion
These findings provide a better understanding of the bioavailability of CUR and CUR-NP following oral administration. To improve the bioavailability of CUR, future studies should focus on enhancing the resistance to metabolic degradation and conversion of CUR to other metabolites, which may lead to novel discoveries regarding food function and disease prevention.
Keywords: absorption, metabolism, bioavailability, mixed micelles, Caco-2, HPLC-MS/MS
Introduction
Curcumin (CUR), also known as diferuloylmethane (IUPAC name (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), is the main polyphenol in turmeric (Curcuma longa L.). CUR is known to exhibit a wide range of effects including anti-inflammatory, antitumor, and lipid-lowering activities, which are attributed primarily to its potent antioxidant capacity.1–3 However, CUR’s ability to exert these biological effects in vivo is undermined by its low bioavailability due to poor intestinal absorption and rapid metabolism.4–6 CUR’s low bioavailability in vivo was confirmed by our previous work in which CUR absorbed through the intestinal cells of rats was predominantly conjugated as curcumin glucuronide (CURG) and low levels of unmetabolized CUR were found in the blood.7,8
We hypothesized that encapsulating CUR with poly-(lactic-co-glycolic acid) (PLGA)-based nanoparticles would enhance the bioavailability of CUR administered orally because of PLGA’s ability to disperse in water, pass through the gut, bypass first-pass metabolism, and endure the harsh external conditions of the gastrointestinal tract (chemical and enzymatic degradation).4,9–13 Our hypothesis is supported by previous studies that reported that encapsulated CUR with PLGA-based nanoparticles administrated orally to rats increased CUR levels in the plasma.14–18 However, to the best of our knowledge, there are no data about CURG, despite the fact that it is the main metabolite of CUR found in the plasma. Therefore, it is not clear whether CUR nanoparticles (CUR-NP) actually improve the intestinal absorption of CUR.
In this study, we aimed to evaluate whether orally administered PLGA-based nanoparticles improved the bioavailability of CUR. We prepared PLGA-based CUR-NP by solvent evaporation. Following oral administration of CUR-NP to rats, we determined the levels of CURG and CUR in the plasma and organs (eg, kidney and liver) using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). After confirming that intestinal absorption of CUR-NP was higher than nonencapsulated CUR, we sought to determine the cause of increased intestinal absorption of CUR-NP by examining micellar solubilization of CUR-NP and CUR in mixed micelles comprised of phosphatidylcholine (PC) and bile salts in vitro. In addition, we investigated the cellular uptake of micelles in differentiated Caco-2 human intestinal cells. Overall, this study provides a better understanding of the bioavailability of CUR and CUR-NP administered orally, which may lead to novel discoveries in food function and disease prevention.
Materials and methods
Materials
CUR was purchased from Wako Pure Chemical Industries, Ltd (Osaka, Japan). CURG was purchased from Toronto Research Chemicals (Toronto, ON, Canada). PLGA (50:50, molecular weight =30,000–60,000 g/mol), polyvinyl alcohol (PVA, molecular weight =30,000–70,000 g/mol, 87%–90% hydrolyzed), and egg yolk PC (purity ≥99%) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Taurocholic acid sodium salt (purity ≥98%) was purchased from Nacalai Tesque (Kyoto, Japan). All other chemicals and reagents used were of analytical grade or higher.
Preparation of CUR-NP
CUR-NP was prepared by solvent evaporation.19 Briefly, 20 mg of CUR and 200 mg of PLGA were dissolved in 2 mL of dichloromethane as an oil phase. Next, 4 mL of 5% (w/v) PVA aqueous solution was added and vortexed. The organic–aqueous mixture was sonicated at 40% amplitude for 2 minutes using a sonic dismembrator and then added dropwise to 100 mL of 0.3% (w/v) PVA aqueous solution under rapid stirring. The resultant oil-in-water emulsion was stirred overnight in a fume hood to allow the dichloromethane solvent to evaporate. The suspension was centrifuged (2,000× g, 10 minutes, 4°C) to remove larger aggregates and CUR not wrapped in the CUR-NP. The supernatant was collected, filtered (Millex-HV, 0.45 μm, 33 mm; EMD Millipore, Billerica, MA, USA), washed twice with Milli-Q water by centrifugation, and finally resuspended in 2 mL of 1% (w/v) sucrose aqueous solution. The CUR-NP suspension was lyophilized and stored in the dark at −30°C.
Nanoparticles characterization
Approximately 5 mg of CUR-NP (lyophilized powder) was resuspended in 5 mL of distilled water. The suspension was analyzed by dynamic light scattering and laser Doppler anemometry using ELS-Z (Otsuka Electronics Co., Ltd, Osaka, Japan) to determine the mean diameters, polydispersity index (PDI), and zeta potentials.
To measure the entrapment efficiency (EE) and the loading efficiency (LE), lyophilized CUR-NP (~10 mg) was dissolved in 10 mL of methanol and sonicated to extract CUR. The solution was then centrifuged (1,000× g, 10 minutes, 4°C), and the supernatant was collected. The amount of CUR in the extract was determined by HPLC-UV. Specifically, a ProC18 column (4.6×150 mm, 5 μm; YMC Co., Ltd, Kyoto, Japan) was used, and the extract was eluted using a binary gradient consisting of the following HPLC solvents: (A) 0.05% formic acid and (B) acetonitrile. The gradient profile was as follows: B linear ratio; 30%–50% at 0–17 minutes, 50%–100% at 17–22 minutes, and 100% at 22–32 minutes. The flow rate was adjusted to 1 mL/min, and the column temperature was maintained at 40°C. The column eluent was sent to a UV detector (UV-2075 PLUS; Jasco, Tokyo, Japan) for CUR monitoring at 420 nm. The concentration of CUR was calculated using the equation corresponding to the standard curve. EE was calculated as follows: EE (%) = CURencapsulated/CURtotal ×100, and LE was calculated as follows: LE (%) = CURencapsulated/CUR-NPtotal ×100.18
Surface morphology of CUR-NP (lyophilized powder) was evaluated by scanning electron microscope (SEM; SU8000 Type II, Hitachi Ltd, Tokyo, Japan) and transmission electron microscope (TEM; H-7650 ZeroA, Hitachi Ltd). Lyophilized CUR-NP powder (containing 3 mg CUR) was suspended in 10 mL of distilled water and photographed. CUR (3 mg) was also photographed.
Animal study
Male Sprague Dawley rats (6 weeks of age, 150–190 g body weight) were purchased from CLEA Japan Inc. (Tokyo, Japan) and housed in cages kept at 24°C with a 12-hour light:dark cycle. The rats were acclimated with commercial rodent chow (CE-2; CLEA Japan Inc.) and water for 1 week. After acclimation, rats were fasted for 16 hours and received CUR (100 mg/kg) by oral gavage using 1% sodium cholate as a vehicle. Similarly, CUR-NP was administrated to rats at a dose equivalent to 100 mg CUR/kg using 1% polysorbate 80 (PS80; Nihon Yushi Co., Ltd, Tokyo, Japan) as a vehicle. Blood samples (~0.3 mL) were collected into heparinized tubes from the jugular vein at 0, 0.5, 1, 1.5, 3, 6, and 24 hours following oral administration and centrifuged (1,000× g, 15 minutes, 4°C) to prepare the plasma samples. In a separate study, the liver and kidney were excised at 1.5 hours after oral administration of CUR or CUR-NP. Harvested plasma and organs were stored at −80°C until further analysis. These protocols were reviewed by the Committee on the Ethics of Animal Experiments and carried out in accordance with the Animal Experiment Guidelines of Tohoku University (Sendai, Japan). The permit number for this animal experiment is 2015–Noudou–015.
Extraction from samples
Plasma (100 μL) was mixed with 200 μL of methanol by vortexing and then centrifuged (5,000× g, 5 minutes, 4°C). The supernatant was collected, mixed with 500 μL of water, and then loaded onto an Oasis HLB 1 cc cartridge (Waters, Milford, MA, USA). The cartridge was washed with 1 mL of water, and the curcuminoids (CUR, CURG, and other metabolites) were eluted with 2 mL of methanol. Organs were homogenized with saline (containing 1 mM ethylenediaminetetraacetic acid) to prepare 30% (w/v) homogenate solution. Curcuminoids in the homogenate solution (100 μL) were extracted in a manner similar to that of plasma. The extracts were dried and redissolved in 1 mL of methanol.
Aliquots of extracts were injected onto an XBridge™ C18 column (2.1×150 mm, 3.5 μm; Waters) maintained at 40°C. The mobile phase consisted of two components: (A) 0.05% formic acid and (B) acetonitrile. The gradient profile was as follows: 0–20 minutes 85%–0% A linear. The flow rate was 0.2 mL/min. Curcuminoids were analyzed using 4000 QTRAP HPLC-MS/MS (AB Sciex, Foster City, CA, USA). MS/MS parameters of CUR, CURG, and tetrahydrocurcumin (THC, purity ≥95%; Sigma-Aldrich Co.) were optimized with their respective standards under electrospray ionization (negative). The parameters were as follows: turbo gas temperature, 600°C; spray voltage, −4,000 V; nebulizer gas, 30 psi; auxiliary gas, 30 psi; curtain gas, 20 psi; collision gas, 6.0. CUR, CURG, and THC were detected using multiple-reaction monitoring (MRM) for the transition of parent ions to product ions: CUR, m/z 367>217 (collision energy [CE], −18 V; declustering potential [DP], −60 V); CURG, m/z 543>134 (CE, −72 V; DP, −85 V); THC, m/z 371>235 (CE, −24 V; DP, −75 V). The concentrations of CUR, CURG, and THC in the extracts were calculated using the standard curves of CUR, CURG, and THC, respectively. Other curcuminoids were analyzed using MRM transitions from the literature (CE, −60 V; DP, −70 V): curcumin sulfate (CURS), m/z 447>134; curcumin glucuronide sulfate (CURGS), m/z 623>134; curcumin diglucuronide (CURDG), m/z 719>134; curcumin disulfate (CURDS), m/z 527>134; dihydrocurcumin (DHC), m/z 369>135; hexahydrocurcumin (HHC), m/z 373>179; octahydrocurcumin (OHC), m/z375 >179; dihydrocurcumin glucuronide (DHCG), m/z 545>135; tetrahydrocurcumin glucuronide (THCG), m/z 547>135; hexahydrocurcumin glucuronide (HHCG), m/z 549>179; octahydrocurcumin glucuronide (OHCG), m/z 551>179; dihydrocurcumin sulfate (DHCS), m/z 449>135; tetrahydrocurcumin sulfate (THCS), m/z 451>135; hexahydrocurcumin sulfate (HHCS), m/z 453>179; octahydrocurcumin sulfate (OHCS), m/z 455>179.20
Micellar solubilization study
Mixed micelles, comprised of PC and bile salts containing either CUR (CUR-MM) or CUR-NP (NP-MM), were prepared as previously described.21 Briefly, micellar solutions were composed of 9.9 mM taurocholic acid sodium salt, 0.9 mM egg yolk PC, 1.5 mM oleic acid, 0.75 mM 1-oleoyl-rac-glycerol, and 3 mg CUR or CUR-NP (containing 3 mg CUR). These micellar solutions were prepared in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 15 mM sodium phosphate, 132 mM NaCl, 1% nonessential amino acid solution (100×, liquid), 1% penicillin/streptomycin/glutamine solution (100×, liquid), and 0.1% insulin solution (pH 7.4). The micelles prepared by sonication were centrifuged (100,000× g, 1 hour, 37°C). After the resultant suspension was filtered (Millex-GV, 0.22 μm, 33 mm) and gassed in an air and 5% CO2 incubator for 2 hours to maintain pH 7.4 at 37°C, the concentration of CUR was analyzed by HPLC-UV as described earlier.
Cell study
Caco-2 cells were grown in DMEM containing 10% fetal bovine serum (FBS), 1% nonessential amino acid solution, 1% penicillin/streptomycin/glutamine solution, and 0.1% insulin solution on 10 cm plastic petri dishes incubated at 37°C and 5% CO2. Cells were subcultured by removing the medium and detaching the cells from the culture dish using 0.05% trypsin in phosphate-buffered saline. Cells were suspended in 1.5 mL of culture medium and seeded on the apical side of Transwell® (Corning Incorporated, Corning, NY, USA). Culture medium (2.5 mL) was added to the basolateral well. Cells were grown to confluency on the apical side for 15 days. After reaching confluency, cells were continuously cultured for 18 days to mature brush border membranes. Culture medium was changed every day.
The micelles (CUR-MM or NP-MM) obtained in the solubilization study were diluted with CUR-free micellar solutions and FBS to achieve the desired final concentration (25 μM CUR and 10% FBS in CUR-MM or NP-MM solutions). These sample micellar solutions (1.5 mL) were added to the apical side of differentiated Caco-2 cells. The basolateral side was filled with 2.5 mL of culture medium. After 3 or 24 hours of incubation, the medium was collected from both sides. The cells were scraped, collected, and subjected to sonication in 200 μL of water. The solution was then mixed with 400 μL of methanol, and the supernatant was collected after centrifugation (20,000× g, 15 minutes, 4°C). The supernatant was mixed with 1 mL of water. The culture medium (100 μL) was mixed with 500 μL of methanol and 1.4 mL of water. The mixture (1.6 mL for cells and 2 mL for medium) was loaded onto an Oasis HLB 1 cc cartridge, washed with 1 mL of water, and eluted with 2 mL of methanol. The extracts were dried and redissolved in 1 mL of methanol. Aliquots of the methanol extracts were analyzed by HPLC-MS/MS as described earlier. The procedures were performed in accordance with the Guidelines for Ethics Committee of Tohoku University.
Statistical analysis
Data are expressed as mean ± standard error (SE). Statistical significance between the two groups was assessed by the Mann–Whitney U-test. Means significantly differed at P<0.05 and P<0.01.
Results
Nanoparticles characterization
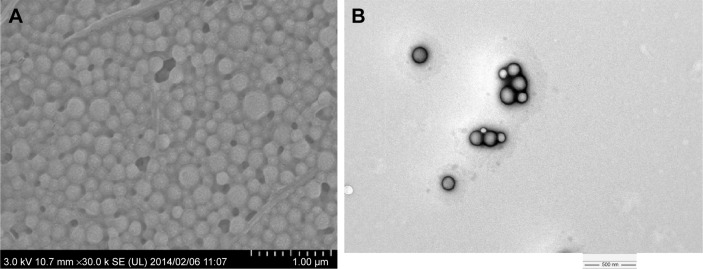
After lyophilization of CUR-NP suspension, ~150 mg of CUR-NP powder was obtained. CUR-NP characteristics: mean diameter, 186.9±2.8 nm; PDI, 0.095±0.011; zeta potential, −16.7 mV; EE, 18.3%±1.1%; LE, 2.65%±0.09% (n=3). For a minimum of 3 months, the concentration of CUR in CUR-NP, its mean diameter, PDI, and zeta potential had not changed in the dark at −30°C (data not shown). Based on the SEM and TEM images of CUR-NP (Figure 1), it appeared that CUR-NP was immobilized in the sucrose matrix without aggregation and around 200 nm spherical CUR-NP was confirmed by TEM with phosphotungstic acid staining.
Figure 1.
(A) SEM and (B) TEM images of CUR-NP.
Notes: (A) Lyophilized CUR-NP was immobilized in the sucrose matrix without aggregation confirmed by SEM (×30,000 magnification). (B) Around 200 nm spherical CUR-NP was confirmed by TEM (×20,000 magnification).
Abbreviations: SEM, scanning electron microscope; TEM, transmission electron microscope; CUR-NP, curcumin nanoparticles.
Dispersion of CUR-NP (lyophilized powder) in distilled water resulted in a homogeneous suspension (Figure 2). These results indicate that CUR was successfully loaded into PLGA-based nanoparticles.
Figure 2.
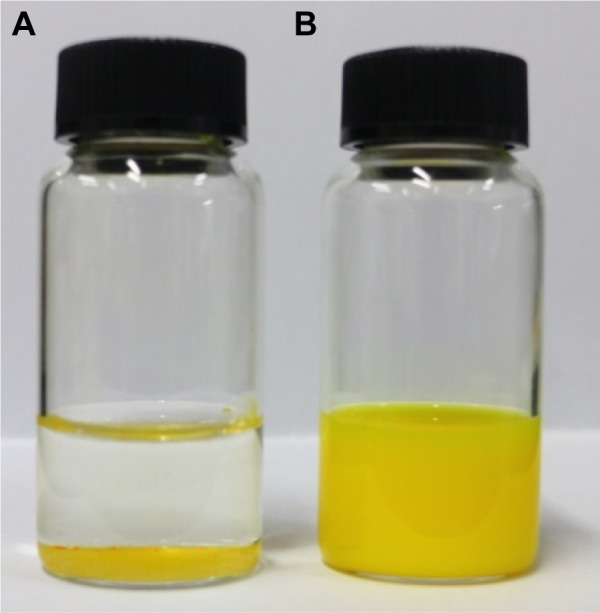
Photographs of CUR and CUR-NP suspensions.
Notes: (A) CUR powder and (B) CUR-NP were redispersed in distilled water, mixed for 5 minutes, and then photographed.
Abbreviations: CUR, curcumin; CUR-NP, curcumin nanoparticles.
Metabolic fate of CUR-NP
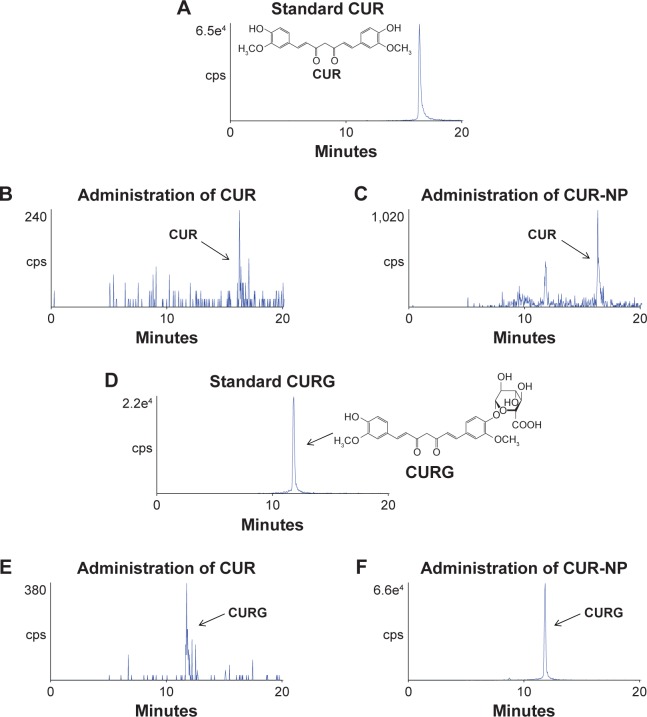
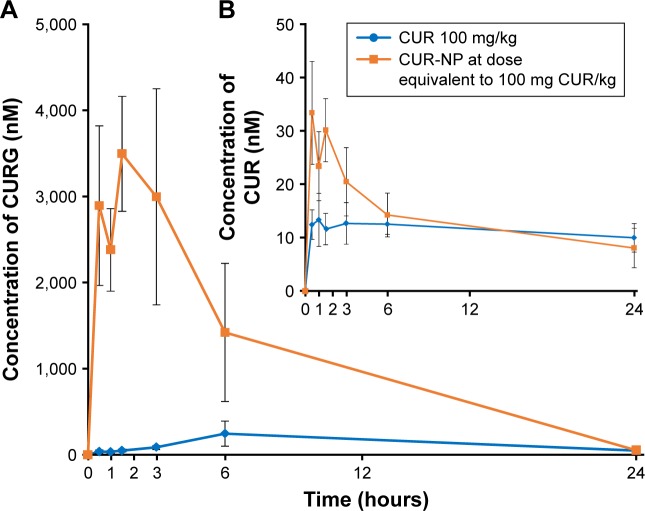
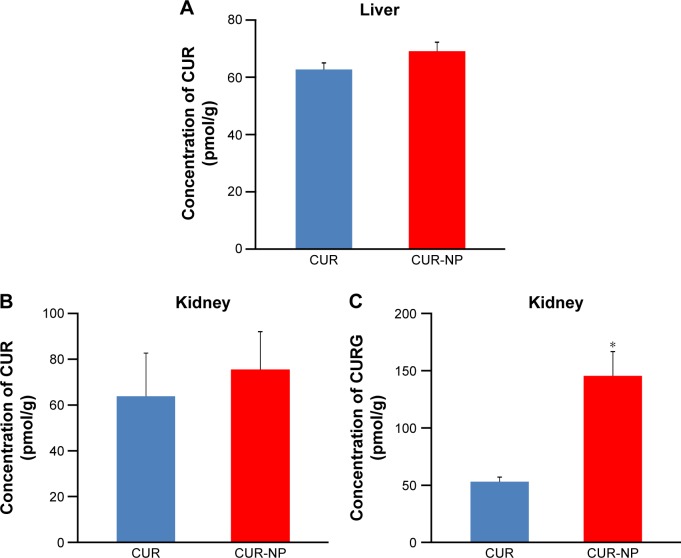
Rats were divided into two groups and administrated orally with suspensions containing 100 mg/kg CUR or CUR-NP (at a dose equivalent to 100 mg CUR/kg). Figure 3 shows representative HPLC-MS/MS chromatograms of plasma extracts at 1.5 hours after oral administration of CUR or CUR-NP. The chromatogram contains two peaks: a large peak attributable to CURG and a small peak attributable to CUR. The maximum concentration (Cmax) of CURG was 3,493±667 nM, and the time of maximum concentration (Tmax) of CURG was 1.5 hours in the CUR-NP-administrated group (Figure 4). The area under the blood concentration–time curve (AUC, calculated by GraphPad Prism 6 for Windows; GraphPad Software, Inc., La Jolla, CA, USA) of CURG in the plasma at 24 hours was significantly higher in the CUR-NP-administrated group compared with the CUR-administrated group (Figure 5). Additionally, other curcuminoids such as CURS, CURGS, DHCG, THCG, HHCG, DHCS, THCS, and HHCS were detected in the plasma, especially in the CUR-NP-administrated group (Figure 6). We analyzed the curcuminoids in the livers and kidneys of rats 1.5 hours after oral administration of CUR or CUR-NP (Figure 7). In contrast to plasma samples, CURG was not detected in the liver. A small amount of CURG was detectable in the kidney with higher amounts in the CUR-NP-administrated group. Similar trends were observed for other metabolites in the liver and kidney (Table 1; Figure S1). However, there was no difference in the levels of CUR between the CUR- and CUR-NP-administrated groups. Taken together, these results suggest that CUR is effectively absorbed in rats following oral administration of CUR-NP and is present mainly as CURG in the plasma with minimal amounts found in other organs. Overall, encapsulating CUR with PLGA-based nanoparticles enhances the absorption of CUR.
Figure 3.
Representative chromatograms of CUR and CURG.
Notes: (A) Standard CUR (1,000 fmol). (B) CUR in rat plasma extracts at 1.5 hours following oral administration of CUR. (C) CUR in rat plasma extracts at 1.5 hours following oral administration of CUR-NP. (D) Standard CURG (1,000 fmol). (E) CURG in rat plasma extracts at 1.5 hours following oral administration of CUR. (F) CURG in rat plasma extracts at 1.5 hours following oral administration of CUR-NP. Each rat plasma sample (100 μL) was extracted in 1 mL of methanol and 10 μL of it was analyzed by HPLC-MS/MS with MRM.
Abbreviations: CUR, curcumin; CURG, curcumin glucuronide; cps, centipoise; CUR-NP, curcumin nanoparticles; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; MRM, multiple-reaction monitoring.
Figure 4.
Concentration–time curves for CURG and CUR.
Notes: Concentration–time curves for (A) CURG and (B) CUR in rat plasma following oral administration of CUR (100 mg/kg) or CUR-NP (at a dose equivalent to 100 mg CUR/kg) (mean ± SE, n=4).
Abbreviations: CUR, curcumin; CURG, curcumin glucuronide; CUR-NP, curcumin nanoparticles; SE, standard error.
Figure 5.
Comparison of AUCs between CUR- and CUR-NP-administrated groups.
Notes: (A) CUR AUC and (B) CURG AUC for 24 hours following oral administration of CUR (100 mg/kg) or CUR-NP (at a dose equivalent to 100 mg CUR/kg) (mean ± SE, n=4. Means significantly differed at *P<0.05, performed by Mann–Whitney U-test).
Abbreviations: CUR, curcumin; CURG, curcumin glucuronide; AUC, area under the blood concentration–time curve; CUR-NP, curcumin nanoparticles; SE, standard error.
Figure 6.
Representative chromatograms of curcuminoids.
Notes: Representative chromatograms of identified curcuminoids (except CUR and CURG) in rat plasma at 1.5 hours following oral administration of CUR (100 mg/kg) or CUR-NP (at a dose equivalent to 100 mg CUR/kg). Each rat plasma sample (100 μL) was extracted in 1 mL of methanol and 10 μL of it was analyzed by HPLC-MS/MS with MRM. The left chromatograms are from the CUR-administrated group and the right chromatograms are from the CUR-NP-administrated group. (A, B) CURS; (C, D) CURGS; (E, F) DHCG; (G, H) THCG; (I, J) HHCG; (K, L) DHCS; (M, N) THCS; (O, P) HHCS.
Abbreviations: CUR, curcumin; CURG, curcumin glucuronide; CUR-NP, curcumin nanoparticles; cps, centipoise; CURS, curcumin sulfate; CURGS, curcumin glucuronide sulfate; DHCG, dihydrocurcumin glucuronide; THCG, tetrahydrocurcumin glucuronide; HHCG, hexahydrocurcumin glucuronide, DHCS, dihydrocurcumin sulfate; THCS, tetrahydrocurcumin sulfate; HHCS, hexahydrocurcumin sulfate; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; MRM, multiple-reaction monitoring.
Figure 7.
Comparison of the concentration of CUR or CURG in organs between CUR- and CUR-NP-administrated groups.
Notes: Concentration of (A) CUR in the rat liver, (B) CUR in the rat kidney, and (C) CURG in the rat kidney at 1.5 hours following oral administration of CUR (100 mg/kg) or CUR-NP (at a dose equivalent to 100 mg CUR/kg) (mean ± SE, n=4. Means significantly differed at *P<0.05, performed by Mann–Whitney U-test).
Abbreviations: CUR, curcumin; CUR-NP, curcumin nanoparticles; CURG, curcumin glucuronide; SE, standard error.
Table 1.
Comparison of peak areas (counts) of curcuminoids in rat organs
| Organ | Administrated sample | HHCG | THCS | HHCS | OHCS# |
|---|---|---|---|---|---|
| Liver | CUR | nd | nd | 14,548±4,379 | nd |
| CUR-NP | 14,563±4,158 | 117,075±40,152 | 183,500±43,929* | 12,480±3,540 | |
| Kidney | CUR | nd | nd | nd | nd |
| CUR-NP | 7,500±1,416 | nd | nd | nd |
Notes: Peak areas of curcuminoids (except CUR and CURG) identified in rat liver and kidney are shown (mean ± SE, n=4. Means significantly differed at *P<0.05, performed by Mann–Whitney U-test). The homogenized organ (100 μL) was extracted in 1 mL of methanol and 10 μL was analyzed by HPLC-MS/MS with MRM.
Representative chromatogram of OHCS in rat liver at 1.5 hours following oral administration of CUR-NP is shown in Figure S1.
Abbreviations: CUR, curcumin; CURG, curcumin glucuronide; CUR-NP, curcumin nanoparticles; SE, standard error; HHCG, hexahydrocurcumin glucuronide; THCS, tetrahydrocurcumin sulfate; HHCS, hexahydrocurcumin sulfate; OHCS, octahydrocurcumin sulfate; nd, not detected; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; MRM, multiple-reaction monitoring.
Micellar solubilization and cell culture studies
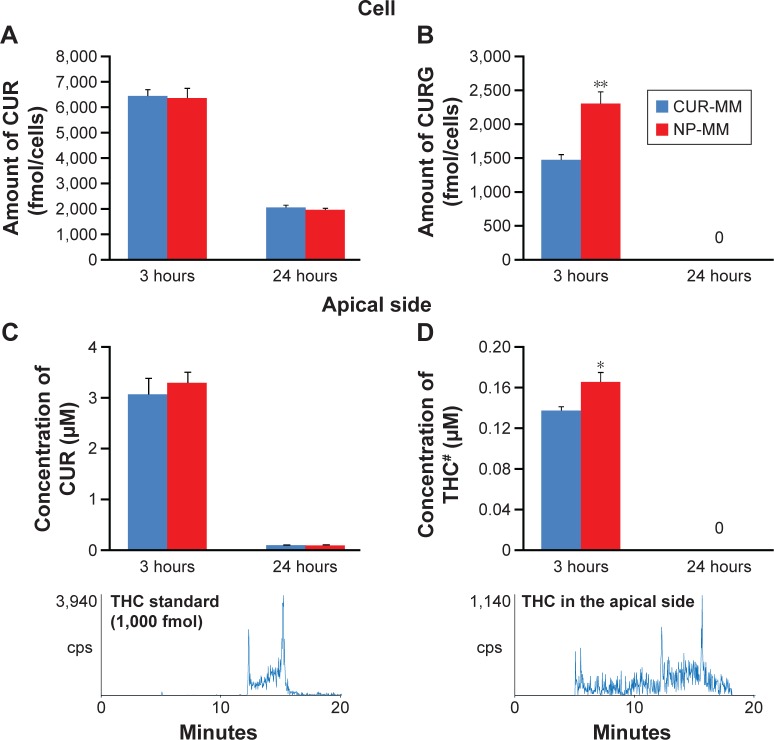
For the micellar solubilization study, CUR-MM and NP-MM were prepared and the concentration of CUR in the mixed micelles was determined by HPLC-UV. NP-MM contained approximately three times the amount of CUR compared with CUR-MM (Figure 8). This suggests that CUR encapsulated in CUR-NP tends to be solubilized in mixed micelles. After adjusting the concentration of CUR in the CUR-MM and NP-MM solutions to 25 μM for the cell culture study, the solutions were added to the apical side of differentiated Caco-2 cells and incubated. HPLC-MS/MS analysis revealed that CUR and certain metabolites were present in the cells and medium (Figure 9; Table 2). Some of the differences between CUR-MM- and NP-MM-treated groups were significant. However, the differences appeared to be small with the exception of CURS in medium (apical side) after 24 hours of treatment. Overall, these results suggest that the high solubilization capacity of CUR-NP in mixed micelles, rather than the cellular uptake efficiency, explains the high intestinal absorption of CUR-NP in vivo.
Figure 8.
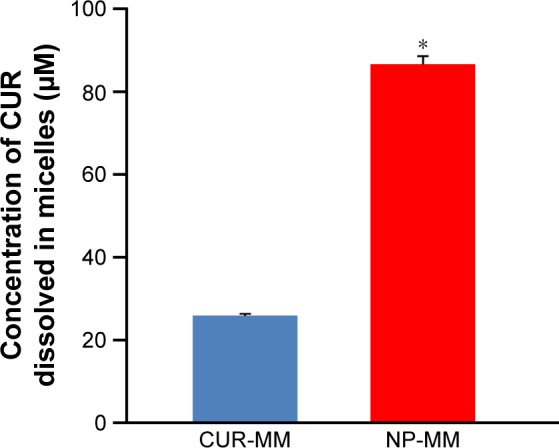
Comparison of the concentrations of CUR dissolved in micelles.
Notes: Comparison of the concentrations of CUR dissolved in CUR-MM and NP-MM (mean ± SE, n=4. Means significantly differed at *P<0.05, performed by Mann–Whitney U-test).
Abbreviations: CUR, curcumin; CUR-MM, mixed micelles of phosphatidylcholine and bile salts containing curcumin; NP-MM, mixed micelles of phosphatidylcholine and bile salts containing curcumin nanoparticles; SE, standard error.
Figure 9.
Comparison of the concentrations of CUR, CURG, and THC.
Notes: Concentration of (A) CUR in Caco-2 cells, (B) CURG in cells, (C) THC in medium of the apical side, and (D) THC in the apical side (mean ± SE, n=6. Means significantly differed at *P<0.05, **P<0.01, performed by Mann–Whitney U-test). #Representative chromatograms of THC are shown underneath the bar graphs. THC in the medium of the apical side was extracted in 1 mL of methanol and 0.1 μL of it was analyzed by HPLC-MS/MS with MRM.
Abbreviations: CUR, curcumin; CURG, curcumin glucuronide; THC, tetrahydrocurcumin; CUR-MM, mixed micelles of phosphatidylcholine and bile salts containing curcumin; NP-MM, mixed micelles of phosphatidylcholine and bile salts containing curcumin nanoparticles; SE, standard error; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; MRM, multiple-reaction monitoring.
Table 2.
Comparison of peak areas of curcuminoids in the cell study
| Time (hours) | Treated sample | CURS | HHCS | OHCS |
|---|---|---|---|---|
| Cell (counts) | ||||
| 3 | CUR-MM | 514,167±34,136 | 79,600±4,725 | 14,767±1,088 |
| NP-MM | 393,167±38,176* | 83,933±4,637 | 16,517±1,076 | |
| 24 | CUR-MM | 61,383±4,393 | 17,850±702 | nd |
| NP-MM | 56,617±4,555 | 15,150±830 | nd | |
| Apical side (counts) | ||||
| 3 | CUR-MM | 40,383±2,646 | 18,267±1,076 | nd |
| NP-MM | 41,750±1,448 | 18,017±802 | nd | |
| 24 | CUR-MM | 16,322±2,198 | 37,300±1,560 | nd |
| NP-MM | 462±43* | 37,400±1,534 | nd | |
| Basolateral side (counts) | ||||
| 3 | CUR-MM | nd | 52,733±1,119 | nd |
| NP-MM | nd | 59,650±3,304 | nd | |
| 24 | CUR-MM | nd | 55,450±1,096 | nd |
| NP-MM | nd | 56,300±1,736 | nd |
Notes: Peak areas of curcuminoids (except CUR, CURG, and THC) identified in Caco-2 cells and medium (apical and basolateral sides) are shown (mean ± SE, n=6. We compared peak areas between CUR-MM and NP-MM in each curcuminoid at the same time. Means significantly differed at *P<0.05, performed by Mann–Whitney U-test). Curcuminoids in cells and medium were extracted in 1 mL of methanol. Extracts were analyzed by HPLC-MS/MS with MRM (10 μL for cells and 0.1 μL for medium).
Abbreviations: CUR, curcumin; CURG, curcumin glucuronide; THC, tetrahydrocurcumin; SE, standard error; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; MRM, multiple-reaction monitoring; CURS, curcumin sulfate; HHCS, hexahydrocurcumin sulfate; OHCS, octahydrocurcumin sulfate; CUR-MM, mixed micelles of phosphatidylcholine and bile salts containing curcumin; NP-MM, mixed micelles of phosphatidylcholine and bile salts containing curcumin nanoparticles; nd, not detected.
Discussion
Although CUR is known to exhibit antioxidative, anti-inflammatory, antitumor, and lipid-lowering activities in vivo, it has limited bioavailability due to poor absorption and rapid metabolism and elimination.1–6,22 A number of studies have suggested ways to overcome the inherent limitations of CUR.23 For example, it has been suggested that encapsulation of CUR in PLGA-based nanoparticles increases the bioavailability of CUR by increasing intestinal permeability and reducing metabolic degradation and conversion to other metabolites. To the best of our knowledge, only four articles have reported the bioavailability of PLGA-based CUR-NP following oral administration.14–16,18 In these studies, PLGA-based CUR-NP was shown to increase the concentration of CUR in the plasma compared with aqueous suspensions of CUR. The results from these studies suggest that nanoparticles increase the relative bioavailability of CUR (comparing AUC/dose) by 5- to 26-fold. Thus, based on these previous studies, PLGA-based nanoparticles are expected to improve the bioavailability of CUR following oral administration. However, these studies have several limitations. For one, the reported CUR parameters14–16,18 (eg, Cmax, Tmax, and AUC) were variable. More importantly, levels of CURG, which is the major metabolite found in the body, were not measured. Finally, it is unclear why encapsulating CUR with PLGA-based nanoparticles increases intestinal absorption. Overall, there is a lack of conclusive evidence that justifies the use of PLGA-based CUR-NP to enhance the absorption and bioavailability of CUR administered orally.
To provide conclusive evidence, we used rat in vivo and in vitro models to investigate intestinal absorption and metabolism of CUR-NP. We chose HPLC-MS/MS as the preferred analytical method because HPLC-MS/MS offers specific advantages over conventional analysis (HPLC-UV) of biomolecules. For instance, HPLC-MS/MS provides neutral loss scanning and MRM, which provides useful structural information even when background contaminants are present.24 We and others have used HPLC-MS/MS with MRM to examine and identify several CUR metabolites in the plasma and tissues of rats and mice supplemented with CUR.8,25–28 HPLC-MS/MS analyses of curcuminoids in liver microsomes and in turmeric have also been reported.29,30 In the present study, we used HPLC-MS/MS to analyze the number of curcuminoids (CUR, CURG, CURS, CURGS, CURDG, CURDS, DHC, THC, HHC, OHC, DHCG, THCG, HHCG, OHCG, DHCS, THCS, HHCS, and OHCS) present in the plasma and organs of rats. The detection of these curcuminoids by HPLC-MS/MS was reproducible and unaltered by the storage of extract samples at temperatures below −80°C for 1 month (data not shown). Based on these findings, rats were administrated orally with suspensions of either 100 mg/kg CUR or CUR-NP (at a dose equivalent to 100 mg CUR/kg). We subsequently analyzed CUR, CURG, and other metabolites in the plasma and organs by HPLC-MS/MS. The results suggest that CUR is effectively absorbed following oral administration of CUR-NP and is predominately found in the plasma as CURG (Figures 3–7; Table 1). Overall, CUR-NP increases intestinal absorption of CUR, but does not appear to prevent metabolic degradation and conversion to other metabolites (eg, glucuronidation). Although encapsulating CUR with PLGA-based NP improves intestinal absorption, there is still a need for formulations that improve resistance to metabolic degradation and conversion to other metabolites.
The CUR-NP prepared in this study had a small diameter (186.9 nm), narrow size distribution (PDI =0.095), and negative zeta potential (−16.7 mV), which were similar to those reported in previous studies.14–18 Some researchers have developed modified forms of PLGA-based CUR-NP. Tsai et al reported that using high-molecular-weight PLGA increases relative bioavailability of CUR 1.67-fold compared to low-molecular-weight PLGA.17 Khalil et al reported that PLGA-polyethylene glycol nanoparticles increase CUR bioavailability by 55.4-fold compared to an aqueous suspension of CUR.15 It is hypothesized that these formulations improve not only the absorbability, but also the resistance to metabolism of CUR.23 Further studies are needed to improve the bioavailability of CUR. In addition, only a few studies have reported the presence of CUR metabolites other than CURG.7,31–33 However, we revealed that other metabolites are present in the plasma and organs of rats following oral administration of CUR-NP (Figure 6; Table 1). It would be interesting to see whether increased levels of CURG and other metabolites contribute to the physiological activity of CUR-NP.
In addition, the low EE may be due to aggregation during preparation. These aggregates appeared to be composed of larger-sized CUR-NP and CUR not wrapped in CUR-NP, and they were removed during centrifugation and filtration. Actually, during the solvent (dichloromethane) evaporation process, visible aggregates of CUR or CUR-NP were observed. Additional studies are needed to focus on optimizing the amounts of PLGA and PVA to overcome this problem.
The mechanism of absorption of CUR in the gastrointestinal tract is still unclear. In the case of carotenoids, which exhibit chemical characteristics similar to those of CUR (eg, polarity and molecular weight), Yonekura et al reported that the absorption of dietary carotenoids involves several steps that begin with the mechanical and enzymatic disruption of the food matrix, release of the carotenoids, and the incorporation into the lipid droplets of gastric emulsions.34 The carotenoids are then transferred from the lipid droplets to mixed micelles produced by the action of bile salts, biliary phospholipids, dietary lipids, and their hydrolysis products. Following solubilization, carotenoids are absorbed by the intestinal cells, packed into chylomicrons, and secreted into the lymphatic system.35,36 Therefore, the major factors that influence intestinal absorption of carotenoids are solubilization capacity in mixed micelles and their incorporation efficiency into intestinal cells, and these factors may be applicable to CUR.
Some studies have focused on the interaction between CUR and bile salts in mixed micelles.37 Based on these studies, we prepared mixed micelles containing CUR or CUR-NP and compared the micellar solubilization to determine why CUR-NP has higher intestinal absorption (Figure 5). We found that NP-MM contained three times more CUR compared to CUR-MM (Figure 8), which suggests that encapsulated CUR enhances solubilization in mixed micelles. Therefore, we suggest that the high solubilization capacity of CUR-NP in mixed micelles results in higher intestinal absorption of CUR-NP in vivo.
We also investigated the cellular incorporation of these micelles into differentiated Caco-2 human intestinal cells, which are useful models for studying the absorption and metabolism of dietary compounds.38,39 We found that CUR and certain metabolites are present in the cells and medium (Figure 9; Table 2). The levels of some of these metabolites were significantly different between the CUR-MM- and NP-MM-treated groups, but the differences appeared to be small, except for CURS in medium (apical side) after 24 hours of treatment. Taken together, these results suggest that the high solubilization capacity of CUR-NP in mixed micelles, rather than cellular uptake efficiency, explains the high intestinal absorption of CUR-NP in vivo. In addition, CUR in the medium (apical side) rapidly degraded (Figure 9C). These results are similar to previous studies40–42 and most likely occurred due to rapid cellular uptake of CUR from the medium and/or CUR degradation in the medium.40–42
Conclusion
We showed that CUR-NP administered orally to rats was effectively absorbed and the predominant CUR metabolite was CURG in the plasma. The results suggest that CUR-NP improves intestinal absorption, but does not improve metabolic degradation and conversion to other metabolites. The in vitro study revealed that CUR-NP is solubilized in mixed micelles and the high solubilization capacity of CUR-NP leads to higher intestinal absorption of CUR following oral administration. Since CURG is less potent in terms of antioxidant activity and antitumor effects compared to CUR,8,43 it is desirable to use a NP formulation that not only increases intestinal absorption but also decreases the metabolic degradation and conversion of CUR to other metabolites to further improve the bioavailability of CUR. Overall, additional in vitro and in vivo studies are needed to develop a better understanding of the absorption, metabolism, and overall effects of CUR and CUR-NP in the body.
Supplementary material
Representative chromatogram of OHCS.
Notes: Representative chromatogram of OHCS in rat liver at 1.5 hours following oral administration of CUR-NP. The homogenized organ (100 μL) was extracted in 1 mL of methanol and 10 μL of it was analyzed by HPLC-MS/MS with MRM.
Abbreviations: OHCS, octahydrocurcumin sulfate; cps, centipoise; CUR-NP, curcumin nanoparticles; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; MRM, multiple-reaction monitoring.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI, 15K14725) of the Japan Society for the Promotion of Science (JSPS), Japan. Taiki Miyazawa was supported by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad (https://www.jsps.go.jp/). We thank Myles Migneault (Vascular Biology Laboratory, Jean Mayer Human Nutrition Research Center on Aging (HNRCA) at Tufts University) and Mayuko Itaya, Reina Kamiyoshihara, Yasuhiko Hanzawa and, Saki Hayasaka (Food and Biodynamic Chemistry Laboratory, Graduate School of Agricultural Science at Tohoku University) for their assistance in the preparation of the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20(5):9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asai A, Miyazawa T. Dietary curcuminoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J Nutr. 2001;131(11):2932–2935. doi: 10.1093/jn/131.11.2932. [DOI] [PubMed] [Google Scholar]
- 4.Mohanty C, Das M, Sahoo SK. Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin. Expert Opin Drug Deliv. 2012;9(11):1347–1364. doi: 10.1517/17425247.2012.724676. [DOI] [PubMed] [Google Scholar]
- 5.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 6.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010;343(9):489–499. doi: 10.1002/ardp.200900319. [DOI] [PubMed] [Google Scholar]
- 7.Asai A, Miyazawa T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000;67(23):2785–2793. doi: 10.1016/s0024-3205(00)00868-7. [DOI] [PubMed] [Google Scholar]
- 8.Shoji M, Nakagawa K, Watanabe A, et al. Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem. 2014;151:126–132. doi: 10.1016/j.foodchem.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Joshi G, Kumar A, Sawant K. Enhanced bioavailability and intestinal uptake of gemcitabine HCl loaded PLGA nanoparticles after oral delivery. Eur J Pharm Sci. 2014;60:80–89. doi: 10.1016/j.ejps.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Zakeri-Milani P, Loveymi BD, Jelvehgari M, Valizadeh H. The characteristics and improved intestinal permeability of vancomycin PLGA-nanoparticles as colloidal drug delivery system. Colloids Surf B Biointerfaces. 2013;103:174–181. doi: 10.1016/j.colsurfb.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Sahana DK, Mittal G, Bhardwaj V, Kumar MN. PLGA nanoparticles for oral delivery of hydrophobic drugs: influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. J Pharm Sci. 2008;97(4):1530–1542. doi: 10.1002/jps.21158. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37(3–4):223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Khalil NM, do Nascimento TC, Casa DM, et al. Pharmacokinetics of curcumin-loaded PLGA and PLGA-PEG blend nanoparticles after oral administration in rats. Colloids Surf B Biointerfaces. 2013;101:353–360. doi: 10.1016/j.colsurfb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YM, Jan WC, Chien CF, Lee WC, Lin LC, Tsai TH. Optimised nano-formulation on the bioavailability of hydrophobic polyphenol, curcumin, in freely-moving rats. Food Chem. 2011;127(3):918–925. doi: 10.1016/j.foodchem.2011.01.059. [DOI] [PubMed] [Google Scholar]
- 17.Tsai YM, Chang-Liao WL, Chien CF, Lin LC, Tsai TH. Effects of polymer molecular weight on relative oral bioavailability of curcumin. Int J Nanomedicine. 2012;7:2957–2966. doi: 10.2147/IJN.S32630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie X, Tao Q, Zou Y, et al. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011;59(17):9280–9289. doi: 10.1021/jf202135j. [DOI] [PubMed] [Google Scholar]
- 19.Miyazawa T, Nakagawa K, Harigae T, et al. Distribution of β-carotene-encapsulated polysorbate 80-coated poly(D,L-lactide-co-glycolide) nanoparticles in rodent tissues following intravenous administration. Int J Nanomedicine. 2015;10:7223–7230. doi: 10.2147/IJN.S94336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa K, Zingg JM, Kim SH, et al. Differential cellular uptake and metabolism of curcuminoids in monocytes/macrophages: regulatory effects on lipid accumulation. Br J Nutr. 2014;112(1):8–14. doi: 10.1017/S0007114514000567. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda I, Matsuoka R, Hamada T, et al. Cholesterol esterase accelerates intestinal cholesterol absorption. Biochim Biophys Acta. 2002;1571(1):34–44. doi: 10.1016/s0304-4165(02)00204-0. [DOI] [PubMed] [Google Scholar]
- 22.Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007;595:453–470. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- 23.Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res Treat. 2014;46(1):2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King R, Fernandez-Metzler C. The use of Qtrap technology in drug metabolism. Curr Drug Metab. 2006;7(5):541–545. doi: 10.2174/138920006777697936. [DOI] [PubMed] [Google Scholar]
- 25.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60(2):171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 26.Marczylo TH, Steward WP, Gescher AJ. Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography (UPLC) method. J Agric Food Chem. 2009;57(3):797–803. doi: 10.1021/jf803038f. [DOI] [PubMed] [Google Scholar]
- 27.Zhongfa L, Chiu M, Wang J, et al. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol. 2012;69(3):679–689. doi: 10.1007/s00280-011-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma MK, Najar IA, Tikoo MK, et al. Development of a validated UPLC-qTOF-MS method for the determination of curcuminoids and their pharmacokinetic study in mice. Daru. 2013;21(1):11. doi: 10.1186/2008-2231-21-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamvakopoulos C, Sofianos ZD, Garbis SD, Pantazis P. Analysis of the in vitro metabolites of diferuloylmethane (curcumin) by liquid chromatography–tandem mass spectrometry on a hybrid quadrupole linear ion trap system: newly identified metabolites. Eur J Drug Metab Pharmacokinet. 2007;32(1):51–57. doi: 10.1007/BF03190990. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H, Timmermann BN, Gang DR. Use of liquid chromatography-electrospray ionization tandem mass spectrometry to identify diarylheptanoids in turmeric (Curcuma longa L.) rhizome. J Chromatogr A. 2006;1111(1):21–31. doi: 10.1016/j.chroma.2006.01.103. [DOI] [PubMed] [Google Scholar]
- 31.Ireson CR, Jones DJ, Orr S, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11(1):105–111. [PubMed] [Google Scholar]
- 32.Ireson C, Orr S, Jones DJ, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–1064. [PubMed] [Google Scholar]
- 33.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27(4):486–494. [PubMed] [Google Scholar]
- 34.Yonekura L, Nagao A. Intestinal absorption of dietary carotenoids. Mol Nutr Food Res. 2007;51(1):107–115. doi: 10.1002/mnfr.200600145. [DOI] [PubMed] [Google Scholar]
- 35.Parker RS, Swanson JE, You CS, Edwards AJ, Huang T. Bioavailability of carotenoids in human subjects. Proc Nutr Soc. 1999;58(1):155–162. doi: 10.1079/pns19990021. [DOI] [PubMed] [Google Scholar]
- 36.Faulks RM, Southon S. Challenges to understanding and measuring carotenoid bioavailability. Biochim Biophys Acta. 2005;1740(2):95–100. doi: 10.1016/j.bbadis.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Patra D, Ahmadieh D, Aridi R. Study on interaction of bile salts with curcumin and curcumin embedded in dipalmitoyl-sn-glycero-3-phosphocholine liposome. Colloids Surf B Biointerfaces. 2013;110:296–304. doi: 10.1016/j.colsurfb.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 38.Sugawara T, Kushiro M, Zhang H, Nara E, Ono H, Nagao A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J Nutr. 2001;131(11):2921–2927. doi: 10.1093/jn/131.11.2921. [DOI] [PubMed] [Google Scholar]
- 39.Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology. 1989;96(3):736–749. [PubMed] [Google Scholar]
- 40.Jaruga E, Salvioli S, Dobrucki J, et al. Apoptosis-like, reversible changes in plasma membrane asymmetry and permeability, and transient modifications in mitochondrial membrane potential induced by curcumin in rat thymocytes. FEBS Lett. 1998;433(3):287–293. doi: 10.1016/s0014-5793(98)00919-3. [DOI] [PubMed] [Google Scholar]
- 41.Dempe JS, Pfeiffer E, Grimm AS, Metzler M. Metabolism of curcumin and induction of mitotic catastrophe in human cancer cells. Mol Nutr Food Res. 2008;52(9):1074–1081. doi: 10.1002/mnfr.200800029. [DOI] [PubMed] [Google Scholar]
- 42.Dempe JS, Scheerle RK, Pfeiffer E, Metzler M. Metabolism and permeability of curcumin in cultured Caco-2 cells. Mol Nutr Food Res. 2013;57(9):1543–1549. doi: 10.1002/mnfr.201200113. [DOI] [PubMed] [Google Scholar]
- 43.Choudhury AK, Raja S, Mahapatra S, Nagabhushanam K, Majeed M. Synthesis and evaluation of the anti-oxidant capacity of curcumin glucuronides, the major curcumin metabolites. Antioxidants (Basel) 2015;4(4):750–767. doi: 10.3390/antiox4040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative chromatogram of OHCS.
Notes: Representative chromatogram of OHCS in rat liver at 1.5 hours following oral administration of CUR-NP. The homogenized organ (100 μL) was extracted in 1 mL of methanol and 10 μL of it was analyzed by HPLC-MS/MS with MRM.
Abbreviations: OHCS, octahydrocurcumin sulfate; cps, centipoise; CUR-NP, curcumin nanoparticles; HPLC-MS/MS, high-performance liquid chromatography-tandem mass spectrometry; MRM, multiple-reaction monitoring.