Abstract
Non-traumatic shoulder instability is frequently associated with chronic disabling pain, altered patterns of motion, dysfunctional muscle strategies and hyperlaxity. Identifying the relationship between potential aetiologies can be challenging. An expanded assessment may be useful to estimate the contribution of each component and offer a framework for targeted rehabilitation.
Keywords: Assessment, instability, non-traumatic, physiotherapy, shoulder
Introduction
Non-traumatic (or atraumatic) shoulder instability (NTSI) is a subclassification of glenohumeral joint (GHJ) instability, encompassing those for whom trauma is not considered the primary aetiology. Beyond this distinction, patients with NTSI have been variously classified according to the presence of dynamic or muscle patterning instability, atraumatic structural or multidirectional instability (MDI), generalized joint hyperlaxity or congenital hyperlaxity, voluntary and involuntary instability and repetitive overuse.1–4 The diagnostic criteria for each subgroup can prove unreliable, lack validity and vastly alter the incidence of a specific diagnosis within a population according to which criteria are used.5
Perhaps the difficulty in classifying NTSI is because it is a clinical manifestation with a spectrum of underlying causes.
Abnormal muscle patterning is considered a highly relevant and perhaps causative phenomenon in NTSI.6–8 Once a significant underlying lesion has been excluded (e.g. labral, capsular, bony or neurological), the management indications are conservative rather than surgical, yet the evidence for specific exercises remains unclear.9 Abnormal patterns of motion may develop for a number of reasons. They may have developed as a result of weakness, avoidance of pain provocation, altered sensory input causing mismatch of anticipated motion/position and reality, or fear-avoidant strategies. Sometimes, it is even deliberate as seen in the “party trick”.
The clinician’s challenge is to identify the driver behind the dysfunction and focus rehabilitation towards the most relevant aspect for the individual.
Here, a framework of clinical reasoning and assessment strategies to aid the clinician is presented, at the same time as acknowledging that we have yet to understand whether these subgroups can be validly tested and reliably identified.
Clinical reasoning framework
Patients with NTSI represented in Figure 1 may experience overt dislocation and subluxation, at rest or during motion, or less pronounced symptoms (e.g. a slipping or wobbly shoulder). The remit of the present review does not represent the athletic repetitive overhead/overuse population, although some of the considerations above will be relevant. There can clearly be overlap in some aetiologies. A painful shoulder can simultaneously become weak, begin ‘popping out’, be associated with fear of motion and develop an ingrained abnormal pattern of movement. The first step for the therapist is to assess motion and establish the potential for movement correction as the primary strategy. In those patients where movement cannot comfortably be modified, the clinician should explore other aetiologies, using the assessment indicated in Figure 3.
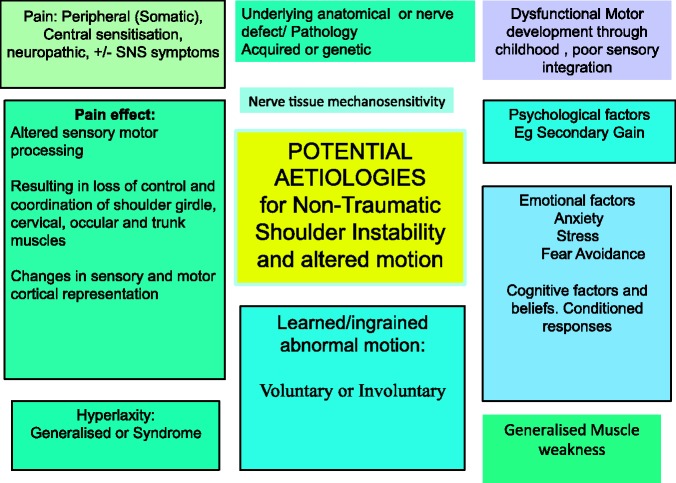
Figure 1.
Potential aetiologies in nontraumatic (or atraumatic) shoulder instability.
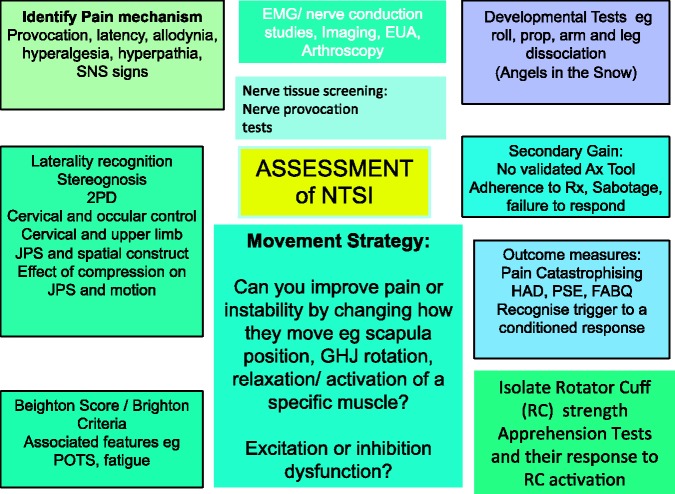
Figure 3.
The assessment of potential aetiologies in non-traumatic (or atraumatic) shoulder instability.
Learned and ingrained abnormal motion in muscle patterning instability
Evidence of altered muscle control in patients with NTSI and generalized joint hyperlaxity has been observed experimentally supporting the clinical notion of altered muscle sequencing and excitation levels. Reduced activity and time domain or reflex activity have been seen in lower trapezius,10 infraspinatus,11 deltoid12 and supraspinatus.13 Excessive activity is observed in latissimus dorsi (LD) and pectoralis major (PM).11
Because electromyography and transcranial magnetic stimulation are not readily available as clinical assessment tools, the ability to assess motion and recognize compensations is crucial.
Motion assessment
It is important to observe the patient’s willingness to move, their movement strategy and functional compromise. Specifically, the clinician should observe the provocative movements and differentiate their suitability for movement retraining alone by correcting the movement strategy and assessing the effect on symptoms. The instability/subluxation may be present at rest or occur during movements of the arm. This is often termed positional instability.
The pelvis, trunk and neck should remain in neutral and the scapula should upwardly rotate during GHJ elevation motion. Aberrant motion of the scapula includes downward rotation, winging and forward tilt. Aberrant trunk and neck motion includes side flexion, lumbar extension and thoracic flexion. Aberrant arm motion includes excessive internal rotation when elevating the arm and hyperextension of the elbow.
Assessment
Observe how the patient performs the actions of flexion, abduction and external rotation, including functional provocative motion.
Repeat the tests with correction of the aberrant action (e.g. help the scapula actively or passively into upward tilt, prevent trunk side flexion or thoracic flexion and maintain external GHJ rotation through elevation).
If this is associated with an improvement in symptoms, it strongly suggests a learned abnormal motion. The focus will then be on retraining the faulty components of motion (Figure 2).
However, if symptoms worsen or are unchanged or it is not possible to facilitate the desired pattern of motion, other differentials would need to be considered and assessed (see below).
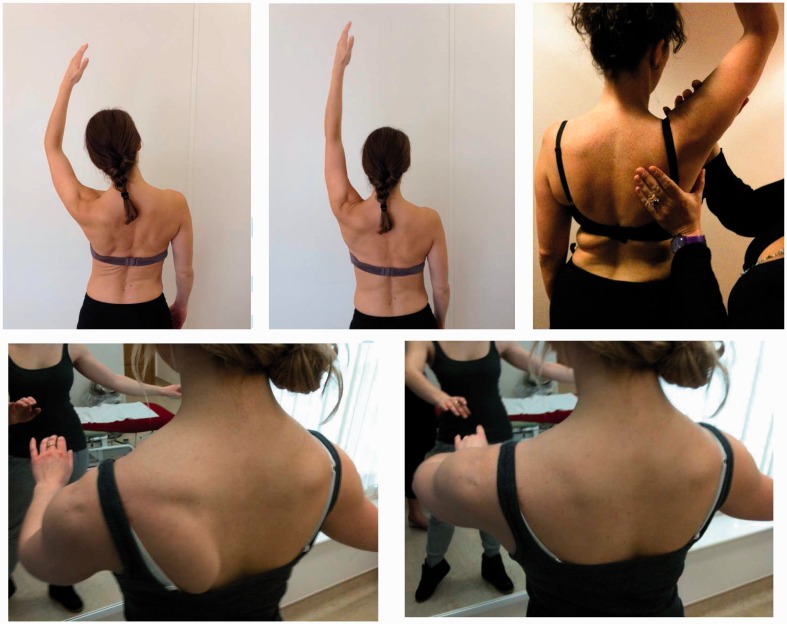
Figure 2.
Movement corrections, of trunk side flexion and upward scapula rotation through flexion, and correction of downward scapula rotation in ballet pose.
Correcting motion
For all tests, in standing, ensure the trunk is in neutral, the head is in neutral and arms are not clamped to the side of the body.
Many patients feel vulnerable in certain positions and describe not knowing where their arm is in space. For those with generalized poor joint position sense, a tubigrip/compression worn on the arm or trunk support may improve the response to all tests.
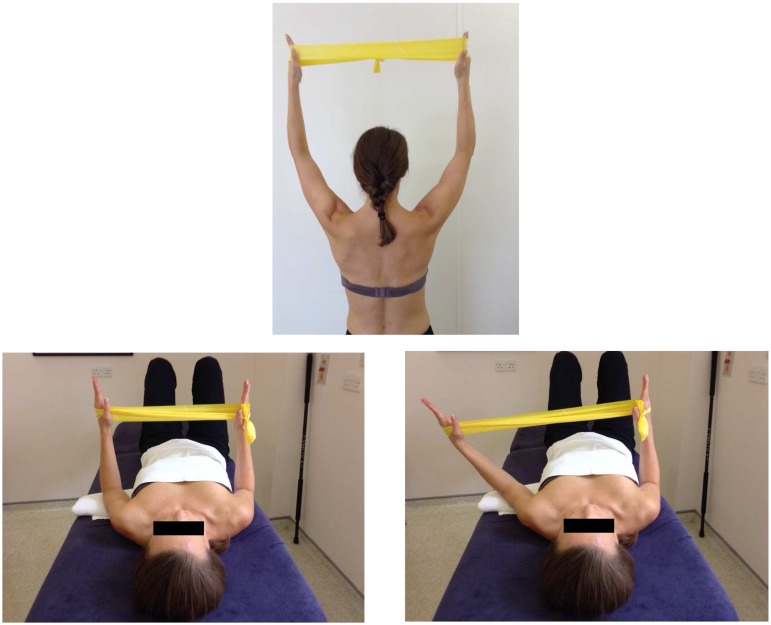
An active shrug motion (Figure 4) can be used to facilitate upward scapula rotation.14
If neural tissue sensitivity is suspected, be cautious about provoking pain.
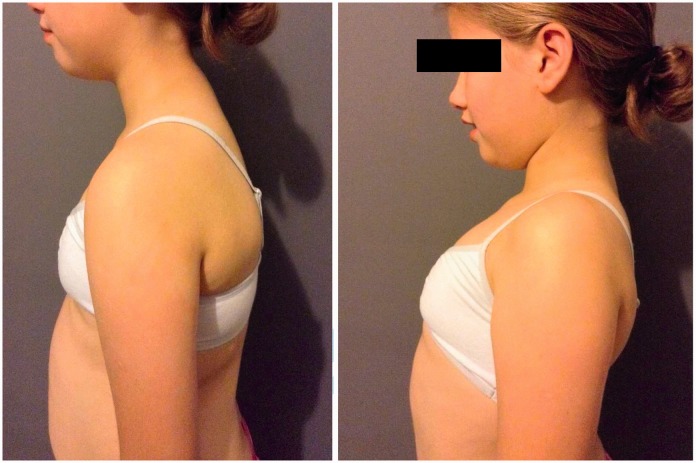
Figure 4.
Images showing how a downwardly rotated scapula (left) can be upwardly orientated into the correct position using the ‘Shrug’ (Watson et al. 2010).
External rotation
If they cannot perform the islolated motion with facilitation and correction in standing, the patient should be assessed in supine with the weight of the arm supported. The instruction is to perform external and internal rotation without allowing the scapula to move.
Apprehension associated with this movement in supine probably represents weakness in the rotator cuff (RC). This may be seen as inability to keep the scapula stable. The scapula motion reduces unstable GHJ motion.
Excessive PM activity may need facilitation of relaxation before motion.
Flexion, abduction and scaption
These movements will often differentiate those with positional instability (PI) as a result of altered muscle patterning from those with weakness and generalized joint hyperlaxity. The former group will usually ‘pop’ either in or out of joint during flexion but less commonly in abduction. The latter group will either be more symptomatic in abduction or equally symptomatic in both directions as a result of multidirectional instability. By contrast, the PI can usually be corrected once aberrant motion is eliminated. Repeat the flexion motion maintaining the trunk, neck and GHJ in neutral, limiting hyperextension at the elbow and ensure the scapula does not depress or tilt forwards.
If the instability symptoms are not revealed during active flexion, abduction or external rotation, further exploration of apprehension and muscle strength should be undertaken for posterior instability (horizontal flexion and internal rotation), inferior instability (axial loading) and anterior instability (abduction, external rotation). The assessment should focus on the inability of the muscles to control the motion as it approaches its position of apprehension and the effect of compression, taping or feedback on joint position sense and symptoms.
Further differentiation
Generalized muscle weakness
In its most simple form, NTSI can occur in a previously asymptomatic shoulder if shoulder muscles decondition and cross the threshold from maintaining humeral head stability within the glenoid to not. The history may include a mild or moderate ‘trauma’ as the precipitating factor where reflex muscle inhibition is the assumed aetiology, or where they have gradually become less active (typically from sport at university and school to less in their working lives). There is often a background of joint hyperlaxity, which may or may not be part of a syndrome. Usually, the arm positions and functions associated with anterior (abduction, external rotation), posterior (horizontal flexion, internal rotation) or inferior instability (axial load) will be reported as provocative. These should be confirmed or excluded with apprehension and relocation tests. The patient may have experienced slipping and apprehension in the shoulder before, and may report hypermobility issues in other joints. It is important therefore to always enquire about family history including siblings. If a hypermobility syndrome is suspected, particularly a painful one, exercise caution with provocative actions and strength testing, and consider pacing the assessment to include rest.
Muscle strength assessment can be misleading because the patient can generate force using compensatory strategies, usually due to scapula faults. The electromyographic (EMG) literature tells us that deltoid and the RC co-activate for all movements during maximal force generation,15 therefore the tests should be considered as the ability to generate forces in a desired direction, rather than specific muscle tests. In patients with MDI, muscle activity has a longer time domain but with lower intensity16 and fewer activity strategies compared to controls,17 although this reverses as function improves. Loss of scapula upward tilt and lateral scapula rotation has been observed in NTSI versus asymptomatic controls.18
The absolute resistance generated on a strength test is less relevant than the way in which it is achieved. Therefore, when performing resistance tests, there is the need to always identify:
Which muscles are being used to generate the resistance to the applied force?
What does the scapula do? Can it maintain a stable base in upward rotation?
To achieve upward scapula rotation effectively as a basis for shoulder motion, the strength in the upper trapezius as part of the upper trapezius/lower trapezius/serratus anterior force couple often needs developing. This is better achieved with a modified shrug at 30° abduction than with the arm by the side.19
The force generated in the directions of external rotation (ER) and flexion should be examined. If correction of scapula position improves force generation at the GHJ (i.e. RC strength), rehabilitation focuses on scapula control of the desired scapula position when practicing the GHJ motion.
If correction of the scapula reduces force generation at the GHJ (i.e. reveals RC weakness), rehabilitation focuses on RC strengthening in positions that facilitate scapula control; e.g. supine lying (Figure 5)
Figure 5.
Strengthening external rotation in supine with the scapula controlled (below). Elevation sequencing in, upward shrug with some resistance being used to preset the posterior rotator cuff (above).
If the patient is unable to control or tolerate the scapula position and achieve isolated GHJ external rotation or flexion, then further assessment is needed. Other differentials would include neural tissue pain, central sensitization, or a dominant muscle activation pattern proving difficult to inhibit.
Neurodynamic tissue mechanosensitivity
Mechanosensitivity in neural tissue as a pain source is a well-recognized entity.20 In the presence of sensitive neural tissue (which may be secondary to previous trauma; e.g. Colles fracture, whiplash, even dislocation), the body will endeavour to protect the threatened tissue and adopt protective postures and motion. In the upper limb, it may involve bending the elbow, protracting the shoulder and elevating the shoulder girdle.21 An anteriorly translating humeral head could be part of this pattern. For this reason, the upper limb neural dynamic tests and nerve palpation20 are indicated in NTSI where neurogenic pain is suspected. These should be performed actively at first and under the patient’s control, with subsequent passive assessment of the potential limiting interface tissues such as the cervical spine, scalenes and first rib.
Hypermobility
Generalized joint hypermobility can be a benign condition or part of a painful connective tissue disorder ranging from joint hypermobility syndrome (JHS) to Ehlers–Danlos syndome and Marfans. The presenting complaint in any of these conditions may be subluxation of the shoulder,22 which may be part of a wider clinical picture of pain and deconditioning, or simply a mono-articular dysfunction within a hypermobile system.
Measures to assess JHS include the Beighton score23 and Brighton (1998) criteria (a system which takes into account major and minor criteria.24 Clinicians should be mindful that JHS can be associated with non-musculoskeletal symptoms, for which patients have often seen a range of specialists. There is association between JHS and bowel dysfunction,25 gynaecological dysfunction,26 chronic fatigue, vascular disorders, skin elasticity and autonomic nervous system dysfunction.27 Additionally, a great challenge in this group is altered pain processing and reduced responsiveness to analgesia28
Assessment for JHS should take into account the full clinical picture so that management planning can be comprehensive.29 However, many of the domains affected by JHS will respond to nonpharmacological input to recondition the musculoskeletal system. For example, postural orthostatic tachycardia syndrome (PoTS) is often identified in patients with hypermobile shoulder dysfunction. PoTS is characterized by an increase in heart rate of 30 beats per minute or more when changing from supine to upright. It can be associated with dizziness and is a great barrier to rehabilitation and daily function.30 An assessment is needed regarding whether the patient is employing strategies to improve PoTS: pharmacological treatment, high salt and fluid intake, wearing compressive stockings and a paced exercise programme.31
Strategies such as the ability to stand on one leg, bear weight on all fours, and step up/down should be achievable before commencing shoulder rehabilitation. During the assessment, a test of the effect of wearing a compression support on either the trunk or the affected arm is required as many patients report a facilitative effect enhancing their ability to know where they are in space and therefore facilitating muscle activity (e.g. tubigrip). Feedback, such as visual cues, tactile cues, tape and demonstration, should be employed to maximize the precision of position and action. Once general conditioning and postural control is established, if the shoulder instability has not resolved, a progressive, paced strengthening programme for the trunk, scapula, neck and RC is balanced against appropriate sequencing.
Voluntary instability
The asymptomatic hypermobile individual can easily develop into a ‘twitcher’ who voluntarily subluxes and twitches the shoulder ‘as a form of relief’. This could be interpreted as a desire to feel where they are in space because they have so little feedback from the capacious joint capsule. Fundamentally, all abnormal voluntary components should be eliminated. Distraction is a key tool, or an alternative action each time they feel the desire. Cognitive techniques are important but the learning and reinforcement of where the joint should be positioned within ball and socket, and not at the front, bottom or back of the socket is vital. Positions that enhance body awareness and improve accuracy should be adopted. Once again, general body position awareness and RC strengthening may be needed as a prelude to training correct movement of the shoulder.
Pain
The contemporary model of pain science explains how pain can extend beyond somatic provocation and local tissue changes. The relationship between nociception and pain is less correlated as pain persists as a result of sensitization at the spinal cord level and beyond into the cortex.
Somatotropic representations for the sensory and motor homunculus are not fixed but change in response to peripheral input including underuse, pain, injury and cognition. Phantom limb pain occurs where a painful limb representation remains in the homunculus despite the anatomical absence of the limb. Cortical reorganization has been observed in response to painful shoulder conditions.32 Part of the reorganizing has been attributed to a rebalance of inhibitory and excitatory inputs,33 which has been specifically observed in NTSI10 and muscle patterning shoulder instability.34 This plasticity is where therapy can be targeted. In painful NTSI, widespread pain can be experienced from neck to hand and the symptoms so easily provoked that any upper limb motion can be intolerable. In an attempt to find solutions for this patient group, we have extrapolated from research in painful upper quadrant models such as phantom limb pain, chronic regional pain syndrome and whiplash associated disorders.
All three models have identified somatosensory changes at a cortical level. These include sensory two-point discrimination and spatial construct, an altered ability to discriminate left/right in the affected limb, motor control changes,35 balance, cervical kinaesthesea and eye control.36,37
Crucially, the somatosensory assessment techniques can be used as the rehabilitation techniques. We found that cervical proprioception training and eye control (smooth pursuit, gaze stability and smooth pursuit neck torsion) had a positive impact on pain, function and two-point discrimination in more than 50% of our patients with severely painful NTSI.38 A more sensitive tool for sensory discrimination should probably be utilized for future research and assessment: two- point orientation discrimination (2POD).39
It is therefore clear that assessment of the chronically painful, unstable shoulder should extend beyond the nociceptive paradigm. Clues in the history that would suggest the need to explore these signs include widespread and disabling pain, dizziness and autonomic features such as ‘the hand feels too big, puffy, swollen’.
Assesssment for painful shoulder with suspected central sensitisation
Assess for allodynia, hyperalgesia, hyperpathia and cold sensitivity
Neuropathic pain screen: PainDETECT40
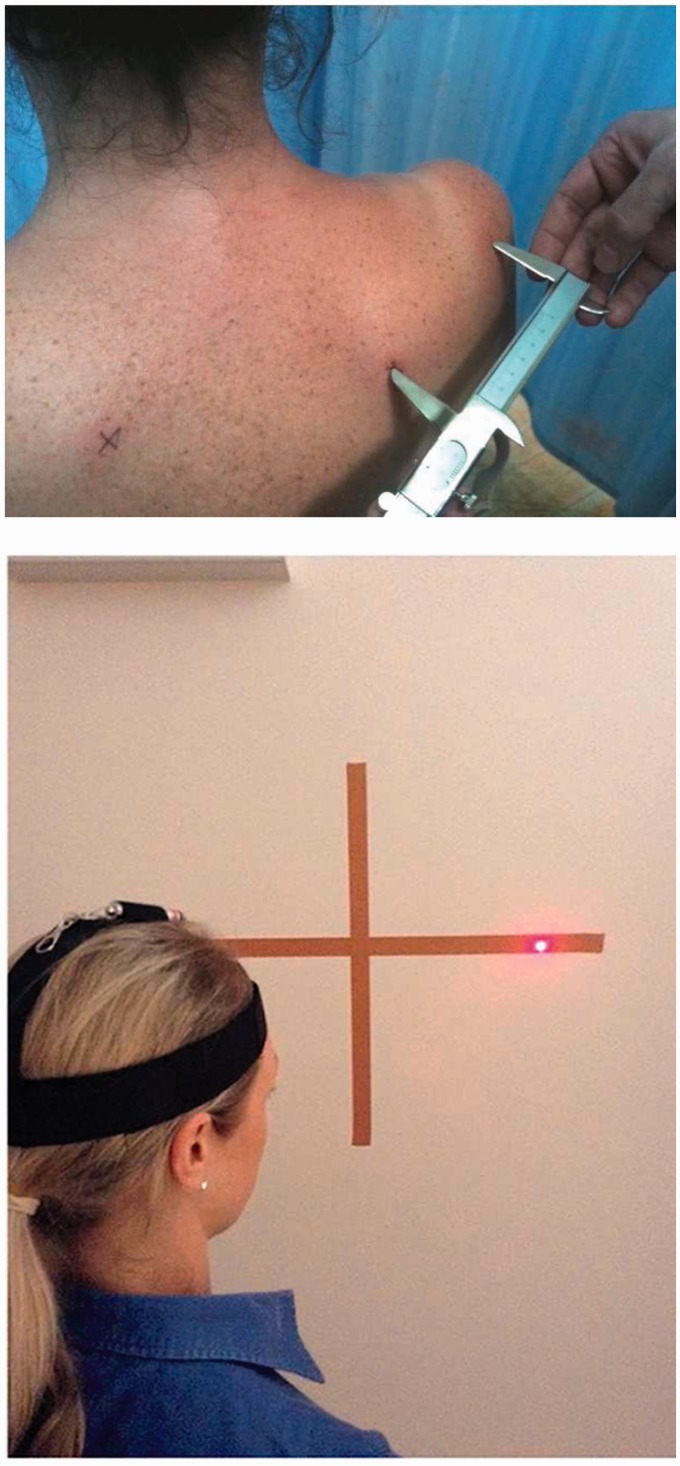
2POD: use calipers along the spine of the scapula, humerus or forearm. Measure the distance at which the patient discriminates correctly whether orientation is horizontal or vertical. Compare side-to-side differences (Figure 6)
Cervical kinaesthesia: test the ability to follow a path of cervical motion and return to the centre with eyes open and closed. Use a laser pointer mounted on a headband for feedback (Figure 6)37
Smooth pursuit and eye follow: follow the ‘H’ pattern with eyes, maintaining still head37
Gaze stability: keep eyes focused on a point when moving head left and right, up and down37
Stereognosis: test the ability to blindly recognize a number of ‘household objects’ through palpation and dexterous manipulation
Laterality discrimination: ‘Recognise’ software (NOI, Adelaide, Australia)
Manual therapy segmental assessment of the cervical spine (nonprovocative) for localized or generalized spasm
Figure 6.
Two-point orientation discrimination and cervical kinaesthesia assessment. The dysfunction can become the treatment but should remain non-provocative. Graded Motor Imagery (GMI) may be indicated in the presence of pain-mediated cortical adaptations (http://www.gradedmotorimagery.com).
Developmental coordination/sensory motor integration
Exploration of the motor developmental milestones through childhood may offer clues to whether there is difficulty with sensory motor integration. A child who went from bottom shuffling to walking, without crawling, may have failed to acquire the crucial art of weight bearing through the shoulders and proprioceptive coordination of the shoulder girdle. Other developmental coordination difficulties and balance issues such as throw and catch should be taken into consideration. Informal sensory motor integration assessments that can inform a rehabilitation route for coordination include: willingness to weight bear through upper limbs, ‘angels in the snow’ patterns, ‘spotty dog’ action and hula hooping. These general activities can act as a prelude to more focused shoulder-specific rehabilitation
Hypnosis
Hypnosis is a set of techniques designed to enhance concentration and heighten one’s responsiveness to suggestions. Thoughts, feelings, behaviour or physiological state can be modified, including the experience of pain. Physiotherapy assessment should take into account whether thought patterns, pain, relaxation and behaviour needs to be modified, and also whether hypnotherapy could be a useful adjunct to the therapy.
Studies using functional magnetic resonance imaging have shown that hypnosis to reduce pain perception is associated with reduced activity in the primary sensory cortex and increased activity in the left anterior cingulate cortex and basal ganglia, suggesting a possible inhibition pathway which blocks pain signals.41 The measurable change in brain activity associated with hypno-analgesia will reassure clinicians that this can be an effective, safe adjunct to physiotherapy, is frequently less expensive than medication, and is feasible in all patients except those who strongly oppose it.
Emotional components of pain and dysfunction
The value of hypnotherapy does however extend beyond pain relief and into other psychological and physiological parameters that are commonly experienced in our nontraumatic instability population. These include anxiety (more prevalent in JHS),42 an inability to relax generally or in specific muscles, fear of motion and beliefs/triggers associated with the condition, and loss of autonomy. Clinicians can screen for how dominant an emotional components is in the aetiology of the condition for an individual by looking at how highly they score on the following outcome measures: Fear-Avoidance Beliefs Questionnaire (FABQ),43 the Pain Catastrophizing Scale,44 Hospital, Anxiety and Depression score (HAD)45 and the personal self-efficacy score (PSE).46
Many of these emotional parameters can be significantly influenced by cognitive therapies, including explanation of pain biology,47 meditative techniques,48 pacing, and strategies to recognize and modify the physiological response to a trigger, thereby taking control of the condition.
Fundamentally, where pharmacological interventions may offer limited analgesic or psychological benefit (and their side effects often are poorly tolerated), cognitive therapies are efficacious and can be employed as an adjunct to physical and pharmacological therapy. Specialized physiotherapists are well placed to screen patients for emotional components of dysfunction, set boundaries and either incorporate this approach to their management or work directly with psychologists and pain teams to plan rehabilitation.
Psychological disorders
Studies have alluded to underlying psychological conditions being relevant in this condition.6 The concept of secondary gain is considered a likely scenario for some patients. The individual will probably not recognize or acknowledge a voluntary component to the instability and express that is it entirely outside their control. However, they have greater motivation psychologically for staying in their present state than for recovering. For example, ongoing illness means that they get sympathy from others, are exempted from some tasks, or have a ‘legitimate reason’ for not succeeding in competition, etc. Given that most individuals genuinely seek independence in function both in terms of employment and recreational activities but many have experienced failed attempts at rehabilitation and sometimes surgery, it can be very hard to identify the few individuals for whom recovery is not desirable. There is no outcome measure to identify secondary gain.
The presence of psychological illness such as depression, bipolar disorder, schizophrenia or personality disorder should probably not be interpreted as causation in this condition because there is likely to be distribution of these disorders across musculoskeletal conditions, as in the general population.
Boundary setting is a useful tool. At the initial assessment, clear rehabilitation parameters are established. The patient should be given achievable ‘homework’, be it cognitive or physical at the end of every session, with the expectation that this should be adhered to. The patient who repeatedly sabotages or fails to complete their homework should raise alarm bells. Our boundary is that within three sessions of treatment (assuming the assessment has prioritized the order of rehabilitation appropriately and identified the correct strategy), progress has been made and maintained. For example, reducing frequency of voluntary twitches, following appropriate pain modifying measures, improved trunk control, and performing a simple hand motion without associated activity in the wrong muscles. The key is that the patient is taking responsibility for self as a condition of ongoing treatment.
The persistently hypertonic muscle
Persistent hypertonicity in a muscle is often encountered in NTSI. The muscles mostly affected are the PM, LD11 and the upper fibres of trapezius. Their action will cause a relative increased translation between scapula and humerus, and associated GHJ subluxation with motion or even at rest. Transcranial Magnetic Stimulation (TMS) studies suggest that there may be a lack of inhibition associated with this disorder, which correlates with reduced function, but which reverses as the clinical and functional picture improves.34
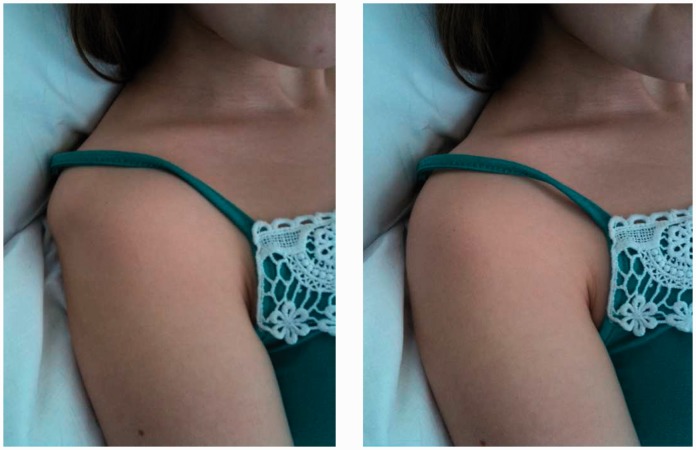
Reducing the excitation drive can be hard to achieve and the assessment process should first aim to train the patient to find their own control (i.e. a centrally achieved ‘brain training’ effect). A comfortable well-supported position of the arm (e.g. supine or crook lying) is required and then relaxation techniques should be employed. These include visualization, demonstration, contract–relax physiological techniques and medical hypnotherapy. The goal is to allow the ball to sit more or less centrally within the socket by reducing adverse tone that dissociates the two (Figure 7).
Figure 7.
A persistently hypertonic pectoralis major causing anterior subluxation (visible and palpable). Relaxation techniques focusing on pectoralis major control facilitates relocation.
Peripheral input to the muscle may be beneficial as an adjunct (e.g. TENS, acupuncture, trigger point therapy) but, ultimately, the central drive towards finding inhibition is the goal.
If the subluxation and pain improves as muscle tone reduces, the direction of treatment should be towards relearning motion patterns at the same time as maintaining the inhibition levels of the dysfunctional muscle. This can be likened to learning the piano, where control over each hand can be learned individually.
Correct motor pattern learning
The efficiency of this skill acquisition is associated with changes in the volume of neuronal activation in the brain as shown by functional imaging studies comparing novice to expert49 and the patient should be encouraged to understand the value of task specific repetition of correct technique50 and the ‘10,000’ hours51 rule for making the transfer to expert in shoulder motion in terms of focused ‘brain training’ rather than general strengthening.
Is there a role for botulinum toxin A (Botox) in the assessment and reduction of muscle tone?
There are mixed reports about the efficacy of Botox for this problem. The evidence in NTSI remains at case report level52 with some studies advocating positive effects as a presurgical management tool53 and others showing short-term benefit but little long term gain. Ultrasound can visualize the fasciculating muscle and allow direct injection into the muscle.54 In the stroke literature for persistent subluxation, Botox is associated with reduced pain and disability but hemiplegic spasticity trials suggest it may not be better over the long term than saline injection.55
The role as a diagnostic tool in reducing subluxation and pain may have greater value than its role as a therapeutic tool. Clinical experience in our shoulder unit has been that some patients find this very helpful but, for some, as the Botox wears off, the muscle continues to fire abnormally, sometimes even more strongly, despite continued rehabilitation. Perhaps the peripheral mechanism of inhibiting the muscle creates an even stronger drive at the cortical level.
Summary
Exclude anatomical or neurological deficit
Ensure cessation of voluntary instability habits including twitches and ticks
Try to facilitate a normal pattern of motion including normal tonicity. If successful, teach the patient how to achieve this, train repetitively and then strengthen into function
- If unsuccessful consider whether the instability is driven by:Pain: central sensitization (pain mechanism)Neural tissue mechanosensitivity (Neural tissue provocation tests)RC weakness: apprehension and relocation tests and strength assessmentA wider hypermobility syndrome (Beighton and Brighton scores, global symptoms and postural coordination/control)Sensory motor processing dysfunction:A: maintained by persistent cervical/shoulder/arm pain: eye control and dissociation from head motion, cervical kinaesthesia, two-point discrimination, laterality discrimination, body schema/spatial constructB: as a result of dysfunctional developmental coordination (angels in the snow)Emotional dysfunction (fear avoidance, pain catastrophizing, anxiety, beliefs, other triggers)Psychological issues (secondary gain; monitor adherence to rehabilitation and be cautious but not dismissive of multiple failed interventions)Fundamental inability to relax a hypertonic muscle (relaxation in supine, visualization, hypnotherapy, meditation, Botox, acupuncture TENS (note that this may be a result of pathology or true RC weakness; therefore, consider Examination Under Anaesthesia and ensure muscle tests conducted in supine)
Proprioception and motor control represent not only peripheral capabilities of joint and muscle tissue, but also integration of cognitive, sensory and motor representation in the brain.56 ‘Use it or lose it’ is an accurate mantra for shoulder stability. There is scope for outcome measures for shoulder instability57 to be compared with other outcome measures for pain and emotional dysfunction and inform our future practice regarding the efficacy of this framework.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Lewis A, Kitamura T, Bayley JIL. Mini symposium: shoulder instability (ii) The classification of shoulder instability: new light through old windows!. Curr Orthop 2004; 18: 97–108. [Google Scholar]
- 2.Neer II CS. Involuntary, inferior and multidirectional instability of the shoulder: etiology, recognition and treatment. J Bone Joint Surg (A) 1980; 62: 897–908. [PubMed] [Google Scholar]
- 3.Calvert P. Mini-symposium: shoulder instability classification and clinical assessment. Curr Orthopaed 1996; 10: 151–7. [Google Scholar]
- 4.Kuhn J. A new classification system for shoulder instability. Br J Sports Med 2010; 44: 341–6. [DOI] [PubMed] [Google Scholar]
- 5.McFarland EG, Kim TK, Park HB, Neira CA, Gutierrez MI. The effect of variation in definition on the diagnosis of multidirectional instability of the shoulder. J Bone Joint Surg Am 2003; 85: 2138–44. [DOI] [PubMed] [Google Scholar]
- 6.Rowe C, Pierce D, Clark J. Voluntary dislocation of the shoulder a preliminary report on a clinical, electromyographic, and psychiatric study of twenty-six patients. J Bone Joint Surg Am 1973; 55: 445–60. [PubMed] [Google Scholar]
- 7.Takwale VJ, Calvert P, Rattue H. Involuntary positional instability of the shoulder in adolescents and young adults. J Bone Joint Surg 2000; 82: 719–23. [DOI] [PubMed] [Google Scholar]
- 8.Jaggi A, Lambert S. Rehabilitation for shoulder instability. Br J Sports Med 2010; 44: 333–40. [DOI] [PubMed] [Google Scholar]
- 9.Warby SA, Pizzari T, Ford JJ, Hahne AJ, Watson L. The effect of exercise-based management for multidirectional instability of the glenohumeral joint: a systematic review. J Shoulder Elbow Surg 2014; 23: 128–42. [DOI] [PubMed] [Google Scholar]
- 10.Alexander CM. Altered control of the trapezius muscle in subjects with non-traumatic shoulder instability. Clin Neurophysiol 2007; 118: 2664–71. [DOI] [PubMed] [Google Scholar]
- 11.Jaggi A, Noorani A, Malone A, Cowan J, Lambert S, Bayley I. Muscle activation patterns in patients with recurrent shoulder instability. Int J Shoulder Surg 2012; 6: 101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronberg M, Brostrom L, Nemeth G. Differences in shoulder muscle activity between patients with generalized joint laxity and normal controls. Clin Orthopaedics and Rel Res 1991; 269: 181–92. [PubMed] [Google Scholar]
- 13.Barden JM, Balyk R, Raso VJ, Moreau M, Bagnall K. Atypical shoulder muscle activation in MDI. Clin Neurophysiol 2005; 116: 1846–57. [DOI] [PubMed] [Google Scholar]
- 14.Watson LA, Pizzari T, Balster S. Thoracic outlet syndrome part 2: conservative management of thoracic outlet. Man Ther 2010; 15: 305–14. [DOI] [PubMed] [Google Scholar]
- 15.Dark A, Ginn KA, Halaki M. Shoulder muscle recruitment patterns during commonly used rotator cuff exercises: an electromyographic study. Phys Ther 2007; 87: 1039–46. [DOI] [PubMed] [Google Scholar]
- 16.Pizzari T, Wickham J, Watson L, Zika M, Hill S. Altered muscle activation patterns in multidirectional shoulder instability during dynamic abduction. J Sci Med Sport 2009; 12: S4–4. [Google Scholar]
- 17.Johnson N, Pizzari T, Watson L, Wickham J, Balster S. Functional improvements and electromyographic (EMG) changes in multidirectional instability of the shoulder after conservative rehabilitation. J Sci Med Sport 2010; 13: e33–34. [Google Scholar]
- 18.Schöttker-Königer T, Schwaller A, Baeyens J-P, Cabri J, Taeymans J. Scapular kinematics in atraumatic shoulder instability 3-D-examination using electromagnetic sensors. Manuelletherapie 2007, pp. 168–76. [Google Scholar]
- 19.Pizzari T, Wickham J, Balster S, Ganderton C, Watson L. Modifying a shrug exercise can facilitate the upward rotator muscles of the scapula. Clin Biomech 2014; 29: 201–5. [DOI] [PubMed] [Google Scholar]
- 20.Butler D S. The sensitive nervous system. NOI Publications, Adelaide, Australia, 2000.
- 21.Coppieters M, Stappaerts K, Wouters L, Janssens K. Aberrant protective force generation during neural provocation testing and the effect of treatment in patients with neurogenic cervicobrachial pain. J Manip Physiol Ther 2003; 26: 99–106. [DOI] [PubMed] [Google Scholar]
- 22.Cameron KL, Duffey ML, DeBerardino TM, Stoneman PD, Jones CJ, Owens BD. Association of generalized joint hypermobility with a history of glenohumeral joint instability. J Athletic Training 2010; 45: 253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beighton PH, Soskolne L, Solomon CL. Articular mobility in an African population. Ann Rheum Dis 1973; 32: 413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grahame R, Bird HA, Child A, et al. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). J Rheumatol 2000; 27: 1777–9. [PubMed] [Google Scholar]
- 25.Fikree A, Aktar R, Grahame R, Aziz Q. Gastrointestinal symptoms in the joint hypermobility syndrome. Gut 2012; 61(Suppl 2): A314–A314DOI: 10.1136/gutjnl-2012-302514d.44. [Google Scholar]
- 26.Mastoroudes H, Giarenis I, Cardozo L, et al. Prolapse and sexual function in women with benign joint hypermobility syndrome. BJOG 2013; 120: 187–92. [DOI] [PubMed] [Google Scholar]
- 27.Mathias C, Low D, Iodice V, Owens A, Kirbis M, Grahame R. Postural tachycardia syndrome – current experience and concepts. Nat Rev Neurol 2012; 8: 22–34. [DOI] [PubMed] [Google Scholar]
- 28.Hakim A, Grahame R, Norris P, Hopper CJ. Local anaesthetic failure in joint hypermobility syndrome. J R Soc Med 2005; 98: 84–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keer R, Simmonds J. Joint protection and physical rehabilitation of the adult with hypermobility syndrome. Curr Opin Rheumatol 2011; 2: 131–6. [DOI] [PubMed] [Google Scholar]
- 30.Grubb BP. Postural tachycardia syndrome. Circulation 2008; 117: 2814–17. [DOI] [PubMed] [Google Scholar]
- 31.Fu Q, Van Gundy TB, Shibata S, et al. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension Aug 2011; 58: 167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngomo S, Mercier C, Bouyer L, Savoie A, Roy JS. Alterations in central motor representation increase over time in individuals with rotator cuff tendinopathy. Clin Neurophysiol. 2014 Jun 21 pii: S1388-2457(14)00316-2. doi: 10.1016/j.clinph.2014.05.035. [Epub ahead of print]. [DOI] [PubMed]
- 33.Kaas JH. Subcortical contributions to massive cortical reorganisations. Neuron 1999; 22: 657–60. [DOI] [PubMed] [Google Scholar]
- 34.Barrett C, Emery R, Wallace A, Davey N. Altered corticospinal control of shoulder musculature in shoulder instability – a case study. Third Proceedings of the International Shoulder Group, 2000, Newcastle Upon Tyne. Publ by Delft University Press Science 2001. The Netherlands.
- 35.Lorimer Moseley G, Flor H. Targeting cortical representations in the treatment of chronic pain: a review. Neurorehabil Neural Repair 2012; 26: 646–52. [DOI] [PubMed] [Google Scholar]
- 36.Treleaven J, Jull G, Grip H. Head eye co-ordination and gaze stability in subjects with persistent whiplash associated disorders. Man Ther 2011; 16: 252–7. [DOI] [PubMed] [Google Scholar]
- 37.Jull G, Sterling M, Falla D, Treleaven J, O’Leary S. Whiplash, headache and neck pain. Research-based directions for physical therapies. Churchill Livingstone, Elsevier, 2008.
- 38.Barrett C, Morgans S. Expanding clinical assessment in persistent, painful shoulder muscle patterning dysfunction. Extrapolation from the whiplash models and pain literature. Poster presentation at International Federation Orthopaedic Manipulative Physical Therapists, Quebec City, Canada 1–5 October 2012.
- 39.Tong J, Mao O, Goldreich D. Two-point orientation discrimination versus the traditional two-point test for tactile spatial acuity assessment. Front Hum Neurosci 2013; 7: 579–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freynhagen R, Baron R, Gockel U, Tölle T. PainDETECT: a new screening questionnaire to identify neuropathic components. Curr Med Res Opin 2006; 22: 1911–1911. [DOI] [PubMed] [Google Scholar]
- 41.Schulz-Stübner S, Krings T, Meister IG, Rex S, Thron A, Rossaint R. Clinical hypnosis modulates functional magnetic resonance imaging signal intensities and pain perception in a thermal stimulation paradigm. Reg Anesth Pain Med 2004; 29: 549–56. [DOI] [PubMed] [Google Scholar]
- 42.Bulbena A, Gago J, Martín-Santos R, et al. Anxiety disorder and Joint laxity: a definitive link. Neural Psychiatry Brain Res 2004; 11: 137–40. [Google Scholar]
- 43.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993; 52: 157–68. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan M, Bishop S, Pivic J. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995; 7: 524–32. [Google Scholar]
- 45.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–70. [DOI] [PubMed] [Google Scholar]
- 46.Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 2007; 11: 153–63. [DOI] [PubMed] [Google Scholar]
- 47.Louw A, Diener I, Butler D, Puentedura E. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch Phys Med Rehabil 2011; 92: 2041–56. [DOI] [PubMed] [Google Scholar]
- 48.Surawy C, McManus F, Muse K, Williams M. Mindfulness-based cognitive therapy (MBCT) for health anxiety (hypochondriasis): rationale, implementation and case illustration mindfulness. This article is published with open access at Springerlink.com 2014. Oxford Mindfulness Centre Publications. DOI: 10.1007/s12671-013-0271-1. [DOI] [PMC free article] [PubMed]
- 49.Leff DR, Orihuela-Espina F, Atallah L, et al. Functional pre-frontal reorganization accompanies learning-associated refinements in surgery: a manifold embedding approach. Comput AidedSurg 2008; 13: 325–39. [DOI] [PubMed] [Google Scholar]
- 50.Ericsson A, Krampe R, Tesch-Romer C. The role of deliberate practice in the acquisition of expert performance. Psychol Rev 1993; 100: 363–406. [Google Scholar]
- 51.Gladwell M. Outliers. The story of success. Little, Brown and Company 2008.
- 52.Sinha A, Higginson DW, Vickers A. Use of botulinum A toxin in irreducible shoulder dislocation caused by spasm of pectoralis major. J Shoulder Elbow Surg 1999; 8: 75–6. [DOI] [PubMed] [Google Scholar]
- 53.Donnellan C, Scott M, Antoun M, Wallace WA. Physiotherapy and botulinum toxin injections prior to stabilization surgery for recurrent atraumatic anteroinferior shoulder dislocation with abnormal muscle patterning. Shoulder Elbow 2012; 4: 287–90. [Google Scholar]
- 54.Khanna M, Walker A, Morgans S, Barrett C, Emery R. Ultrasound-guided injection of botulinum toxin in the treatment of shoulder muscle patterning instability: experience in 3 cases and its advantages over nonimage-guided techniques. Abstract Arch RSNA 2011, pp. SSA13–07. [Google Scholar]
- 55.Marciniak CM, Harvey RL, Gagnon CM, et al. Does botulinum toxin type A decrease pain and lessen disability in hemiplegic survivors of stroke with shoulder pain and spasticity?: a randomized, double-blind, placebo-controlled trial. Am J Phys Med Rehabil 2012; 91: 1007–19. [DOI] [PubMed] [Google Scholar]
- 56.Van Vliet P, Heneghan N. Motor control and the management of musculoskeletal dysfunction. Manl Ther 2006; 11: 208–13. [DOI] [PubMed] [Google Scholar]
- 57.Rouleau D, Faber K, MacDermid J. Systematic review of patient-administered shoulder functional scores on instability. J Shoulder Elbow Surg 2010; 19: 1121–28. [DOI] [PubMed] [Google Scholar]