Abstract
Background
Recent studies show that epithelial–mesenchymal transition (EMT) and tumor-associated macrophages (TAMs) contribute to the progression and poor prognosis of carcinoma through multiple mechanisms. Both inflammation and changing of epithelium have a close relationship with tumorigenesis of gastric cancer. However, the relevance between EMT and TAMs is still unclear in gastric cancer and needs more scientific research. This study is designed to explore the relationship between EMT and TAMs in gastric cancer.
Materials and methods
Immunohistochemistry was used to detect the expression of EMT-related proteins and TAM markers in cancer tissues and normal gastric tissues.
Results
High levels of EMT and TAMs infiltration are related to aggressive features and independent prognostic factors in gastric cancer, respectively. In addition, expression of the two indicators is associated with expression of transforming growth factor-β1 (TGF-β1). Infiltration of TAMs is also associated with EMT-related marker in gastric cancer.
Conclusion
Our results suggest that high levels of EMT and TAMs infiltration are related to aggressive features and independent prognostic factors in gastric cancer, respectively. A correlation was found between EMT- and TAM-related indicators, which may be associated with TGF-β signaling pathway. The level of TAMs infiltration plays an important role in gastric cancer, the markers of which can be used as prognostic indicators.
Keywords: gastric cancer, prognosis, tumor-associated macrophages, E-cadherin, TGF-β1
Introduction
There are approximately one million patients diagnosed with gastric cancer every year worldwide.1 Although the incidence rate of gastric cancer is continually decreasing in recent years, it is still the second leading cause of cancer-related deaths.1,2 So, it is necessary for researchers to figure out new targets and therapies for gastric cancer. Since this is a typical inflammation-associated epithelial cancer,3 cancer-related inflammation and the change of normal epithelium are two crucial points that need to be focused on. Both invasiveness and metastasis are the characteristic features of malignant cancer and are the main cause of cancer-related deaths. Recent researches suggest that tumorigenesis has a close relationship with cancer-related inflammation and epithelial–mesenchymal transition (EMT), both of which contribute to invasiveness and metastasis of various cancers, especially in gastric cancer.4–7 Macrophages appear in almost 50% of the solid tumors, and tumor-associated macrophages (TAMs), no doubt, act as one of the vital components of cancer-related inflammation in gastric cancer.8
The process of epithelial cells transforming into mesenchymal cells is defined as EMT, and the reverse process is known as mesenchymal–epithelial transition.9 Both of them, especially the progress of EMT, hold an important position in embryonic development, fibrosis, pathogenesis of cancer, and progression of other diseases.10–12 EMT has been proved to be one of the crucial processes of tumorigenesis in various cancers, and high expression of EMT-related proteins indicates a poor prognosis in cancer patients.13–15 It has been confirmed that in the process of EMT, deconstruction of cell polarity, reorganization of cytoskeleton, and changes of the signaling programs improve motility and invasiveness of cancer cells through multiple mechanisms.16,17 Extracellular cues stimulate the correlative signaling pathways (including Hedgehog, integrin, transforming growth factor-β [TGF-β], Wnt, platelet-derived growth factor, Notch, Akt, PI3K, NF-κB, Ras, and so on), which may reprogram the gene expression in EMT.18–25 Then, the transcription factors that mediate the whole switch of EMT are finely adjusted at different levels.26 Though the combined action of all the related signaling pathways is necessary, TGF-β family signaling pathway plays a primary role in EMT.26,27
TAM is one of the dominating inflammatory components of immune cell infiltration in cancer stroma, which is discovered in tumor microenvironment of many cancers, including gastric cancer.28 Furthermore, a high-level infiltration of TAMs is always related to poor prognosis of cancer.29,30 Considerable evidences indicate that cancer cell is implicated in many critical respects of tumor progression by changing the phenotype of TAMs and remodeling TAM activities.31,32 Cytotoxic activity of TAMs is enhanced or suppressed by molecular products derived from tumor. TAMs facilitate growth, migration, and metastasis of tumor cells under the influence of these released products, including TGF-β1.33–35 In addition, previous studies have shown that TGF-β1 can promote cancer invasiveness and metastasis through effects on the tumor microenvironment, ie, by immune suppression, angiogenesis, and so on.36,37 However, the relationship between TGF-β signaling pathways and TAMs in the tumor is not clear.38
TGF-β family is a huge cloud of extracellular growth factors including TGF-βs, activins, bone morphogenetic proteins, and so on.39 TGF-β1 is the key cytokine with dual and inverse functions in cancer biology that regulates growth, migration, angiogenesis, and immune response to cancer.40 It has an inhibiting effect in the early stage of oncogenesis, while in the later stage acts as a tumor promoter.41 Accumulating evidences have shown that high expression of TGF-β1 is associated with a poor prognosis in patients with cancers, including gastric cancer.42–44 Furthermore, TGF-β1 expressed during immune response contributes to the transformation of TAMs and tumor microenvironment.45,46 Moreover, TGF-β signaling pathway has been reported to be a dominating signaling pathway of EMT, and TGF-β1 can activate some EMT-related pathways, such as IL-6/STAT3 and PI-3/Akt.26 As TGF-β1 plays an important role both in EMT and TAM, it may have a close relationship with tumorigenesis of gastric cancer. In this study, we demonstrate a connection between EMT and TAM related to TGF-β1. High expression of TGF-β1 induced by TAM in tumor microenvironment may facilitate the progression of EMT, which is associated with the poor prognosis of gastric carcinoma.
Materials and methods
Clinical and tissue samples
A total of 178 gastric cancer tumor samples were collected from the First Affiliated Hospital, Medical School, Xi’an Jiaotong University and the 215th Hospital in Shaanxi Province from 2004 to 2009. The patients included 125 males and 53 females, with age ranging from 25 to 81 years. All the gastric patients were diagnosed with gastric adenocarcinoma, with a complete clinical history and detailed follow-up information. Clinicopathological parameters of all the patients were assessed in detail according to the tumor node metastasis (TNM) staging mentioned in the AJCC Cancer Staging Manual.47 The clinicopathological data of 178 patients are documented in Table 1. None of the patients accepted chemotherapy or radiation therapy before surgery.
Table 1.
Expression of CD163 and TGF-β1 in gastric cancer and normal tissues
| Group | CD163
|
TGF-β1
|
||||
|---|---|---|---|---|---|---|
| High | Low | P-value | High | Low | P-value | |
| Gastric cancer tissue | 84 | 94 | <0.001 | 111 | 67 | <0.001 |
| Normal tissue | 38 | 140 | 37 | 141 | ||
Abbreviation: TGF-β1, transforming growth factor-β1.
Immunohistochemistry and scoring
Formalin-fixed and paraffin-embedded tissue specimens (4 μm in thickness) of cancer and pericarcinous tissues from each patient were used. Tissue sections were placed on charged glass slides, and then were subjected to deparaffinization and rehydration. Then, antigen retrieval was performed using citrate buffer at pH 6.0. Antibodies against E-cadherin (ZA-0565-3), CD163 (ZM-0428), and TGF-β1 (bs-0086R), purchased from Beijing Zhongshan Biotechnology (Beijing, People’s Republic of China), were used for blocking. Streptavidin–peroxidase technique was used for the purpose of staining. The sections were stained with 0.02% diaminobenzidine solution followed by counterstaining with hematoxylin for 2 minutes. Irrelevant rabbit antiserum was used as a first antibody for the duplicated section as a negative control.
The indicator expression was evaluated, respectively, by two pathologists who were blinded to the clinical data. The staining results of three indicators were scored semi-quantitatively on the basis of immunostaining intensity and percentage of positive tumor cells. Sections were observed with a light microscope at high power (200×) in at least five areas. The mean staining results of E-cadherin and TGF-β1 were scored and placed into five categories: 0 point for <5%, 1 point for 5%–25%, 2 points for 26%–50%, 3 points for 51%–75%, and 4 points for >75%. The staining intensity was scored as 0, 1, 2, and 3, corresponding to no coloring, slightly yellow, brown yellow, and tan, respectively. Sum of the two aforementioned scores was calculated as the final staining score which was defined as follows: 0–5, low expression and ≥6, high expression. However, for the positivity of TAM markers (CD163), staining density was estimated by the mean count of the stained cells in five areas. If the average number in each case was less than the mean, the section was allocated to the group of low expression; otherwise, it was allocated to the group of high expression.
Ethics statement
The Protection of Human Subjects Committee of The First Affiliated Hospital, Medical School, Xi’an Jiaotong University, approved this study which complies with the Declaration of Helsinki. Informed consent was obtained from the patients.
Statistical analysis
Associations between clinicopathologic variables and expressions of CD163, TGF-β1, and E-cadherin were examined by the chi-square test. The Spearman’s rank correlation coefficient was used to confirm the association among E-cadherin, CD163, and TGF-β1. Kaplan–Meier test was used to calculate survival curves, which were compared using the log-rank test. Factors showing prognostic significance were evaluated with the multivariate Cox regression model. The normally distributed variables were checked by Student’s t-test or one-way analysis of variance. P-values <0.05 and 0.01were considered to be statistically significant. Data were analyzed by SPSS software package (Version 16.0; SPSS Inc., Chicago, IL, USA).
Results
Differential expression of CD163 and TGF-β1 markers in cancer tissues and normal mucosae and their association with clinicopathologic features in gastric cancer
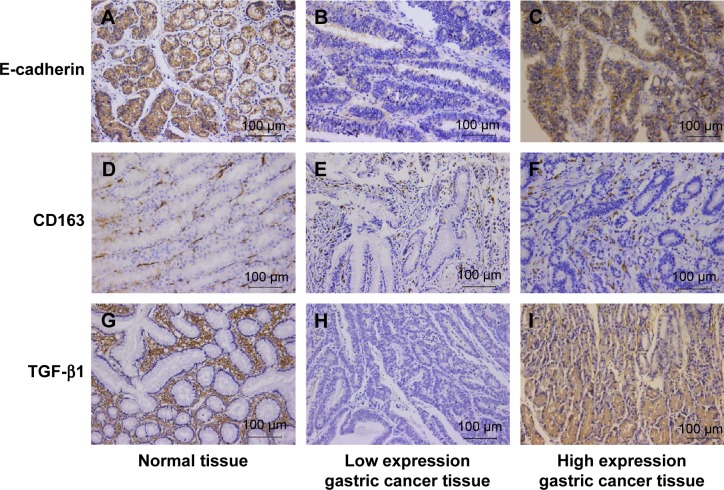
Expression of CD163, TGF-β1, and E-cadherin was significantly different in gastric cancer tissues and normal gastric tissues (Table 1). The clinicopathologic features of gastric cancer patients and their correlation with the expression of CD163, TGF-β1, and E-cadherin are described in Table 2. High and low expressions of CD163, TGF-β1, and E-cadherin, according to typical immunohistochemical staining patterns, in gastric cancer and paracancer normal tissues are shown in Figure 1. High expression of TGF-β1 was observed in the cytoplasm of tumor cells in 111 cases (62.4%) and in the cytoplasm of normal cells in 37 cases (20.8%), which shows a statistical significance (P<0.001). TGF-β1 expression was found to be higher in low- and advanced-grade tumors, and statistical analysis revealed that high expression of TGF-β1 was related to tumor size, differentiation, Borrmann type, TNM stage, nodal involvement, and metastasis (P<0.05). High expression of CD163 was detected in 94/178 (52.8%) cases of gastric cancers and in 38 (21.3%) cases with normal tissues. The data show statistical significance (P<0.05). Significant associations were also seen with respect to tumor size, differentiation, Borrmann type, TNM stage, nodal involvement, and metastasis.
Table 2.
Correlations of TGF-β1 and CD163 expressions with clinicopathologic characteristics of gastric cancer patients
| Variable | n | TGF-β1
|
CD163
|
||||
|---|---|---|---|---|---|---|---|
| High | Low | P-value | High | Low | P-value | ||
| Sex | 0.738 | 0.623 | |||||
| Male | 125 | 79 | 46 | 68 | 57 | ||
| Female | 53 | 32 | 21 | 26 | 27 | ||
| Age (years) | 0.536 | 0.548 | |||||
| ≤60 | 94 | 61 | 33 | 42 | 42 | ||
| >60 | 84 | 50 | 34 | 52 | 42 | ||
| Tumor size (cm) | 0.015 | 0.020 | |||||
| <3 | 44 | 22 | 22 | 17 | 27 | ||
| 3–5 | 54 | 30 | 24 | 26 | 28 | ||
| >5 | 80 | 59 | 21 | 51 | 29 | ||
| Borrmann | 0.032 | 0.029 | |||||
| I | 22 | 11 | 11 | 10 | 12 | ||
| II | 55 | 32 | 23 | 21 | 34 | ||
| III | 62 | 36 | 26 | 38 | 24 | ||
| IV | 39 | 32 | 7 | 25 | 14 | ||
| Differentiation | <0.001 | <0.001 | |||||
| High | 12 | 3 | 9 | 3 | 9 | ||
| Moderate | 57 | 24 | 33 | 20 | 37 | ||
| Poor | 109 | 84 | 25 | 71 | 38 | ||
| Depth of invasion | 0.033 | 0.002 | |||||
| T1 | 16 | 5 | 11 | 5 | 11 | ||
| T2 | 18 | 10 | 8 | 3 | 15 | ||
| T3 | 67 | 47 | 20 | 39 | 28 | ||
| T4 | 77 | 49 | 28 | 47 | 30 | ||
| Lymph node metastasis | <0.001 | <0.001 | |||||
| N0 | 49 | 18 | 31 | 13 | 36 | ||
| N1 | 36 | 17 | 19 | 13 | 23 | ||
| N2 | 35 | 26 | 9 | 11 | 13 | ||
| N3 | 58 | 50 | 8 | 46 | 12 | ||
| Distant metastasis | 0.003 | 0.019 | |||||
| M0 | 138 | 78 | 60 | 66 | 72 | ||
| M1 | 40 | 33 | 7 | 28 | 12 | ||
| TNM stage | 0.002 | <0.001 | |||||
| I | 21 | 8 | 13 | 5 | 16 | ||
| II | 34 | 17 | 17 | 10 | 24 | ||
| III | 83 | 53 | 30 | 51 | 32 | ||
| IV | 40 | 33 | 7 | 28 | 12 | ||
Abbreviations: TGF, transforming growth factor; TNM, tumor node metastasis.
Figure 1.
Immunohistochemical results of E-cadherin, TGF-β1, and CD163 in the gastric cancer and paracancer tissue.
Notes: (A) Typical high expression of E-cadherin in paracancer tissue of gastric cancer. Staining was localized predominantly in the cytomembrane. (B) Typical low expression of E-cadherin in gastric cancer tissue. (C) Infrequent high expression of E-cadherin in gastric cancer tissue. (D) Low expression of CD163 in normal tissue. Staining was localized predominantly in the cytosol. (E) Low expression of CD163 in gastric cancer tissue. (F) High expression of CD163 in gastric cancer tissue. (G) Low expression of TGF-β1 in paracancer tissue. Staining was localized predominantly in the cytosol. (H) Low expression of TGF-β1 in gastric cancer. (I) High expression of TGF-β1 in gastric cancer. Magnification, 200×.
Abbreviation: TGF-β1, transforming growth factor-β1.
Association among expression of EMT, TGF-β1 markers, and TAMs infiltration
Significant associations among the expression of CD163, TGF-β1, and E-cadherin are described in Tables 3 and 4. High expression of CD163 was related to low expression of E-cadherin (r=−0.421, P<0.001) and high expression of TGF-β1 (r=−0.427, P<0.001) in gastric cancer tissues. High expression of TGF-β1 was correlated with a loss of E-cadherin expression (r=−0.452, P<0.001) in the same samples. In order to have a better illustration, the relationships between expression of TGF-β1 and E-cadherin were calculated by different CD163 infiltration group in gastric cancer tissues, and we found almost exactly the same result (Table 5).
Table 3.
Association between CD163 infiltration with expression of E-cadherin and TGF-β1 in gastric cancer tissues
| Group | CD163
|
r | P-value | |
|---|---|---|---|---|
| High | Low | |||
| E-cadherin | −0.421 | <0.001 | ||
| High | 14 | 46 | ||
| Low | 80 | 38 | ||
| TGF-β1 | 0.427 | <0.001 | ||
| High | 77 | 34 | ||
| Low | 17 | 50 | ||
Abbreviation: TGF-β1, transforming growth factor-β1.
Table 4.
Expression correlation of TGF-β1 with E-cadherin in gastric cancer tissues
| Group | TGF-β1
|
r | P-value | |
|---|---|---|---|---|
| High | Low | |||
| E-cadherin | −0.452 | <0.001 | ||
| High | 19 | 41 | ||
| Low | 92 | 26 | ||
Abbreviation: TGF-β1, transforming growth factor-β1.
Table 5.
Association between expression of E-cadherin and TGF-β1 of differential CD163 infiltration in gastric cancer tissues
| Group | TGF-β1 | CD136 high
|
CD136 low
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| High | Low | r | P-value | High | Low | r | P-value | ||
| E-cadherin | −0.385 | <0.001 | −0.440 | <0.001 | |||||
| High | 12 | 13 | 8 | 28 | |||||
| Low | 59 | 10 | 32 | 16 | |||||
Abbreviation: TGF-β1, transforming growth factor-β1.
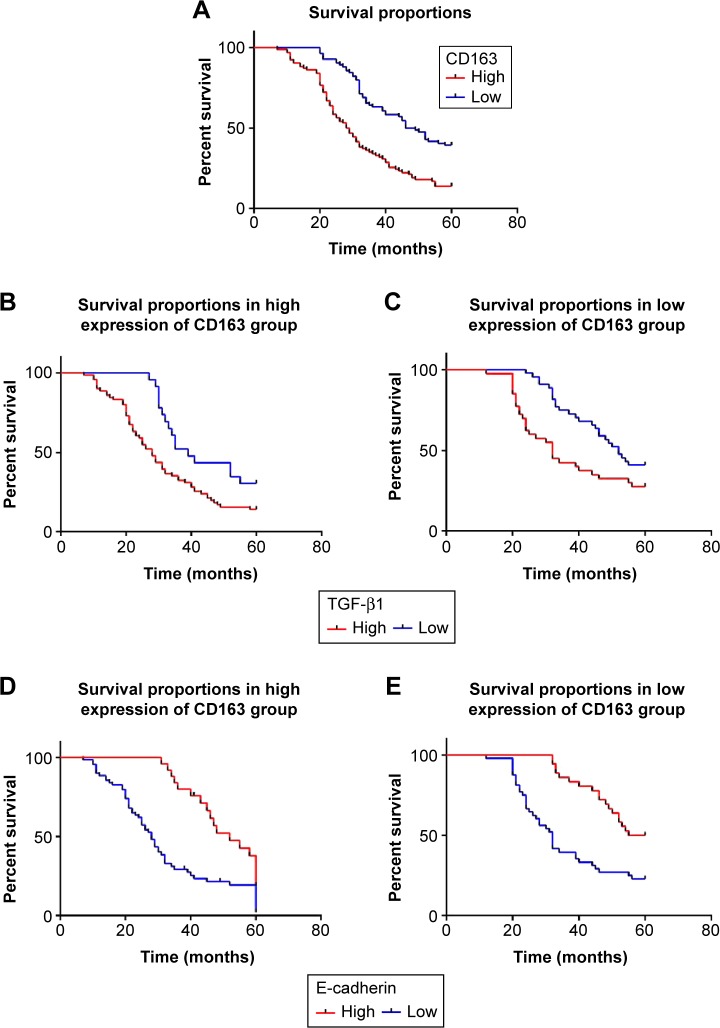
Association of TGF-β1 and CD163 expression with survival times of gastric cancer patients
Survival curves were calculated by the Kaplan–Meier method and log-rank test. Patients with high expression of CD163 have an unfavorable overall survival (28.00±2.20 months, P<0.001) compared to those with low CD163 expression (46.00±3.05 months, P<0.001) (Table 6; Figure 2A). The same is true for the expression of TGF-β1 in the groups exhibiting differential expression of CD163 (P<0.001) (Table 6; Figure 2B and C). Instead, the low expression of E-cadherin has a statistically significant correlation with poor overall survival (P<0.001) (Figure 2D and E) and with poor prognosis (P<0.001) in CD163 differential expression groups. Multivariate Cox proportional hazard analysis of overall survival reveals that low expression of E-cadherin and high expression of CD163 are independent prognostic factors in patients with gastric cancer (P<0.05) (Table 7).
Table 6.
Univariate analysis of overall survival
| Variable | N | Overall survival
|
||
|---|---|---|---|---|
| Median ± SE | 95% CI | P-value | ||
| Sex | 0.973 | |||
| Male | 125 | 34.00±2.97 | 28.16–39.83 | |
| Female | 53 | 38.00±3.63 | 30.86–45.13 | |
| Age (years) | 0.223 | |||
| ≤60 | 94 | 40.00±3.87 | 32.40–47.59 | |
| >60 | 84 | 32.00±1.13 | 29.77–34.22 | |
| Size (cm) | 0.019 | |||
| <3 | 44 | 46.00±5.20 | 35.79–56.20 | |
| 3–5 | 54 | 34.00±1.83 | 30.40–37.51 | |
| >5 | 80 | 32.00±0.98 | 30.06–33.93 | |
| Borrmann | 0.502 | |||
| I | 22 | 39.00±7.62 | 24.06–53.93 | |
| II | 55 | 40.00±3.69 | 32.76–47.23 | |
| III | 62 | 34.00±3.50 | 27.14–40.85 | |
| IV | 39 | 31.00±1.77 | 27.51–34.48 | |
| Differentiation | 0.107 | |||
| High | 12 | 40.00±6.06 | 28.11–51.88 | |
| Moderate | 57 | 39.00±6.11 | 26.97–51.02 | |
| Poor | 109 | 32.00±1.74 | 28.59–35.41 | |
| Depth of invasion | 0.005 | |||
| T1 | 16 | 46.00±8.66 | 29.01–62.98 | |
| T2 | 18 | 52.00 ±15.91 | 20.81–83.18 | |
| T3 | 67 | 35.00±4.60 | 25.98–44.01 | |
| T4 | 77 | 31.00±1.45 | 28.14–33.86 | |
| Lymph node metastasis | <0.001 | |||
| N0 | 49 | 55.00±9.79 | 35.79–74.20 | |
| N1 | 36 | 43.00±7.80 | 27.22–58.78 | |
| N2 | 35 | 36.00±3.54 | 29.04–42.95 | |
| N3 | 58 | 29.00±2.53 | 24.03–33.96 | |
| Distant metastasis | <0.001 | |||
| M0 | 138 | 40.00±3.91 | 32.32–47.67 | |
| M1 | 40 | 29.00±1.57 | 25.91–32.08 | |
| TNM stage | <0.001 | |||
| I | 21 | 49.00±3.21 | 42.69–66.71 | |
| II | 34 | 45.00±5.83 | 33.57–56.42 | |
| III | 83 | 34.00±2.27 | 29.53–38.41 | |
| IV | 40 | 29.00±1.57 | 25.91–32.08 | |
| E-cadherin | <0.001 | |||
| High | 60 | 54.00±2.08 | 48.52–59.48 | |
| Low | 118 | 29.00±1.45 | 26.15–31.85 | |
| TGF-β1 | <0.001 | |||
| High | 111 | 31.00±1.31 | 28.41–33.58 | |
| Low | 67 | 50.00±5.26 | 39.68–60.31 | |
| CD163 | <0.001 | |||
| High | 94 | 28.00±2.20 | 23.68–32.31 | |
| Low | 84 | 46.00±3.05 | 40.01–51.98 | |
Abbreviations: SE, standard error; CI, confidence interval; TNM, tumor node metastasis; TGF-β1, transforming growth factor-β1.
Figure 2.
Kaplan–Meier curves for differential expression of E-cadherin, TGF-β1, and CD163 in gastric cancer.
Notes: Differential expression of three indicators showed a significant difference in cumulative overall survival. (A) Patients with high expression of CD163 showed a poor overall survival (P<0.001). (B, C) Patients with high expression of TGF-β1 had a poor overall survival in high/low expression of CD163 group (P<0.001). (D, E) Patients with low expression of E-cadherin had a poor overall survival in high/low expression of CD163 group (P<0.001).
Abbreviation: TGF-β1, transforming growth factor-β1.
Table 7.
Multivariate Cox proportional hazard analysis of overall survival
| Variable | Overall survival
|
||
|---|---|---|---|
| HR | 95% CI | P-value | |
| Lymph node metastasis | 1.238 | 1.050–1.460 | 0.011 |
| E-cadherin | 2.559 | 1.711–3.829 | <0.001 |
| TGF-β1 | 0.651 | 0.430–0.985 | 0.042 |
| CD163 | 0.597 | 0.406–0.878 | 0.009 |
Abbreviations: HR, hazards ratio; CI, confidence interval; TGF-β1, transforming growth factor-β1.
Discussion
Being the second leading cause of cancer-related deaths, gastric cancer is still one of the major public health problems worldwide.1,48 Because tumorigenesis of gastric cancer has a close relationship with inflammation and changing of epithelium, it is necessary to figure out whether there is a correlation between these two aspects in gastric cancer. Recent studies showed that both EMT and TAMs contribute to progress of gastric cancer, which is related to epithelial change and inflammation, respectively.20,49 However, the relevance between EMT and TAMs in gastric cancer still needs more attention. This study was designed to explore the relationship between EMT and TAMs in gastric cancer.
The present study showed a high expression of TGF-β1 (62.36%) and CD163 (52.8%), but a low expression of E-cadherin (33.71%) in gastric cancer tissues. We demonstrated a significant relationship between TAMs and EMT based on the expression of typical indicators CD163 and E-cadherin. Furthermore, both high expression of CD163 and low expression of E-cadherin were correlated to high expression of TGF-β1 in gastric cancer. High expression of TGF-β1 and CD163 was related to tumor size, differentiation, Borrmann type, TNM stage, nodal involvement, and distant metastasis of gastric cancer. Significant association of low expression of E-cadherin was also seen with tumor size, differentiation, Borrmann type, TNM stage, nodal involvement, distant metastasis, and poor prognosis. High expression of both TGF-β1 and CD163 indicates a poor prognosis. Our findings manifest a connection between TAMs and EMT in the tissues of gastric cancer, both of which are associated with TGF-β1.
The mechanism by which EMT and TAMs interact with each other in gastric cancer tissues has not been clear yet. Accumulating evidence shows, in lung cancer and hepatocellular carcinoma, TGF-β1 is a major inducer of EMT, which can activate other EMT-related signaling pathways.50,51 So, TGF-β1 originated from TAMs and tumor-related microenvironment, and TGF-β signaling pathway may be one of the key ways by which TAMs may cause an effect on the progress of EMT in gastric cancer. It has been reported that TAMs promote progress of EMT through activation of Gas6/Axl-NF-κB17 in oral cancer.52 Moreover, a recent study demonstrated that CCL18 secreted by TAMs downregulates miR98 and miR27b to promote EMT in breast cancer.53 More intensive studies are needed to figure out the mechanism by which EMT and TAMs interact with each other.
In general, our study demonstrated a relationship between EMT and TAMs, associated with TGF-β1. High infiltration of TAMs and expression of TGF-β1 are related to aggressive features and poor prognosis of gastric cancer, respectively. The expression levels of E-cadherin, TGF-β1, and CD163 may serve as prognostic factors for gastric cancer.
Acknowledgments
This work was supported by the Scientific and Technological Planning Project of Shaanxi Province (No 2014KW23-02).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clini. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, Chatenoud L, Levi F, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125(3):666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 3.Chung HW, Jang S, Kim H, Lim JB. Combined targeting of high-mobility group box-1 and interleukin-8 to control micrometastasis potential in gastric cancer. Int J Cancer. 2015;137(7):1598–1609. doi: 10.1002/ijc.29539. [DOI] [PubMed] [Google Scholar]
- 4.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TL, Dipaolo RJ. A new mouse model of inflammation and gastric cancer. Oncoimmunology. 2013;2(10):25911. doi: 10.4161/onci.25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qadri Q, Rasool R, Gulzar GM, Naqash S, Shah ZA. H. pylori infection, inflammation and gastric cancer. J Gastrointest Cancer. 2014;45(2):126–132. doi: 10.1007/s12029-014-9583-1. [DOI] [PubMed] [Google Scholar]
- 7.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 9.Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19(3):294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Hugo H, Ackland ML, Blick T, et al. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 11.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7(2):131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Weinberg RA. Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 14.Lyons JG, Lobo E, Martorana AM, Myerscough MR. Clonal diversity in carcinomas: its implications for tumour progression and the contribution made to it by epithelial-mesenchymal transitions. Clin Exp Metastasis. 2008;25(6):665–677. doi: 10.1007/s10585-007-9134-2. [DOI] [PubMed] [Google Scholar]
- 15.Takai M, Terai Y, Kawaguchi H, et al. The EMT (epithelial-mesenchymal-transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res. 2014;7:76. doi: 10.1186/1757-2215-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang Y, Massagué J. Epithelial–mesenchymal transitions: twist in development and metastasis. Cell. 2004;118(3):277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Son H, Moon A. Epithelial–mesenchymal transition and cell invasion. Toxicol Res. 2010;26(4):245–252. doi: 10.5487/TR.2010.26.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 19.Yanaka Y, Muramatsu T, Uetake H, Kozaki KI, Inazawa J. miR-544a induces epithelial-mesenchymal transition through the activation of WNT signaling pathway in gastric cancer. Carcinogenesis. 2015;36(11):1363–1371. doi: 10.1093/carcin/bgv106. [DOI] [PubMed] [Google Scholar]
- 20.Gao Q, Yuan Y, Gan HZ, Peng Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol Lett. 2015;9(5):2381–2387. doi: 10.3892/ol.2015.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Ye S, Xiao W, Wang W, Luo L, Liu Y. ERK1/2 pathway mediates epithelial-mesenchymal transition by cross-interacting with TGFbeta/Smad and Jagged/Notch signaling pathways in lens epithelial cells. Int J Mol Med. 2014;33(6):1664–1670. doi: 10.3892/ijmm.2014.1723. [DOI] [PubMed] [Google Scholar]
- 22.Jin C, Liang R. miR-205 promotes epithelial-mesenchymal transition by targeting AKT signaling in endometrial cancer cells. J Obstet Gynaecol Res. 2015;41(10):1653–1660. doi: 10.1111/jog.12756. [DOI] [PubMed] [Google Scholar]
- 23.Xu W, Yang Z, Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9(4):317–324. doi: 10.1080/19336918.2015.1016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Mallen-St CJ, Luo J, Sharma S, Dubinett S, John MS. p53 modulates NF-kappaB mediated epithelial-to-mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2015;51(10):921–928. doi: 10.1016/j.oraloncology.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Lei Y, Gao X, et al. p53 attenuates the oncogenic Ras-induced epithelial-mesenchymal transition in human mammary epithelial cells. Biochem Biophys Res Commun. 2013;434(3):606–613. doi: 10.1016/j.bbrc.2013.03.124. [DOI] [PubMed] [Google Scholar]
- 26.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196(3):254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metast Rev. 2006;25(3):315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 30.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 31.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 32.Li MO, Wan YY, Sanjabi S, Robertson AL, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 34.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56(20):4625–4629. [PubMed] [Google Scholar]
- 35.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Massague J. TGFbeta in cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13(18 Pt 1):5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 38.Takamiya R, Ohtsubo K, Takamatsu S, Taniguchi N, Angata T. The interaction between Siglec-15 and tumor-associated sialyl-Tn antigen enhances TGF-beta secretion from monocytes/macrophages through the DAP12-Syk pathway. Glycobiology. 2013;23(2):178–187. doi: 10.1093/glycob/cws139. [DOI] [PubMed] [Google Scholar]
- 39.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23(6):787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 40.Standiford TJ, Kuick R, Bhan U, Chen J, Newstead M, Keshamouni VG. TGF-beta-induced IRAK-M expression in tumor-associated macrophages regulates lung tumor growth. Oncogene. 2011;30(21):2475–2484. doi: 10.1038/onc.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang KS, Hu ZL, Li JH, Xiao DS, Wen JF. Enhancement of metastatic and invasive capacity of gastric cancer cells by transforming growth factor-beta 1. Acta Biochim Biophys Sin (Shanghai) 2006;38(3):179–186. doi: 10.1111/j.1745-7270.2006.00151.x. [DOI] [PubMed] [Google Scholar]
- 42.Kong FM, Jirtle RL, Huang DH, Clough RW, Anscher MS. Plasma transforming growth factor-beta 1 level before radiotherapy correlates with long term outcome of patients with lung carcinoma. Cancer. 1999;86(9):1712–1719. [PubMed] [Google Scholar]
- 43.Wu CT, Chang YH, Lin WY, Chen WC, Chen MF. TGF beta1 expression correlates with survival and tumor aggressiveness of prostate cancer. Ann Surg Oncol. 2015;22(Suppl 3):s1587–s1593. doi: 10.1245/s10434-015-4804-9. [DOI] [PubMed] [Google Scholar]
- 44.Ma Y, Liu H, Zhang H, Shao RG. The TGF-beta signaling pathway induced EMT in breast cancer. Yao Xue Xue Bao. 2015;50(4):385–392. [PubMed] [Google Scholar]
- 45.Shen Z, Seppanen H, Kauttu T, et al. Vasohibin-1 expression is regulated by transforming growth factor-beta/bone morphogenic protein signaling pathway between tumor-associated macrophages and pancreatic cancer cells. J Interferon Cytokine Res. 2013;33(8):428–433. doi: 10.1089/jir.2012.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russell D. Leek ALH. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol. 2002;7(2):117–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 47.Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A. AJCC Cancer Staging Handbook. New York: Springer; 2010. [Google Scholar]
- 48.Shi D, Wang Y, Xing A, et al. C/EBPα-induced miR-100 expression suppresses tumor metastasis and growth by targeting ZBTB7A in gastric cancer. Cancer Lett. 2015;369(2):376–385. doi: 10.1016/j.canlet.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 49.Wu H, Xu JB, He YL, et al. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol. 2012;106(4):462–468. doi: 10.1002/jso.23110. [DOI] [PubMed] [Google Scholar]
- 50.Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan QM, Jing YY, Yu GF, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352(2):160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 52.Lee CH, Liu SY, Chou KC, et al. Tumor-associated macrophages promote oral cancer progression through activation of the Axl signaling pathway. Ann Surg Oncol. 2014;21(3):1031–1037. doi: 10.1245/s10434-013-3400-0. [DOI] [PubMed] [Google Scholar]
- 53.Lin X, Chen L, Yao Y, et al. CCL18-mediated down-regulation of miR98 and miR27b promotes breast cancer metastasis. Oncotarget. 2015;6(24):20485–20499. doi: 10.18632/oncotarget.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]