Abstract
Background
Cementless surface replacement hemiarthroplasty (CSRHA) is an established treatment for glenohumeral osteoarthritis; however, studies evaluating its role in arthritis with rotator cuff deficiency are limited. This study reviews the outcomes of CSRHA for glenohumeral osteoarthritis with and without rotator cuff tears.
Methods
41 CSRHA (Mark III Copeland prosthesis) were performed for glenohumeral osteoarthritis with intact rotator cuffs (n = 21) and cuff-deficient shoulders (n = 20). Patients were assessed using Oxford and Constant questionnaires, patient satisfaction, range of motion measurements and by radiography.
Results
Mean age and follow-up were 75 years and 5.1 years, respectively. Functional gains were significantly higher in patients with intact rotator cuffs compared to cuff-deficient shoulders, with Oxford Shoulder Score improving from 18 to 37.5 and 15 to 27 and forward flexion improved from 60° to 126° and 44° to 77° in each group, respectively. Two patients with deficient cuffs had deficient subscapularis tendons; one of which was dislocated anteriorly.
Conclusions
CSRHA provides significant improvements in pain and function in patients with glenohumeral osteoarthritis. In patients with deficient cuffs, functional gains are limited, and should be considered in low-demand patients where pain is the primary problem. Caution should be taken in patients with a deficient subscapularis as a result of the risk of dislocation.
Keywords: Arthritis, CSRA, cuff deficient, cuff intact, shoulder, Shoulder resurfacing glenohumeral arthritis cuff deficiency
Introduction
Primary glenohumeral osteoarthritis that is resistant to conservative therapy has been treated successfully with shoulder arthroplasty including hemiarthroplasty, total shoulder replacement and, more recently, humeral head resurfacing arthroplasty.1–3 The rotator cuff is often intact in primary glenohumeral osteoarthritis.4 This contrasts with cuff tear arthropathy (CTA) where there is superior migration of the humeral head as a result of a lack of dynamic stability. Although CTA is a negative prognostic indicator in arthroplasty surgery, it has been treated successfully with hemiarthroplasty and reverse shoulder arthroplasty.1,5 Less is known about the management of shoulder arthroplasty in osteoarthritic cuff-deficient shoulders with surface replacement hemiarthroplasty.6,7
Cementless surface replacement hemiarthroplasty (CSRHA) is an alternative to traditional stemmed implants, and aims to retain the patient’s own anatomy and preserves bone stock.8 The Mark III Copeland CSRHA (Biomet Merck, Swindon, UK) has been used since 1993, and consists of a pegged humeral component with hydroxyapatite coating and has been used successfully to treat primary glenohumeral osteoarthritis.1
Although few studies have described the outcomes of CSRHA in cuff tear arthropathy,9–12 none have evaluated its outcome in primary glenohumeral osteoarthritis with full-thickness degenerative tears of their rotator cuffs. The present study aimed to report the clinical and functional outcomes of the Mark III Copeland CSRHA, in patients with primary glenohumeral osteoarthritis with intact and deficient rotator cuffs presenting to a UK district general hospital.
Materials and methods
Between 2006 and 2010, 53 consecutive Mark III Copeland CSR hemiarthroplasties were performed in 43 patients for glenohumeral osteoarthritis with and without rotator cuff tears. Four patients died and two were not contactable, leaving 37 patients for follow-up. Four patients had bilateral procedures.
There were 20 shoulders with glenohumeral arthritis associated with rotator cuff deficiency without superior migration of the humeral head (Group 1) and 21 with primary glenohumeral osteoarthritis with intact rotator cuffs (Group 2). Indications for implantation included glenohumeral degenerative changes in the presence of severe pain associated with functional limitation, resistant to non-operative measures. Patients with active sepsis, avascular necrosis, fracture sequelae, rheumatoid arthritis or inflammatory arthritis were excluded. The integrity of the rotator cuff was assessed clinically, radiologically and intra-operatively.
All patients were routinely assessed with the Oxford Shoulder Score (OSS)13, both pre- and postoperatively. Postoperative Constant14 shoulders scores were obtained additionally. In cuff-deficient patients (Group 1), power was not assessed as patients were unable to abduct their shoulder to 90°.
Additionally, patients were asked to rate their satisfaction with the outcome of their shoulder surgery as either very happy, happy, unsure or worse. Statistical analysis on function was performed using the paired t-test (SPSS Statistics for Windows, version 17.0; SPSS, Inc., Chicago, IL, USA). p < 0.05 was considered statistically significant.
Anteroposterior (AP) and lateral radiographs were obtained in all patients immediately postoperatively, 6 months and at final follow-up. Serial radiographs were evaluated for component malposition, sizing inaccuracy, subsidence, periprosthetic radiolucency, and glenoid erosion by two independent assessors. Radiolucent lines and glenoid erosion were measured according to the criteria as described by Sirveaux et al.15 (type E0, no glenoid erosion; E1, concentric erosion of the glenoid; E2, erosion of the superior glenoid; E3, erosion extended to the inferior glenoid).
The Copeland Mark III hydroxyapatite coated resurfacing hemiarthroplasty was used in all cases and performed by one of the senior surgeons (AJ, IL or DAW). An inter-scalene block was performed for most patients to provide adequate postoperative pain relief. All cases were performed through a standard deltopectoral or MacKenzie approach as described by Copeland et al.16 The subscapularis was identified and if intact was detached near its lateral insertion, and repaired at the end of the procedure. All patients in group 1 had a completely ruptured supraspinatus and two patients had deficient subscapularis tendons.
Tenotomy of the long head of biceps was not routinely performed. In no cases was the glenoid resurfaced. In cases where the glenoid had grade 4 arthritic changes, it was drilled with multiple 2-mm drill holes. A capsular release was performed if there was a restriction in rotation, and subscapularis lengthening was only performed if <30° of external rotation was possible on the table. In 18 patients (10 in group 1, eight in group 2) excision of the acromioclavicular joint (ACJ) was performed if it was degenerative.
Postoperatively, the arm was placed in a sling, with physiotherapy starting on day 1 and including passive assisted mobilization, progressing to active-assisted mobilization at 2 weeks. Concentric strengthening started at 6 weeks. The sling was worn for 6 weeks, after which the patients were allowed full, unrestricted range of motion exercises.
No Institutional Review Board/Ethical approval was required for the present study.
Results
Patient follow-up, mean age and sex are shown in Table 1.
Table 1.
Mean follow-up, age and sex.
| Group 1 (cuff-deficient) | Group 2 (cuff-intact) | |
|---|---|---|
| Follow-up (range) in years | 5.0 (2.5 to 7.6) | 5.3 (2.5 to 6.7) |
| Mean age | 75 (52 to 88) | 75 (58 to 93) |
| Sex | 12 female, 8 male | 16 female, 5 male |
Fuctional outcome
Pre- and postoperative functional score and range of motion are shown in Table 2
Table 2.
Functional and satisfaction outcomes.
| Pre-operative | Postoperative | Satisfaction | |
|---|---|---|---|
| Group 1: Cuff-deficient (n = 20) | 16 Very happy/happy | ||
| Oxford | 15 | 27 | 4 Unhappy |
| Constant (out of 75) | NA | 35 | |
| Forward flexion (°) | 44° | 77 | |
| Abduction (°) | 32° | 76 | |
| Group 2: Cuff-intact (n = 21) | 18 Very happy/happy | ||
| Oxford | 18 | 37.5 | 3 Unsure |
| Constant (out of 100) | NA | 62.6 | |
| Forward flexion (°) | 60 | 126 | |
| Abduction (°) | 65 | 117 |
Patients with intact rotator cuff (Group 2) had significantly greater improvement in OSS and achieved greater range of movement at final follow-up compared to the cuff-deficient Group 1 (p = 0.01). No significant differences in outcome where noted in patients who had ACJ resection compared to those who did not.
Further analysis of the four questions directly related to pain within the OSS (worse pain, usual pain, activity interference due to pain and night pain) was performed (maximum score 16). Pre-operative scores rose from 4 and 5 to 11 and 13 postoperatively for groups 1 and 2, respectively. Additionally, postoperative pain scores were 11 in Group 1 and 12 in Group 2 out of 15 on analysis of the Constant Score (None = 15, Mild = 10, Moderate = 5, Severe = 0).
Postoperative radiological assessment
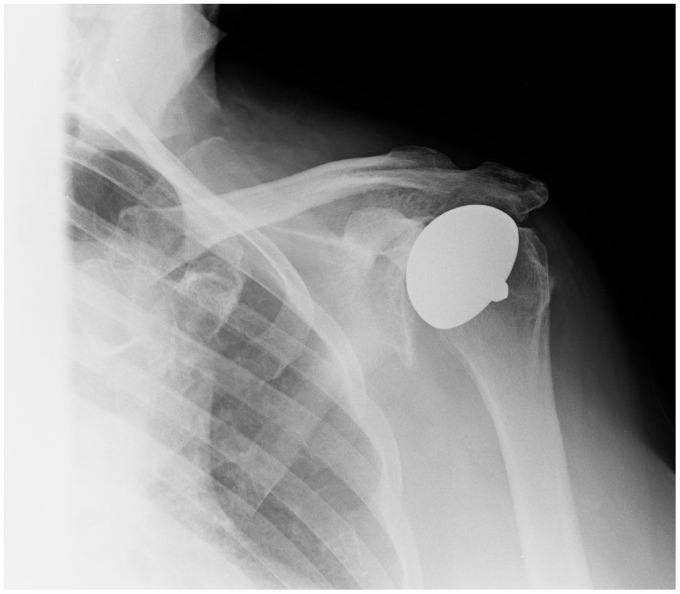
There were no cases of aseptic loosening or progressive radiolucent lines. In group 1, six had no glenoid erosion, five patients had E1 glenoid erosion and nine had E2. In group 2, 10 patients had no glenoid erosion (E0), nine had E1 and two had E2. Postoperatively, progression of superior glenoid erosion was noted in 12 cases at final follow-up (Figure 1). There was no correlation between the progression of glenoid erosion and functional/satisfaction outcomes. Two components were oversized; however, the patients functional scores did not reflect this.
Figure 1.
Presence of superior glenoid erosion (E2).
We had two cases of acromial fractures that occurred after falls, and these were treated non-operatively and went on to unite without further problems.
Complications
Re-operation was required in three patients. Two patients with intact cuffs (Group 2) had stiff shoulders and poor function despite no obvious radiological complications and underwent manipulations under anaesthesia. Their forward flexion improved from an average of 40° to 75° following the procedure and were satisfied with their outcome at final follow-up.
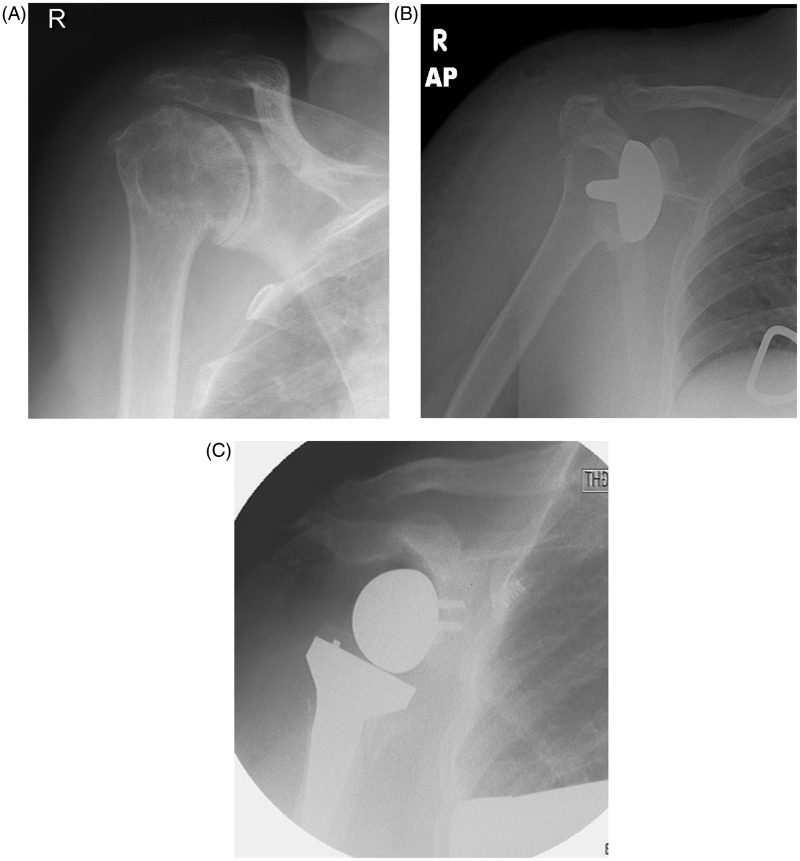
One patient with a deficient supraspinatus and subscapularis (Figure 2A) dislocated anteriorly on day 1 postoperatively. A closed reduction was attempted (Figure 2B); however, she dislocated a second time and was subsequently revised to a reverse shoulder prosthesis (Figure 2C).
Figure 2.
(A) shows an anteroposterior radiograph with concentric osteoarthritis in a patient with a full-thickness cuff tear. The patient was found to have a deficient subscapularis intra-operatively. At 1 day postoperatively (B), she dislocated and was found to be unstable and was therefore revised to a reverse shoulder arthroplasty (C).
There were no cases of deep infection and one case of superficial wound infection, which responded to oral antibiotics.
Assessment of satisfaction outcomes
Of the four patients who were unhappy in Group 1, one was a result of instability/dislocation and three were a result of persistent pain and poor function. All had repeat inflammatory markers and subsequent clinical and radiological assessment; however, no cause of the persistent pain was found. At final follow-up, they had a mean OSS of 16 (range 15 to 19).
Of the three patients who were unsure about their shoulders in Group 2, all had significant gains in range of motion, and functional scores compared to their pre-operative function. All three had bilateral replacements and although noted considerable improvements in their pain and function, felt that one shoulder was worse than the contralateral side, with mean OSS rising from 15 to 35.
Discussion
Glenohumeral osteoarthritis with or without associated rotator cuff tears causes significant morbidity and loss of function. Our study shows that good pain reduction can be achieved following CSRHA in patients with glenohumeral osteoarthritis, however with less consistent gains in range of motion and inferior results in the cuff-deficient shoulder. Although reverse shoulder arthroplasty provides a more consistent increase in range of motion in the cuff-deficient shoulder due to an increase in deltoid function,17 resurfacing arthroplasty has several key advantages including preservation of bone stock, independency of glenoid bone stock, a shorter, less technically demanding operation and a lower complication rate. We had three re-operations with one patient requiring revision in the cuff-deficient group (<10%), which compares favourably with the quoted complication rates for reverse shoulder arthroplasty.18
Edwards et al.6 evaluated the outcome of shoulder arthroplasty in patients with glenohumeral osteoarthritis and compared patients with intact rotator cuffs (n = 472), partial tears (n = 41) or full-thickness (n = 42) supraspinatus tears without superior migration of the humeral head. Although they found that patients with full-thickness tears had reduced postoperative strength, they had better pain relief compared to the other patient groups, and therefore the supraspinatus tear did not influence the total Constant score. Our patients with deficient cuffs had similar improvements in pain but, in contrast to Edwards et al.,6 and in agreement with other studies,19–21 we found that arthroplasty did not improve function in patients with a full-thickness rotator cuff tears. It is important to note that in the study by Edwards et al.,6 patients were younger (67.6 years) compared to our series (74 years) and had a greater pre-operative range of motion (forward flexion of 89° compared to 42° in our series). Furthermore, surgical techniques and treatments were not standardized because it was a multicentred study and included variability including whether the rotator cuff was repaired (n = 11) or glenoid resurfaced (n = 64).
Unlike Edwards et al.,6 we did not attempt repair of the supraspinatus tendon in any case because we found it either too retracted or attenuated and had concerns about the survival of the repair as a result of the quality of the tendon and the patients age. Little has been published on concurrent rotator cuff repair; Goldberg22 and Pollock et al.7 suggested that it improves outcome; however, they found no statistical difference in their series of cuff-deficient arthritis treated with stemmed hemiarthroplasty.
Few studies have evaluated the role of humeral surface replacement in cuff-deficient shoulders. Pape et al.12 reviewed 24 patients who underwent resurfacing arthroplasty with the Aequalis Resurfacing Head (Tournier Inc., Edina, MN, USA) and had greater mean forward flexion and abduction of 109° and 101° than patients in our study (77° and 76°). This may be attributed to their patients having a greater pre-operative range of motion, a slightly younger patient group or shorter follow-up period of 2 years compared to our mean follow-up of 5 years. Pape et al.12 reported small improvements in range of motion following resurfacing arthroplasty in cuff-deficient shoulders. In their study, one patient had anterosuperior instability which was attributed to a deficient subscapularis tendon. In our series, we had one patients in group 1 (5%) who had an anterior dislocation and was also noted to have a deficient subscapularis tendon. Several studies have evaluated the use of reverse shoulder arthroplasty in patients with cuff tear arthropathy and had greater functional gains compared to our study.1,15,17 However, concerns remain with reverse shoulder arthroplasty due to its longevity, and the difficulty of revision as a result of bone loss both on the humeral but particularly the glenoid side.18 We found small improvements in range of motion in patients with cuff-deficient shoulders undergoing resurfacing arthroplasty and therefore suggest that resurfacing arthroplasty should only be undertaken in low-demand patients where pain is the predominant problem. In the high-demand patient with a deficient rotator cuff, other surgical treatments should be considered.
Sanchez-Sotelo et al.23 discussed factors that lead to anterosuperior instability in their series of 33 shoulders with cuff tear arthropathy treated with stemmed hemiarthroplasty at a mean follow-up of 5 years. Twenty-one percent had anterosuperior instability and this was attributed to a prior history of subacromial decompression and a loss of the coracoacromial arch, which has also been found in other studies.24,25 No comment was made on the relationship between patients with a deficient subcapularis tendon and anterior instability; however, it was deficient or partially torn in 52% of their patients and this may have acted as a confounding factor. More recently, concerns have been raised about anterior instability in patients with deficient subscapularis tendons in patients undergoing reverse shoulder arthroplasty (RSA). Edwards et al.26 reviewed 138 patients undergoing RSA, with 76 having an irreparable subscapularis tendon. There were seven cases of anterior dislocations, all occurring in patients with irreparable supscapularis ruptures; however, none of the dislocations occurred in the cuff tear arthropathy group (n = 60) but occurred in more complex diagnoses such as failed arthroplasty and fixed dislocations. All dislocations were stable after revision, which allowed re-tensioning of the deltoid with prosthetic augmentation.
We had similar gains in outcome scores in patients with primary glenohumeral osteoarthritis (group 2) compared to other studies evaluating resurfacing hemiarthroplasty. Al-Hadithy et al.27 reported gains in OSS from 22 to 42 and forward flexion of 66° to 115° in their series of 50 patients at 4-year follow-up, which is comparable to our results. Similarly, we had 11 cases of glenoid erosion; however, this did not correlate with function.
The present study has several limitations. It is a retrospective review with no true control group and no randomization, making it prone to confounding factors and measurement bias. We minimized measurement bias by having three researchers who were independent to the index procedure assess outcomes. We also performed ACJ resections in just under half of the patients, almost equally in both groups, and although no significant difference was seen, it may have confounded the results.
Conclusions
Humeral head resurfacing hemiarthroplasty is a viable treatment option for glenohumeral osteoarthritis. In patients with deficient cuffs, functional gains are limited, and suggest that it should be considered in low-demand patients where pain is the primary problem. We have changed our practice to routinely perform an ultrasound scan to assess the state of the rotator cuff pre-operatively. Caution should be taken in patients with a deficient subscapularis as a result of the high risk of dislocation and other treatment options should be considered.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Al-Hadithy N, Domos P, Sewell MD, Pandit R. Reverse shoulder arthroplasty in 41 patients with cuff tear arthropathy with a mean follow-up period of 5 years. J Shoulder Elbow Surg 2014; 23: 1662–8. [DOI] [PubMed] [Google Scholar]
- 2.Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicentre study. J Shoulder Elbow Surg 2002; 11: 130–135. [DOI] [PubMed] [Google Scholar]
- 3.Radnay CS, Setter KJ, Chambers L, Levine WN, Bigliani LU, Ahmad CS. Total shoulder replacement compared with humeral head replacement for the treatment of primary glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg 2007; 16: 396–402. [DOI] [PubMed] [Google Scholar]
- 4.Neer CS., II Replacement arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am 1974; 56: 1–13. [PubMed] [Google Scholar]
- 5.Hurov J. Anatomy and mechanics of the shoulder: review of current concepts. J Hand Ther 2009; 22: 328–42. [DOI] [PubMed] [Google Scholar]
- 6.Edwards TB, Boulahia A, Kempf JF, Boileau P, Nemoz C, Walch G. Cuff disease on the results of shoulder arthroplasty for primary osteoarthritis. J Bone Joint Surg Am 2002; 84A: 2240–8. [DOI] [PubMed] [Google Scholar]
- 7.Pollock RG, Deliz ED, McIlveen ST, Flatow EL, Bigliani LU. Prosthetic replacement in rotator cuff-deficient shoulders. J Shoulder Elbow Surg 1992; 1: 173–86. [DOI] [PubMed] [Google Scholar]
- 8.Levy O, Copeland SA. Cementless surface replacement arthroplasty of the shoulder. 5 to 10 year results with the Copeland Mark 2 prosthesis. J Bone Joint Surg Br 2001; 83: 213–21. [DOI] [PubMed] [Google Scholar]
- 9.Alizadehkhaiyat O, Kyriakos A, Singer MS, Frostick SP. Outcome of Copeland shoulder resurfacing arthroplasty with a 4-year mean follow-up. J Shoulder Elbow Surg 2013; 22: 1352–8. [DOI] [PubMed] [Google Scholar]
- 10.Levy O, Copeland SA. Cementless surface replacement arthroplasty (Copeland CSRA) for osteoarthritis of the shoulder. J Shoulder Elbow Surg 2004; 13: 266–71. [DOI] [PubMed] [Google Scholar]
- 11.Mullett H, Levy O, Raj D, Even T, Abraham R, Copeland SA. Copeland surface replacement of the shoulder. Results of an hydroxyapatite-coating cementless implant in patients over 80 years of age. J Bone Joint Surg Br 2007; 89: 1466–9. [DOI] [PubMed] [Google Scholar]
- 12.Pape G, Bruckner T, Loew M, Zeifang F. Treatment of severe cuff tear arthropathy with humeral head resurfacing arthroplasty: two-year minimum follow-up. J Shoulder Elbow Surg 2013; 22: e1–7. [DOI] [PubMed] [Google Scholar]
- 13.Dawson J, Fitzpatrick R, Carr A. Questionnaire on the perception of patients about shoulder surgery. J Bone Joint Surg Br 1996; 78: 593–600. [PubMed] [Google Scholar]
- 14.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res 1987; 214: 160–4. [PubMed] [Google Scholar]
- 15.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg [Br] 2004; 86B: 388–95. [DOI] [PubMed] [Google Scholar]
- 16.Copeland SA, Funk L, Levy O. Surface replacement arthroplasty of the shoulder. Corr Orthop 2002; 16: 21–31. [Google Scholar]
- 17.Nolan BM, Anderson E, Wiater JM. Reverse total shoulder arthroplasty improves function in cuff tear arthropathy. Clin Orthop Relat Res 2011; 469: 2476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nam D, Kepler CK, Neviaser AS, et al. Reverse total shoulder arthroplasty: current concepts, results and component wear analysis. J Bone Joint Surg Am 2010; 92: 23–35. [DOI] [PubMed] [Google Scholar]
- 19.Arntz CT, Jackins S, Matsen FA., III Prosthetic replacement of the shoulder for the treatment of defects in the rotator cuff and the surface of the glenohumeral joint. J Bone Joint Surg Am 1993; 75: 485–91. [DOI] [PubMed] [Google Scholar]
- 20.Hawkins RJ, Bell RH, Jallay B. Total shoulder arthroplasty. Clin Orthop 1989; 242: 188–94. [PubMed] [Google Scholar]
- 21.Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg 1997; 6: 495–505. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg SS, Bell JE, Kim HJ, Bak SF, Levine WN, Bigliani LU. Hemiarthroplasty for the rotator cuff-deficient shoulder. J Bone Joint Surg Am 2008; 90: 554–9. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Sotelo J, Cofield RH, Rowland CM. Shoulder hemiarthroplasty for glenohumeral arthritis associated with severe rotator cuff deficiency. J Bone Joint Surg Am 2001; 83: 1814–22. [DOI] [PubMed] [Google Scholar]
- 24.Field LD, Dines DM, Zabinski SJ, Warren RF. Hemiarthroplasty of the shoulder for rotator cuff arthropathy. J Shoulder Elbow Surg 1997; 6: 18–23. [DOI] [PubMed] [Google Scholar]
- 25.Wiley AM. Superior humeral dislocation. A complication following decompression and debridement for rotator cuff tears. Clin Orthop 1991; 263: 135–41. [PubMed] [Google Scholar]
- 26.Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009; 18: 892–6. [DOI] [PubMed] [Google Scholar]
- 27.Al-Hadithy N, Domos P, Sewell MD, Naleem A, Papanna MC, Pandit R. Cementless surface replacement arthroplasty of the shoulder for osteoarthritis: results of fifty Mark III Copeland prosthesis from an independent centre with four-year mean follow up. J Shoulder Elbow Surg 2012; 21: 1776–81. [DOI] [PubMed] [Google Scholar]