Abstract
Background
We present the largest series of surgically treated primary bone tumours of the elbow in the English literature (75 cases). We sought to identify characteristics specific to these lesions and recommend an investigatory protocol.
Methods
The national registry and case notes were reviewed between 1954-2014. Tumours were classified according to Enneking's spectrum.
Results
There were no benign latent cases in this series as these were managed locally. All patients presented with persistent rest pain, with or without swelling. The distal humerus, in contrast to the proximal radius and ulna, was responsible for the majority and the more aggressive cases. Misdiagnosis was evident in 13% of cases; most of which were attributed to simple bone cysts. All patients that were referred required surgical intervention to either establish the diagnosis or for treatment. Benign tumours had a 19% recurrence rate, with giant cell tumour the most aggressive. Malignant tumours carried 39% local recurrence rate and a 5-year mortality of 61%.
Conclusions
The suspicion of a tumour should be raised in the patient with unremitting, unexplained, non-mechanical bony elbow pain. These echo the NICE recommendations and we recommend prompt specialist referral. With high rates of local recurrence, we recommend close postoperative monitoring.
Keywords: Elbow, experience, primary, registry, tumour
Introduction
Primary bony tumours of the elbow are uncommon and account for approximately 1% of all osseous tumours.1 The majority of the literature in this area describes individual osseous lesions and their treatments, with one case series consisting of 25 patients.2
The present study was conducted by retrospective review of the Scottish Bone Tumour Registry (SBTR), founded in 1954. An independent clerk prospectively enters the data of patients that present with a primary bone tumour throughout Scotland. The tumours are confirmed by the orthopaedic oncology multidisciplinary team. The SBTR receives approximately 100 new cases per annum. There are currently over 5000 cases logged in the registry. Each case includes data concerning patient demographics, diagnosis, radiological imaging, pathology and clinical course.
Delayed diagnosis is a recurring theme frequently attributed to a lack of familiarity of tumours in this region.3 We sought to identify characteristics in epidemiology and clinical course specific to primary bone tumours affecting the elbow. We report how the cases presented and how they were subsequently treated. In addition, we recommend a protocol for the diagnosis and management of these rare tumours.
Materials and methods
The anatomical boundary of the elbow is defined as the medial and lateral epicondyles of the distal humerus, the capitellum, the radial head and the olecranon.4 Accordingly, all cases of benign and malignant primary bone tumours involving these regions were included in the present study. The SBTR was retrospectively reviewed from January 1954 until June 2014. A total of 12 cases were excluded from the registry because when they were seen initially, their elbow pathology was found to be a result of either: post-traumatic cysts, metastases or multiple myeloma. This left 75 cases of primary osseous tumours of the elbow.
Benign lesions were sub-classified as latent, active or aggressive according to the spectrum of disease described by Enneking et al.5 This classification was described in 1980 and therefore, in cases earlier to this year, the senior author (AM) sub-classified the tumour retrospectively by examining the case notes, histology slides and pathology reports. Malignant lesions were classed as either low or high grade. Surgical treatment modality was classified as intralesional with or without bone graft or cement, marginal excision, radical excision, endoprosthetic replacement or amputation.
The clinical course, adjuvant therapy, local recurrence, distant metastases, imaging and notes from the follow-up consultations were evaluated. Surgery varied depending on tumour type, grade, treatment modality and current guidelines at the time. All patients were followed-up for a minimum of 2 years until discharge or death. No patients were lost to follow-up (median 8 years, range 2 years to 18 years).
Results
There were 47 (63%) benign and 28 (37%) malignant primary bone tumours. There were no latent cases in the benign group. The commonest benign tumour was fibrous dysplasia with 15 (32%) cases. This was followed by giant cell tumour with nine (19%) cases, osteoid osteoma with eight (17%) cases and seven (15%) cases were aneurysmal bone cysts. Within the benign group, 43% were on the aggressive side of the spectrum. With respect to the malignant elbow bone tumours, eight cases (29%) were low grade, whereas 20 cases (71%) were high grade. There were 17 cases (61%) of osteosarcoma and seven cases (25%) of Ewing’s sarcoma. The demographics and the characteristics of the presentations are summarized in Table 1.
Table 1.
Demographics and characteristics of the tumours at presentation to the tertiary service.
| Demographics and presentation | Distal humerus |
Proximal ulna and radius |
||
|---|---|---|---|---|
| Benign | Malignant | Benign | Malignant | |
| Number of patients | 23 | 26 | 24 (12 ulna:12 radius) | 7 (4 ulna: 3 radius) |
| Male: female | 10: 13 | 13: 13 | 12: 12 | 5: 2 |
| Mean age at presentation (years, range) | 23 (3–62) | 49 (1–87) | 34 (6–83) | 31 (8–51) |
| Mean length of symptoms (months, range) | 6 (1–24) | 6 (1–12) | 9 (1–72) | 6 (1–12) |
| Pain as presenting symptom | 23 (100%) | 22 (76%) | 23 (96%) | 6 (75%) |
| Swelling as presenting symptom | 16 (70%) | 22 (76%) | 14 (58%) | 6 (75%) |
| Fracture at presentation | 6 (26%) | 7 (24%) | 10 (42%) | 3 (38%) |
There were 38 male and 37 female cases in this series. With respect to benign tumours, the ratio of male: female was 22: 25 and 16: 12 for malignant tumours. The age at presentation ranged from 11 years to 87 years with a mean age of 34 years. Benign tumours presented in patients at a mean of 29 years (range 12 years to 83 years) and malignant tumours presented at 40 years of age (range 11 years to 82 years). The length of symptoms prior to presentation to the treating orthopaedic oncologist surgeon varied from 1 month to 72 months. In the benign tumour group, this mean time was 8 months compared to 6 months for the malignant tumours. Rest pain was the most frequent presenting symptom, noted in 87% of all tumours. Swelling was also common with 70% of cases having this as a presenting complaint.
Depending on the decade of presentation, pre-operative imaging for staging included plain radiographs, bone scans, computed tomography (CT) and/or magnetic resonance imaging (MRI). Needle and/or open biopsies were obtained to confirm the diagnosis in all cases. Adjuvant therapy was administered when recommended by an oncologist.
All patients had a surgical intervention to establish a diagnosis or for treatment of symptoms. The treatment for primary bone tumours varied depending on histological type, available treatments and individual patient factors. Similarly, the clinical course varied depending on the personality of the tumour. We present these as individual cases, divided into benign and malignant tumours in Tables 2 and 3, respectively. In this series, the majority of tumours originated in the distal humerus. These were generally noted to be more aggressive compared to the proximal radius and ulna.
Table 2.
Benign elbow lesions.
| Case | Spectrum | Location | Sex/age | Year | Histological diagnosis | Surgical treatment | Recurrence and surgery | Misdiagnosis |
|---|---|---|---|---|---|---|---|---|
| 1 | Active | D Humerus | M/18 | 1996 | SBC | ILC & BG | N | N |
| 2 | Active | D Humerus | M/56 | 1968 | FD | ILC | N | N |
| 3 | Active | D Humerus | M/29 | 1980 | FD | ME | N | N |
| 4 | Active | D Humerus | F/13 | 2008 | SBC | ILC | N | N |
| 5 | Active | D Humerus | F/14 | 1965 | OO | ME | N | N |
| 6 | Active | D Humerus | F/13 | 1960 | OO | ILC | Y, RE | Y |
| 7 | Active | D Humerus | F/15 | 1964 | FD | None | N | N |
| 8 | Active | D Humerus | F/27 | 1970 | SBC | ILC & BG | Y, ILC & ORIF | Y |
| 9 | Active | D Humerus | F/13 | 1977 | SBC | ILC & BG | N | N |
| 10 | Active | D Humerus | F/24 | 1968 | FD | ILC | N | N |
| 11 | Active | D Humerus | M/16 | 1968 | SBC | None | N | N |
| 12 | Active | P Radius | M/20 | 1976 | FD | ILC | N | N |
| 13 | Active | P Radius | M/23 | 1977 | FD | ILC | N | N |
| 14 | Active | P Radius | M/44 | 1957 | FD | ILC & CP | N | N |
| 15 | Active | P Radius | M/29 | 1938 | FD | ME | N | N |
| 16 | Active | P Radius | M/29 | 1967 | FD | ME | N | N |
| 17 | Active | P Radius | F/43 | 1980 | OO | RE | N | N |
| 18 | Active | P Radius | F/37 | 1958 | FD | ILC | N | N |
| 19 | Active | P Radius | M/19 | 1984 | OO | ILC & BG | N | N |
| 20 | Active | P Radius | F/43 | 1980 | OO | RE | N | N |
| 21 | Active | P Radius | M/15 | 1982 | FD | ILC | N | N |
| 22 | Active | P Ulna | M/30 | 1973 | FD | ILC & CP | N | N |
| 23 | Active | P Ulna | F/33 | 1955 | SBC | None | N | N |
| 24 | Active | P Ulna | F/50 | 1995 | FD | ME | N | N |
| 25 | Active | P Ulna | M/45 | 1997 | FD | ILC & BG | N | N |
| 26 | Active | P Ulna | F/45 | 1987 | FD | ILC & ORIF | N | N |
| 27 | Active | P Ulna | M/39 | 2012 | OO | OK & RFA | N | Y |
| 28 | Aggressive | D Humerus | M/23 | 1967 | CB | ILC | N | N |
| 29 | Aggressive | D Humerus | F/62 | 1963 | ABC | ILC & ORIF | N | N |
| 30 | Aggressive | D Humerus | F/22 | 1963 | ABC | ILC | Y, RE &BG | Y |
| 31 | Aggressive | D Humerus | M/31 | 1963 | CB | ILC & BG | N | N |
| 32 | Aggressive | D Humerus | M60 | 1959 | GCT | ILC & BG | N | N |
| 33 | Aggressive | D Humerus | F/22 | 1979 | GCT | ILC & CP | Y, RE & BG | N |
| 34 | Aggressive | D Humerus | F/24 | 1984 | GCT | ILC & BG | N | N |
| 35 | Aggressive | D Humerus | M/21 | 1989 | OO | ILC | N | N |
| 36 | Aggressive | D Humerus | F/12 | 1960 | OO | ILC | N | N |
| 37 | Aggressive | D Humerus | F/20 | 1958 | GCT | ILC | Y, AMP | Y |
| 38 | Aggressive | D Humerus | M/16 | 1968 | GCT | ILC & BG | Y, RE & BG | N |
| 39 | Aggressive | D Humerus | F/16 | 1969 | ABC | ILC | N | N |
| 40 | Aggressive | P Radius | M/44 | 1954 | ABC | ILC & BG | N | N |
| 41 | Aggressive | P Radius | F/23 | 1956 | GCT | RE | N | N |
| 42 | Aggressive | P Radius | F/36 | 1964 | GCT | ILC & BG | N | N |
| 43 | Aggressive | P Ulna | M/12 | 1979 | ABC | ILC | Y, ILC | Y |
| 44 | Aggressive | P Ulna | F/31 | 1966 | GCT | ILC & CP | N | N |
| 45 | Aggressive | P Ulna | F/13 | 1987 | ABC | ILC & BG | N | N |
| 46 | Aggressive | P Ulna | F/18 | 2002 | ABC | ILC & BG | Y, RE | Y |
| 47 | Aggressive | P Ulna | F/51 | 1991 | GCT | RE | Y | Y |
P, proximal; D, distal; M, male; F, female; Y, yes; N, no; ABC, aneursymal bone cyst; SBC, simple bone cyst; GCT, giant cell tumour; FD, fibrous dysplasia; CB, chondroblastoma; OO, osteoid osteoma; ILC, intralesional curettage; BG, bone grafting; ORIF, open reduction internal fixation; ME, marginal excision; CP, cementoplasty; RE, radical excision; RT, radiotherapy; RFA, radiofrequency ablation; OK, Outerbridge and Kashiwagi procedure; AMP, amputation.
Table 3.
Malignant elbow lesions.
| Case | Spectrum | Location | Sex/age | Year | Histological diagnosis | Surgical treatment | Adjuvant treatment | Recurrence and surgery | Metastases | Follow-up (years) | Death from disease | Misdiagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Low | D Humerus | F/77 | 1987 | OS | N | RT | N | N | 5 | N | N |
| 2 | Low | D Humerus | M/28 | 1980 | CS | RE | RT | N | N | 30 | N | N |
| 3 | Low | D Humerus | F/80 | 1985 | NHL | N | RT | N | N | 2 | 2 years | N |
| 4 | Low | D Humerus | F/23 | 1962 | OS | ILC | RT | N | N | 12 | N | N |
| 5 | Low | D Humerus | F/70 | 1986 | NHL | ILC & ORIF | RT | N | N | 2 | N | N |
| 6 | Low | D Humerus | F/72 | 1997 | NHL | ILC & ORIF | RT | N | N | 16 | N | N |
| 7 | Low | D Humerus | F/37 | 1962 | CS | ILC | N | Y | N | 5 | N | N |
| 8 | Low | P Radius | F/50 | 1975 | ES | RE | N | N | N | 5 | N | N |
| 9 | High | D Humerus | F/57 | 1990 | OS | RE | N | Y | Y | 1 | 1 years | N |
| 10 | High | D Humerus | F/58 | 1967 | CS | RE | N | N | N | 6 | 6 years | N |
| 11 | High | D Humerus | F/69 | 1969 | OS | N | RT | N | Y | 1 | 1 years | N |
| 12 | High | D Humerus | F/62 | 1968 | OS | N | RT | N | Y | 1 | 1 year | N |
| 13 | High | D Humerus | M/13 | 1970 | OS | AMP | RT | Y | Y | 1 | 1 year | N |
| 14 | High | D Humerus | M/19 | 1972 | OS | RE | RT | Y | Y | 2 | 2 years | N |
| 15 | High | D Humerus | M/6 | 1967 | CS | N | RT | Y | Y | 2 | 2 year | N |
| 16 | High | D Humerus | F/6 | 1973 | OS | AMP | N | N | N | 5 | N | N |
| 17 | High | D Humerus | F/20 | 1960 | OS | AMP | N | N | N | 5 | N | N |
| 18 | High | D Humerus | M/82 | 1989 | OS | ILC & BG | N | Y | Y | 2 | 2 years | N |
| 19 | High | D Humerus | M/59 | 1986 | OS | AMP | N | Y | N | 1 | 1 year | N |
| 20 | High | D Humerus | M/58 | 1983 | OS | AMP | RT | N | Y | 4 | 4 years | N |
| 21 | High | D Humerus | M/19 | 1977 | OS | ILC & BG | RT | N | N | 6 | 6 yeass | N |
| 22 | High | D Humerus | F/67 | 1997 | OS | RE | NO | N | N | 4 | 4 years | N |
| 23 | High | D Humerus | M/54 | 2001 | NHL | ILC & BG | NO | Y | Y | 2 | 2 years | N |
| 24 | High | D Humerus | M/21 | 1984 | OS | AMP | RT | Y | N | 1 | 1 years | N |
| 25 | High | D Humerus | M/8 | 2006 | ES | RE | CH | Y | Y | 4 | 4 years | N |
| 26 | High | D Humerus | M/77 | 2000 | NHL | N | RT | N | N | 5 | N | N |
| 27 | High | D Humerus | M/16 | 2009 | ES | EPR | CH | N | N | 4 | N | N |
| 28 | High | P Radius | M/11 | 1978 | ES | AMP | CH | Y | Y | 3 | 3 years | N |
| 29 | High | P Radius | M/12 | 1977 | ES | ILC & BG | CH | N | N | 5 | N | N |
| 30 | High | P Ulna | M/23 | 1961 | OS | RE, AMP | RT | Y | Y | 1 | 1 year | N |
| 31 | High | P Ulna | F/68 | 1997 | OS | RE | N | N | Y | 1 | 1 year | Y |
| 32 | High | P Ulna | M/19 | 1988 | ES | RE | CH | Y | Y | 2 | 2 years | N |
| 33 | High | P Ulna | M/18 | 2008 | ES | EPR | CH | N | N | 5 | N | N |
P, proximal; D, distal; M, male; F, female; Y, yes; N, no; OS, osteosarcoma; CS, chondrosarcoma; NHL, Non-Hodgkin’s lymphoma; ES, Ewing’s sarcoma; ILC, intralesional curettage; BG, bone grafting; ORIF, open reduction internal fixation; ME, marginal excision; CP, cementoplasty; RE, radical excision; RT, radiotherapy; CH, chemotherapy; EPR, endoprosthetic replacement; AMP, amputation.
There was a 19% (n = 9) local recurrence rate at a mean of 3 years in the benign tumour cohort. Comparatively, 39% (n = 11) of the malignant group had a local recurrence. Within the malignant group, the low grade tumours recurred less than the high grade group (low grade 22%; high grade 48%). The high grade tumours recurred at a mean of 1.5 years postoperatively. In addition, 43% (n = 12) of the primary malignant elbow tumours presented with distant metastases. The overall 5-year mortality in the malignant group was 61% (low grade 12%; high grade 68%). In this series, 13% of all tumours were initially misdiagnosed, with management altered as a consequence.
Discussion
With 75 tumours identified in a period of 60 years, the rarity of these conditions is apparent. Relevant literature on the subject is sparse. These cases were from a national database, which serves a population of approximately 5 million people. The aims were therefore to identify the types and demographics of primary osseous elbow tumours and to recommend an investigatory and treatment protocol.
There were no benign latent cases identified in this series because these lesions were managed locally, without referral to the tertiary service. In addition, this suggests that benign latent lesions are less likely to lead to significant pain requiring referral. We identified more malignant tumours in the distal humerus and more benign tumours affecting the proximal ulna and radius. Patients with malignant tumours affecting the distal humerus presented at an older age, at an average of 49 years compared to 23 years in benign conditions. A similar trend was found by Bruguera et al. in their review from 1998.6
This series highlights the significant morbidity and mortality associated with elbow tumours. Over a quarter of the benign group had recurrences which resulted in further surgery while the 5-year mortality for the high grade malignancies was 68%.
Benign active lesions
The commonest tumour in this series was fibrous dysplasia representing 32% of all the benign tumours. In patients with severe fibrous dysplasia, the peak fracture rate is reported between 6 years to 10 years of age, although it is reported in adulthood.7–9 All patients in our cohort presented with pain initially but no swelling around the elbow. A pathological fracture at presentation was evident in 20% of these cases. In our series, cases were treated surgically with intralesional curettage and cementoplasty or bone autograft. None of these patients re-presented with recurring symptoms, there were no malignant transformations in these patients and there were no misdiagnoses here. Another large series does, however, report that malignant transformation of fibrous dysplasia can occur in up to 4%.10
Osteoid osteomas were responsible for 17% of the benign elbow tumour group. These typically presented with elbow pain and stiffness. Classically, as was apparent in the majority of our cases, these lesions are more painful at night and relieved by salicylates.11 CT scanning has been suggested as the best imaging modality to confirm the diagnosis.12 However, when the nidus is subperiosteal and small, MRI may be of more benefit as it shows additional signs such as oedema, effusion and synovitis. The majority of our osteoid osteomas were treated with intralesional curettage or image guided radiofrequency ablation.13
With any tumour of the elbow, we recommend seeking advice from regional elbow subspecialists. One of our cases of osteoid osteoma was treated with an Outerbridge and Kashiwagi procedure.14 This allowed easy access to both the anterior and posterior aspects of the humerus and removal of the entire tumour without extensive soft tissue dissection.
Benign aggressive lesions
Giant cell tumour (GCT) of the elbow was present in 19% of the benign cases. The vast majority of cases were female patients and this follows the documented trend of female preponderance with GCT.15 They were all managed with a biopsy followed by intralesional curettage and bone grafting, with a 50% recurrence rate observed. Previous reports have suggested a higher recurrence rate for primary treatment of GCT when curettage was used instead of radical excision, making the latter a more suitable and reliable option in aggressive lesions.16 Our recurrent cases of disease were successfully treated with radical excision. However, one patient eventually had a forequarter amputation and one patient died from metastases. Both cases were initially misdiagnosed. It is recommended that all patients with benign aggressive lesions such as this or aneurysmal bone cysts (our third most common benign tumour) are closely followed-up with serial imaging.
Malignant lesions
Malignant tumours of the elbow are challenging in terms of aggressiveness, regional anatomy and their rarity. There were approximately equal numbers of males to females and the mean age of presentation was 40 years. Our series found high-grade osteosarcoma to account for 61% of the malignancies. The majority of our cases presented with a painful swelling, but one presented with a fungating mass. Several of the patients had metastatic spread at the time of diagnosis and therefore radiotherapy was used in these cases as a palliative measure. Over half of the operations here were amputations, and only 14% of these patients survived more than 5 years.
Ewing’s sarcoma was responsible for 25% of malignant cases, with the majority presenting in adolescence. These aggressive tumours resulted in death in 50% of the cases. The majority of these tumours were treated with neoadjuvant chemotherapy and radical excision. All of the fatalities had chest metastases within 2 postoperative years. Our institution has issued national guidelines regarding the follow-up of malignant tumours. We recommend that chest radiographs are requested at 6-monthly intervals after surgery for all high grade malignant tumours for the first 5 postoperative years, then annually until the tenth postoperative year. This is in addition to the routine surgical follow-up.17
Elbow reconstruction
Endoprosthetic replacement (EPR) may bring more favourable outcomes for patients. Ayoub et al.18 looked at eight cases of Ewing’s sarcoma affecting the skeletally immature humerus. The tumours were resected and the limbs salvaged with extensible EPRs, with a 90% 5 year survival. This was in stark contrast to our largely non-EPR group. Two of our entire series underwent EPR for primary Ewing’s sarcoma. Both cases had neoadjuvant chemotherapy followed with custom-made total elbow replacements and have had no recurrence. The main reason for our largely non-EPR treatment involving the remaining cases was the fact that several of the cases were treated in the advanced metastatic stage where extensive reconstructive surgery was not deemed appropriate. There were two cases of Ewing’s sarcoma that did not present with metastases and did not receive an EPR. These two cases both presented in the 1970s and we propose that EPR treatment was not commonplace at that time.
Infection is clearly a major concern in the immunocompromised patients undergoing prolonged surgery with large metallic prosthesis. Therefore, we use antibiotic impregnated cement and more recently have been requesting silver-coated EPRs. There is emerging evidence that this can reduce postoperative infection in orthopaedic oncology as silver has low toxicity and excellent antimicrobial activity.19,20
A limitation of the present study is that patients with historical investigations and treatments are included in the series. We acknowledge that investigatory and treatment modalities have evolved over this period, which present difficulties in the analysis of this data. Hence, the data is presented in a qualitative form. However, the study is strengthened by the fact that no patients were lost to follow-up.
Recommendations
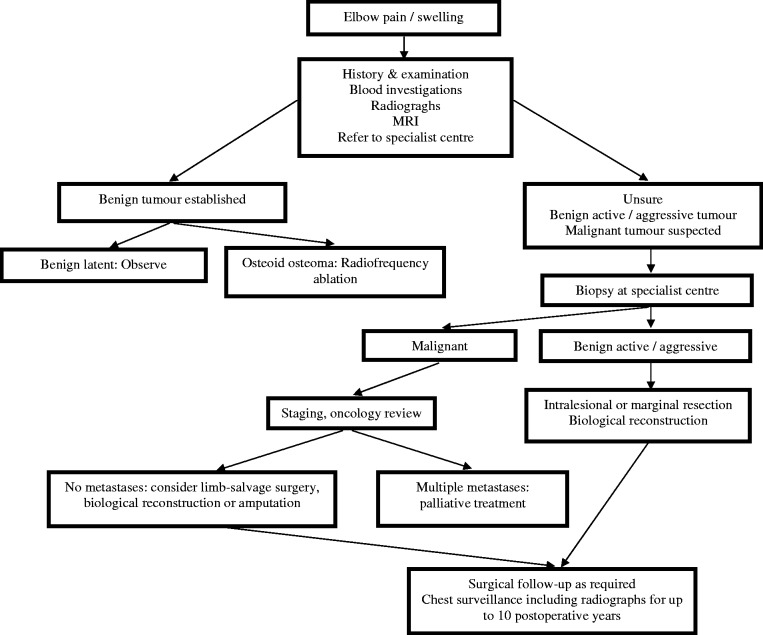
Misdiagnosis remains a problem and was evident with 10 cases (13%) identified on the database. The majority were concerning benign lesions that were attributed to simple bone cysts. However, two were incorrect initial diagnoses in the malignant group. We recommend that patients presenting with persistent and moderate elbow pain should be investigated, especially if the onset is insidious and nonresolving (Figure 1). In our experience, this pain was not only functional but often present at rest. The National Institute of Clinical Excellence (NICE) guidelines state that patients with increasing, unexplained or persistent bone pain or tenderness, particularly pain at rest should be investigated by the primary healthcare professional urgently.21 We consider that our study reinforces this.
Figure 1.
Management protocol for the suspicion of an elbow tumour.
It is recommended that histological diagnosis can be obtained by image-guided core biopsy, which is less invasive and appropriate in lesions with an easily penetrable outer cortex or soft tissue component. Where this is not possible, open biopsy is recommended. All biopsies should be carried out after consultation with the musculoskeletal orthopaedic specialist to ensure that the definitive surgical treatment is not jeopardized. It is emphasized that the biopsy should be carried out by the team carrying out the definitive surgical intervention. This recommendation follows guidelines from Members of the Musculoskeletal Tumour Society.22 In our centre, all biopsies are reviewed by two separate pathologists who are members of the national musculoskeletal sarcoma team.
All patients are discussed at the weekly national sarcoma meeting prior to decision on surgical treatment. This weekly meeting involves the three centres, via a video link, that are treating bone tumours in Scotland. There are, however, no formal weekly meetings with the five other treating centres from the rest of the UK (Birmingham, Newcastle, Oswestry, Oxford and Stanmore). Occasionally, if specialist advice is required from a Consultant outside of Scotland, an e-mail referral will be made to that particular specialist.
The operative approach to the elbow will depend on the anatomical location of the tumour as well as pathological type and local staging. The surgical exposure of the elbow is limited by several factors including surrounding neurovascular structures. In addition, the articular cartilage of the various bones can limit the location and size of bone windows for intralesional procedures. Our management protocol for the suspicion of an elbow tumour is summarized in Figure 1.
Conclusions
Clinicians should note that elbow tumours present with unexplained and unremitting non-mechanical pain, swelling or fracture. Early specialist referral is the quickest means to deliver expert care from the multidisciplinary team with these rare tumours. Misdiagnosis occurs most frequently in the benign group and thus a high degree of suspicion should be advised. In our series, all benign lesions required surgical intervention. Malignant tumours are associated with significant morbidity and historically have led to death or amputation. With specialist centres now being referred to more frequently, reconstruction of the elbow is an expanding field. Complex cases require input from both elbow specialists and musculoskeletal oncologists.
Acknowledgments
We thank Mrs Jean Campbell for her assistance with the data collection. The results of this paper were presented at the annual Musculoskeletal Tumour Society in 2013. They have no rights over the paper and the results have not been previously published or being considered by another Journal.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Ayoub KS, Fiorenza F, Grimer RJ, Tillman RM, Carter SR. Extensible endoprostheses of the humerus after resection of bone tumours. J Bone Joint Surg Br 1999; 81: 495–500. [DOI] [PubMed] [Google Scholar]
- 2.Bruguera JA, Newman RJ. Primary tumors of the elbow: a review of the Leeds Regional Bone Tumour Registry. Orthopedics 1998; 21: 551–3. [PubMed] [Google Scholar]
- 3.Cassard X, Accadbled F, Gauzy JS, Cahuzac JP. Osteoid osteoma of the elbow in children: a report of three cases and a review of the literature. J Pediatr Orthop B 2002; 11: 240–4. [DOI] [PubMed] [Google Scholar]
- 4.Chanson P, Dib A, Visot A, Derome PJ. McCune-Albright syndrome and acromegaly: clinical studies and responses to treatment in five cases. Eur J Endocrinol 1994; 131: 229–34. [DOI] [PubMed] [Google Scholar]
- 5.Chapurlat RD, Orcel P. Fibrous dysplasia of bone and McCune-Albright syndrome. Best Pract Res Clin Rheumatol 2008; 22: 55–69. [DOI] [PubMed] [Google Scholar]
- 6.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980; 153: 106–20. [PubMed] [Google Scholar]
- 7.Hardes J, von Eiff C, Streitbuerger A, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol 2010; 101: 389–95. [DOI] [PubMed] [Google Scholar]
- 8.Leet AI, Chebli C, Kushner H, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune–Albright syndrome. J Bone Miner Res 2004; 19: 571–7. [DOI] [PubMed] [Google Scholar]
- 9.Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am 1996; 78: 656–63. [DOI] [PubMed] [Google Scholar]
- 10.O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am 1994; 76: 1827–33. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira VC, van der Heijden L, van der Geest IC, et al. Giant cell tumours of the small bones of the hands and feet: long-term results of 30 patients and a systematic literature review. Bone Joint J 2013; 95B: 838–45. [DOI] [PubMed] [Google Scholar]
- 12.Peyser A, Applbaum Y, Khoury A, Liebergall M, Atesok K. Osteoid osteoma: CT-guided radiofrequency ablation using a water-cooled probe. Ann Surg Oncol 2007; 14: 591–6. [DOI] [PubMed] [Google Scholar]
- 13.Pritchard DJ, Dahlin DC. Neoplasms of the elbow. In: Morrey BF. (ed). The elbow and its disorders, Philadelphia, PA: WB Saunders, 1985, pp. 713–35. [Google Scholar]
- 14.Rachel JN, Kurt PT, Heck RK. Osteoid osteoma. Pract Orthop Pathol 2010; 21: 105–7. [Google Scholar]
- 15.NICE. Referral Guidelines for Suspected Cancer in Adults and Children. NICE Clinical Guidelines, No. 27. Clinical Governance Research and Development Unit (CGRDU), Department of Health Sciences, University of Leicester (June 2005). https://www.nice.org.uk/guidance/cg27.
- 16.Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer 1994; 73: 1411–24. [DOI] [PubMed] [Google Scholar]
- 17.White J. Sarcoma NMCN 2012–13 Activity Report, version 1.0 (May 2013). http://www.ssn.scot.nhs.uk.
- 18.Steinberg B, Plancher K. Clinical anatomy of the wrist and elbow. Clin Sports Med 1995; 14: 299–313. [PubMed] [Google Scholar]
- 19.Tobin EJ, Bambauer R. Silver coating of dialysis catheters to reduce bacterial colonization and infection. Ther Apher Dial 2003; 7: 504–9. [DOI] [PubMed] [Google Scholar]
- 20.Tow BP, Tan MH. Delayed diagnosis of Ewing's sarcoma of the right humerus initially treated as chronic osteomyelitis: a case report. J Orthop Surg (Hong Kong) 2005; 13: 88–92. [DOI] [PubMed] [Google Scholar]
- 21.Wada T, Isogai S, Ishii S, Yamashita T. Debridement arthroplasty for primary osteoarthritis of the elbow. Surgical technique. J Bone Joint Surg Am 2005; 87(Suppl 1): 95–105. [DOI] [PubMed] [Google Scholar]