Abstract
Elevations of plasma concentrations of branched-chain amino acids (BCAAs) are observed in human insulin resistance and type 2 diabetes mellitus (T2DM); however, there has been some controversy with respect to the passive or causative nature of the BCAA phenotype. Using untargeted metabolomics, plasma BCAA and other metabolites were assessed in lean control Sprague-Dawley rats (LC) and temporally during diabetes development in the UCD-T2DM rat model, i.e., prediabetic (PD) and 2 wk (D2W), 3 mo (D3M), and 6 mo (D6M) post-onset of diabetes. Plasma leucine, isoleucine, and valine concentrations were elevated only in D6M rats compared with D2W rats (by 28, 29, and 30%, respectively). This was in contrast to decreased plasma concentrations of several other amino acids in D3M and/or D6M relative to LC rats (Ala, Arg, Glu, Gln, Met, Ser, Thr, and Trp). BCAAs were positively correlated with fasting glucose and negatively correlated with plasma insulin, total body weight, total adipose tissue weight, and gastrocnemius muscle weight in the D3M and D6M groups. Multivariate analysis revealed that D3M and D6M UCD-T2DM rats had lower concentrations of amino acids, amino acid derivatives, 1,5-anhydroglucitol, and conduritol-β-opoxide and higher concentrations of uronic acids, pantothenic acids, aconitate, benzoic acid, lactate, and monopalmitin-2-glyceride relative to PD and D2W UCD-T2DM rats. The UCD-T2DM rat does not display elevated plasma BCAA concentrations until 6 mo post-onset of diabetes. With the acknowledgement that this is a rodent model of T2DM, the results indicate that elevated plasma BCAA concentrations are not necessary or sufficient to elicit an insulin resistance or T2DM onset.
Keywords: branched-chain amino aicds, diabetes, University of California at Davis Type 2 Diabetes rat model, amino acids, branched-chain amino acids
alterations of branched-chain amino acid (BCAA) metabolism and increased circulating BCAA concentrations are associated with insulin resistance and type 2 diabetes mellitus (T2DM), based on studies utilizing untargeted and targeted metabolite analyses in human cohorts (11–13, 24, 29, 41, 45, 51). Furthermore, circulating concentrations of BCAAs in the obese insulin-resistant state are correlated with indices of insulin resistance and compromised blood glucose control (3, 13, 32), whereas fasting plasma BCAA concentrations predicted T2DM development in the Framingham Offspring Study (45). It has since been proposed that excess BCAAs can contribute to development of insulin resistance, at least under conditions of high-fat feeding or elevated tissue fatty acid availability (29). Based on the weight of published evidence, we favor the perspective that insulin resistance and/or T2DM lead to alterations in efficient tissue BCAA catabolism, which contributes to increased circulating BCAA levels (2, 26) and may promote “anaplerotic stress” (1, 13). Still, the exact mechanism behind this association, whether causative or casual, has yet to be conclusively defined.
Metabolomics analysis has provided much evidence to allow a global view of the metabolic landscape of T2DM as well as identify biomarkers that differentiate T2DM and insulin resistance conditions from otherwise healthy phenotypes. In addition to BCAAs, several other prognostic biomarkers of insulin resistance and/or T2DM that are currently being investigated were identified and originally characterized using metabolomics approaches (12, 41, 45, 46). The vast majority of these studies were derived from cross-sectional studies with heterogeneous populations that include a broad range of metabolic outcomes associated with this disease (42). Only a few studies have assessed the circulating metabolome longitudinally before and/or after the clinical diagnosis of T2DM has been established (12, 14, 45–47). This is especially critical since the progression and development of T2DM is not a static occurrence and can take upwards of 10 yr (20). Thus, characterization of noninvasive biomarkers that track specific and temporal metabolic disturbances early in the progression of T2DM is needed.
To address this issue, we have utilized the University of California at Davis Type 2 Diabetes Mellitus (UCD-T2DM) rat model to investigate the changes in the plasma metabolome during the progression from prediabetes to diabetes onset and on to a state of advanced T2DM. The UCD-T2D rat spontaneously develops polygenic adult-onset obesity and insulin resistance accompanied by β-cell dysfunction, leading to overt diabetes. However, unlike most other rat models of T2DM, the UCD-T2DM rat does not have a genetic defect in the leptin signaling pathway (9). In addition, the UCD-T2DM rat develops diabetes with ad libitum access to a standard chow diet and at a later age compared with other rodent models of T2DM (9). These characteristics of the UCD-T2DM rat model more closely resemble the pathophysiology of T2DM in humans. Thus, this model will allow us to carefully observe the metabolome during an experimentally controlled progression from prediabetes to newly diagnosed T2DM and to the insulinopenic, later stages of fulminant T2DM, which is difficult or impossible in a human research trial. In the current study, we addressed two primary aims: 1) to evaluate the hypothesis that worsening metabolic status drives BCAA changes, and not vice-versa, by determining the temporal patterns of plasma BCAA during the progression of insulin resistance and diabetes and 2) to identify novel metabolite signatures that characterize the progression of metabolic changes before and after the onset of overt long-term diabetes.
RESEARCH DESIGN AND METHODS
Animals.
All animal studies were approved by the University of California Davis Institutional Animal Care and Use Committee. Rats were maintained on a 14:10-h light-dark schedule with ad libitum access to standard chow (2018 Teklad Global; Harlan Laboratories, Indianapolis, IN). The UCD-T2DM rat model was created originally by crossing obese Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) prone to adult-onset obesity and insulin resistance with Zucker diabetic fatty lean rats (ZDF-lean; Charles River Laboratories). ZDF-lean rats were homozygous wild-type (+/+) at the leptin receptor locus (9, 31), ensuring functional leptin receptors. ZDF-lean rats have an autosomal recessive β-cell defect, causing impairment of β-cell function (16); however, the impairment is not sufficient to disrupt normoglycemia in the absence of obesity-induced insulin resistance (9). In-depth details of the breeding strategy have been described previously (9). Unlike other rodent models of T2DM, UCD-T2DM rats are fertile and produce offspring homozygous for the β-cell defect (9). Lean Sprague-Dawley control (LC; n = 7) rats (Harlan Laboratories, Indianapolis, IN) were used for comparison.
Experimental design.
Blood and tissues were collected from male UCD-T2DM rats after a 13-h fast (1900-0800) prior to the onset of diabetes (PD; n = 8) and 2 wk (D2W; n = 9), 3 mo (D3M; n = 14), and 6 mo (D6M; n = 16) after the onset of diabetes. Rats were given a 200 mg/kg ip dose of pentobarbital sodium and then exsanguinated via cardiac puncture between ca. 0800–1100. LC, PD, D2W, and D3M rats were age-matched at time of tissue harvest (165.5 ± 1.3 days; means ± SE). D6M rats were not age-matched at tissue harvest due to age-related logistics; therefore, D6M rats had a similar age of diabetes onset to D3M rats but aged ∼90 days longer than D3M animals. Onset of diabetes was defined as nonfasted blood glucose concentrations >200 mg/dl on 2 consecutive wk (9); therefore, inclusion as PD was determined in rats that met the age requirements and had nonfasting blood glucose concentrations < 200 mg/dl. Weekly determination of nonfasting blood glucose concentrations were taken in the afternoon between 1300 and 1600 with a glucose meter (LifeScan One-Touch Ultra, Milpitas, CA) from a drop of tail blood collected using a lancet.
Body composition.
Subcutaneous, mesenteric, epididymal, and retroperitoneal adipose depots, gasatrocnemius muscle, and liver were dissected and weighed immediately. Total adiposity is defined as a summed total of subcutaneous, mesenteric, epididymal, and retroperitoneal depots.
Plasma samples.
Blood was collected in EDTA-treated tubes from the tail prior to pentobarbital administration and used for assessment of plasma insulin, glucose, and triglycerides. Plasma was separated by centrifugation and stored in a −80°C freezer until analysis. Fasting plasma glucose was assessed with an enzymatic colorimetric assay (Fisher Diagnostics, Middletown, VA). Plasma insulin concentrations were measured with a rat-specific radioimmunoassay (EMD Millipore, Billerica, MA). Plasma triglycerides were measured with an enzymatic colorimetric assay (L-type TG H kit; Wako Chemicals, Richmond, VA). Plasma samples used for untargeted metabolomics of primary metabolism were delivered to the West Coast Metabolomics Center at the University of California Davis Genome Center on dry ice. In-depth details of the analytical methods have been described previously (13). Briefly, samples were thawed, extracted, and derivatized by silylation/methyloximation before analysis by gas chromatography quadrupole time-of-flight mass spectrometry. Results were processed by the Fiehn lab metabolomics BinBase database and matched against spectrum from the Fiehn mass spectral library of 1,200 authentic metabolite spectra, using retention index and mass spectrum information or the NIST05 commercial library. Metabolite data provided as peak heights of quantifier heights normalized to the sum intensities of all reported metabolites. Ions lacking structural identification were assigned BinBase identification numbers (36) and were included in all statistical analyses. All metabolomics data are provided as Supplemental Material (Supplemental Material for this article is available at the AJP-Endocrinology and Metabolism web site).
Statistical analysis.
All statistical analyses, figures, and tables were conducted in R (version 3.1.2, http://www.r-project.org/). Alpha was determined at 0.05 for all statistical tests unless alternatively specified.
Group differences among plasma nonfasting and fasting glucose, insulin, triglycerides, total weight, adiposity measurements, gastrocnemius muscle, liver, and plasma amino acid and amino acid derivatives were assessed with a one-way ANOVA. Measurements with a nonnormal distribution were log transformed before ANOVA. Post hoc analysis of group means was measured by Tukey's honestly significantly different test. Associations among selected variables were assessed with Spearman's correlations. Univariate tests were corrected for multiple comparisons using false methods by Benjamini and Hochberg (6).
Data preprocessing treatment for multivariate analyses.
Data were first transformed with a natural log function, and then each metabolite was assessed for univariate outliers by Grubbs' Test for Outliers (21) at α = 0.01. In total, 68 measurements were identified as an outlier and removed from analysis (0.34% of all data). Of the metabolites with removed univariate outliers, none had >10% of their data removed. Data were then randomly split into two-thirds and one-third training and test sets, respectively. Centering, scaling, and imputation of missing data (i.e., data removed during outlier identification) by K-nearest neighbor averaging were first assessed on training data and then applied on the training and test data sets separately using the preProcess function from the caret package (23).
Partial least squares-discriminant analysis (PLS-DA) model development and feature selection.
PLS-DA models were constructed to identify metabolites that discriminate LC, PD, D2W, D3M, and D6M groups (model 1) or only UCD-T2DM rat groups (model 2). Training data were used exclusively to develop PLS-DA models and select metabolites that contained discriminant class information. Test data were used only to assess PLS-DA model prediction accuracy through prediction of test set class membership. Models, including all metabolites, were initially constructed using functions from the caret package (23). Variable importance measurements based on weighted sums of the absolute regression coefficients (22, 23) were assessed on the initial models that included all metabolites. We then used a backward iterative selection strategy to identify metabolites that contained important discriminant information. First, variable important measurements were ordered, then a PLS-DA model was fit based on that order, and then class discrimination was predicted in the test data and compared with the actual class membership. Models were then fit iteratively, with the next top feature removed (as assessed by the variable important measurement), followed by class discrimination in the test set. Classification rates of all models were plotted and visually assessed to determine the point at which the classification rate regressed. At that point, all metabolites that had previously been removed were considered as metabolites with important discriminant information and used to develop final models. Final models were then used to predict the classification group of the remaining animals (“test” set). Ward's hierarchical clustering method was used to cluster the Euclidean distances of Spearman's correlations among featured metabolites.
RESULTS
Characteristics of UCD-T2DM rats during uncontrolled progression of diabetes.
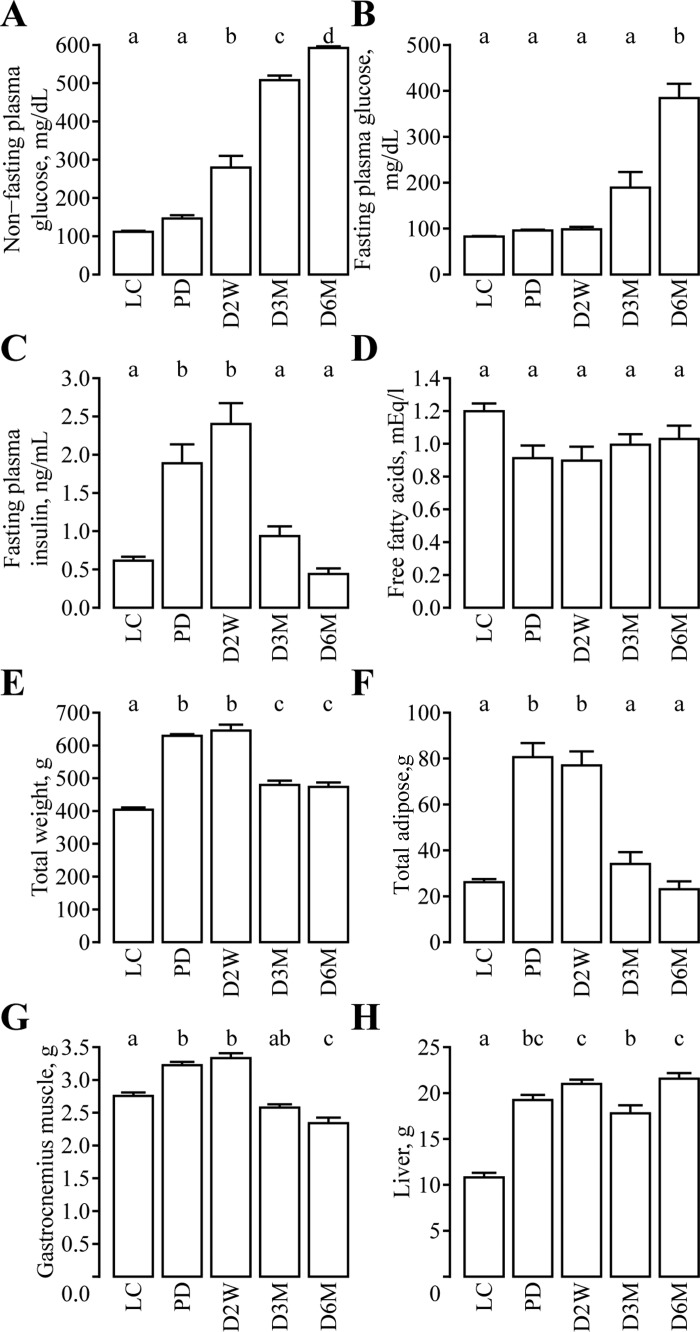
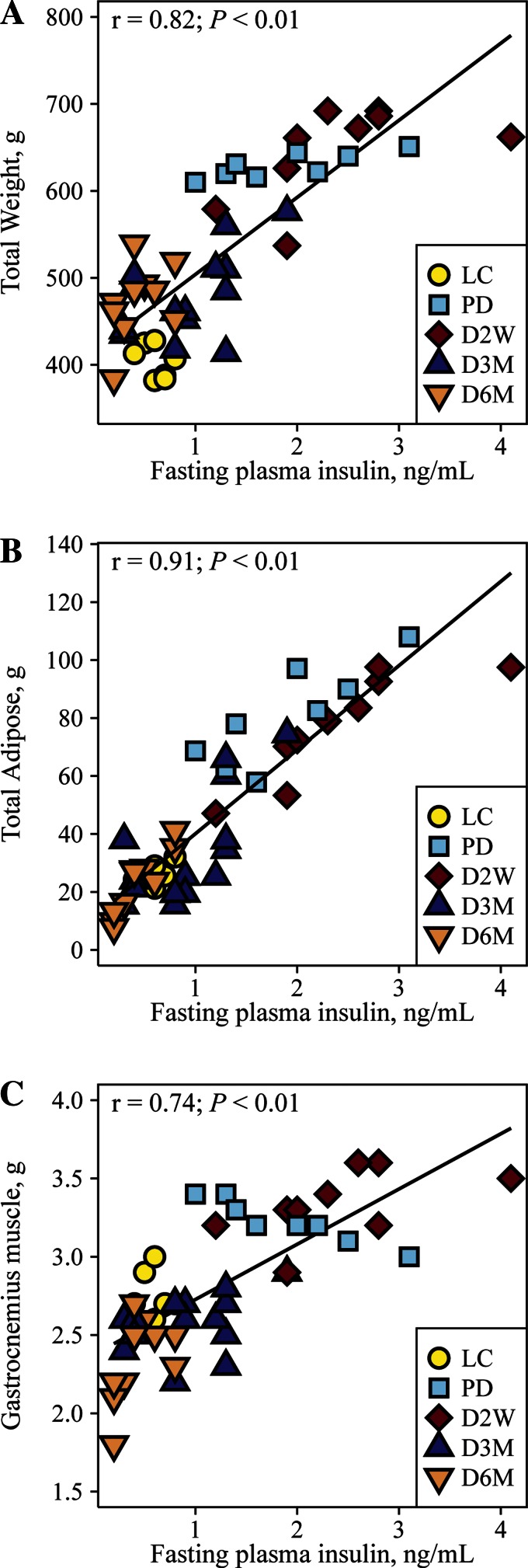
Similarly to previous reports (9), nonfasting plasma glucose was similar in PD rats compared with LC but significantly increased as diabetes progressed (Fig. 1A). Fasting plasma glucose was significantly elevated only at D6M (Fig. 1B). Fasting insulin was greater in PD and D2W rats compared with controls but declined in D3M and D6M rats, consistent with insulin resistance in PD and D2W followed by a deterioration of β-cell function evident in this model during the later stages of diabetes (Fig. 1C). No differences in fasting fatty acids were observed (Fig. 1D). UCD-T2DM rats at all diabetic stages had greater total body weight compared with LC animals, but D3M and D6M rats had significantly less body weight relative to PD and D2W rats (Fig. 1E). Similar to the observations in fasting insulin, total adiposity was greater in PD and D2W rats compared with LC rats but regressed in D3M and D6M to weights comparable with LC rats (Fig. 1F). Gastrocnemius muscle weight was also greater in PD and D2W rats relative to LC animals but also regressed in D3M and D6M rats, with D6M rats having a significantly lower weight compared with LC rats (Fig. 1G). Liver weights were greater in UCD-T2DM rats compared with LC and were lower in D3M relative to the PD, D2W, and D6M groups (Fig. 1H). Total body weight, total adiposity, and gastrocnemius muscle weight were strongly correlated with fasting plasma insulin across the groups (Fig. 2).
Fig. 1.
Progression of diabetes and adiposity in male University of California at Davis Type 2 Diabetes Mellitus (UCD-T2DM) rats. Plasma nonfasting glucose (A), fasting plasma glucose (B), fasting insulin (C), fasting free fatty acid concentrations (D), total body weight (E), total adipose tissue weight (F), gastrocnemius muscle weight (G), and liver weight (H) in lean control Sprague-Dawley rats (LC; n = 7) and UCD-T2DM rats before the onset [prediabetic (PD); n = 8] of diabetes and 2 wk (D2W; n = 9), 3 mo (D3M; n = 14), and 6 mo (D6M; n = 10) post-onset. Values are means ± SE. Group differences were assessed by 1-factor ANOVA. Means without a common letter differ significantly by Tukey's honestly significantly different (HSD) test. Statistical significance determined at P ≤ 0.05.
Fig. 2.
Correlation among fasting insulin and total weight (A), total adipose (B), and gastrocnemius muscle (C) in lean Sprague-Dawley controls (LC; n = 7) and UCD-T2DM rats prior to the onset of diabetes (PD; n = 8) and UCD-T2DM rats 2 wk (D2W; n = 9), 3 mo (D3M; n = 14), and 3 mo (D6M, n = 10) post-onset of diabetes. Total adiposity is defined as a summed total of subcutaneous, mesenteric, epididymal, and retroperitoneal adipose depots.
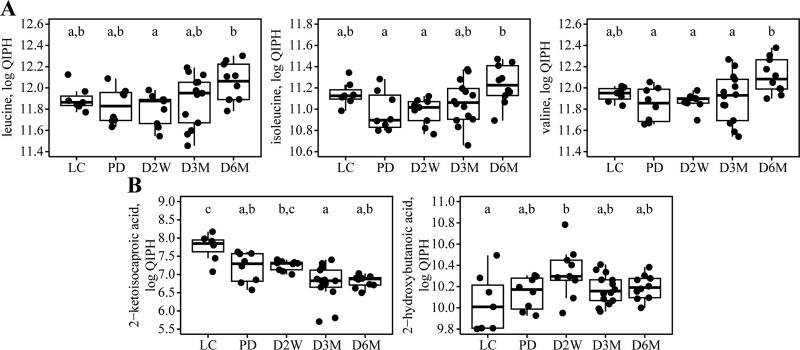
Plasma BCAAs are increased in later stages of diabetes, in contrast to a sharp decline in most other amino acids. We assessed the temporal differences in BCAAs and related metabolites due to the numerous reports of elevated circulating BCAAs under conditions of insulin resistance in humans (12, 13, 29, 41, 45, 51). As depicted in Fig. 3A, concentrations of all BCAAs were significantly greater in overnight-fasted D6M rats compared with PD or D2W groups but were not elevated in PD, D2W, or D3M rats relative to LC rats. Plasma abundance of Leu was 28% greater in D6M rats relative to D2W rats, whereas plasma abundances of Ile and Val were ∼30% greater in D6M rats compared with PD rats, respectively. Plasma abundances of 2-ketoisocaproate, the branched-chain keto-acid product of leucine, were significantly lower in PD, D3M, and D6M rats compared with the LC rats; additionally, rats in the D3M group had lower concentrations than D2W rats (Fig. 3B). Plasma levels of the methionine/cysteine-derived derivative 2-hydroxybutanoic acid [a.k.a. 2-hydroxybutyrate, implicated as a marker of increased risk for T2D risk (12, 13, 15)] were significantly greater only in rats at D2W vs. LC, although the absolute mean concentration trended higher in the UCD-T2D rats compared with LC controls (Fig. 3B). It was notable, from the standpoint of a putative T2DM-predictive biomarker, that 2-hydroxybutanoic acid correlated with fasting glucose and insulin in PD rats (Fig. 4).
Fig. 3.
Temporal changes in plasma abundances of branched-chain amino acids and branched-chain keto acid dehydrogenase substrates in male UCD-T2DM rats. The BCAA (A) and detected branched-chain keto acid dehydrogenase substrates (B) are shown for lean Sprague-Dawley control rats (LC; n = 7) and UCD-T2DM rats before the onset (PD; n = 8) of diabetes and 2 wk (D2W; n = 9), 3 mo (D3M; n = 14), and 6 mo (D6M; n = 10) post-onset of diabetes. Upper and lower hinges (box) are data within the interquartile range (IQR) and are values between the 1st and 3rd quartile. The median value is represented by a solid line. Whiskers include data based on (IQR × 1.5) + upper hinge and (IQR × 1.5) − lower hinge. ●, Actual data that are jittered for clarity. Units are log-transformed quantifier ion peak heights (QIPH). Group differences assessed by 1-factor ANOVA. Means without a common letter differ significantly by Tukey's HSD test. Statistical significance determined at P ≤ 0.05.
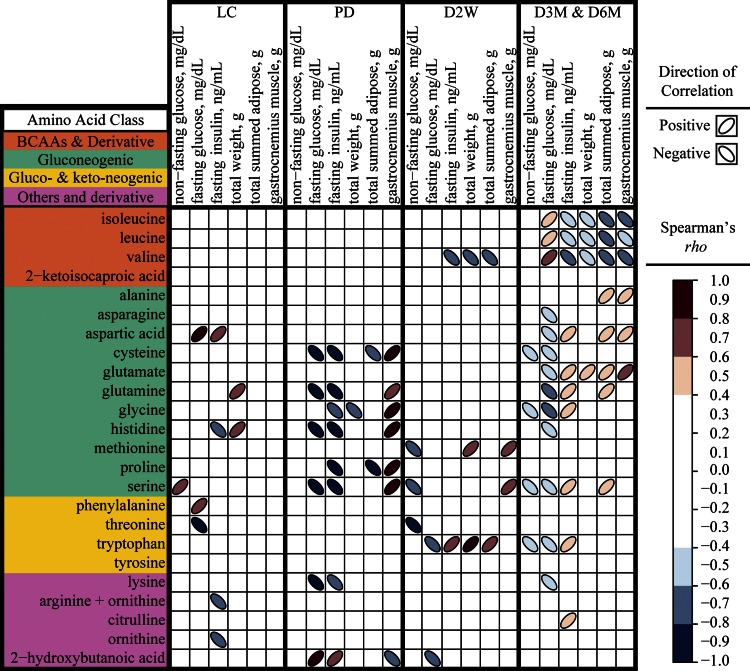
Fig. 4.
Spearman's correlations among clinical parameters and plasma amino acids stratified by diabetes status in rats. Correlations are indicated for lean Sprague-Dawley controls (n = 7), UCD-T2DM rats prior to the onset of diabetes (PD; n = 8), UCD-T2DM rats 2 wk post-onset of diabetes (D2W; n = 9), and UCD-T2DM rats in the later stages of diabetes [3 (D3M) and 6 mo (D6M) post-onset of diabetes; n = 24]. Significant correlations are presented as colored ellipses (P ≤ 0.05). Red ellipses positioned in a positive orientation are positive Spearman's rho correlation coefficients, and blue ellipses positioned in a negative orientation are negative Spearman's rho correlation coefficients. Darker ellipses represent a greater absolute rho correlation coefficient. BCAAs, branched-chain amino acids.
In marked contrast to the BCAAs, several gluconeogenic and ketogenic amino acids were lower in D3M and D6M UCD-T2DM animals compared with LC animals (Table 1). For example, Ala, Gln, Gly, Met, Ser, and Trp were all reduced by between 16 and 36% in D6M rats relative to LC rats. Only Asp, Trp, and citrulline differed in PD and D2W rats compared with LC rats; all were higher, whereas citrulline remained higher throughout the progression of diabetes (Table 1).
Table 1.
Fold difference of plasma amino acids and amino acid derivatives in UCD-T2DM rats relative to lean Sprague-Dawley controls
| Parameter | PD | D2W | D3M | D6M |
|---|---|---|---|---|
| Branched-chain amino acids and derivative | ||||
| Isoleucine | 0.85 (0.7, 1.1) | 0.85 (0.8, 0.9) | 0.93 (0.8, 1.1) | 1.10 (1.0, 1.3) |
| Leucine | 0.95 (0.8, 1.1) | 0.91 (0.8, 1.0) | 1.01 (0.9, 1.2) | 1.17 (1.0, 1.3) |
| Valine | 0.92 (0.8, 1.0) | 0.94 (0.9, 1.0) | 0.99 (0.9, 1.1) | 1.20 (1.1, 1.4) |
| 2-KIC | 0.58 (0.4, 0.8)* | 0.58 (0.5, 0.8)* | 0.39 (0.3, 0.5)* | 0.38 (0.3, 0.5)* |
| Gluconeogenic amino acids | ||||
| Alanine | 1.18 (1.0, 1.4) | 1.05 (0.9, 1.2) | 0.81 (0.7, 0.9) | 0.78 (0.7, 0.9)* |
| Asparagine | 1.33 (1.2, 1.6)* | 1.31 (1.1, 1.5)* | 1.02 (0.9, 1.1) | 0.97 (0.8, 1.2) |
| Aspartic acid | 1.18 (1.0, 1.4) | 1.18 (1.0, 1.5) | 1.10 (0.9, 1.4) | 0.87 (0.7, 1.3) |
| Cysteine | 1.03 (0.8, 1.3) | 1.25 (1.0, 1.6) | 1.09 (0.9, 1.3) | 0.77 (0.6, 1.2) |
| Glutamate | 1.34 (1.1, 1.7) | 1.32 (1.1, 1.6) | 1.02 (0.9, 1.2) | 0.91 (0.8, 1.1) |
| Glutamine | 1.07 (0.9, 1.3) | 1.13 (1.0, 1.3) | 0.87 (0.7, 1.0) | 0.74 (0.6, 0.9)* |
| Glycine | 1.18 (1.1, 1.4) | 1.09 (1.0, 1.2) | 0.93 (0.8, 1.1) | 0.69 (0.6, 0.8)* |
| Histidine | 1.05 (0.8, 1.3) | 1.01 (0.9, 1.1) | 0.85 (0.8, 1.0) | 0.80 (0.7, 0.9) |
| Methionine | 1.02 (0.9, 1.1) | 0.96 (0.9, 1.1) | 0.83 (0.8, 0.9)* | 0.84 (0.8, 1.0)* |
| Proline | 2.28 (1.1, 5.7) | 1.11 (0.5, 3.0) | 1.56 (0.8, 4.7) | 1.80 (0.8, 4.8) |
| Serine | 1.06 (1.0, 1.2) | 1.01 (0.9, 1.1) | 0.72 (0.7, 0.8)* | 0.64 (0.6, 0.7)* |
| Gluconeogenic and ketogenic amino acids | ||||
| Phenylalanine | 1.08 (1.0, 1.2) | 1.10 (1.0, 1.2) | 0.94 (0.9, 1.0) | 0.96 (0.9, 1.1) |
| Threonine | 1.37 (1.3, 1.5)* | 1.30 (1.1, 1.5)* | 0.82 (0.7, 1.0) | 0.83 (0.7, 1.0) |
| Tryptophan | 1.13 (1.0, 1.3) | 1.16 (1.0, 1.4) | 0.88 (0.7, 1.1) | 0.71 (0.6, 0.8)* |
| Tyrosine | 1.06 (0.9, 1.3) | 1.22 (1.0, 1.4) | 1.10 (0.9, 1.3) | 1.04 (0.9, 1.2) |
| Other amino acids and derivatives | ||||
| Lysine | 1.08 (0.9, 1.3) | 1.07 (1.0, 1.2) | 0.90 (0.8, 1.0) | 0.81 (0.7, 0.9) |
| Arginine + ornithine | 1.02 (0.6, 1.7) | 0.80 (0.5, 1.3) | 0.56 (0.4, 0.9)* | 0.66 (0.4, 1.0) |
| Citrulline | 1.18 (0.9, 1.4) | 1.34 (1.1, 1.6)* | 1.33 (1.1, 1.6)* | 1.32 (1.1, 1.5)* |
| Ornithine | 1.02 (0.7, 1.6) | 0.84 (0.6, 1.3) | 0.62 (0.4, 0.9)* | 0.70 (0.5, 1.1) |
| 2-HB | 1.07 (0.9, 1.3) | 1.32 (1.0, 1.7)* | 1.11 (0.9, 1.4) | 1.12 (0.9, 1.4) |
Data are mean fold difference [95% confidence interval (CI)] of University of California at Davis Type 2 Diabetes Mellitus (UCD-T2DM) rats prior to the onset of diabetes (PD; n = 8) and 2 wk (D2W; n = 9), 3 mo (D3M; n = 14), and 6 mo (D6M; n = 10) post-onset of diabetes relative to lean Sprague-Dawley controls (n = 7).
2-KIC, 2-ketoisocaproic acid; 2-HB, 2-hydroxybutanoic acid. CIs are adjusted bootstrap percentile (BCa) intervals.
Values that are significantly different (P < 0.05) from lean Sprague-Dawley controls (Dunnett's test).
Using the prediabetic UCD-T2D rats as a reference, there were very few alterations in D2W rats, but several amino acids were significantly altered in D3M and D6M rats (Table 2). BCAA concentrations are only higher in D6M rats relative to PD rats, but all other significantly altered amino acids decreased relative to PD concentrations. Non-BCAA amino acids and BCAA derivatives (2-ketoisocaproic acid, Ala, Asn, Gln, Gly, Met, Ser, Thr, Trp, and ornithine) were all decreased in both D3M and D6M rats, whereas glutamate and lysine were decreased only in D6M rats.
Table 2.
Fold difference of plasma amino acids and amino acid derivatives in diabetic UCD-T2DM rats relative to prediabetic UCD-T2DM rats
| Parameter | D2W | D3M | D6M |
|---|---|---|---|
| Branched-chain amino acids and derivatives | |||
| Isoleucine | 0.99 (0.85, 1.13) | 1.10 (0.93, 1.28) | 1.29 (1.08, 1.51)* |
| Leucine | 0.96 (0.82, 1.09) | 1.07 (0.91, 1.25) | 1.24 (1.05, 1.43)* |
| Valine | 1.02 (0.92, 1.14) | 1.07 (0.93, 1.25) | 1.30 (1.15, 1.53)* |
| 2-KIC | 0.99 (0.79, 1.32) | 0.67 (0.49, 0.9)* | 0.64 (0.51, 0.87)* |
| Gluconeogenic amino acids | |||
| Alanine | 0.89 (0.75, 1.06) | 0.69 (0.6, 0.78)* | 0.66 (0.57, 0.77)* |
| Asparagine | 0.98 (0.84, 1.15) | 0.76 (0.66, 0.88)* | 0.73 (0.61, 0.88)* |
| Aspartic acid | 1.00 (0.89, 1.17) | 0.94 (0.8, 1.12) | 0.74 (0.59, 1.09) |
| Cysteine | 1.21 (0.99, 1.63) | 1.05 (0.84, 1.42) | 0.75 (0.56, 1.14) |
| Glutamate | 0.99 (0.81, 1.32) | 0.76 (0.61, 0.97) | 0.68 (0.54, 0.9)* |
| Glutamine | 1.05 (0.92, 1.24) | 0.81 (0.68, 0.97)* | 0.69 (0.58, 0.82)* |
| Glycine | 0.92 (0.82, 1.02) | 0.78 (0.66, 0.92)* | 0.59 (0.51, 0.65)* |
| Histidine | 0.96 (0.76, 1.32) | 0.81 (0.64, 1.12) | 0.76 (0.6, 1.06) |
| Methionine | 0.94 (0.83, 1.06) | 0.81 (0.75, 0.9)* | 0.82 (0.75, 0.95)* |
| Proline | 0.49 (0.28, 0.97) | 0.68 (0.41, 1.39) | 0.79 (0.43, 1.47) |
| Serine | 0.95 (0.82, 1.1) | 0.68 (0.6, 0.77)* | 0.6 (0.53, 0.7)* |
| Gluconeogenic and ketogenic amino acids | |||
| Phenylalanine | 1.02 (0.92, 1.13) | 0.87 (0.78, 0.98) | 0.89 (0.8, 1.03) |
| Threonine | 0.95 (0.83, 1.1) | 0.60 (0.54, 0.66)* | 0.61 (0.54, 0.69)* |
| Tryptophan | 1.03 (0.9, 1.17) | 0.78 (0.67, 0.93)* | 0.63 (0.54, 0.72)* |
| Tyrosine | 1.15 (0.95, 1.37) | 1.04 (0.84, 1.25) | 0.99 (0.82, 1.14) |
| Other amino acids and derivatives | |||
| Lysine | 0.99 (0.82, 1.17) | 0.83 (0.67, 1) | 0.75 (0.59, 0.91)* |
| Arginine + ornithine | 0.78 (0.54, 1.14) | 0.56 (0.41, 0.78)* | 0.65 (0.45, 0.97)* |
| Citrulline | 1.13 (0.98, 1.39) | 1.13 (0.98, 1.37) | 1.12 (0.98, 1.33) |
| Ornithine | 0.82 (0.62, 1.14) | 0.60 (0.46, 0.83)* | 0.68 (0.5, 0.94)* |
| 2-HB | 1.24 (1.06, 1.52)* | 1.04 (0.92, 1.17) | 1.05 (0.94, 1.2) |
Data are mean fold difference (95% CI) of UCD-T2DM rats at 2 wk (D2W; n = 9), 3 mo (D3M; n = 14), and 6 mo (D6M; n = 10) post-onset of diabetes relative to prediabetic UCD-T2DM rats (n = 8).
2-KIC, 2-ketoisocaproic acid; 2-HB, 2-hydroxybutanoic acid. CIs are adjusted bootstrap percentile (BCa) intervals.
Values that are significantly different (P < 0.05) from prediabetic UCD-T2DM rats (Dunnett's test).
Plasma concentrations of all BCAAs are correlated to total adiposity, fasting plasma glucose, and insulin in later but not earlier stages of diabetes. Significant associations among all measured amino acids and non-fasting glucose, fasting glucose, fasting insulin, total body weight, total adiposity, and gastrocnemius muscle in LC, PD, D2W, and late stages of diabetes (combined D3M and D6M) are provided in Fig. 4. No BCAAs were correlated to these parameters in LC or PD animals. Only fasting plasma insulin, total body weight, and total adipose weight are significantly and negatively correlated to Val at D2W, but all BCAAs are positively correlated with fasting plasma glucose and negatively correlated with fasting plasma insulin, total body weight, total adipose tissue weight, and gastrocnemius muscle weight in the later stages of diabetes. Distinct correlation patterns emerged (i.e., multiple indices correlated with several amino acids) in PD and in the late stages of diabetes, which were not apparent in LC and D2W groups. Except for the correlations between fasting plasma insulin and His in LC and PD groups, there were no similarities in significant correlation between those two groups. A pattern emerged in PD rats, where Cys, Gln, Glyc, His, Pro, and Ser were all positively correlated to gastrocnemius weight and negatively correlated with both fasting glucose and fasting insulin or just fasting insulin alone. All of these correlations were not observed in D2W rats, with the exception of the correlation between Ser and gastrocnemius weight. Many of the gluconeogenic amino acids (Asp, Glu, Gln, Gly, Ser, and Trp) in the later stages of diabetes had opposite relationships to indices that were correlated to BCAAs.
Metabolites discriminant of progression of diabetes.
In addition to examining BCAAs and other amino acids, a secondary aim was to characterize the “global” metabolic profile of diabetes progression. A total of 414 metabolites were observed in the untargeted metabolomics analysis; 146 were annotated metabolites based on their structural identification and 268 were unknown (i.e., metabolites that did not have full structural identification). The unknown metabolites were assigned BinBase identification numbers (36) and were included in multivariate statistical analyses.
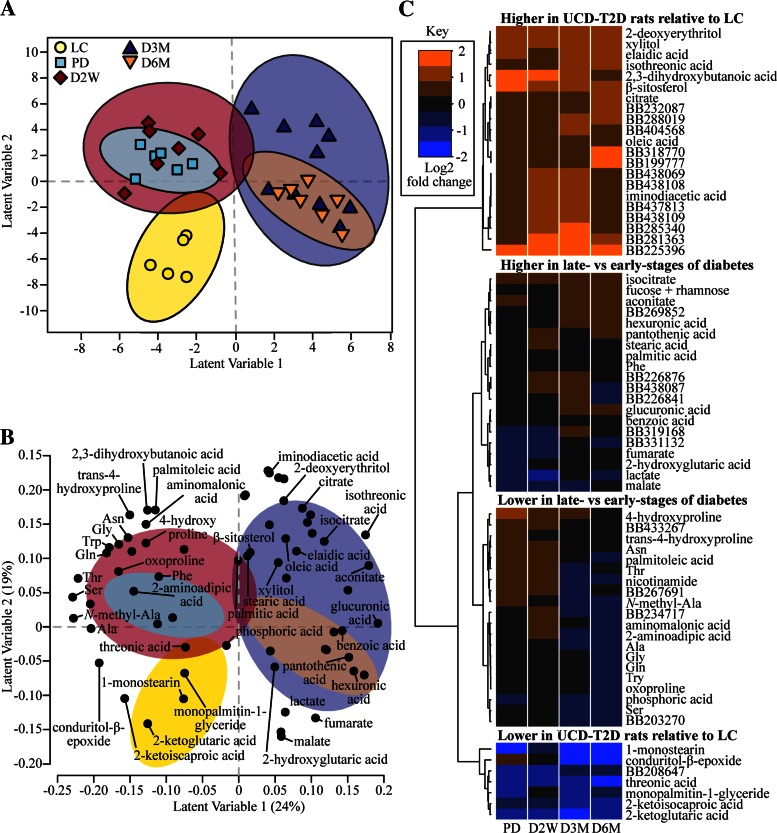
We found that 68 metabolites discriminated LC, PD, D2W, D3M, and D6M rats (model 1) based on an iterative backwards selection strategy for the PLS-DA model. Model 1 correctly classified 85% of the 11 rats in the test data using four latent variable dimensions. Separation of animal group clusters along latent variable dimensions 1 and 2 is portrayed in a PLS-DA scores plot, in which each symbol represents an individual rat (Fig. 5A). We then used the metabolites featured in model 1 (Fig. 5B) and constructed a heat map of the log2 fold difference of the metabolites in UCD-T2DM rats relative to the LC rats (Fig. 5C). Metabolites clustered within the top branch of the dendrogram in Fig. 5C are generally higher in all UCD-T2DM rat groups relative to LC animals, whereas metabolites in the bottom branch were all generally lower in the UCD-T2DM rats relative to LC rats. These metabolites correspond to the separation of LC rats from UCD-T2DM rats along latent variable 2 in model 1 (Fig. 5A).
Fig. 5.
Variance in select plasma metabolites discriminates rats by diabetes status and progression. A: partial least squares-discriminant analysis (PLS-DA) scores plot of lean Sprague-Dawley rats (LC; n = 5) and UCD-T2DM rats before the onset (PD; n = 6) of diabetes and 2 wk (D2W; n = 7), 3 mo (D3M; n = 10), and 6 mo (D6M; n = 7) post-onset of diabetes. Scores from individual rats are indicated as symbols. Confidence regions of group clusters are presented as 95% confidence ellipses based on Hotelling's T2 statistic. B: PLS-DA projections of discriminating metabolite factors in latent variables 1 and 2. Confidence ellipses from A are overlayed to help distinguish metabolites associated with group clusters from the scores plot. Only metabolites that have structural identification were annotated in the loadings plot for clarity. C: the log2 fold differences of UCD-T2DM rat groups relative to LC in metabolites selected in PLS-DA analyses are presented as a heat map. Known and unknown metabolites are provided in the heat map; unknowns are indicated by their BinBase (BB) number.
The two middle branches (Fig. 5C) provide more information regarding differences between early and late stages of diabetes and correspond to the separation of early and late stages of diabetes along latent variable 1 from model 1 (Fig. 5A). For example, the majority of the amino acids and amino acid derivatives are found in the lower-middle branch and have a larger negative fold difference in D3M and D6M (relative to LC) rats compared PD and D2W (i.e., blue shading in later-stage groups compared with black or orange shading in early-stage groups). The upper-middle branch (Fig. 5C) contains metabolites that generally have a greater log2 fold change relative to LC in D3M and D6M rats compared with PD and D2W, viz. they are increased in late diabetes vs. early diabetes. These metabolites include tricarboxylic acid cycle intermediates (isocitrate, aconitate, fumarate, and malic acid), hexuronic and glucuronic acids, stearic and palmitic acids, pantothenic acid, benzoic acid, 2-hydroxyglutaric acid, and lactate.
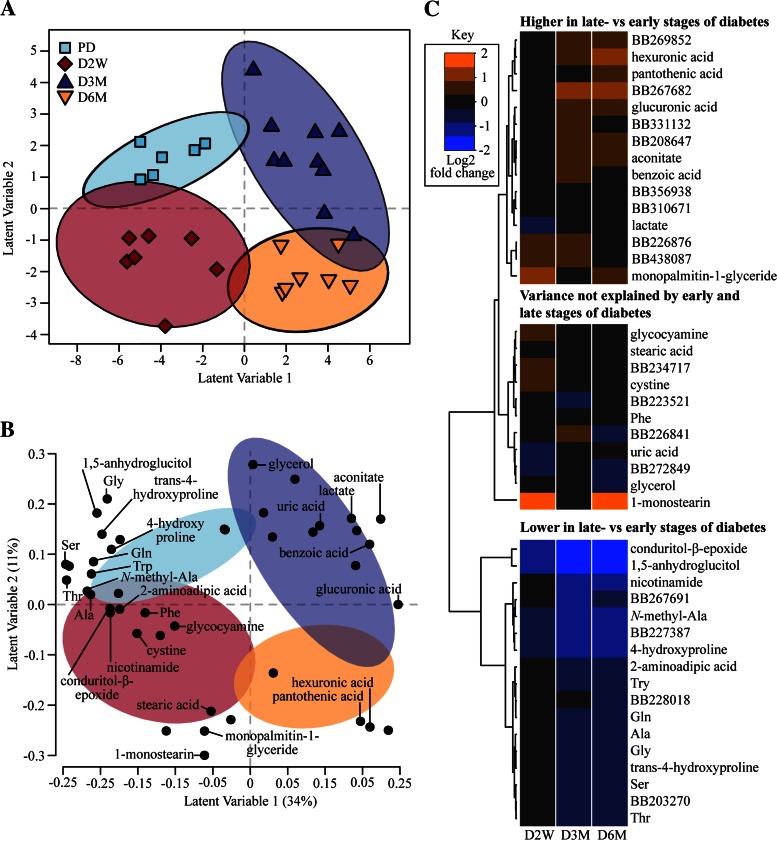
Much of the variance in model 1 explains the discrimination of LC rats from UCD-T2DM rats, raising the question of whether some metabolites depicted in Fig. 5 reflect an animal strain effect rather than the onset and progression of diabetes per se. Indeed, a post hoc analysis revealed that a total of 31 metabolites differed between LC and PD (Table 3). The relatively high number of metabolites statistically different between LC and PD prompted us to produce a second PLS-DA model, including only UCD-T2DM rat groups to differentiate prediabetic vs. diabetic groups within rat strain, and to better understand metabolites that track diabetes progression without the potential strain confounder (model 2). In this model, 82% of rats in the test set were correctly classified with four latent variables, and spatial separation of all groups was visualized along latent variables 1 and 2 (Fig. 6A). Most annotated metabolites in model 2 were also found in model 1, except for 1,5-anhydroglucitol, glucosamine, glycerol, cysteine, and uric acid (Fig. 6B). Using a similar heat map, but based on the log2 fold metabolite difference of D2W, D3M, and D6M rats relative to PD rats (Fig. 6C), we found that the majority of metabolites explain the differences between early and later stages of diabetes (upper and lower branches). There was also a group of metabolites whose variance does not appear to be explained by the early and late stages of diabetes (middle branch). Again, plasma concentrations of amino acids and amino acid derivatives are featured prominently in model 2 and are lower in the later stages of diabetes (D3M and D6M) relative to PD (Fig. 6C). Interestingly, monopalmitin-1-glyceride, 1-monostearin, and stearic acid were the only lipid-derived metabolites featured in model 2. In contrast to a high abundance of tricarboxylic acid cycle intermediates found in model 1, aconitate was the only one also identified in model 2 (Fig. 6B). Similarly to model 1, plasma concentrations of hexuronic and glucuronic acid, lactate, and benzoic acid were also identified by model 2 (Fig. 6B) and also greater in D3M and D6M (Fig. 6C).
Table 3.
Significantly different metabolites among LC and PD
| Parameter | LC | PD | FC | P Value |
|---|---|---|---|---|
| BB307889 | 450 ± 51 | 2,082 ± 1163 | 2.210 | ≤0.01 |
| BB225396 | 318 ± 34 | 1,455 ± 338 | 2.194 | ≤0.05 |
| β-Sitosterol | 958 ± 68 | 2,848 ± 238 | 1.572 | ≤0.01 |
| 3-Phenyl-3-hydroxypropanoic acid | 279 ± 21 | 764 ± 74 | 1.453 | ≤0.01 |
| 2,3-Dihydroxybutanoic acid | 572 ± 91 | 1,516 ± 177 | 1.406 | ≤0.01 |
| 4-Hydroxyproline | 3,397 ± 254 | 8,804 ± 1,075 | 1.374 | ≤0.05 |
| Xylitol | 2,679 ± 241 | 5,953 ± 688 | 1.152 | ≤0.05 |
| BB199250 | 1,502 ± 161 | 3,258 ± 238 | 1.117 | ≤0.01 |
| 2-Deoxyerythritol | 2,202 ± 172 | 4,113 ± 193 | 0.901 | ≤0.01 |
| BB280602 | 179 ± 19 | 319 ± 26 | 0.834 | ≤0.05 |
| Citric acid | 51,760 ± 4,875 | 82,715 ± 3,460 | 0.676 | ≤0.05 |
| BB199794 | 9,865 ± 920 | 15,443 ± 889 | 0.647 | ≤0.05 |
| BB270003 | 1,123 ± 137 | 1,753 ± 127 | 0.642 | ≤0.05 |
| BB232087 | 53,012 ± 4,418 | 82,559 ± 3,476 | 0.639 | ≤0.05 |
| BB310006 | 374 ± 26 | 579 ± 48 | 0.631 | ≤0.05 |
| BB433267 | 20,248 ± 1,970 | 30,905 ± 1,611 | 0.610 | ≤0.05 |
| BB404568 | 925 ± 70 | 1,355 ± 79 | 0.551 | ≤0.05 |
| Isothreonic acid | 700 ± 64 | 1,012 ± 49 | 0.532 | ≤0.05 |
| Indole-3-acetate | 295 ± 12 | 426 ± 28 | 0.530 | ≤0.01 |
| N-methylalanine | 8,660 ± 451 | 12,442 ± 824 | 0.523 | ≤0.05 |
| BB199777 | 50,042 ± 3,656 | 71,849 ± 3,438 | 0.522 | ≤0.05 |
| BB318770 | 49,822 ± 3,812 | 71,548 ± 3,421 | 0.522 | ≤0.05 |
| Threonine | 62,697 ± 2,290 | 85,811 ± 2,652 | 0.453 | ≤0.01 |
| Phosphoric acid | 152,729 ± 6,372 | 121,622 ± 5,615 | −0.329 | ≤0.05 |
| Urea | 495,391 ± 4,4187 | 286,409 ± 16,521 | −0.790 | ≤0.05 |
| α-Ketoglutaric acid | 3,766 ± 391 | 2,059 ± 175 | −0.871 | ≤0.05 |
| BB208647 | 2,964 ± 260 | 1,384 ± 91 | −1.099 | ≤0.01 |
| Monopalmitin-1-glyceride | 371 ± 26 | 172 ± 24 | −1.109 | ≤0.05 |
| 1, 5-anhydroglucitol | 23,197 ± 1,682 | 10,561 ± 2,033 | −1.135 | ≤0.05 |
| Threonic acid | 4,907 ± 664 | 2,207 ± 333 | −1.153 | ≤0.05 |
| 1-Monostearin | 1,993 ± 364 | 142 ± 10 | −3.811 | ≤0.05 |
Values in LC and PD columns are means ± SE.
LC, lean Sprague-Dawley controls (n = 7); PD, prediabetic UCD-T2DM rats (n = 8); FC, log2 fold change of PD relative to LC means; BB, BinBase. Unidentified metabolites indicated with their BB nomenclature. Units are quantifier ion peak heights from time-of-flight/gas chromatography/mass spectrometry. Mann-Whitney U-tests were used to compare LC and PD groups. False discovery rate-adjusted P values (Benjamini and Hochberg) are given.
Fig. 6.
Variance in select plasma metabolites discriminates rats by diabetes status and progression when comparing only prediabetic with diabetic UCD-T2DM rats. A: partial least squares-discriminant analysis (PLS-DA) scores plot characterization of UCD-T2DM rats before the onset (PD; n = 6) of diabetes and 2 wk (D2W; n = 7), 3 mo (D3M; n = 10), and 6 mo (D6M; n = 7) post-onset of diabetes. PLS-DA projections of individual rats from latent variables 1 and 2 are presented as symbols. Confidence regions of group clusters are presented as 95% confidence ellipses based on Hotelling's T2 statistic. B: PLS-DA projections of metabolites in latent variables 1 and 2 are presented as a loadings plot. Confidence ellipses from A are overlayed to help distinguish metabolites associated with group clusters from the scores plot. Only metabolites that have structural identification were annotated in the loadings plot. C: the log2 fold differences of UCD-T2DM rat groups relative to LC in metabolites selected in PLS-DA analyses are presented as a heat map. Known and unknown metabolites are provided in the heat map; unknowns are indicated by their BinBase (BB) number.
DISCUSSION
In this study, an untargeted metabolomics approach was used to investigate changes of plasma BCAAs and other amino acids during the onset and progression of diabetes in the UCD-T2DM rat model, and in addition we identified non-amino acid metabolites that exhibited temporal changes with diabetes progression. We did not observe significant differences in plasma concentrations of any BCAA in PD or D2W rats compared with LC animals, and only UCD-T2DM rats with diabetes of 6 mo duration (D6M) had increased circulating BCAA levels. Thus, the increase in BCAA at 6 mo post-onset of diabetes occurs well after the UCD-T2DM rat develops peripheral insulin resistance and progressive β-cell dysfunction (9). We interpret this to indicate that systemic perturbations of BCAA metabolism are a consequence of impaired metabolic regulation rather than a causative factor in the progression of insulin resistance and diabetes in this model.
We utilized the UCD-T2DM rat model to assess changes in plasma BCAA amino acids because the UCD-T2DM rat phenotype more closely resembles clinical T2DM in humans compared with other rodent models. Although many features of the UCD-T2DM rat model are consistent with the human diabetic phenotype relative to other rodent models (e.g., functional leptin signaling, female fertility, and later ages of diabetes onset), our results suggest divergent plasma BCAA phenotypes. Higher BCAA concentrations are regularly observed in individuals with insulin resistance or T2DM relative to control groups in human clinical studies (12, 13, 29, 41, 45, 51, 52), leading some to hypothesize that elevated BCAAs contribute or are causative of insulin resistance and/or deterioration of metabolic health (30). This association is less consistent in rodent models of insulin resistance and diabetes, since it has been reported in some cases (10, 39, 40) but not in others (19, 35). Results from the current study are more consistent with the latter studies, and the increase in circulating BCAA levels in the UCD-T2DM rat model appears to coincide with late-stage fulminant diabetic phenotype rather than insulin resistance. Thus, the BCAA metabolism in the UCD-T2DM rat model and other rat models of diabetes may have limited direct applicability to human T2DM. However, the results from this model still provide important new evidence that elevations in plasma BCAAs are not necessary to induce or further aggravate insulin resistance.
Our observation of elevated plasma BCAAs coupled to the reduction of most other plasma amino acids in unregulated late-stage diabetes is not without precedent. Wijekoon et al. (50) and Belabed et al. (5) observed similar plasma amino acid trends in ZDF rats compared with age-matched lean counterparts. The elevation of plasma BCAAs, in contrast to all other amino acids, supports the view that the BCAAs are differentially regulated in the later stages of uncontrolled T2DM. This would imply that the rate-limiting step in BCAA mitochondrial metabolism, branched-chain ketoacid dehydrogenase complex (BCKDC), may have a reduced enzymatic activity. It has long been known that BCKDC is reduced during starvation and type 1 diabetes mellitus (17, 34), which, again, are conditions that may be more reflective of the uncontrolled diabetes seen in D3M and D6M rats herein. Teleologically, reduced BCKDC activity under these conditions serves to attenuate irreversible losses of these essential amino acids (34). Countering this viewpoint, 2-ketoisocaproic acid, the branched-chain keto-acid (BCKA) derivative of leucine, was relatively unchanged between the early and later stages of diabetes. A reduction in BCKDC activity should increase plasma branched-chain keto-acid concentrations; however, we suspect that significant reductions in tissues containing high concentrations of branched-chain aminotransferase (e.g., skeletal muscle and adipose tissue) resulted in a diminution in the initial step in BCAA catabolism. Although unavailable for the current study, information regarding BCAA and amino acid enzyme activities would be an important area for future studies in the UCD-T2DM model.
The premise that blood BCAA patterns reflect metabolic perturbations associated with T2D development or insulin resistance suggests that amelioration of insulin resistance through interventions would normalize BCAA concentrations. Significant decreases in plasma BCAA concentrations have been widely observed after gastric bypass surgery (25, 38, 43), which is known to dramatically improve insulin sensitivity within 6 days after surgery (49). Interestingly, Laferrère et al. (25) compared individuals with equal weight loss from either gastric bypass surgery or caloric restriction and found that only the individuals treated with gastric bypass surgery had a significant reduction in plasma BCAA concentrations. We have found that baseline BCAA concentrations were significantly correlated to HOMA-IR in obese women with metabolic syndrome, but these correlations were no longer significant after a weight loss intervention and improvements in insulin sensitivity (32). Pharmacological treatment of insulin resistance or T2DM with thiazolidinedione (TZD) treatment decreased serum Val concentrations (4) and increased adipose BCKDH gene expression (37). In contrast, Irving et al., found no changes in plasma BCAA concentrations after 12-wk of combined thiazolidinedione and metformin treatments (18). Metformin treatment alone had no effect on plasma BCAA (33) and increased plasma Leu and Iso after 2 days of treatment (44). Collectively, these studies indicate that plasma BCAA concentrations and BCAA pathways are linked to insulin sensitivity, but these relationships are context specific. Future studies in the UCD-T2DM rat that involve pharmacological and nonpharmacologic interventions to normalize metabolic homeostasis may help elucidate further the connections between systemic BCAAs and metabolic health.
A secondary aim of this study was to characterize more globally which metabolic pathways best discriminate progression of T2DM-like phenotype in the UCD-T2DM rat model. We found that most of the variance in the metabolome could be explained by differences between either LC and early (PD and D2W as a group) relative to later stages of diabetes (D3M and D6M as a group), as highlighted in Figs. 5 and 6. Amino acids (e.g., Thr, Ser, Ala, Gly, Gln, Trp, and Phe) and amino acid derivatives were the most striking and abundant features in both PLS-DA models and were associated with the early stages of diabetes (i.e., greater in PD and D2W animals compared with D3M and D6M animals). Two amino acid derivatives of particular interest are 2-aminoadipic acid and aminomalonic acid; both were reduced in the later stages of diabetes. Part of the Lys synthesis pathway, 2-aminoadipic has been identified in several metabolomics studies. Wang et al. (46) reported that individuals from the Framingham Heart Study with the highest concentrations of plasma 2-aminoadipic acid had a significantly greater risk of later developing diabetes. Conversely, oral supplementation of 2-aminoadipic acid in C56BL/6 male mice fed a high-fat diet increased glucose clearance without altering insulin secretion (46), suggesting an important role for this metabolite in marking and modifying glucose disposal. Aminomalonic acid has not been well-characterized in metabolic disease, but has been isolated from Escherichia coli (7). Although speculative, these results suggest an interaction between the metabolic health of the host and the microbiome, as revealed previously in metabolomics studies in insulin-resistant women (8). Future work will need to be done to identify the origin of aminomalonic acid and its relationship to insulin resistance. Considering the deterioration of glucose control that defines progression from prediabetes to frank diabetes, it is not surprising that several metabolites related to carbohydrate metabolism were identified prominently in multivariate statistical analyses discriminating early and late diabetes. Plasma 1,5-anhydroglucitol concentration is considered as a biomarker for short-term glycemic control (27, 28, 41, 53). Renal reabsorption of 1,5-anhydroglucitol is inhibited under conditions of hyperglycemia, thus explaining the lower plasma concentration at the later stages of diabetes compared with prediabetes stages. Higher glucuronic and hexuronic acids were associated with later stages of diabetes. There is very little known about how T2DM affects these metabolites, but plasma glucuronic acid was also found to be increased in obese women with T2DM relative to weight-matched control subjects (13).
Compared with lean Sprague-Dawley rats, prediabetic UCD-T2DM rats have greater adiposity, are hyperphagic, are insulin resistant, and display abnormal β-cell morphology (9); thus, it was not surprising to identify metabolites that differentiate these animals. The groups of rats also shared housing and dietary conditions and were of similar age, which were strengths of the study design. However, we acknowledge that comparing the UCD-T2DM rats with a lean Sprague-Dawley strain introduces genetic strain differences that confound interpretations specifically related to metabolic status. Nevertheless, further evaluations compared the within-strain transition from prediabetes to frank diabetes, thus enabling a unique opportunity to determine the metabolite landscape of diabetes development in this model.
Untargeted metabolomics was used to determine temporal differences in metabolites in a relatively small set of UCD-T2DM rats. Thus, a limitation of this study is that it involves a high variable-to-sample ratio, which substantially increases the chances of false discoveries. To counter this, we used a PLS-DA modeling paradigm since it is robust to small sample sizes and collinearity. We also used a training/test data scheme to protect to validate PLS-DA models and minimize overfitting (48). Our findings revealed that despite differences in some metabolite patterns compared with human diabetes, there are many plasma metabolites in the UCD-T2DM rat model that have been identified previously in human cohorts. Therefore, the UCD-T2DM rat may be an adequate model to validate and explore mechanistic pathways associated with these potential biomarkers of type 2 diabetes.
In conclusion, we utilized untargeted metabolomics to identify changes in plasma BCAAs and other amino acids during the progression of uncontrolled diabetes in age-matched UCD-T2DM rats. Plasma concentrations of BCAA did not increase until 6 mo post-onset of diabetes and were inversely correlated with adiposity and plasma insulin levels, suggesting that the increases in BCAA are likely due to insulin deficiency and the catabolic state of uncontrolled diabetes. In contrast to plasma BCAAs, most non-BCAAs decreased during the progression of diabetes. The increase in BCAAs relative to the decrease in other amino acids in the later stages of diabetes is possibly coupled to a negative feedback mechanism that limits BCAA oxidation to mitigate protein losses. Multivariate analysis identified several other diabetes-regulated plasma metabolites that included amino acids, amino acid derivatives, and carbohydrate derivatives. A clear alteration in circulating amino acids was a dominant feature of diabetes progression in the UCD-T2DM rat, highlighting that the metabolic dysregulation associated with T2DM includes marked perturbations in circulating amino acids. The late-stage plasma elevations of BCAAs established that an increase in circulating BCAAs is not necessary or sufficient to induce insulin resistance and diabetes in the UCD-T2DM rat, and elevated BCAA levels were a consequence, not a cause, of progressive metabolic deterioration.
GRANTS
This research was supported by an intramural US Department of Agriculture-Agricultural Research Service Project (5306-51530-019-00), National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) Grant R01-DK-078328-01 (S. H. Adams), and the West Coast Comprehensive Metabolomics Center funded by the NIH/NIDDK (U24-DK-097154; O. Fiehn). P. J. Havel's laboratory also received funding during the project period from NIH grants R01-HL-091333, R01-HL-107256, R01-HL-121324, U24-DK-092993, RC1-DK-087307, and R01-DK-095060 and a Multicampus Award from the University of California, Office of the President (award no. 142691).
DISCLOSURES
No potential conflicts of interest relevant to this article, financial or otherwise, were reported.
AUTHOR CONTRIBUTIONS
B.D.P. analyzed data; B.D.P. and S.H.A. interpreted results of experiments; B.D.P. prepared figures; B.D.P. and S.H.A. drafted manuscript; B.D.P., J.L.G., K.L.S., O.F., P.J.H., and S.H.A. edited and revised manuscript; B.D.P., J.L.G., K.L.S., O.F., P.J.H., and S.H.A. approved final version of manuscript; J.L.G., K.L.S., O.F., and P.J.H. performed experiments; O.F., P.J.H., and S.H.A. conception and design of research.
Supplementary Material
REFERENCES
- 1.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139: 1073–1081, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr 2: 445–456, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badoud F, Lam KP, DiBattista A, Perreault M, Zulyniak MA, Cattrysse B, Stephenson S, Britz-McKibbin P, Mutch DM. Serum and adipose tissue amino acid homeostasis in the metabolically healthy obese. J Proteome Res 13: 3455–3466, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Bao Y, Zhao T, Wang X, Qiu Y, Su M, Jia W, Jia W. Metabonomic variations in the drug-treated type 2 diabetes mellitus patients and healthy volunteers. J Proteome Res 8: 1623–1630, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Belabed L, Senon G, Blanc MC, Paillard A, Cynober L, Darquy S. The equivocal metabolic response to endotoxaemia in type 2 diabetic and obese ZDF rats. Diabetologia 49: 1349–1359, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met 57: 289–300, 1995. [Google Scholar]
- 7.Van Buskirk JJ, Kirsch WM, Kleyer DL, Barkley RM, Koch TH. Aminomalonic acid: identification in Escherichia coli and atherosclerotic plaque. Proc Natl Acad Sci USA 81: 722–725, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell C, Grapov D, Fiehn O, Chandler CJ, Burnett DJ, Souza EC, Casazza GA, Gustafson MB, Keim NL, Newman JW, Hunter GR, Fernandez JR, Garvey WT, Harper ME, Hoppel CL, Meissen JK, Take K, Adams SH. Improved metabolic health alters host metabolism in parallel with changes in systemic xeno-metabolites of gut origin. PLoS One 9: e84260, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am J Physiol Regul Integr Comp Physiol 295: R1782–R1793, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doisaki M, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Goto H, Fujita Y, Kadota Y, Kitaura Y, Bajotto G, Kazama S, Tamura T, Tamura N, Feng GG, Ishikawa N, Shimomura Y. Regulation of hepatic branched-chain alpha-keto acid dehydrogenase kinase in a rat model for type 2 diabetes mellitus at different stages of the disease. Biochem Biophys Res Commun 393: 303–307, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Felig P, Marliss E, Cahill GF. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 281: 811–816, 1969. [DOI] [PubMed] [Google Scholar]
- 12.Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, Milburn MV, Kastenmüller G, Adamski J, Tuomi T, Lyssenko V, Groop L, Gall WE. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 62: 1730–1737, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One 5: e15234, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG, Fritsche A, Häring HU, Hrabě de Angelis M, Peters A, Roden M, Prehn C, Wang-Sattler R, Illig T, Schulze MB, Adamski J, Boeing H, Pischon T. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 62: 639–648, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, Ryals JA, Milburn MV, Nannipieri M, Camastra S, Natali A, Ferrannini E. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 5: e10883, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffen SC, Wang J, German MS. A genetic defect in beta-cell gene expression segregates independently from the fa Locus in the ZDF rat. Diabetes 50: 63–68, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Harris RA, Goodwin GW, Paxton R, Dexter P, Powell SM, Zhang B, Han A, Shimomura Y, Gibson R. Nutritional and hormonal regulation of the activity state of hepatic branched-chain alpha-keto acid dehydrogenase complex. Ann NY Acad Sci 573: 306–313, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Irving BA, Carter RE, Soop M, Weymiller A, Syed H, Karakelides H, Bhagra S, Short KR, Tatpati L, Barazzoni R, Nair KS. Effect of insulin sensitizer therapy on amino acids and their metabolites. Metabolism 64: 720–728, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacob RJ, Sherwin RS, Greenawalt K, Shulman GI. Simultaneous insulinlike growth factor I and insulin resistance in obese zucker rats. Diabetes 41: 691–697, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Kendall DM, Cuddihy RM, Bergenstal RM. Clinical application of incretin-based therapy: therapeutic potential, patient selection and clinical use. Am J Med 122: S37–S50, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Komsta L. Outliers: Tests for Outliers (Online). R Package version 0.14. http://CRAN.R-project.org/package=outliers [2011]. [Google Scholar]
- 22.Kuhn M, Johnson K. Applied Predictive Modeling. New York: Springer Science & Business Media, 2013. [Google Scholar]
- 23.Kuhn M. Caret: Classification and Regression Training (Online). R package version 6.0–41. http://CRAN.R-project.org/package=caret [2015]. [Google Scholar]
- 24.Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, Karpe F, Humphreys S, Bedinger DH, Dunn TN, Thomas AP, Oort PJ, Kieffer DA, Amin R, Bettaieb A, Haj FG, Permana P, Anthony TG, Adams SH. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 304: E1175–E1187, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laferrère B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, Bose M, Teixeira J, Stevens RD, Wenner BR, Bain JR, Muehlbauer MJ, Haqq A, Lien L, Shah SH, Svetkey LP, Newgard CB. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med 3: 80re2, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10: 723–736, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menni C, Fauman E, Erte I, Perry JR, Kastenmüller G, Shin SY, Petersen AK, Hyde C, Psatha M, Ward KJ, Yuan W, Milburn M, Palmer CN, Frayling TM, Trimmer J, Bell JT, Gieger C, Mohney RP, Brosnan MJ, Suhre K, Soranzo N, Spector TD. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 62: 4270–4276, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mook-Kanamori DO, Selim MM, Takiddin AH, Al-Homsi H, Al-Mahmoud KA, Al-Obaidli A, Zirie MA, Rowe J, Yousri NA, Karoly ED, Kocher T, Sekkal Gherbi W, Chidiac OM, Mook-Kanamori MJ, Abdul Kader S, Al Muftah WA, McKeon C, Suhre K. 1,5-Anhydroglucitol in saliva is a noninvasive marker of short-term glycemic control. J Clin Endocrinol Metab 99: E479–E483, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9: 311–326, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15: 606–614, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, Hess JF. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet 13: 18–19, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Piccolo BD, Comerford KB, Karakas SE, Knotts TA, Fiehn O, Adams SH. Whey protein supplementation does not alter plasma branched-chained amino acid profiles but results in unique metabolomics patterns in obese women enrolled in an 8-week weight loss trial. J Nutr 145: 691–700, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Preiss D, Rankin N, Welsh P, Holman RR, Kangas AJ, Soininen P, Würtz P, Ala-Korpela M, Sattar N. Effect of metformin therapy on circulating amino acids in a randomized trial: the CAMERA study: Metabolism. Diabet Med. In press. [DOI] [PubMed] [Google Scholar]
- 34.Randle PJ. Alpha-ketoacid dehydrogenase complexes and respiratory fuel utilization in diabetes. Diabetologia 28: 479–484, 1985. [DOI] [PubMed] [Google Scholar]
- 35.Sailer M, Dahlhoff C, Giesbertz P, Eidens MK, de Wit N, Rubio-Aliaga I, Boekschoten MV, Müller M, Daniel H. Increased plasma citrulline in mice marks diet-induced obesity and may predict the development of the metabolic syndrome. PLoS One 8: e63950, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scholz M, Fiehn O. SetupX—a public study design database for metabolomic projects. Pac Symp Biocomput 2007: 169–180, 2007. [PubMed] [Google Scholar]
- 37.Sears DD, Hsiao G, Hsiao A, Yu JG, Courtney CH, Ofrecio JM, Chapman J, Subramaniam S. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc Natl Acad Sci USA 106: 18745–18750, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrere B, Gorroochurn P, Teixeira J, Brantley PJ, Stevens VJ, Hollis JF, Appel LJ, Lien LF, Batch B, Newgard CB, Svetkey LP. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55: 321–330, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab 293: E1552–E1563, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.She P, Olson KC, Kadota Y, Inukai A, Shimomura Y, Hoppel CL, Adams SH, Kawamata Y, Matsumoto H, Sakai R, Lang CH, Lynch CJ. Leucine and protein metabolism in obese Zucker rats. PLoS One 8: e59443, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suhre K, Meisinger C, Döring A, Altmaier E, Belcredi P, Gieger C, Chang D, Milburn MV, Gall WE, Weinberger KM, Mewes HW, Hrabé de Angelis M, Wichmann HE, Kronenberg F, Adamski J, Illig T. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One 5: e13953, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suhre K. Metabolic profiling in diabetes. J Endocrinol 221: R75–R85, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Tan HC, Khoo CM, Tan MZW, Kovalik JP, Ng ACM, Eng AKH, Lai OF, Ching JH, Tham KW, Pasupathy S. The Effects of Sleeve Gastrectomy and Gastric Bypass on Branched-Chain Amino Acid Metabolism 1 Year After Bariatric Surgery. Obes Surg. In press. [DOI] [PubMed] [Google Scholar]
- 44.Walford GA, Davis J, Warner AS, Ackerman RJ, Billings LK, Chamarthi B, Fanelli RR, Hernandez AM, Huang C, Khan SQ, Littleton KR, Lo J, McCarthy RM, Rhee EP, Deik A, Stolerman E, Taylor A, Hudson MS, Wang TJ, Altshuler D, Grant RW, Clish CB, Gerszten RE, Florez JC. Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism 62: 1772–1778, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med 17: 448–453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang TJ, Ngo D, Psychogios N, Dejam A, Larson MG, Vasan RS, Ghorbani A, O'Sullivan J, Cheng S, Rhee EP, Sinha S, McCabe E, Fox CS, O'Donnell CJ, Ho JE, Florez JC, Magnusson M, Pierce KA, Souza AL, Yu Y, Carter C, Light PE, Melander O, Clish CB, Gerszten RE. 2-Aminoadipic acid is a biomarker for diabetes risk. J Clin Invest 123: 4309–4317, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, Heim K, Campillos M, Holzapfel C, Thorand B, Grallert H, Xu T, Bader E, Huth C, Mittelstrass K, Doring A, Meisinger C, Gieger C, Prehn C, Roemisch-Margl W, Carstensen M, Xie L, Yamanaka-Okumura H, Xing G, Ceglarek U, Thiery J, Giani G, Lickert H, Lin X, Li Y, Boeing H, Joost HG, de Angelis MH, Rathmann W, Suhre K, Prokisch H, Peters A, Meitinger T, Roden M, Wichmann HE, Pischon T, Adamski J, Illig T. Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol 8: 615, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westerhuis JA, Hoefsloot HC, Smit S, Vis DJ, Smilde AK, van Velzen EJJ, van Duijnhoven JP, van Dorsten FA. Assessment of PLSDA cross validation. Metabolomics 4: 81–89, 2008. [Google Scholar]
- 49.Wickremesekera K, Miller G, Naotunne TD, Knowles G, Stubbs RS. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg 15: 474–481, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Wijekoon EP, Skinner C, Brosnan ME, Brosnan JT. Amino acid metabolism in the Zucker diabetic fatty rat: effects of insulin resistance and of type 2 diabetes. Can J Physiol Pharmacol 82: 506–514, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Würtz P, Tiainen M, Mäkinen VP, Kangas AJ, Soininen P, Saltevo J, Keinänen-Kiukaanniemi S, Mäntyselkä P, Lehtimäki T, Laakso M, Jula A, Kähönen M, Vanhala M, Ala-Korpela M. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care 35: 1749–1756, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Yan L, Chen W, Lin L, Song X, Yan X, Hang W, Huang B. Metabonomics research of diabetic nephropathy and type 2 diabetes mellitus based on UPLC-oaTOF-MS system. Anal Chim Acta 650: 16–22, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Wang S, Puhl MD, Jiang X, Hyrc KL, Laciny E, Wallendorf MJ, Pappan KL, Coyle JT, Wice BM. Global biochemical profiling identifies β-hydroxypyruvate as a potential mediator of type 2 diabetes in mice and humans. Diabetes 64: 1383–1394, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.