Abstract
Contact between β-cells is necessary for their normal function. Identification of the proteins mediating the effects of β-cell-to-β-cell contact is a necessary step toward gaining a full understanding of the determinants of β-cell function and insulin secretion. The secretory machinery of the β-cells is nearly identical to that of central nervous system (CNS) synapses, and we hypothesize that the transcellular protein interactions that drive maturation of the two secretory machineries upon contact of one cell (or neural process) with another are also highly similar. Two such transcellular interactions, important for both synaptic and β-cell function, have been identified: EphA/ephrin-A and neuroligin/neurexin. Here, we tested the role of another synaptic cleft protein, CADM1, in insulinoma cells and in rat and human islet β-cells. We found that CADM1 is a predominant CADM isoform in β-cells. In INS-1 cells and primary β-cells, CADM1 constrains insulin secretion, and its expression decreases after prolonged glucose stimulation. Using a coculture model, we found that CADM1 also influences insulin secretion in a transcellular manner. We asked whether extracellular CADM1 interactions exert their influence via the same mechanisms by which they influence neurotransmitter exocytosis. Our results suggest that, as in the CNS, CADM1 interactions drive exocytic site assembly and promote actin network formation. These results support the broader hypothesis that the effects of cell-cell contact on β-cell maturation and function are mediated by the same extracellular protein interactions that drive the formation of the presynaptic exocytic machinery. These interactions may be therapeutic targets for reversing β-cell dysfunction in diabetes.
Keywords: cell adhesion molecule 1, SynCam, pancreatic islet, insulin secretion
β-cells require contact with other β-cells to mature and function normally (12, 19, 27). This contact gives rise to transcellular protein interactions that drive the maturation and help regulate the function of the insulin secretory machinery (19, 27, 31, 40, 50). Consistent with an essential role of interactions between β-cells, insulin exocytic complexes assemble under the plasma membrane at sites of β-cell-to-β-cell contact (16). Identifying the transcellular protein interactions that mediate the effects of β-cell-to-β-cell contact and help guide assembly and functioning of the insulin secretory machinery is crucial for understanding how contact between β-cells promotes functional maturation and helps to control insulin secretion.
β-Cells and neurons are very much alike, with similar patterns of protein expression and shared developmental pathways, and likely derive from a common evolutionary ancestral cell type in the primitive central nervous system (CNS) (1, 2, 41, 58). The insulin secretory machinery in particular bears a striking resemblance to the synaptic machinery for neurotransmitter release, and the width of the interstitial space between β-cells approximates that of the synaptic cleft (1, 2, 29, 48). Synapse formation (synaptogenesis) is triggered by direct interactions between proteins on the surfaces of contacting neural processes (13, 47). Given the parallels between the synaptic and β-cell exocytic machinery, the cell surface proteins mediating the effects of contact between β-cells may be the same as those that guide synaptogenesis (50). Previously, we described one such synaptogenic protein interaction, neuroligin-neurexin, that influences β-cell function; another, EphA-ephrin-A, was described elsewhere (31, 40, 50).
Like members of the neuroligin/neurexin and Eph/ephrin protein families, members of the CADM (cell adhesion molecule) protein family are synaptogenic: transcellular interactions between CADM proteins on contacting neural processes trigger pre- and postsynaptic differentiation (7). CADMs are their own extracellular binding partners; interactions are either homophilic or heterophilic with other CADM isoforms (14). Previously, we found that CADM1 (also referred to as SynCAM1, Necl2, TSLC1, and IGSF4) is expressed in islet α- and β-cells (48). Subsequently, CADM1 was found to be a key target of the microRNA miR-375 (51, 52). This is the most abundant β-cell microRNA and participates in the regulation of islet function, including insulin and glucagon secretion, and α- and β-cell proliferation (42, 51, 52). Regulation of CADM1 expression by miR-375 underscores the potential importance of the protein in β-cell development and function.
In α-cells, CADM1 helps constrain glucagon secretion (23). Enhanced insulin secretion in CADM1 global knockout mice suggests that CADM1 similarly inhibits insulin exocytosis (38). Alternatively, the increased secretion in this mouse model could reflect an effect of CADM1 deficiency on the CNS or some other tissue. The subplasmalemmal insulin secretory machinery includes a set of proteins that constitute a mechanism for halting insulin secretion just prior to insulin release (26, 40, 63). Determination that CADM1 inhibited insulin exocytosis would implicate it in this regulatory mechanism.
Here, we investigated the role of CADM in β-cell function. We found that CADM1 is the predominant CADM isoform in human islets and, along with CADM4, one of two predominant isoforms in INS-1 cells and rat islets. We show that insulin secretion varies inversely with CADM1 expression. Furthermore, we show that β-cell expression of CADM1 decreases after glucose stimulation and that CADM1 binds essential components of the β-cell secretory machinery. Asking whether, as in the synapse, transcellular interactions contribute to the effect of CADM1 on exocytic function, we found that transcellular CADM1 interactions do indeed influence insulin secretion, and we provide evidence that, as in the synapse, they do so through effects on assembly of the secretory machinery and the cortical actin network. These results bring to three the number of synaptic cleft, synaptogenic protein interactions known to also help determine insulin secretion via extracellular interactions. They provide further evidence that parallel sets of transcellular protein interactions organize the synaptic neurotransmitter secretory machinery and the submembrane β-cell insulin secretory apparatus.
RESEARCH DESIGN AND METHODS
Antibody and plasmid reagents.
Antibodies used were rabbit anti-CADM1 and mouse anti-GADPH, anti-FLAG, anti-syntaxin-1, and anti-CASK (all from Sigma, St. Louis, MO); mouse anti-synaptophysin and anti-Munc18 (BD, Franklin Lakes, NJ); rabbit anti-EPB41L3/DAL-1 (ThermoFisher, Waltham, MA); IRDye 680-conjugated anti-mouse IgG and IRDye 800CW-conjugated anti-rabbit IgG (LI-COR); and Alexa Fluor 488 anti-rabbit and 594 anti-mouse IgG (Life Technologies, Carlsbad, CA). The expression construct for FLAG-tagged CASPR1 was generously provided by Davide Comoletti (Robert Wood Johnson Medical School). The expression plasmid encoding FLAG-tagged CADM1 was generated by adding a FLAG-tag to full-length CADM1 cDNA (kindly provided by Thomas Biederer, Tufts University) and insertion into pcDNA4 (Life Technologies).
Cell culture and transfection.
INS-1 cells were cultured in RPMI 1640 medium containing 10% FBS, 2 mM l-glutamine, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, and penicillin-streptomycin. Islets were cultured in the same medium without 2-mercaptoethanol or sodium pyruvate. COS-7 cells were cultured in DMEM containing 10% FBS, 2 mM l-glutamine, and penicillin-streptomycin. COS cells were also cocultured with INS-1 cells or islet cells in a 1:1 mixture of the RPMI- and DMEM-based media. For dissociation, islets were incubated overnight and then washed with Hanks' buffered saline solution without calcium or magnesium. Islets were then treated with 0.01% trypsin solution in Hanks' buffered saline solution for 3 min at 37°C, followed by mechanical disruption using a P200 pipette. Cocultures were seeded with cells from ∼50 islets (rat) or islet equivalents (human) per well. Details regarding this coculture method are available in video and print (61). Cells were maintained in a humidified 37°C incubator with 5% CO2. Transfections took place in 24-well plates using DNA constructs or siRNA duplexes mixed with Lipofectamine 2000 (Life Technologies) according to the manufacturer's protocols. RNA interference experiments used pooled siRNAs and a nontargeting control siRNA pool (Dharmacon, Lafayette, CO). Knockdown was quantified by quantitative PCR (qPCR) analysis. COS cells were transfected at 100% confluency. INS-1 cells were transfected at 30% confluency and harvested 24 or 72 h after transfection with plasmid or siRNA, respectively.
Immunoblotting.
Protein extracts were prepared by lysing cells in RIPA buffer (150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 50 mM Tris·HCl, 2 mM EDTA, 1 mM phenylmethanesulfonylfluoride, and protease inhibitor cocktail; Sigma). Protein was quantified using the DC Protein Assay (Bio-Rad, Irvine, CA). Proteins (20 μg/lane) were electrophoresed in 4–12% Bis-Tris NuPAGE gels with an IR protein ladder (LI-COR) and then transferred to PVDF membranes. Membranes were blocked with 5% milk in PBS and probed with primary antibodies in Odyssey Blocking Buffer (LI-COR) overnight followed by IRDye-conjugated secondary antibodies in 5% milk in PBS with 0.1% Tween-20. Membranes were imaged and band density quantified using an Odyssey Infrared Imaging System (LI-COR).
Immunoprecipitation.
Cells were lysed in 150 mM NaCl, 1% Nonidet P-40, 50 mM Tris (pH 8), 1 mM phenylmethanesulfonylfluoride, and a protease inhibitor cocktail (Sigma). Lysates were precleared using protein G-Sepharose beads and then incubated overnight at 4°C with 5 μg of anti-CADM1 or purified rabbit nonspecific IgG. Next, incubation with protein G-Sepharose beads at 4°C for 2 h was followed by thorough washing with PBS. Samples were denatured in LDS sample buffer (Life Technologies) and dithiothreitol prior to Western blotting.
Islets.
Islets were isolated from adult male Sprague-Dawley rats (Harlan, Indianapolis, IN), as described previously (49), with adherence to University of California Irvine guidelines for the use and care of laboratory animals and under an Institutional Animal Care and Use Committee-approved protocol. Human islets were provided by the Integrated Islet Distribution Program (coordinated at the City of Hope, Duarte, CA; sponsor: National Institute of Diabetes and Digestive and Kidney Diseases).
Real-time qPCR.
Total RNA was isolated using GenElute mammalian RNA kit (Sigma) and then reverse-transcribed. Brain RNA was obtained from Clontech Laboratories. qPCR was performed using PerfeCTa SYBR Green FastMix (Quanta BioSciences, Gaithersburg, MD) on an ABI 7500 Fast Real-Time qPCR system. Samples were analyzed in duplicate alongside no-RT and no-template controls; values were normalized to 18S RNA. Primers were designed using Primer3 software and are shown in Table 1 (56). Analysis of qPCR results to yield relative change in message levels was by calculation of 2−ΔΔCT (36).
Table 1.
qRT-PCR primer sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| hCADM1 | GGTGATGGGCAGAATCTGTT | ACCAGGACTGTGATGGTGGT |
| hCADM2 | ATCCAGAAACGCAGGTGTTC | CGCCGCTAAGTACACATTGA |
| hCADM3 | GTGCTCAAGTGCCAAGTGAA | GGCTGTGTCTTTTTCCCGTA |
| hCADM4 | GGTTCCTATCTGACCCACGA | CCTCACTTCTGGCCCTTACA |
| rCADM1 | GAAGGACAGCAGGTTTCAGC | GCCTTTGAGTTCCTTGTTCC |
| rCADM2 | GACCGTAGCGATGATGGAGT | CAGGTTCTGGCAGTGGTTTT |
| rCADM3 | GGACCGCCAAGTCCCTCGTC | ATTCGCGTCTGGTCCCCGTG |
| rCADM4 | GTCATCTGTGAAGCGCAGAA | AGCACATGTCAGCACCAGAG |
qRT-PCR, quantitative real time-PCR; h, human; r, rat.
Flow cytometry.
FACS analysis was kindly overseen by Alberto Hayek (University of California, San Diego, CA) and carried out by Orion BioSolutions (Vista, CA), using a chicken anti-CADM1 monoclonal antibody (CM004-3; MBL International, Woburn, MA) as described previously (28). Briefly, dissociated islet cells were fixed in 2% paraformaldehyde, washed in ice-cold PBS, and permeabilized in 0.05% Triton X-100. Cells were labeled with antibodies to CADM1, proinsulin (Abcam), and amylase (Sigma) or nonimmune chicken IgY (MBL International). Antibody-labeled cells were stained with fluorescein isothiocyanate (FITC)- or R-phycoerythrin (PE)-labeled secondary antibodies. Cells were washed and resuspended in PBS and analyzed using a BD FacsScan instrument and CellQuest software (Cytometri Research).
Insulin secretion and glucose stimulation.
INS-1 cells were preincubated with 2.75 mM glucose in Krebs-Ringer bicarbonate buffer for 1 h and next incubated for 1 h in fresh Krebs-Ringer bicarbonate buffer containing either 2.75 mM glucose alone (basal conditions) or 16.7 mM glucose with 0.1 mM IBMX (stimulating conditions). IBMX was added along with glucose to potentiate glucose-stimulated insulin secretion, which is otherwise reduced substantially below physiological (in vivo) levels in insulinoma cells such as INS-1 cells and dissociated primary β-cells. Use of IBMX in this manner has been described previously and is fairly common in tissue culture studies of β-cell function (e.g., see Refs. 9, 20, 54, and 59). For potassium stimulation studies, 30 mM KCl instead of glucose was used. When indicated, latrunculin-B (10 μM; Adipogen, San Diego, CA) was added to the medium. After 1 h, the medium was collected, and cell lysates were prepared by 30-min incubation in RIPA buffer at 4°C. Insulin was measured by RIA (Millipore, Billerica, MA). Secreted insulin was normalized to total insulin content determined from cell lysates.
Syntaxin clustering.
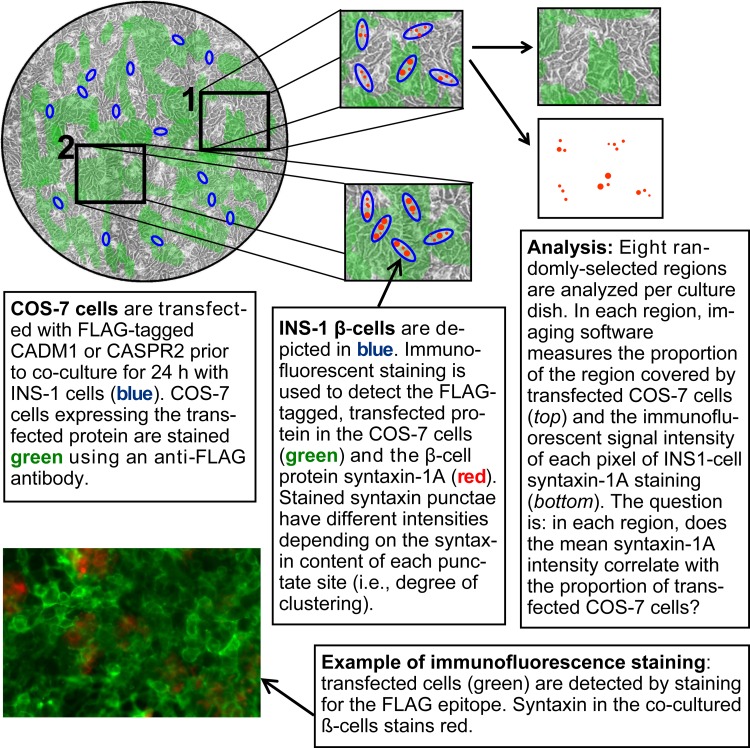
Quantitative immunofluorescence analysis of syntaxin-1 clustering in INS-1 cells was carried out in cocultures exactly as described before (50), except for the use of transfected COS-7 cells in place of human embryonic kidney (HEK)-293 cells (see Fig. 10 for an explanatory diagram). COS-7 cells were pretransfected to express FLAG-tagged CADM1 or FLAG-tagged CASPR2. The latter is an unrelated, neuronal, nonsynaptogenic transmembrane protein (57). After a 24-h coculture, cells were washed with PBS, fixed with 4% paraformaldehyde for 1 h, and then washed with 1% BSA in PBS containing 0.1% Tween 20. Cells were stained for 1 h with anti-FLAG primary antibody (1:500 dilution) to label transfected COS-7 cells and with anti-syntaxin-1A antibody (1:100). After washing with 1% BSA in PBS containing 0.1% Tween 20, cells were incubated with 1:200 Alexa Fluor-488 anti-rabbit IgG and 1:500 Alexa Fluor-594 anti-mouse IgG. To quantify clustering of syntaxin-1 using immunofluorescence, imaging software was employed for pixel-by-pixel determination of the signal intensity of syntaxin-positive pixels. Images of eight random, nonoverlapping regions within each culture well were captured using a Zeiss LSM 700 Confocal Microscope (UCI Optical Biology Core Facility) and analyzed using Zeiss Zen Digital Imaging software, as described previously (50).
Fig. 10.
Method of analysis of syntaxin-1A clustering. COS-7 cells in culture dishes (top left; illustration not to scale) were transfected with FLAG-tagged CADM1 or with the nonsynaptogenic, transmembrane protein CASPR2 (control), also FLAG epitope tagged. INS-1 cells were cultured for 24 h on the COS-7 cells. Eight randomly selected regions were analyzed per dish in blinded fashion. If expression of the FLAG-tagged protein by COS-7 cells increases the intensity of syntaxin-1A punctae in contacting INS-1 cells, then mean syntaxin-1A intensity in each region will vary as a function of the proportion of the region stained positively with an anti-FLAG antibody. Details regarding coculture and use of this assay to analyze neuroligin-2 have been published previously (50, 61). The assay is adapted from the artificial synapse formation/coculture assay originally described by Schieffele et al. (45a) in 2000 and now routinely utilized to identify and study synaptogenic proteins (such as CADM1) (13).
Statistical analysis.
Data are presented as means ± SE. Differences between quantitative data sets were analyzed by two-tailed Student's t-test. Linear regression from syntaxin clustering data and slope analysis by F-test were performed using GraphPad Prism 5 software. P < 0.05 was considered statistically significant.
RESULTS
CADM expression in human and rat islets.
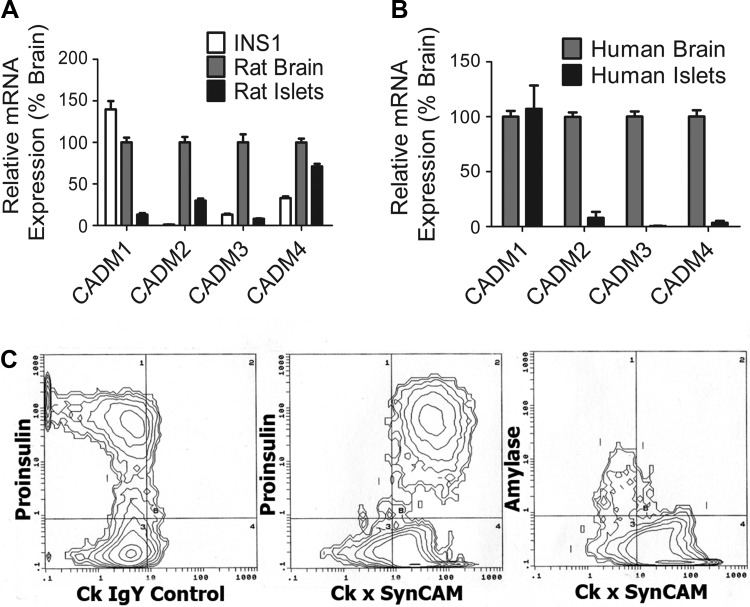
There are four CADM protein family members; all are expressed and functional in the brain (7, 14). Previously, we determined that CADM1 is expressed on the surface of α- and β-cells in rat and human islets (48). Because islet expression of the other three isoforms was not characterized previously, we used qPCR to analyze transcript levels in rat and human islets and in INS-1 β-cells, comparing levels with those in the brain. CADM1 was the predominant transcript in INS-1 cells, with levels closest to those in rat brain, whereas CADM2 was not detectable (Fig. 1A). All four transcripts were detected in rat islets (Fig. 1A). Here, CADM4 levels were closest to those in brain. In human islets, CADM1 transcript levels were comparable with those in brain, whereas levels of the other three transcripts were substantially lower (Fig. 1B).
Fig. 1.
CADM expression in rat and human β-cells. A: mRNAs from INS-1 cells and rat brain and islets were reverse-transcribed, and the expression of each CADM isoform was quantified by quantitative PCR (qPCR). Results are presented as β-cell expression and %brain expression (means ± SE). B: mRNAs from human brain and islets were analyzed as in A. C: FACS analysis of dissociated human islet cells. Antibodies to proinsulin were used to tag β-cells. Antibodies to amylase were used to tag pancreatic exocrine cells remaining in the islet preparations. Left: results with a nonimmune control IgY; middle and right: results with anti-CADM1 IgY antibody. The populations of cells tagged by the anti-CADM1 antibody appear to the right of the vertical line at the middle and right. The populations of cells tagged by the anti-proinsulin antibody appear above the horizontal line at the left and middle. (quantitative PCR; n = 3 individual preparations assayed in duplicate).
We also analyzed CADM expression data yielded by previous transcriptome-wide microarray and RNA sequencing (RNA-seq) studies. These results, unlike our qPCR results, are not normalized to brain expression, and therefore, they provide further insights into CADM isoform expression. The Beta Cell Gene Atlas, a compilation of integrated gene expression data calculated from 27 microarray studies (32), confirms that CADM1 transcript levels are enriched in human islets and β-cells (Table 2). In rat islets, purified rat β-cells, and INS-1 cells, there is predominant expression of CADM1 and CADM4 (CADM2 data not available). In addition to providing further evidence of the predominance of CADM1 expression, RNA-seq results (Table 2) indicate that CADM1 is the most abundant CADM isoform in rat islets and in both human α- and β-cells. CADM4 mRNA is relatively more abundant in rat islet cells than in human β-cells.
Table 2.
CADM isoform gene expression
| CADM1 | CADM2 | CADM3 | CADM4 | Ref. No. | |
|---|---|---|---|---|---|
| Integrated microarray results | |||||
| Human islets | +++ | + | + | + | 32 |
| Human β-cells | +++ | NM | ++ | ++ | |
| Rat islets | +++ | NM | − | +++ | |
| Rat β-cells | +++ | NM | − | +++ | |
| INS-1 cells | +++ | NM | + | +++ | |
| RNA-seq | |||||
| Human α-cells | 23.89 | 0.01 | 0.00 | 4.04 | 8 |
| Human β-cells | 66.10 | 0.04 | 0.03 | 1.00* | |
| Rat islets | 7.17 | 0.28 | 0.67 | 1.00* | 24 |
Transcript levels yielded from analysis and integration of data from 27 microarray studies; cutoff values as defined in the Beta Cell Gene Atlas: −, no expression; + low expression; ++, moderate expression; +++, enriched expression; NM, not measured (for details regarding the Beta Cell Gene Atlas, see Ref. 32). mRNA expression data (RNA-seq) from highly purified human islet α- and β-cells and from whole rat islets. Average fragments per kilobase of exon per million reads (normalized as described in the references listed) were obtained from data sets deposited in NCBI GEO (8, 24).
To help interpret relative isoform abundance, expression levels are shown normalized to human β-cell or rat islet CADM4 values.
Normalization of islet CADM1 transcript levels to levels in the brain in our qPCR study (Fig. 1A) might have masked the relative abundance in rat islets evident in Table 2. Together, the results in Fig. 1 and Table 2 indicate that CADM1 is the predominant islet isoform and that CADM4 is also enriched in islet cells.
We used FACS analysis to confirm human β-cell expression of CADM1 protein and, since human β-cells are heterogeneous, to ask whether there is a population of β-cells lacking CADM1 expression (4). Human β-cells were uniformly positive for CADM1 expression (Fig. 1C, middle). A proinsulin-negative population of cells was also CADM1 positive (Fig. 1C, middle, bottom right quadrant) whereas amylase-positive cells were CADM1 negative (Fig. 1C, right). These results are consistent with prior immunostaining studies showing CADM1 expression in β-cells and other islet endocrine cell types but not in exocrine tissue (30).
CADM1 constrains insulin secretion in INS-1 β-cells.
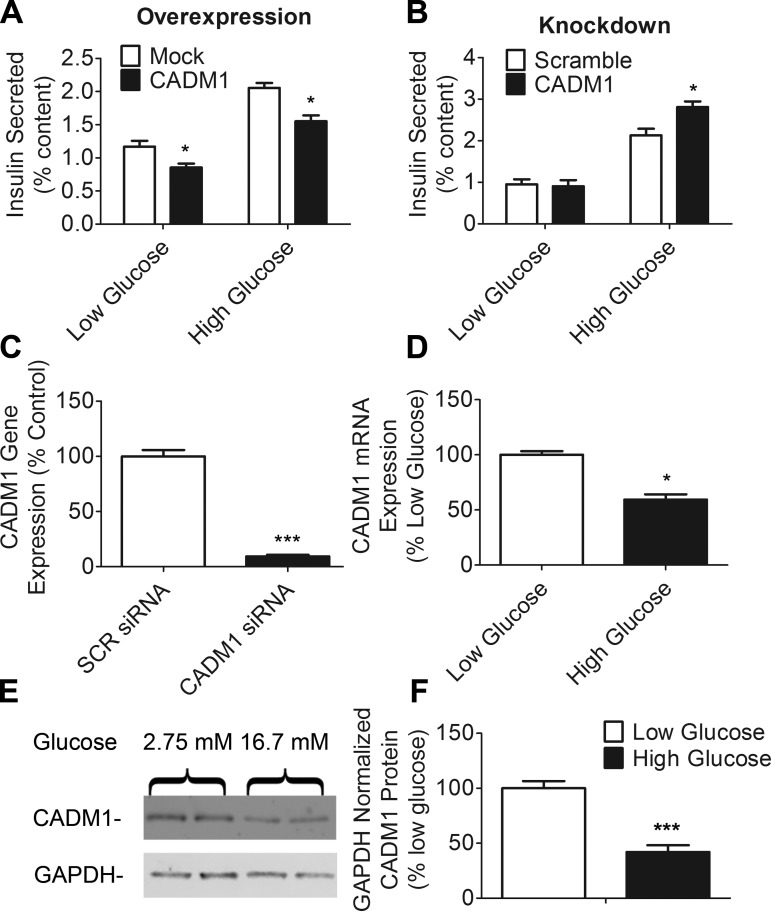
We next asked whether alterations in CADM1 expression levels would affect insulin secretion. As noted earlier, increased insulin secretion in whole body CADM1 knockout mice suggests an inhibitory effect (38). Although it seems paradoxical that a component of the membrane secretory apparatus would function to inhibit secretion, granuphilin, tomosyn-2, and a number of other constituents of the submembrane insulin secretory machinery have such an effect, most likely because they participate in a late-stage regulatory mechanism that constrains secretion (17, 26, 40, 63). Our results indicate that CADM1 behaves like these other proteins; its overexpression in INS-1 cells resulted in decreased insulin secretion at basal and stimulating glucose levels (Fig. 2A). Conversely, siRNA-mediated CADM1 knockdown, yielding a mean 91% reduction in CADM1 transcript levels, increased glucose-stimulated insulin secretion (Fig. 2, B and C).
Fig. 2.
Effect of CADM1 expression level on insulin secretion; glucose-sensitive expression of CADM1. A: INS-1 cells were transfected with a CADM1 expression plasmid (black bars) or mock-transfected with empty vector (open bars) and incubated for 48 h, followed by 1 h of incubation in either 2.75 (low) or 16.7 mM (high) glucose with 0.1 mM IBMX. Insulin secretion is shown normalized to total cellular insulin content. B: INS-1 cells were transfected for 72 h with pools of either nontargeting, scrambled (open bars), or CADM1 (black bars) siRNAs and then insulin secretion analyzed as in A. C: RNA was isolated from siRNA-treated INS-1 cells and degree of CADM1 knockdown determined by qPCR. Data are shown normalized to control values obtained using scrambled (SCR) siRNA. D: INS-1 cells were incubated for 18 h in 2.75 (low) or 16.7 mM (high) glucose. RNA was isolated from cells and CADM1 transcript levels measured by qPCR. Data are shown as expression level normalized to that in low-glucose samples (2.75 mM). E: INS-1 cells were incubated in 2.75 or 16.7 mM glucose as in D, and then CADM1 protein levels were analyzed by immunoblot analysis of cell lysates. GAPDH protein was probed as a loading control. F: bands from E were quantitated. GAPDH-normalized CADM1 levels were normalized to control levels. All data are represented as means ± SE from 6 samples and representative of 3 experiments; qPCR and insulin RIA samples were assayed in duplicate. *P < 0.05; ***P < 0.005.
Because CADM1 inhibits insulin secretion, its expression may fall in response to glucose stimulation. Such is the case with tomosyn-2 and neurexin; expression of both declines in response to glucose (5, 40). We found that, likewise, CADM1 transcript and protein levels also fall at stimulating glucose concentrations (Fig. 2, D–F).
CADM1 constrains insulin secretion in primary rat β-cells.
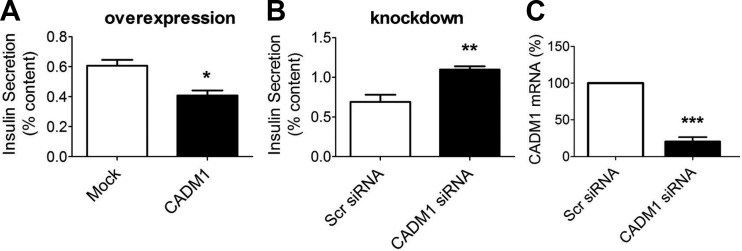
We next tested the effect of CADM1 overexpression and knockdown in primary rat islet β-cells (Fig. 3). The results paralleled that in INS-1 β-cells; overexpression decreased glucose-stimulated insulin secretion (Fig. 3A), whereas knockdown increased secretion (Fig. 3B). As in α-cells then, CADM1 in islet β-cells appears to function as an inhibitor of hormone release (23).
Fig. 3.
Effect of altering CADM1 expression on insulin secretion by primary rat islet cells. A: dispersed rat islets were transfected with a CADM1-expressing plasmid or mock-transfected (Mock) with empty vector and then incubated for 48 h. Insulin secretion was then measured during a 1-h incubation in 16.7 mM glucose with 0.1 mM IBMX. B: dispersed rat islets were transfected for 72 h with pool of either scrambled (Scr) or CADM1 siRNA. Insulin secretion was measured over a 1-h incubation in 16.7 mM glucose with 0.1 mM IBMX. Total insulin secreted is shown normalized to total cellular insulin content. C: to verify effectiveness of CADM1 siRNA, CADM1 mRNA was measured by RT-qPCR. CADM1 mRNA level after treatment with CADM1 siRNA is shown relative to level after treatment with control (scrambled) siRNA. Data are represented as the mean ± SE of 3 experiments. *P < 0.05; **P < 0.01; ***P < 0.005.
Time course of decrease in CADM1 protein levels.
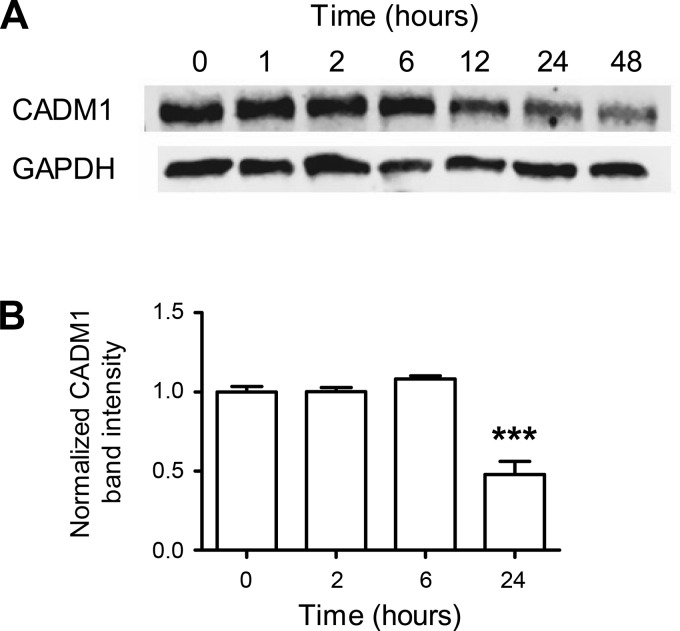
We next asked whether the decrease in CADM1 expression at elevated glucose concentrations (Fig. 2, D–F) could be part of the acute response of the β-cell to increased ambient glycemia or a longer-term adaptation. We analyzed CADM1 protein content in INS-1 cells at different times after exposure to a raised, stimulating glucose concentration (Fig. 4). The time course reveals that CADM1 protein levels did not decline immediately; there was a lag of ≥6 h. Subsequently, levels reach ∼50% (48 ± 8%) of starting levels by 24 h (corresponding change in CADM1 transcript shown in Fig. 2D) (Fig. 4).
Fig. 4.
Kinetics of glucose-induced downregulation of CADM1 protein. INS-1 cells were incubated overnight in 5 mM glucose, followed by exposure (at time t = 0) to 16.7 mM glucose. A: cell lysates at various time points were collected and equal amounts of protein immunoblotted for CADM1 and a loading control, GAPDH. The immunoblot shown is representative of 4 independent experiments. B: time points repeated at least 3 times over the course of the separate time course experiments were quantitated by infrared fluorescent imaging of the immunoblots using a LiCor Odyssey imaging system. Data are shown as CADM1 expression levels normalized to GAPDH and relative to the zero time point (mean normalized CADM1 band intensity at time 0 is 1). Bars are means ± SE of 3 experiments. ***P < 0.005
Effect of transcellular CADM1 interactions on insulin secretion.
In the CNS, CADM molecules on the surface of one neural process bind across the synaptic cleft to CADM molecules on the surface of an apposed process to drive synaptogenesis (13, 47). Such interactions are transcellular, occurring between proteins situated on the surfaces of neighboring cells, and can be studied in vitro, as has been shown previously, by coculture with transfected HEK-293 or COS-7 cells (13, 35, 50).
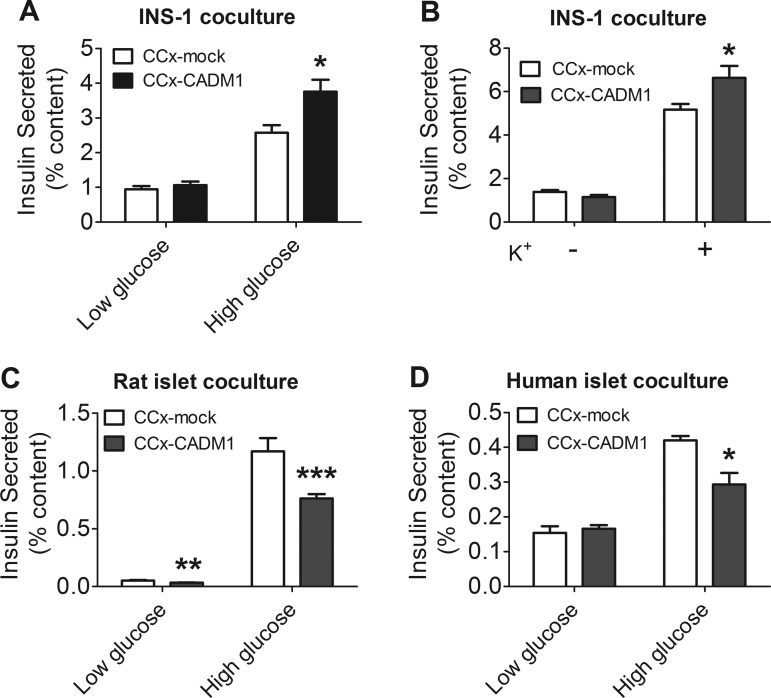
We cocultured β-cells in contact with COS-7 cells pretransfected with CADM1 or, as a control, with empty vector. Coculture of INS-1 cells with COS-7 cells expressing CADM1 increased both glucose-stimulated insulin secretion (Fig. 5A) and potassium-stimulated insulin secretion (Fig. 5B), which was indicative of an effect on insulin secretion downstream of glucose sensing. CADM1 also acted in a transcellular manner to influence insulin secretion by rat and human islet β-cells; however, here the result was a decrease rather than increase in insulin secretion (Fig. 5, C and D).
Fig. 5.
Insulin secretion by β-cells cocultured with COS-7 cells expressing CADM1. A and B: INS-1 cells were cocultured (CCx) with COS-7 cells pretransfected with CADM1 expression vector (black bars) or mock-transfected (mock) with empty (control) vector (open bars). After 24 h, the cocultures were incubated for 1 h in 2.75 mM (low) glucose or in 16.7 mM (high) glucose with 0.1 mM IBMX (A) or in 2.74 mM glucose supplemented with either 0 (−) or 30 mM (+) KCl (B). Insulin secreted during this last hour is shown as %cellular insulin content. C: islets were isolated from male Sprague-Dawley rats and dispersed. Islets were then cocultured with COS-7 cells as in A for 24 h, followed by 1-h incubation in low or high glucose with 0.1 mM IBMX. D: human islets were dispersed and then cocultured as in A for 24 h, followed by 1 h incubation in low or high glucose with 0.1 mM IBMX. Insulin secreted was normalized to total cellular insulin content. All data are represented as means ± SE from 6 samples assayed in duplicate and representative of 3 experiments. *P < 0.05; **P < 0.01; ***P < 0.005.
Association of CADM1 with the subplasmalemmal insulin secretory apparatus.
The short, cytoplasmic, carboxyl-terminal tails of the CADM family members contain motifs for binding to synaptic scaffolding molecules with PDZ type II domains and to members of the protein 4.1 family (6). The latter function as subplasmalemmal hubs that help anchor and organize the submembrane actin cytoskeleton and bind a number of membrane-associated proteins, including regulators of cytoskeleton formation and proteins that drive clustering of exocytic proteins (21). CADM1 promotes assembly of the presynaptic neurotransmitter secretory apparatus through binding to CASK, a PDZ domain-containing scaffolding protein (25, 45). Previously, we found that CASK is expressed in β-cells and, as in neurons, interacts with neurexin (40).
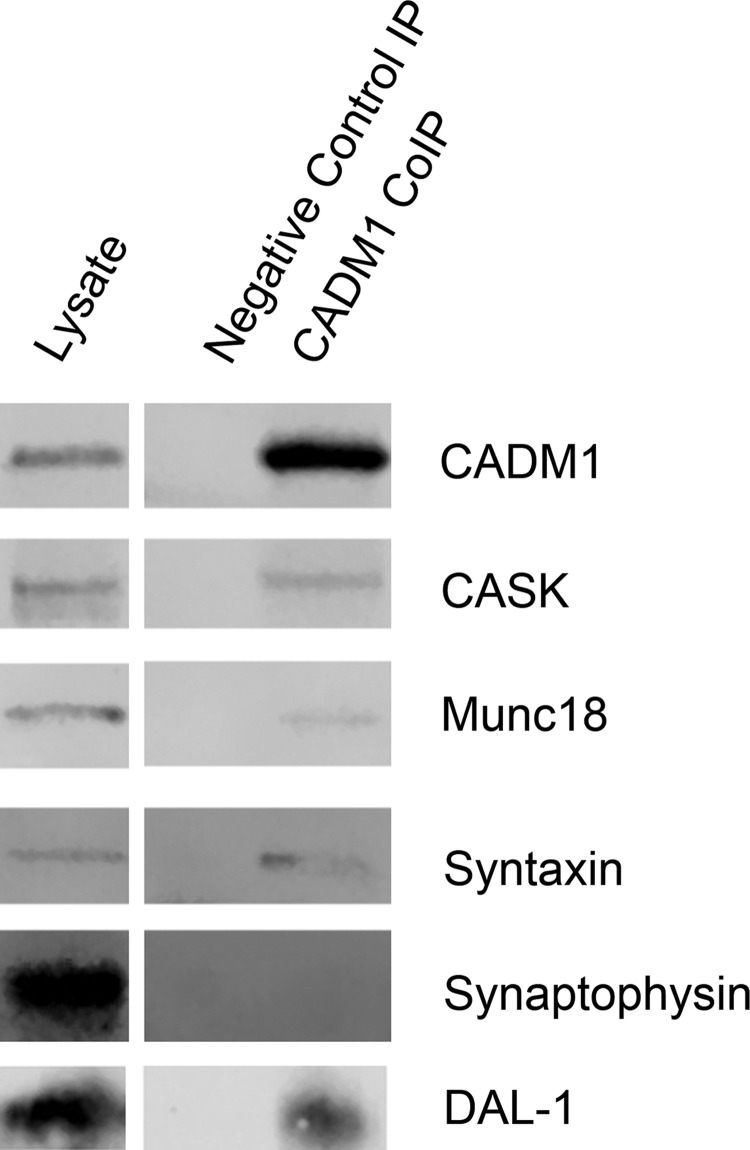
To help determine whether CADM1 affects insulin secretion through direct interactions with the secretory machinery and, in β-cells, whether CADM1 similarly associates with CASK, we immunoprecipitated CADM1 from INS-1 cell lysates. Figure 6 shows that CADM1 coprecipitated with CASK as well as Munc18 and syntaxin-1A, two additional constituents of the submembrane insulin secretory assembly. Thus, in both β-cells and neurons, CADM1 interacts with constituents of the submembrane protein assemblies that mediate regulated insulin or neurotransmitter secretion (25, 45).
Fig. 6.
CADM1 interacts with components of the insulin exocytic assembly and the actin-binding protein DAL-1. Immunoprecipitates were prepared from INS-1 cell lysates using an anti-CADM1 antibody or nonimmune rabbit IgG (control). Immunoprecipitated proteins were analyzed by Western blotting, with probing for the proteins indicated to the right of each row. CASK, Munc18, and syntaxin-1 are constituents of the subplasmalemmal insulin secretory machinery. The F-actin-binding protein DAL-1, also known as EPB41L3 and protein 4.1B, is known to bind to CADM1 in other cell types. Synaptophysin, unlike the other proteins, is a vesicle-associated protein, not a component of the submembrane secretory apparatus, and no corresponding band was detected, even after adjusting the fluorescent imaging system for maximal sensitivity, during immunoblot analysis of precipitated proteins (results are representative of 3 separate experiments).
CADM1, as has been demonstrated in a variety of cell types, plays an essential role in cytoskeletal organization and remodeling (10, 11, 39, 44). It helps anchor F-actin to subplasmalemmal sites and binds proteins that regulate actin cytoskeletal dynamics (11, 39). Consistent with CADM1 having parallel function in β-cells, CADM1 coimmunoprecipitated DAL-1 (Fig. 6), a protein 4.1 family member also known as EPB41L3 and protein 4.1B and shown previously to interact with CADM1 in other cell types (10, 44).
Transcellular CADM1 interactions enhance syntaxin-1 clustering.
In studies of synapse formation, analysis of the punctate immunofluorescent staining of pre- or postsynaptic components of the neurotransmitter signaling machinery is used to follow synaptic maturation. Assembly of the presynaptic exocytic protein complexes is accompanied by the clustering of syntaxin-1 or synapsin at discrete sites, and this clustering is signaled by the resultant increased intensity of punctate staining (3, 13, 46). A defining property of synaptogenic proteins such as CADM1 is the induction of such clustering (13, 47).
As in neurons, membrane-associated SNARE and other exocytic proteins cluster during maturation of the islet β-cell secretory machinery (33, 50). Previously, we found that neuroligin-2 increases syntaxin-1 clustering in β-cells (50). Using the same approach, we asked whether transcellular CADM1 interactions would do the same.
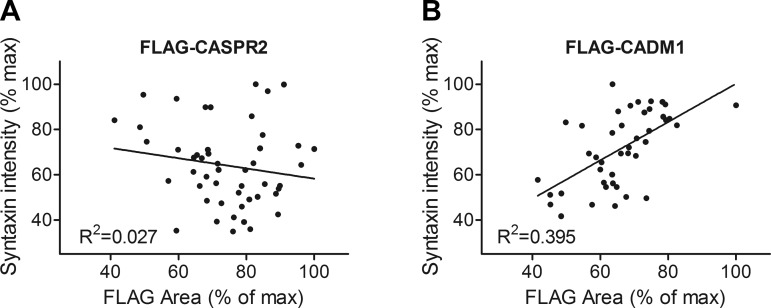
A schematic of the “artificial synapse formation assay” used to determine whether proteins drive secretory machinery assembly, adapted as described previously to β-cells, is included in Fig. 10 (50). INS-1 cells were seeded onto pretransfected COS-7 cells and cocultured overnight. The intensity of INS-1 cell syntaxin-1 puncta in different regions was determined, as was the efficiency of COS-7 cell transfection in the same regions. Image analysis showed that the intensity of syntaxin-1 puncta in INS-1 cells increased in proportion to the level of CADM1 expression in the underlying COS-7 cells (Fig. 7). This suggests that transcellular CADM1 interactions drive syntaxin-1 clustering.
Fig. 7.
Coculture with CADM1-expressing COS-7 cells promotes clustering of syntaxin-1. INS-1 cells were cocultured with COS-7 cells expressing FLAG-tagged CADM1 (FLAG-CADM1) or, as a negative control, with FLAG-tagged CASPR2 (FLAG-CASPR2). After 24 h, cells were fixed and stained for syntaxin-1 and for the FLAG epitope. Stained cocultures were imaged in small, nonoverlapping fields, and the following were determined for each field: 1) %field area staining positive for FLAG and 2) the average intensity of the syntaxin puncta. These values were normalized to their respective maximum values and plotted for the FLAG-CASPR2 (A) and the FLAG-CADM1 (B) cocultures. There was no correlation between the intensity of the syntaxin puncta in INS-1 cells (y-axis) and the level of FLAG-CASPR2 expression by the cocultured COS-7 cells (x-axis; A). In contrast, with FLAG-CADM1-expressing COS-7 cells, the average immunofluorescent intensity of the syntaxin puncta in INS-1 cells increased in proportion to the FLAG-CADM1 expression level (the percentage of the underlying area staining for CADM1); P < 0.01. Data from 3 separate experiments are shown together here; the same relationship between transfection efficiency and intensity of syntaxin-1 punctae is also present when the 3 experiments are analyzed separately.
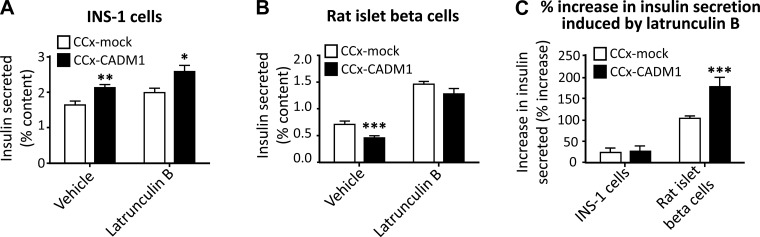
Latrunculin counteracts transcellular CADM1 inhibition of insulin secretion.
CADM1 promotes F-actin assembly and helps anchor the cortical actin network to the plasma membrane. As a result, exocytic sites that assemble around the CADM1 cytoplasmic domain are also sites of actin filament nucleation and membrane-tethering of the cytoskeleton (11, 39, 60). Because the actin network helps regulate insulin granule trafficking, we asked whether enhancement of local actin network formation might be an additional mechanism through which CADM1 influences insulin secretion.
The effects of the cortical actin network on glucose-stimulated insulin secretion vary between cell types. In primary β-cells, the actin mesh impedes insulin granule trafficking, and F-actin depolymerization contributes to increased glucose-stimulated insulin secretion (22, 53). This is in contrast to poorly granulated β-cell lines such as INS-1, where the actin network does not hinder granule trafficking and its depolymerization does not significantly increase insulin secretion (18, 34, 55). These divergent effects of actin mesh on secretion could help explain why transcellular CADM1 interactions decrease insulin secretion by primary β-cells while increasing secretion by INS-1 cells.
We used latrunculin, an inhibitor of actin polymerization, to test the role of F-actin in CADM1-mediated changes in insulin secretion. In cocultures with INS-1 cells, latrunculin did not change the stimulatory effect of transcellular CADM1 interactions on insulin secretion (Fig. 8A). In primary rat β-cells, on the other hand, latrunculin markedly attenuated the inhibitory effect on insulin exocytosis of extracellular CADM1 interactions (Fig. 8B). This suggests that transcellular CADM1-mediated inhibition of insulin secretion in primary β-cells is brought about at least in part by effects on the actin network.
Fig. 8.
Effect of latrunculin on CADM1-induced changes in insulin secretion. A and B: INS-1 cells (A) and cells from dissociated rat islets (B) were cocultured (CCx) with COS-7 cells expressing CADM1 (CCx-CADM1; black bars) or with mock-transfected COS-7 cells (CCx-mock; open bars). After 24 h, cells were incubated for 30 min in high (16.7 mM) glucose with 0.1 mM IBMX with or without latrunculin. Insulin secretion (as %cellular content) is shown. C: %increase in insulin secretion caused by latrunculin in control cocultures (mock-transfected; open bars) and CADM1 cocultures (black bars) is shown. The left 2 bars and the right 2 bars in C show the latrunculin-induced %increase in insulin secretion by INS-1 cells and by rat islet cells, respectively. Note that latrunculin caused a greater %increase in insulin secretion by rat islet β-cells in CADM1 cocultures than by rat islet β-cells in control cocultures (compare the 2 right bars in C). What appears to be a slight latrunculin-induced increase in insulin secretion by INS-1 cells (left 2 bars) was not statistically significant. All data are represented as means ± SE from 6 samples and representative of 3 experiments; insulin RIA samples were assayed in duplicate; *P < 0.05; **P < 0.01; ***P < 0.005.
Latrunculin increased insulin secretion in rat β-cells cocultured with control COS-7 cells by ∼100% (Fig. 8C, right open bar). This effect was augmented to ∼175% by coculture with CADM1-transfected COS-7 cells (Fig. 8C, right closed bar). Inhibition of actin polymerization, in other words, caused a much greater increase in insulin secretion in primary β-cells cocultured with CADM1-expressing COS-7 cells than with control COS-7 cells. Taken together, these data indicate that transcellular CADM1 interactions did indeed influence insulin secretion by primary β-cells in part through effects on the actin cytoskeleton.
DISCUSSION
The submembrane protein complexes that mediate insulin exocytosis in the pancreatic islets and neurotransmitter exocytosis in the brain are nearly identical, so it is natural to wonder whether the mechanisms guiding their formation are also the same (1, 2, 41, 58). In the CNS, assembly of these submembrane protein complexes is guided by “synaptogenic” proteins that interact across the nascent synaptic cleft. There is evidence to suggest that similar transcellular interactions help direct the formation of the insulin exocytic machinery. This evidence includes the dependence of β-cell function and maturation on contact between β-cells as well as the tendency for exocytic complexes to form beneath the β-cell plasma membrane at sites where such cell-to-cell contact occurs (12, 16, 27).
Our results show that the synaptogenic protein CADM1 interacts with the submembrane secretory machinery in β-cells and constrains insulin secretion. After an at least 6-h lag, CADM1 protein levels fall following glucose stimulation. Our coculture experiments reveal that transcellular CADM1 interactions also influence insulin secretion. Therefore, CADM1 provides the third example of a synaptogenic extracellular protein interaction that modulates insulin secretion. The two previously identified examples involve the proteins neuroligin/neurexin and EphA/ephrin-A (31, 40).
Extracellular CADM1 interactions are either homophilic or heterophilic with CADM2 (7, 14). We have found that CADM1 is the predominant islet β-cell isoform transcript. CADM4 expression tends also to be enriched in islet cells. In contrast to brain, expression of CADM2 and CADM3 is markedly lower.
Synaptogenic protein interactions are defined by their ability to trigger the formation of pre- or postsynaptic sites (“hemi-synapses”) in coculture experiments closely akin to those employed here (13, 45a, 47). In neuronal coculture assays, and in the β-cell coculture assays that we adapted from the neuronal system, maturation of submembrane secretory complexes is assessed by using immunofluorescence to analyze punctae of syntaxin-1 or other membrane-associated SNARE proteins. Punctate staining intensity, which increases as exocytic sites assemble, is enhanced by transcellular CADM1 interactions in both neural processes and, as now revealed here, in β-cells (3, 7, 13, 46).
Transcellular CADM1 interactions likely promote assembly of the submembrane β-cell secretory complexes, sometimes referred to as “excitosomes,” at sites of β-cell-to-β-cell contact (37, 43). CADM1 functions in this regard similarly to neurexin, which is also expressed in both β-cells and brain (40). Both proteins recruit CASK and other exocytic scaffolding proteins to the submembrane exocytic assemblies via a cytoplasmic PDZ-binding motif. Both also coimmunoprecipitate with the key t-SNARE syntaxin-1 and also with constituents of the secretory machinery, such as Munc-18, that enable a late-stage mechanism-constraining insulin secretion (40, 62). Consistent with participation in this constraining mechanism, decreased expression of either CADM1 or neurexin increases insulin secretion, whereas overexpression of either has the opposite effect (40). CADM1 expression, like that of neurexin, decreases in response to raised glucose levels (40). This glucose effect on CADM1 expression could serve to enhance insulin secretion when ambient glucose levels are persistently elevated.
Coculture experiments allowed the effects of transcellular CADM interactions to be observed without directly altering β-cell CADM1 protein expression. β-Cell gene expression and secretory mechanisms were not manipulated, and the observed effects on insulin secretion and sytaxin-1A clustering reflected the endogenous β-cell response to CADM1 expression by neighboring cells. With INS-1 cells, contact with CADM1-expressing COS-7 cells promoted, in addition to syntaxin-1 clustering, increased glucose-stimulated insulin secretion. Consistent with an effect downstream of glucose sensing, potassium-stimulated insulin secretion was also increased. With rat and human primary β-cells, as is discussed below, glucose-stimulated insulin secretion decreased in CADM1 coculture experiments.
Overexpression and gene silencing experiments yielded the same result in INS-1 cells and primary β-cells; insulin secretion increased as CADM1 expression decreased. In contrast, insulin secretion by INS-1 cells and primary β-cells (rat and human) responded differently in the coculture experiments with CADM1-expressing COS-7 cells. Several explanations for this divergence can be envisioned. First, granuphilin, Munc-18, and other constituents of the submembrane secretory apparatus impart on the exocytic machinery an ability to constrain insulin secretion (17, 26, 40, 63). This built-in inhibitory mechanism provides a potential brake on insulin release: a final control point where insulin release can be checked just prior to membrane fusion (26). Our results suggest that, like neurexin, CADM1 both participates in this inhibitory mechanism and also interacts with and helps drive assembly of the rest of the secretory assembly (40). Because INS-1 cells differ from primary β-cells in important ways, such as having far fewer granules and responding less robustly to glucose, it seems likely that the complex interplay between the prosecretory and secretion-constraining activities of CADM1 in the coculture experiments could favor the former in INS-1 cells and the latter in primary β-cells (17, 26, 40, 63). As another explanation for the divergent insulin secretion responses, CADM1 is an anchor point for the cortical actin network and promotes its formation at exocytic sites that assemble around the CADM1 cytoplasmic domain (11, 39, 60). The actin network impedes insulin exocytosis in primary β-cells, and in these cells we found that pharmacological actin depolymerization rescued insulin secretion from inhibition by CADM1-expressing COS cells. In contrast to primary β-cells, insulin secretion from INS-1 cells is subject to, at most, only minimal inhibition by actin. It is likely that CADM1-influenced F-actin assembly at exocytic sites inhibited insulin secretion by primary β-cells while having no or minimal impact on secretion by INS-1 cells.
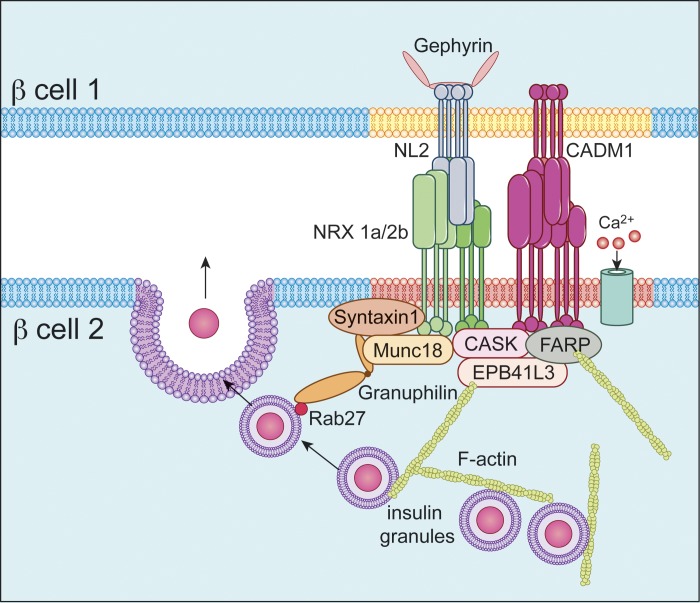
The findings reported here, together with our prior findings regarding neurexin and neuroligin, are consistent with the overall hypothesis that β-cell excitosome assembly, in parallel with formation of the closely related presynaptic active sites of secretion, occurs around the intracellular domains of neurexin, CADM1, and perhaps other presynaptic, synaptogenic proteins (Fig. 9) (48, 50). Four such synaptogenic transmembrane proteins have been found to interact with the insulin secretory machinery: neurexin-1α, neurexin-2β, ephrin-A (not shown in Fig. 9), and now CADM1 (31, 40). Neurexin-neuroligin and CADM1 interactions across the synaptic cleft induce “lateral” clustering, meaning lateral movement of these proteins through the plasma membrane, leading to accumulation at discrete sites, which in turn triggers synaptogenesis (15, 47). The model in Fig. 9 posits that, in β-cells, similar interactions drive formation of the secretory microdomains (as was tested using the approach shown in Fig. 10).
Fig. 9.
Proposed model of a site of cell-cell contact between β-cells. Transcellular CADM1-CADM1 and neuroligin (NL)-neurexin (NRX) binding interactions are depicted in the extracellular space. Also shown is the interaction of CADM1 and neurexin-1α and -2β (NRX 1a/2b) with submembrane exocytic proteins such as syntaxin-1 and with other constituents of the exocytic complex, such as Munc18, that can constrain secretion. The membrane domain in red (through which neurexin passes) and the underlying exocytic proteins represent an exocytic microdomain (excitosome). CADM1, like neurexin, binds the scaffolding protein CASK and, both directly and indirectly, other components of the submembrane insulin exocytic machinery. Granuphilin, a component of the secretory protein complex that acts to constrain insulin secretion, is also associated either directly or indirectly with CADM1 and neurexin. CADM1 also binds proteins (EPB41L3/DAL-1 and FARP) that regulate the assembly of and help anchor the cortical actin network. Neuroligin-2 passes through an as-yet-unidentified membrane domain (yellow) and binds the postsynaptic scaffolding protein gephyrin.
CADM1 helps organize, anchor, and promote formation of the cortical actin network. Interaction with F-actin-binding proteins and inducers of actin filament formation, including DAL-1 (EPB41L3), has been demonstrated here and elsewhere (11, 39, 60). This aspect of CADM1 function is also incorporated in the model (Fig. 9). The model is derived largely from findings in the neurobiology field and provides a framework for further investigations.
In conclusion, our results suggest that, like neurexin-neuroligin and EphA-ephrin-A, CADM1 molecules engage in transcellular interactions between β-cells paralleling identical interactions in the central nervous system. As occurs during maturation of the presynaptic machinery for neurotransmitter exocytosis, extracellular CADM1 interactions drive clustering of syntaxin-1, and CADM1 associates with CASK and other components of the submembrane insulin secretory machinery. As in other cell types, CADM1 interacts with the actin-binding protein DAL-1 and by inference with the cortical actin network. CADM1 has a constraining effect on insulin secretion analogous to that of neurexin, granuphilin, and other components of the insulin exocytic machinery, and its expression decreases after glucose stimulation. These results support the idea that functional maturation of the β-cell insulin secretory machinery is guided by a set of the same transcellular interactions that trigger the formation and drive the maturation of presynaptic exocytic sites in the CNS. The importance of such interactions for normal β-cell maturation and insulin secretion is underscored by the dependence of β-cell function on contact with other β-cells.
GRANTS
S. D. Chessler was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-080971. N. -W. Chi was supported by Grant 5-I01-BX000702 from the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors
AUTHOR CONTRIBUTIONS
C.Z. and S.D.C. conception and design of research; C.Z., T.A.C., M.R.M., D.D., and E.J.P. performed experiments; C.Z., T.A.C., M.R.M., D.D., and S.D.C. analyzed data; C.Z., T.A.C., M.R.M., N.-W.C., and S.D.C. interpreted results of experiments; C.Z. and S.D.C. prepared figures; C.Z. and S.D.C. drafted manuscript; C.Z., T.A.C., N.-W.C., and S.D.C. edited and revised manuscript; C.Z., T.A.C., M.R.M., D.D., E.J.P., N.-W.C., and S.D.C. approved final version of manuscript.
REFERENCES
- 1.Arntfield ME, van der Kooy D. β-Cell evolution: How the pancreas borrowed from the brain: The shared toolbox of genes expressed by neural and pancreatic endocrine cells may reflect their evolutionary relationship. Bioessays 33: 582–587, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Atouf F, Czernichow P, Scharfmann R. Expression of neuronal traits in pancreatic beta cells. Implication of neuron-restrictive silencing factor/repressor element silencing transcription factor, a neuron-restrictive silencer. J Biol Chem 272: 1929–1934, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Basarsky TA, Parpura V, Haydon PG. Hippocampal synaptogenesis in cell culture: developmental time course of synapse formation, calcium influx, and synaptic protein distribution. J Neurosci 14: 6402–6411, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benninger RK, Piston DW. Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics. Trends Endocrinol Metab 25: 399–406, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatnagar S, Soni MS, Wrighton LS, Hebert AS, Zhou AS, Paul PK, Gregg T, Rabaglia ME, Keller MP, Coon JJ, Attie AD. Phosphorylation and degradation of tomosyn-2 de-represses insulin secretion. J Biol Chem 289: 25276–25286, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biederer T. Bioinformatic characterization of the SynCAM family of immunoglobulin-like domain-containing adhesion molecules. Genomics 87: 139–150, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science 297: 1525–1531, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Blodgett DM, Nowosielska A, Afik S, Pechhold S, Cura AJ, Kennedy NJ, Kim S, Kucukural A, Davis RJ, Kent SC, Greiner DL, Garber MG, Harlan DM, diIorio P. Novel Observations From Next-Generation RNA Sequencing of Highly Purified Human Adult and Fetal Islet Cell Subsets. Diabetes 64: 3172–3181, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns SM, Vetere A, Walpita D, Dancik V, Khodier C, Perez J, Clemons PA, Wagner BK, Altshuler D. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metab 21: 126–137, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Busam RD, Thorsell AG, Flores A, Hammarström M, Persson C, Öbrink B, Hallberg BM. Structural basis of tumor suppressor in lung cancer 1 (TSLC1) binding to differentially expressed in adenocarcinoma of the lung (DAL-1/4.1B). J Biol Chem 286: 4511–4516, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheadle L, Biederer T. The novel synaptogenic protein Farp1 links postsynaptic cytoskeletal dynamics and transsynaptic organization. J Cell Biol 199: 985–1001, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Begum S, Opare-Addo L, Garyu J, Gibson TF, Bothwell AL, Papaioannou VE, Herold KC. Promotion of beta-cell differentiation in pancreatic precursor cells by adult islet cells. Endocrinology 150: 570–579, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig AM, Graf ER, Linhoff MW. How to build a central synapse: clues from cell culture. Trends Neurosci 29: 8–20, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci 27: 12516–12530, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogel AI, Stagi M, Perez de Arce K, Biederer T. Lateral assembly of the immunoglobulin protein SynCAM 1 controls its adhesive function and instructs synapse formation. EMBO J 30: 4728–4738, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geron E, Boura-Halfon S, Schejter ED, Shilo BZ. The Edges of Pancreatic Islet β Cells Constitute Adhesive and Signaling Microdomains. Cell Rep (January 13, 2015). doi: 10.1016/j.celrep.2014.12.031 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Gomi H, Mizutani S, Kasai K, Itohara S, Izumi T. Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol 171: 99–109, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaslip AT, Nelson SR, Lombardo AT, Beck Previs S, Armstrong J, Warshaw DM. Cytoskeletal dependence of insulin granule movement dynamics in INS-1 beta-cells in response to glucose. PLoS One 9: e109082, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoang Do O, Thorn P. Insulin secretion from beta cells within intact islets: Location matters. Clin Exp Pharmacol Physiol 42: 406–414, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol 228: 121–128, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Hoover KB, Bryant PJ. The genetics of the protein 4.1 family: organizers of the membrane and cytoskeleton. Curr Opin Cell Biol 12: 229–234, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Howell SL, Tyhurst M. The cytoskeleton and insulin secretion. Diabetes Metab Rev 2: 107–123, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Ito A, Ichiyanagi N, Ikeda Y, Hagiyama M, Inoue T, Kimura KB, Sakurai MA, Hamaguchi K, Murakami Y. Adhesion molecule CADM1 contributes to gap junctional communication among pancreatic islet alpha-cells and prevents their excessive secretion of glucagon. Islets 4: 49–55, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Jacovetti C, Matkovich SJ, Rodriguez-Trejo A, Guay C, Regazzi R. Postnatal beta-cell maturation is associated with islet-specific microRNA changes induced by nutrient shifts at weaning. Nat Commun 6: 8084, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kakunaga S, Ikeda W, Itoh S, Deguchi-Tawarada M, Ohtsuka T, Mizoguchi A, Takai Y. Nectin-like molecule-1/TSLL1/SynCAM3: a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. J Cell Sci 118: 1267–1277, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kasai K, Fujita T, Gomi H, Izumi T. Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic 9: 1191–1203, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Kelly C, McClenaghan NH, Flatt PR. Role of islet structure and cellular interactions in the control of insulin secretion. Islets 3: 41–47, 2011. [DOI] [PubMed] [Google Scholar]
- 28.King CC, Beattie GM, Lopez AD, Hayek A. Generation of definitive endoderm from human embryonic stem cells cultured in feeder layer-free conditions. Regen Med 3: 175–180, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Koh DS, Cho JH, Chen L. Paracrine interactions within islets of Langerhans. J Mol Neurosci 48: 429–440, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Koma Y, Furuno T, Hagiyama M, Hamaguchi K, Nakanishi M, Masuda M, Hirota S, Yokozaki H, Ito A. Cell adhesion molecule 1 is a novel pancreatic-islet cell adhesion molecule that mediates nerve-islet cell interactions. Gastroenterology 134: 1544–1554, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Konstantinova I, Nikolova G, Ohara-Imaizumi M, Meda P, Kucera T, Zarbalis K, Wurst W, Nagamatsu S, Lammert E. EphA-Ephrin-A-mediated beta cell communication regulates insulin secretion from pancreatic islets. Cell 129: 359–370, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Kutlu B, Burdick D, Baxter D, Rasschaert J, Flamez D, Eizirik DL, Welsh N, Goodman N, Hood L. Detailed transcriptome atlas of the pancreatic beta cell. BMC Med Genomics 2: 3, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J 20: 2202–2213, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, Rungger-Brändle E, Just I, Jonas JC, Aktories K, Wollheim CB. Effect of disruption of actin filaments by Clostridium botulinum C2 toxin on insulin secretion in HIT-T15 cells and pancreatic islets. Mol Biol Cell 5: 1199–1213, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu DS, Loh KH, Lam SS, White KA, Ting AY. Imaging transcellular neurexin-neuroligin interactions by enzymatic probe ligation. PLoS One 8: e52823, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald PE. Signal integration at the level of ion channel and exocytotic function in pancreatic β-cells. Am J Physiol Endocrinol Metab 301: E1065–E1069, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Matthäus D. The Role of CADM1 in Energy and Glucose Homeostasis. Berlin: Humboldt-Universität zu Berlin, 2014. [Google Scholar]
- 39.Moiseeva EP, Straatman KR, Leyland ML, Bradding P. CADM1 controls actin cytoskeleton assembly and regulates extracellular matrix adhesion in human mast cells. PLoS One 9: e85980, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosedale M, Egodage S, Calma RC, Chi NW, Chessler SD. Neurexin-1α contributes to insulin-containing secretory granule docking. J Biol Chem 287: 6350–6361, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nica AC, Ongen H, Irminger JC, Bosco D, Berney T, Antonarakis SE, Halban PA, Dermitzakis ET. Cell-type, allelic, and genetic signatures in the human pancreatic beta cell transcriptome. Genome Res 23: 1554–1562, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poy MN, Hausser J, Trajkovski M, Braun M, Collins S, Rorsman P, Zavolan M, Stoffel M. miR-375 maintains normal pancreatic alpha- and beta-cell mass. Proc Natl Acad Sci USA 106: 5813–5818, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutter GA, Tsuboi T, Ravier MA. Ca2+ microdomains and the control of insulin secretion. Cell Calcium 40: 539–551, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Sakurai-Yageta M, Masuda M, Tsuboi Y, Ito A, Murakami Y. Tumor suppressor CADM1 is involved in epithelial cell structure. Biochem Biophys Res Commun 390: 977–982, 2009. [DOI] [PubMed] [Google Scholar]
- 45.Samuels BA, Hsueh YP, Shu T, Liang H, Tseng HC, Hong CJ, Su SC, Volker J, Neve RL, Yue DT, Tsai LH. Cdk5 promotes synaptogenesis by regulating the subcellular distribution of the MAGUK family member CASK. Neuron 56: 823–837, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101: 657–669, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Shi P, Scott MA, Ghosh B, Wan D, Wissner-Gross Z, Mazitschek R, Haggarty SJ, Yanik MF. Synapse microarray identification of small molecules that enhance synaptogenesis. Nat Commun 2: 510, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siddiqui TJ, Craig AM. Synaptic organizing complexes. Curr Opin Neurobiol 21: 132–143, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suckow AT, Comoletti D, Waldrop MA, Mosedale M, Egodage S, Taylor P, Chessler SD. Expression of neurexin, neuroligin, and their cytoplasmic binding partners in the pancreatic beta-cells and the involvement of neuroligin in insulin secretion. Endocrinology 149: 6006–6017, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suckow AT, Sweet IR, Van Yserloo B, Rutledge EA, Hall TR, Waldrop M, Chessler SD. Identification and characterization of a novel isoform of the vesicular gamma-aminobutyric acid transporter with glucose-regulated expression in rat islets. J Mol Endocrinol 36: 187–199, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Suckow AT, Zhang C, Egodage S, Comoletti D, Taylor P, Miller MT, Sweet IR, Chessler SD. Transcellular neuroligin-2 interactions enhance insulin secretion and are integral to pancreatic beta cell function. J Biol Chem 287: 19816–19826, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tattikota SG, Rathjen T, McAnulty SJ, Wessels HH, Akerman I, van de Bunt M, Hausser J, Esguerra JL, Musahl A, Pandey AK, You X, Chen W, Herrera PL, Johnson PR, O'Carroll D, Eliasson L, Zavolan M, Gloyn AL, Ferrer J, Shalom-Feuerstein R, Aberdam D, Poy MN. Argonaute2 mediates compensatory expansion of the pancreatic beta cell. Cell Metab 19: 122–134, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tattikota SG, Sury MD, Rathjen T, Wessels HH, Pandey AK, You X, Becker C, Chen W, Selbach M, Poy MN. Argonaute2 regulates the pancreatic beta-cell secretome. Mol Cell Proteomics 12: 1214–1225, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thurmond DC, Gonelle-Gispert C, Furukawa M, Halban PA, Pessin JE. Glucose-stimulated insulin secretion is coupled to the interaction of actin with the t-SNARE (target membrane soluble N-ethylmaleimide-sensitive factor attachment protein receptor protein) complex. Mol Endocrinol 17: 732–742, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Tomas A, Meda P, Regazzi R, Pessin JE, Halban PA. Munc 18-1 and granuphilin collaborate during insulin granule exocytosis. Traffic 9: 813–832, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Uenishi E, Shibasaki T, Takahashi H, Seki C, Hamaguchi H, Yasuda T, Tatebe M, Oiso Y, Takenawa T, Seino S. Actin dynamics regulated by the balance of neuronal Wiskott-Aldrich syndrome protein (N-WASP) and cofilin activities determines the biphasic response of glucose-induced insulin secretion. J Biol Chem 288: 25851–25864, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3—new capabilities and interfaces. Nucleic Acids Res 40: e115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varea O, Martin-de-Saavedra MD, Kopeikina KJ, Schürmann B, Fleming HJ, Fawcett-Patel JM, Bach A, Jang S, Peles E, Kim E, Penzes P. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc Natl Acad Sci USA 112: 6176–6181, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, Tulina N, Carlin DL, Rulifson EJ. The origin of islet-like cells in Drosophila identifies parallels to the vertebrate endocrine axis. Proc Natl Acad Sci USA 104: 19873–19878, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weir GC, Halban PA, Meda P, Wollheim CB, Orci L, Renold AE. Dispersed adult rat pancreatic islet cells in culture: A, B, and D cell function. Metabolism 33: 447–453, 1984. [DOI] [PubMed] [Google Scholar]
- 60.Yageta M, Kuramochi M, Masuda M, Fukami T, Fukuhara H, Maruyama T, Shibuya M, Murakami Y. Direct association of TSLC1 and DAL-1, two distinct tumor suppressor proteins in lung cancer. Cancer Res 62: 5129–5133, 2002. [PubMed] [Google Scholar]
- 61.Zhang C, Suckow AT, Chessler SD. Coculture analysis of extracellular protein interactions affecting insulin secretion by pancreatic beta cells. J Vis Exp e50365, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W, Efanov A, Yang SN, Fried G, Kolare S, Brown H, Zaitsev S, Berggren PO, Meister B. Munc-18 associates with syntaxin and serves as a negative regulator of exocytosis in the pancreatic beta -cell. J Biol Chem 275: 41521–41527, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Zhang W, Lilja L, Mandic SA, Gromada J, Smidt K, Janson J, Takai Y, Bark C, Berggren PO, Meister B. Tomosyn is expressed in beta-cells and negatively regulates insulin exocytosis. Diabetes 55: 574–581, 2006. [DOI] [PubMed] [Google Scholar]