Abstract
Many low-birth weight infants are at risk for poor growth due to an inability to achieve adequate protein intake. Administration of the amino acid leucine stimulates protein synthesis in skeletal muscle of neonates. To determine the effects of enteral supplementation of the leucine metabolite β-hydroxy-β-methylbutyrate (HMB) on protein synthesis and the regulation of translation initiation and degradation pathways, overnight-fasted neonatal pigs were studied immediately (F) or fed one of five diets for 24 h: low-protein (LP), high-protein (HP), or LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol HMB·kg body wt−1·day−1. Cell replication was assessed from nuclear incorporation of BrdU in the longissimus dorsi (LD) muscle and jejunum crypt cells. Protein synthesis rates in LD, gastrocnemius, rhomboideus, and diaphragm muscles, lung, and brain were greater in HMB80 and HP and in brain were greater in HMB40 compared with LP and F groups. Formation of the eIF4E·eIF4G complex and S6K1 and 4E-BP1 phosphorylation in LD, gastrocnemius, and rhomboideus muscles were greater in HMB80 and HP than in LP and F groups. Phosphorylation of eIF2α and eEF2 and expression of SNAT2, LAT1, MuRF1, atrogin-1, and LC3-II were unchanged. Numbers of BrdU-positive myonuclei in the LD were greater in HMB80 and HP than in the LP and F groups; there were no differences in jejunum. The results suggest that enteral supplementation with HMB increases skeletal muscle protein anabolism in neonates by stimulation of protein synthesis and satellite cell proliferation.
Keywords: amino acid, protein metabolism, translation initiation, infant
low-birth weight infants constitute ∼10% of all births in the US. Most experience extrauterine growth restriction by hospital discharge and remain small throughout their lives (25, 62). The neonatal period is a critical time in development, as an infant's rate of growth is higher at this stage than at any other period of postnatal development (18). Postnatal nutrition has a critical impact on the short- and long-term outcomes of the low-birth weight infant (76). Because of concerns for feeding intolerance and other issues that limit protein intake, current feeding strategies are often unable to provide the adequate nutrition that is needed to optimize the growth of the low-birth weight infant (1, 38, 49, 72). Understanding the mechanisms underlying growth in the neonatal period is important to optimize nutritional strategies to improve growth and to decrease adverse long-term developmental outcomes of low-birth weight infants (11).
Previous studies using the neonatal pig as a model of the human neonate have shown that the postprandial increases in amino acids and insulin independently stimulate muscle protein synthesis by modulating the signaling pathways that regulate translation initiation (19, 57, 81). Supplementation with the branched-chain amino acid leucine also enhances protein synthesis during the neonatal period (14, 29). Leucine is an important substrate for protein synthesis, but it also functions as a signaling molecule to enhance muscle protein synthesis (4, 15) through activation of the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway (3, 28, 29). The activation of mTORC1 results in phosphorylation and activation of ribosomal protein S6 kinase 1 (S6K1), leading to the activation of ribosomal protein S6 (S6), which promotes mRNA translation of proteins involved in the regulation of translation (60). mTORC1 also phosphorylates the eukaryotic initiation factor (eIF) repressor 4E-binding protein (4E-BP1), leading to the release of eukaryotic initiation factor-4E (eIF4E), which can then bind to eIF4G (41). The formation of the active eIF4E·eIF4G complex, which mediates the binding of mRNA to the 43S ribosomal complex, and the phosphorylation of S6 stimulate translation initiation and protein synthesis.
A balance between protein synthesis and degradation is critical in protein homeostasis and is required for normal muscle development. Of the several proteolytic systems that regulate protein degradation, the ubiquitin-proteasome and autophagy-lysosome systems are thought to be the two most important (26, 44, 63). In the ubiquitin-proteasome system, two muscle-specific ubiquitin ligases, muscle atrophy F-box (atrogin-1) and muscle RING finger protein-1 (MuRF1), are critical to the control of muscle proteolysis (8). The autophagy-lysosome system requires activation of the ubiquitin-like molecule microtubule-associated protein light chain 3 (LC3), followed by the formation of autophagosomes leading to autophagy (63). This system is vital for the generation of free amino acids and is crucial for the survival of neonatal animals in catabolic conditions (12, 39).
Muscle growth in the postnatal period also requires an accretion of myonuclei through the proliferative activity of satellite cells (18). Satellite cell proliferation in the immature muscle is relatively high, decreasing as the muscles undergo maturation and eventually becoming quiescent (18). How specific nutrients regulate this process is unclear. Undernutrition during this period reduces myonuclear accretion presumably as a result of reduced satellite cell proliferation (7). Leucine can stimulate cell division of isolated satellite cells (16, 36), but whether it is effective when administered orally in vivo and whether it also promotes intestinal crypt cell proliferation has not been described.
Recently, there has been increased interest in evaluating leucine metabolites as possible adjuncts to nutritional therapies to increase muscle growth. One such metabolite, β-hydroxy-β-methylbutyrate (HMB), has been reported to enhance muscle growth and improve strength in conjunction with resistance exercise in adults (33, 54) and to slow muscle protein breakdown (54). In our previous study, we showed that acute parenteral infusion of HMB increases protein synthesis in skeletal muscle of the neonatal pig (77). The maximum stimulation of muscle protein synthesis occurred at circulating concentrations of ∼90 nmol/ml, with higher concentrations of HMB being ineffective (77). No studies have evaluated the effects of enteral HMB supplementation on protein synthesis, protein degradation, or nuclear accretion in the neonate. We hypothesized that enteral supplementation with HMB in a low-protein diet would stimulate protein synthesis to the rate achieved with feeding a high-protein diet through activation of the mTORC1 signaling pathway. It was also hypothesized that HMB would downregulate protein degradation pathways and promote cell proliferation.
MATERIALS AND METHODS
Animals and surgery.
Experiments were approved by the Animal Care and Use Committee of the Baylor College of Medicine and were conducted in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. Forty-six piglets (1.8 kg ± 0.3 kg, 36 males and 10 females, Yorkshire × Landrace × Duroc × Hampshire; Rosenbaum Farms, Burton, TX) were weaned from sows at 2 days of age and housed in individual steel cages in an environmentally controlled room maintained at 28°C. At 2 days of age, indwelling catheters were surgically inserted into the jugular vein and carotid artery under general anesthesia, as described previously (17). At weaning, piglets were fed a commercially available milk replacement formula ad libitum (Soweena Litter Life; Merrik's, Middleton, WI) and transitioned to the experimental formula (Table 1) over 3 days. Piglets remained on the experimental formula until the experimental period.
Table 1.
Composition of the experimental diets for the 24-h feeding period
| HP | LP | |
|---|---|---|
| Ingredient, %ingredient, %as fed | ||
| Whey protein concentratea | 7.64 | 3.5 |
| Casein, hydrolyzedb | 1.53 | 0.70 |
| Lactosec | 1.59 | 2.02 |
| Fat pak 80d | 0.59 | 0.59 |
| Trace mineral premixc | 0.91 | 0.91 |
| Vitamin premixc | 0.21 | 0.21 |
| Xanthan gum | 0.10 | 0.10 |
| Corn oil | 3.73 | 5.99 |
| Water | 83.7 | 86.0 |
| Macronutrient supply | ||
| Total protein, g·kg body wt−1·day−1 | 18.0 | 8.3 |
| Total carbohydrate, g·kg body wt−1·day−1 | 6.0 | 6.0 |
| Total fat, g·kg body wt−1·day−1 | 11.0 | 15.3 |
| Metabolizable energy, kcal·kg body wt−1·day−1 | 195.0 | 195.0 |
HP, high protein; LP, low protein.
Nutrabio, Middlesex, NJ;
HCA-411, American Casein Company, Burlington, NJ;
Dyets, Bethlehem, PA;
Milk Specialties Global Animal Nutrition, Carpentersville, IL.
Pilot study.
A pilot study was performed to determine HMB clearance and, thence, the optimal timing for administration of HMB in the feeding trial. Piglets (n = 3–4/treatment) were fasted overnight and then randomly assigned to one of four treatment groups and fed a single meal comprised of either 1) a low-protein (LP) diet (Table 1) supplemented with 2 μmol/kg body wt HMB (LP + HMB2), 2) a low-protein diet supplemented with 20 μmol/kg body wt HMB (LP+ HMB20), 3) a low-protein diet supplemented with 40 μmol/kg body wt HMB (LP + HMB40), or 4) water supplemented with 40 μmol/kg body wt HMB (H20 + HMB40). Blood samples were collected for determination of circulating HMB at 0, 15, 30, 60, 90, 120, 240, 480, 720, and 1,440 min relative to the time of the feed, and the plasma was frozen at −20°C until further processing.
Treatments and infusions.
At 5–7 days of age, overnight-fasted (12–14 h) piglets (2.2 kg ± 0.4 kg) were placed in a sling restraint system and underwent orogastric feeding tube insertion or were studied immediately in the fasted state (F). Piglets were randomly assigned to one of five dietary treatment groups and fed (n = 7–9/treatment; Table 1) 1) a low-protein diet without HMB supplementation (LP), 2) a low-protein diet supplemented with 4 μmol·kg body wt−1·day−1 HMB (HMB4), 3) a low-protein diet supplemented with 40 μmol·kg body wt−1·day−1 HMB (HMB40), 4) a low-protein diet supplemented with 80 μmol·kg body wt−1·day−1 HMB (HMB80), or 5) a high-(HP) protein diet. The HMB was given twice daily in the diet in two equal doses every 12 h at 0, 12, and 24 h of study based on the results of the pilot study. The LP and HP diets provided 8.3 and 18.0 g·kg body wt−1·day−1 protein, respectively. The diets were isocaloric (195 kcal·kg body wt−1·day−1) and contained the same concentration of lactose (Table 1). The piglets were bolus fed with their respective diet in six equal meals via orogastric tube at a rate of 40 ml/kg body wt per feed over 24 h. Feed was delivered over 30 min every 4 h using a syringe pump (PHD 2000 infusion; Harvard Apparatus, Holliston, MA). 5-Bromo-2′-deoxyuridine (BrdU) was administered intravenously at 0 and 12 h (25 mg·kg body wt−1·dose−1) for measurement of cell proliferation. Blood samples were collected at 24, 24.5, 25, 25.75, and 26.25 h during the study for measurement of plasma HMB, individual plasma amino acids, insulin, and glucose concentrations. Plasma was obtained by centrifugation at 12,000 g and frozen at −20°C until further processing. At 25.75 h, piglets were injected with a flooding dose of l-[4-3H]phenylalanine for measurement of fractional protein synthesis rates (35). Following euthanasia at 26.25 h, tissue samples were collected from skeletal muscles [longissimus dorsi (LD), gastrocnemius, soleus, rhomboideus, and diaphragm], heart, lung, liver, spleen, stomach, duodenum, jejunum, ileum, colon, kidney, pancreas, brain (frontal cortex), and skin. All tissue samples were quickly removed and immediately frozen in liquid nitrogen. Tissue samples were stored at −80°C until analysis of protein synthesis rates, translation initiation and degradation of signaling proteins, or histology.
Plasma and tissue hormone and substrate analysis.
Plasma HMB was analyzed by gas chromatography-mass spectrometry, as described elsewhere (53). A modification to the plasma HMB assay was used for analysis of muscle intracellular HMB content (77). Analysis of plasma α-keto acids was performed using high-performance liquid chromatography, as described previously (55). Individual plasma amino acid concentrations were analyzed by using high-performance liquid chromatography (PICO-TAG reverse phase column; Waters, Milford, MA) after deproteinization and derivatization with phenylisothiocyanate (11). Plasma radioimmunoreactive insulin concentrations were analyzed using a porcine insulin immunoassay kit (EMD Millipore, Billerica, MA). Plasma glucose was determined using the glucose oxidase method (model 2300; Yellow Springs Instruments, Yellow Springs, OH).
Tissue fractional protein synthesis rates.
Fractional rates of protein synthesis were measured with a flooding dose of l-[4-3H]phenylalanine infusion (1.5 mmol/kg, 0.5 mCi/kg; American Radiolabeled Chemicals, St. Louis, MO) injected 30 min prior to euthanasia and tissue collection (35). Blood samples were collected 5, 15, and 30 min after l-[4-3H]phenylalanine infusion and frozen at −20°C for subsequent analysis. Piglets were euthanized and tissue samples obtained, immediately frozen in liquid nitrogen, and stored at −70°C until analysis (20). Fractional rates of protein synthesis (Ks, %protein mass synthesized in a day) in tissues were calculated as KS (%/day) = [(SAbound phe/SAfree phe) × 1,440/t] × 100, as described previously (31), where SAbound phe and SAfree phe (in disintegrations·min−1·nmol−1) are the specific radioactivity of the protein-bound and the tissue free phenylalanine, respectively, t is the time of labeling in minutes, and 1,440 is the minutes-to-day conversion. SAfree phe was corrected for the linear regression of the blood-specific radioactivity of each pig 5, 15, and 30 min after injection of the tracer.
Protein immunoblot analysis.
Proteins from tissue homogenates were separated on polyacrylamide gels (polyacrylamide gel electrophoresis). For each assay, samples were run concurrently on triple-wide gels to eliminate interassay variation (CBS Scientific, San Diego, CA). Proteins were electrophoretically transferred to polyvinylidene difluoride transfer membranes (Pall, Jersey Village, TX), incubated with primary antibodies, washed, and exposed to secondary antibody (21). Phosphorylated forms of the signaling proteins were normalized to the total abundance of the respective proteins. Total abundance of proteins was normalized with β-actin abundance. Primary antibodies used were β-actin (Santa Cruz Biotechnology, Dallas, TX), total S6K1 (Santa Cruz Biotechnology), S6K1 Thr389 (Cell Signaling Technology, Danvers, MA), total 4E-BP1 (Novus Biologicals, Littleton, CO), 4E-BP1 Thr46 (Invitrogen, Carlsbad, CA), total eIF2α (Cell Signaling Technology), eIF2α Ser51 (Cell Signaling Technology), total eEF2 (Cell Signaling Technology), eEF2 Thr56 (Cell Signaling Technology), total protein kinase B (PKB; Cell Signaling Technology), PKB Thr308 and Ser473 (Cell Signaling Technology), muscle RING finger 1 (MuRF1; R & D Systems, Minneapolis, MN), atrogin-1 (ECM Biosciences, Versailles, KY), SNAT2 (Santa Cruz Biotechnology), LAT1 (MBL International, Woburn, MA), and microtubule-associated protein-1 light chain 3 (LC3; Cell Signaling Technology).
Quantification of the eIF4E·eIF4G complex.
eIF4E·eIF4G complexes were immunoprecipitated from fresh tissue homogenates using an anti-eIF4E monoclonal antibody (Dr. Leonard Jefferson, Penn State University College of Medicine, Hershey, PA). Abundance of eIF4G in the complexes was analyzed using eIF4G antibody (EMD Millipore, Billerica, MA) and normalized to eIF4E (Cell Signaling Technology) recovered in the immunoprecipitate, as described previously (26, 28, 30).
BrdU labeling and tissue collection.
Samples for histological determination of BrdU incorporation into nuclei were either oriented in gum tragacanth and frozen in liquid nitrogen-cooled isopentane (longissimus dorsi muscle) or fixed in 10% buffered formalin (jejunum).
Histology and morphometry.
LD muscles were cryosectioned (10 μm) perpendicular to the muscle fibers and, after fixing in 3% paraformaldehyde, were subjected to antigen retrieval for 60 min in 1 M HCl-1% Triton X-100 at room temperature, followed by neutralization in 0.1 M borate buffer, pH 8.5. After blocking in 10% goat serum, sections were incubated overnight at 4°C with dystrophin antibody (H300, 4 μg/ml; Santa Cruz Biotechnology), followed by Alexa Fluor 488-conjugated secondary antibody (20 μg/ml; Life Technologies, Grand Island, NY). The sections were then incubated for 30 min with BrdU antibody (clone G3G4, 2.5 μg/ml; Developmental Studies Hybridoma Bank, University of Iowa), followed by biotinylated goat anti-mouse antibody (6 μg/ml; Vector Laboratories, Burlingame, CA), and finally stretpavidin-conjugated Alexa Fluor 647 (10 μg/ml; Life Technologies). Nuclei were visualized by staining with Sytox Orange (0.25 μM; Life Technologies). Sections were visualized by confocal laser microscopy, and captured images were analyzed using Image-Pro software (MediaCybernetics). BrdU-positive nuclei contained within the sarcolemma were defined as myonuclei added during the experiment; ≥2,000 fibers/pig were evaluated.
For jejunum, samples were paraffin-embedded and cross-sections (3–4 μm) were made. After deparaffinization, antigen retrieval (followed by 2 M HCl for 1 h, Diva Decloaker; Biocare Medical, Concord, CA) and endogenous peroxidase inactivation sections were incubated with a biotinylated streptavidin (4+ streptavidin horseradish peroxidase label; Biocare Medical). To visualize the antibody, 3,3-diaminobenzidine was applied to react with the horseradish peroxidase; the tissue was then counterstained with hematoxylin. Sections were observed by light-microscopy and images were captured. For each pig, ∼10 crypts were selected that were oriented so that the entire crypt from base to the crypt-villus junction could be seen. The number of BrdU-positive nuclei were counted and expressed as a percentage of the total number of epithelial cells in the crypt. Only samples from LP, LP + HMB80, and HP pigs were analyzed.
Statistics.
Data were analyzed using the mixed-model procedure (PROC MIXED) of the SAS statistical program (SAS 9.3; SAS Institute, Cary, NC), with diet included as a fixed effect and pig included as a random effect, where appropriate. A correction for repeated measurements was included for circulating concentration data collected over time. Differences between means were determined using the Tukey test and were considered significantly different at P ≤ 0.05. A trend toward significance was also considered at P < 0.10. For BrdU, differences among treatments were analyzed by ANOVA using a general linear model. Dunnett's test was used to determine whether there was any positive effect of HMB or protein supplementation of the LP diet on the number of BrdU+ nuclei. Data are presented as least square means ± SE.
RESULTS
Pilot study.
Peak plasma HMB values were attained 30–90 min after ingestion and were ∼30, 50, and 120 nmol/ml in piglets provided a low-protein feed supplemented with 2, 20, or 40 μmol/kg body wt HMB, respectively (Fig. 1). The values declined only by ∼25% after 4 h and returned to baseline at or before 12 h. Thus, in the full study reported herein, HMB was given every 12 h to maintain pulsatility. Because previous studies showed that parenteral infusion of HMB maximally stimulated protein synthesis when plasma levels increased to ∼90 nmol HMB/ml (77), in the full study, piglets were supplemented with 2, 20, and 40 μmol HMB/kg body wt HMB every 12 h to provide 4, 40, and 80 μmol·kg body wt−1·day−1, respectively.
Fig. 1.

Plasma β-hydroxy-β-methylbutyrate (HMB) concentrations in piglets fasted overnight and then fed low-protein diets supplemented with 2 (LP + HMB2), 20 (LP + HMB20), or 40 (LP + HMB40) μmol/kg body wt HMB or water with 40 μmol/kg HMB (H2O + HMB40). Values are means ± SE; n = 3–4.
Plasma and tissue hormone and substrate analysis.
Plasma concentrations of HMB were 3 ± 3 nmol/ml at baseline in the piglets fasted overnight and 2 ± 2 and 4 ± 2 nmol/ml at 25.75 h after the initiation of the study in those fed LP and HP diets, respectively (Fig. 2). Plasma HMB concentrations attained maximum levels of 8 ± 2, 43 ± 2, and 90 ± 2 nmol/ml at 25.75 h (1.75 h after the last HMB dose) in pigs fed the HMB4, HMB40, and HMB80 diets, respectively.
Fig. 2.
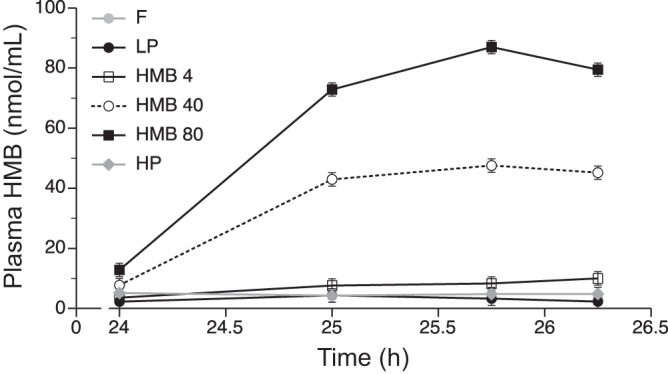
Plasma HMB concentrations in piglets fasted overnight (F) or fed a low-protein diet (LP), LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or high-protein diet (HP). Values are means ± SE; n = 7–9. Time is given as hours from initiation of supplementation.
The HMB40 and HMB80 treatments increased HMB concentrations above fasting levels in the LD muscle (P < 0.05; Table 2). The circulating concentrations of α-ketoisocaproic acid (KIC), α-ketoisovalerate, and α-ketomethylvalerate did not differ between those fed LP and those supplemented with HMB but were generally higher in F and HP fed pigs (Table 2). Circulating levels of the branched-chain amino acids and essential amino acids were higher in those pigs fed the HP diet compared with the LP diet alone, the LP diet supplemented with HMB, or the fasted group (P < 0.05; Table 2). There was no effect of dietary treatment on the circulating concentrations of the nonessential amino acids (Table 2). Plasma glucose concentrations increased with feeding and were greater in all fed groups than in fasted pigs (P < 0.01; Table 2). Plasma insulin concentrations increased with feeding but did not differ among dietary treatments (Table 2).
Table 2.
Longissimus dorsi muscle HMB concentrations and plasma α-keto acid, amino acid, glucose, and insulin concentrations after enteral supplementation with HMB for 24 h
| Measurement | F | LP | HMB4 | HMB40 | HMB80 | HP |
|---|---|---|---|---|---|---|
| Muscle HMB* | 4.2 ± 2.4a | 6.7 ± 2.3a | 8.2 ± 2.3a | 14.5 ± 2.2b | 26.8 ± 2.2c | 8.5 ± 2.3a |
| Plasma KIV† | 33.3 ± 3.0a | 14.3 ± 2.8b | 13.8 ± 2.8b | 13.6 ± 2.6b | 16.7 ± 2.5b | 27.6 ± 2.8a |
| Plasma KMV† | 33.2 ± 6.1a,b | 24.1 ± 5.9b | 21.9 ± 5.9b | 24.5 ± 5.8b | 28.2 ± 5.7b | 41.6 ± 5.9a |
| Plasma KIC† | 38.9 ± 7.3a,b | 31.3 ± 7.1a | 29.8 ± 7.1a | 24.7 ± 6.9a | 40.1 ± 6.7a,b | 57.3 ± 7.1b |
| Plasma leucine† | 125 ± 61.4a | 245 ± 51.2a | 196 ± 51.2a | 250 ± 49.1a | 214 ± 47.5a | 566 ± 51.2b |
| Plasma BCAA† | 554 ± 153a | 676 ± 129a | 522 ± 129a | 661 ± 124a | 591 ± 120a | 1,555 ± 129b |
| Plasma EAA† | 2,750 ± 312a | 2,839 ± 264a | 2,287 ± 264a | 2,910 ± 254a | 2,756 ± 247a | 4,316 ± 264b |
| Plasma NEAA† | 1,629 ± 232 | 2,052 ± 175 | 1,655 ± 175 | 2,141 ± 164 | 2,010 ± 154 | 2,583 ± 175 |
| Plasma glucose‡ | 69.4 ± 11.8a | 125.4 ± 8.9b | 136.5 ± 9.0b | 137.0 ± 8.4b | 122.0 ± 7.9b | 137.3 ± 9.0b |
| Plasma insulin§ | 1.7 ± 6.6a | 10.8 ± 5.1b | 11.5 ± 5.1b | 8.0 ± 4.8b | 13.4 ± 4.6b | 18.5 ± 5.1b |
Data are given as means ± SE; n = 7–9. Longissimus dorsi muscle concentrations in piglets fasted overnight (F) or fed a low-protein diet (LP), LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or a high-protein diet (HP). Values obtained at 26.25 h after the initiation of the study.
HMB, β-hydroxy-β-methylbutyrate; KIV, α-ketoisovalerate; KMV, α-ketomethylvalerate; KIC, α-ketoisocaproic acid; BCAA, branched-chain amino acids; EAA, essential amino acids; NEAA, nonessential amino acids.
Skeletal muscle concentrations in nmol/g tissue;
plasma concentrations in nmol/ml;
plasma concentrations in mg/dl;
plasma concentrations in μU/ml.
Labeled means in a row without a common letter differ, P < 0.05.
Protein synthesis in skeletal and cardiac muscles.
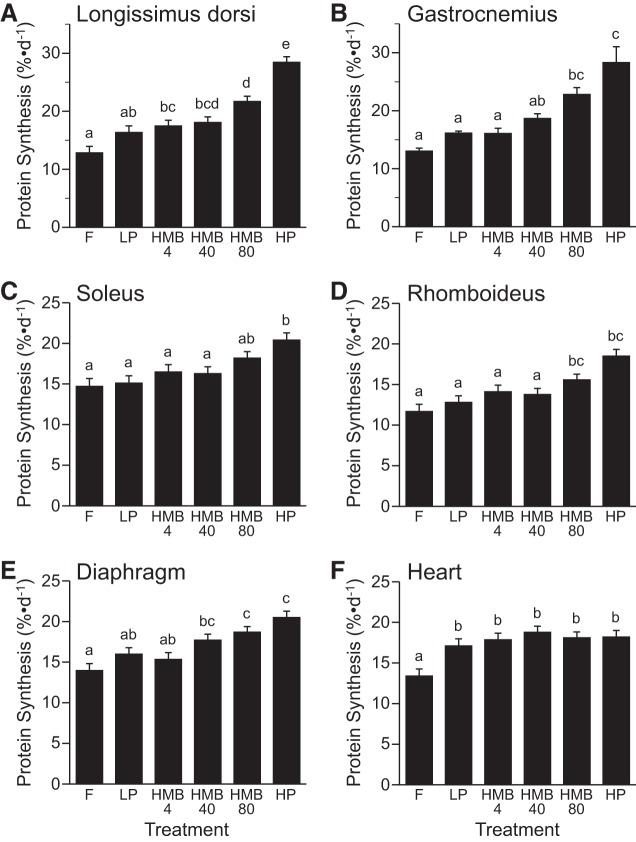
The fractional rates of protein synthesis in the LD, gastrocnemius, rhomboideus, and diaphragm muscles were greater in the HMB80 group than the LP or F groups (P < 0.05; Fig. 3, A, B, D, and E). Protein synthesis rates in the LD, gastrocnemius, soleus, rhomboideus, and diaphragm muscles were greater in piglets fed the HP diet than an LP diet and those that were fasted (P < 0.05; Fig. 3, A–E). In the heart, protein synthesis increased with feeding (P < 0.05), but there was no effect of the other treatments (Fig. 3F).
Fig. 3.
Fractional rates of protein synthesis in longissimus dorsi (A), gastrocnemius (B), soleus (C), rhomboideus (D), diaphragm (E), and heart (F) muscles of piglets that were fasted overnight (F) or fed a low-protein diet (LP), LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or high-protein diet (HP). Values are means ± SE; n = 7–9. a,b,c,d,eValues not sharing lowercase letters differ significantly, P < 0.05.
Protein synthesis in the intestine.
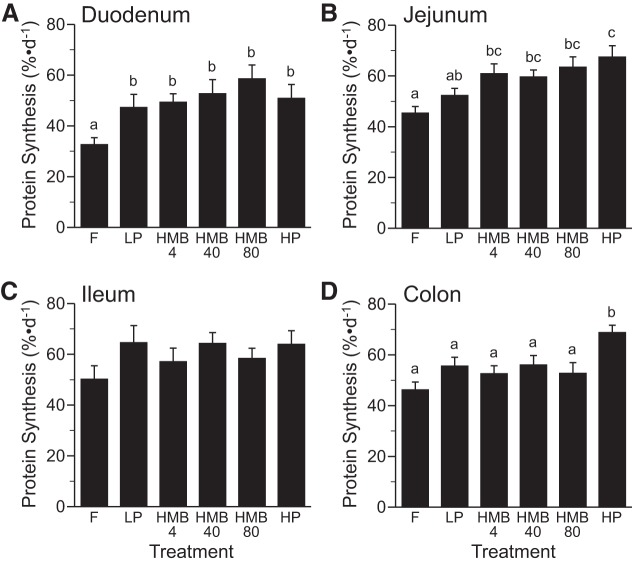
The fractional rate of protein synthesis in the duodenum was greater in the fed than in overnight-fasted piglets (P < 0.05) and did not differ among fed groups (Fig. 4A). Feeding a HP diet increased protein synthesis in the jejunum compared with feeding the LP diet (P < 0.05) and those supplemented with HMB4, -40, and -80 were intermediate between the LP and HP groups (Fig. 4B). Protein synthesis in the ileum did not differ between treatment groups (Fig. 4C). Protein synthesis in the colon was greater in the HP group than all other groups (P < 0.05; Fig. 4D).
Fig. 4.
Fractional rates of protein synthesis in duodenum (A), jejunum (B), ileum (C), and colon (D) of piglets that were fasted overnight (F) or fed a low-protein diet (LP), LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or high-protein diet (HP). Values are means ± SE; n = 7–9. a,b,cValues not sharing lowercase letters differ significantly, P < 0.05.
Protein synthesis in other organs and tissues.
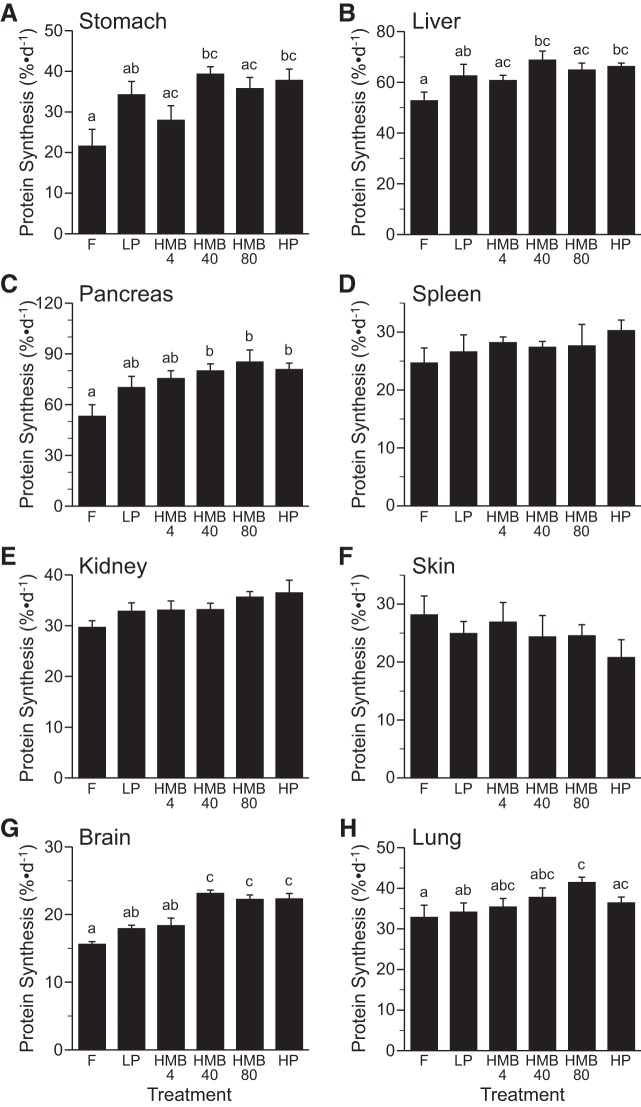
The fractional rates of protein synthesis in the stomach and liver did not differ among dietary treatments (Fig. 5, A and B); however, protein synthesis was greater in the HMB40 and HP diet compared with the fasted group (P < 0.05). Similarly, protein synthesis in the pancreas did not differ among all groups that were fed but was higher in those supplemented with HMB40 and -80 and the HP diet compared with the fasted piglets (P < 0.05; Fig. 5C). There were no differences among treatment groups in the fractional rates of protein synthesis in the spleen, kidney, or skin (Fig. 5, D–F).
Fig. 5.
Fractional rates of protein synthesis in stomach (A), liver (B), pancreas (C), spleen (D), kidney (E) skin, brain (G), and lung (H) of piglets that were fasted overnight (F) or fed a low-protein diet (LP), LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or high-protein diet (HP). Values are means ± SE; n = 7–9. a,b,cValues not sharing lowercase letters differ significantly, P < 0.05.
The fractional rates of protein synthesis in the brain were greater in the piglets supplemented with HMB40 and -80 or fed the HP diet compared with feeding the LP diet alone or the fasting condition (P < 0.05; Fig. 5G). The increase in protein synthesis in the brain in the HMB40- and HMB80-supplemented groups was similar to that achieved with feeding the HP diet. In the lung, HMB80 supplementation increased the fractional rate of protein synthesis compared with feeding a LP diet alone or the fasting condition (P < 0.05; Fig. 5H). The values were not significantly different among HMB groups.
Translation initiation and degradation signaling.
Phosphorylation of S6K1 and 4E-BP1 (Table 3) as well as formation of the active eIF4E·eIF4G complex (Fig. 6, A, B, and D) in the LD, gastrocnemius, and rhomboideus muscles were greater (P < 0.05) in the HMB80 compared with the LP and F groups. Active eIF4E·eIF4G complex formation in LD and gastrocnemius muscles was similar in the HMB80 and HP groups. In the soleus muscle, phosphorylation of 4E-BP1 and S6K1 (Table 3) was greater in the HP compared with the LP group (P < 0.05) and was intermediary in those fed the HMB80-supplemented diet. Formation of the active eIF4E·eIF4G complex (Fig. 6C) in soleus muscle was higher in the HMB80 compared with the LP and F groups (P < 0.05) but was greatest in those fed the HP diet. In the diaphragm, phosphorylation of S6K1 (Table 3) and the association of eIF4E with eIF4G (Fig. 6E) were greater in the HMB80 than the LP group (P < 0.05) and tended to be greater for 4E-BP1 phosphorylation (Table 3). The phosphorylation of 4E-BP1 and S6K1 (Table 3) and active eIF4E·eIF4G complex formation (Fig. 6F) in the heart increased with feeding (P < 0.05) and was greatest in the HP group (P < 0.05); however, there was no effect of HMB supplementation. The phosphorylation of eIF2α and eEF2 in the LD, gastrocnemius, soleus, rhomboideus, diaphragm, and heart was unaffected by feeding the LP or HP diet or by HMB supplementation (Table 4). Phosphorylation of PKB in the LD muscle was higher in the fed groups than in the fasted group (P < 0.05) but was unaffected by HMB supplementation (data not shown).
Table 3.
Phosphorylation of S6K1 (Thr389) and 4E-BP1 (Thr37/46) in muscles of piglets after enteral supplementation with HMB for 24 h
| F | LP | HMB4 | HMB40 | HMB80 | HP | |
|---|---|---|---|---|---|---|
| p-S6K1, AU | ||||||
| LD | 0.03 ± 0.01a | 0.19 ± 0.06a,b | 0.20 ± 0.03a,b | 0.26 ± 0.04b | 0.54 ± 0.05c | 0.72 ± 0.07d |
| Gastrocnemius | 0.16 ± 0.03a | 0.54 ± 0.08a,b | 0.72 ± 0.13b,c | 0.84 ± 0.08b,c | 1.25 ± 0.09c,d | 1.65 ± 0.21d |
| Soleus | 0.05 ± 0.02a | 0.54 ± 0.10a,b,c | 0.77 ± 0.18a,b,c | 0.76 ± 0.13a,b,c | 1.27 ± 0.25b,c | 2.25 ± 0.37d |
| Rhomboideus | 0.02 ± 0.01a | 0.13 ± 0.03a,b | 0.17 ± 0.05a,b | 0.25 ± 0.07a,b,c | 0.43 ± 0.07c | 0.74 ± 0.10d |
| Diaphragm | 0.09 ± 0.03a | 0.47 ± 0.07a,b | 0.58 ± 0.13a,b | 0.72 ± 0.12b,c | 1.17 ± 0.12c,d | 1.55 ± 0.20d |
| Heart | 0.09 ± 0.02a | 0.55 ± 0.10b | 0.54 ± 0.14b | 0.67 ± 0.14b | 0.93 ± 0.15b | 1.85 ± 0.30c |
| p-4E-BP1, AU | ||||||
| LD | 0.04 ± 0.01a | 0.21 ± 0.09a | 0.40 ± 0.07a,b | 0.56 ± 0.14b | 0.74 ± 0.12b,c | 0.86 ± 0.13c |
| Gastrocnemius | 0.03 ± 0.01a | 0.11 ± 0.02a,b | 0.16 ± 0.03a,b | 0.22 ± 0.03c | 0.45 ± 0.04d | 0.65 ± 0.06e |
| Soleus | 0.12 ± 0.03a | 0.36 ± 0.09a,b,c | 0.46 ± 0.09a,b,c | 0.58 ± 0.12b,c | 0.98 ± 0.15b,c | 1.67 ± 0.30d |
| Rhomboideus | 0.23 ± 0.01a | 0.18 ± 0.04a,b,c | 0.23 ± 0.05a,b,c | 0.31 ± 0.09b,c | 0.57 ± 0.07c | 0.99 ± 0.19d |
| Diaphragm | 0.18 ± 0.05a | 0.96 ± 0.20a,b | 1.32 ± 0.25a,b | 1.43 ± 0.26a,b | 1.95 ± 0.24b,c | 2.74 ± 0.45c |
| Heart | 0.06 ± 0.03a | 0.46 ± 0.13b | 0.44 ± 0.13b | 0.50 ± 0.13b | 0.53 ± 0.10b | 1.21 ± 0.18c |
Data are given as means ± SE; n = 7–9. Piglets were fasted overnight (F) or fed a low-protein diet (LP), a LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or a high-protein diet (HP).
HMB, β-hydroxy-β-methylbutyrate; AU, arbitrary units; LD, longissimus dorsi; S6K1, ribosomal protein S6 kinase-1; 4E-BP1, eukaryotic initiation factor 4E-binding protein 1.
Labeled means in a row without a common letter differ, P < 0.05.
Fig. 6.
Abundance of eukaryotic initiation factor (eIF)4G·eIF4E in longissimus dorsi (A), gastrocnemius (B), soleus (C), rhomboideus (D), diaphragm (E), and heart (F) muscles of piglets that were fasted overnight (F) or fed a low-protein diet (LP), LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or high-protein diet (HP). Black lines between bands indicate where images from the same blots were spliced to adjust sample order on the membrane for presentation. Values are means ± SE; n = 7–9. a,b,c,d,eValues not sharing lowercase letters differ significantly, P < 0.05. AU, arbitrary units.
Table 4.
Phosphorylation of eIF2α (Ser51) and eEF2 (Thr56) in muscles of piglets after enteral supplementation with HMB for 24 h
| F | LP | HMB4 | HMB40 | HMB80 | HP | |
|---|---|---|---|---|---|---|
| p-eIF2α, AU | ||||||
| LD | 0.90 ± 0.16 | 1.06 ± 0.13 | 0.99 ± 0.15 | 1.02 ± 0.18 | 0.98 ± 0.17 | 1.01 ± 0.15 |
| Gastrocnemius | 0.68 ± 0.11 | 0.82 ± 0.16 | 0.82 ± 0.12 | 0.85 ± 0.15 | 0.80 ± 0.14 | 0.82 ± 0.14 |
| Soleus | 1.19 ± 0.31 | 1.01 ± 0.26 | 1.28 ± 0.22 | 1.33 ± 0.24 | 0.94 ± 0.13 | 0.96 ± 0.29 |
| Rhomboideus | 0.75 ± 0.14 | 0.69 ± 0.11 | 0.77 ± 0.21 | 0.83 ± 0.20 | 0.91 ± 0.14 | 0.86 ± 0.11 |
| Diaphragm | 0.99 ± 0.21 | 0.89 ± 0.16 | 0.92 ± 0.12 | 1.05 ± 0.20 | 1.01 ± 0.15 | 0.96 ± 0.19 |
| Heart | 1.44 ± 0.34 | 1.15 ± 0.33 | 1.00 ± 0.15 | 1.16 ± 0.29 | 1.41 ± 0.33 | 1.37 ± 0.28 |
| p-eEF2, AU | ||||||
| LD | 1.42 ± 0.23 | 1.59 ± 0.28 | 1.52 ± 0.35 | 1.54 ± 0.28 | 1.69 ± 0.26 | 1.56 ± 0.20 |
| Gastrocnemius | 0.98 ± 0.15 | 0.83 ± 0.18 | 0.96 ± 0.19 | 0.77 ± 0.14 | 0.99 ± 0.17 | 0.99 ± 0.18 |
| Soleus | 1.22 ± 0.26 | 1.05 ± 0.24 | 0.98 ± 0.32 | 1.11 ± 0.23 | 0.98 ± 0.15 | 1.26 ± 0.24 |
| Rhomboideus | 0.76 ± 0.17 | 0.99 ± 0.19 | 0.84 ± 0.14 | 0.96 ± 0.19 | 0.95 ± 0.17 | 0.86 ± 0.21 |
| Diaphragm | 1.45 ± 0.25 | 1.29 ± 0.21 | 1.32 ± 0.25 | 1.25 ± 0.24 | 1.03 ± 0.22 | 1.12 ± 0.23 |
| Heart | 0.58 ± 0.16 | 0.63 ± 0.14 | 0.62 ± 0.15 | 0.60 ± 0.12 | 0.57 ± 0.12 | 0.54 ± 0.18 |
Data are given as means ± SE; n = 7–9. Piglets were fasted overnight (F) or fed a low-protein diet (LP), a LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or a high-protein diet (HP).
HMB, β-hydroxy-β-methylbutyrate; AU, arbitrary units; LD, longissimus dorsi; eIF2α, eukaryotic factor 2α; eEF2, eukaryotic elongation factor 2.
P < 0.05.
The abundance of atrogin-1 and MuRF1 in the LD, gastrocnemius, soleus, rhomboideus, diaphragm, and heart was unaffected by treatment (Table 5). The LC3-II/total LC3 ratio in the muscles also did not differ between groups (Table 5). There was no effect of HMB supplementation or feeding the LP or HP diets on the abundance of the amino acid transporters LAT1 and SNAT2 in the LD, gastrocnemius, soleus, rhomboideus, diaphragm, or heart (Table 6).
Table 5.
Relative abundance of protein degradation signaling proteins in muscles of piglets after enteral supplementation with HMB for 24 h
| F | LP | HMB4 | HMB40 | HMB80 | HP | |
|---|---|---|---|---|---|---|
| Atrogin-1, AU | ||||||
| LD | 1.77 ± 0.43 | 1.75 ± 0.28 | 1.67 ± 0.43 | 2.21 ± 0.31 | 1.89 ± 0.26 | 1.76 ± 0.23 |
| Gastrocnemius | 0.66 ± 0.15 | 0.61 ± 0.10 | 0.59 ± 0.10 | 0.48 ± 0.15 | 0.53 ± 0.07 | 0.50 ± 0.06 |
| Soleus | 1.25 ± 0.34 | 1.43 ± 0.33 | 1.16 ± 0.26 | 1.12 ± 0.26 | 1.39 ± 0.32 | 1.01 ± 0.26 |
| Rhomboideus | 0.89 ± 0.19 | 0.75 ± 0.12 | 0.74 ± 0.21 | 0.84 ± 0.17 | 0.74 ± 0.17 | 0.97 ± 0.23 |
| Diaphragm | 0.72 ± 0.15 | 0.91 ± 0.18 | 0.75 ± 0.17 | 0.75 ± 0.12 | 0.89 ± 0.18 | 0.76 ± 0.18 |
| Heart | 0.88 ± 0.70 | 0.91 ± 0.27 | 1.03 ± 0.28 | 0.90 ± 0.29 | 0.85 ± 0.22 | 0.82 ± 0.13 |
| MuRF1, AU | ||||||
| LD | 2.32 ± 0.35 | 2.04 ± 0.37 | 1.89 ± 0.43 | 1.87 ± 0.32 | 2.09 ± 0.29 | 1.91 ± 0.30 |
| Gastrocnemius | 2.32 ± 0.35 | 2.04 ± 0.37 | 1.89 ± 0.43 | 1.87 ± 0.32 | 2.09 ± 0.29 | 1.91 ± 0.26 |
| Soleus | 0.68 ± 0.21 | 0.65 ± 0.11 | 0.55 ± 0.11 | 0.54 ± 0.11 | 0.64 ± 0.14 | 0.64 ± 0.18 |
| Rhomboideus | 0.43 ± 0.08 | 0.45 ± 0.11 | 0.53 ± 0.10 | 0.53 ± 0.09 | 0.58 ± 0.12 | 0.50 ± 0.13 |
| Diaphragm | 0.84 ± 0.18 | 0.91 ± 0.18 | 0.86 ± 0.21 | 0.74 ± 0.12 | 0.87 ± 0.18 | 0.96 ± 0.21 |
| Heart | 0.43 ± 0.08 | 0.45 ± 0.11 | 0.53 ± 0.10 | 0.53 ± 0.09 | 0.58 ± 0.12 | 0.50 ± 0.13 |
| LC3II/total LC3 ratio | ||||||
| LD | 0.68 ± 0.10a | 0.13 ± 0.05b | 0.13 ± 0.03b | 0.13 ± 0.08b | 0.14 ± 0.07b | 0.10 ± 0.04b |
| Gastrocnemius | 0.34 ± 0.05a | 0.06 ± 0.01b | 0.05 ± 0.01b | 0.08 ± 0.01b | 0.06 ± 0.01b | 0.08 ± 0.02b |
| Soleus | 0.17 ± 0.05a | 0.03 ± 0.003b | 0.04 ± 0.01b | 0.03 ± 0.01b | 0.03 ± 0.01b | 0.04 ± 0.01b |
| Rhomboideus | 0.27 ± 0.04a | 0.09 ± 0.02b | 0.10 ± 0.03b | 0.10 ± 0.02b | 0.09 ± 0.02b | 0.10 ± 0.03b |
| Diaphragm | 0.37 ± 0.06a | 0.13 ± 0.03b | 0.08 ± 0.01b | 0.11 ± 0.03b | 0.12 ± 0.03b | 0.09 ± 0.03b |
| Heart | 0.44 ± 0.14a | 0.10 ± 0.03b | 0.12 ± 0.02b | 0.13 ± 0.03b | 0.10 ± 0.02b | 0.13 ± 0.03b |
Data are given as means ± SE; n = 7–9. Piglets were fasted overnight (F) or fed a low-protein diet (LP), a LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or a high-protein diet (HP).
HMB, β-hydroxy-β-methylbutyrate; AU, arbitrary units; LD, longissimus dorsi; atrogin-1, muscle atrophy F-box; MuRF1, muscle RING finger 1 protein; LC3, microtubule-associated protein light chain 3.
Labeled means in a row without a common letter differ, P < 0.05.
Table 6.
Relative abundance of amino acid transporters in muscles of piglets after enteral supplementation with HMB for 24 h
| F | LP | HMB4 | HMB40 | HMB80 | HP | |
|---|---|---|---|---|---|---|
| LAT1, AU | ||||||
| LD | 1.01 ± 0.12 | 1.24 ± 0.17 | 1.11 ± 0.19 | 0.95 ± 0.17 | 1.19 ± 0.18 | 1.13 ± 0.18 |
| Gastrocnemius | 0.91 ± 0.14 | 0.90 ± 0.16 | 0.88 ± 0.13 | 0.78 ± 0.13 | 0.98 ± 0.15 | 0.93 ± 0.15 |
| Soleus | 0.74 ± 0.20 | 0.59 ± 0.17 | 0.66 ± 0.15 | 0.56 ± 0.13 | 0.61 ± 0.11 | 0.67 ± 0.19 |
| Rhomboideus | 0.96 ± 0.25 | 0.87 ± 0.20 | 0.89 ± 0.18 | 0.86 ± 0.18 | 1.14 ± 0.25 | 0.93 ± 0.20 |
| Diaphragm | 0.69 ± 0.19 | 0.76 ± 0.17 | 0.88 ± 0.20 | 0.70 ± 0.13 | 0.73 ± 0.18 | 0.73 ± 0.15 |
| Heart | 0.23 ± 0.05 | 0.25 ± 0.08 | 0.28 ± 0.08 | 0.23 ± 0.06 | 0.24 ± 0.05 | 0.24 ± 0.07 |
| SNAT2, AU | ||||||
| LD | 1.71 ± 0.30 | 1.51 ± 0.25 | 1.55 ± 0.31 | 1.46 ± 0.25 | 1.88 ± 0.21 | 1.50 ± 0.22 |
| Gastrocnemius | 0.98 ± 0.17 | 0.94 ± 0.15 | 0.90 ± 0.15 | 0.84 ± 0.11 | 0.86 ± 0.11 | 1.01 ± 0.14 |
| Soleus | 0.32 ± 0.09 | 0.36 ± 0.11 | 0.29 ± 0.04 | 0.30 ± 0.07 | 0.32 ± 0.07 | 0.27 ± 0.05 |
| Rhomboideus | 0.71 ± 0.15 | 0.57 ± 0.12 | 0.64 ± 0.12 | 0.65 ± 0.11 | 0.67 ± 0.10 | 0.69 ± 0.16 |
| Diaphragm | 0.94 ± 0.21 | 0.89 ± 0.18 | 0.81 ± 0.16 | 0.95 ± 0.14 | 0.87 ± 0.17 | 0.80 ± 0.13 |
| Heart | 0.22 ± 0.06 | 0.17 ± 0.03 | 0.24 ± 0.05 | 0.14 ± 0.04 | 0.22 ± 0.05 | 0.18 ± 0.03 |
Data are given as means ± SE; n = 7–9. Piglets were fasted overnight (F) or fed a low-protein diet (LP), a LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or a high-protein diet (HP).
HMB, β-hydroxy-β-methylbutyrate; AU, arbitrary units; LD, longissimus dorsi; LAT1, system L amino acid transporter 1; SNAT2, system A amino acid transporter 2.
P < 0.05.
Satellite cell proliferation in longissimus dorsi.
There was a significant treatment effect (P < 0.05) on the number of BrdU+ myonuclei per fiber cross-section (Fig. 7), with a higher number present in piglets fed the HMB80 (P < 0.05) and HP diets (P < 0.05) than the LP diet. The number of BrdU+ myonuclei was similar in the HMB80 group and the HP group.
Fig. 7.
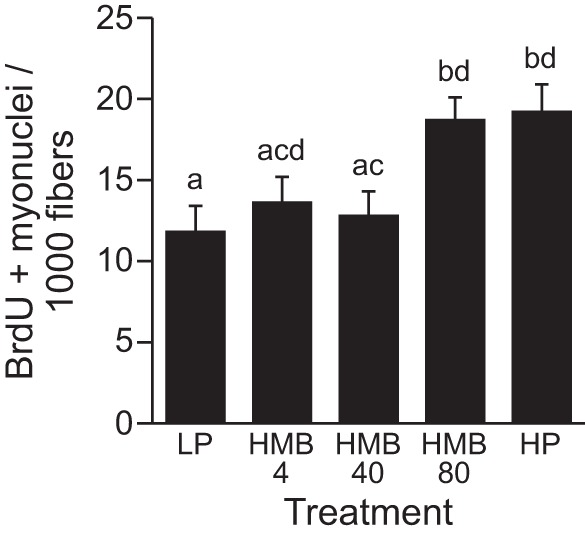
No. of 5-bromo-2′-deoxyuridine (BrdU) + myonuclei per fiber cross-section in longissimus dorsi muscles of piglets fed a low-protein diet (LP), LP diet supplemented with 4 (HMB4), 40 (HMB40), or 80 (HMB80) μmol·kg−1·day−1 HMB for 24 h, or high-protein diet (HP). Values are means ± SE; n = 7–9. a,b,c,dValues not sharing lowercase letters differ significantly, P < 0.05.
Jejunum crypt cell proliferation.
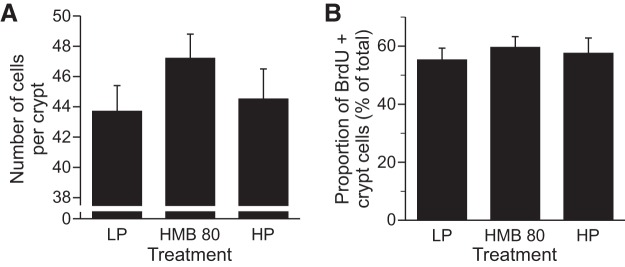
There was no difference among groups in the average number of epithelial cells per crypt longitudinal section (Fig. 8A) or in the proportion of epithelial cells within a crypt section that were BrdU+ (Fig. 8B).
Fig. 8.
Average no. of epithelial cells per crypt longitudinal section and the proportion of epithelial cells within a crypt section that were BrdU positive in the jejunum of piglets that were fed a low-protein diet (LP), LP diet supplemented with 80 μmol·kg−1·day−1 HMB for 24 h (HMB80), or high-protein diet (HP). Values are means ± SE; n = 7–9.
DISCUSSION
The rapid growth that occurs during the neonatal period is dominated by the gain in skeletal muscle mass that is supported by an elevated rate of protein synthesis (13, 17, 18, 22). Amino acids, particularly leucine and insulin, have been shown to independently stimulate skeletal muscle protein synthesis (17, 19, 29, 56, 81). Leucine and its metabolites are now being investigated as possible adjuncts in nutritional programs to promote muscle growth. A recent study demonstrated that acute parenteral infusion of HMB increases protein synthesis in skeletal muscle of neonatal pigs (77). Therefore, we hypothesized that enteral supplementation of HMB in a milk replacement formula would be effective in stimulating protein synthesis. Our results revealed that enteral HMB supplementation for 24 h resulted in a positive anabolic response in skeletal muscle, brain, and lung tissues of the neonate. These effects were associated with the activation of the mTORC1-signaling pathway and stimulation of muscle satellite cell proliferation.
Endogenous production of HMB results from the irreversible oxidation by α-ketoisocaproatedioxygenase of the KIC produced from the transamination of leucine (74). Physiological levels of HMB typically range from 1 to 4 nmol/ml (52, 54). In the present study, the stimulation of protein synthesis by enteral HMB supplementation was observed at circulating concentrations of 90 nmol/ml, and lower doses of HMB were not as effective. In our previous parenteral HMB infusion study, stimulation of muscle protein synthesis was also achieved at circulating HMB concentrations of ∼90 nmol/ml, with higher doses being less effective (77). This circulating HMB level of 90 nmol/ml was accompanied by about a fourfold increase in the intracellular HMB level in the current and previous HMB infusion studies (77) and was similar to the circulating level achieved in HMB supplementation studies in adult humans (83).
Previous studies in neonatal pigs have shown that administration of either α-ketoisocaproic acid (KIC) or leucine increases muscle protein synthesis but can be accompanied by a fall in the circulating levels of the other branched-chain amino acids, which can become rate limiting for protein synthesis (9, 31). This phenomenon, known as the “leucine paradox,” likely occurs due to increased activation of the branched-chain-α-keto acid dehydrogenase complex that promotes branched-chain amino acid oxidation and results in reduction of the circulating levels of the α-keto acids of valine and isoleucine (66). The potential anabolic properties of HMB have been studied in adult animals and humans (37, 43, 51, 52, 54, 59, 78, 83). In our study, muscle protein synthesis increased in response to HMB supplementation for 24 h without a fall in the concentrations of circulating amino acids or α-keto acids and, therefore, may be a more useful adjunct in nutritional therapies than leucine or KIC. It is notable that the breakdown of leucine to HMB is irreversible (52, 61, 74), and therefore, the observed responses from HMB supplementation cannot be ascribed to the production of leucine or KIC. Moreover, plasma insulin and glucose concentrations, which increased after feeding, did not differ between groups, and thus the increase in protein synthesis in response to HMB supplementation cannot be attributed to changes in insulin or glucose concentrations.
Although leucine is well known to stimulate protein synthesis in skeletal muscle (69), its effects in other tissues are controversial. Some studies showed that leucine supplementation stimulated protein synthesis in the myocardium of rats (75) and neonatal pigs (30, 49, 70, 82), whereas other studies in rats have shown no effect (45, 46). We demonstrated previously that parenteral administration of HMB for 60 min increased protein synthesis in glycolytic, oxidative, and mixed fiber-type skeletal muscles (77), but there was no effect on protein synthesis in the heart muscle (77). Similarly in our study, enteral supplementation with HMB for 24 h increased protein synthesis in muscles of glycolytic, mixed, and oxidative fiber types. The greatest response occurred in muscles containing predominantly fast-twitch glycolytic fibers, and there was no effect in heart muscle.
Studies in neonatal (70) and weanling (82) pigs have shown that enteral leucine supplementation can increase protein synthesis in organs such as the kidney, liver, jejunum, pancreas, spleen, and stomach, but this is not always the case (45, 46). In a previous study, parenteral HMB infusion for 1 h did not alter protein synthesis in visceral tissues, except for the lungs and spleen (77). In the present study, enteral HMB supplementation also did not affect protein synthesis in visceral tissues except for the brain and lungs, with an effect on the brain occurring even at circulating levels of 43 nmol/ml. Long-term studies are warranted to determine whether the increase in protein synthesis in the lungs and brain is maintained with prolonged HMB administration.
As with other studies evaluating the effectiveness of leucine (4, 28–31, 49, 67, 68, 70) and HMB (5, 27, 55, 58, 77, 78) administration, this study is in agreement that the mechanism resulting in the increase in skeletal muscle protein synthesis involves activation of mTORC1-dependent translation initiation. Our results showed that HMB increased the phosphorylation of S6K1 and 4E-BP1 as well as formation of the active eIF4E·eIF4G complex that activates the binding of mRNA to the ribosome. These responses occurred in all skeletal muscles, but not in the heart. Of the groups receiving HMB supplementation, the maximal increase in mTORC1 signaling occurred at a circulating concentration of 90 nmol/ml. Increased PKB phosphorylation has been reported in myoblasts treated with HMB in culture (42). However, in the current study we found that HMB supplementation in vivo did not activate PKB, and thus HMB, like leucine (68), appears to activate mTORC1 signaling through a pathway independent of insulin signaling. The exact molecular mechanism by which HMB activates mTORC1 and protein synthesis is currently unknown. However, an important milestone was recently achieved in the nutrient signaling pathway field that suggests that leucine binds to a sensor called sestrin2 and activates mTORC1 resulting in the stimulation of protein synthesis (64, 80). Similar studies are needed to determine whether HMB has a similar action as leucine or HMB has a completely different mode of mTORC1 activation.
Comparable with previous studies that evaluated short-term leucine (68, 70) and HMB (77) administration, our study showed that enteral HMB supplementation had no effect on the phosphorylation of eIF2α, which regulates tRNA binding to the ribosome and inhibits protein synthesis. A high proportion of cellular energy is spent on protein synthesis and the majority of it on the polypeptide elongation process (40). The regulation of the translation elongation pathway is in part regulated by the eEF2 pathway (10). In catabolic states, eEF2 is phosphorylated and leads to inhibition of elongation and protein synthesis and thereby promotes the conservation of energy (10). Previous studies also have shown no effect of leucine (68, 69, 79) or HMB (77) supplementation on eEF2 phosphorylation in neonatal pigs, in agreement with the current study.
Specific amino acid transporters such as SNAT2 and LAT1 are needed to transport amino acids into the cell (50). Only a handful of these amino acid transporters have been associated with mTORC1 activation (71). Inhibition of SNAT2 is known to cause the depletion of intracellular glutamine, which results in the depletion of other amino acids, particularly leucine (32), and leads to a reduction in mTORC1 activation and decreased protein synthesis rates. LAT1 is also expressed in various tissues with high rates of growth and protein turnover (24, 65), including skeletal muscle, and facilitates the transport of large neutral amino acids such as leucine, tryptophan, and tyrosine (73). Similarly, LAT1 has been shown to have a positive association with the activation of mTORC1 (66). In a previous study, no effect of amino acid transporter abundance was seen with acute parenteral HMB supplementation (77). Likewise, in this current study, we did not see an effect of HMB on SNAT2 and LAT1 abundances. This lack of effect of HMB supplementation may not be surprising since enteral HMB supplementation had no effect on the circulating concentrations of individual essential amino acids, nonessential amino acids, or the branched-chain keto acids.
The rapid accretion of protein that occurs in the neonatal period is accompanied by an increase in the myonuclear content of muscle fibers (18). Satellite cells are adult muscle progenitor cells that play a crucial role in the addition of new myonuclei to the growing fibers since myonuclei are postmitotic (47, 48). In the neonatal period, satellite cells account for a significant proportion of total muscle nuclei, but their absolute and relative numbers decrease with increasing maturity as the cells become quiescent (18, 23). BrdU, a synthetic thymidine analog nucleoside, is incorporated into nuclei during DNA synthesis (34). Because skeletal muscle nuclei are postmitotic and cannot incorporate BrdU into their DNA, only those myonuclei that were generated by the proliferation and differentiation of satellite cells during the labeling administration period are labeled (2). Thus, the relative number of BrdU+ myonuclei (nuclei enclosed within the dystrophin positive sarcolemma) provides a measure of satellite cell proliferative activity. In the current study, we found that HMB supplementation increased the number of BrdU+ myonuclei in the longissimus dorsi, a fast-twitch muscle, suggesting that HMB may stimulate satellite cell proliferation and also contribute to increased muscle growth in neonatal muscle. These results are in agreement with a previous study showing that HMB supplementation enhances stem cell proliferation in fast-twitch muscles of aged rats as well as in isolated satellite cells (2, 42). In the jejunum, there was no effect of HMB treatment on the number of cells per crypt or the number that were BrdU positive, which suggest that the enhancement of satellite cell proliferation by HMB is not a general effect on all actively proliferating cells.
In previous studies evaluating the mechanisms by which HMB promotes strength and muscle hypertrophy in adults, it has been shown that HMB may suppress proteolysis by inhibiting the ubiquitin-proteasome system (6). In the current study, we did not identify effects of HMB on the abundance of the ubiquitin ligases atrogin-1 and MuRF1, which are indices of the ubiquitin-proteasome proteolytic system. We also found no effect of HMB supplementation on the LC3-II/LC3 ratio, a marker of the autophagy-lysosome system. These results are similar to our previous findings with parenteral HMB supplementation (77) and suggest that in neonatal muscle the primary effect of HMB supplementation is to enhance the muscle anabolic pathways rather than to reduce the catabolic pathways.
In conclusion, the results of the current study suggest that HMB merits consideration as a nutritional supplement to enhance protein synthesis and muscle accretion in the neonate. Enteral HMB supplementation of a milk-based formula enhanced muscle protein synthesis by activating mTORC1-regulated processes, including translation initiation, and promoted accretion of muscle cell nuclei through stimulation of satellite cell proliferation. The effects of HMB appear to be more specific to muscles rather than visceral organs, although the effect of HMB on protein synthesis in the lung and brain warrants further study. Furthermore, the results suggest that HMB supplementation had no effect on protein degradation through either the ubiquitin-proteasome or autophagy-lysosome pathway. Although the results of this study are suggestive, further work is needed to determine whether the effect of enteral HMB supplementation on protein synthesis and myonuclear accretion are sustained with long-term HMB administration to increase muscle growth.
GRANTS
This project was supported by Abbott Nutrition, National Institute of Child Health and Human Development Grant HD-072891, US Department of Agriculture (USDA)/National Institute of Food and Agriculture Grant 2013-67015-20438, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-044474 and AR-046308, and USDA Current Research Information System Grant 6250-51000-055. This work is a publication of the USDA/Agricultural Research Service Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, and Abbott Nutrition. The contents of this publication do not necessarily reflect the views or politics of the USDA, nor does the mention of trade names, commercial products, or organizations imply endorsements by the US Government.
DISCLOSURES
M. Kao, D. A. Columbus, A. Suryawan, J. Steinhoff-Wagner, A. Hernandez-Garcia, H. V. Nguyen, M. L. Fiorotto, and T. A. Davis have no conflicts of interest.
AUTHOR CONTRIBUTIONS
M.K., D.A.C., M.L.F., and T.A.D. conception and design of research; M.K., D.A.C., A.S., J.S.-W., A.H.-G., and H.V.N. performed experiments; M.K., D.A.C., A.S., H.V.N., M.L.F., and T.A.D. analyzed data; M.K., D.A.C., A.S., M.L.F., and T.A.D. interpreted results of experiments; M.K. prepared figures; M.K. drafted manuscript; M.K., D.A.C., A.S., J.S.-W., A.H.-G., H.V.N., M.L.F., and T.A.D. edited and revised manuscript; M.K., D.A.C., A.S., J.S.-W., A.H.-G., H.V.N., M.L.F., and T.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully thank Rose Almonaci and Ryan Fleischman for expert technical assistance.
REFERENCES
- 1.Abdelhamid AE, Chuang SL, Hayes P, Fell JM. In vitro cow's milk protein-specific inflammatory and regulatory cytokine responses in preterm infants with necrotizing enterocolitis and sepsis. Pediatr Res 69: 165–169, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Alway SE, Pereira SL, Edens NK, Hao Y, Bennett BT. beta-Hydroxy-beta-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp Gerontol 48: 973–984, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 131: 856S–860S, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130: 139–145, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Aversa Z, Bonetto A, Costelli P, Minero VG, Penna F, Baccino FM, Lucia S, Rossi Fanelli F, Muscaritoli M. Beta-hydroxy-beta-methylbutyrate (HMB) attenuates muscle and body weight loss in experimental cancer cachexia. Int J Oncol 38: 713–720, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Baptista IL, Leal ML, Artioli GG, Aoki MS, Fiamoncini J, Turri AO, Curi R, Miyabara EH, Moriscot AS. Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve 41: 800–808, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Beerman DH, Hood LF, Liboff M. Satellite cell and myonuclei populations in rat soleus and extensor digitorum longus muscles after maternal nutritional deprivation and realimentation. J Anim Sci 57: 1618–1625, 1983. [DOI] [PubMed] [Google Scholar]
- 8.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Boutry C, El-Kadi SW, Suryawan A, Wheatley SM, Orellana RA, Kimball SR, Nguyen HV, Davis TA. Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am J Physiol Endocrinol Metab 305: E620–E631, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem 269: 5360–5368, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S. Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 37: 593–599, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Cecconi F, Levine B. The role of autophagy in mammalian development: cell makeover rather than cell death. Dev Cell 15: 344–357, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien PF, Smith K, Watt PW, Scrimgeour CM, Taylor DJ, Rennie MJ. Protein turnover in the human fetus studied at term using stable isotope tracer amino acids. Am J Physiol Endocrinol Metab 265: E31–E35, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Columbus DA, Fiorotto ML, Davis TA. Leucine is a major regulator of muscle protein synthesis in neonates. Amino Acids 47: 259–270, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 135: 376–382, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Dai JM, Yu MX, Shen ZY, Guo CY, Zhuang SQ, Qiu XS. Leucine Promotes Proliferation and Differentiation of Primary Preterm Rat Satellite Cells in Part through mTORC1 Signaling Pathway. Nutrients 7: 3387–3400, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7-than in 26-day-old pigs. Am J Physiol Endocrinol Metab 270: E802–E809, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care 12: 78–85, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282: E880–E890, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol Regul Integr Comp Physiol 257: R1141–R1146, 1989. [DOI] [PubMed] [Google Scholar]
- 21.Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279: E1226–E1234, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Denne SC, Kalhan SC. Leucine metabolism in human newborns. Am J Physiol Endocrinol Metab 253: E608–E615, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol 15: 666–673, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Edinger AL. Controlling cell growth and survival through regulated nutrient transporter expression. Biochem J 406: 1–12, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Ehrenkranz RA. Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol 31: 48–55, 2007. [DOI] [PubMed] [Google Scholar]
- 26.El-Kadi SW, Suryawan A, Gazzaneo MC, Srivastava N, Orellana RA, Nguyen HV, Lobley GE, Davis TA. Anabolic signaling and protein deposition are enhanced by intermittent compared with continuous feeding in skeletal muscle of neonates. Am J Physiol Endocrinol Metab 302: E674–E686, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. Signaling pathways initiated by β-hydroxy-β-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab 293: E923–E931, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab 293: E1615–E1621, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab 288: E914–E921, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290: E612–E621, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr 140: 1418–1424, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A. Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol 18: 1426–1436, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 20: 445–451, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Gallegly JC, Turesky NA, Strotman BA, Gurley CM, Peterson CA, Dupont-Versteegden EE. Satellite cell regulation of muscle mass is altered at old age. J Appl Physiol (1985) 97: 1082–1090, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J 192: 719–723, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han B, Tong J, Zhu MJ, Ma C, Du M. Insulin-like growth factor-1 (IGF-1) and leucine activate pig myogenic satellite cells through mammalian target of rapamycin (mTOR) pathway. Mol Reprod Dev 75: 810–817, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Holecek M, Muthny T, Kovarik M, Sispera L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem Toxicol 47: 255–259, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Johnson JD, Albritton WL, Sunshine P. Hyperammonemia accompanying parenteral nutrition in newborn infants. J Pediatr 81: 154–161, 1972. [DOI] [PubMed] [Google Scholar]
- 39.Kadowaki M, Kanazawa T. Amino acids as regulators of proteolysis. J Nutr 133: 2052S–2056S, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Kaul G, Pattan G, Rafeequi T. Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem Funct 29: 227–234, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Kimball SR. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am J Clin Nutr 99: 237S–242S, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornasio R, Riederer I, Butler-Browne G, Mouly V, Uni Z, Halevy O. Beta-hydroxy-beta-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta 1793: 755–763, 2009. [DOI] [PubMed] [Google Scholar]
- 43.Kovarik M, Muthny T, Sispera L, Holecek M. Effects of beta-hydroxy-beta-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J Physiol Biochem 66: 311–319, 2010. [DOI] [PubMed] [Google Scholar]
- 44.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17: 1807–1819, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Lynch CJ, Hutson SM, Patson BJ, Vaval A, Vary TC. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am J Physiol Endocrinol Metab 283: E824–E835, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Lynch CJ, Patson BJ, Anthony J, Vaval A, Jefferson LS, Vary TC. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am J Physiol Endocrinol Metab 283: E503–E513, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol 44: 459–462, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murgas Torrazza R, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr 140: 2145–2152, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136: 521–534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC Jr, Connelly AS, Abumrad N. Effect of leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol (1985) 81: 2095–2104, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Nissen S, Sharp RL, Panton L, Vukovich M, Trappe S, Fuller JC Jr. beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr 130: 1937–1945, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Nissen S, Van Koevering M, Webb D. Analysis of beta-hydroxy-beta-methyl butyrate in plasma by gas chromatography and mass spectrometry. Anal Biochem 188: 17–19, 1990. [DOI] [PubMed] [Google Scholar]
- 54.Nissen SL, Abumrad NN. Nutritional role of the leucine metabolite β-hydroxy-β-methylbutyrate (HMB). J Nutr Biochem 8: 300–311, 1997. [Google Scholar]
- 55.Nissen SL, Van Huysen C, Haymond MW. Measurement of branched chain amino acids and branched chain alpha-ketoacids in plasma by high-performance liquid chromatography. J Chromatogr 232: 170–175, 1982. [DOI] [PubMed] [Google Scholar]
- 56.O'Connor PM, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 284: E100–E119, 2003. [DOI] [PubMed] [Google Scholar]
- 57.O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 285: E40–E53, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Pimentel GD, Rosa JC, Lira FS, Zanchi NE, Ropelle ER, Oyama LM, Oller do Nascimento CM, de Mello MT, Tufik S, Santos RV. β-Hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr Metab (Lond) 8: 11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russell ST, Tisdale MJ. Mechanism of attenuation by beta-hydroxy-beta-methylbutyrate of muscle protein degradation induced by lipopolysaccharide. Mol Cell Biochem 330: 171–179, 2009. [DOI] [PubMed] [Google Scholar]
- 60.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31: 342–348, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Sabourin PJ, Bieber LL. Formation of beta-hydroxyisovalerate by an alpha-ketoisocaproate oxygenase in human liver. Metabolism 32: 160–164, 1983. [DOI] [PubMed] [Google Scholar]
- 62.Saigal S, Stoskopf BL, Streiner DL, Burrows E. Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics 108: 407–415, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Sandri M. Autophagy in health and disease. 3. Involvement of autophagy in muscle atrophy. Am J Physiol Cell Physiol 298: C1291–C1297, 2010. [DOI] [PubMed] [Google Scholar]
- 64.Saxton RA, Knockenhauer KE, Wolfson RL, Chantranupong L, Pacold ME, Wang T, Schwartz TU, Sabatini DM. Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway. Science 351: 53–58, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Storey BT, Fugere C, Lesieur-Brooks A, Vaslet C, Thompson NL. Adenoviral modulation of the tumor-associated system L amino acid transporter, LAT1, alters amino acid transport, cell growth and 4F2/CD98 expressionwith cell-type specific effects in cultured hepatic cells. Int J Cancer 117: 387–397, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Suryawan A, Davis TA. Regulation of protein synthesis by amino acids in muscle of neonates. Front Biosci (Landmark Ed) 16: 1445–1460, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab 295: E868–E875, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suryawan A, Nguyen HV, Almonaci RD, Davis TA. Differential regulation of protein synthesis in skeletal muscle and liver of neonatal pigs by leucine through an mTORC1-dependent pathway. J Anim Sci Biotechnol 3: pii: 3. doi: 10.1186/2049-1891-3-3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suryawan A, Orellana RA, Fiorotto ML, Davis TA. Triennial Growth Symposium: leucine acts as a nutrient signal to stimulate protein synthesis in neonatal pigs. J Anim Sci 89: 2004–2016, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA. Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res 71: 324–331, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taylor PM. Amino acid transporters: eminences grises of nutrient signalling mechanisms? Biochem Soc Trans 37: 237–241, 2009. [DOI] [PubMed] [Google Scholar]
- 72.Thureen PJ, Melara D, Fennessey PV, Hay WW Jr. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res 53: 24–32, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Uchino H, Kanai Y, Kim DK, Wempe MF, Chairoungdua A, Morimoto E, Anders MW, Endou H. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol 61: 729–737, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Van Koevering M, Nissen S. Oxidation of leucine and α-ketoisocaproate to β-hydroxy-β-methylbutyrate in vivo. Am J Physiol Endocrinol Metab 262: E27–E31, 1992. [DOI] [PubMed] [Google Scholar]
- 75.Vary T. Oral leucine enhances myocardial protein synthesis in rats acutely administered ethanol. J Nutr 139: 1439–1444, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vlaardingerbroek H, van Goudoever JB, van den Akker CH. Initial nutritional management of the preterm infant. Early Hum Dev 85: 691–695, 2009. [DOI] [PubMed] [Google Scholar]
- 77.Wheatley SM, El-Kadi SW, Suryawan A, Boutry C, Orellana RA, Nguyen HV, Davis SR, Davis TA. Protein synthesis in skeletal muscle of neonatal pigs is enhanced by administration of β-hydroxy-β-methylbutyrate. Am J Physiol Endocrinol Metab 306: E91–E99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, Etheridge T, Rathmacher JA, Smith K, Szewczyk NJ, Atherton PJ. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 591: 2911–2923, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA. Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140: 264–270, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolfson RL, Chantranupong L, Saxton RA, Shen K, Scaria SM, Cantor JR, Sabatini DM. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351: 43–48, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab 275: E602–E609, 1998. [DOI] [PubMed] [Google Scholar]
- 82.Yin Y, Yao K, Liu Z, Gong M, Ruan Z, Deng D, Tan B, Liu Z, Wu G. Supplementing L-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39: 1477–1486, 2010. [DOI] [PubMed] [Google Scholar]
- 83.Zanchi NE, Gerlinger-Romero F, Guimaraes-Ferreira L, de Siqueira Filho MA, Felitti V, Lira FS, Seelaender M, Lancha AH Jr. HMB supplementation: clinical and athletic performance-related effects and mechanisms of action. Amino Acids 40: 1015–1025, 2011. [DOI] [PubMed] [Google Scholar]