Abstract
The pain phenotype in sickle cell disease (SCD) patients is highly variable. A small percentage of SCD patients experience many vaso-occlusive crises/year, 5% of patients account for over 30% of pain episodes, while 39% report few episodes of severe pain. Clearly, a better understanding of the pathobiology of SCD is needed to improve its therapy. Humanized sickle cell mice recapitulate several phenotypes of SCD patients and provide a model for the study of SCD pain. Researchers have shown that one strain of humanized SCD mice, the BERK strain, has abnormal pain phenotype. However, the nociception phenotype of another humanized SCD mouse strain, the Townes strain, has not been described. In a large cross-sectional study of BERK and Townes SCD mice, we examined thermosensory response and sensory nerve fiber function using sine-wave electrical stimulation at 2000, 250, and 5 Hz to stimulate preferentially Aβ, Aδ, and C sensory nerve fibers, respectively. We found that BERK and Townes mice, compared to respective controls, had decreases in 2000, 250, and 5 Hz current vocalization thresholds in patterns that suggest sensitization of a broad spectrum of sensory nerve fibers. In addition, the pattern of sensitization of sensory fibers varied according to strain, sex, age, and mouse genotype. In a similarly variable pattern, Townes and BERKs also had significantly altered sensitivity to noxious thermal stimuli in agreement with what has been shown by others. In summary, the analysis of somatosensory function using sine-wave electrical stimulation in humanized sickle cell mice suggests that in SCD, both myelinated and unmyelinated, fibers are sensitized. The pattern of sensory fiber sensitization is distinct from that observed in pain models of neuropathic and inflammatory pain. These findings raise the possibility that sensitization of a broad spectrum of sensory fibers might contribute to the altered and variable nociception phenotype in SCD.
Keywords: Sickle cell, pain, nociception, sine wave, somatosensory, fetal hemoglobin
Introduction
Patients with sickle cell disease (SCD) face a lifelong challenge of living with pain that is associated with healthcare disparities. In these patients, the pain can be incapacitating and is associated with decreased quality of life and neurodevelopmental deficits.1–4 The spectrum of pain phenotype in SCD patients is highly variable in that a few patients have many recurrent vaso-occlusive crises/year, 5% account for over 30% of pain episodes, whereas 39% report few pain episodes.2–5 While the mechanism for such variability in SCD-associated pain (intensity, distribution, chronicity, and pattern of pain) is poorly understood, researchers have shown that it is associated with factors such as genotype, age, and disease severity.2,3,6 Further, a growing body of literature suggests that SCD patients may have three distinct pain types that include acute pain during vaso-occlusive crisis, chronic pain, and neuropathic pain.7–9 Clearly, the pathobiology of SCD pain is incompletely understood and as a result, its treatment is often less than adequate.
Humanized sickle cell mice provide a model to study SCD pain and allow for evaluation of somatosensory function associated with the disease.10–14 Using mechanical and thermal stimuli, researchers have shown that the BERK strain of SCD mice displays thermal and mechanical hyperalgesia, which are associated with disruptions of the architecture of small sensory fibers in the dermis.10 These findings in BERK mice partially mirror those in patients with SCD who have been shown to have increased sensitivity to heat and cold.9,15 Further, using ex vivo skin-nerve preparations, others have shown that mechanical allodynia in BERK mice is associated with increases in number of evoked potentials to suprathreshold forces which suggests that light-touch cutaneous afferents (Aβ fibers) are sensitized to mechanical forces.13 Therefore, several studies suggest that SCD is associated with somatosensory dysfunction that is likely to involve small and large sensory nerve fibers.
Evaluation of somatosensory function and analgesic fiber selectivity can be performed with quantitative evaluation of nocifensive behavior in response to activation of Aβ (large myelinated), Aδ (lightly myelinated), and C (small, unmyelinated) sensory fibers. Such evaluation can be done using variable rates of noxious radiant heat as Aδ fibers are activated by high rate and C fibers by low rate of skin heating.16,17 Alternatively, sensory fiber function can be examined with sine-wave electrical stimulation at 2000, 250, and 5 Hz to stimulate preferentially Aβ, Aδ, and C sensory nerve fibers, respectively.18–22 This preferential stimulation derives from distinct electrophysiological characteristics (refractory period, diameter, conduction velocity) of afferent neurons.19–21,23 A number of animal and human studies suggest that sine-wave stimulation may be used to evaluate distinct classes of sensory fibers, to examine quantitatively somatosensory function, and to evaluate fiber selectivity of analgesics.19,23–26
In this investigation, we used sine-wave stimulation in a large cross-sectional study to examine the effect of SCD, strain, genotype, age, and sex on somatosensory fiber function and nocifensive behavior in two strains of humanized SCD mice.
Material and methods
This study was conducted in strict adherence to the recommendations in the Guide for the Care and Use of Laboratory Animals, National Institutes of Health policies, and making every effort to minimize distress. The investigational protocol was approved by the Animal Care and Use Committees from the National Institutes of Health Clinical Center, NIH, Bethesda, MD, and from the Children’s National Medical Center, Washington, DC.
Animals
In this study, we adhered to recently published guidelines to optimize the predictive value of preclinical research.27 Mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred at our animal facility. We examined two strains of humanized SCD mice. The first strain was generated by Dr Tim M. Townes at the University of Alabama (here referred to as the Townes strain): B6;129-Hbatm1(HBA)Tow Hbbtm2(HBG1,HBB*)Tow/Hbbtm3(HBG1,HBB)Tow/J (Jackson Laboratory, stock number 013071).28,29 Townes SCD mice do not express any mouse hemoglobin as mouse α- and β-globin genes were replaced with human α- and β-globin genes as indicated below.28,29 Homozygous sickle Townes carry a mutation designed with the human hemoglobin α-gene (Hbatm1(HBA)Tow, hα) and the Hbbtm2(HBG1,HBB*)Tow mutation, a 9.7-kb DNA fragment containing the human Aγ-globin gene and human sickle hemoglobin beta (βS) that replaced mouse major and minor β-globin genes (hα/hα::βS/βS). Heterozygous Townes mice carry the Hbatm1(HBA)Tow (hα) mutation, one copy of human sickle hemoglobin beta (βS), and one copy of the Hbbtm3(HBG1,HBB)Tow mutation, a DNA fragment consisting of human hemoglobin gamma (Aγ) gene and human wild-type hemoglobin beta (βA) genes (hα/hα::βA/βS). Townes control animals carry two copies of the human α-globin gene and two copies of the Hbbtm3(HBG1,HBB)Tow mutation (hα/hα::βA/βA).28,29 Homozygous (hα/hα::βS/βS) sickle Townes mice recapitulate hematologic defects of human SCD (anemia, reticulocytosis, leukocytosis, sickling) as well as liver and kidney disease.28,29
The second strain was generated by Dr Paszty at Berkley University (here referred to as the BERK strain): Hbatm1Paz Hbbtm1Tow Tg(HBA-HBBs)41Paz/J, stock number 003342).30 Homozygous BERK mice do not express mouse hemoglobin and carry copies of a transgene (Tg(HBA-HBBs)41Paz) that contains human HBA1 (hemoglobin, alpha 1), HBG2 (hemoglobin, gamma G, fetal component), HBG1 (hemoglobin, gamma A, fetal component), HBD (hemoglobin, delta), and HBBS (hemoglobin, beta, sickle allele) genes.30 Heterozygous BERK do not express mouse Hba and have one copy of mouse Hbb and the Tg (HBA-HBBs)41Paz. We used C57BL/6 as control strain for BERK mice.
Study design and experimental protocol
We evaluated nocifensive behavior using electrical and thermal stimulation in a cross-sectional fashion in different cohorts of animals. Mice were housed in ventilated cages in a temperature-controlled environment (21℃), kept in standard 12-h light–dark cycle, and had unrestricted access to regular mouse chow and water. In order to control for variability and synchronize estrous cycles, female mice from all genotypes were housed together. All experiments were performed between 6:30 AM and 12:00 PM in a quiet room with one animal present during measurements. During any given experiment of nocifensive behavior, SCD mice and respective controls were examined during each session in order to control for the effect of time and operator variability. Nocifensive behavior was evaluated using hot plate latency, cold plate sensitivity, and current vocalization threshold (sensory fiber interrogation) in this sequence. Only one of the three testing paradigms was conducted per day and the investigator conducting quantitative sensory testing was unaware of the animals’ genotype. Male and female mice of each strain and genotype were examined during one of the following age intervals: young age (6–10 weeks), middle age (12 < age < 16 weeks), and older age (20 < age < 27 weeks). These age intervals were selected to capture representative ages in mouse lifespan and avoid selection bias given that homozygous sickle mice have a mortality rate of 20% between weaning and 14 weeks. In this cross-sectional study, animals were studied only once after being randomly assigned to be studied in only one of the three age groups.
Nocifensive behavior studies
Sensory nerve fiber interrogation using sine-wave electrical stimulation
In order to evaluate somatosensory fiber function, we used sine-wave electrical stimulation delivered at three different frequencies: 2000, 250, and 5 Hz that preferentially stimulate Aβ, Aδ, and C fibers, respectively, as described.26,31,32 The sine-wave electrical stimuli generated by a neurostimulator (Neurotron, Inc, Baltimore, MD) are controlled by custom software and are delivered to the tail of gently restrained mice as previously described.26,31,32 Briefly, stimuli at each frequency (2000, 250, and 5 Hz) are delivered in a stepwise fashion at increasing intensities and last 1 s on a 50% duty cycle as each stimulus is followed by a 1-s stimulus-free interval. There was a 1-min rest period between stimulation with different frequencies. When vocalization occurs, the electrical stimulus that elicited it could be shorter than 1 s, as vocalization prompts termination of the stimulus. For 2000 Hz, stimuli intensities are set from 0.4 to 1.6 mA with stepwise increases of 0.1 mA, for 250 Hz from 0.14 to 0.5 mA with stepwise increases of 0.04 mA, and for 5 Hz from 0.05 to 0.5 mA with stepwise increases of 0.05 mA. Current thresholds for each frequency were determined as the average of five consecutive measurements and were obtained sequentially in response to 2000, 250, and 5 Hz. The order of stimuli frequency delivery is based on studies showing that vocalization thresholds for 2000 and 250 Hz frequencies may be affected when they follow the 5 Hz electrical stimulus.31 For each frequency, the amperage of the electrical stimulus that elicited audible vocalization (nocifensive behavior) or the maximum amperage delivered for each frequency was defined as current threshold.26,31,32 The unit of measurement of current threshold is “unit” (U) and corresponds to 100 times the current intensity (amperage) that elicited audible vocalization.
Thermosensory studies
Hot plate latency
In order to evaluate nocifensive response to thermal stimuli, mice were placed on a 55℃ hot plate (Harvard Apparatus, Holliston, MA) and latency time for pain-avoiding behaviors (jumping, stomping, or repeated lifting or licking of hind or front paws) was measured. Once one of these behaviors was noted, mice were removed from the hot plate and time in seconds was recorded as heat response latency.33
Cold plate sensitivity
For cold plate experiments, we used a Peltier cooler to generate a cold surface maintained at 2℃ (Harvard Apparatus, Holliston, MA). Mice were placed on the cold plate and for 5 min, their behavior was captured on video. The time animals spent withdrawing from the cold surface (lifting front or hind paws, rubbing of front paws) was recorded and the percentage of time withdrawing from the cold plate surface during the observation period was calculated and interpreted to reflect cold plate sensitivity.34
Hemoglobin analysis and quantitation
Hemoglobin analysis and quantitation from mouse red cell hemolysate was performed with capillary zone electrophoresis using the hemoglobin assay program on MINICAP™ (Sebia, Norcross, GA) according to the manufacturer’s recommendations. Red blood cell hemolysates were injected by aspiration at the anionic side of the capillary. High voltage protein separation was performed and hemoglobin fractions were detected at 415 nm. Fetal hemoglobin levels are expressed as % of total hemoglobin.
Statistical analysis
Descriptive statistics and graphics were used to describe the outcome measures on the response to sine-wave electrical stimulation using 2000, 250, and 5 Hz to stimulate preferentially Aβ, Aδ, and C sensory fibers, respectively, as well heat and cold sensitivity. Because BERK and Townes are two different strains of mice, it is inappropriate to analyze the two samples of mice in one regular regression or analysis of variance (ANOVA) model. To avoid such an issue, one might implement the same regression model separately for each of the two strains. However, this approach does not provide significance testing for differences in the parameter estimates between the two models. In this study, to model the response measured with each of the five stimulation paradigm (i.e., 2000, 250, 5 Hz, cold and heat sensitivity), we conducted the same multiple regression model among both BERK and Townes mice simultaneously. Such a model, called multigroup models, is a special case of structural equation model.35–37 With such an approach, we can include the BERK and Townes mice as different populations in our modeling and test invariance of genotype effect, as well as age and gender effect, across different strains. The independent variables included in the model are: sex (0: female; 1: male), middle age (1: [12 < age < 16 weeks]; 0: otherwise), older age (1: [20 < age < 27 weeks]; 0: otherwise), heterozygous (1: heterozygous genotype; 0: otherwise), and homozygous (1: homozygous genotype; 0: otherwise). Each multigroup model produced two sets (one for Townes and one for BERK) of slope coefficients that can be interpreted exactly in the same way as in multiple regressions. The two sets of slope coefficients were compared across strains with respect to their signs, statistical significance, and magnitudes. For example, to assess the effect of heterozygous genotype among Townes and BERK mice, we checked whether its slope coefficients in regressions of the two strains were in the same direction (i.e. both positive or both negative), whether its slopes were both statistically significant. If the slopes had the same sign and were statistically significant among both Townes and BERK mice, difference in effect magnitude between the strains was tested using Wald test. As such, the multigroup model enabled us to test whether the relationships of genotypes with the response measured under different stimulation paradigm were invariant across mice (Townes versus BERK) strains, controlling for age and sex. The models were estimated using Mplus 7.1.38 In consideration of possible nonnormality in the data, an estimator for maximum likelihood estimation with robust standard errors (MLR) was used for model estimation.38 Similar to the Yuan and Bentler’s method39 MLR adjusts the ML Chi-square by a constant, called scaling correction factor, to account for nonnormality of the observed measures and provides robust standard errors that are used to conduct significance testing for individual parameter estimates.
Results
Eight hundred and forty six animals were enrolled in this investigation and sample statistics are presented in Table 1.
Table 1.
Number of animals enrolled in a large cross-sectional study of current vocalization threshold and quantitative thermosensory testing in two strains of sickle cell mice.a
| Variable | Townes Strain | BERK Strain |
|---|---|---|
| Current Vocalization Threshold (N = 774) | ||
| Genotype | ||
| Control | 80 | 204 |
| Hetero | 161 | 108 |
| Homo | 137 | 84 |
| Sex | ||
| Male | 166 | 172 |
| Female | 212 | 224 |
| Age | ||
| Young | 122 | 179 |
| Middle | 144 | 100 |
| Older | 112 | 117 |
| Total | 378 | 396 |
| Quantitative Sensory Testing (N = 503) | ||
| Genotype | ||
| Control | 80 | 139 |
| Hetero | 79 | 67 |
| Homo | 80 | 58 |
| Sex | ||
| Male | 97 | 116 |
| Female | 142 | 148 |
| Age | ||
| Young | 82 | 141 |
| Middle | 88 | 58 |
| Older | 69 | 65 |
| Total | 239 | 264 |
Young indicates age < 10 weeks, middle 12 < age < 16 weeks, and older 20 < age < 27 weeks. Some animals underwent both current vocalization threshold and quantitative thermosensory testing. Animals were studied only one time at the age group determined at time of randomization.
Cross-sectional study of the effect of SCD on nocifensive behavior in response to sine-wave electrical stimulation
Selected results of multigroup regression models are presented in Tables 2 and 3. Adjusted outcome means for BERKs and Townes were calculated by genotype, age, and gender groups using the regression coefficients in Tables 2 and 3 and shown in Figures 1, 2, and 3, respectively.
Table 2.
Selected results of multigroup model by current vocalization threshold.a
| 2000 Hz Current vocalization threshold |
250 Hz Current vocalization threshold |
5 Hz Current vocalization threshold |
||||
|---|---|---|---|---|---|---|
| Variables | Townes | BERK | Townes | BERK | Townes | BERK |
| Intercept | 81.8 ( < 0.001) | 77.4 (<0.001) | 33.1 (<0.001) | 31.9 (<0.001) | 24.2 (<0.001) | 18.1 (<0.001) |
| GENOTYPE | ||||||
| Control | – | – | – | – | – | – |
| Hetero | –1.1 (0.654) | ─3.8 (0.135) | –1.1 (0.414) | –1.0 (0.391) | –2.1 (0.219) | 7.4 (<0.001) |
| Homo | –6.3 (0.014) | –1.6 (0.513) | –3.7 (0.003) | –2.6 (0.029) | –5.8 (<0.001) | 2.4 (0.156) |
| AGE | ||||||
| Young | – | – | – | – | – | – |
| Middle | –1.2 (0.681) | 12.1 (0.001) | 1.1 (0.431) | 3.4 (0.043) | 0.4 (0.847) | –2.7 (0.190) |
| Older | –6.4 (0.015) | 3.6 (0.129) | –2.5 (0.066) | 1.8 (0.116) | –5.1 (0.003) | –5.8 (<0.001) |
| SEX | ||||||
| Female | – | – | – | – | – | – |
| Male | 11.9 (<0.001) | 13.0 (<0.001) | 7.0 (<0.001) | 7.0 (<0.001) | 9.5 (<0.001) | 4.4 (<0.001) |
| R2 | 0.21 (<0.001) | 0.22 (<0.001) | 0.24 (<0.001) | 0.24 (<0.001) | 0.29 (<0.001) | 0.22 (0.001) |
The results show the current vocalization threshold differences (p value) comparing a given group with the respective reference group. Intercept indicates the intercept coefficient of the regression model, i.e. the value at which the fitted line in the model crosses the y-axis. – indicates the reference group, hetero indicates heterozygous, homo homozygous, young indicates age < 10 weeks, middle 12 < age < 16 weeks, and older 20 < age < 27 weeks. R2 indicates R square.
Table 3.
Selected results of multigroup models by thermosensory testing.a
| Hot Plate Latency (s) |
Cold Plate Sensitivity (%) |
|||
|---|---|---|---|---|
| Variables | Townes | BERK | Townes | BERK |
| Intercept | 8.9 (<0.001) | 9.2 (<0.001) | 21.7 (<0.001) | 44.9 (<0.001) |
| GENOTYPE | ||||
| Control | – | – | – | – |
| Hetero | 0.86 (0.009) | –2.0 (<0.001) | 11.8 (<0.001) | –29.0 (<0.001) |
| Homo | 0.05 (0.892) | –2.4 (<0.001) | 19.1 (<0.001) | –23.0 (<0.001) |
| AGE | ||||
| Young | – | – | – | – |
| Middle | –0.9 (0.009) | –0.6 (0.180) | –2.5 (0.297) | 4.6 (0.298) |
| Older | –0.3 (0.363) | –0.7 (0.042) | 6.0 (0.066) | 5.5 (0.080) |
| SEX | ||||
| Female | – | – | – | – |
| Male | –1.0 (<0.001) | 0.3 (0.258) | –1.2 (0.633) | 3.3 (0.172) |
| R2 | 0.12 (0.014) | 0.34 (<0.001) | 0.23 (<0.001) | 0.39 (<0.001) |
The results show the current vocalization threshold differences (p value) comparing a given group with the respective reference group. Intercept indicates the intercept coefficient of the regression model, i.e. the value at which the fitted line in the model crosses the y-axis. – indicates the reference group, hetero indicates heterozygous, homo homozygous, young indicates age < 10 weeks, middle 12 < age < 16 weeks, and older 20 < age < 27 weeks. R2 indicates R square.
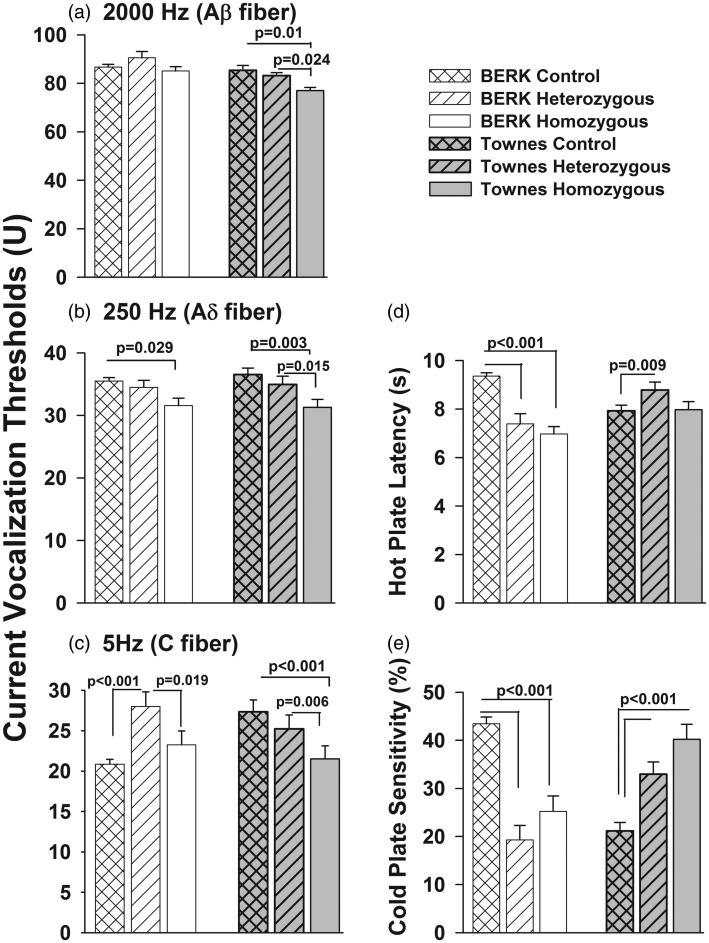
Figure 1.
Effect of genotype of BERK (three left bars in each panel) and Townes mice (three right bars in each panel) on current vocalization threshold to 2000 Hz (panel a), 250 Hz (panel b), and 5 Hz (panel c) and thermosensory response to hot (panel d) and cold plate (panel e) controlling for sex and age. C57BL/6 mice were used as control BERK animals. Townes controls were nonsickling littermates of heterozygous and homozygous Townes mice that expressed normal human hemoglobin. P values reflect comparison of groups indicated by horizontal bars
Figure 2.
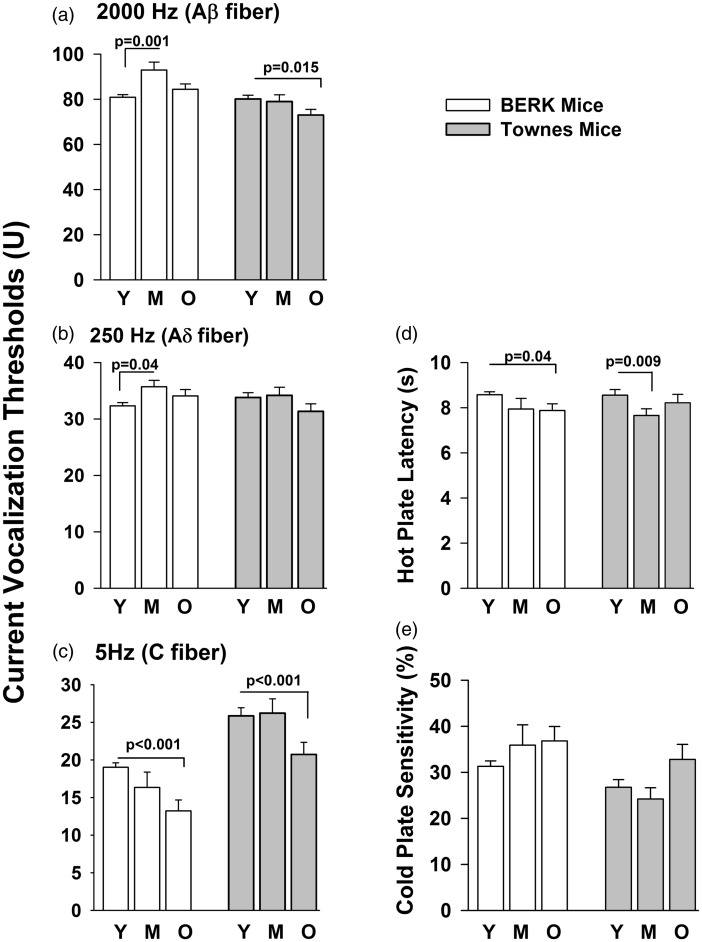
Effect of age of BERK (three left bars in each panel) and Townes mice (three right bars in each panel) on current vocalization threshold to 2000 Hz (panel a), 250 Hz (panel b), and 5 Hz (panel c) and thermosensory response to hot (panel d) and cold plate (panel e) controlling for sex and genotype. C57BL/6 mice were used as control BERK and Townes mice expressing normal human hemoglobin as control Townes. Animals were studied once at either young (Y, 6 < age < 10 weeks), middle (M, 12 < age < 16 weeks), or older (O, 20 < age < 27 weeks) ages. P values reflect comparisons of groups indicated by horizontal bars
Figure 3.
Effect of sex (M = male, F = female) of BERK (two left bars in each panel) and Townes mice (two right bars in each panel) on current vocalization threshold to 2000 Hz (panel a), 250 Hz (panel b), and 5 Hz (panel c) and thermosensory response to hot (panel d) and cold plate (panel e) controlling for genotype and age. C57BL/6 mice were used as control BERK and Townes mice expressing normal human hemoglobin as control Townes. P values reflect comparison of groups indicated by horizontal bars
Effect of genotype on current thresholds and thermosensory response of SCD mice
The pattern of genotype effect on current vocalization thresholds in response to 2000, 250, and 5 Hz differed across Townes and BERK strains (Figure 1, Table 2). Specifically, among BERK mice, controlling for age and sex, homozygous BERK had similar current vocalization thresholds in response to 2000 Hz (p = 0.51) and 5 Hz (p = 0.16) compared with C57BL/6 controls (Figure 1, Table 2). However, in response to 250 Hz stimulation, homozygous BERK mice had lower current vocalization thresholds compared with C57BL/6 controls (Figure 1, p = 0.029). In addition, heterozygous BERK had significantly higher 5 Hz current vocalization threshold compared with both, C57BL/6 controls (p < 0.001) and homozygous BERK (Wald test χ2 = 5.51, p = 0.019), Figure 1, Table 2.
In contrast, controlling for age and sex, homozygous (sickle, hα/hα::βS/βS) Townes had significantly lower current vocalization thresholds in response to 2000 Hz (p = 0.01), 250 Hz (p = 0.003), and 5 Hz (p < 0.001) compared to Townes control (hα/hα::βA/βA) mice (Figure 1, Table 2). In addition, heterozygous (hα/hα::βA/βS) Townes had similar current vocalization thresholds (2000 Hz [p = 0.654], 250 Hz [p = 0.414], and 5 Hz [p = 0.219]) compared to Townes controls but significantly higher thresholds compared to homozygous Townes (2000 Hz [Wald test χ2 = 5.08, p = 0.024], 250 Hz [Wald test χ2 = 5.92, p = 0.015], and 5 Hz [Wald test χ2 = 7.58, p = 0.006]). Homozygous genotype had a significant effect on 250 Hz current vocalization threshold in both Townes and BERK mice, and such an effect remained invariant (similar, Wald test χ2 = 0.427, p = 0.514) across the two strains. However, the effects of homozygous genotype on 2000 and 5 Hz current vocalization thresholds were only statistically significant (p < 0.05) for Townes, but not for BERKs.
Concerning thermosensory response, the pattern of genotype effect on hot plate latency and cold plate sensitivity differed across Townes and BERK strains (Figure 1, Table 3). Specifically, controlling for age and sex, homozygous and heterozygous BERK had significantly lower hot plate latency and cold plate sensitivity compared with C57BL/6 (Figure 1, all p < 0.001). In contrast, among Townes mice, controlling for age and sex, heterozygous Townes had significantly higher hot plate latency (p = 0.009) and cold plate sensitivity (p < 0.001) compared to Townes controls mice (Figure 1, Table 3). In addition, homozygous Townes had similar hot plate latency (p = 0.892) but significantly higher cold plate sensitivity (p < 0.001) compared to Townes controls mice (Figure 1, Table 3).
Effect of age on current thresholds and thermosensory response of SCD mice
The pattern of age effect on current vocalization thresholds in response to 2000, 250, and 5 Hz and on thermosensory response differed across Townes and BERK strains (Figure 2, Table 2, and Table 3). Specifically, among BERK mice, controlling for genotype and sex, middle age (12 < age < 16 weeks) animals had significantly higher current vocalization thresholds in response to 2000 Hz (p = 0.001) and 250 Hz (p = 0.043) compared with younger (age < 10 weeks) BERK mice (Figure 2, Table 2). With regards to 5 Hz sine-wave stimulation, middle age BERKs had similar (p = 0.190), but older (20 < age < 27 weeks) BERKs had significantly lower current vocalization thresholds (p < 0.001) compared to younger BERK mice (Figure 2, Table 2). In contrast, among Townes, controlling for genotype and sex, older Townes mice (20 < age < 27 weeks) had significantly lower current vocalization threshold in response to 2000 Hz (p = 0.015) and 5 Hz (p = 0.003), but not 250 Hz, compared to younger (age < 10 weeks) animals (Figure 2, Table 2). The significant negative effect of older age on current vocalization thresholds in response to 5 Hz was invariant (similar) between the two strains (Wald test χ2 = 0.090, p = 0.765).
Similarly, the pattern of age effect on thermosensory response to hot plate differed across Townes and BERK strains (Figure 2, Table 3). Specifically, among BERK mice, controlling for genotype and sex, older BERK mice (20 < age < 27) had significantly lower hot plate latency (p = 0.042) compared with younger animals (Figure 2, Table 3). In contrast, among Townes mice, controlling for genotype and sex, middle-aged, but not older, Townes mice had significantly lower hot plate latency (p = 0.009) than did younger animals (Figure 2, Table 3). There were no significant effects of age on cold plate sensitivity among BERK or Townes mice.
Effect of sex on current thresholds and thermosensory response of SCD mice
The effect of sex on current vocalization thresholds in response to 2000, 250, and 5 Hz was similar across Townes and BERK strains (Figure 3, Table 2). Specifically, among BERK mice, controlling for age and genotype, males had significantly higher current vocalization thresholds in response to 2000, 250, and 5 Hz compared to female mice (all p < 0.001, Figure 3, Table 2). Similarly, among Townes mice, controlling for age and genotype, males had significantly higher current vocalization thresholds in response to 2000, 250, and 5 Hz compared to female mice (all p < 0.0001, Figure 3, Table 2). The effect of sex on current vocalization thresholds in response to 2000 and 250 Hz remained invariant across strains (Wald test χ2 = 0.193, p = 0.716; Wald test χ2 = 0.000, p = 0.985, respectively), but the sex effect on current vocalization thresholds in response to 5 Hz was stronger among Townes compared to BERKs (Wald test χ2 = 7.872, p = 0.005).
In contrast, the pattern of sex effect on thermosensory response to hot plate differed across Townes and BERK strains (Figure 3, Table 3). Specifically, among BERKs, controlling for genotype and age, males had similar hot plate latency (p = 0.258) compared with female mice (Figure 3, Table 3). In contrast, among Townes mice, controlling for genotype and age, males had significantly lower (p < 0.001) hot plate latency compared to female mice (Figure 3). There were no significant effects of sex on cold plate sensitivity among BERK or Townes mice (Figure 3, Table 3).
Quantitative sensory testing in control strains (Townes controls and C57BL/6 animals)
We found that there were some differences in quantitative sensory test results comparing C57BL/6 and Townes control, the respective control groups for BERK and Townes mice enrolled in this study (Table 4). Briefly, Townes control animals had similar mean 2000 Hz (p = 0.54) and 250 Hz (p = 0.34) but higher 5 Hz current vocalization thresholds compared to C57BL/6 mice (Figure 1, Table 4, p < 0.0001). In addition, Townes control animals had significantly lower hot plate latency (Figure 1, Table 4, p < 0.0001) and cold plate sensitivity (Figure 1, Table 4, p < 0.0001) compared with C57BL/6.
Table 4.
Mean (SD) current vocalization thresholds, hot plate latency, and cold plate sensitivity for Townes and BERK strains control groups.a
| Current vocalization threshold (Units) |
Thermosensory testing |
||||
|---|---|---|---|---|---|
| 2000 Hz |
250 Hz |
5 Hz |
Hot Plate Latency (s) |
Cold Plate sensitivity (%) |
|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Townes controls | 85.4 (17.3) | 36.5 (9.1) | 27.3 (13.1) | 7.9 (2.1) | 21.2 (15.3) |
| BERK controls (C57BL/6) | 86.7 (15.6) | 35.5 (7.9) | 20.9 (8.6) | 9.4 (1.6) | 43.4 (16.0) |
| P value | 0.5375 | 0.3355 | <0.0001 | <0.0001 | <0.0001 |
SD represents standard deviation.
Hemoglobin analysis in Townes mice
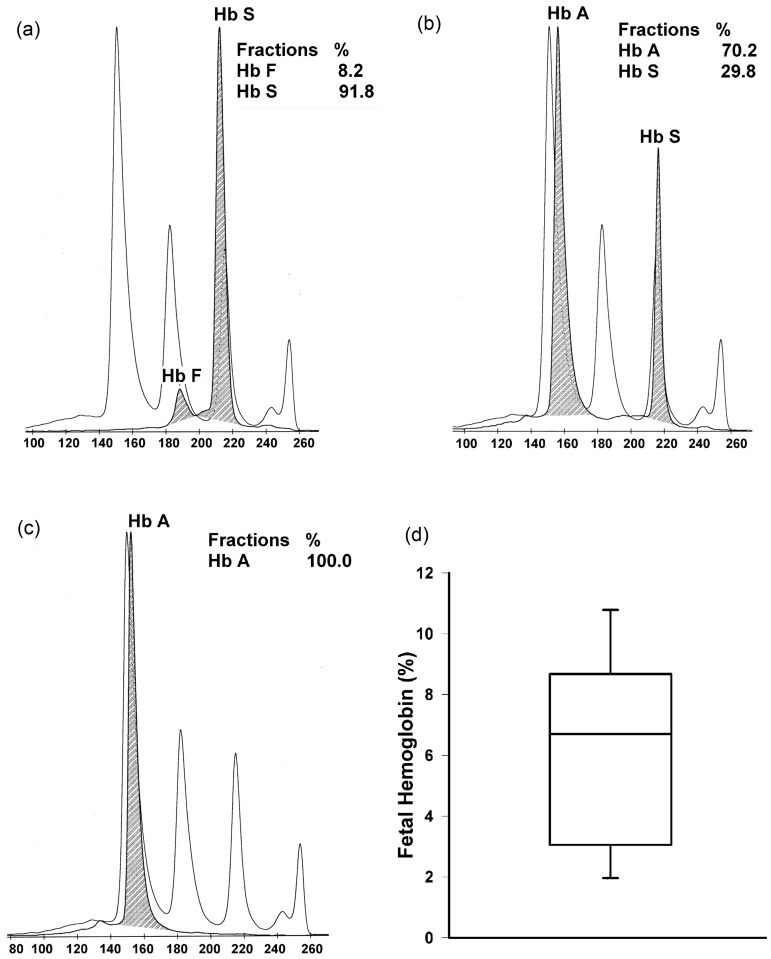
Once we determined the somatosensory phenotype in Townes mice, in order to determine whether adult mice express fetal hemoglobin, we performed hemoglobin analysis in red blood cell hemolysate of 20-week-old Townes mice (Figure 4). Homozygous but not heterozygous or control adult Townes mice had measurable levels of human fetal hemoglobin.
Figure 4.
Hemoglobin analysis of red blood cell hemolysate of 20-week-old Townes mice. Panels a, b, and c show representative hemoglobin analysis of homozygous (hα/hα::βS/βS, a), heterozygous (hα/hα::βA/βS, b), and Townes control (hα/hα::βA/βA, c) mice. The clear curves show hemoglobin concentrations in standards and shaded curves show human hemoglobin fractions in Townes mice hemolysates. The percentages of each hemoglobin type are shown in respective tables. Panel d shows medians, interquartile range (box plot), and 5th and 95th percentiles (whiskers) of fetal hemoglobin (percentage of total hemoglobin) in homozygous Townes mice (N = 10)
Discussion
A number of in vitro and in vivo studies support the use of sine-wave stimulation for evaluation of the function of sensory fiber types. In electrophysiologic studies, intracellular recordings of rat dorsal root ganglia show that sine-wave stimulation at 2000, 250, and 5 Hz, respectively, recruits Aβ, Aδ (myelinated fibers), and C (unmyelinated) axons.19 Researchers have shown that rodent models of inflammatory and neuropathic pain are associated with changes in current threshold of sensory fibers (Aβ, Aδ, and C fibers) that vary according to various disease processes. For example, in models of inflammatory pain (carrageenan injection), animals develop decreases in 250 Hz, and 5 Hz and increases in 2000 Hz current thresholds.40 In models of neuropathic pain, rodents develop decreases in 2000 and 250 Hz and increases or no changes in 5 Hz current thresholds.20,41 In postoperative pain models, animals develop significant decreases in current thresholds in all frequencies (2000, 250, and 5 Hz) after surgical incision, which suggests that postincisional pain is associated with sensitization of all sensory fiber types.21 In chemotherapy-induced neuropathic pain, current threshold for 250 Hz (Aδ fiber) and 2000 Hz (Aβ fiber), but not 5 Hz (C fiber), is reduced, thus suggesting that chemotherapy-induced neuropathic pain is associated with sensitization of myelinated, but not unmyelinated, sensory fibers.42 Taken together these studies suggest that the sine wave stimulation paradigm can be helpful for the evaluation of sensory fiber type in various rodent pain models.
Researchers have also used sine wave stimulation to determine the effect of various analgesic modalities on sensitization of specific fiber types. For example, intrathecal administration of morphine, an analgesic often used to treat surgery-associated pain, in rodent models of postoperative pain, attenuates the sensitization of Aδ (250 Hz) and C fibers, but not of Aβ fibers (2000 Hz).21 In animal models of chemotherapy-induced neuropathic pain, the analgesic effect of the anticonvulsant gabapentin, which is often used to treat neuropathic pain, was associated with decreases in current thresholds in Aβ (2000 Hz) and Aδ (250 Hz) but not in C (5 Hz) fibers.42 Therefore, the sine wave stimulation paradigm can be valuable for the study of sensory fiber sensitization, nociception, and of fiber-specific effects of various analgesic modalities in vivo.
In this study, we found that mice from two humanized strains of SCD, BERK and Townes, have decreased current thresholds in response to sine-wave electrical stimulation at 2000, 250, and 5 Hz which have been shown to preferentially stimulate Aβ, Aδ, and C sensory fibers, respectively. In addition, we found that some of these alterations in vocalization thresholds differ across strains of SCD mice and vary according to genotype, sex, and age intervals studied. These findings thus suggest that in humanized SCD mice, there are alterations in somatosensory function that involve sensitization of a broad spectrum of sensory nerve fibers (C, Aδ, and Aβ).
These results are in concert with findings from electrophysiologic studies in sickle cell mice.13 Using an ex vivo saphenous nerve-skin fiber preparation, researchers have shown that the mechanical allodynia seen in vivo was associated with increased sensitization of rapidly adapting Aβ and Aδ fibers in homozygous BERK mice.13 Our findings appear to reflect the behavior correlates of those electrophysiologic findings ex vivo given that in BERK and Townes mice, there was evidence of myelinated fibers sensitization as the 2000 Hz (Aβ-fiber) and 250 Hz (Aδ-fiber) current vocalization thresholds were decreased. Taken together, these studies thus support the use of sine-wave stimulation for in vivo investigations of sensory fiber function in SCD mice. In addition, our findings and those of others, suggesting the presence of mechanical allodynia in mice10,13 and humans43 with SCD as well as the evidence of myelinated nerve fiber sensitization support the hypothesis that altered nocifensive behavior in SCD mice might be associated with myelinated sensory fiber sensitization and possible neuropathic processes.
Using different nociception evaluation paradigms than those used here, the nocifensive behavior in several SCD mouse strains has been previously reported.10–14,44 Here, we characterize a formerly undescribed nocifensive phenotype and somatosensory function in BERK and another humanized SCD mouse, the Townes strain. Homozygous Townes had lower current vocalization thresholds in response to 2000, 250, and 5 Hz and altered thermal sensitivity in patterns that varied according to genotype, age, and sex. Further, Townes mice had alterations in nocifensive phenotype in patterns that were somewhat distinct from those observed in BERKs. An important characteristic of Townes mice is that they express human fetal hemoglobin (Figure 4). It is possible that the nociception phenotype differences between Townes and BERK mice could be partially explained by the expression of fetal hemoglobin in Townes mice. In humans, therapies that alter fetal hemoglobin levels are effective and disease-modifying strategies to treat SCD.45,46 Therefore, the availability of a SCD strain that expresses fetal hemoglobin offers the unique advantage of allowing for the preclinical evaluation of therapies that alter fetal hemoglobin on SCD-associated pain phenotype.
Our findings using sine-wave electrical stimulation to interrogate the function of Aβ, Aδ, and C sensory nerve fibers add to the understanding of somatosensory function of SCD mice. Using sine-wave electrical stimulation, one bypasses peripheral nociceptors and directly stimulates large and small afferents fibers.19,21–23,32,40 Here we found that SCD mice had altered tolerance thresholds in response to 2000, 250, and 5 Hz stimulations. Therefore, our findings coupled with those of others suggest that in various strains of SCD mice the previously reported alterations in heat, cold, and mechanical sensitivity are associated with alterations in somatosensory function that involve several types of large sensory fibers. Based on the similarities between patterns of altered response to thermal and electrical stimuli and age and sex-dependent variability of nocifensive behavior, it is possible that sensitization of large sensory nerve fibers pathways might play a role in nocifensive response to thermal stimuli in SCD mice and in small fiber alterations as previously described by others.10–14,44
That BERK mice have sex- and age-dependent alterations on nocifensive response to noxious heat and cold has been previously described.10–14 While we used different thermal quantitative sensory testing assays, our results are in concert with those of others indicating that homozygous BERK mice have increased sensitivity to noxious heat stimuli that varies according to age and sex.14 Our findings that BERK mice had lower sensitivity to cold compared to controls are in contrast with other sickle cell mouse studies showing that BERK mice have increased cold sensitivity.10,14 While there is some debate as to the best method to measure cold plate sensitivity, we postulate that these discrepant results can be partially explained by the differences in methodologies used to examine cold sensitivity.34,47,48 Here we measured the duration of paw withdrawal (paw lift duration) from the cold plate over 5 min of observation, whereas others measured cold plate withdrawal latency and number of paw lifts10,14 over a 2 min observation. It is noteworthy that results of quantitative sensory testing in humans with SCD have also yielded some discrepant results as it refers to cold sensitivity. One study in children showed that SCD patients are less sensitive to heat and cold detection compared to controls,15 whereas another showed that SCD patients have increased sensitivity to cold and heat.9 This variability in quantitative sensory testing results in human and animal studies suggests that nocifensive and pain phenotypes can vary depending on quantitative sensory testing paradigm used and can be significantly affected by age, sex, and genotype in SCD.
That the SCD pain phenotype varies with age has been shown both in humans and in animals.2,14 An earlier cross-sectional study showed that pain rates among SCD patients increase until the third decade of life and decline after that.2 The authors conclude that this reflects higher early mortality among patients with higher rates of pain.2 Others have shown that in SCD mice, the nocifensive phenotype measured by some methods such as grip force and cold sensitivity also varies according to age.14 In this cross-sectional mouse study, taking into account sex, and genotype, we observed that the effect of age on current vocalization threshold varies according to the frequency studied (sensory fiber). For example in Townes mice, the threshold to 2000 and 5 Hz was lower in older compared to younger SCD mice but was unchanged in response to 250 Hz across all ages. In BERK mice, the age-related pattern of changes in thresholds also varied according to the frequency studied. These findings suggest that the effect of SCD on sensory nerve fibers function and in turn on nocifensive behavior could vary with aging.
We recognize that our study has limitations. For the study of the BERK strain, we used C57BL/6 as controls because of a lack of availability of animals that express a transgene with normal human hemoglobin.30 However, this limitation only pertains to the analysis of the BERK animals as the control animals for the Townes mice are littermates of homozygous and heterozygous Townes. Even if we were to disregard the comparisons between C57BL/6 and BERK mice, the comparisons between homozygous and heterozygous (littermates) BERK mice are valid and informative. Further, our findings in BERK animals are in concert with those of others using mice that express human hemoglobin as BERK controls. Therefore, despite some limitations, the results of this investigation add to our understanding of sensory fiber function in SCD. Another consideration is that C57BL/6 animals had similar responses to 2000 and 250 Hz stimulation, lower current vocalization threshold in response to 5 Hz, longer hot plate latencies, and increased sensitivity to cold plate compared with Townes controls. These findings thus suggest that the expression of normal human hemoglobin might also alter the nocifensive response of normal mice and should be taken into account when interpreting results from humanized SCD mice.
In summary, the analysis of somatosensory function using sine-wave electrical stimulation in humanized sickle cell mice suggests that in SCD there is sensitization of a broad spectrum of sensory, both myelinated and unmyelinated, fibers. This pattern of sensory fiber sensitization appears to be distinct from those observed in pain models of neuropathic and inflammatory pain. These findings thus suggest that SCD-associated changes in nociception are distinct from other models of altered nociception especially as it relates to sensory fiber sensitization. Further, these findings also raise the possibility that sensitization of a broad spectrum of sensory fibers might contribute to the altered and variable nociception phenotype in SCD.
Acknowledgements
The authors are grateful to LaShon Middleton and Sayuri Kamimura for expert technical support during experiments, to Aral C. Greene and Nina Afsar for editorial help, and to Edward C.C. Wong, MD and Nannette Poidven for measurements of fetal hemoglobin concentration. This work was supported by the Intramural Program from the National Institutes of Health Clinical Center, NIH and the Sheikh Zayed Institute for Pediatric Surgical Innovation.
Author contributions
Conceived and designed the experiments: NK, VG, and ZMNQ. Performed the experiments: NK, LW, NS, AK, LEFA, VG, and ZMNQ. Analyzed the data: YC, JW, and ZMNQ. Contributed reagents/materials/analysis tools: VG, JCF, and ZMNQ. Wrote the paper: NK and ZMNQ. Reviewed the manuscript: NK, LW, NS, AK, LEFA, YC, JW, VG, JCF, and ZMNQ.
References
- 1.Glass P, Brennan T, Wang J, Luchtman-Jones L, Hsu L, Bass CM, Rana S, Martin B, Reed C, Cheng YI, Gordeuk V. Neurodevelopmental deficits among infants and toddlers with sickle cell disease. J Dev Behav Pediatr 2013; 34: 399–405. [DOI] [PubMed] [Google Scholar]
- 2.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR. Pain in sickle cell disease. Rates and risk factors. N Engl J Med 1991; 325: 11–16. [DOI] [PubMed] [Google Scholar]
- 3.Smith WR, Penberthy LT, Bovbjerg VE, McClish DK, Roberts JD, Dahman B, Aisiku IP, Levenson JL, Roseff SD. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med 2008; 148: 94–101. [DOI] [PubMed] [Google Scholar]
- 4.Darbari DS, Onyekwere O, Nouraie M, Minniti CP, Luchtman-Jones L, Rana S, Sable C, Ensing G, Dham N, Campbell A, Arteta M, Gladwin MT, Castro O, Taylor JGt, Kato GJ, Gordeuk V. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr 2012; 160: 286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Firth PG, Head CA. Sickle cell disease and anesthesia. Anesthesiology 2004; 101: 766–85. [DOI] [PubMed] [Google Scholar]
- 6.McClish DK, Smith WR, Dahman BA, Levenson JL, Roberts JD, Penberthy LT, Aisiku IP, Roseff SD, Bovbjerg VE. Pain site frequency and location in sickle cell disease: The PiSCES project. Pain 2009; 145: 246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: A critical reappraisal. Blood 2012; 120: 3647–56. [DOI] [PubMed] [Google Scholar]
- 8.Brandow AM, Farley RA, Panepinto JA. Neuropathic pain in patients with sickle cell disease. Pediatr Blood Cancer 2013;61:512–7. [DOI] [PMC free article] [PubMed]
- 9.Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol 2013; 88: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: Modulation by cannabinoids. Blood 2010; 116: 456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood 2013; 122: 1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood 2011; 118: 3376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrison SR, Kramer AA, Gerges NZ, Hillery CA, Stucky CL. Sickle cell mice exhibit mechanical allodynia and enhanced responsiveness in light touch cutaneous mechanoreceptors. Mol Pain 2012; 8: 62–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: Effects of strain, age, and acuteness. Br J Haematol 2012; 156: 535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Leary JD, Crawford MW, Odame I, Shorten GD, McGrath PA. Thermal pain and sensory processing in children with sickle cell disease. Clin J Pain 2013;30:244–50. [DOI] [PubMed]
- 16.Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain 1996; 68: 141–50. [DOI] [PubMed] [Google Scholar]
- 17.Yeomans DC, Cooper BY, Vierck CJ., Jr Effects of systemic morphine on responses of primates to first or second pain sensations. Pain 1996; 66: 253–63. [DOI] [PubMed] [Google Scholar]
- 18.Raj PP, Chado HN, Angst M, Heavner J, Dotson R, Brandstater ME, Johnson B, Parris W, Finch P, Shahani B, Dhand U, Mekhail N, Daoud E, Hendler N, Somerville J, Wallace M, Panchal S, Glusman S, Jay GW, Palliyath S, Longton W, Irving G. Painless electrodiagnostic current perception threshold and pain tolerance threshold values in CRPS subjects and healthy controls: a multicenter study. Pain Pract 2001; 1: 53–60. [DOI] [PubMed] [Google Scholar]
- 19.Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain 2005; 1: 13–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto M, Inoue M, Hald A, Yamaguchi A, Ueda H. Characterization of three different sensory fibers by use of neonatal capsaicin treatment, spinal antagonism and a novel electrical stimulation-induced paw flexion test. Mol Pain 2006; 2: 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagakura Y, Jones TL, Malkmus SA, Sorkin L, Yaksh TL. The sensitization of a broad spectrum of sensory nerve fibers in a rat model of acute postoperative pain and its response to intrathecal pharmacotherapy. Pain 2008; 139: 569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watabiki T, Nagakura Y, Wegner K, Kakimoto S, Tozier NA, Malkmus SA, Yaksh TL. Assessment of canine sensory function by using sine-wave electrical stimuli paradigm. Physiol Behav 2010; 101: 327–30. [DOI] [PubMed] [Google Scholar]
- 23.Katims J. Electrodiagnostic functional sensory evaluation of the patient with pain: A review of the neuroselective current perception threshold and pain tolerance threshold. Pain Digest 1998; 8: 219–30. [Google Scholar]
- 24.Kiso T, Nagakura Y, Toya T, Matsumoto N, Tamura S, Ito H, Okada M, Yamaguchi T. Neurometer measurement of current stimulus threshold in rats. J Pharmacol Exp Ther 2001; 297: 352–6. [PubMed] [Google Scholar]
- 25.Oda M, Kitagawa N, Yang BX, Totoki T, Morimoto M. Quantitative and fiber-selective evaluation of dose-dependent nerve blockade by intrathecal lidocaine in rats. J Pharmacol Exp Ther 2005; 312: 1132–7. [DOI] [PubMed] [Google Scholar]
- 26.Spornick N, Guptill V, Koziol D, Wesley R, Finkel J, Quezado ZM. Mouse current vocalization threshold measured with a neurospecific nociception assay: The effect of sex, morphine, and isoflurane. J Neurosci Methods 2011; 201: 390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012; 490: 187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu LC, Sun CW, Ryan TM, Pawlik KM, Ren J, Townes TM. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood 2006; 108: 1183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 2007; 318: 1920–3. [DOI] [PubMed] [Google Scholar]
- 30.Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science 1997; 278: 876–8. [DOI] [PubMed] [Google Scholar]
- 31.Finkel JC, Besch VG, Hergen A, Kakareka J, Pohida T, Melzer JM, Koziol D, Wesley R, Quezado ZM. Effects of aging on current vocalization threshold in mice measured by a novel nociception assay. Anesthesiology 2006; 105: 360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finkel J, Guptill V, Khaibullina A, Spornick N, Vasconcelos O, Liewehr DJ, Steinberg SM, Quezado ZM. The three isoforms of nitric oxide synthase distinctively affect mouse nocifensive behavior. Nitric Oxide 2012; 26: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev 2001; 53: 597–652. [PubMed] [Google Scholar]
- 34.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994; 59: 369–76. [DOI] [PubMed] [Google Scholar]
- 35.Hayduk LA. Structural equations modeling with lisrel, Baltimore, MD: Johns Hopkins University Press, 1987. [Google Scholar]
- 36.Jöreskog KG, Sörbom D. LISREL 8 user’s reference guide, Uppsala, Sweden: Scientific Software International, 1996. [Google Scholar]
- 37.Wang J, Wang X. Structural equation modeling: Applications using Mplus, West Sussex, United Kingdom: Higher Education Press, 2012. [Google Scholar]
- 38.Muthen LK, Muthen BO. Mplus user’s guide, 7th ed Los Angeles, CA: Muthén & Muthén, 1998–2012. [Google Scholar]
- 39.Yuan KH, Bentler PM. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. In: Sociological Methodology, Washington, DC: The American Sociological Association, 2000, pp. 165–200. [Google Scholar]
- 40.Nagakura Y, Malkmus S, Yaksh TL. Determination of current threshold for paw withdrawal with sine-wave electrical stimulation in rats: Effect of drugs and alteration in acute inflammation. Pain 2008; 134: 293–301. [DOI] [PubMed] [Google Scholar]
- 41.Halder SK, Yano R, Chun J, Ueda H. Involvement of LPA1 receptor signaling in cerebral ischemia-induced neuropathic pain. Neuroscience 2013; 235: 10–15. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto M, Inoue M, Hald A, Xie W, Ueda H. Inhibition of paclitaxel-induced A-fiber hypersensitization by gabapentin. J Pharmacol Exp Ther 2006; 318: 735–40. [DOI] [PubMed] [Google Scholar]
- 43.Jones S, Duncan ER, Thomas N, Walters J, Dick MC, Height SE, Stephens AD, Thein SL, Rees DC. Windy weather and low humidity are associated with an increased number of hospital admissions for acute pain and sickle cell disease in an urban environment with a maritime temperate climate. Br J Haematol 2005; 131: 530–3. [DOI] [PubMed] [Google Scholar]
- 44.Lunzer MM, Yekkirala A, Hebbel RP, Portoghese PS. Naloxone acts as a potent analgesic in transgenic mouse models of sickle cell anemia. Proc Natl Acad Sci USA 2007; 104: 6061–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, Witkop C, Bass EB, Segal JB. Systematic review: Hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med 2008; 148: 939–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGann PT, Ware RE. Hydroxyurea for sickle cell anemia: What have we learned and what questions still remain? Curr Opin Hematol 2011; 18: 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jasmin L, Kohan L, Franssen M, Janni G, Goff JR. The cold plate as a test of nociceptive behaviors: Description and application to the study of chronic neuropathic and inflammatory pain models. Pain 1998; 75: 367–82. [DOI] [PubMed] [Google Scholar]
- 48.Brenner DS, Golden JP, Gereau RWt. A novel behavioral assay for measuring cold sensation in mice. PLoS One 2012; 7: e39765–e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]