Abstract
Both prostacyclin analogs and phosphodiesterase 5 (PDE5) inhibitors are effective treatments for pulmonary arterial hypertension (PAH). In addition to direct effects on vascular smooth muscle, prostacyclin analogs increase cAMP levels and ATP release from healthy human erythrocytes. We hypothesized that UT-15C, an orally available form of the prostacyclin analog, treprostinil, would stimulate ATP release from erythrocytes of humans with PAH and that this release would be augmented by PDE5 inhibitors. Erythrocytes were isolated and the effect of UT-15C on cAMP levels and ATP release were measured in the presence and absence of the PDE5 inhibitors, zaprinast or tadalafil. In addition, the ability of a soluble guanylyl cyclase inhibitor to prevent the effects of tadalafil was determined. Erythrocytes of healthy humans and humans with PAH respond to UT-15C with increases in cAMP levels and ATP release. In both groups, UT-15C-induced ATP release was potentiated by zaprinast and tadalafil. The effect of tadalafil was prevented by pre-treatment with an inhibitor of soluble guanylyl cyclase in healthy human erythrocytes. Importantly, UT-15C-induced ATP release was greater in PAH erythrocytes than in healthy human erythrocytes in both the presence and the absence of PDE5 inhibitors. The finding that prostacyclin analogs and PDE5 inhibitors work synergistically to enhance release of the potent vasodilator ATP from PAH erythrocytes provides a new rationale for the co-administration of these drugs in this disease. Moreover, these results suggest that the erythrocyte is a novel target for future drug development for the treatment of PAH.
Keywords: Tadalafil, zaprinast, treprostinil, red blood cell, cAMP, cGMP
Introduction
In spite of the limited understanding of the fundamental pathophysiology underlying the development and progression of pulmonary arterial hypertension (PAH), it is clear that treatment of PAH with phosphodiesterase 5 (PDE5) inhibitors and prostacyclin or its analogs, alone or in combination, improves survival.1,2 Several reports have demonstrated structural changes in the pulmonary vasculature as well as abnormal pulmonary vasoreactivity in humans with PAH.3,4 However, in addition to these abnormalities, in contrast to healthy human erythrocytes, PAH erythrocytes do not release the potent vasodilator, ATP, when mechanically deformed,5 demonstrating another defect in PAH that may contribute to increased pulmonary vascular resistance.
Studies in PAH patients have shown that the use of PDE5 inhibitors and prostacyclin analogs in combination results in improved hemodynamics, lengthened time to clinical worsening, increased survival, and increased exercise capacity.1,2,6,7 Although both PDE5 inhibitors and prostacyclin analogs can clearly dilate blood vessels directly, an alternative mechanism of vasodilation when these drugs are co-administered has been described.8,9 Similar to vascular smooth muscle cells, human erythrocytes express prostacyclin receptors (IPRs).10 The binding of active analogs of prostacyclin to erythrocyte IPRs results in stimulation of a signaling pathway that culminates in the release of ATP.10,11 ATP release requires increases in intracellular cAMP .10–12 The level of this cyclic nucleotide is modulated by phosphodiesterase 3 (PDE3).12 The critical role of PDE3 is demonstrated by the observation that, in healthy human erythrocytes, pharmacological inhibitors of PDE3 increase both intracellular cAMP and ATP release in response to IPR activation.12
In addition to the use of pharmacological agents, PDE3 activity can also be inhibited by local increases in cGMP which, in human erythrocytes, is regulated by the activity of PDE5.13 Indeed, two selective yet chemically dissimilar PDE5 inhibitors, zaprinast and tadalafil,14,15 were shown to potentiate increases in cAMP and ATP release from healthy human erythrocytes stimulated by the prostacyclin analog, UT-15C.8 Here we investigated the hypotheses that (1) erythrocytes of humans with PAH also release ATP in response to IPR activation and (2) that PDE5 inhibitors enhance this response.
Methods
Isolation of human erythrocytes
Blood obtained from healthy volunteers and patients with PAH by venipuncture was collected into heparin5,8,16 and centrifuged at 500xg at 4℃ for 10 min. The plasma, buffy coat, and uppermost layer of erythrocytes were removed by aspiration. The remaining erythrocytes were resuspended and washed three times in a physiological buffer containing 21.0 tris(hydroxymethyl) aminomethane, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, and 5.5 glucose with 0.5% bovine serum albumin fraction V, final pH 7.4 with care to remove a minimal number of additional erythrocytes. Erythrocytes were prepared on the day of use. Informed consent was obtained from all subjects. The protocol for blood removal was approved by the Institutional Review Board of Saint Louis University. All record keeping was in strict compliance with the Health Insurance Portability and Accountability Act regulations.
Measurement of cAMP
Washed erythrocytes were diluted to a 50% hematocrit in physiological buffer. Samples were centrifuged at 14,000xg for 10 min at 4℃. The supernatant was removed and stored overnight at −20℃ to precipitate the remaining proteins. The samples were centrifuged at 3700xg for 10 min at 4℃ and the supernatant was removed. Samples were then dried under vacuum centrifugation and reconstituted in assay buffer. The concentration of cAMP was determined using an enzyme immunoassay (GE Healthcare, Piscataway, NJ). Levels of cAMP are reported as picomoles per 5 x 108 erythrocytes. Cells were counted using a hemocytometer.
Measurement of ATP
Washed erythrocytes were diluted to a 20% hematocrit in physiological buffer. ATP was measured by the luciferin–luciferase technique.17 Briefly, a 200 µL sample of the erythrocyte suspension (0.04% hematocrit) was injected into a cuvette containing 100 µL of 10 mg/mL crude firefly lantern extract (Sigma, St. Louis, MO) and 100 µL of 0.5 mg/mL d-luciferin (Research Products International, Mt. Prospect, IL). The light emitted was detected using a luminometer (Turner Designs, Sunnyvale, CA). An ATP standard curve was generated on the day of each study. ATP levels measured at baseline and the peak value after administration of UT-15C are reported as nanomoles per 4 x 108 erythrocytes. Cells were counted using a hemocytometer.
Measurement of free hemoglobin
To exclude the presence of hemolysis, samples were centrifuged at 500xg for 10 min at 4℃. The presence of free hemoglobin in the supernatant was determined by light absorption at 405 nm.18 If any increase in free hemoglobin was detected, the study was excluded.
Determination of the effect of UT-15C on cAMP levels in the presence and absence of tadalafil
Erythrocytes were diluted to a 50% hematocrit in physiological buffer and incubated for 30 min with tadalafil (10 µmol/L) or its vehicle (dimethylformamide, DMF; Sigma). Erythrocytes were then incubated with UT-15C (1 µmol/L) for 15 min and cAMP levels were determined.
Determination of the effect of UT-15C on ATP release in the presence and absence of PDE5 inhibitors
Erythrocytes were diluted to a 20% hematocrit in physiological buffer. ATP was measured prior to and at 5, 10, and 15 min after UT-15C (1 µmol/L, United Therapeutics, Research Triangle Park, NJ). The peak response is reported. In separate studies, erythrocytes were pre-treated for 30 min with one of two selective yet chemically dissimilar inhibitors of PDE5,14,15 zaprinast (ZAP, 10 µmol/L; Sigma) or tadalafil (TAD, 10 µmol/L; Eli Lily, Indianapolis, IN) or their vehicle (DMF) followed by UT-15C.
Determination of ATP release from erythrocytes pre-treated with tadalafil in the presence and absence of the soluble guanylyl cyclase inhibitor ODQ
Erythrocytes were diluted to a 20% hematocrit in physiological buffer and incubated with the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one19 (ODQ, 10 µmol/L, Sigma) for 10 min prior to the addition of tadalafil (10 µmol/L) for 30 min. ATP was measured prior to and at 5, 10, and 15 min after UT-15C (1 µmol/L). The peak response is reported.
Data analysis
Statistical significance among groups was determined using an analysis of variance followed by a Fisher’s least significant difference (LSD) test. Results are reported as mean ± the standard error (SE).
Results
Characteristics of subjects
Patients with PAH were identified as WHO group I by physicians in the Pulmonary Clinic of Saint Louis University. Healthy volunteers were faculty, staff, and students at Saint Louis University Medical Center. A history form that included a detailed listing of all medical conditions, medications, and age was completed for each subject. The mean age for the 23 healthy volunteers (10 males, 13 females) was 43 ± 3 (range 24–67 years) and was 51 ± 4 for the 14 patients with PAH (7 males, 7 females; range 18–65 years). Other conditions present in the PAH patients included systemic lupus erythematosus (3), positive HIV viral titer (2), and cirrhosis (2). Medications received by PAH patients included angiotensin converting enzyme inhibitors (3), calcium channel blockers (6), coumadin (2), prostacyclin analogs (2), low-dose aspirin (3), diuretics (6), beta-blockers (6), endothelin receptor antagonists (4), and PDE5 inhibitors (6). It is not possible to withdraw such medications from humans with PAH for the purpose of this study. However, there are no published reports that, after the washing of erythrocytes, any of the medications listed above alter prostacyclin analog-induced ATP release from these cells. Importantly, in the absence of drugs, baseline values for ATP from healthy humans and humans with PAH did not differ (6.1 ± 0.6 and 6.7 ± 0.9 nmol per 4 x 108 erythrocytes, respectively).
Effect of UT-15C on cAMP levels and ATP release in erythrocytes of healthy humans or humans with PAH
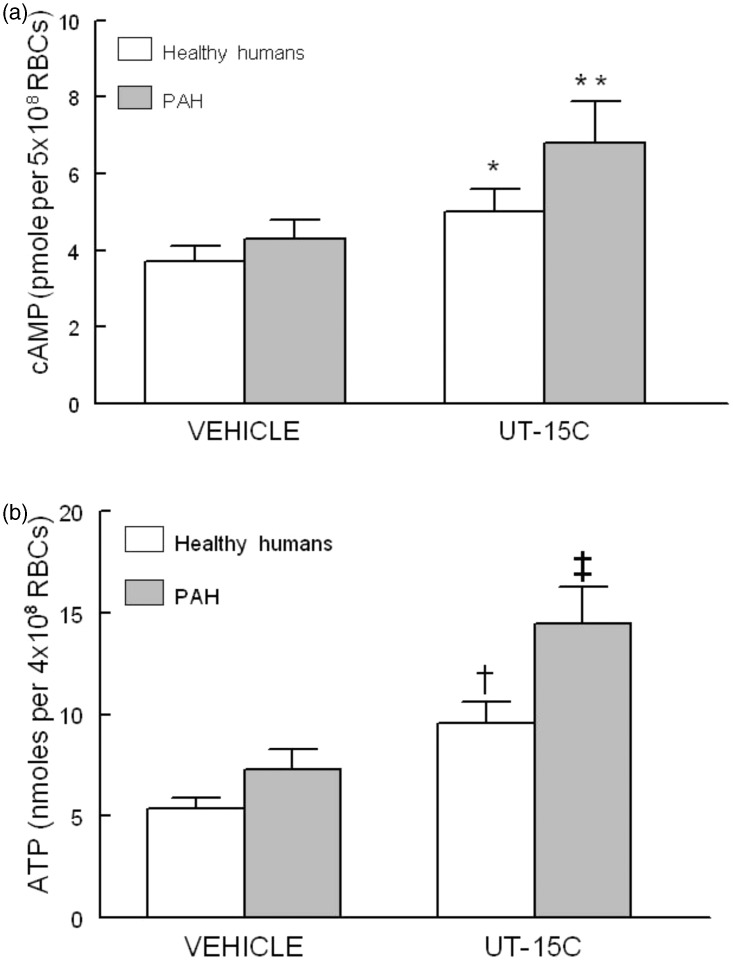
Incubation of erythrocytes from both healthy humans (n = 7) and humans with PAH (n = 7) with the prostacyclin analog UT-15C (1 µmol/L) resulted in significant increases in cAMP accumulation (Figure 1a) (P < 0.05). The same concentration of UT-15C stimulated significant increases in ATP release from healthy human (n = 15) and PAH (n = 13) erythrocytes (Figure 1b) (P < 0.01). Importantly, both increases in cAMP and ATP release were greater in erythrocytes from humans with PAH (Figure 1a: P < 0.05, and Figure 1b: P < 0.01).
Figure 1.
Panel a: Effect of UT-15C (1 µmol/L) on cAMP levels of healthy human (open bars, n = 7) and PAH erythrocytes (RBCs) (filled bars, n = 7). Cells were incubated with UT-15C for 15 min before the reaction was stopped. Values are means ± SE. *Different from respective control (P < 0.05); **different from all other values (P < 0.05). Panel b: Effect of UT-15C (1 µmol/L) on ATP release from healthy human (open bars, n = 15) and PAH erythrocytes (filled bars, n = 13). Values are means ± SE. †Different from respective control (P < 0.01); ‡different from all other values (P < 0.01)
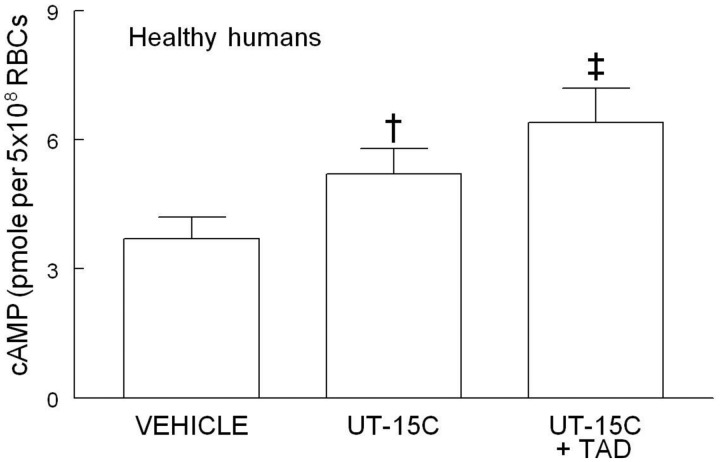
Effect of the PDE5 inhibitor tadalafil on basal and UT-15C-induced cAMP accumulation in healthy human erythrocytes
It has been shown that cGMP inhibits the hydrolysis of cAMP by PDE3.12,13 Since human erythrocytes possess PDE5, a PDE that hydrolyzes cGMP, we hypothesized that inhibition of PDE5 would increase cAMP levels in these cells in response to activation of the IPR by the prostacyclin analog, UT-15C. As depicted in Figure 2, in the presence of the PDE5 inhibitor tadalafil (10 µmol/L, n = 7), UT-15C-stimulated increases in cAMP were potentiated (P < 0.01). In the absence of UT-15C, tadalafil had no effect on basal cAMP levels. (Data not shown.)
Figure 2.
Effect of UT-15C (1 µmol/L) on cAMP accumulation in the presence and absence of tadalafil (TAD, 10 µmol/L) in healthy human erythrocytes (RBCs) (n = 7). Cells were treated with tadalafil 30 min before the addition of UT-15C. The reaction was stopped 15 min after the addition of UT-15C and cAMP was determined. Values are means ± SE. †Different from control (P < 0.01); ‡different from all other values (P < 0.01)
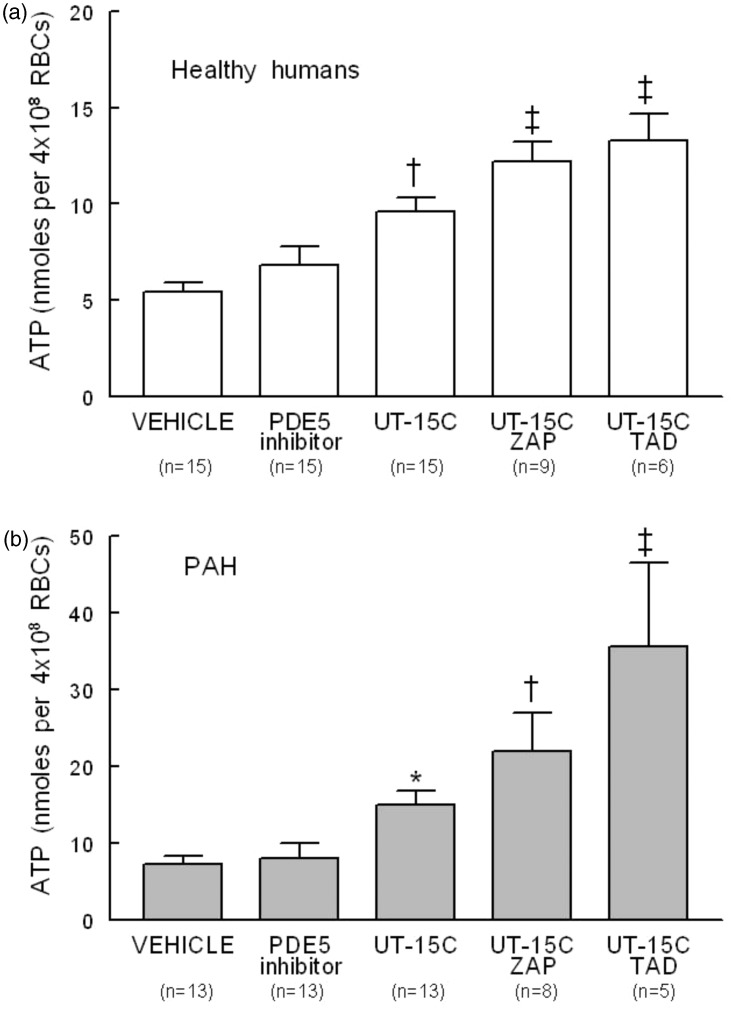
Effect of PDE5 inhibitors on basal and UT-15C-stimulated ATP release from erythrocytes of healthy humans and humans with PAH
The effect of UT-15C on ATP release from erythrocytes in the presence and absence of one of two chemically dissimilar PDE5 inhibitors, zaprinast (10 µmol/L) or tadalafil (10 µmol/L), was determined. Neither zaprinast nor tadalafil had an effect on basal ATP release in healthy human or PAH erythrocytes and thus were combined in Figure 3a and b (PDE5 inhibitor). However, both zaprinast and tadalafil potentiated UT-15C-induced ATP release from erythrocytes of both healthy humans (Figure 3a) and humans with PAH (Figure 3b). As was reported for cAMP, UT-15C-stimulated increases in ATP release from PAH erythrocytes were greater than those seen in healthy human erythrocytes in the presence and absence of zaprinast or tadalafil (P < 0.01). It is of interest that in PAH cells, tadalafil was more effective than zaprinast in augmenting UT-15C-induced increases in ATP release (Figure 3b, P < 0.01).
Figure 3.
Effect of UT-15C (1 µmol/L) on ATP release in the presence and absence of the PDE5 inhibitors zaprinast (ZAP, 10 µmol/L) or tadalafil (TAD, 10 µmol/L) on healthy human (Panel a) (open bars) or PAH (Panel b) (filled bars) erythrocytes (RBCs). Cells were treated for 30 min with zaprinast, tadalafil, or their vehicle (DMF) prior to the addition of UT-15C. Values are reported as means ± SE. Samples containing either TAD or ZAP alone were not different and thus were combined (PDE5 inhibitor). Panel a; †different from vehicle (DMF) alone (P < 0.01); ‡different from vehicle and UT-15C alone (P < 0.01). Panel b; *different from vehicle (DMF) alone (P < 0.05); †different from vehicle and UT-15C alone (P < 0.01); ‡different from all other values (P < 0.01)
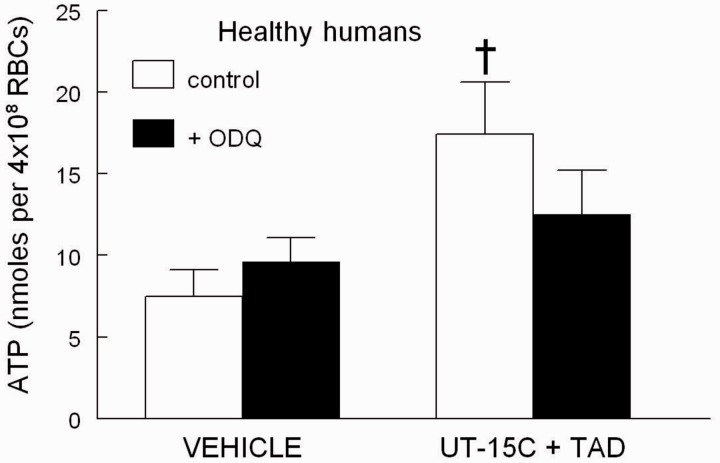
Effect of the soluble guanylyl cyclase inhibitor ODQ on UT-15C-induced ATP release from healthy human erythrocytes pre-treated with tadalafil
Healthy human erythrocytes were incubated with UT-15C and tadalafil in the presence and absence of the soluble guanylyl cyclase inhibitor ODQ (10 µmol/L).19 ODQ inhibited the ability of tadalafil to augment UT-15C-induced ATP release (n = 5), providing support for the hypothesis that tadalafil increases ATP release via a cGMP-dependent mechanism (Figure 4).
Figure 4.
Effect of UT-15C (1 µmol/L) and tadalafil (TAD, 10 µmol/L) on ATP release (n = 5) from healthy human erythrocytes (RBCs) in the presence (filled bars) and absence (open bars) of ODQ (10 µmol/L). Cells were incubated with ODQ or its vehicle (DMF) for 10 min prior to the addition of TAD followed 30 min later by UT-15C. †Different from all other values (P < 0.01). Values are reported as means ± SE
Discussion
PAH is characterized by the sustained elevation of pulmonary vascular resistance which leads to right ventricular heart failure and death.1,2 Despite considerable research efforts, the fundamental mechanisms responsible for the development of PAH have yet to be established. Although numerous studies have focused on understanding the abnormalities of the pulmonary vasculature that are associated with PAH,3,4 erythrocyte abnormalities could also contribute to the development and progression of the disease.5,20
Erythrocytes contribute actively to the control of local vascular caliber in the lung via their release of the powerful vasodilator ATP5,9,17 in response to mechanical deformation.16,21,22 When released from circulating erythrocytes, ATP binds to purinergic receptors on the pulmonary endothelium leading to the local synthesis of vasodilators including nitric oxide.9,23 These findings demonstrate that the erythrocyte participates in the maintenance of low vascular resistance in the pulmonary circulation. Although healthy human erythrocytes release ATP when exposed to mechanical deformation, erythrocytes of humans with PAH do not.5 Thus, in PAH, in addition to altered blood vessel reactivity,3 an important erythrocyte-mediated stimulus for pulmonary vasodilation is defective.5,20
In the absence of a clear understanding of the pathophysiology of PAH, treatment has been primarily directed toward the pharmacological reduction of pulmonary vascular resistance. The administration of either prostacyclin or its active analogs has been highly successful in the treatment of PAH.24–26 It has been assumed that the sole mechanism by which prostacyclin and its analogs produce vasodilation is via direct relaxation of vascular smooth muscle.23,27 However, a second mechanism by which prostacyclin could stimulate vasodilation was proposed following the report that human erythrocytes possess IP receptors10 that, when activated, stimulate increases in cAMP and ATP release from these cells.8,10,28
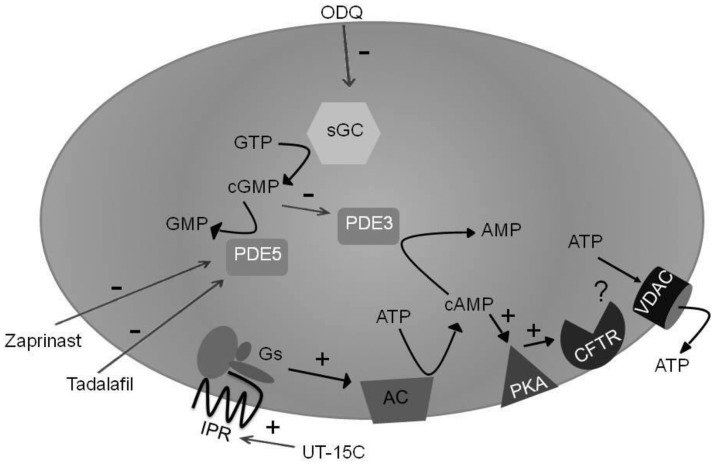
As depicted in Figure 5, IPR-mediated ATP release from human erythrocytes initiates a signaling pathway that requires increases in cAMP,8,10,28 the concentration of which is determined by the balance between its synthesis by adenylyl cyclases29 and its hydrolysis by PDE3.12,13,30 PDE3-mediated hydrolysis of cAMP is inhibited by cGMP.30 Inhibition of PDE5 in erythrocytes results in increases in cGMP13 which could then inhibit PDE3 activity in these cells. The interrelationship between the effects of prostacyclin analogs and PDE5 inhibitors on this signaling pathway is illustrated by the finding that PDE5 inhibitors augment increases in cAMP and ATP release from healthy human erythrocytes stimulated by incubation with prostacyclin analogs.8
Figure 5.
Proposed model of the signal transduction pathway through which phosphodiesterase 5 (PDE5) inhibitors potentiate prostacyclin analog-induced stimulation of cAMP accumulation and ATP release from human erythrocytes. ODQ: 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, sGC: soluble guanylyl cyclase, GTP: guanosine 5’-triphosphate, cGMP: cyclic guanosine 3’,5’-monophosphate, GMP: guanosine 5’-monophosphate, PDE5: phosphodiesterase 5, PDE3: phosphodiesterase 3, AMP: adenosine 5’-monophosphate, cAMP: cyclic adenosine 3’,5’-monophosphate, ATP: adenosine 5’-triphosphate, IPR: prostacyclin receptor, Gs: heterotrimeric G protein Gs, AC: adenylyl cyclase, PKA: protein kinase A, CFTR: cystic fibrosis transmembrane conductance regulator, VDAC: voltage-dependent anion channel, +: activation, −: inhibition
Here we report that UT-15C, a water soluble salt of the prostacyclin analog treprostinil that is available for oral administration, stimulates increases in cAMP and ATP release from erythrocytes of humans with PAH as well as those of healthy humans (Figure 1). Importantly, when compared with healthy human cells, PAH erythrocytes demonstrated greater increases in cAMP and ATP release in response to the IPR agonist.
Previously it was shown that the PDE5 inhibitor zaprinast augments UT-15C-induced increases in cAMP in healthy human erythrocytes.8 However, to establish that this effect of zaprinast was due to inhibition of PDE5, we performed additional studies using tadalafil, another selective yet chemically dissimilar PDE5 inhibitor. Similar to the results obtained with zaprinast, tadalafil potentiated UT-15C-induced increases in cAMP in healthy human erythrocytes (Figure 2). Although one interpretation of these results is that zaprinast and tadalafil are not selective PDE5 inhibitors, this is not supported by other studies.14,15 Rather, we propose that these results demonstrate that increases in cGMP in human erythrocytes lead to inhibition of PDE3, resulting in increases in cAMP.
Although augmentation of UT-15C-induced increases in cAMP by PDE5 inhibitors is important in terms of defining a mechanism by which PDE5 and PDE3 can interact, of greater clinical importance is the effect of PDE5 inhibitors on UT-15C-induced ATP release. When healthy human and PAH erythrocytes were pre-treated with either zaprinast or tadalafil, UT-15C-induced increases in ATP release were augmented (Figure 3a and b). It is important to note that the absolute values for ATP release were significantly greater in PAH than in healthy human erythrocytes in response to both UT-15C alone (Figure 1b) and UT-15C in the presence of tadalafil (Figure 3a and b).
Finally, we determined if PDE5 inhibitors augment UT-15C-induced ATP release via a cGMP-dependent mechanism. When erythrocytes were pre-incubated with the soluble guanylyl cyclase inhibitor, ODQ,19 the ability of a PDE5 inhibitor (tadalafil) to augment ATP release from healthy human erythrocytes was prevented (Figure 4).
The results presented here suggest a previously unrecognized mechanism by which the combined use of a prostacyclin analog and a PDE5 inhibitor could be particularly advantageous for the treatment of humans with PAH. It has been previously shown in isolated perfused lungs and intact rabbits that pulmonary vasodilation is enhanced when PDE inhibitors are administered in combination with inhaled iloprost.31,32 In studies in humans, reductions in pulmonary vascular resistance produced by the PDE5 inhibitor sildenafil and inhaled iloprost were shown to be additive.6,33,34 The potential for improved survival in PAH patients treated with this approach is suggested by studies in rats in which pulmonary hypertension was induced by monocrotaline. In these studies, rats that received both a prostacyclin analog and a PDE5 inhibitor showed increased survival.1
In summary, we present evidence for the first time that the prostacyclin analog UT-15C and PDE5 inhibitors interact to augment cAMP accumulation and ATP release from erythrocytes of humans with PAH. Some of the beneficial effects of the administration of these drugs in combination in PAH patients may be explained by the release of this potent vasodilator and stimulus for nitric oxide synthesis in the pulmonary vasculature. Synergism between PDE5 inhibitors and prostacyclin analogs in stimulating release of the potent vasodilator ATP from PAH erythrocytes provides a new rationale for the use of these drugs in combination in this clinical condition. Moreover, these results suggest that the erythrocyte is a novel target for future drug development for the treatment of PAH.
Acknowledgments
The authors thank JL Sprague for inspiration. We thank Eli Lily for the generous gift of tadalafil. This study was supported by NIH (HL-089094) and grants from United Therapeutics and Saint Louis University.
Author contributions
RSS contributed to the study concept and design; GNM, YY, and RSS acquired patients; GNM, YY, RSS, and EAB participated in data acquisition and analysis; RSS and EAB wrote the manuscript; AHS and MLE assisted in manuscript review.
REFERENCES
- 1.Itoh T, Nagaya N, Fujii T, Iwase T, Nakanishi N, Hamada K, Kangawa K, Kimura H. A combination of oral sildenafil and beraprost ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med 2004; 169: 34–8. [DOI] [PubMed] [Google Scholar]
- 2.Simoneau G, Rubin LJ, Galie N, Barst RJ, Fleming TR, Frost AE, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch DB. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension. Ann Intern Med 2008; 149: 521–30. [DOI] [PubMed] [Google Scholar]
- 3.Palevsky HI, Schloo BL, Pietra GG, Weber KT, Janicki JS, Ruben E, Fishman AP. Primary pulmonary hypertension: vascular structure, mophometry, and responsiveness to vasodilator agents. Circulation 1989; 80: 1207–21. [DOI] [PubMed] [Google Scholar]
- 4.Rubin LJ. Pathology and pathophysiology of primary pulmonary hypertension. Am J Cardiol 1995; 75: 51A–4A. [DOI] [PubMed] [Google Scholar]
- 5.Sprague RS, Stephenson AH, Ellsworth ML, Keller C, Lonigro AJ. Impaired release of ATP from red blood cells of humans with primary pulmonary hypertension. Exp Biol Med 2001; 226: 434–9. [DOI] [PubMed] [Google Scholar]
- 6.Ghofrani HA, Rose F, Schermuly RT, Olschewski H, Widemann R, Kreckel A, Weissmann N, Ghofrani S, Enke B, Seeger W, Grimminger F. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol 2003; 42: 158–64. [DOI] [PubMed] [Google Scholar]
- 7.Bendayan D, Shitrit D, Kramer MR. Combination therapy with prostacyclin and tadalafil for severe pulmonary arterial hypertension: a pilot study. Respirology 2008; 13: 916–8. [DOI] [PubMed] [Google Scholar]
- 8.Knebel SM, Elrick MM, Bowles EA, Zdanovec AK, Stephenson AH, Ellsworth ML, Sprague RS. Synergistic effects of prostacyclin analogs and phosphodiesterase inhibitors on cyclic adenosine 3’,5’ monophosphate accumulation and adenosine 3’5’ triphosphate release from human erythrocytes. Exp Biol Med 2013; 238: 1069–74. [DOI] [PubMed] [Google Scholar]
- 9.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, Lonigro AJ. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol 2003; 285: H693–700. [DOI] [PubMed] [Google Scholar]
- 10.Sprague RS, Bowles EA, Hanson MS, DuFaux EA, Sridharan M, Adderley S, Ellsworth M, Stephenson AH. Prostacyclin analogs stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes. Microcirculation 2008; iFirst: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprague RS, Goldman D, Bowles EA, Achilleus D, Stephenson AH, Ellis CG, Ellsworth ML. Divergent effects of low-O2 tension and iloprost on ATP release from erythrocytes of humans with type 2 diabetes: implications for O2 supply to skeletal muscle. Am J Physiol Heart Circ Physiol 2010; 299: H566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson MS, Stephenson AH, Bowles EA, Sridhara M, Adderley S, Sprague RS. Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP. Am J Physiol Heart Circ Physiol 2008; 295: H786–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adderley SP, Thuet KM, Sridharan M, Bowles EA, Stephenson AH, Ellsworth ML, Sprague RS. Identification of cytosolic phosphodiesterases in the erythrocyte: a possible role for PDE5. Med Sci Monit 2011; 17: CR241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Feng Q, Cote RH. Efficacy and selectivity of phosphodiesterase-targeted drugs in inhibiting photoreceptor phosphodiesterase (PDE6) in retinal photoreceptors. Invest Opthalmol Vis Sci 2005; 46: 3060–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res 2004; 16: S11–14. [DOI] [PubMed] [Google Scholar]
- 16.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 1998; 275: H1726–32. [DOI] [PubMed] [Google Scholar]
- 17.Strehler B, McElroy W. Assay of adenosine triphosphate. In: Colowick S, Kaplan N. (eds). Methods in enzymology, New York, NY: Academic Press, 1957. [Google Scholar]
- 18.Holownia P, Bishop E, Newman DJ, John WG, Price CP. Adaptation of latex-enhanced assay for percent glycohemoglobin to a Dade Dimension analyzer. Clin Chem 1997; 43: 76–84. [PubMed] [Google Scholar]
- 19.Pyriochou A, Beis D, Koika V, Potytarchou C, Papadimitriou E, Zhou Z, Papapetropoulos A. Soluble guanylyl cyclase activation promotes angiogenesis. JPET 2006; 319: 663–71. [DOI] [PubMed] [Google Scholar]
- 20.Persson SU, Gustavasson CG, Larsson H, Persson S. Studies on blood rheology in patients with primary pulmonary hypertension. Angiology 1991; 42: 836–42. [DOI] [PubMed] [Google Scholar]
- 21.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol 2001; 281: C1158–64. [DOI] [PubMed] [Google Scholar]
- 22.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Increases in perfusate flow rate stimulate ATP release from red blood cells in isolated rabbit lungs. Exp Clin Cardiol 1998; 3: 73–7. [Google Scholar]
- 23.Sprague RS, Ellsworth ML, Stephenson AJ, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol 1996; 271: H2717–22. [DOI] [PubMed] [Google Scholar]
- 24.Galiè N, Humbert M, Vachiéry J-L, Vizza CD, Kneussl M, Manes A, Sitbon O, Torbicki A, Delcroix M, Naeije R, Hoeper M, Chaouat A, Morand S, Besse B, Simonneau G. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 2002; 39: 1496–502. [DOI] [PubMed] [Google Scholar]
- 25.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretski BF, Clayton LM, Jöbsis MM, Blackburn SD, Shortino D, Crow JW. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334: 296–301. [DOI] [PubMed] [Google Scholar]
- 26.Hoeper MM, Olschewski H, Ghofrani HA, Wilkens H, Winkler J, Borst MM, Niedermeyer J, Fabel H, Seeger W. A comparison of the acute hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in primary pulmonary hypertension. J Am Coll Cardiol 2000; 35: 176–82. [DOI] [PubMed] [Google Scholar]
- 27.Özkan M, Dweik RA, Laskowski D, Arroliga AC, Erzurum SC. High levels of nitric oxide in individuals with pulmonary hypertension receiving epoprostenol therapy. Lung 2002; 179: 233–43. [DOI] [PubMed] [Google Scholar]
- 28.Sridharan M, Bowles EA, Richards JP, Krantic M, Davis KL, Dietrich KA, Stephenson AH, Ellsworth ML, Sprague RS. Prostacyclin receptor-mediated ATP release from erythrocytes requires the voltage-dependent anion channel. Am J Physiol Heart Circ Physiol 2012; 302: H553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sprague R, Bowles E, Stumpf M, Ricketts G, Friedman A, Hou W-H, Stephenson A, Lonigro A. Rabbit erythrocytes possess adenylyl cyclase type II that is activated by the heterotrimeric G proteins Gs and Gi. Pharmacol Rep 2005; 57(suppl): 222–8. [PubMed] [Google Scholar]
- 30.Adderley SP, Sprague RS, Stephenson AH, Hanson MS. Regulation of cAMP by phosphodiesterases in erythrocytes. Pharmacol Rep 2010; 62: 475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schermuly RT, Krupnik E, Tenor H, Schudt C, Weissmann N, Rose F, Grimminger F, Seeger W, Walmrath D, Ghofrani HA. Coaerosolization of phosphodiesterase inhibitors markedly enhances the pulmonary vasodilatory response to inhaled iloprost in experimental pulmonary hypertension. Am J Respir Crit Care Med 2001; 164: 1694–700. [DOI] [PubMed] [Google Scholar]
- 32.Schermuly RT, Inholte C, Ghofrani HA, Gall H, Weissmann N, Weidenbach A, Seeger W, Grimminger F. Lung vasodilatory response to inhaled iloprost in experimental pulmonary hypertension: amplification by different type phosphodiesterase inhibitors. Respir Res 2005; 6: 76–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkens H, Guth A, König J, Forestier N, Cremers B, Hennen B, Böhm M, Sybrecht GW. Effect of inhaled iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation 2001; 104: 1218–22. [DOI] [PubMed] [Google Scholar]
- 34.Ghofrani HA, Widemann R, Rose F, Olschewski H, Schermuly RT, Weissmann N, Seeger W, Grimminger F. Combination therapy with oral sildenafil and inhaled iloprost for severe pulmonary hypertension. Ann Intern Med 2002; 136: 515–22. [DOI] [PubMed] [Google Scholar]