Abstract
Sepsis is the most common cause of acute respiratory distress syndrome, a severe lung inflammatory disorder with an elevated morbidity and mortality. Sepsis and acute respiratory distress syndrome involve the release of inflammatory mediators to the systemic circulation, propagating the cellular and molecular response and affecting distal organs, including the brain. Since it has been reported that sepsis and acute respiratory distress syndrome contribute to brain dysfunction, we investigated the brain-lung crosstalk using a combined experimental in vitro airway epithelial and brain cell injury model. Conditioned medium collected from an in vitro lipopolysaccharide-induced airway epithelial cell injury model using human A549 alveolar cells was subsequently added at increasing concentrations (no conditioned, 2%, 5%, 10%, 15%, 25%, and 50%) to a rat mixed brain cell culture containing both astrocytes and neurons. Samples from culture media and cells from mixed brain cultures were collected before treatment, and at 6 and 24 h for analysis. Conditioned medium at 15% significantly increased apoptosis in brain cell cultures 24 h after treatment, whereas 25% and 50% significantly increased both necrosis and apoptosis. Levels of brain damage markers S100 calcium binding protein B and neuron-specific enolase, interleukin-6, macrophage inflammatory protein-2, as well as matrix metalloproteinase-9 increased significantly after treating brain cells with ≥2% conditioned medium. Our findings demonstrated that human epithelial pulmonary cells stimulated with bacterial lipopolysaccharide release inflammatory mediators that are able to induce a translational clinically relevant and harmful response in brain cells. These results support a brain-lung crosstalk during sepsis and sepsis-induced acute respiratory distress syndrome.
Keywords: Sepsis, lung injury, apoptosis, acute respiratory distress syndrome, brain injury, inflammation
Introduction
Sepsis is a systemic inflammatory response resulting from severe infections. Sepsis is the leading cause of acute respiratory distress syndrome (ARDS), multiple system organ dysfunction, and death among non-cardiac critically ill patients.1 Despite advances in understanding the host immunologic response and pathophysiology of sepsis and ARDS, it still lacks a specific therapy.2 Mechanisms underlying lung injury in sepsis and ARDS are coordinated by a cascade of inflammatory mediators that eventually lead to structural alterations and the disruption of the alveolar epithelial-endothelial capillary barrier, increasing lung permeability and contributing to edema formation.3,4
There is ample evidence showing that injured lung cells release inflammatory mediators to the systemic circulation, contributing to spread the damage and exerting a deleterious effect on distal organs.5 This systemic propagation of the inflammatory response may cause tissue damage leading to significant, often unappreciated, long-term consequences secondary to effects on various organ systems that ultimately lead to higher mortality.5 In this context, sepsis-associated encephalopathy represents a severe brain dysfunction caused by systemic inflammation without clinical or laboratory evidence of direct brain infection and it is associated with a poor prognosis.6 Several studies suggest that ARDS may be responsible for brain dysfunction and poor neurocognitive outcomes. In animal models, acute lung injury led to enhanced brain damage,7 and increased brain cytokines impair neurocognitive features.8 Interleukin (IL)-6, one of the pro-inflammatory cytokines with ample roles in the central nervous system (CNS), has been the focus of therapeutic approaches to reduce neuroinflammation associated with brain dysfunction and cognitive impairment.8 Specific markers of CNS injury, such as the glycolytic isoenzyme neuron-specific enolase (NSE) or the calcium-binding protein S100B, have been studied in sepsis and are currently considered useful biomarkers in the diagnosis of septic-associated encephalopathy.6,9 Macrophage inflammatory protein-2 (MIP-2) plays an essential role in the neuroinflammatory response during sepsis since they modulate neutrophil infiltration into the brain,10 and MMP-9 plays a crucial role in the blood–brain barrier (BBB) integrity.11
Up to 71% of septic patients develop potentially irreversible acute cerebral dysfunction,9 and survivors are often discharged with debilitating cognitive and behavioural problems that persist for years or are irreversible, with impaired quality of life.12–15 Since cognitive disturbances after sepsis and ARDS are common and potentially modifiable, there is a remarkable growing interest in elucidating mechanisms underlying the interaction between the lung and the brain14,15 during critical illness.
Our study was aimed to explore the response of astrocytes and cortical neurons to mediators released by lipopolysaccharide (LPS)-activated human lung epithelial cells, in a well-established in vitro approach based on the first steps in the development of sepsis and sepsis-induced ARDS.16 We postulated that inflammatory mediators released during LPS-induced lung epithelial cell injury may cause brain cell injury, contributing to the brain dysfunction found in patients with sepsis and sepsis-induced ARDS.
Materials and methods
Lung alveolar epithelial and primary mixed brain cell cultures
We used A549 cells (human pulmonary alveolar epithelial carcinoma cells), a cell line that retain features of type II alveolar epithelial cells. A549 cells were obtained from American Type Culture Collection (ATCC®, USA), grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% v/v fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (all from Gibco, Madrid, Spain) and maintained in an incubator at 37℃ with a humidified atmosphere containing 95% air/5% CO2. Once the culture was established, cells were seeded at a density of 5 × 104 cells/cm2 in multiwell plates (Corning, Madrid, Spain) and maintained in 2% FBS for subsequent experiments.
Primary rat mixed cerebral cell cultures were prepared as described previously by a member of our group.17 Briefly, astrocytes were obtained by dissecting the brain cortical area of 1-day-old Sprague-Dawley rat pups and seeded in 75 cm2 flasks. Ten days after plating, astrocytes were collected and seeded at 2 × 105 cells per cm2 in poly-D-lysine-precoated multiwell plates (BD Biocoat, Spain) in DMEM containing 25 mM glucose, 10% v/v FBS, 100 U/mL penicillin and 100 µg/mL streptomycin. Four days after plating, cortical neurons were obtained from E16-17 rat embryos and 2 × 105 cells were seeded on top of the astrocytes homogeneous feeder layer. This mixed culture contains 45 ± 5% astrocytes and 55 ± 5% neurons.17 All procedures were approved by the Animal Care Committee of the Hospital Universitario of Santiago de Compostela, according to European Union rules (86/609/EEC, 2003/65/EC, RD 1201/2005).
LPS stimulation of A549 cells
We used an in vitro LPS-induced lung epithelial cell injury model validated by our group.16 A549 cells were treated with LPS and their conditioned media was subsequently placed over mixed primary brain cells. Pilot experiments testing increasing doses of LPS from Escherichia coli 055:B5 (Sigma-Aldrich, Madrid, Spain) were performed to determine the optimal LPS concentration that activates A549 without causing significant cell death, resulting to be 100 ng/mL. A549 cells were placed in medium containing 2% FBS, 100 ng/mL LPS was added to culture medium, and cells were returned to the incubator. Twelve hours after treatment, culture medium was collected and passed through a 0.22 µm-pore-size filter (Millipore, Madrid, Spain) to remove remaining cells and stored at −80℃ until analysis or prepared for subsequent treatment of neural cells. All experiments were performed three times in triplicate.
Treatment of brain cells with A549 conditioned medium
Filtered collected medium from A549 cells was incubated for 90 min at 37℃ with LPS inhibitor polymyxin B sulphate (10 µg/mL, Sigma, Spain) to exclude the possibility that LPS could activate mixed brain cells. Polymyxin B is a cationic polypeptide that binds to the lipid A, the most toxic portion of LPS, resulting in LPS inactivation.18 At the tested dose, polymyxin B did not affect brain cells viability, as previously reported.19 This was designated as “A549 conditioned medium”, and contained inactivated LPS, DMEM, FBS, LPS and any other factors secreted by LPS-stimulated A549 cells.
Subsequently, culture medium of mixed brain cells was replaced by A549 conditioned medium at different percentages: 0% (no conditioned medium), 2%, 5%, 10%, 15%, 25%, and 50%. Supernatant and cell lysates from mixed brain cells were collected for analysis before treatment with conditioned medium (t = 0), at 6 h (t = 6), and at 24 h (t = 24). Controls for each group were established as mixed brain cell cultures whose medium was replaced by medium from non-stimulated A549 cells.
Trypan blue exclusion assay
Twenty-four hours after adding conditioned medium, brain cell death was determined by Trypan blue dye exclusion test, which does not discriminate between necrosis and apoptosis. Cells from each group were detached by trypsinization and subsequently collected by centrifugation. After resuspension in phosphate buffered saline, equal volumes of cell suspension and 0.4% Trypan blue (Invitrogen, Madrid, Spain) were mixed and incubated for 10 min at room temperature. The number of dead cells (blue stained) was counted using a hemocytometer from five random fields with counting grid. Data are expressed as percent of total cells/field ± SD.
Lactate dehydrogenase determination
Lactate dehydrogenase (LDH) released from damaged brain cells was measured to determine cell necrosis. LDH activity was analyzed in culture media of cerebral mixed culture collected 24 h after adding conditioned medium using a commercial kit (Sigma) and a microplate reader (Biotek, USA) for spectrophotometric measurements. LDH release is expressed as percentage of total cell LDH and plotted as percentage of LDH release induced by treatment with A549 conditioned medium. Basal LDH release was 8 ± 2% (n = 9).
Apoptosis assessment by caspase-3 activity
Caspase-3 activity in mixed brain cell lysates collected 24 h after adding conditioned medium was determined as an apoptosis marker in a fluorometric assay by measuring the extent of cleavage of the fluorescent peptide substrate (Z-DEVC-AMC) using a commercial kit following manufacturer’s instructions (Invitrogen). The amount of the released fluorescence product was determined in a fluorescent microplate reader (Biotek).
Biomarkers implicated in inflammation, chemotaxis, and cell integrity
IL-6, MIP-2, and MMP-9 were measured in A549 conditioned medium and in mixed brain cells medium collected 6 and 24 h after treatment. In addition, secreted NSE and S100 calcium binding protein B (S100B) were measured at t = 6 and t = 24 in supernatant of brain mixed cultures as markers of neuronal damage and astroglial activation, respectively. ELISA commercial kits were used following manufacturer’s instructions (IL-6 and MMP-9 from R&D Systems, USA; MIP-2, NSE and S100B from Cusabio, China). Lower detection limits of the assays were 36 pg/mL, 0.03 ng/mL, 6.25 pg/mL, 0.09 ng/mL, and 3.12 pg/mL for IL-6, MMP-9, MIP-2, NSE, and S100B, respectively. Inter- and intra-assay coefficients of variation were below 7% for all analysis.
Statistical analysis
All data are from nine independent experiments. Comparisons among experimental groups were performed with ANOVA following post-hoc test for linear trends. Pairwise comparisons between treated and control groups were made with Student's t-test. Statistical software Prism 5 (GraphPad, USA) was used. A two-sided P value <0.05 was considered significant.
Results
Effects of A549 conditioned media on brain cells
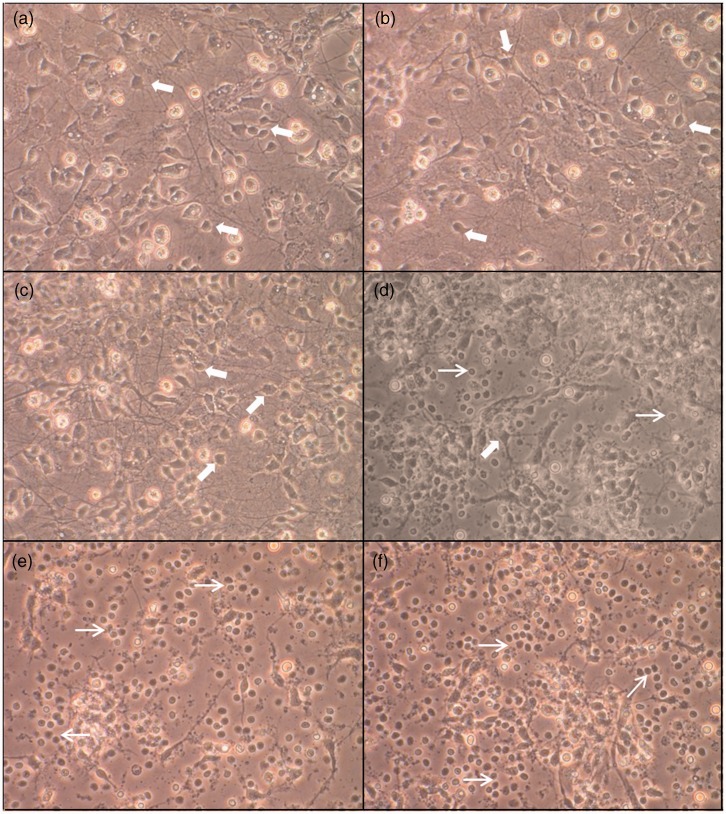
Treatment with 2% or 5% of A549 conditioned media did not induce morphological changes in brain cell cultures (Figure 1b and c). At 15%, some damaged neurons were visible, while others remained morphologically unaltered (Figure 1d). Neurons treated with 25% and 50% conditioned media lost their neurites and became shrunken and rounded, all typical morphological features of apoptosis (Figure 1e and f).
Figure 1.
Morphological changes and death in rat brain cells exposed to A549 conditioned media. Phase-contrast microscopic observation (×20) of primary rat mixed cortical cultures 24 h after treatment with 2% (b), 5% (c), 15% (d), 25% (e), or 50% (f) A539 conditioned medium as well as controls (a). Thick arrows show normal viable neuronal bodies while thin arrows show round damaged or dead cells. See text for details. (A color version of this figure is available in the online journal.)
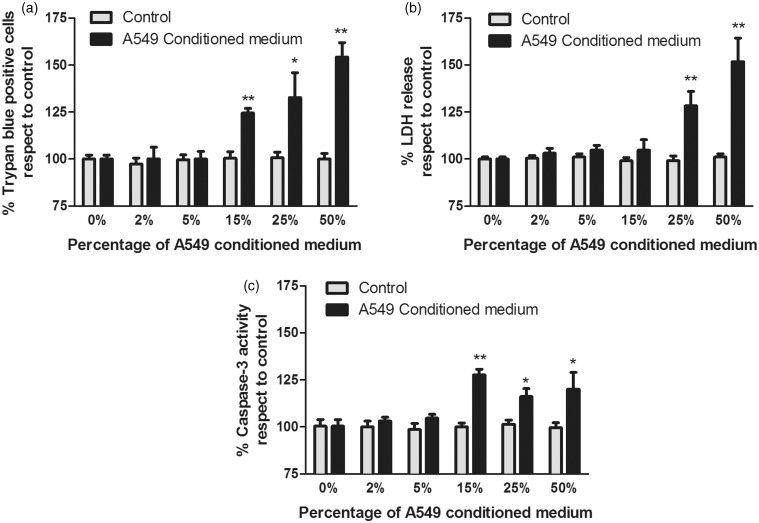
A549 conditioned media at 2% or 5% did not increase necrosis or apoptosis in brain cells compared to control (Figure 2a–c). Conditioned medium at 15% did not increase brain cell necrosis (Figure 2b) but it yielded higher apoptosis compared to control (Figure 2a and c) (P < 0.005). Conditioned media at 25% or 50% led to a significant increase of brain cell death (P < 0.05 and P < 0.005, respectively) (Figure 2a), both for necrosis (Figure 2b, P < 0.005) and apoptosis (Figure 2c, P < 0.05) compared to controls.
Figure 2.
Effects of A549 conditioned medium on brain cells viability at 24 h. (a) Trypan blue assay, (b) effects on lactate dehydrogenase (LDH) release, (c) effects on caspase-3 activity. *P < 0.05, **P < 0.005 compared to controls. n = 9
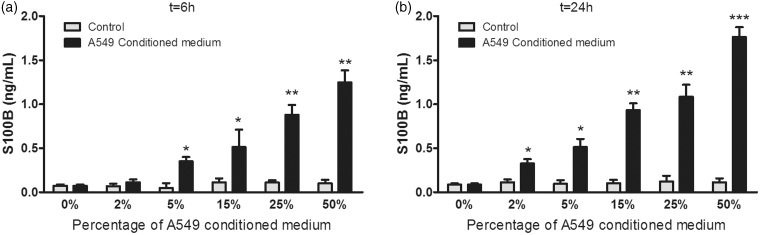
S100B levels were different among treated brain cell groups and showed a significant increasing linear trend with A549 conditioned medium, both at 6 h (P < 0.005) and 24 h (P < 0.001) (Figure 3a and b).
Figure 3.
Effects of A549 conditioned medium on S100B levels in neural cells at 6 h (a) and at 24 h (b). *P < 0.05, **P < 0.005, ***P < 0.001 compared to controls. n = 9
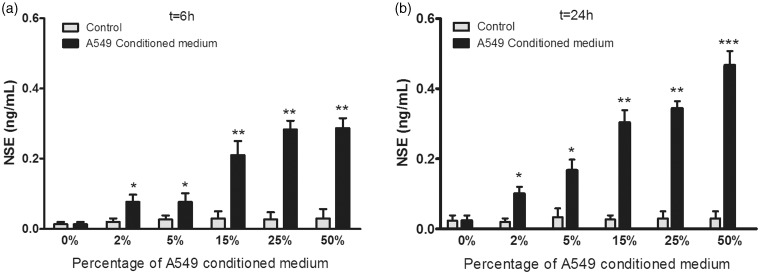
NSE levels differed among brain cell groups, showing a significant linear trend with percentage of A549 conditioned media, both at 6 h (P < 0.001) and 24 h (P < 0.001) (Figure 4a and b). Levels of S100B and NSE differed at 6 versus 24 h only with A549 conditioned medium at 50% (P < 0.05).
Figure 4.
Effects of A549 conditioned medium on neuron-specific enolase (NSE) levels in brain cells at 6 h (a) and at 24 h (b). *P < 0.05, **P < 0.005, ***P < 0.001 compared to controls. n = 9
IL-6, MIP-2, and MMP-9 in A549 conditioned media
Twelve hours after LPS stimulation, supernatant of A549 cells had 5024.6 ± 187.5 pg/mL of IL-6, 821.6 ± 38.0 pg/mL of MIP-2, and 632.2 ± 20.2 pg/mL of MMP-9, while levels in untreated culture media were below detection limit.
Effects of A549 conditioned medium on IL-6, MIP-2, and MMP-9 levels in brain cells
IL-6, MIP-2, and MMP-9 levels differed among groups of brain cells and showed a significant increasing linear trend with percentage of A549 conditioned media, both at 6 h (P < 0.001, P < 0.001, P < 0.05, respectively) and 24 h (P < 0.001, P < 0.001, P < 0.01, respectively). IL-6, MIP-2, and MMP-9 increased significantly at 6 and 24 h compared to controls (Table 1, all P < 0.0001). Levels of IL-6, MIP-2, and MMP-9 were higher at 24 compared to 6 h in all groups (Table 1, all P < 0.05).
Table 1.
Levels of interleukin (IL)-6, macrophage inflammatory protein-2 (MIP-2), and matrix metalloproteinase-9 (MMP-9) in brain cell cultures
| A549 Conditioned medium | t = 6 h |
t = 24 h |
|||||
|---|---|---|---|---|---|---|---|
| IL-6 | MIP-2 | MMP-9 | IL-6 | MIP-2 | MMP-9 | ||
| 2% | C | BDL | BDL | BDL | BDL | BDL | BDL |
| T | 327.3 ± 77.5* | 25.4 ± 7.6* | 29.3 ± 3.2* | 780.8 ± 104.5*† | 84.2 ± 11.7*† | 60.2 ± 18.0*† | |
| 5% | C | BDL | BDL | BDL | BDL | BDL | BDL |
| T | 734.3 ± 65.7* | 60.1 ± 10.4* | 49.2 ± 9.7* | 1134.6 ± 244.8*† | 105.1 ± 23.6*† | 90.5 ± 14.3*† | |
| 15% | C | BDL | BDL | BDL | BDL | BDL | BDL |
| T | 1051.7 ± 301.4* | 201.3 ± 30.5* | 66.5 ± 15.1* | 2244.3 ± 280.1*† | 353.2 ± 26.9*† | 138.3 ± 66.1*† | |
| 25% | C | BDL | BDL | BDL | BDL | BDL | BDL |
| T | 1945.8 ± 206.9* | 362.2 ± 35.9* | 203.1 ± 14.8* | 3175.8 ± 301.4*† | 585.3 ± 22.1*† | 511.8 ± 36.2*† | |
| 50% | C | BDL | BDL | BDL | BDL | BDL | BDL |
| T | 3608.6 ± 205.2* | 521.8 ± 20.6* | 353.9 ± 39.2* | 4703.6 ± 217.7*† | 799.5 ± 17.2*† | 603.4 ± 30.9*† | |
C: control (neural cells treated with non-stimulated A549 medium, as explained in Methods); T: treated (with LPS-stimulated A549 2%, 5%, 15%, 25%, or 50% A549 conditioned medium). Results are expressed as mean ± SD; n = 9. Each T group is compared with its corresponding C group. Levels are expressed as picograms per milliliter. BDL: below the detection limit of the assay; *P < 0.0001 respect to each control group, †P < 0.05 t = 24 h vs. t = 6 h.
Discussion
Our findings suggest that lung epithelial cells when stimulated by LPS may exert significant biological effects on brain cells. Brain cells subjected to products released by stimulated lung alveolar cells caused (1) apoptosis and necrosis, (2) synthesis of specific markers of neuronal damage and astroglial activation, and (3) release of IL-6, MIP-2, and MMP-9. According to our findings, mediators released by pulmonary cells after LPS stimulation are toxic for brain cells in a dose-dependent manner, inducing from a tightly regulated process as cellular apoptosis to an uncontrolled release of harmful cellular content causing brain cell necrosis. Inflammatory molecules released by stimulated pulmonary cells might account for the observed effects. Of note, the selected dose of LPS did not cause cell death of A549 cells; therefore, no molecules released by necrotic or apoptotic cells, which could affect and/or mask neural response, are expected to be present in conditioned media.
The inflammatory cascade of LPS-stimulated A549 cells involves cytokines such as IL-8,16 IL-1β, or TNF-α,20 which induce neuronal death in vitro.21,22 The increase of S100B at 6 h indicates an early response of brain cells, sustained and pronounced at 24 h. S100B is a multifaceted protein produced mainly by astrocytes with an important role in neuroinflammation by signaling tissue damage and participating in the inflammatory response by activating microglia.23 The marked increase of S100B observed in our experiments suggests that mediators released from stimulated alveolar cells might include regulators of S100B. Serum S100B levels were elevated in an animal model of ARDS where hippocampal cells showed damage.7 S100B has been suggested as a candidate biomarker for cerebral dysfunction in septic shock.24 High concentrations of extracellular S100B stimulate the expression of pro-inflammatory cytokines and induce neuronal apoptosis.25 Also, conditioned media yielded to higher NSE levels suggesting that cortical neurons are very sensitive to mediators released by LPS-stimulated A459 cells. NSE is a glycolytic isoenzyme located almost exclusively in neurons and it is considered a reliable, quantitative, and specific marker of neuronal injury in vitro.26 NSE serum levels have been extensively investigated as markers of poor outcome in patients with stroke or neurological dysfunction after surgery.27,28 Both S100B and NSE have been explored as markers of brain injury in severe sepsis, and may help to diagnose sepsis-associated encephalopathy.6 Serum S100B and NSE are increased in children with septic shock29 and in patients with brain injury and severe sepsis.30 Of note, S100B has been reported as the strongest predictor of survival in patients with severe sepsis.30
In our study, increasing concentrations of conditioned media were associated with increasing levels of IL-6, MIP-2, and MMP-9 in brain cells. Although we did not explore the cellular origin of these mediators, it has been reported that both neurons and astrocytes express IL-6,31 MIP-2,32 and MMP-9.33 A549 cells release IL-1β and TNF-α after LPS stimulation, which exert a strong and synergic inducing signal for IL-6 in neurons34 and astrocytes.35 Pro-inflammatory cytokines are increased in patients with septic-associated encephalopathy and might contribute to the development of long-term neurological deficits8,36 since chronic or high increases of IL-6 levels are associated with neuronal and cognitive impairment.37 Therefore, acute lung inflammation during ARDS might contribute to cerebral dysfunction by promoting neuroinflammation. Although lung-brain crosstalk in critical patients is still poorly understood, it has been reported that mechanical ventilation might activate intracellular signalling pathways in the brain, leading to the release of inflammatory mediators to the bloodstream that may activate neurons38 and also triggering hippocampal apoptosis.39
Chemokines, such as MIP-2, are expressed in the brain during sepsis and seems to be necessary for neutrophil transmigration into the brain parenchyma. High levels of MIP-2 have been associated with severity of sepsis40 and shock survival in mice.41 MMP-9 plays a crucial role in the integrity of the BBB as a main component of the extracellular matrix in the neurovascular unit.11,42 Degradation of the basal lamina in brain tissue often results in BBB leakage, edema, and hemorrhage, and MMP-9 levels are considered markers of poor prognosis in brain ischemia.43 Although there is evidence that BBB is affected in septic patients44 and it is thought to contribute to development and progression of septic-associated encephalopathy,45 mechanisms of BBB damage in sepsis have been poorly explored. Dal-Pizzol et al.46 reported that the increase of BBB permeability in septic rats is time-related to the increase of MMP-9 and MMP-2 in the microvessels, and this effect was reversed by MMPs inhibitors. Thus, by increasing MMP-9 in brain cells, mediators released by stimulated lung epithelial cells might contribute to BBB damage in sepsis. Pro-inflammatory mediators released by injured lungs during pneumonia, sepsis, or sepsis-induced ARDS may reach the brain contributing to cerebral dysfunction and development of sepsis-associated encephalopathy, a clinical condition associated with poor prognosis.9
Our study has limitations and strengths. First, although our in vitro approach is based on the first steps in the development of sepsis and sepsis-induced ARDS,16,20 cell culture stimulation with LPS does not completely replicate sepsis. Second, although we have used a brain cellular model including neurons and astrocytes, we did not study the LPS effects on microglia, another key player of neuroinflammation. However, we do not think that the addition of microglia would have weakened our results since levels of mediators would be likely greater when microglia cells are added, but this would not change our conclusions regarding lung-brain crosstalk. Third, although we cannot extrapolate the concentrations of conditioned media with a plausible clinical neurophysiological deterioration, several reports in septic patients with acute lung injury have shown that S100B and NSE are markedly increased and predicted outcome.29,30 We do not think that the lack of BBB in our cellular model constitutes a major limitation, due to the previously reported evidence of BBB disruption in sepsis, allowing substances to reach the brain. Finally, we have used brain cells from the cortical area; therefore, we cannot state for certain that the same response to injurious stimuli could be observed in other brain regions, due to somatotopic organization of the brain. A clinically relevant strength of our study is that we have analyzed the response of brain cells caused by LPS-induced lung inflammation at an early time-period. Usually patients with sepsis and/or ARDS are mechanically ventilated, a procedure that affects brain function.15,38,39 However, our findings suggest that early lung inflammation might cause deleterious effects in the brain. Due to the complexity of the interaction between organs, further research is needed to validate our findings using in vivo models of sepsis-induced ARDS.
In summary, our study showed that epithelial pulmonary cells after stimulation with LPS release different mediators that significantly affect brain cells. These findings seem to support the concept that a severely damaged organ might cause a biological response in a distal one, in line with emerging ideas regarding lung-brain crosstalk in sepsis and ARDS.
Acknowledgements
Supported by grants from the Instituto de Salud Carlos III, Spain (PI10/0393, CB06/06/1088). RRG is recipient of a “Sara Borrell” postdoctoral contract (CD11/00104) from Instituto de Salud Carlos III, Spain.
Author contributions
RRG, ARN, JLMB, and AB conceived and conducted the experiments, RRG, JLA, JA, PRMR, PP, and JV analyzed the data, RRG, JLA, JA, PRMR, PP, and JV wrote the manuscript or revised it for important intellectual content. All authors read and approved the final manuscript.
References
- 1.Blanco J, Muriel-Bombin A, Sagredo V, Taboada F, Gandía F, Tamayo L, Collado J, García-Labattut A, Carriedo D, Valledor M, De Frutos M, López MJ, Caballero A, Guerra J, Alvarez B, Mayo A, Villar J. Grupo de Estudios y Análisis en Cuidados Intensivos. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care 2008; 12: R158–R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skrupky LP, Kerby PW, Hotchkiss RS. Advances in the management of sepsis and the understanding of key immunologic defects. Anesthesiology 2011; 115: 1349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 2010; 6: 147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villar J. What is the acute respiratory distress syndrome? Respir Care 2011; 56: 1539–45. [DOI] [PubMed] [Google Scholar]
- 5.Seeley EJ, Matthay MA, Wolters PJ. Inflection points in sepsis biology: from local defense to systemic organ injury. Am J Physiol Lung Cell Mol Physiol 2012; 303: L355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siami S, Annane D, Sharshar T. The encephalopathy in sepsis. Crit Care Clin 2008; 24: 67–82. [DOI] [PubMed] [Google Scholar]
- 7.Fries M, Bickenbach J, Henzler D, Beckers S, Dembinski R, Sellhaus B, Rossaint R, Kuhlen R. S-100 protein and neurohistopathologic changes in a porcine model of acute lung injury. Anesthesiology 2005; 102: 761–7. [DOI] [PubMed] [Google Scholar]
- 8.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson JX, Young GB. Progress in clinical neurosciences: Sepsis-associated encephalopathy: evolving concepts. Can J Neurol Sci 2003; 30: 98–105. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci 2008; 13: 2400–7.. [DOI] [PubMed] [Google Scholar]
- 11.del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Ann N Y Acad Sci 2010; 1207: 46–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med 2010; 38: 1276–83. [DOI] [PubMed] [Google Scholar]
- 13.Leibovici L. Long-term consequences of severe infections. Clin Microbiol Infect 2013; 19: 510–2. [DOI] [PubMed] [Google Scholar]
- 14.Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, Localio AR, Demissie E, Hopkins RO, Angus DC. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 2013; 185: 1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quílez ME, López-Aguilar J, Blanch L. Organ crosstalk during acute lung injury, acute respiratory distress syndrome, and mechanical ventilation. Curr Opin Crit Care 2012; 18: 23–8. [DOI] [PubMed] [Google Scholar]
- 16.Cabrera-Benitez NE, Pérez-Roth E, Casula M, Ramos-Nuez A, Ríos-Luci C, Rodríguez-Gallego C, Sologuren I, Jakubkiene V, Slutsky AS, Padrón JM, Villar J. Anti-inflammatory activity of a novel family of aryl ureas compounds in an endotoxin-induced airway epithelial cell injury model. PLoS One 2012; 7: e48468–e48468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodríguez-González R, Agulla J, Pérez-Mato M, Sobrino T, Castillo J. Neuroprotective effect of neuroserpin in rat primary cortical cultures after oxygen and glucose deprivation and tPA. Neurochem Int 2011; 58: 337–43. [DOI] [PubMed] [Google Scholar]
- 18.Cooperstock MS. Inactivation of endotoxin by polymyxin B. Antimicrob Agents Chemother 1974; 6: 422–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Kim JH, Kim JH, Seo JW, Han HS, Lee WH, Mori K, Nakao K, Barasch J, Suk K. Lipocalin-2 is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem 2011;286:43855–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng T, Yunfeng N, Jinbo Z, Zhipei Z, Huizhong Z, Li L, Tao J, Yunjie W. Single immunoglobulin IL-1 receptor-related protein attenuates the lipopolysaccharide-induced inflammatory response in A549 cells. Chem Biol Interact 2010; 183: 442–9. [DOI] [PubMed] [Google Scholar]
- 21.Thirumangalakudi L, Yin L, Rao HV, Grammas P. IL-8 induces expression of matrix metalloproteinases, cell cycle and pro-apoptotic proteins, and cell death in cultured neurons. J Alzheimers Dis 2007; 11: 305–11. [DOI] [PubMed] [Google Scholar]
- 22.Ye L, Huang Y, Zhao L, Li Y, Sun L, Zhou Y, Qian G, Zheng JC. IL-1β and TNF-α induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J Neurochem 2013; 125: 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchi R, Adami C, Giambanco I, Donato R. S100B binding to RAGE in microglia stimulates COX-2 expression. J Leukoc Biol 2007; 81: 108–18. [DOI] [PubMed] [Google Scholar]
- 24.Lipcsey M, Olovsson M, Larsson E, Einarsson R, Qadhr GA, Sjölin J, Larsson A. The brain is a source of S100B increase during endotoxemia in the pig. Anesth Analg 2010; 110: 174–80. [DOI] [PubMed] [Google Scholar]
- 25.Rothermundt M, Peters M, Prehn JH, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech 2003; 60: 614–32. [DOI] [PubMed] [Google Scholar]
- 26.Hans P, Bonhomme V, Collette J, Moonen G. Neuron-specific enolase as a marker of in vitro neuronal damage. Part I: assessment of neuron-specific enolase as a quantitative and specific marker of neuronal damage. J Neurosurg Anesthesiol 1993; 5: 111–6. [DOI] [PubMed] [Google Scholar]
- 27.Brea D, Sobrino T, Blanco M, Cristobo I, Rodríguez-González R, Rodríguez-Yañez M, Moldes O, Agulla J, Leira R, Castillo J. Temporal profile and clinical significance of serum neuron-specific enolase and S100 in ischemic and hemorrhagic stroke. Clin Chem Lab Med 2009; 47: 1513–8. [DOI] [PubMed] [Google Scholar]
- 28.Cata JP, Abdelmalak B, Farag E. Neurological biomarkers in the perioperative period. Br J Anaesth 2011; 107: 844–58. [DOI] [PubMed] [Google Scholar]
- 29.Hsu AA, Fenton K, Weinstein S, Carpenter J, Dalton H, Bell MJ. Neurological injury markers in children with septic shock. Pediatr Crit Care Med 2008; 9: 245–51. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, Huyghens L. Elevated serum levels of S-100beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med 2006; 34: 1967–74. [DOI] [PubMed] [Google Scholar]
- 31.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 2012; 8: 1254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes JK, Sharkey J, Andrews PJ. The temporal expression, cellular localization, and inhibition of the chemokines MIP-2 and MCP-1 after traumatic brain injury in the rat. J Neurotrauma 2009; 26: 507–25. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia 2005; 50: 329–39. [DOI] [PubMed] [Google Scholar]
- 34.Ringheim GE, Burgher KL, Heroux JA. Interleukin-6 mRNA expression by cortical neurons in culture: evidence for neuronal sources of interleukin-6 production in the brain. J Neuroimmunol 1995; 63: 113–23. [DOI] [PubMed] [Google Scholar]
- 35.Aloisi F, Carè A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol 1992; 149: 2358–66. [PubMed] [Google Scholar]
- 36.Wratten ML. Therapeutic approaches to reduce systemic inflammation in septic-associated neurologic complications. Eur J Anaesthesiol 2008; 42(Suppl): 1–7. [DOI] [PubMed] [Google Scholar]
- 37.Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci USA 1997; 94: 1500–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quilez ME, Fuster G, Villar J, Flores C, Martí-Sistac O, Blanch L, López-Aguilar J. Mechanical ventilation affects neuronal activation in ventilated rats. Crit Care 2011; 15: R124.–R124.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González-López A, López-Alonso I, Aguirre A, Amado-Rodríguez L, Batalla-Solís E, Astudillo A, Tomás-Zapico C, Fueyo A, dos Santos CC, Talbot K, Albaiceta GM. Mechanical ventilation triggers hippocampal apoptosis by vagal and dopaminergic pathways. Am J Respir Crit Care Med 2013; 188: 693–702. [DOI] [PubMed] [Google Scholar]
- 40.Walley KR, Lukacs NW, Standiford TJ, Strieter RM, Kunkel SL. Elevated levels of macrophage inflammatory protein 2 in severe murine peritonitis increase neutrophil recruitment and mortality. Infect Immun 1997; 65: 3847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krakauer T, Buckley MJ, Fisher D. Proinflammatory mediators of toxic shock and their correlation to lethality. Mediators Inflamm 2010; 2010: 517594–517594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol 1998; 274: 1203–11. [DOI] [PubMed] [Google Scholar]
- 43.Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, Dávalos A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke 2003; 34: 40–6. [PubMed] [Google Scholar]
- 44.Davies DC. Blood–brain barrier breakdown in septic encephalopathy and brain tumours. J Anat 2002; 200: 639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gofton TE, Young GB. Sepsis-associated encephalopathy. Nat Rev Neurol 2012; 8: 557–66. [DOI] [PubMed] [Google Scholar]
- 46.Dal-Pizzol F, Rojas HA, Dos Santos EM, Vuolo F, Constantino L, Feier G, Pasquali M, Comim CM, Petronilho F, Gelain DP, Quevedo J, Moreira JC, Ritter C. Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood-brain barrier dysfunction in an animal model of severe sepsis. Mol Neurobiol 2013; 48: 62–70. [DOI] [PubMed] [Google Scholar]