Abstract
Amiodarone, a common and effective antiarrhythmic drug, has been reported to have anti-inflammatory effects such as reducing the activation and movement of neutrophils. However, its effects on human T cells remain unclear. The aim of this study was to elucidate the effects and possible underlying mechanisms of amiodarone on human T cells. We isolated human primary T cells from the peripheral blood of healthy volunteers and performed enzyme-linked immunosorbent assay (ELISA), flow cytometry, electrophoretic mobility shift assay, luciferase assay, and Western blotting to evaluate the modulatory effects of amiodarone on human T cells. We found that amiodarone dose dependently inhibited the production of cytokines, including interleukin-2 (IL-2), IL-4, tumor necrosis factor-alpha, and interferon-gamma in activated human T cells. By flow cytometry, we demonstrated that amiodarone suppressed the expression of IL-2 receptor-alpha (CD25) and CD69, the cell surface markers of activated T cells. Moreover, molecular investigations revealed that amiodarone down-regulated activator protein-1 (AP-1) and nuclear factor kappa-B (NF-κB) DNA-binding activities in activated human T cells and also inhibited DNA binding and transcriptional activities of both AP-1 and NF-κB in Jurkat cells. Finally, by Western blotting, we showed that amiodarone reduced the activation of c-Jun NH2-terminal protein kinase and P38 mitogen-activated protein kinase, and suppressed stimuli-induced I-kappa B-alpha degradation in activated human T cells. Through regulation of AP-1 and NF-κB signaling, amiodarone inhibits cytokine production and T cell activation. These results show the pleiotropic effects of amiodarone on human T cells and suggest its therapeutic potential in inflammation-related cardiovascular disorders.
Keywords: Amiodarone, activator protein-1, cytokine, nuclear factor kappa-B, T cells
Introduction
Atrial fibrillation (Af) is one of the most common arrhythmias in clinical practice, accounting for approximately one-third of admissions resulting from cardiac rhythm disturbances, and about 1% of the adult population in the United States has been reported to suffer from Af.1–3 Af frequently coexists with heart failure, affecting about 30% of all individuals with heart failure.4–7 Moreover, Af is a common clinical complication of acute coronary syndrome. In the Cooperative Cardiovascular Project, Af was found to be present in 22% of 106,780 persons aged 65 years and older with acute myocardial infarction.8 Additionally, in the Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy trial, Af was found to be present in 6.4% of 9432 patients with non-ST segment elevation myocardial infarction or unstable angina.9 It has also been reported that Af increases the risk of mortality after myocardial infarction or coronary artery bypass grafting.10,11 These results highlight the importance of Af and its close association with heart failure and acute coronary syndrome.
Although the mechanisms and specific factors leading to this kind of atrial arrhythmia have not been completely determined, previous studies have reported on signaling transduction systems, inflammation, oxidative stress, atrial stretching, and/or ischemia as factors related to the cascade of events causing Af.3,12–15 Bruins et al. first indicated the relationship of Af with an inflammatory process, after patients who developed Af after coronary artery bypass surgery were found to have increased levels of plasma C-reactive protein.16 More recently, increasing evidence suggests that inflammation is a risk factor for developing new-onset Af.17
Inflammation plays an important role in the pathogenesis of various diseases, and its characteristics include redness, heat, pain, and swelling, which result from local responses of immune, vascular, and parenchymal cells to infection or injury.18 Interestingly, during the inflammatory cascade, T cells are known to be a key factor in the progression of atherosclerosis, and they have been linked to acute onset of coronary events.19 Activated T cells, especially CD4+ T-helper type 1 cells, are major producers of interferon-γ (IFN-γ), which is known to induce the expression of class II major histocompatibility complex antigens on macrophages and vascular smooth muscle cells and to promote atherosclerosis.20 It is therefore reasonable to hypothesize that the activation of T cells plays a crucial role in the pathogenesis of both Af and atherosclerosis.
Amiodarone is the most effective antiarrhythmic drug for preventing the recurrence of Af and has limited proarrhythmic toxicity.7,21 It is categorized as a class III antiarrhythmia agent (a multichannel blocker) and also has the ability to block alpha- and beta-receptors in cardiac cells.22 Clinically, it is used to treat various kinds of ventricular and atrial arrhythmias, including suppression of ventricular tachycardia and complex arrhythmias associated with heart disease.23–25 Intriguingly, many pleiotropic effects of amiodarone have been reported, with the most important being to reduce inflammation.22 Wilson et al. suggested that amiodarone inhibits the phospholipase C signaling pathway which plays a major role in the production of inflammatory mediators in arachidonic acid metabolism.26 Moreover, amiodarone has been reported to modify phospholipid metabolism in Jurkat cells and to have phospholipidogenic effects on CD3+ T lymphocytes.27,28 Furthermore, amiodarone has been reported to reduce the production of tumor necrosis factor-alpha (TNF-α) in lipopolysaccharide-stimulated human mononuclear cells.29 All of these studies point to the anti-inflammatory nature of amiodarone in treating cardiovascular disorders.
In this study, we investigated whether one of the effects of amiodarone on slowing and even reversing the incidence of Af in ischemic heart disease is by reducing activated T cell-related inflammation. Our novel findings demonstrate that amiodarone effectively reduces T cell activation and suppresses the production of inflammatory cytokines in human primary T cells. The mechanisms are likely to be mediated through activator protein-1 (AP-1) and nuclear factor kappa-B (NF-κB) signaling pathways. These results suggest that amiodarone has a therapeutic potential in treating inflammation-related cardiovascular disorders.
Materials and methods
Preparation of amiodarone
The purified form of amiodarone (provided by Sanofi-Synthelabo Pharmaceutical) was dissolved in ethanol. The defined concentrations were added to culture media with immune cells to test its effects. The final concentrations in the media were about 1–5 µM.
Isolation of human primary T cells from human blood
Human peripheral blood mononuclear cells were collected from whole blood buffy coat of healthy volunteers as described previously (more than 40 men or women participants with an age from 30 to 60 years).30 The layer of purified mononuclear cells was collected and incubated with antibodies including L243 (anti-DR; American Type Culture Collection (ATCC), Rockville, MD), OKMI (anti-CD11b; ATCC), and LM2 (anti-MacI; ATCC) for 30 min at 4℃. The cells were then washed with medium containing 0.1% fetal bovine serum and incubated with magnetic beads conjugated with goat anti-mouse IgG (R&D, Minneapolis, MN). The antibody-stained cells were then removed with a magnet. Following a repeat of the above procedures, T cells were obtained with a purity of more than 98% as determined by the percentage of CD3+ cells in flow cytometry (Beckton Dickinson, Mountain View, CA).
Cell treatment and stimulation
For cell activation, the following stimuli were used: 5–20 ng/mL of phorbol 12-myristate 13-acetate (PMA, Sigma, St. Louis, MO), 1 µM of ionomycin (Sigma), 10 µg/mL of immobilized anti-CD3 mAb (OKT3, ATCC), l µg/mL of soluble anti-CD-28 mAb (clone 9.3, kindly provided by Di-Carl June, Naval Institute, Bethesda, NIH), and 2 ng/mL of TNF-α (Sigma, USA).
Measurement of nonspecific cytotoxicity of the drugs
Several assays were used to examine drug cytotoxicity on the cells. The release of lactate dehydrogenase (LDH), as an indicator of damage to the plasma membrane and cell death, was measured according to the assay manufacturer’s instructions (Roche, Indianapolis, IN, USA). The percent of cytotoxicity was calculated as ([sample value – medium control]/[high control – medium control]) × 100, where the sample values were the averages of the absorbance values from triplicate measurements of the indicated dose of amiodarone or vehicle-treated cell culture supernatants after the subtraction of the absorbance values from the background control. The average absorbance values of untreated cell culture supernatants, used as the medium control, were calculated in a similar manner. An equal number of cells treated with 1% Triton X-100 was used as the high control. 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assays were performed as previously described.31,32 In brief, T cells at 1 × 106 mL−1 in 100 µL volume were incubated in the presence or absence of amiodarone for 24 h. Twenty-five microliters of MTT (5 mg/mL in H2O) was then added, and the cells were incubated at 37℃ for 2 h followed by the addition of 100 µL of lysis buffer containing 20% sodium dodecyl sulfate and 50% dimethylformamide. After incubation at 37℃ for another 6 h, the amount of dissolved reduced MTT crystals was measured with an enzyme-linked immunosorbent assay (ELISA) reader (Dynatech, Chantilly, VA).
Cytokine production assay
The determination of cytokine concentrations was performed according to our previous report.30 Briefly, a 96-well flat-bottom plate was coated with anti-cytokine mAb (100 µL at 4 mg/mL) in phosphate-buffered saline (PBS) pH 7.3 at room temperature overnight. The plate was then washed with PBS containing 0.05% Tween 20 (PBS-T) three times, followed by incubation with a blocking solution containing 1% bovine serum albumin, 5% sucrose, and 0.05% NaN3 in PBS for more than 1 h. After washing with PBS-T, the collected 100 µL of supernatant was added into each well for 24 h. The plates were then washed with PBS-T three times and then incubated with biotinylated anticytokine detection antibodies (l00 µL at 12.5 ng/mL) for 2 h at room temperature. Following washing, 100 µL of streptavidin horseradish peroxidase (1:2000 dilution of a 1.25 mg/mL solution) was added and incubated for 20 min at room temperature. After washing three times, 100 µL of substrate solution containing a 1:1 mixture of H2O2 and tetramethylbenzidine was added and incubated for another 20 min at room temperature. The reaction was stopped by adding stop solution, and the cytokine concentrations were measured with a microplate reader (Dynatech).
Measurement of cell surface molecule expressions
Human peripheral blood T cells at a concentration of 1 × 106 mL−1 were preincubated with various concentrations of amiodarone for 2 h. After adding stimuli for another 24 h, the cells were collected and washed with PBS. After washing, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-interleukin-2 (IL-2) receptor alpha (anti-IL-2Rα, anti-CD25) and anti-CD69 monoclonal antibodies (mAbs) or FITC-conjugated isotype-matched mAb (PharMingen, San Diego, LA), and the expressions of these surface molecules were determined with a flow cytometer (Becton Dickinson).
Nuclear extract preparation
Nuclear extracts were prepared according to our previous study.33 Briefly, the treated cells were left at 4℃ in 70 µL of buffer A (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.9, 10 mM KCl, 1.5 mM MgC12, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 3.3 µg/mL aprotinin) for 15 min with occasional gentle vortexing. The swollen cells were then centrifuged at 15,000 r/min for 3 min. After removal of the supernatants (cytoplasmic extract), the pelleted nuclei were washed with 70 µL buffer A and centrifuged at 15,000 r/min for 10 min. Subsequently, the nuclear pellets were resuspended in 25 µL buffer C (20 mM HEPES [pH 7.9], 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM ethylenediaminetetraacetic acid (EDTA), 25% glycerol, 1 mM DTT, 0.5 mM PMSF, and 3.3 µg/mL aprotinin) and incubated at 4℃ for 30 min with occasional vigorous vortexing. The mixtures were then centrifuged at 15,000 r/min for 20 min and the supernatants were used as the nuclear extracts.
Electrophoresis mobility shift assay (EMSA)
The oligonucleotides containing NF-κB and AP-1 binding sites were purchased and used as the DNA probes (Promega) (NF-κB binding site [5′-AGT TGA GGG GAC TTT CCC AGG C-3′] and AP-1 binding site [5′-CGC TTG ATG AGT CAG CCG GAA-3′]). The DNA probes were radiolabeled with [γ-32P]ATP using T4 kinase (Promega). For the binding reaction, the radiolabeled NF-κB and AP-1 probes were incubated with 5 µg of nuclear extracts prepared from the treated cells. In some experiments, a competition study was done with a 100-fold molar excess of unradiolabeled wild-type or mutant NF-κB or AP-1 oligonucleotides. The competitors were added 10 min before the addition of the radiolabeled NF-κB or AP-1 probe. The binding buffer contained 10 mM Tris–HCl (pH 7.5), 50 mM NaCl, 1 mM EDTA, 1 mM DTT, 5% glycerol, and 2 µg poly(dI-dC). The reaction mixture was left at room temperature to allow binding for 20 min. The final reaction mixture was analyzed in a 6.6% nondenaturing polyacrylamide gel with 0.5 × Tris–borate–EDTA as an electrophoresis buffer.
Transient transfections and luciferase assays
We used the transfection reagent TransFast™ (Promega) to transfect plasmids into the cells instead of using electroporation. Briefly, on the day of the transfection, Jurkat T cells were adjusted to 1 × 106 mL−1 in serum-free media and evenly mixed with 2 mg of reporter plasmid (Stratagene, La Jolla, CA) and TransFast™ transfectant (6 mL) in triplicate. Forty-eight hours after transfection, the cells were equally distributed and pretreated or not with various concentrations of amiodarone and then stimulated with or without PMA (5 ng/mL) and ionomycin (1 mM) for 24 h. Subsequently, the cell pellets were collected, the total cell lysates were prepared, and the luciferase activity was determined and assayed according to the manufacturer’s instructions (Promega). Jurkat cells transfected with reporter construct alone were taken as basal activity. Transfections were performed in at least three independent experiments of triplicate determinations. Data are expressed relative to control luciferase activity, normalized to protein, and presented as means +/− SD.
Western blot (Immunoblot) analysis
ECL Western blotting (Amersham, Arlington Heights, IL) was done according to the manufacturer’s instruction and our previous work.33 Briefly, after extensive washing, the treated and untreated cells were pelleted and resuspended in lysis buffer (20 mM HEPES [pH 7.9], 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, and 3.3 µg of aprotinin per mL). After periodic vortexing for 1 h, the mixture was centrifuged at 16,000 × g for 20 min, and the supernatant was collected and the protein concentration measured with Lowry assays. Equal amounts (25 µg) of whole cellular extracts were analyzed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein was then transferred to a nitrocellulose filter. For immunoblotting, the nitrocellulose filter was incubated with TBS-T containing 5% nonfat milk (milk buffer) for 1–2 h and then blotted with antisera against p-ERK (extracellular signal-regulated kinase), p-P38, p-JNK (c-Jun NH2-terminal protein kinase) (Calbiochem, La Jolla, CA), IκBα, or beta-actin (PharMingen) overnight at 4℃. After washing with milk buffer twice for 20 min, the filter was incubated with donkey anti-mouse IgG (secondary antibody) conjugated to horseradish peroxidase at concentration of 1:5000 for 1 h. The filter was then incubated with the substrate for 1 min and exposed to an X-ray film.
Statistics
The results were expressed as means ± standard deviation (SD). A paired or unpaired Student’s t-test was used to determine the significance of differences, and a value of P less than 0.05 was considered to be statistically significant.34
Results
Cytotoxic effects of amiodarone on human T cells
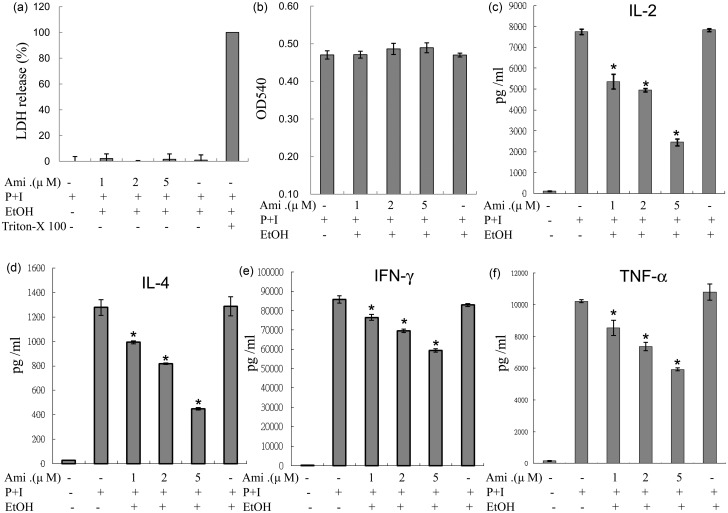
The results of LDH and MTT assays performed on human T lymphocytes indicated that the concentrations of amiodarone used in the experiments were not cytotoxic and did not affect cell viability (Figure 1(a) and (b)).
Figure 1.
Amiodarone (Ami) suppressed IL-2, IL-4, IFN-γ, and TNF-α production from activated human T cells. Human T cells at 1 × 106 mL–1 were pretreated with various concentrations of amiodarone (1–5 µM) for 2 h and then stimulated with PMA + ionomycin (P + I) for another 24 h. (a) The supernatants were collected to determine the possible cytotoxic effects of amiodarone with LDH release assays, in which cells treated with 1% Triton X-100 were the positive controls. (b) Cells were incubated with MTT and then analyzed in a microplate reader as described in “Materials and methods” section. (c–f) The supernatants were collected for cytokine measurements. The representative data of at least three to five different donor cells are shown as means±SD. An asterisk (*) denotes statistical significance (p < 0.05) compared with the cells that were stimulated only. EtOH: ethanol
Amiodarone reduced cytokine production from activated human T cells
To examine the effects of amiodarone on the production of cytokines from human peripheral T cells, human T cells were pretreated with amiodarone for 2 h, and then PMA + ionomycin was used to activate human T cells for another 24 h. We found that amiodarone inhibited the stimuli-induced production of cytokines including IL-2, IL-4, IFN-γ, and TNF-α, in human T cells in a dose-dependent manner (Figure 1(c) to (f)).
Amiodarone inhibited T cell activation
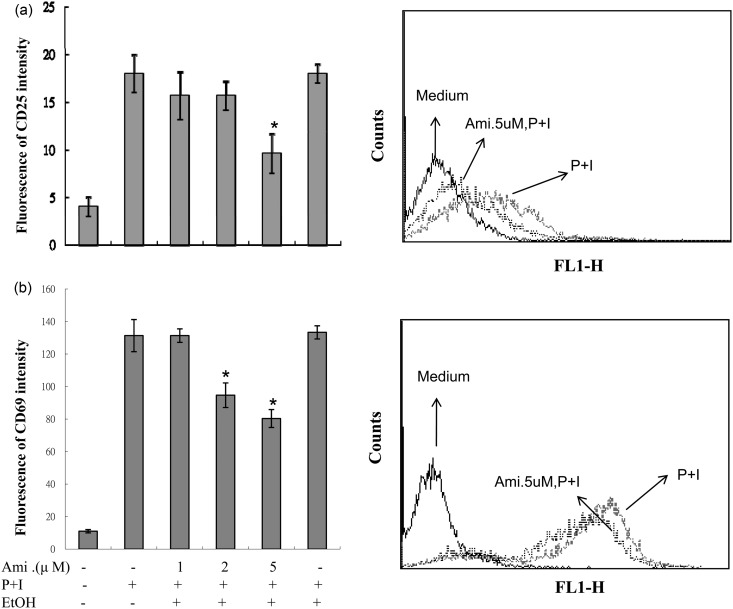
Since many T-lymphocyte cytokines were inhibited by amiodarone, we then investigated whether amiodarone affects T cell activation. As shown in Figure 2, the expressions of the cell surface markers of activated T cells, CD25 and CD69, were substantially increased after stimulation with PMA + ionomycin, and this increase was dose dependently decreased by the pretreatment of amiodarone.
Figure 2.
Amiodarone (Ami) inhibited the expressions of CD25 and CD69 in activated human T cells. Human peripheral blood T cells at 1 × 106 mL–1 were treated with 1–5 µM amiodarone for 2 h and then stimulated with PMA + ionomycin (P + I) for another 24 h. The expressions of CD25 and CD69 were measured by flow cytometry. The representative data of at least six different donor cells are shown. An asterisk (*) denotes statistical significance (p < 0:05) compared with the cells that were stimulated only
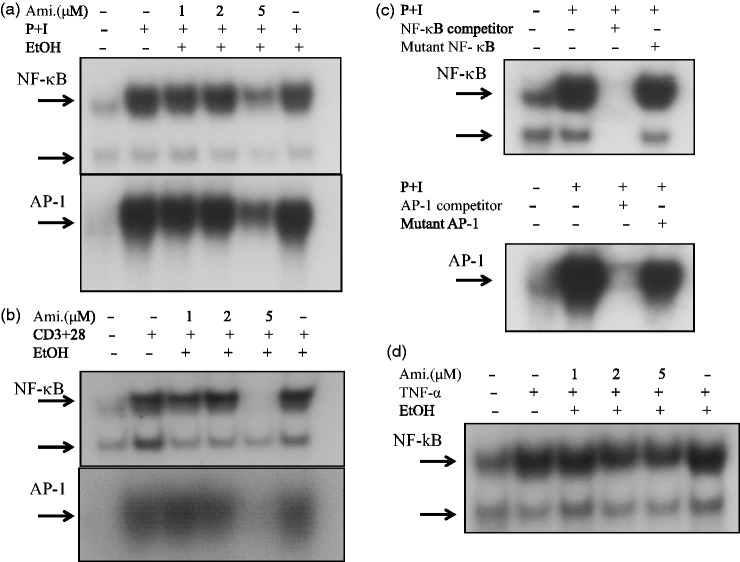
Amiodarone down-regulated AP-1 and NF-κB DNA-binding activities in activated human T cells
Due to the ability of amiodarone to inhibit cytokine production, we hypothesized that amiodarone may modulate some signaling molecules that are commonly involved in T-cell activation. Therefore, we examined the effects of amiodarone on the activation of transcription factors AP-1 and NF-κB. The results showed that amiodarone effectively inhibited AP-1 and NF-κB DNA-binding activities in human T cells activated with PMA + ionomycin (Figure 3(a)). For a more physiological approach, we chose anti-CD3 + anti-CD28 mAbs to stimulate T cells. The results revealed that amiodarone down-regulated AP-1 and NF-κB DNA-binding activities in activated T cells (Figure 3(b)). In competition assays, wild-type AP-1 and NF-κB caused complete disappearance of the band whereas the mutant type did not, indicating the specificity of AP-1 and NF-κB (Figure 3(c)). We further examined whether amiodarone could modulate the AP-1 and NF-κB DNA-binding activities induced by the proinflammatory mediator, TNF-α. As shown in Figure 3(d), amiodarone suppressed TNF-α-induced NF-κB DNA-binding activity, but not AP-1 (data not shown).
Figure 3.
Amiodarone (Ami) blocked AP-1 and/or NF-κB DNA-binding activity in human T cells. Human T cells at 2 × 107 mL−1 were pretreated with the indicated concentrations of amiodarone for 2 h and then stimulated with (a) PMA + ionomycin (P + I), or (b) anti-CD3 + anti-CD28 mAbs (CD3 + CD28) for 1 h. Nuclear extracts were then collected. AP-1 and NF-κB DNA-binding activities were determined by EMSA. The 32P-labeled oligonucleotides containing the NF-κB or AP-1 site were used as probes. The representative data of at least three independent experiments are shown. (c) The specificity of AP-1 or NF-κB binding activity was assessed by competition with the 100 × unlabeled AP-1 or NF-κB as indicated in “Materials and methods” section. Inhibition of the retarded AP-1 or NF-κB band was verified by co-incubation of nuclear extracts with 100-fold molar excess of wild-type AP-1 or NF-κB. In contrast, nuclear extracts co-incubated with mutant-type AP-1 or NF-κB did not affect the band density. (d) Similar to (a) and (b), the effect of amiodarone on NF-κB DNA-binding activity in TNF-α stimulated human T cells was examined by EMSA. The representative data of three different donor cells are shown
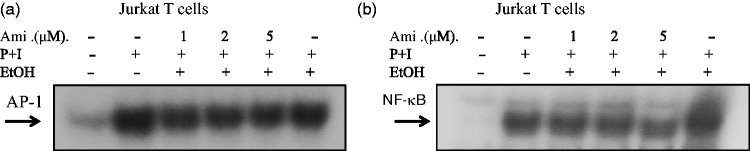
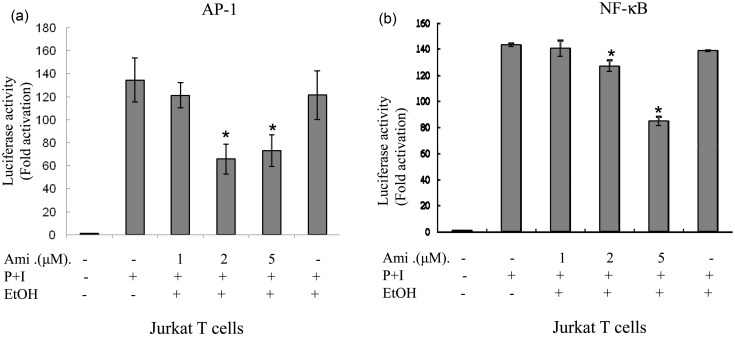
Amiodarone down-regulated AP-1 and NF-κB DNA-binding activities and transcriptional activity in Jurkat cells
To further confirm the effects of amiodarone on AP-1 and NF-κB transcriptional activities, we examined the effects on Jurkat T cells. We transiently transfected AP-1 or NF-κB luciferase reporter plasmids into Jurkat T cells. Forty-eight hours after transfection, the cells were treated with amiodarone at various concentrations and then stimulated with PMA + ionomycin to induce AP-1 and NF-κB transcriptional activity. Consistent with the human primary T-cell observations, amiodarone significantly and dose dependently inhibited both AP-1 and NF-κB DNA binding (Figure 4) and transcriptional activities (Figure 5) in Jurkat cells.
Figure 4.
Amiodarone (Ami) inhibited DNA-binding activities of both AP-1 and NF-κB in Jurkat cells. Human T cell line (Jurkat cells) at 2 × 106 mL−1 was pretreated with the indicated concentrations of amiodarone for 2 h and then stimulated with PMA + ionomycin (P + I) for 1 h. Nuclear extracts were then collected. (a) AP-1 or (b) NF-κB DNA-binding activities were determined by EMSA. The representative data of at least three independent experiments are shown
Figure 5.
Amiodarone (Ami) inhibited transcriptional activities of both AP-1 and NF-κB in Jurkat cells. Human T cell line (Jurkat cells) at 1 × 106 mL−1 was mixed together with (a) pAP-1-Luc (labeled as AP-1) or (b) pNF-κB-Luc (labeled as NF-κB) reporter plasmids and the transfection reagent TransFast™ as described in “Materials and methods” section. At 48 h after transfection, the cells were equally divided and pretreated or not with amiodarone at various dosages for 2 h. After stimulation with PMA + ionomycin for another 24 h, the cells were collected and the total cell lysates were analyzed for luciferase activity. Values are expressed as luciferase activity (fold activation) compared with unstimulated cells. The representative data of at least three independent experiments are shown as means±SD. An asterisk (*) denotes statistical significance (p < 0.05) compared with the cells that were stimulated only
Selective inhibitory effects of amiodarone on JNK and P38 MAPK in activated human T cells
Due to the inhibition of AP-1 DNA-binding activity by amiodarone, we hypothesized whether mitogen-activated protein kinases (MAPKs) would also be affected. Therefore, we evaluated the effects of amiodarone on the activation of MAPK under PMA + ionomycin stimulation. As shown in Figure 6, treatment with amiodarone inhibited the expressions of p-JNK and p-P38 (Figure 6(a) and (b)), but not p-ERK in PMA + ionomycin-activated T cells (Figure 6(c)).
Figure 6.
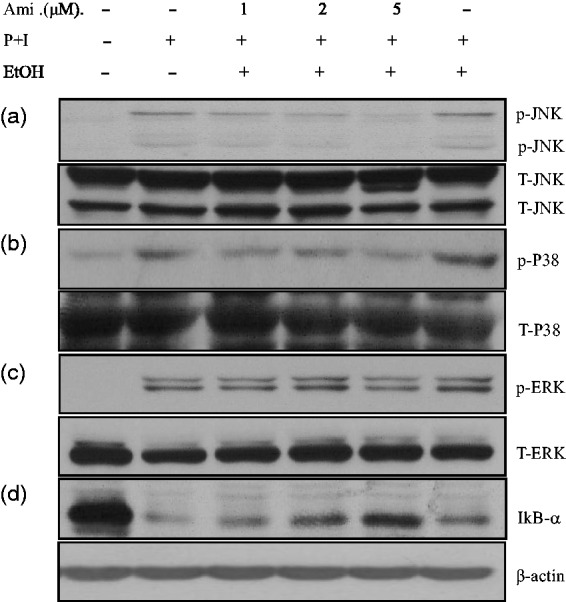
Amiodarone (Ami) inhibited JNK and P38 MAPK signaling pathways and prevented IκBα degradation. Human T cells at 2 × 107 mL−1 were pretreated with the indicated doses of amiodarone for 2 h and then stimulated with PMA + ionomycin (P + I) for another 15–20 min. After washing, the cell pellets were collected and the total cell lysates were analyzed for the protein level. Amiodarone pretreatment suppressed the activity of JNK (a) and P38 MAPK (b), but not ERK (c). Under these conditions, amiodarone had no effect on total JNK, P38, or ERK protein levels. Amiodarone prevented the degradation of IκBα (d). The representative data of four to six experiments are shown. p: phosphorylated; T: total
Amiodarone blocked IκBα degradation
The inhibition of NF-κB activity by amiodarone suggested that it may have an effect on the NF-κB-associated protein, IκBα, which retains NF-κB in an inactive status in the cytosol. We further demonstrated that amiodarone significantly suppressed IκBα degradation in PMA + ionomycin-activated T cells (Figure 6(d))
Discussion
Clinically, amiodarone is prescribed for the treatment of Af. One of its therapeutic roles is an anti-inflammatory effect via reducing the activation and movement of neutrophils.35 Previous studies have suggested that amiodarone alters T cell subsets and regulates T cell functions, which may lead to target organ toxicity such as autoimmune thyroid disease and hypersensitivity pneumonitis in susceptible patients.36,37 In the current study, we showed that one of the beneficial effects of amiodarone is to reduce the activation of stimulated human primary T cells, which may have additional inhibitory effects on immune-related atherogenesis. Further, amiodarone may be able to treat Af in acute coronary syndrome or other clinical conditions related to T cell activation.
The current understanding of the complex mechanisms of Af was summarized in a recent consensus statement published by the Heart Rhythm Society.38 Although enhanced automaticity, triggered activity, or reentry is thought to induce cardiac arrhythmias, multiple factors from anatomic changes to substrates at the cellular and electrophysiological level also play crucial roles. Among these, inflammation has been highlighted as a risk factor for Af.17,39 As reported in the pathogenesis of postoperative Af, some degree of inflammation and/or oxidative stress may be important.40 During open heart surgery, the occurrence of Af is related to activation of the complement system, release of proinflammatory cytokines, and increased C-reactive protein levels. Therefore, an inflammatory mechanism in patients with Af has been postulated in light of the high incidence (25–40%) of Af after cardiac surgery.41,42 Furthermore, there is increasing evidence linking inflammation and Af,43 and this has also been confirmed in several in vivo experiments.44–48 These results suggest that medications inhibiting inflammatory pathways could possibly prevent or treat Af, which further indicates the vital role that inflammation plays in the pathogenesis of Af.
In patients with persistent Af, histopathological evidence points to inflammatory infiltrates as well as increased tissue factor expressions in left atrial appendages.49 Frustaci et al. reported endomyocardial biopsies of 12 patients with refractory lone Af and found that eight had evidence of inflammation, including lymphomononuclear infiltration and necrosis of the adjacent myocytes in the myocardium.43 Cytokines are known to be the major mediators in this process. Sata et al. demonstrated that inflammatory markers, including IL-6, TNF-α, and C-reactive protein are elevated in the plasma of patients with paroxysmal Af.50 In addition, IL-2, secreted by T-helper type 1 lymphocytes, has been reported to be correlated with recent onset Af.51 IL-2 can also induce the production of IL-6 and activate other cells such as macrophages and vascular cells in the vessels to trigger cytotoxic molecules.52 Other proinflammatory mediators such as IFN-γ and TNF-α may also trigger detrimental biochemical reactions in chronic cardiovascular events and then induce production of oxygen and nitrogen radicals. Furthermore, IL-4 has been reported to enhance oxidative effects with inflammatory properties.53,54 Therefore, proinflammatory cytokines can be regarded as promoting factors in the progression of Af. Our results demonstrated that amiodarone, an antiarrhythmic agent, could inhibit these inflammatory cytokines and therefore possibly halt the inflammatory pathway of Af.
AP-1 and/or NF-κB could be induced to regulate the gene expression of many cytokines during the inflammatory process.55 This suggests that the suppressors of cytokine-related signaling pathways may reduce cytokine-induced chronic inflammation. Thus, drug therapy targeted toward minimizing inflammation may be effective in the prevention of Af and its complications.56 Although several mechanisms are related to the up- and down-regulation of AP-1 activity, MAPKs including ERKs, JNKs, and P38 kinases, are known to be common signaling pathways mediating AP-1 activity.
The activation of NF-κB is considered to be part of a stress response as it is activated by a variety of stimuli. In its inactive form, NF-κB is sequestered in the cytoplasm, bound by members of the IkB family of inhibitor proteins. After cell activation, the IκB proteins are rapidly phosphorylated due to the binding of certain cytokines to their surface receptors. Previous reports have suggested that NF-κB can also be activated in the signaling pathways involved in MAPKs.57,58 Our results demonstrated that amiodarone could suppress AP-1 and NF-κB DNA-binding activity in activated human T cells. Furthermore, we found that amiodarone down-regulated AP-1 and NF-κB transcriptional activity in Jurkat T cells. These results emphasize the anti-inflammatory effects of amiodarone and provide detailed mechanisms of how amiodarone suppresses inflammatory cytokine production.
The limitation of this study is that we only showed ex vivo effects of amiodarone on activated human T cells, and thus making clinical and specific conclusions is beyond the scope of this study. Additionally, the actual role of T cells in atrial arrhythmia during acute coronary events should be further clarified. Nevertheless, our results demonstrate that amiodarone can regulate the processes associated with AP-1 and NF-κB activity in activated human T lymphocytes. In particular, our findings showed that amiodarone exerts these immunomodulatory effects in a therapeutic concentration, which implies that the anti-inflammatory properties of amiodarone will affect T cells in patients with Af.59,60 To clarify these effects, future studies are needed with the inclusion of parallel analysis of T cells from patients who exhibit Af. Moreover, gene expression array analysis in amiodarone-treated human primary T cells as well as Jurkat cells will provide a more complete picture of the anti-inflammatory effects of amiodarone on T cells. This study provides novel concepts regarding the clinical application of amiodarone.
Conclusions
In conclusion, Af is increasingly recognized as a multifactorial disorder, and systemic inflammation may play a significant role in Af. This is the first study to provide data on the potential anti-inflammatory effects of amiodarone on activated primary human T cells. We suggest that amiodarone exerts pleiotropic effects to modulate the function of human T cells and reduce the inflammatory processes of Af. Further in vivo and human clinical studies are needed to elucidate the effects of amiodarone on Af and inflammation-related cardiovascular disorders.
ACKNOWLEDGEMENTS
The authors are grateful for the kind gift (amiodarone) from Sanofi-Synthelabo Pharmaceutical and also gratefully acknowledge Hui-Tzu Tan for his technique assistance. This work was supported by Tri-Service General Hospital grants (TSGH-C98-4-S03 and TSGH-C96-020).
Author contributions
Conceived and designed the experiments: SMC, WHL, CHW, SPY. Performed the experiments: SMC, CSL, LJH, TNT. Analyzed the data: SMC, LJH, JHL, SPY. Wrote the manuscript: SMC, CSL, SPY.
REFERENCES
- 1.Curtis AB. Practice implications of the atrial fibrillation guidelines. Am J Cardiol 2013; 111: 1660–70. [DOI] [PubMed] [Google Scholar]
- 2.Friberg J, Buch P, Scharling H, Gadsbphioll N, Jensen GB. Rising rates of hospital admissions for atrial fibrillation. Epidemiology 2003; 14: 666–672. [DOI] [PubMed] [Google Scholar]
- 3.Magnani JW, Rienstra M, Lin H, Sinner MF, Lubitz SA, McManus DD, Dupuis J, Ellinor PT, Benjamin EJ. Atrial fibrillation: Current knowledge and future directions in epidemiology and genomics. Circulation 2011; 124: 1982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaikh AY, Maan A, Heist EK, Tighe DA, Aurigemma GP, McManus DD. Management strategies in atrial fibrillation in patients with heart failure. Cardiol Rev 2012; 20: 288–96. [DOI] [PubMed] [Google Scholar]
- 5.McManus DD, Shaikh AY, Abhishek F, Vasan RS. Atrial fibrillation and heart failure parallels: Lessons for atrial fibrillation prevention. Crit Pathw Cardiol 2011; 10: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevenson WG, Stevenson LW, Middlekauff HR, Fonarow GC, Hamilton MA, Woo MA, Saxon LA, Natterson PD, Steimle A, Walden JA, Tillisch JH. Improving survival for patients with atrial fibrillation and advanced heart failure. J Am Coll Cardiol 1996; 28: 1458–63. [DOI] [PubMed] [Google Scholar]
- 7.Chinitz JS, Vaishnava P, Narayan RL, Fuster V. Atrial fibrillation through the years: Contemporary evaluation and management. Circulation 2013; 127: 408–16. [DOI] [PubMed] [Google Scholar]
- 8.Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, Solomon AJ. Acute myocardial infarction complicated by atrial fibrillation in the elderly: Prevalence and outcomes. Circulation 2000; 101: 969–74. [DOI] [PubMed] [Google Scholar]
- 9.Al-Khatib SM, Pieper KS, Lee KL, Mahaffey KW, Hochman JS, Pepine CJ, Nykanen DG, McCrindle BW, Freedom RM, Benson LN. Atrial fibrillation and mortality among patients with acute coronary syndromes without ST-segment elevation: Results from the PURSUIT trial. Am J Cardiol 2001; 88: 76–79. [DOI] [PubMed] [Google Scholar]
- 10.El-Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. New-onset atrial fibrillation predicts long-term mortality after coronary artery bypass graft. J Am Coll Cardiol 2010; 55: 1370–6. [DOI] [PubMed] [Google Scholar]
- 11.Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: A systematic review and meta-analysis. Circulation 2011; 123: 1587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corradi D, Callegari S, Maestri R, Benussi S, Alfieri O. Structural remodeling in atrial fibrillation. Nat Clin Pract Cardiovasc Med 2008; 5: 782–96. [DOI] [PubMed] [Google Scholar]
- 13.Sinno H, Derakhchan K, Libersan D, Merhi Y, Leung TK, Nattel S. Atrial ischemia promotes atrial fibrillation in dogs. Circulation 2003; 107: 1930–6. [DOI] [PubMed] [Google Scholar]
- 14.Hammwohner M, Ittenson A, Dierkes J, Bukowska A, Klein HU, Lendeckel U, Goette A. Platelet expression of CD40/CD40 ligand and its relation to inflammatory markers and adhesion molecules in patients with atrial fibrillation. Exp Biol Med (Maywood) 2007; 232: 581–9. [PubMed] [Google Scholar]
- 15.Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J 2006; 27: 136–49. [DOI] [PubMed] [Google Scholar]
- 16.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, Wildevuur CR, Eijsman L, Trouwborst A, Hack CE. Activation of the complement system during and after cardiopulmonary bypass surgery: Postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation 1997; 96: 3542–8. [DOI] [PubMed] [Google Scholar]
- 17.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation 2003; 108: 3006–10. [DOI] [PubMed] [Google Scholar]
- 18.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013; 339: 166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gotsman I, Sharpe AH, Lichtman AH. T-cell costimulation and coinhibition in atherosclerosis. Circ Res 2008; 103: 1220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012; 32: 2045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev 2007:CD005049. [DOI] [PubMed]
- 22.Singh BN. Amiodarone as paradigm for developing new drugs for atrial fibrillation. J Cardiovasc Pharmacol 2008; 52: 300–305. [DOI] [PubMed] [Google Scholar]
- 23.Greene HL. The CASCADE Study: randomized antiarrhythmic drug therapy in survivors of cardiac arrest in Seattle. CASCADE Investigators. Am J Cardiol 1993; 72: 70F–74F. [DOI] [PubMed] [Google Scholar]
- 24.Singh SN, Fletcher RD, Fisher SG, Singh BN, Lewis HD, Deedwania PC, Massie BM, Colling C, Lazzeri D. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med 1995; 333: 77–82. [DOI] [PubMed] [Google Scholar]
- 25.Cairns JA, Connolly SJ, Roberts R, Gent M. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet 1997; 349: 675–82. [DOI] [PubMed] [Google Scholar]
- 26.Wilson BD, Clarkson CE, Lippmann ML. Amiodarone causes decreased cell-mediated immune responses and inhibits the phospholipase C signaling pathway. Lung 1993; 171: 137–48. [DOI] [PubMed] [Google Scholar]
- 27.Aussel C, Pelassy C. Effects of antiarrhythmic drugs on phospholipid metabolism in Jurkat T cells. The potassium channel blocker, clofilium, specifically increases phosphatidylserine synthesis. FEBS Lett 1992; 304: 281–4. [DOI] [PubMed] [Google Scholar]
- 28.Perico ME, Crivellente F, Faustinelli I, Suozzi A, Cristofori P. Flow cytometry, with double staining with Nile red and anti-CD3 antibody, to detect phospholipidosis in peripheral blood lymphocytes of rats treated with amiodarone. Cell Biol Toxicol 2009; 25: 587–98. [DOI] [PubMed] [Google Scholar]
- 29.Matsumori A, Ono K, Nishio R, Nose Y, Sasayama S. Amiodarone inhibits production of tumor necrosis factor-alpha by human mononuclear cells: A possible mechanism for its effect in heart failure. Circulation 1997; 96: 1386–9. [DOI] [PubMed] [Google Scholar]
- 30.Cheng SM, Chu KM, Lai JH. The modulatory mechanisms of fenofibrate on human primary T cells. Eur J Pharm Sci 2010; 40: 316–24. [DOI] [PubMed] [Google Scholar]
- 31.Hung LF, Huang KY, Yang DH, Chang DM, Lai JH, Ho LJ. Advanced glycation end products induce T cell apoptosis: Involvement of oxidative stress, caspase and the mitochondrial pathway. Mech Ageing Dev 2010; 131: 682–91. [DOI] [PubMed] [Google Scholar]
- 32.Lai JH, Ho LJ, Kwan CY, Chang DM, Lee TC. Plant alkaloid tetrandrine and its analog block CD28-costimulated activities of human peripheral blood T cells: potential immunosuppressants in transplantation immunology. Transplantation 1999; 68: 1383–92. [DOI] [PubMed] [Google Scholar]
- 33.Cheng SM, Lai JH, Yang SP, Tsao TP, Ho LJ, Liou JT, Cheng CC. Modulation of human T cells signaling transduction by lovastatin. Int J Cardiol 2010; 140: 24–33. [DOI] [PubMed] [Google Scholar]
- 34.Ho LJ, Juan TY, Chao P, Wu WL, Chang DM, Chang SY, Lai JH. Plant alkaloid tetrandrine downregulates IkappaBalpha kinases-IkappaBalpha-NF-kappaB signaling pathway in human peripheral blood T cell. Br J Pharmacol 2004; 143: 919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozbakis-Dengiz G, Halici Z, Akpinar E, Cadirci E, Bilici D, Gursan N. Role of polymorphonuclear leukocyte infiltration in the mechanism of anti-inflammatory effect of amiodarone. Pharmacol Rep 2007; 59: 538–44. [PubMed] [Google Scholar]
- 36.Rabinowe SL, Larsen PR, Antman EM, George KL, Friedman PL, Jackson RA, Eisenbarth GS. Amiodarone therapy and autoimmune thyroid disease. Increase in a new monoclonal antibody-defined T cell subset. Am J Med 1986; 81: 53–57. [DOI] [PubMed] [Google Scholar]
- 37.Akoun GM, Gauthier-Rahman S, Milleron BJ, Perrot JY, Mayaud CM. Amiodarone-induced hypersensitivity pneumonitis. Evidence of an immunological cell-mediated mechanism. Chest 1984; 85: 133–5. [DOI] [PubMed] [Google Scholar]
- 38.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2007; 4: 816–61. [DOI] [PubMed] [Google Scholar]
- 39.Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. Serum long-chain n-3 polyunsaturated fatty acids and risk of hospital diagnosis of atrial fibrillation in men. Circulation 2009; 120: 2315–21. [DOI] [PubMed] [Google Scholar]
- 40.Elahi MM, Flatman S, Matata BM. Tracing the origins of postoperative atrial fibrillation: The concept of oxidative stress-mediated myocardial injury phenomenon. Eur J Cardiovasc Prev Rehabil 2008; 15: 735–41. [DOI] [PubMed] [Google Scholar]
- 41.Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004; 291: 1720–29. [DOI] [PubMed] [Google Scholar]
- 42.Halonen J, Halonen P, Jarvinen O, Taskinen P, Auvinen T, Tarkka M, Hippelainen M, Juvonen T, Hartikainen J, Hakala T. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA 2007; 297: 1562–7. [DOI] [PubMed] [Google Scholar]
- 43.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997; 96: 1180–4. [DOI] [PubMed] [Google Scholar]
- 44.Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res 2004; 62: 105–11. [DOI] [PubMed] [Google Scholar]
- 45.Shiroshita-Takeshita A, Schram G, Lavoie J, Nattel S. Effect of simvastatin and antioxidant vitamins on atrial fibrillation promotion by atrial-tachycardia remodeling in dogs. Circulation 2004; 110: 2313–9. [DOI] [PubMed] [Google Scholar]
- 46.Shiroshita-Takeshita A, Brundel BJ, Lavoie J, Nattel S. Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc Res 2006; 69: 865–75. [DOI] [PubMed] [Google Scholar]
- 47.Dernellis J, Panaretou M. Effect of C-reactive protein reduction on paroxysmal atrial fibrillation. Am Heart J 2005; 150: 1064–1064. [DOI] [PubMed] [Google Scholar]
- 48.Dernellis J, Panaretou M. Relationship between C-reactive protein concentrations during glucocorticoid therapy and recurrent atrial fibrillation. Eur Heart J 2004; 25: 1100–7. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura Y, Nakamura K, Fukushima-Kusano K, Ohta K, Matsubara H, Hamuro T, Yutani C, Ohe T. Tissue factor expression in atrial endothelia associated with nonvalvular atrial fibrillation: Possible involvement in intracardiac thrombogenesis. Thromb Res 2003; 111: 137–42. [DOI] [PubMed] [Google Scholar]
- 50.Sata N, Hamada N, Horinouchi T, Amitani S, Yamashita T, Moriyama Y, Miyahara K. C-reactive protein and atrial fibrillation. Is inflammation a consequence or a cause of atrial fibrillation? Jpn Heart J 2004; 45: 441–5. [DOI] [PubMed] [Google Scholar]
- 51.Rizos I, Tsiodras S, Rigopoulos AG, Dragomanovits S, Kalogeropoulos AS, Papathanasiou S, Sakadakis EA, Kremastinos DT. Interleukin-2 serum levels variations in recent onset atrial fibrillation are related with cardioversion outcome. Cytokine 2007; 40: 157–64. [DOI] [PubMed] [Google Scholar]
- 52.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol 2011; 12: 204–12. [DOI] [PubMed] [Google Scholar]
- 53.Leskinen MJ, Kovanen PT, Lindstedt KA. Regulation of smooth muscle cell growth, function and death in vitro by activated mast cells—a potential mechanism for the weakening and rupture of atherosclerotic plaques. Biochem Pharmacol 2003; 66: 1493–8. [DOI] [PubMed] [Google Scholar]
- 54.Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J Clin Invest 2004; 114: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 1997; 9: 240–6. [DOI] [PubMed] [Google Scholar]
- 56.Bachmann JM, Majmudar M, Tompkins C, Blumenthal RS, Marine JE. Lipid-altering therapy and atrial fibrillation. Cardiol Rev 2008; 16: 197–204. [DOI] [PubMed] [Google Scholar]
- 57.Bode AM, Dong Z. Signal transduction pathways: targets for chemoprevention of skin cancer. Lancet Oncol 2000; 1: 181–8. [DOI] [PubMed] [Google Scholar]
- 58.Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L, Liao W, Chen Z, Liu Z, Su B. The essential role of MEKK3 in TNF-induced NF-kappaB activation. Nat Immunol 2001; 2: 620–4. [DOI] [PubMed] [Google Scholar]
- 59.Naccarelli GV, Rinkenberger RL, Dougherty AH, Giebel RA. Amiodarone: Pharmacology and antiarrhythmic and adverse effects. Pharmacotherapy 1985; 5: 298–313. [DOI] [PubMed] [Google Scholar]
- 60.Rotmensch HH, Belhassen B, Swanson BN, Shoshani D, Spielman SR, Greenspon AJ, Greenspan AM, Vlasses PH, Horowitz LN. Steady-state serum amiodarone concentrations: relationships with antiarrhythmic efficacy and toxicity. Ann Intern Med 1984; 101: 462–9. [DOI] [PubMed] [Google Scholar]