Abstract
Oxidative stress and inflammation are implicated in tissue remodeling, hypertrophy, and organ malfunction. Since heme-oxygenase (HO) is a cytoprotective enzyme with effects against oxidative stress and inflammation, we investigated the effects of upregulating HO with hemin on adipocyte hypertrophy, proteins of repair/regeneration including beta-catenin, Oct3/4 and Pax2 as well as pro-fibrotic/remodeling proteins like osteopontin and transforming growth factor-beta (TGF-β) in pericardial adipose tissue from obese Zucker rats (ZRs). Treatment with hemin significantly reduced pericardial adipose tissue inflammation/oxidative stress, suppressed osteopontin and TGF-β, and attenuated pericardial adipocyte hypertrophy in obese ZRs. These were associated with enhanced expression of the stem/progenitor-cell marker cKit; the potentiation of several proteins of regeneration including beta-catenin, Oct3/4, Pax2; and improved pericardial adipocyte morphology. Interestingly, the amelioration of adipocyte hypertrophy in hemin-treated animals was accompanied by improved adipocyte function, evidenced by increased levels of pericardial adipose tissue adiponectin. Furthermore, hemin significantly reduced hypertriglyceridemia and hypercholesteromia in obese ZRs. The protective effects of hemin were accompanied by robust potentiation HO activity and the total antioxidant capacity, whereas the co-administration of hemin with the HO inhibitor, stannous mesoporphyrin abolished the effects of hemin. These data suggest that hemin improves pericardial adipocyte morphology and function by enhancing proteins of repair and regeneration, while concomitantly abating inflammatory/oxidative insults and suppressing extracellular-matrix/profibrotic and remodeling proteins. The reduction of hypertriglyceridemia, hypercholesteromia, pericardial adiposity, and pericardial adipocyte hypertrophy with corresponding improvement of adipocyte morphology/function in hemin-treated animals suggests that HO inducers may be explored for the design of novel remedies against cardiac complications arising from excessive adiposity.
Keywords: Inflammation, heme oxygenase, adipocytes, obesity, adiponectin, oxidative stress
Introduction
The adipose tissue is an important metabolic organ that produces a wide range of substances with both physiological and pathophysiological functions. For example, the adipose tissue produces adiponectin, a substance with insulin-sensitizing and anti-inflammatory effects.1,2 On the other hand, the adipose tissue produces a variety of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IL-1β.2 In many chronic diseases including obesity and insulin resistance, adipocyte function may be impaired due to hypertrophy3 and/or excessive production of IL-6 and TNF-α.4 Adipocyte hypertrophy may lead to reduced production of adiponectin, exacerbation of inflammatory insults, and impairment of glucose metabolism.1,5,6 These pathophysiological changes in adipocytes contribute to insulin resistance and related cardiometabolic complications in many obese subjects. Moreover, in obesity, elevated oxidative stress, increased inflammatory insults, insulin resistance, dysfunctional lipid metabolism, and impaired glucose metabolism are among the pathological characteristics implicated in the conundrum of cardiometabolic complication. These pathophysiological conditions are aggravated by excessive pericardial adiposity. For example, visceral adiposity like pericardial fat is correlated to insulin resistance and cardiac diseases,7 although the specific role of pericardial adiposity above and beyond that of visceral adiposity remains largely unclear.7
Pericardial adiposity, in comparison to central obesity is a greater risk factor for cardiac complications.8 By virtue of its anatomical and functional proximity to the coronary circulation, pericardial adipose tissue may be even more malignant than central adiposity. Pericardial adiposity can lead to myocardial inflammation, left ventricular hypertrophy, and coronary artery disease through paracrine mechanisms that include increased production of inflammatory cytokines, reactive oxygen species, and other atherogenic factors.1,9 Thus, novel strategies capable of reducing pericardial adiposity, inflammation, and oxidative stress may be useful to improve the cardiometabolic profile of obese subjects.
The cytoprotective role of the heme-oxygenase (HO) system has been widely acknowledged.10–17 HO is a protective enzyme with inducible (HO-1) and constitute (HO-2 and HO-3) isoforms that degrades the pro-oxidant, heme, to generate carbon monoxide, an anti-inflammatory/vasodilatory gas, bilirubin/biliverdin with antioxidant properties, and iron that is rapidly converted to ferritin, an antioxidant.18,19 HO activity is largely derived from HO-1 and HO-2 isoforms because HO-3 is a pseudo-transcript of HO-2.19 Generally, HO-2 contributes to basal physiological functions while HO-1 serves as a sensitive index that is stimulated by a wide variety of different physical, chemical, and pathophysiological stimuli.19 However, the pathophysiological activation of HO-1 results only to a transient or marginal increase in HO activity that is below the threshold necessary to trigger the downstream signaling components through which the HO system elicits its effects, so more robust enhancement of HO-1 by HO inducers is needed to surmount the threshold.20,21 Therefore, in the present study, the HO inducer, hemin was used to enhance the HO system in obese Zucker rats and the morphology and function of pericardial adipose tissue examined since pericardial adiposity plays a major pathophysiological role in heart disease and related complications.1,8,9 Since osteopontin is known to promote inflammation, extracellular matrix invasion, and hypertrophy,22–31 the effects of an upregulated HO system on the expression of osteopontin in pericardial adipocyte were assessed. Furthermore, we also investigated whether treatment with hemin improves pericardial adipocyte morphology and function by abating hypertrophy, oxidative stress, and inflammation, while concomitantly enhancing proteins of regeneration such as beta-catenin, Oct3/4, and pax232–34 in the adipose tissue. Moreover, the effects of the HO system on the expression of Oct3/4 and pax2 and osteopontin in pericardial adipose tissue have not been reported. Therefore, the finding reported in the present manuscript offers novel insights on the role of hemin on adipose tissue repair.
Materials and methods
Animals and treatment groups and biochemical assays
The experimental protocol for this study is in conformity with the Guide for Care and Use of Laboratory Animals stipulated by the Canadian Council on Animal Care and the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by University of Saskatchewan Animal Ethics Committee. Male Zucker fatty (ZF) rats of age 12 weeks and their corresponding sex/age-matched Zucker lean (ZL) rats were obtained from Charles River Laboratories (Willington, MA, USA). The animals were housed at 21℃ with 12-h light/dark cycles, had access to drinking water ad libitum, and were fed with standard laboratory chow.
Hemin (30 mg/kg i.p., Sigma, St Louis, MO) was administered to induce HO, while stannous mesoporphyrin ([SnMP] 20 mg/kg i.p., Porphyrin Products [Logan, UT, USA]) was given to inhibit HO. Hemin and SnMP were prepared as we previously reported and administered biweekly for 8 weeks.10,35,36 At 16 weeks of age, the animals were randomly divided into several experimental groups (n = 6 per group) including: (a) controls (ZF and ZL), (c) hemin-treated ZF and ZL, and (c) ZF + hemin + SnMP.
During the treatment period body weight and fasting glucose were measured weekly. Body weight was measured using a digital balance (Model Mettler PE1600, Mettler Instruments Corporation, Greifensee, Zurich, Switzerland), while fasting glucose was measured using a diagnostic auto-analyzer (BD, Franklin Lakes, NJ) after 6 h of fasting as previously reported.35,37 At the end of the 8-week treatment period, the animals were 24 weeks old, and the study was terminated. Prior to killing, the animals were weighed, anesthetized with pentobarbital sodium (50 mg/kg i.p.), and the pericardial fat pad isolated and weighed with an analytical balance (Precisa Instruments Ltd, Dietikon, Switzerland) as previously reported.38
HO activity in the pericardial adipose tissue was determined spectrophotometrically as we previously reported,10,38,39 while enzyme-linked immunosorbent assay (ELISA) kits were used for measuring HO-1 (Stressgen-Assay Design, Ann Arbor, MI, USA), adiponectin (Phenix Pharmaceuticals, Inc., Burlingame, CA, USA), TNF-α, IL-6, and IL-1β (Immuno-Biological Laboratories Co. Ltd, Gunma, Japan), and enzyme immuno-assay (EIA) kits for 8-isoprostane and total antioxidant capacity (Cayman Chemical, Ann Arbor, MI, USA).10,38,39 Similarly, cholesterol and triglyceride were determined using fluorimetric assay kits for cholesterol and triglyceride (Cayman Chemical, Ann Arbor, MI, USA) as we previously reported.37,40
Histological, morphological, and immunohistochemical analyses of pericardial adipocytes
Pericardial adipose tissue was fixed in 10% formalin phosphate buffer for 48 h, processed and paraffin embedded as we previously reported,2,13 and sections of 5 µm thicknesses were cut and stained with hematoxylin and eosin for histological and morphological analyses. The pericardial adipose tissue was collected from same area of the heart from all animals in the different experimental groups. Adipocytes were scanned using a microscope (Aperio Scan Scope Model CS, Aperio Technology Inc, CA, USA) and analyzed with Aperio Image Scope V11.2.0.780 software (Aperio, e-Pathology Solution, CA, USA).
At 100 × optical zoom, several areas of groups of pericardial adipocyte were outlined (approximately 5–15 adipocytes per area) using image analysis tools from Aperio Image Scope (Aperio, e-Pathology Solution, CA, USA), and the total number of pericardial adipocytes per group outlined was divided by the total area. By so doing, the areas of adipocytes from the pericardial fat pad were calculated. A total of five areas were randomly outlined per slide and the average of adipocytes from these areas recorded as we previously reported.2
Immunohistochemistry was done as we previously reported.39,41 Sections of 5 µm of pericardial adipose tissue were treated with bovine serum albumin in phosphate buffered saline to block nonspecific staining. After blocking, the sections were incubated overnight with ED1 (1:500 dilution, sc-59103, Santa Cruz Biotechnology, CA, USA). Thereafter, the pericardial adipose tissue sections were incubated with goat anti-mouse IgG for 30 min (1:200 dilution; Jackson ImmunoResearch Laboratories, Inc., ME, USA). Subsequently, immunohistochemical staining was done using standard avidin–biotin complex method with the chromagen 3,3'-diaminobenzidine used at the final detection step.
The pericardial adipose tissue sections were scanned using a microscope (Aperio Scan Scope Model CS, Aperio Technology Inc., CA) and macrophages (brown from immune-stained sections) were quantified by manually counting the positively stained cells in several randomized nonoverlapping fields as we previously reported.39,41
Western immunoblotting
Pericardial fat was homogenized as previously reported.10,35,36,38,42 Primary antibodies such as antibeta catenin antibody ([E247] ab32572, Abcam Inc., Cambridge, MA, USA) and ([Santa Cruz Biotechnology, Santa Cruz, CA, USA], ED1 [CD68] [sc-59103], ED2 [CD163] [sc-58956], CD-206 [sc-48758], osteopontin [sc-21742], Oct-3/4 [sc-5279], c-Kit [sc-365504], transforming growth factor [TGF]-β1/2/3 [sc7892]) and nitrotyrosine (sc-32757) were used. Densitometric analysis was done with UN-SCAN-IT software (Silk Scientific Inc., Orem, UT, USA). G6PDH antibody (A9521, Sigma St Louis, MO, USA) was used as a control to ascertain equivalent loading.
Statistical analysis
All data are expressed as means ± standard error of mean from at least four independent experiments unless otherwise stated. Statistical analyses were done using two-way analysis of variance (ANOVA), using Statistical Analysis System (SAS), software Version 9.3 (SAS Institute Inc., Cary, NC, USA) and Student’s t-test. Group differences of p < 0.05 were considered statistically significant.
Results
Treatment with hemin upregulates pericardial HO-1 and HO activity but reduced pericardial adiposity in ZF
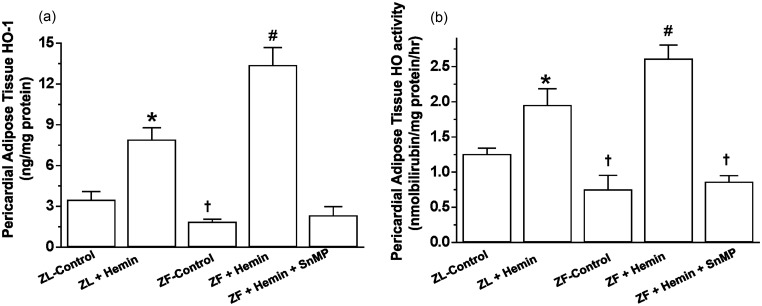
The administration of hemin to ZF significantly enhanced the depressed basal HO-1 and HO-activity in pericardial adipose tissue (Figure 1(a) and (b)), while treatment with the HO inhibitor, SnMP reversed the effects of hemin. The potentiation of HO-1 and HO activity in ZF was accompanied by significant reduction of pericardial adiposity (2.9 ± 0.2 versus 1.8 ± 0.4 g/kg body weight, p < 0.05), whereas the co-administering the HO inducer, hemin and the HO blocker, SnMP abolished the effect of hemin (Table 1). Hemin also enhanced HO-1 and HO activity and reduced pericardial adiposity in ZL-control rats, although the effects of hemin on these parameters were more accentuated in ZF.
Figure 1.
Effects of the HO inducer, hemin, and the HO inhibitor, SnMP on HO-1 and HO activity in the pericardial adipose tissue obtained from ZLs and ZFs. (a) Hemin enhanced HO-1, whereas SnMP annulled the effects of hemin. (b) Hemin increased HO activity, while SnMP nullified the hemin effect. Bars represent means ± SEM; n = 6 rats per group (*p < 0.01 versus ZL control; †p < 0.01 versus ZL control; #p < 0.01 versus ZF + Hemin + SnMP or ZF control)
Table 1.
Effect of hemin and stannous mesoporphyrin (SnMP) on physiological variables in Zucker fatty (ZF) and Zucker lean (ZL) rats
| Animal groups |
|||||
|---|---|---|---|---|---|
| Parameters | ZL Control | ZL + Hemin | ZF Control | ZF + Hemin | ZF + Hemin + SnMP |
| Body weight (g) | 467.3. ± 5.9 | 440.9 ± 7.3@ | 735.4 ± 15.2* | 678.5 ± 12.6@ | 685.8 ± 9.3# |
| Fasting glucose (mmol/L) | 7.7 ± 0.3 | 6.8 ± 0.2@ | 8.8 ± 0.4* | 7.1 ± 0.5@ | 9.1 ± 0.4* |
| Pericardial adiposity (g/kg body weight) | 1.7 ± 0.3 | 1.2 ± 0.1@ | 2.9 ± 0.2* | 1.8 ± 0.4@ | 3.0 ± 0.8* |
| Total Cholesterol (mmol/L) | 1.9 ± 0.4 | 1.6 ± 0.2 | 8.6 ± 0.7* | 3.2 ± 0.4@@ | 9.1 ± 1.3** |
| Triglycerides (mmol/L) | 0.9 ± 0.08 | 0.7 ± 0.05 | 5.7 ± 0.8* | 2.8 ± 0.2@@ | 5.9 ± 0.7** |
@p < 0.05, @@p < 0.01 versus ZL control or ZF control; *p < 0.05, **p < 0.01 versus ZL control or ZF + Hemin; #p < 0.05 versus ZF control, n = 6 per group.
Since hypertriglyceridemia and hypercholesteromia are among the pathophysiological driving force in many cardiometabolic diseases43 including heart failure,44–46 we determined the effect of hemin total cholesterol and triglycerides. In ZF-control the levels of total cholesterol and triglycerides were significantly elevated as compared to ZL control (Table 1). However, treatment with hemin significantly abated hypertriglyceridemia and hypercholesteromia in ZF. On the other hand, the co-administration of the HO inducer, hemin with the HO inhibitor, SnMP resulted in the annulment of the hemin-lowering effects of hypertriglyceridemia and hypercholesteromia (Table 1), suggesting a role of the HO system in the modulation of hypertriglyceridemia and hypercholesteromia. Hemin also reduced hypertriglyceridemia and hypercholesteromia in ZL although the hemin effect was greater in ZF (Table 1).
Our results also indicate that hemin and SnMP caused a slight loss of body weight of 5.6 and 7.7% in ZL control and ZF control, respectively (Table 1). The slight loss of weight may not be due to toxicity because our recent data showed that several indices of toxicity including plasma gamma-glutamyltransferase, aspartate aminotransferase, and alanine aminotransferase were within normal range.10 Our results also indicate that fasting glucose levels in ZFs were within normal range (Table 1). However, treatment with hemin caused a slight but significant reduction of glycemia, while the co-administration of hemin and SnMP annulled the effect (Table 1). Similarly, hemin administration also reduced fasting blood glucose in ZL controls. The vehicle dissolving hemin and SnMP did not affect any of the measured parameters.
Hemin administration enhanced the antioxidant status in ZF and reduced oxidative stress
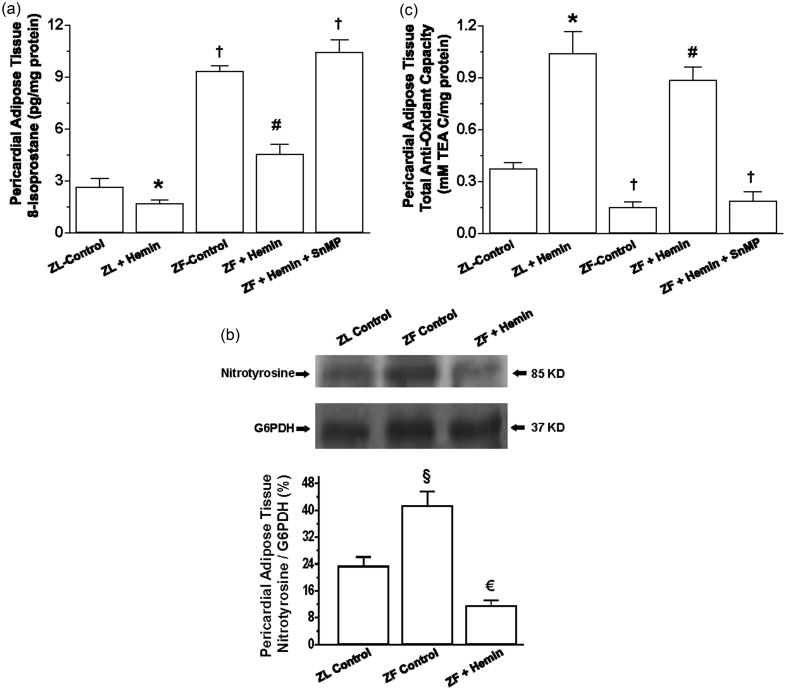
Since oxidative stress is among the major pathophysiological driving forces that compromise adipocyte function,47,48 we measured the levels of 8-isoprostane, an important marker of oxidative stress.49 In ZF, the basal level of 8-isoprostane in the pericardial adipose tissue was significantly elevated as compared to ZL control (Figure 2(a)), but was reduced by hemin, although ZL control levels were not attained. In contrast, the HO inhibitor, SnMP annulled the effects of hemin and reinstated the high levels of 8-isoprostane. Hemin administration also reduced 8-isoprostane in ZL controls. Besides 8-isoprostane, we also measured the expression of nitrotyrosine, another important marker of oxidative stress.50–53 Our results indicate that in ZF-control rats, the basal expression of nitrotyrosine was markedly elevated as compared to ZL controls (Figure 2(b)). Interestingly, treatment with hemin significantly reduced the expression of nitrotyrosine to levels even below the ZL controls.
Figure 2.
Effects of the HO inducer, hemin, and the HO inhibitor, SnMP on 8-isoprostane and the total antioxidant capacity in pericardial adipose tissue obtained from ZLs and ZFs. Hemin administration (a) reduced 8-isoprostane, (b) abated nitrotyrosine, and (c) increased the total antioxidant capacity, whereas SnMP nullified the hemin effects. Bars represent means ± SEM; n = 4-6 rats per group (*p < 0.01 versus ZL control; †p < 0.01 versus ZL control; #p < 0.01 versus ZF + Hemin + SnMP or ZF control; €p < 0.05 versus all groups; §p < 0.01 versus all groups)
To further explore the effects of hemin on oxidative stress we measured the total antioxidant capacity. In ZFs, the basal level of the total antioxidant capacity was significantly lower than the ZL control, but was greatly enhanced by hemin (Figure 2(c)). In contrast, the co-administration of hemin and SnMP annulled the effect, suggesting a role of the HO system in the modulation of oxidative stress. Treatment with hemin also enhanced the total antioxidant capacity in ZL rats albeit to a lesser extent as compared to ZFs.
Hemin administration suppressed pro-inflammatory cytokines in pericardial adipose tissue
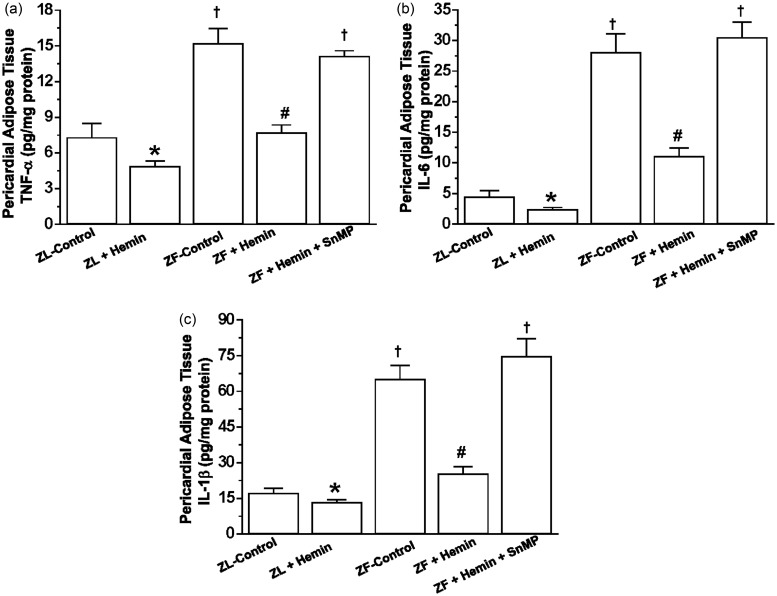
Since excessive inflammation is known to compromise adipocyte function,54 we investigated the effects of hemin on pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β in the pericardial adipose tissue. In ZFs, the basal levels of TNF-α, IL-6, and IL-1β in the pericardial fat were significantly elevated as compared to the ZL control (Figure 3(a) to (c)). However, treatment with hemin greatly reduced TNF-α, IL-6, and IL-1β, whereas the co-administration of hemin and SnMP abolished the effects of hemin. Hemin also reduced TNF-α, IL-6, and IL-1β in ZL controls, albeit less intensely than in ZFs (Figure 3).
Figure 3.
Effects of hemin on TNF-α, IL-6, and IL-1β in the pericardial adipose tissue from ZLs and ZFs. Treatment with hemin (a) suppressed TNF-α, (b) abated IL-6, and (c) attenuated IL-1β, while SnMP reversed the hemin effects. Bars represent means ± SEM; n = 6 rats per group (*p < 0.05 versus ZL control; †p < 0.01 versus ZL control; #p < 0.01 versus ZF + Hemin + SnMP or ZF control)
Hemin administration suppressed the pro-inflammatory macrophage M1 phenotype, but enhanced the anti-inflammatory M2 phenotype in pericardial adipose tissue of ZFs
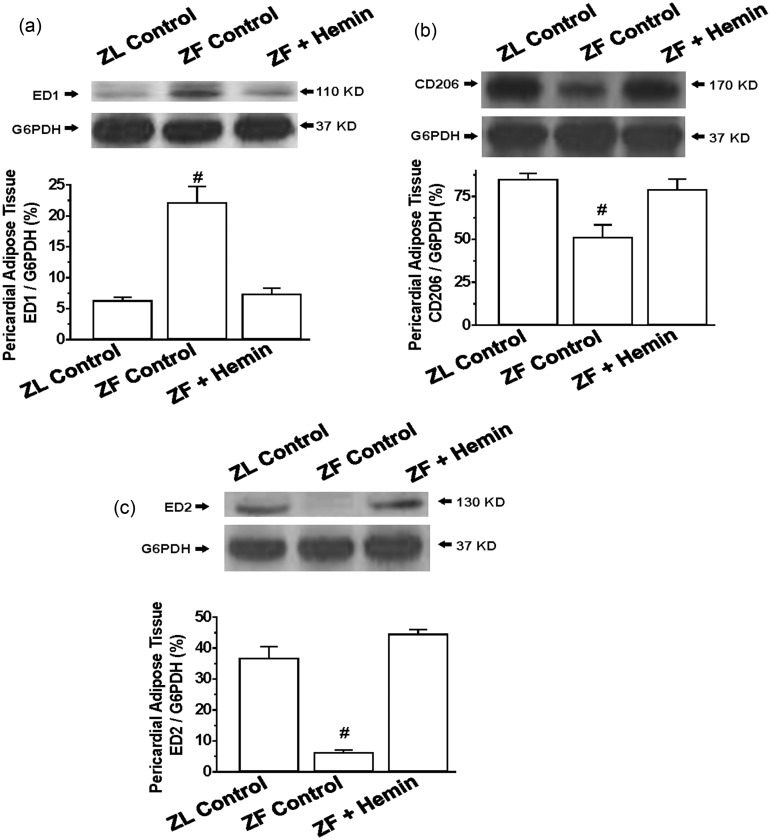
To further investigate the effects of hemin on pericardial adipocyte inflammation, we used Western immunoblotting to measure the pro-inflammatory macrophage M1 phenotype using a specific marker such as ED155 and markers for the anti-inflammatory M2 phenotype such as ED255 and CD 206.56
Our results indicate that the basal expression of the pro-inflammatory macrophage M1 phenotype marker, ED1, in pericardial adipose tissue of ZFs was significantly elevated as compared to ZL control (Figure 4(a)), but was reduced by hemin. In contrast, the basal expression of different markers of the anti-inflammatory macrophage M2 phenotype including ED2 and CD206 was markedly reduced in the pericardial fat of ZFs (Figure 4(b) and (c)), but were robustly increased by hemin, suggesting that hemin may abate inflammation by selectively enhancing the anti-inflammatory M2 phenotype.
Figure 4.
Effects of hemin on ED1, ED2, and CD206 in the pericardial adipose tissue from ZLs and ZFs. Hemin administration (a) abated the expression of ED1, but (b) enhanced the expression of CD206, and (c) increased the expression of ED2 in ZFs. Bars represent means ± SEM; n = 4 rats per group (#p < 0.01 versus all groups).
Hemin suppressed macrophage infiltration in pericardial adipose tissue by abating ED1
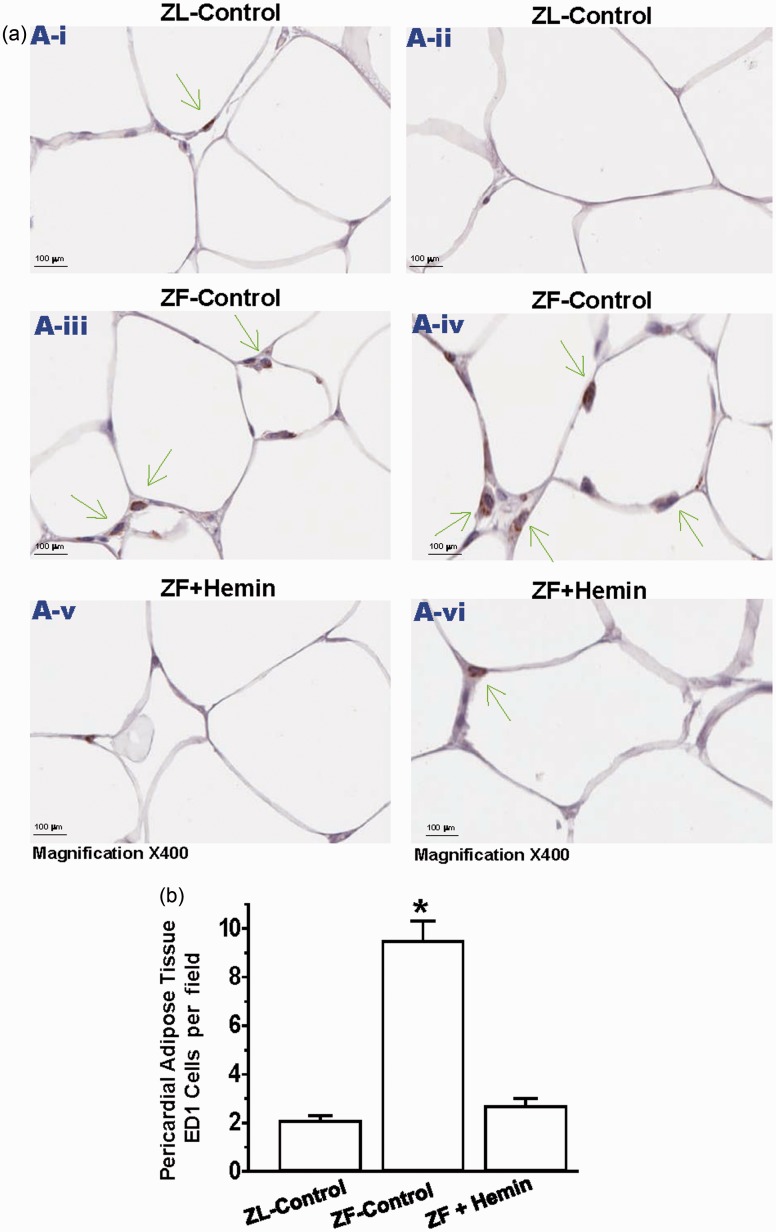
Given that our Western immunoblotting data revealed that hemin abated ED1 pericardial adipose tissue (Figure 4(a)), we use the ED1 antibody to assess macrophage infiltration in the pericardial adipose tissue by immunohistochemistry (Figure 5(a)). Our results indicate that sections of pericardial adipose from ZL controls were almost devoid of the dark brown ED1 positive staining that characterizes macrophage infiltration. However, in untreated ZF controls, there was a marked increase in the number of ED1 positive staining for macrophage (Figure 5(a)). However, treatment with hemin significantly reduced the number of ED1 stained macrophage, suggesting reduction of macrophage infiltration. Correspondingly, hemin significantly reduced the quantitative ED1 score of pericardial adipose tissue sections (Figure 5(b)).
Figure 5.
Effects of hemin on macrophage infiltration in the pericardial adipose tissue. (a) Representative images of pericardial adipose tissue section from different rats. The images reveal that macrophage infiltration (ED1 positive cells stained dark brown in pericardial adipose tissue sections were elevated in ZF controls (panels a-iii and a-iv) as compared to the ZL controls (panels a-i and a-ii), but interestingly were significantly reduced by hemin (panels a-v and a-vi). (Magnification × 400). (b) Quantitative analyses per field showing that in ZF controls, macrophage infiltration was significantly elevated as compared to ZL control, but interestingly was significantly attenuated by hemin. Bars represent means ± SEM; n = 6 rats per group (*p < 0.01 versus all groups). (A color version of this figure is available in the online journal.)
Treatment with hemin improved adipocyte morphology, ameliorated pericardial adipocyte hypertrophy, and improved its function
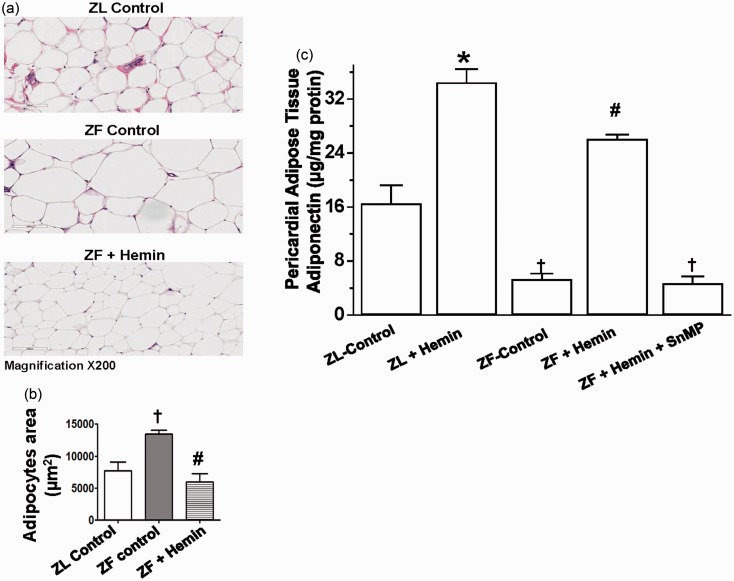
Since adipocyte hypertrophy is associated with elevated inflammation and oxidative stress,1,5,6,57 and after observing the significant reduction of pro-inflammatory cytokines and the potentiation of the antioxidant capacity in hemin-treated animals, we investigated whether these protective effects of hemin will be accompanied by improved adipocyte morphology and function. Given that the size and area of adipocytes are important indices of adipocyte hypertrophy,58 histological and morphometric analyses were used to assess pericardial adipocyte hypertrophy (Figure 6(a) and (b)). Histological and morphological analyses revealed that the size of pericardial adipocytes in untreated ZFs (ZF controls) was significantly enlarged as compared to normal-size adipocytes in the ZL controls, suggesting the pathophysiology of adipocyte hypertrophy in untreated ZF controls (Figure 6(a) and (b)). Interestingly, the hemin abated adipocyte hypertrophy and reinstated comparable adipocyte area in control ZFs as observed in the ZL control (Figure 6(a) and (b)).
Figure 6.
Effects of hemin treatment on pericardial adipocyte histology and function in ZFs.
(a) Representative hematoxylin and eosin staining shows significantly enlarged adipocyte size, and thus adipocyte hypertrophy in ZF (Magnification × 200). (b) Morphometric analyses revealed that the adipocyte area in ZF control was significantly elevated as compared to ZL control, but was restored to comparable levels as observed in ZL control by hemin. (c) Treatment with hemin increased the depressed levels of adiponectin, whereas SnMP nullified the hemin effects. Bars represent means ± SEM; n = 6 rats per group (*p < 0.01 versus ZL control; †p < 0.01 versus ZL control; #p < 0.01 versus ZF + Hemin + SnMP or ZF control). (A color version of this figure is available in the online journal)
Given that adipocyte hypertrophy is known to compromise the production of adiponectin and exacerbate inflammatory insults,1,5,6 it was necessary to measure the levels of adiponectin to ascertain that the hemin-induced amelioration of adipocyte morphology was accompanied by improved adipocyte function.
Our results indicate that in control ZFs, the basal levels of pericardial adipose tissue adiponectin were significantly reduced (Figure 6(c)). However, the administration of hemin robustly increased the levels of adiponectin, whereas the co-treatment of hemin and the HO inhibitor, SnMP nullified the effects of hemin.
Hemin administration enhanced proteins of regeneration in the pericardial adipose tissue
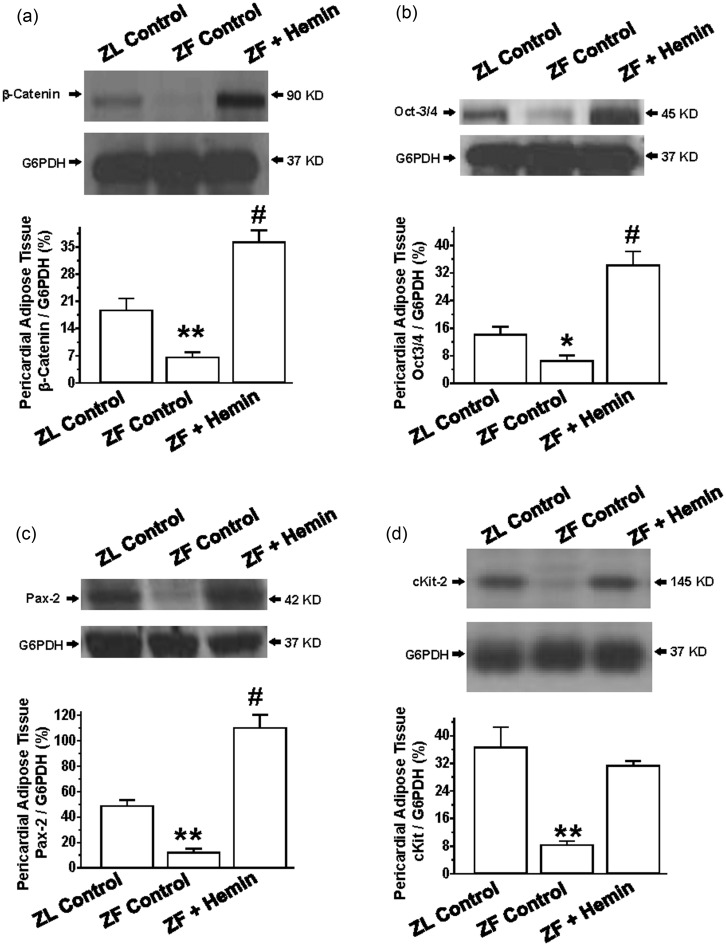
To investigate the mechanisms by which hemin improves pericardial adipocyte morphology, we measured the expression of proteins of regeneration such as beta-catenin, Oct3/4, and pax232–34 in the adipose tissue.
Our results indicate that the expressions of beta-catenin, Oct3/4, and pax2 in ZFs were significantly reduced as compared to the ZL control (Figure 7(a) to (c)). Interestingly, treatment with hemin greatly enhanced the expressions of beta-catenin, Oct3/4, and pax2.
Figure 7.
Effects of hemin on proteins of regeneration in pericardial adipose tissue. Representative Western immunoblotting and relative densitometry of the expressed proteins normalized by G6PHD indicates that hemin (a) increased the expression of β-catenin, (b) enhanced the expression of Oct-3/4, (c) upregulated the expression of Pax-2, and (d) potentiated the expression of cKit in ZFs. Bars represent means ± SEM; n = 4 rats per group (*p < 0.05, **p < 0.01 versus all groups; #p < 0.01 versus all groups)
To further explore the effects of hemin on adipocyte regeneration, we measured the effects of hemin on c-Kit, a stem/progenitor cell marker.59 Our results indicate that the expression of cKit in ZFs was markedly reduced as compared to the ZL control (Figure 7(d)), but was significantly increased by hemin.
Hemin administration suppresses extracellular matrix and profibrotic proteins in the pericardial adipose tissue
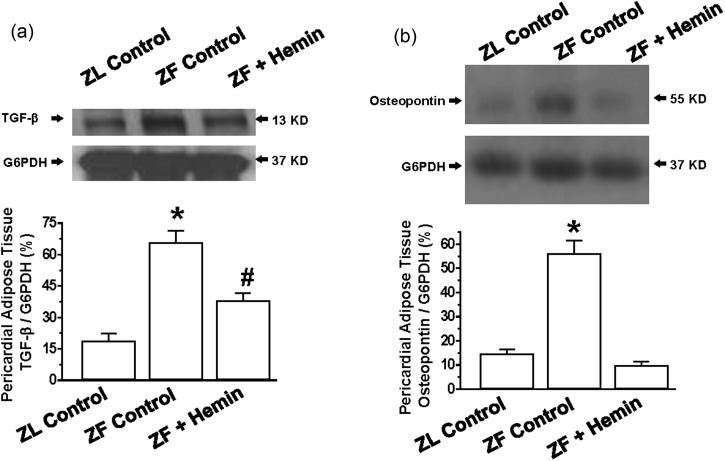
Since osteopontin is known to promote inflammation, extracellular matrix invasion, and hypertrophy,22–31 and in a similar way, transforming growth factor-beta (TGF-β) has been shown to promote fibrosis and hypertrophy,60,61 we investigated the effects of hemin on the expression of osteopontin and TGF-β. Moreover, TGF-β is known to interact with osteopontin.62
Our results indicate that the expressions of osteopontin and TGF-β in pericardial adipose tissue in ZFs were markedly elevated as compared to the ZL control, but were significantly reduced by hemin treatment (Figure 8(a) and (b)).
Figure 8.
Effects of hemin on the expression of transforming growth factor beta (TGF-β) and osteopontin of the pericardial adipose of ZFs. Representative Western immunoblotting and relative densitometry of the expressed proteins normalized by G6PHD shows that hemin (a) abated the expression of TGF-β and (b) reduced the expression of osteopontin. Bars represent means ± SEM; n = 4 rats per group (#p < 0.01 versus all groups)
Discussion
The present study underscores the protective effects of the HO system in obesity. Particularly, this study indicates that treatment with hemin: (i) suppresses hypertriglyceridemia and hypercholesteromia, (ii) reduces pericardial adiposity, (iii) abates pericardial adipocyte hypertrophy, (iv) attenuates adipocyte inflammation/oxidative insults, (v) decreases the excessive levels of extracellular matrix/profibrotic, while concomitantly potentiating the HO system, stem/progenitor cells, and proteins of regeneration in the pericardial adipose tissue. Importantly, these attributes of hemin were accompanied by the restoration of adipocyte morphology and improved adipocyte function as evidenced by increased adiponectin levels. Given that hypertriglyceridemia, hypercholesteromia, and excessive pericardial adiposity are major pathophysiological causes of heart failure and related cardiac complications,8,43–46 the suppression of hypertriglyceridemia, hypercholesteromia, and pericardial adiposity in hemin-treated ZFs, and the corresponding decline of adipocyte hypertrophy, TGF-β, osteopontin, pro-inflammatory macrophage M1 phenotype, TNF-α, IL-6, IL-1β, and 8-isoprostane,22–31,54,60–63 which were associated with the potentiation of the anti-inflammatory macrophage M2 phenotype, proteins of regeneration such as beta-catenin, Oct3/4, and pax232–34 are among the multifaceted mechanisms by which the HO system restores adipocyte morphology and improved adipocyte function. Moreover, the effect of the HO system on the expression of proteins of regeneration such as Oct3/4 and pax2 is a novel mechanism unveiled by this study through which hemin may restore adipocyte morphology and function.
The improvement of pericardial adipocyte morphology and function reported in the present study is an important finding because by virtue of its anatomical and functional proximity to the coronary circulation, pericardial adiposity has been implicated in coronary artery disease and cardiac hypertrophy through paracrine mechanisms that include increased production of inflammatory cytokines, reactive oxygen species, and other atherogenic factors.1,9 Therefore, the hemin-dependent suppression of pericardial adiposity/inflammation may offset this pathophysiological inter-organ cross-talk between the heart and pericardial adipose tissue. We recently showed that hemin administration abates inflammation by modulating macrophage polarization toward the anti-inflammatory M2 phenotype in the myocardium.35,64 Similarly, other reports have acknowledged the role of the HO system on macrophage polarization.65 Therefore, the present study alongside our recent publications and previous reports in literature strongly suggests that the suppression of pericardial adiposity/inflammation may limit the damaging effects of inflammatory mediators from the pericardial adipose tissue to the myocardium via paracrine mechanisms. Moreover, our recent findings indicate that the suppression of pericardial adiposity is associated with improved cardiac function.35,64
Hemin also enhanced HO-1, HO activity, and the total antioxidant capacity in ZL-control rats and correspondingly reduced pericardial adiposity, 8-isoprostane, TNF-α, IL-6, IL-1β, and fasting glucose. However, the magnitude of hemin effects on ZL controls was smaller as compared to ZFs with aberrant HO-1 and HO activity. Although the reasons for this selective effect of hemin on HO-1 and HO activity remain challenging and poorly understood, it is possible that because ZLs are healthy animals, their HO systems are more stable as compared to the aberrant and vulnerable HO system in ZFs, so treatment with hemin more potently enhance the depressed HO system in ZFs. Correspondingly, the greater potentiation of the HO system in ZFs may be responsible for the more pronounced physiological effects in hemin-treated ZFs. Importantly, the higher selectivity of hemin in diseased conditions could be explored in the design of novel remedies against complications arising from excessive pericardial adiposity.
Oxidative stress is a pathophysiological driving force that is implicated in tissue remodeling, hypertrophy, and organ malfunction. Therefore, the potentiation of the antioxidant defense system is important to safeguard tissue and maintain optimal physiological function. The antioxidant effect of the HO system has been widely acknowledged by many authors using different methodologies including lucigenin-enhanced chemiluminescence,62 the 2′,7′-dichlorodihydrofluorescein diacetate probe,63 and flow cytometry.64 Interestingly, the results obtained in the present study using EIA for the assessment of 8-isoprostane and total antioxidant capacity are consistent with the antioxidant effect of the HO system reported by many authors in literature.
Although the mechanisms by which hemin restores adipocyte morphology are multifaceted and complex, the present study suggests a role of stem/progenitor cells and proteins of regeneration. However, our findings represent the tip of an iceberg and further studies would be needed to fully characterize the role of the HO system in tissue regeneration. Nevertheless, the significance of our findings in the pathophysiology of adipocyte dysfunction and obesity-related complications cannot be overemphasized.
Given that hypertriglyceridemia, hypercholesteromia, and excessive pericardial adiposity are major causes of heart failure and related cardiac complications,8,43–46 the concomitant suppression of hypertriglyceridemia, hypercholesteromia, and pericardial adiposity with associated reduction of adipocyte hypertrophy, inflammation/oxidative stress, and extracellular matrix/profibrotic agents, alongside the potentiation of proteins of regeneration, restoration of adipocyte morphology, and improved adipocyte function in hemin-treated ZFs, HO inducers may be explored as a novel class of drugs against cardiac complications arising from excessive adiposity.
Acknowledgements
This work was supported by a grant from the Heart & Stroke Foundation of Saskatchewan, Canada to Dr Joseph Fomusi Ndisang.
Author contribution
JFN: Conceived the idea, designed the study, did experiments, analyzed data, and wrote the manuscript. ST: Did experiments and analyzed data.
References
- 1.Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes 2009; 117: 241–50. [DOI] [PubMed] [Google Scholar]
- 2.Ndisang JF, Jadhav A. Hemin therapy suppresses inflammation and retroperitoneal adipocyte hypertrophy to improve glucose metabolism in obese rats co-morbid with insulin-resistant type-2 diabetes. Diabetes Obes Metab 2013; 15: 1029–39. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Eliasson B, Smith U, Cushman SW, Sherman AS. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetic patients. Obesity (Silver Spring) 2012; 20: 932–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993; 259: 87–91. [DOI] [PubMed] [Google Scholar]
- 5.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev 2006; 2: 367–73. [DOI] [PubMed] [Google Scholar]
- 6.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444: 860–7. [DOI] [PubMed] [Google Scholar]
- 7.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O’Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008; 117: 605–13. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Fox CS, Hickson DA, May WL, Ding J, Carr JJ, Taylor HA. Pericardial fat and echocardiographic measures of cardiac abnormalities: The Jackson Heart Study. Diabetes Care 2011; 34: 341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb 2010; 17: 332–41. [DOI] [PubMed] [Google Scholar]
- 10.Ndisang JF, Lane N, Jadhav A. The heme oxygenase system abates hyperglycemia in Zucker diabetic fatty rats by potentiating insulin-sensitizing pathways. Endocrinology 2009; 150: 2098–108. [DOI] [PubMed] [Google Scholar]
- 11.Mosen H, Salehi A, Alm P, Henningsson R, Jimenez-Feltstrom J, Ostenson CG, Efendic S, Lundquist I. Defective glucose-stimulated insulin release in the diabetic Goto-Kakizaki (GK) rat coincides with reduced activity of the islet carbon monoxide signaling pathway. Endocrinology 2005; 146: 1553–8. [DOI] [PubMed] [Google Scholar]
- 12.Mosen H, Salehi A, Henningsson R, Lundquist I. Nitric oxide inhibits, and carbon monoxide activates, islet acid alpha-glucoside hydrolase activities in parallel with glucose-stimulated insulin secretion. J Endocrinol 2006; 190: 681–93. [DOI] [PubMed] [Google Scholar]
- 13.Ndisang JF, Jadhav A. Heme oxygenase system enhances insulin sensitivity and glucose metabolism in streptozotocin-induced diabetes. Am J Physiol Endocrinol Metab 2009; 296: E829–41. [DOI] [PubMed] [Google Scholar]
- 14.Ndisang JF, Lane N, Jadhav A. Upregulation of the heme oxygenase system ameliorates postprandial and fasting hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 2009; 296: E1029–41. [DOI] [PubMed] [Google Scholar]
- 15.Ndisang JF, Jadhav A. Upregulating the heme oxygenase system suppresses left ventricular hypertrophy in adult spontaneously hypertensive rats for 3 months. J Card Fail 2009; 15: 616–28. [DOI] [PubMed] [Google Scholar]
- 16.Ndisang JF, Wang R. Age-related alterations in soluble guanylyl cyclase and cGMP pathway in spontaneously hypertensive rats. J Hypertens 2003; 21: 1117–24. [DOI] [PubMed] [Google Scholar]
- 17.Ndisang JF, Wang R. Alterations in heme oxygenase/carbon monoxide system in pulmonary arteries in hypertension. Exp Biol Med (Maywood) 2003; 228: 557–63. [DOI] [PubMed] [Google Scholar]
- 18.Ndisang JF, Tabien HE, Wang R. Carbon monoxide and hypertension. J Hypertens 2004; 22: 1057–74. [DOI] [PubMed] [Google Scholar]
- 19.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 2008; 60: 79–127. [DOI] [PubMed] [Google Scholar]
- 20.Ndisang JF. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators Inflamm 2010; 2010: 359732–359732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndisang JF, Lane N, Syed N, Jadhav A. Up-regulating the heme oxygenase system with hemin improves insulin sensitivity and glucose metabolism in adult spontaneously hypertensive rats. Endocrinology 2010; 151: 549–60. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Kim KH, Seo KW, Bae JU, Kim YH, Lee SJ, Lee WS, Kim CD. Resistin derived from diabetic perivascular adipose tissue upregulates vascular expression of osteopontin via AP-1 signaling pathway. J Pathol 2014; 232: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panda D, Kundu GC, Lee BI, Peri A, Fohl D, Chackalaparampil I, Mukherjee BB, Li XD, Mukherjee DC, Seides S, Rosenberg J, Stark K, Mukherjee AB. Potential roles of osteopontin and alphaVbeta3 integrin in the development of coronary artery restenosis after angioplasty. Proc Natl Acad Sci USA 1997; 94: 9308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JJ, Zhu CG, Yu B, Liu YX, Yu MY. The role of inflammation in coronary artery calcification. Ageing Res Rev 2007; 6: 263–70. [DOI] [PubMed] [Google Scholar]
- 25.Chapman J, Miles PD, Ofrecio JM, Neels JG, Yu JG, Resnik JL, Wilkes J, Talukdar S, Thapar D, Johnson K, Sears DD. Osteopontin is required for the early onset of high fat diet-induced insulin resistance in mice. PLoS One 2010; 5: e13959–e13959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh M, Foster CR, Dalal S, Singh K. Role of osteopontin in heart failure associated with aging. Heart Fail Rev 2010; 15: 487–94. [DOI] [PubMed] [Google Scholar]
- 27.Rall DP. Problems remain to be resolved in the area of quantitative risk assessment. Regul Toxicol Pharmacol 1992; 15: 104–5. [DOI] [PubMed] [Google Scholar]
- 28.Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, Liu L, Morimoto J, Rittling SR, Denhardt D, Kitabatake A, Uede T. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension 2004; 43: 1195–201. [DOI] [PubMed] [Google Scholar]
- 29.Zeyda M, Gollinger K, Todoric J, Kiefer FW, Keck M, Aszmann O, Prager G, Zlabinger GJ, Petzelbauer P, Stulnig TM. Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology 2011; 152: 2219–27. [DOI] [PubMed] [Google Scholar]
- 30.Dahiya S, Givvimani S, Bhatnagar S, Qipshidze N, Tyagi SC, Kumar A. Osteopontin-stimulated expression of matrix metalloproteinase-9 causes cardiomyopathy in the mdx model of Duchenne muscular dystrophy. J Immunol 2011; 187: 2723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azibani F, Benard L, Schlossarek S, Merval R, Tournoux F, Fazal L, Polidano E, Launay JM, Carrier L, Chatziantoniou C, Samuel JL, Delcayre C. Aldosterone inhibits antifibrotic factors in mouse hypertensive heart. Hypertension 2012; 59: 1179–87. [DOI] [PubMed] [Google Scholar]
- 32.Heneidi S, Simerman AA, Keller E, Singh P, Li X, Dumesic DA, Chazenbalk G. Awakened by cellular stress: Isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PLoS One 2013; 8: e64752–e64752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villanueva S, Carreno JE, Salazar L, Vergara C, Strodthoff R, Fajre F, Céspedes C, Sáez PJ, Irarrázabal C, Bartolucci J, Figueroa F, Vio CP. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clin Sci (Lond) 2013; 125: 199–210. [DOI] [PubMed] [Google Scholar]
- 34.Sodhi K, Puri N, Kim DH, Hinds TD, Stechschulte LA, Favero G, Rodella L, Shapiro JI, Jude D, Abraham NG. PPARdelta binding to heme oxygenase 1 promoter prevents angiotensin II-induced adipocyte dysfunction in Goldblatt hypertensive rats. Int J Obes (Lond) 2014; 38: 456–65. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Jadhav A, Tiwari S, Lee P, Ndisang JF. The heme oxygenase system selectively enhances the anti-inflammatory macrophage-m2 phenotype, reduces pericardial adiposity, and ameliorated cardiac injury in diabetic cardiomyopathy in zucker diabetic Fatty rats. J Pharmacol Exp Ther 2013; 345: 239–49. [DOI] [PubMed] [Google Scholar]
- 36.Ndisang JF, Jadhav A. Hemin therapy suppresses inflammation and retroperitoneal adipocyte hypertrophy to improve glucose metabolism in obese rats co-morbid with insulin resistant type-2 diabetes. Diabetes Obes Metab 2013;15:1029–39. [DOI] [PubMed]
- 37.Salley TN, Mishra M, Tiwari S, Jadhav A, Ndisang JF. The heme oxygenase system rescues hepatic deterioration in the condition of obesity co-morbid with type-2 diabetes. PLoS One 2013; 8: e79270–e79270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jadhav A, Torlakovic E, Ndisang JF. Interaction among heme oxygenase, nuclear factor-{kappa}B, and transcription activating factors in cardiac hypertrophy in hypertension. Hypertension 2008; 52: 910–7. [DOI] [PubMed] [Google Scholar]
- 39.Jadhav A, Torlakovic E, Ndisang JF. Hemin therapy attenuates kidney injury in deoxycorticosterone acetate-salt hypertensive rats. Am J Physiol Renal Physiol 2009; 296: F521–F34. [DOI] [PubMed] [Google Scholar]
- 40.Ndisang JF, Mishra M. The heme oxygenase system selectively suppresses the proinflammatory macrophage m1 phenotype and potentiates insulin signaling in spontaneously hypertensive rats. Am J Hypertens 2013; 26: 1123–31. [DOI] [PubMed] [Google Scholar]
- 41.Ndisang JF, Jadhav A, Mishra M. The heme oxygenase system suppresses perirenal visceral adiposity, abates renal inflammation and ameliorates diabetic nephropathy in zucker diabetic fatty rats. PLoS One 2014; 9: e87936–e87936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jadhav A, Ndisang JF. Treatment with heme arginate alleviates adipose tissue inflammation and improves insulin sensitivity and glucose metabolism in a rat model of human primary aldosteronism. Free Radic Biol Med 2012; 53: 2277–86. [DOI] [PubMed] [Google Scholar]
- 43.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med 2005; 47: 201–10. [PubMed] [Google Scholar]
- 44.Rubinstein J, Pelosi A, Vedre A, Kotaru P, Abela GS. Hypercholesterolemia and myocardial function evaluated via tissue doppler imaging. Cardiovasc Ultrasound 2009; 7: 56–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yunoki K, Nakamura K, Miyoshi T, Enko K, Kubo M, Murakami M, Hata Y, Kohno K, Morita H, Kusano KF, Ito H. Impact of hypertriglyceridemia on endothelial dysfunction during statin +/- ezetimibe therapy in patients with coronary heart disease. Am J Cardiol 2011; 108: 333–9. [DOI] [PubMed] [Google Scholar]
- 46.Asselin C, Ducharme A, Ntimbane T, Ruiz M, Fortier A, Guertin MC, Lavoie J, Diaz A, Levy E, Tardif JC, Des Rosiers C. Circulating levels of linoleic acid and HDL-cholesterol are major determinants of 4-hydroxynonenal protein adducts in patients with heart failure. Redox Biol 2014; 2: 148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yano M, Yamamoto T, Nishimura N, Gotoh T, Watanabe K, Ikeda K, Garan Y, Taguchi R, Node K, Okazaki T, Oike Y. Increased oxidative stress impairs adipose tissue function in sphingomyelin synthase 1 null mice. PLoS One 2013; 8: e61380–e61380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crujeiras AB, Diaz-Lagares A, Carreira MC, Amil M, Casanueva FF. Oxidative stress associated to dysfunctional adipose tissue: a potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic Res 2013; 47: 243–56. [DOI] [PubMed] [Google Scholar]
- 49.Delanty N, Reilly MP, Pratico D, Lawson JA, McCarthy JF, Wood AE, Ohnishi ST, Fitzgerald DJ, FitzGerald GA. 8-epi PGF2 alpha generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation 1997; 95: 2492–9. [DOI] [PubMed] [Google Scholar]
- 50.Julius U, Drel VR, Grassler J, Obrosova IG. Nitrosylated proteins in monocytes as a new marker of oxidative-nitrosative stress in diabetic subjects with macroangiopathy. Exp Clin Endocrinol Diabetes 2009; 117: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lagoda G, Sezen SF, Hurt KJ, Cabrini MR, Mohanty DK, Burnett AL. Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. FASEB J 2014;28:76–84. [DOI] [PMC free article] [PubMed]
- 52.Darwish RS, Amiridze N, Aarabi B. Nitrotyrosine as an oxidative stress marker: Evidence for involvement in neurologic outcome in human traumatic brain injury. J Trauma 2007; 63: 439–42. [DOI] [PubMed] [Google Scholar]
- 53.Lagoda G, Sezen SF, Hurt KJ, Cabrini MR, Mohanty DK, Burnett AL. Sustained nitric oxide (NO)-releasing compound reverses dysregulated NO signal transduction in priapism. FASEB J 2014; 28: 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno-Navarrete JM, Escote X, Ortega F, Serino M, Campbell M, Michalski MC, Laville M, Xifra G, Luche E, Domingo P, Sabater M, Pardo G, Waget A, Salvador J, Giralt M, Rodriguez-Hermosa JI, Camps M, Kolditz CI, Viguerie N, Galitzky J, Decaunes P, Ricart W, Frühbeck G, Villarroya F, Mingrone G, Langin D, Zorzano A, Vidal H, Vendrell J, Burcelin R, Vidal-Puig A, Fernández-Real JM. A role for adipocyte-derived lipopolysaccharide-binding protein in inflammation- and obesity-associated adipose tissue dysfunction. Diabetologia 2013; 56: 2524–37. [DOI] [PubMed] [Google Scholar]
- 55.Aki K, Shimizu A, Masuda Y, Kuwahara N, Arai T, Ishikawa A, Fujita E, Mii A, Natori Y, Fukunaga Y, Fukuda Y. ANG II receptor blockade enhances anti-inflammatory macrophages in anti-glomerular basement membrane glomerulonephritis. Am J Physiol Renal Physiol 2010; 298: F870–82. [DOI] [PubMed] [Google Scholar]
- 56.Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C, Higashida M, Mikasa H, Nakaya Y, Takanashi S, Igarashi T, Kitagawa T, Sata M. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol 2011; 58: 248–55. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, Wang Z, Li J, Gu D, Li S, Shen C, Song Z. Increased 4-hydroxynonenal formation contributes to obesity-related lipolytic activation in adipocytes. PLoS One 2013; 8: e70663–e70663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burgess AP, Vanella L, Bellner L, Gotlinger K, Falck JR, Abraham NG, Schwartzman ML, Kappas A. Heme oxygenase (HO-1) rescue of adipocyte dysfunction in HO-2 deficient mice via recruitment of epoxyeicosatrienoic acids (EETs) and adiponectin. Cell Physiol Biochem 2012; 29: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin J, Fernandez I, Roy K. Development of feeder-free culture systems for generation of ckit+sca1+ progenitors from mouse iPS cells. Stem Cell Rev 2011; 7: 736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park JK, Ki MR, Lee EM, Kim AY, You SY, Han SY, Lee EJ, Hong IH, Kwon SH, Kim SJ, Rando TA, Jeong KS. Losartan improves adipose tissue-derived stem cell niche by inhibiting transforming growth factor-beta and fibrosis in skeletal muscle injury. Cell Transplant 2012; 21: 2407–24. [DOI] [PubMed] [Google Scholar]
- 61.Huang H, Tang QZ, Wang AB, Chen M, Yan L, Liu C, Jiang H, Yang Q, Bian ZY, Bai X, Zhu LH, Wang L, Li H. Tumor suppressor A20 protects against cardiac hypertrophy and fibrosis by blocking transforming growth factor-beta-activated kinase 1-dependent signaling. Hypertension 2010; 56: 232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi X, Bai S, Li L, Cao X. Hoxa-9 represses transforming growth factor-beta-induced osteopontin gene transcription. J Biol Chem 2001; 276: 850–5. [DOI] [PubMed] [Google Scholar]
- 63.Fujisaka S, Usui I, Ikutani M, Aminuddin A, Takikawa A, Tsuneyama K, Mahmood A, Goda N, Nagai Y, Takatsu K, Tobe K. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1alpha-dependent and HIF-1alpha-independent manner in obese mice. Diabetologia 2013; 56: 1403–12. [DOI] [PubMed] [Google Scholar]
- 64.Tiwari S, Mishra M, Jadhav A, Gerger C, Lee P, Weber L, Ndisang JF. The risk of heart failure and cardiometabolic complications in obesity may be masked by an apparent healthy status of normal blood glucose. Oxid Med Cell Longev 2013; 2013: 253657–253657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weis N, Weigert A, von Knethen A, Brune B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell 2009; 20: 1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]