Abstract
The aim of this study was to define the effects of polysulfide on intracellular Ca2+ concentration ([Ca2+]i) and the underlying machinery, especially from the hydrogen sulfide (H2S) and nitric oxide (NO) perspectives, in rat peritoneal mast cells. We found that a polysulfide donor, Na2S4, increased [Ca2+]i, which is both extracellular and intracellular Ca2+ dependent. Intracellular Ca2+ release induced by Na2S4 was attenuated by the addition of a ryanodine receptor blocker. A slow-releasing H2S donor, GYY4137, dose dependently increased [Ca2+]i that was independent from extracellular Ca2+ influx. The GYY4137-induced [Ca2+]i release was partially attenuated in the presence of the ryanodine receptor blocker. Both polysulfide and H2S donors increased the intracellular NO levels in DAF-2-loaded mast cells, which were abolished by an NO scavenger, cPTIO. Inhibition of NO synthase (NOS) significantly abolished the polysulfide- or H2S-donor-induced [Ca2+]i elevation in the absence of extracellular Ca2+. An NO donor, diethylamine (DEA) NONOate, increased [Ca2+]i in a concentration-dependent manner, in which both extracellular and intracellular Ca2+ are associated. At higher concentrations, the DEA NONOate-induced [Ca2+]i increases were attenuated in the absence of extracellular Ca2+ and by the addition of the ryanodine receptor blocker. H2S and NO dose dependently induced polysulfide production. Curiously, polysulfide, H2S, and NO donors had no effect on mast cell degranulation. Among synthases, cystathionine-γ-lyase, and neuronal NOS seemed to be the major H2S- and NO-producing synthases, respectively. These results indicate that polysulfide acts as a potential signaling molecule that regulates [Ca2+]i homeostasis in rat peritoneal mast cells via a cross talk with NO and H2S.
Keywords: polysulfide, hydrogen sulfide, nitric oxide, Ca2+, mast cells
polysulfides, a group of chemical compounds that contain sulfur atom chains of varying length, can be formed from hydrogen sulfide (H2S) in the presence of oxygen (28, 41). Polysulfide formation from H2S solutions at a physiological pH and on biological time scales has been reported (13). Cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), common H2S-producing synthases, can produce polysulfides via H2S synthesized from l-cysteine as a substrate (19). In a classical study, enzymatic production of polysulfides from mercaptopyruvate was reported (18), and this was later identified as a reaction via a third H2S-producing synthase, 3-mercaptopyruvate sulfurtransferase. Conversely, organic polysulfides act as H2S donors via glucose-supported and thiol, as well as glutathione-dependent, cellular reactions (3). Polysulfides can be produced from a chemical interaction between H2S and nitric oxide (NO) at a physiological pH (6).
H2S has been identified as the third cell-signaling molecule with intensive physiological and pathophysiological effects on various cellular functions; H2S induces vasorelaxation (44, 47, 48); protects the cell against oxidative stress, apoptosis, and necrosis (27); and is involved in nociception and hyperalgesia (26). It has been proposed as an inflammatory mediator, as were carbon monoxide (CO) and NO (33). It has regulatory roles in intracellular Ca2+ homeostasis in pancreatic acinar cells, parotid acinar cells, and pancreatic β-cells (37, 38, 39). With this wide array of effects exerted by H2S, it is assumed that polysulfides are not completely, but at least partly, involved in the H2S effects serving as actual mediators of in vivo signaling (13). Polysulfides have been demonstrated to induce Ca2+ influx by activating transient receptor potential A1 (TRPA1) channels in rat astrocytes, suggesting that polysulfides are among the possible H2S-derived signaling molecules in the brain (29). Moreover, polysulfides were postulated to function as a pronociceptive substance through the activation of TRPA1 in sensory neurons (17). They have also been reported to protect neural cells from oxidative damage through the activation of nuclear factor erythroid-2-related factor-2 (31). Thus the effects of H2S and of polysulfides overlap extensively, indicating that some of the effects previously thought to originate from H2S might originate from polysulfides and vice versa.
In a series of reports from our laboratory, the potential roles of the gaseous molecules NO, H2S, and CO in intracellular Ca2+ homeostasis were proposed in various exocrine and endocrine cells, including pancreatic exocrine and endocrine cells as well as parotid acinar cells (36, 37, 38, 39, 40). Mast cells release intragranular contents, histamine, and other entities by exocytosis in an intracellular Ca2+ concentration ([Ca2+]i)-dependent manner. A previous study from our laboratory demonstrated that a [Ca2+]i increase in the limited region just beneath the plasma membrane of the cell induces massive degranulation when stimulated with a direct G-protein stimulant, compound 48/80 (15). The results suggested that the first phase of [Ca2+]i increase, which might result from intracellular Ca2+ release lasting only a few seconds after the stimulant application, is followed by the second phase, initiated by extracellular Ca2+ entry and mandatory for triggering massive exocytosis. Using mast cells is advantageous for investigating the potential mechanisms underlying stimulus-secretion coupling. Thus, in this study, an attempt was made to elucidate the putative roles of polysulfide in [Ca2+]i homeostasis in rat peritoneal mast cells.
MATERIALS AND METHODS
Materials.
GYY4137 [morpholin-4-ium 4 methoxphenyl (morpholino) phosphinodithionate], fluo-4/AM, HEPES, NG-monomethyl-l-arginine (l-NMMA), carboxy-PTIO (cPTIO), and SSP4 [sulfane sulfur probe 4, 3′,6′-di(O-thiosalicyl)fluorescein] were obtained from Dojindo (Kumamoto, Japan). Sodium tetrasulfide (Na2S4) was a generous gift from Dojindo. DAF-2/DA was purchased from Daiichikagaku (Tokyo, Japan). RPMI 1640, penicillin, streptomycin, and heat-inactivated fetal bovine serum (FBS) were obtained from Life Technologies Japan (Tokyo, Japan). Cell-Tak was obtained from BD Biosciences (San Jose, CA). Diethylamine NONOate (DEA NONOate) was sourced from Enzo Life Science, ruthenium red (RR) was purchased from Cayman Chemicals (Ann Arbor, MI), while sulforhodamine-B (SFRM-B) was from Tokyo Chemical Industry (Tokyo, Japan). Compound 48/80, cremophor EL, and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, MO). Abcam mouse monoclonal antibody against tryptase was a generous gift from the Laboratory of Anatomy of our Graduate School. Other chemicals were obtained from Wako Pure Chemicals (Osaka, Japan).
Isolation of rat peritoneal mast cells.
Adult male SPF Wistar rats weighing 250–300 g were purchased from Clea Japan (Tokyo, Japan), maintained in a controlled environment at an ambient temperature of 22°C and a 12:12-h light-dark cycle, and deprived of food but allowed free access to water overnight before the experiment. All rats were handled according to the guidelines for the ethical use of animals as set by the U.S. National Institutes of Health, and all experiments were approved by the institutional Animal Care and Use Committee of the Graduate School of Veterinary Medicine, Hokkaido University. To isolate mast cells, rats were anesthetized by CO2 inhalation and euthanized by exsanguination. Mast cells were obtained by peritoneal lavage after a 2-min massage of the abdomen with an intraperitoneal injection of 10 ml ice-cold phosphate-buffered saline (PBS) (15). The abdominal cavity was opened, peritoneal exudates were harvested into 15-ml Falcon tubes, and they were centrifuged at 1,400 g for 5 min after which the cell pellets were resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Mast cells were morphologically identified under a light microscope by their large size and dense content of granules, whereas others, including leucocytes, were significantly smaller, and erythrocytes did not emit fluo-4 fluorescence for lack of esterase.
Measurement of [Ca2+]i.
The isolated mast cells were loaded with fluo-4/AM at a final concentration of 10 μM for 45 min at 37°C with constant mild shaking in the dark. After incubation, the cells were washed and resuspended in RPMI 1640 medium, transferred to a recording chamber, on the bottom of which a cover glass previously coated with Cell-Tak was attached, and left for at least 5 min. The chamber was placed on the stage of an inverted microscope (IX70; Olympus, Tokyo, Japan). Intracellular Ca2+ imaging was performed using a confocal laser scanning microscope (Fluoview FV500; Olympus) as reported previously (36, 37, 38, 39). The cells were illuminated at 488 nm with a krypton/argon laser, and the emission light (>505 nm) was guided through a ×40 water immersion objective to a pinhole diaphragm. Photodamage was minimized by attenuating the laser intensity by interposing a neutral density filter into the illumination path. Usually, 1% transmission was sufficient to obtain fluorescence. Confocal images of mast cells were taken at 10-s intervals. The time courses of changes in fluorescence intensity (FI) in the region of interest (ROI) were analyzed using bundled software, Fluoview 5.0 with Tiempo. The changes in FI were expressed as the percentage of basal FI by setting the fluorescence before the application of drugs at 100% (baseline) (F/F0 ×100). For quantitative analyses, the changes in [Ca2+]i were estimated by calculating the summed area of fluorescence changes (SFC) above the baseline during treatment or stimulation. Standard HEPES-buffered solution contained the following (mM): 138 NaCl, 4.7 KCl, 1.3 CaCl2, 1.13 MgCl2, 1 Na2HPO4, 5.5 d-glucose, and 10 HEPES supplemented with 1 mg/ml BSA was used throughout imaging. Mast cells were continuously perfused at a flow rate of 1 ml/min before and during the experiments.
Determination of intracellular NO production.
Real-time monitoring of the changes in intracellular NO levels was carried out using DAF-2/DA, a cell permeable, NO-sensitive fluorescent probe, as previously reported (36, 37, 38, 39). The isolated mast cells were suspended in RPMI 1640 medium containing 1.149 mM l-arginine that is an intrinsic substrate of NO synthase (NOS) and loaded with DAF-2/DA at a final concentration of 10 μM at 37°C for 45 min with mild shaking in the dark. The cells were then washed, resuspended in l-arginine-containing RPMI 1640 medium and transferred to the recording chamber, and the DAF-2T FI was detected by the same protocol as described above for [Ca2+]i imaging. Subsequently, SFC was calculated. Standard HEPES-buffered solution was used throughout the experiments.
Measurement of intracellular levels of polysulfide.
Intracellular production of polysulfide was monitored using a newly developed fluorescent probe, SSP4 (30), with slight modifications. Briefly, isolated mast cells were loaded with 50 μM SSP4 in a serum-free RPMI 1640 medium containing 0.003% Cremophor EL for 15 min at 37°C in the dark. After being washed, SSP4 FI was detected using the confocal laser scanning microscope (FluoView FV500; Olympus) with the same protocol and optical conditions as applied for the [Ca2+]i analysis. Standard HEPES-buffered solution was used throughout the experiments.
Immunofluorescent staining of isolated mast cells.
The isolated peritoneal mast cells that were attached to Cell-Tak-coated cover glasses were immunostained. The cells were fixed with 4% paraformaldehyde in PBS for 30 min at room temperature, washed with cold PBS, preincubated with a blocking buffer for 45 min at 4°C, and incubated with a rabbit antibody against each of CSE (sc-135203), CBS (sc-6715), endothelial (e)NOS (sc-654), neuronal (n)NOS (sc-648), or inducible (i)NOS (sc-650) (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at 4°C. The mast cells were identified using Abcam mouse monoclonal antibody against tryptase (1:250, code ab2378). Following incubation with the primary antibodies, the cells were rinsed with PBS and incubated with the secondary antibodies, donkey anti-mouse IgG (1:500; AlexaFluor 488, green; code A21202; Life Technologies) and donkey anti-rabbit IgG (1:500; AlexaFluor 546, red; code A10040; Life Technologies) for 1 h at 4°C. The cells were washed, and the nuclei were counterstained with 4,6-diamidino-2-phenylindole dihydrochloride (DAPI; 1:2,000; KPL). Negative controls were processed without primary antibodies. A fluorescent microscope with an imaging device (BIOREVO BZ-9000) was used to identify respective synthase expressions. The number of immunostained positive cells was counted, and the percentage was calculated against the total number of the cells used for analyses in the field.
Estimation of degranulation with SFRM-B.
SFRM-B, a water-soluble red fluorescent probe, was used for detecting exocytosis (16). Mast cells were continuously perfused with standard HEPES-buffered solution contained 20% (wt/vol) SFRM-B at a flow rate of 1 ml/min. The cells were illuminated at 543 nm with a He/Ne laser, and the emission (>560 nm) from the cell of interest was recorded every 10 s for 20 min. The changes in the magnitude of exocytosis were expressed as the FI change in the same way as described for fluo-4.
Statistical analysis.
Our data are presented as means ± SE, and n denotes the number of cells analyzed. Statistical significance was analyzed using the Student's t-test or ANOVA. Significance was recognized when P < 0.05.
RESULTS
Polysulfide induces [Ca2+]i increases in mast cells.
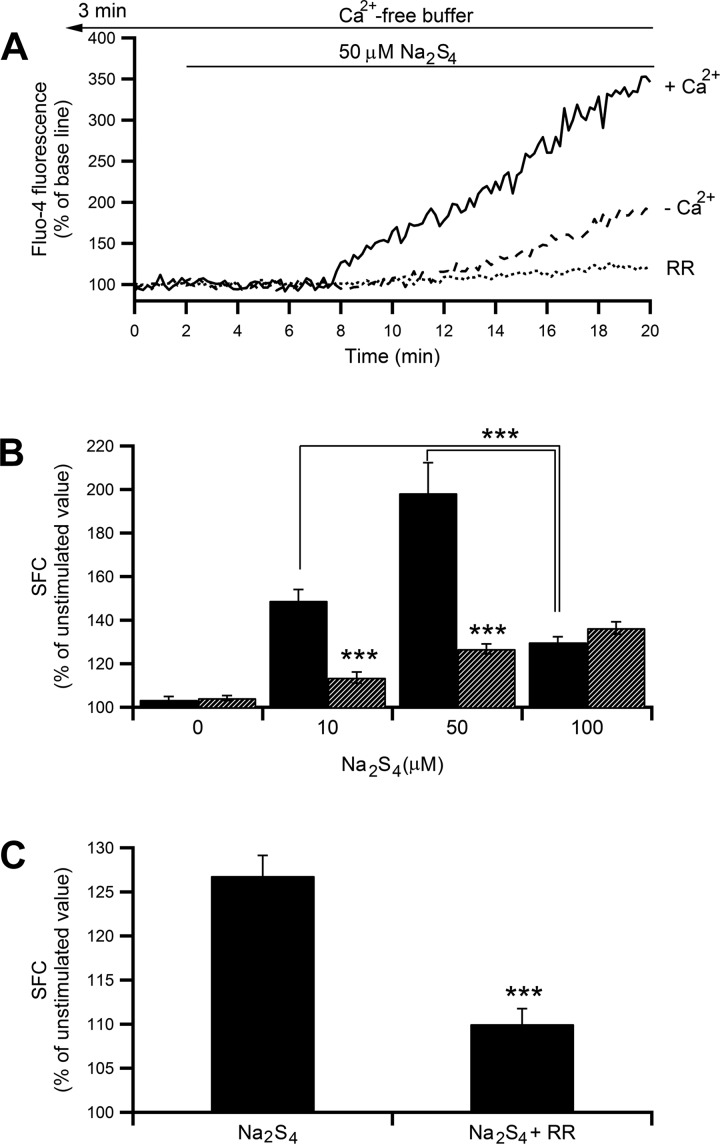
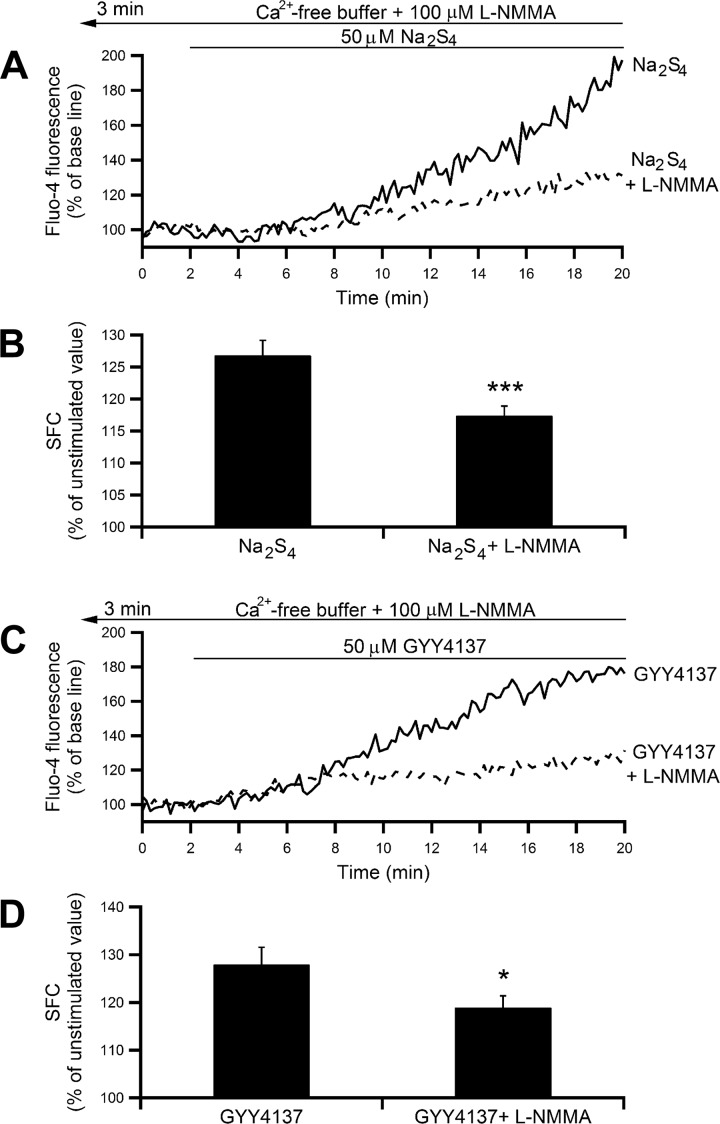
As shown in Fig. 1A, a polysulfide donor, Na2S4 (50 μM), gradually increased [Ca2+]i. Na2S4 appeared to have a concentration-dependent dual effect on [Ca2+]i with a maximum increase at 50 μM Na2S4 (Fig. 1B) followed by attenuation at 100 μM (P < 0.001 by Student's t-test, 100 μM vs. 10 or 50 μM Na2S4). In the absence of extracellular Ca2+, the 10 and 50 μM Na2S4-induced [Ca2+]i increase was largely inhibited (the increment from the unstimulated value was decreased by 72 and 73%, respectively; P < 0.001 by Student's t-test, Fig. 1B) but showed no increase at 100 μM Na2S4. These findings imply that Ca2+ influx is the main source of [Ca2+]i increases at concentrations in the lower and moderate ranges of Na2S4 used and intracellular Ca2+ release only partly contributes to the increase. This does not seem to be the case at the higher concentration, where intracellular Ca2+ release is likely to be the sole contributor to [Ca2+]i increase. As shown in Fig. 1C, in the absence of extracellular Ca2+, the 50 μM Na2S4-induced [Ca2+]i increase was significantly attenuated by the presence of 30 μM RR (the increment from the unstimulated value was decreased by 61%, P < 0.001 by Student's t-test). These results indicate that a large part of the [Ca2+]i increase is associated with extracellular Ca2+ entry, especially at the lower and moderate concentrations, and a remaining part (up to ∼35% of unstimulated value) results from Ca2+ release via ryanodine receptors (RyRs).
Fig. 1.
Effect of Na2S4 on intracellular Ca2+ concentration ([Ca2+]i) in mast cells. A: a representative tracing showing temporal changes in fluo-4 fluorescence intensity (FI) induced by 50 μM Na2S4 in the presence (solid line) or absence (dashed line) of extracellular Ca2+ or in the absence of extracellular Ca2+ but presence of ruthenium red (RR; 30 μM, dotted line). Changes in FI are shown by the percentage against the prestimulation intensity. In the presence of extracellular Ca2+, the cells were perfused with standard HEPES-buffered solution containing 1.3 mM CaCl2 throughout the experiments, and Na2S4 was applied after 2 min. In the absence of extracellular Ca2+, CaCl2 was omitted. Instead, 1 mM EGTA was added, and the cells were perfused with the nominally Ca2+-free buffer for 3 min before starting image acquisition and throughout the experiments. B: dose-response relationship of Na2S4-induced changes in mean (±SE) summed area of fluorescence changes (SFC) in the presence (black bars; n = 12–62) or absence (hatched bars; n = 34–117) of extracellular Ca2+. P < 0.001 by ANOVA; ***P < 0.001 by Student's t-test. C: effect of 30 μM RR on 50 μM Na2S4-induced [Ca2+]i in the absence of extracellular Ca2+. ***P < 0.001 by Student's t-test.
An H2S donor elevates [Ca2+]i in mast cells.
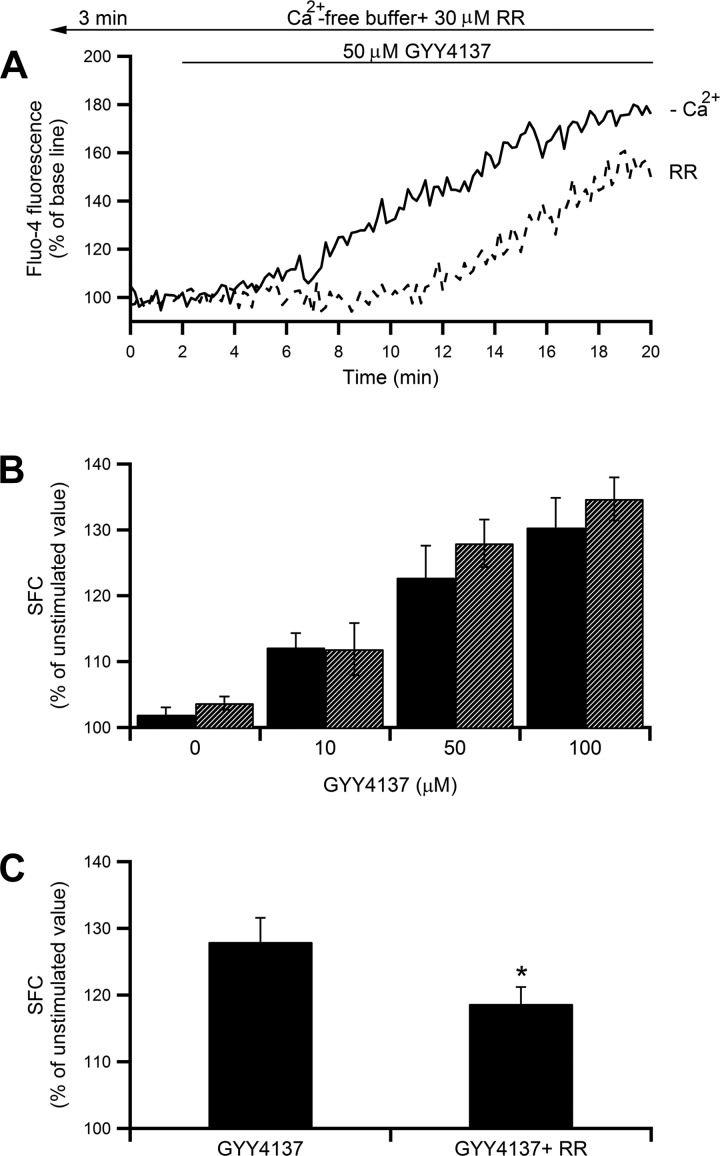
A water-soluble slow-releasing H2S donor, GYY4137, that mimics the endogenously produced H2S (34) was utilized. GYY4137 induced [Ca2+]i increases in concentration-dependent manners (Fig. 2B, black bars, P < 0.001 by ANOVA). The increment induced by 100 μM GYY4137 was ∼35% of the unstimulated value, which seems to be equivalent to the extent found when 100 μM Na2S4 was administered. None of the GYY4137-induced [Ca2+]i increases were affected by the removal of extracellular Ca2+ (Fig. 2B, hatched bars, P < 0.001 by ANOVA), indicating that GYY4137 mainly induces [Ca2+]i elevations via Ca2+ release from intracellular stores. The potential involvement of RyRs in the H2S-induced intracellular Ca2+ release was examined. As shown in Fig. 2, A and C, 50 μM GYY4137-induced intracellular Ca2+ release were partially decreased in the presence of 30 μM RR (Fig. 2C, the increment from the unstimulated value was decreased by 33%, P < 0.05 by Student's t-test).
Fig. 2.
Effect of GYY4137 on [Ca2+]i in mast cells. A: time course of fluo-4 FI changes during treatment with 50 μM GYY4137 in the presence (solid line) or absence (dashed line) of 30 μM RR. Cells were perfused with nominally Ca2+-free buffer containing RR for 3 min before starting image acquisition and throughout the experiments. B: dose-response relationship between increasing concentrations of GYY1437 (10–100 μM) and [Ca2+]i in the presence (black bars; n = 13–56) or absence (hatched bars; n = 9–73) of extracellular Ca2+. P < 0.001 by ANOVA. C: summarized summed area of fluorescence changes (SFC) (means ± SE) of [Ca2+]i increase induced by 50 μM GYY4137 in Ca2+-free buffer in the presence (n = 52) or absence (n = 73) of RR. *P < 0.05 by Student's t-test.
Synthase expression in mast cells.
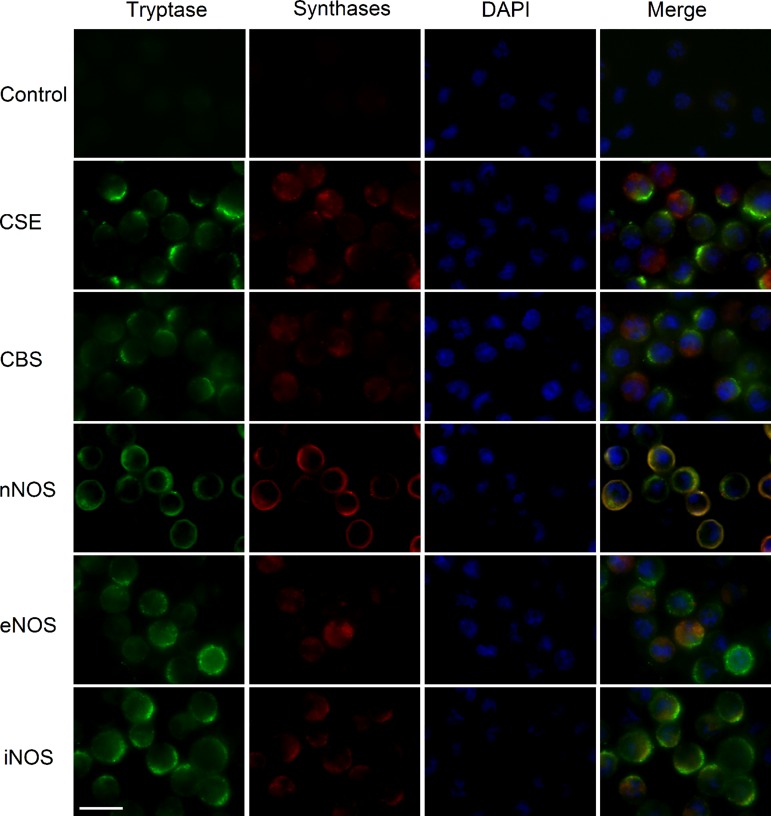
Based on findings that the polysulfide donor and H2S donor could increase [Ca2+]i, the expression of H2S-producing synthases in addition to NO-producing synthases was examined because it has been reported that NO/H2S interaction leads to formation of polysulfide at a physiological pH (6). Immunohistochemical detection revealed the expression of two major H2S-producing synthases, CBS and CSE, and all NO-producing synthases, nNOS, eNOS, and iNOS, in rat peritoneal mast cells (Fig. 3). The percentage of CSE-positive cells tended to be higher than the CBS-positive cells (31 ± 3.3 vs. 22 ± 1.5%). The percentages of nNOS- and eNOS-immunoreactive cells were significantly higher than of iNOS-immunoreactive cells (41 ± 9.7, 26 ± 4.6, and 14 ± 2.01%, respectively; P < 0.01 and P < 0.05, respectively, by Student's t-test). From these results, we presumed that nNOS is the major NOS expressed in rat peritoneal mast cells. Taken together, these results indicate that peritoneal mast cells can synthesize NO and H2S, from which polysulfide can be generated.
Fig. 3.
Expression of NO- and H2S-producing synthases in mast cells. Expression of cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and neuronal nitric oxide synthase (nNOS), endothelial (e)NOS, and inducible (i)NOS in rat peritoneal mast cells was detected. Mast cells were identified by a specific tryptase antibody, and the nuclei were stained with DAPI. Scale bar = 10 μm.
H2S and NO donors stimulate polysulfide production.
As both H2S- and NO-producing synthases are found to be expressed in mast cells, whether H2S and NO can stimulate polysulfide production was examined using SSP4, a polysulfide sensitive fluorescent probe. As shown Fig. 4, A–D, GYY4137 dose dependently increased the SSP4 FI, suggesting the stimulation of the polysulfide production (P < 0.001 by ANOVA), and DEA NONOate, an NO donor increased the SSP4 FI in a concentration-dependent manner (P < 0.001 by ANOVA).
Fig. 4.
Effect of GYY4137 and diethylamine (DEA) NONOate on polysulfide production. A and C: typical examples of temporal changes in SSP4 FI induced by 50 μM of GYY4137 or DEA NONOate. Cells were perfused with standard HEPES-buffered solution throughout the experiments. B and D: dose-response relationships of GYY4137- and DEA NONOate-induced changes in SSP4 FI (n = 9–25 and 19–38, respectively). Each column represents mean of summed area of fluorescence changes (SFC) ± SE. P < 0.001 by ANOVA.
Polysulfide donor and H2S donor stimulate NO production in mast cells.
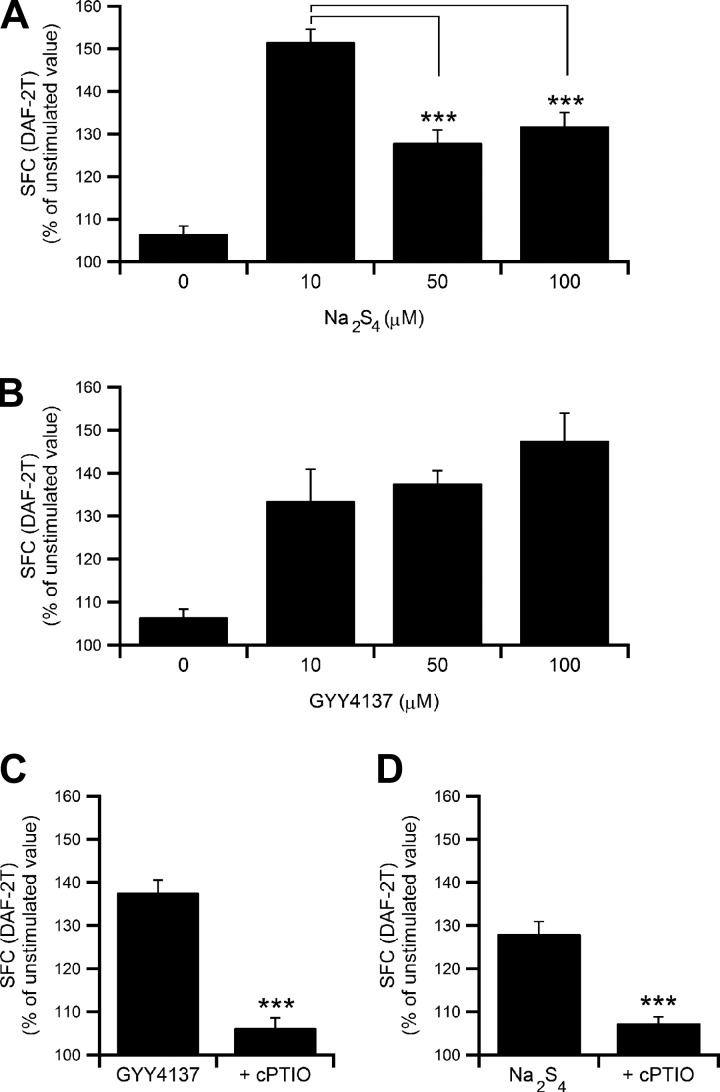
Whether NO is produced after application of a polysulfide or H2S donor was then assessed. When mast cells were treated with various concentrations of Na2S4 or GYY4137 in standard HEPES-buffered solution, Na2S4 (10–100 μM) induced a biphasic increase in the DAF-2T fluorescence, indicating that NO is produced in isolated mast cells, and the maximum response was at 10 μM (Fig. 5A). The Na2S4-induced elevation in NO levels was somewhat less at 50 and 100 μM than at 10 μM (Fig. 5A, P < 0.001 by Student's t-test). GYY4137 also induced NO production in a concentration-dependent manner (10–100 μM, Fig. 5B, P < 0.05 by ANOVA). To assess whether the increase in DAF-2T fluorescence is attributed to the increase in the intracellular NO levels, an NO scavenger, cPTIO (300 μM), was utilized. As shown in Fig. 5, C and D, the GYY4137 (50 μM)- or Na2S4 (50 μM)-induced NO production was largely inhibited in the presence of cPTIO (the increment from the unstimulated value was decreased by 83% and 75% respectively, P < 0.001 by Student's t-test). The finding indicates that DAF-2 can be utilized for quantitative measurement of endogenously produced NO and unexpected interference by other unknown substances would be minor even if it were the case.
Fig. 5.
Effect of Na2S4 and GYY4137 on intracellular NO levels in mast cells. A: summarized summed area of fluorescence changes (SFC) (means ± SE) of NO production induced by increasing concentrations of Na2S4 (10–100 μM, n = 8–46). P < 0.001 by ANOVA; ***P <0.001 by Student's t-test for 50 and 100 μM Na2S4 vs. 10 μM. B: Concentration-dependent effect of NO production induced by increasing concentrations of GYY4137 (10–100 μM, n = 8–55). Each column represents mean SFC ± SE of DAF-2T fluorescence intensity. P < 0.05 by ANOVA. C and D: mean SFC (±SE) of 50 μM of GYY4137- or Na2S4-induced changes in NO production in the presence (n = 17 and 39) or absence (n = 51 and 45) of cPTIO (300 μM). ***P < 0.001 by Student's t-test.
Polysulfide and H2S induce intracellular Ca2+ release via NOS activation.
Based on the findings that polysulfide and H2S donors induce the [Ca2+]i increase partly via Ca2+ release through RyRs and that both donors stimulate intracellular NO production, a possibility that NO thus produced would be involved in both polysulfide- and H2S-induced [Ca2+]i release was examined using a nonspecific inhibitor of all NOS isoforms, l-NMMA. As illustrated in Figs. 6, A and B, pretreatment of mast cells with 100 μM l-NMMA in the Ca2+-free medium significantly attenuated the [Ca2+]i release induced by 50 μM Na2S4 (the increment from the unstimulated value was decreased by 34%, P < 0.001 by Student's t-test, Fig. 6B). The [Ca2+]i release induced by GYY4137 (50 μM) was partially attenuated as well by pretreatment with l-NMMA (Fig. 6, C and D; the increment from the unstimulated value was decreased by 33%, P < 0.05 by Student's t-test). These findings indicate that NOS activation and subsequent NO production, at least in part, contribute to the intracellular Ca2+ release accelerated by polysulfide and H2S.
Fig. 6.
Effect of NOS inhibitor on Na2S4- and GYY4137-induced [Ca2+]i increase. A and C: representative tracings showing the effect of Na2S4 (50 μM) or GYY4137 (50 μM) on [Ca2+]i in the presence (solid line) or absence (dashed line) of 100 μM NG-monomethyl-l-arginine (l-NMMA). Cells were perfused with nominally Ca2+-free buffer containing l-NMMA for 3 min before starting image acquisition and throughout the experiments. Either a polysulfide donor or H2S donor was added to the perfusate 2 min after starting image acquisition. B and D: mean ± SE summed area of fluorescence changes (SFC) of [Ca2+]i increase induced by Na2S4 or GYY4137 in the presence (n = 102 and 76) or absence (n = 117 and 73) of l-NMMA. ***P < 0.001 and *P < 0.05 by Student's t-test, respectively.
NO increases [Ca2+]i in mast cells.
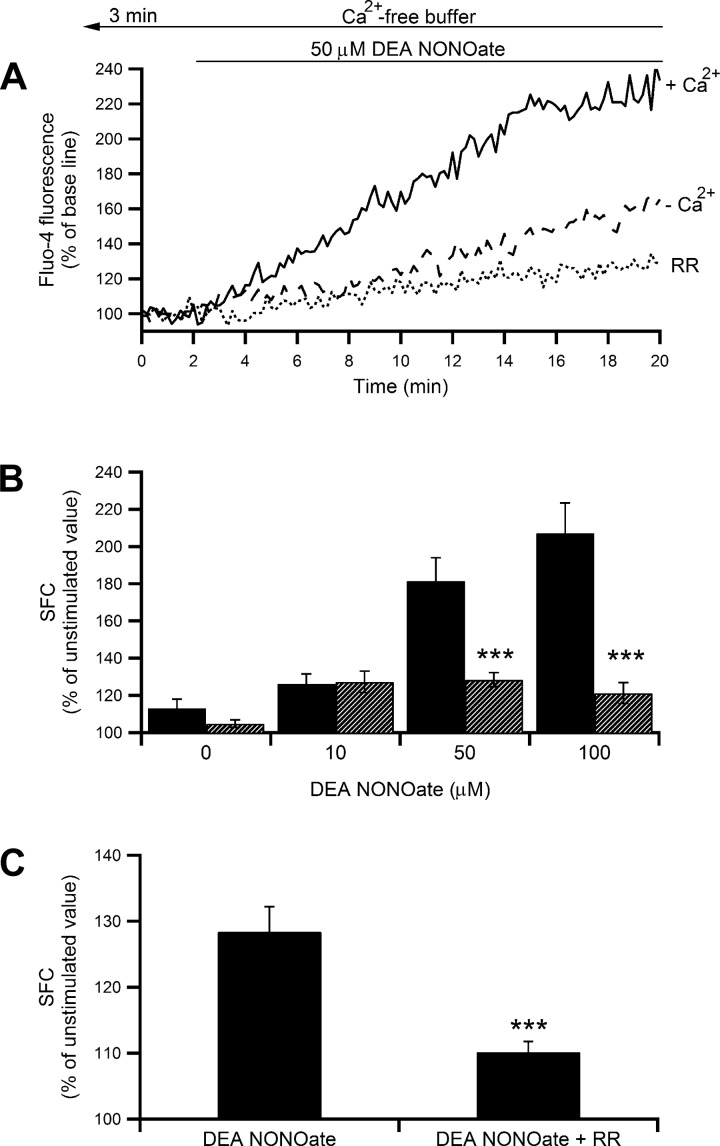
Mast cells were treated with increasing concentrations of DEA NONOate (10–100 μM) in standard HEPES-buffered solution. The donor evoked a dose-dependent [Ca2+]i increase up to a maximum increment of ∼110%, as shown in Fig. 7, A and B (solid line and black bars, P < 0.001 by ANOVA). The [Ca2+]i increase induced by 10 μM DEA NONOate, which was ∼30% of the unstimulated level, persisted and was unaffected even after the removal of extracellular Ca2+. However, at higher concentrations (50 and 100 μM), the DEA NONOate-induced [Ca2+]i increase was significantly less in the absence of extracellular Ca2+, which implies that the major part of the [Ca2+]i increase is via Ca2+ influx, and Ca2+ release represents ∼30% (Fig. 7, A and B; dashed line and hatched bars, P < 0.001 by Student's t-test). Pretreatment of mast cells with RR (30 μM) in the absence of extracellular Ca2+ significantly decreased the [Ca2+]i increase induced by the donor (Fig. 7, A and C, dotted line; the increment from the unstimulated value was decreased by 64%, P < 0.001 by Student's t-test). These findings suggest that NO causes intracellular Ca2+ release via RyRs channels.
Fig. 7.
Effect of DEA NONOate on [Ca2+]i. A: temporal changes of fluo-4 fluorescence intensity in mast cells stimulated with 50 μM DEA NONOate in the presence (solid line) or absence (dashed line) of extracellular Ca2+ or absence of extracellular Ca2+ but presence of 30 μM ruthenium red (RR; dotted line). Cells were perfused with nominally Ca2+-free buffer with RR for 3 min before starting image acquisition and throughout experiments, and treatment with DEA NONOate was 2 min after starting image acquisition. B: summarized data showing the dose-dependent effect of increasing concentrations of DEA NONOate (10–100 μM) on [Ca2+]i in the presence (black bars; n = 21–31) or absence (hatched bars; n = 13–41) of extracellular Ca2+. Each column represents mean ± SE summed area of fluorescence changes (SFC). P < 0.001 by ANOVA; ***P < 0.001 by Student's t-test for 50 and 100 μM DEA NONOate in the presence vs. absence of extracellular Ca2+. C: mean ± SE SFC of 50 μM DEA NONOate-induced [Ca2+]i increase in the presence (n = 65) or absence of RR (n = 41). ***P < 0.001 by Student's t-test.
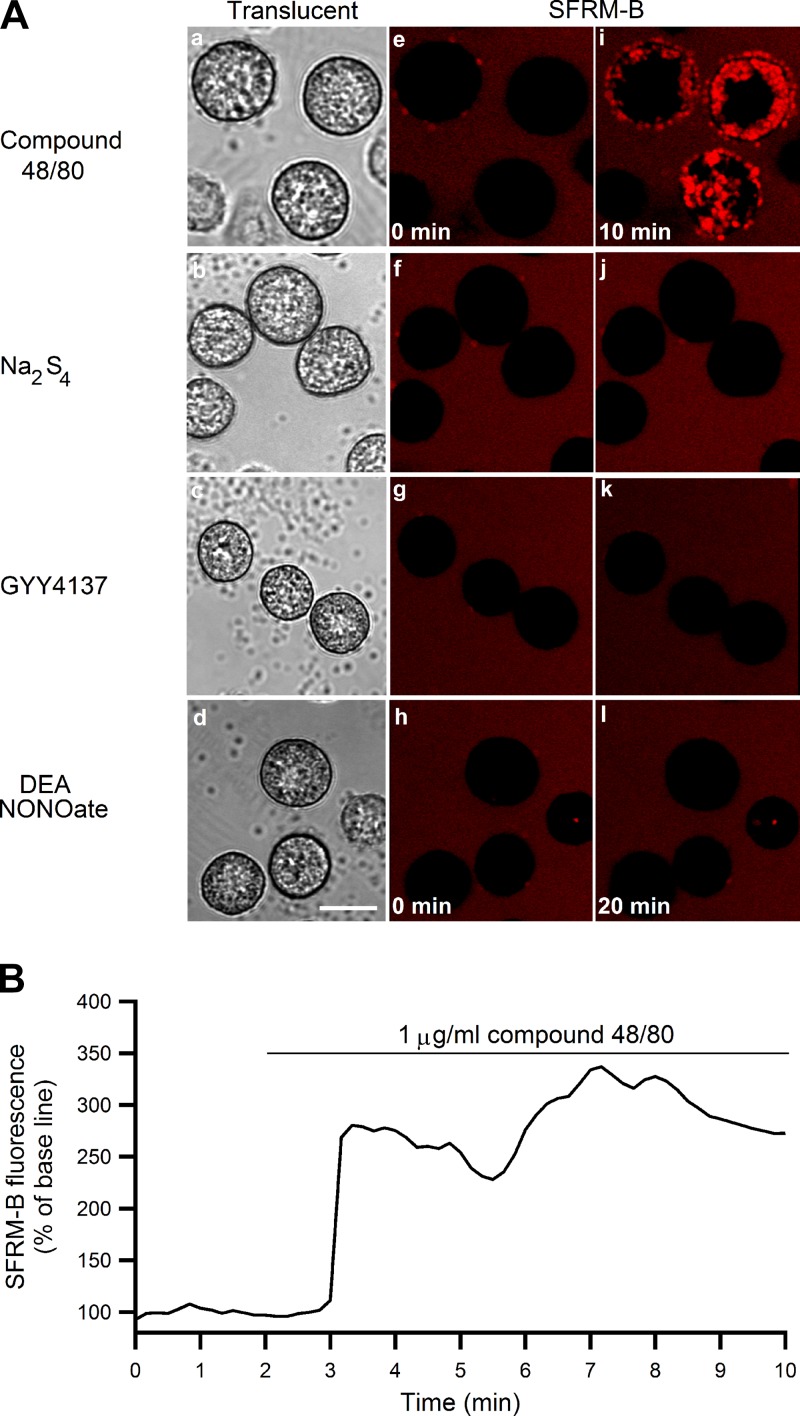
Polysulfide, H2S, and NO donors have no effect on mast cell degranulation.
As shown in Fig. 8A, compound 48/80 (1 μg/ml) stimulated degranulation (Fig. 8Ai). Stimulation of mast cells with Na2S4, GYY4137, or DEA NONOate (50 μM each) showed no obvious effect on exocytosis induction up to 20 min after stimulation (Fig. 8, Aj-Al). Figure 8B shows a time course of 1 μg/ml compound 48/80-induced SFRM-B fluorescence change. Whereas compound 48/80 triggered exocytosis starting one min after application of the chemical (Fig. 8B), Na2S4, GYY4137, and DEA NONOate could not elicit degranulation even after 20 min of application.
Fig. 8.
Effect of compound 48/80, Na2S4, GYY4137, and DEA NONOate on mast cell degranulation. A: translucent (a–d) and sulforhodamine-B (SFRM-B)-loaded fluorescent images of mast cells before (e–h) and after (i–l) stimulation with compound 48/80 for 10 min (1 μg/ml), Na2S4, GYY4137, and DEA NONOate for 20 min (50 μM each) in standard HEPES-buffered solution. Appearance of red fluorescence represents occurrence of exocytosis. Scale bar = 10 μm B: An example of time course changes of SFRM-B fluorescence intensity in mast cells stimulated with compound 48/80 (1 μg/ml) for 10 min in standard HEPES-buffered solution.
DISCUSSION
In general, it is well accepted that increases in [Ca2+]i mediate stimulus-secretion coupling in various secretory cells. In mast cells, exocytosis is also closely associated with the increase in [Ca2+]i (25), the source of which is initially intracellular Ca2+ release followed by extracellular Ca2+ entry, inducing massive exocytosis (15). Inositol trisphosphate receptors (IP3Rs) have been proposed to play a role in inducing Ca2+ release, resulting in the initiation of exocytosis in mast cells (12). The involvement of RyRs was also demonstrated in mast cells (14, 23, 46). A series of studies from our laboratory demonstrated that gaseous molecules such as H2S, NO, and CO could modulate [Ca2+]i in exocrine and endocrine cells (36, 37, 38, 39, 40), suggesting that IP3Rs mainly mediate Ca2+ release in pancreatic acinar cells, but RyRs are involved in the release in parotid acinar cells when stimulated with sodium hydrosulfide, a fast-releasing H2S donor (37, 38). Immunohistochemical detection of H2S synthases, CSE and CBS, implied that H2S is endogenously produced in mast cells (Fig. 3). The present study additionally demonstrated that H2S increased [Ca2+]i in a dose-dependent manner, in which Ca2+ release from the ryanodine-sensitive store is mostly the entire source of [Ca2+]i increases in mast cells (Fig. 2). Thus, we presume that, as in other secretory cells, endogenously produced H2S is involved in the modulation of [Ca2+]i homeostasis in mast cells.
It has been reported that exogenously applied H2S is absorbed and stored as bound sulfane sulfur (22) in tissues, which in turn releases H2S once the cell is activated in the presence of a reducing agent (28). In addition to this reaction, polysulfides can be generated from H2S in the presence of oxidant (41) and from NO/H2S interaction (6). In support of above reactions, the present study using SSP4 also suggested that polysulfide could be accumulated after H2S or NO application (Fig. 4). In an attempt to explore the possible role of polysulfide in the regulation of [Ca2+]i in isolated rat peritoneal mast cells, we found that, like the H2S donor, the polysulfide donor could also induce the [Ca2+]i increase in mast cells, and the maximum effect was at 50 μM. The polysulfide-induced [Ca2+]i increase was dependent on both Ca2+ influx and Ca2+ release, especially at lower and moderate concentrations, but the increase was solely result from intracellular Ca2+ release at the highest concentration, which was likely to be hampered by unknown mechanism(s) (Fig. 1B). Like H2S, RR significantly inhibited the Ca2+ release elicited by the polysulfide donor (Fig. 1B). In astrocytes, it has been reported that the polysulfide-produced [Ca2+]i increase was abolished in the absence of extracellular Ca2+ or by the addition of RR, which was utilized as a nonspecific TRP channel blocker (29). Our experiments were performed using RR in the absence of extracellular Ca2+ to expect blocking the RyRs channels. Although RR may have dual effects on TRP channels and RyRs, the hypothesis that Ca2+ release occurs through RyRs would not be excluded because, under our experimental condition with the nominally Ca2+-free solution, TRP channels, which can be inhibited by RR, are no longer functionally involved in the [Ca2+]i increase, even if it is activated. Taken together, these findings indicate that polysulfide can modulate Ca2+ homeostasis in mast cells by inducing both Ca2+ release and Ca2+ influx and the latter to be considered the main source of Ca2+ elevations. In mouse sensory neurons, rat astrocytes, and neuroblastoma cells, TRPA1 channels have been shown to be involved in the polysulfide-elicited [Ca2+]i increase by accelerating Ca2+ influx (17, 29, 32). A functional role for TRP channel family was suggested in mast cell-derived cell lines or primary mast cells (8). A major portion of the [Ca2+]i increase found in the present experiment is most likely to be due to polysulfide-induced Ca2+ influx via TRP channels except for the highest concentration of polysulfide, while Ca2+ influx was likely to be hampered by unknown mechanism(s) at higher concentrations. What is suggested here is that polysulfide can be an additional signaling molecule in mast cell.
Reportedly, NO is produced by its synthases in mast cells that can be a source and target cells of NO (4). Ubiquitous expression of eNOS and variable expressions of nNOS were demonstrated in a RT-PCR study, but iNOS expression was not detected in human mast cell lines (10). Another study showed eNOS and nNOS but not iNOS mRNA was present (45). In rat peritoneal mast cells, a limited amount of eNOS mRNA was detected in unstimulated cells, but neither nNOS nor iNOS mRNA was identified (11). In the present study, nNOS was found to be predominant and eNOS was less, while iNOS was least predominant (Fig. 3). This result would be supportive of endogenous production of NO. In mast cells, NO is presumed to be a modulatory molecule that inhibits multitudinous allergy-associated activities but enhances other non-allergy-associated responses (35). It has also been reported that NO suppresses mast cell activation and antigen-induced degranulation (7). NO produced via the phosphatidylinositol 3-kinase-Akt-eNOS pathway is assumed to be involved in protecting mast cells from cell death (20). Together, it seems highly possible that not only H2S, but also NO, can be endogenously produced, which modulates mast cell functions.
In our recent studies, functional synergism between H2S and NO was proposed (37, 38, 39). The present finding that both polysulfide and H2S could induce NO production (Fig. 5) and that the polysulfide- or H2S-induced [Ca2+]i increase was attenuated by the NOS inhibitor (Fig. 6) would be indicative of polysulfide or H2S-induced NO production. Hence, the [Ca2+]i increase induced by polysulfide or H2S could partly be attributed to concomitantly produced NO. As shown in Fig. 7, NO from DEA NONOate was found to dose dependently increase [Ca2+]i by both Ca2+ release and Ca2+ influx. Like polysulfide and H2S, the NO-induced Ca2+ release was RyR dependent. Interestingly, when the Ca2+ release is highlighted, one consistency was that the magnitudes of Ca2+ release, expressed as percentages of unstimulated basal levels, were at most 30–35% for polysulfide, H2S, and even NO (Figs. 1, 2, and 7), which was also equal to the response observed in other secretory cells (37, 38, 39). Given that NO plays a central role in Ca2+ release induced by polysulfide and H2S, a question one may have would be why the extracellular Ca2+ dependency is unequal between the polysulfide donor (Fig. 1B) and the H2S donor (Fig. 2B) even though both produce the same amount of NO (Fig. 5, A and B). Although we do not have a clear answer at present, the notion that polysulfide induces Ca2+ influx ∼300 times as efficiently as H2S (28) may hint an explanation as to why, in this study, polysulfide could induce both Ca2+ influx and Ca2+ release but H2S could evoke only Ca2+ release. The indication that H2S generation from GYY4137 occurs in a slow manner and only 4–5% H2S will be generated from a starting concentration of 1 mM within 25 min (34), in addition the exposure time in our experiments was only for 20 min, makes us presume that the final concentration of H2S after application of GYY4137 was not enough to induce Ca2+ influx.
We recently reported a curious phenomenon exerted by an NO donor and an H2S donor in such a way that the former increased, but the latter hardly increased basal insulin release, yet both donors can clearly increase [Ca2+]i in single pancreatic β-cells (39). Similarly, we found that a CO donor could increase [Ca2+]i in pancreatic endocrine and exocrine cells, but the donor dose dependently inhibited basal insulin release and secretagogues-induced amylase release (39, 36). Moreover in this study, all polysulfide, H2S, and NO donors were found to induce [Ca2+]i increase (Figs. 1, 2, and 7) but had no obvious effect on mast cell degranulation, while the G-protein stimulant compound 48/80 could trigger exocytosis (Fig. 8). It seems undoubtable that the [Ca2+]i increase is essential, but under some specific conditions, the increase by itself is not enough to induce exocytosis. Some unknown machinery, in addition to the [Ca2+]i increase, is presumably mandatory for linking the [Ca2+]i increase with exocytosis. In this context, it should be note that, although paradoxical, NO somewhat suppresses antigen-induced mast cell degranulation and H2S abrogates in vitro degranulation in mast cell lines (5, 43). The gaseous molecules aside, it was reported that intracellular alkalinization is associated with compound 48/80-induced histamine release without the [Ca2+]i increase (24, 9) and that cytosolic alkalinization, but not intracellular calcium release, is a sufficient signal for degranulation in mast cells (1, 2). Furthermore, it is claimed that the increase in [Ca2+]i is neither necessary nor sufficient for secretion from mast cells but acts synergistically with other stimuli to promote secretion (42). Although our results showed that the increase in [Ca2+]i by polysulfide, NO, and H2S did not induce exocytosis, we speculate that these substances may have a permissive effect on other secretagogue-induced exocytosis by enhancing the intracellular [Ca2+]i levels, and the interaction among biogases would regulate various cellular functions that are related with intracellular Ca2+ homeostasis. Further studies are required to explore such possibility.
In summary, it is conceivable that NO, H2S, and polysulfide are endogenously produced gases, functioning as signaling molecules that regulate [Ca2+]i homeostasis in rat peritoneal mast cells. However, for unknown reasons, such [Ca2+]i changes are not simply associated with the degranulation process under the present experimental conditions. In the regulation of [Ca2+]i homeostasis, polysulfide and H2S most likely act via a cross talk among them and NO may play a central role in the effects exerted by sulfur compounds in rat peritoneal mast cells.
GRANTS
This study was supported by the F3 Project at Hokkaido University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.M. and Y.H. conception and design of research; A.M. performed experiments; A.M. analyzed data; A.M. and Y.H. interpreted results of experiments; A.M. and Y.H. prepared figures; A.M. and Y.H. drafted manuscript; A.M. and Y.H. edited and revised manuscript; A.M. and Y.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank M. Okawa and K. Ozaki for assistance.
REFERENCES
- 1.Alfonso A, Cabado AG, Vieytes MR, Botana LM. Calcium-pH crosstalks in rat mast cells: cytosolic alkalinization, but not intracellular calcium release, is a sufficient signal for degranulation. Br J Pharmacol 130: 1809–1816, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfonso A, Vieytes MR, Botana LM. Calcium-pH crosstalks in rat mast cells: modulation by transduction signals show non-essential role for calcium in alkaline-induced exocytosis. Biochem Pharmacol 69: 319–327, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, Darley-Usmar VM, Doeller JE, Kraus DW. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA 104: 17977–17982, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidri M, Féger F, Varadaradjalou S, Ben Hamouda N, Guillosson JJ, Arock M. Mast cells as a source and target for nitric oxide. Int Immunopharmacol 1: 1543–1558, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Coleman JW. Nitric oxide: a regulator of mast cell activation and mast cell-mediated inflammation. Clin Exp Immunol 129: 4–10, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortese-Krott MM, Kuhnle GG, Dyson A, Fernandez BO, Grman M, DuMond JF, Barrow MP, McLeod G, Nakagawa H, Ondrias K, Nagy P, King SB, Saavedra JE, Keefer LK, Singer M, Kelm M, Butler AR, Feelisch M. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc Natl Acad Sci USA 112: E4651–E4660, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eastmond NC, Banks EM, Coleman JW. Nitric oxide inhibits IgE-mediated degranulation of mast cells and is the principal intermediate in IFN-gamma-induced suppression of exocytosis. J Immunol 159: 1444–14450, 1997. [PubMed] [Google Scholar]
- 8.Freichel M, Almering J, Tsvilovskyy V. The role of TRP proteins in mast cells. Front Immunol 3: 150, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friis UG, Johansen T. Dual regulation of the Na+/H+-exchange in rat peritoneal mast cells: role of protein kinase C and calcium on pHi regulation and histamine release. Br J Pharmacol 118: 1327–1334, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilchrist M, McCauley SD, Befus AD. Expression, localization, and regulation of NOS in human mast cell lines: effects on leukotriene production. Blood 104: 462–469, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Gilchrist M, Savoie M, Nohara O, Wills FL, Wallace JL, Befus AD. Nitric oxide synthase and nitric oxide production in in vivo-derived mast cells. J Leukoc Biol 71: 618–624, 2002. [PubMed] [Google Scholar]
- 12.Gilfillan AM, Tkaczyk C. Integrated signaling pathways for mast-cell activation. Nat Rev Immunol 6: 218–230, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Greiner R, Pálinkás Z, Bäsell K, Becher D, Antelmann H, Nagy P, Dick TP. Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19: 1749–1765, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzmán RE, Bolaños P, Delgado A, Rojas H, DiPolo R, Caputo C, Jaffe EH. Depolymerisation and rearrangement of actin filaments during exocytosis in rat peritoneal mast cells: involvement of ryanodine-sensitive calcium stores. Pflügers Arch 454: 131–141, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Habara Y, Kanno T. Perimetric [Ca2+]i rise and exocytosis detected by ultraviolet laser scanning confocal microscopy in rat peritoneal mast cells. Exp Physiol 81: 319–328, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Hashikura S, Satoh Y, Cui ZJ, Habara Y. Photodynamic action inhibits compound 48/80-induced exocytosis in rat peritoneal mast cells. Jpn J Vet Res 49: 239–247, 2001. [PubMed] [Google Scholar]
- 17.Hatakeyama Y, Takahashi K, Tominaga M, Kimura H, Ohta T. Polysulfide evokes acute pain through the activation of nociceptive TRPA1 in mouse sensory neurons. Mol Pain 11: 24, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hylin JW, Wood JL. Enzymatic formation of polysulfides from mercaptopyruvate. J Biol Chem 234: 2141–2144, 1959. [PubMed] [Google Scholar]
- 19.Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci USA 111: 7606–7611, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue T, Suzuki Y, Yoshimaru T, Ra C. Nitric oxide protects mast cells from activation-induced cell death: the role of the phosphatidylinositol-3 kinase-Akt-endothelial nitric oxide synthase pathway. J Leukoc Biol 83: 1218–1229, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Inoue T, Suzuki Y, Yoshimaru T, Ra C. Nitric oxide positively regulates Ag (I)-induced Ca2+ influx and mast cell activation: role of a nitric oxide synthase-independent pathway. J Leukoc Biol 86: 1365–1375, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11: 205–214, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe EH, Bolaños P, Galvis G, Caputo C. Ryanodine receptors in peritoneal mast cells: possible role in the modulation of exocytotic activity. Pflügers Arch 447: 377–386, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Jensen TB, Friis UG, Johansen T. Role of physiological HCO3−buffer on intracellular pH and histamine release in rat peritoneal mast cells. Pflügers Arch 436: 357–364, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Kassel O, Amrani Y, Landry Y, Bronner C. Mast cell activation involves plasma membrane potential- and thapsigargin-sensitive intracellular calcium pools. Fundam Clin Pharmacol 9: 531–539, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Kawabata A, Ishiki T, Nagasawa K, Yoshida S, Maeda Y, Takahashi T, Sekiguchi F, Wada T, Ichida S, Nishikawa H. Hydrogen sulfide as a novel nociceptive messenger. Pain 132: 74–81, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kimura H, Shibuya N, Kimura Y. Hydrogen sulfide is a signaling molecule and a cytoprotectant. Antioxid Redox Signal 17: 45–57, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide 41: 4–10, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Kimura Y, Mikami Y, Osumi K, Tsugane M, Oka J, Kimura H. Polysulfides are possible H2S-derived signaling molecules in rat brain. FASEB J 27: 2451–2457, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Kimura Y, Toyofuku Y, Koike S, Shibuya N, Nagahara N, Lefer D, Ogasawara Y, Kimura H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci Rep 5: 14774, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koike S, Ogasawara Y, Shibuya N, Kimura H, Ishii K. Polysulfide exerts a protective effect against cytotoxicity caused by t-buthylhydroperoxide through Nrf2 signaling in neuroblastoma cells. FEBS Lett 587: 3548–3555, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Koike S, Shibuya N, Kimura H, Ishii K, Ogasawara Y. Polysulfide promotes neuroblastoma cell differentiation by accelerating calcium influx. Biochem Biophys Res Commun 459: 488–492, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Bhatia M, Moore PK. Hydrogen sulphide a novel mediator of inflammation? Curr Opin Pharmacol 6: 125–129, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH, Moore PK. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation 117: 2351–2360, 2008. [DOI] [PubMed] [Google Scholar]
- 35.McCauley SD, Gilchrist M, Befus AD. Nitric oxide: a major determinant of mast cell phenotype and function. Mem Inst Oswaldo Cruz 100, Suppl 1: 11–14, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Moustafa A, Habara Y. A novel role for carbon monoxide as a potent regulator of intracellular Ca2+ and nitric oxide in rat pancreatic acinar cells. Am J Physiol Cell Physiol 307: C1039–C1049, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Moustafa A, Habara Y. Hydrogen sulfide regulates Ca2+ homeostasis mediated by concomitantly produced nitric oxide via a novel synergistic pathway in exocrine pancreas. Antioxid Redox Signal 20: 747–758, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moustafa A, Habara Y. Hydrogen sulfide: a novel gaseous signaling molecule and intracellular Ca2+ regulator in rat parotid acinar cells. Am J Physiol Cell Physiol 309: C480–C490, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Moustafa A, Habara Y. Reciprocal interaction among gasotransmitters in isolated pancreatic β-cells. Free Radic Biol Med 90: 47–58, 2016. [DOI] [PubMed] [Google Scholar]
- 40.Moustafa A, Sakamoto KQ, Habara Y. A fundamental role for NO-PLC signaling pathway in mediating intracellular Ca2+ oscillation in pancreatic acini. Nitric Oxide 24: 139–150, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Nagy P, Winterbourn CC. Rapid reaction of hydrogen sulfide with the neutrophil oxidant hypochlorous acid to generate polysulfides. Chem Res Toxicol 23: 1541–1543, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol 395: 193–214, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roviezzo F, Bertolino A, Sorrentino R, Terlizzi M, Matteis M, Calderone V, Mattera V, Martelli A, Spaziano G, Pinto A, D'Agostino B, Cirino G. Hydrogen sulfide inhalation ameliorates allergen induced airway hypereactivity by modulating mast cell activation. Pharmacol Res 100: 85–92, 2015. [DOI] [PubMed] [Google Scholar]
- 44.Siebert N, Cantré D, Eipel C, Vollmar B. H2S contributes to the hepatic arterial buffer response and mediates vasorelaxation of the hepatic artery via activation of KATP channels. Am J Physiol Gastrointest Liver Physiol 295: G1266–G1273, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki Y, Inoue T, Ra C. Endothelial nitric oxide synthase is essential for nitric oxide generation, L-type Ca2+ channel activation and survival in RBL-2H3 mast cells. Biochim Biophys Acta 1803: 372–385, 2010. [DOI] [PubMed] [Google Scholar]
- 46.Takei M, Ueno M, Endo K. Effect of ryanodine on histamine release from rat peritoneal mast cells induced by anti-IgE. J Pharm Pharmacol 44: 523–525, 1992. [DOI] [PubMed] [Google Scholar]
- 47.Webb GD, Lim LH, Oh VM, Yeo SB, Cheong YP, Ali MY, El Oakley R, Lee CN, Wong PS, Caleb MG, Salto-Tellez M, Bhatia M, Chan ES, Taylor EA, Moore PK. Contractile and vasorelaxant effects of hydrogen sulfide and its biosynthesis in the human internal mammary artery. J Pharmacol Exp Ther 324: 876–882, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]