Abstract
Plasma membrane-localized CaV1.2 channels are the primary calcium (Ca2+) influx pathway in arterial smooth muscle cells (myocytes). CaV1.2 channels regulate several cellular functions, including contractility and gene expression, but the trafficking pathways that control the surface expression of these proteins are unclear. Similarly, expression and physiological functions of small Rab GTPases, proteins that control vesicular trafficking in arterial myocytes, are poorly understood. Here, we investigated Rab proteins that control functional surface abundance of CaV1.2 channels in cerebral artery myocytes. Western blotting indicated that Rab25, a GTPase previously associated with apical recycling endosomes, is expressed in cerebral artery myocytes. Immunofluorescence Förster resonance energy transfer (immunoFRET) microscopy demonstrated that Rab25 locates in close spatial proximity to CaV1.2 channels in myocytes. Rab25 knockdown using siRNA reduced CaV1.2 surface and intracellular abundance in arteries, as determined using arterial biotinylation. In contrast, CaV1.2 was not located nearby Rab11A or Rab4 and CaV1.2 protein was unaltered by Rab11A or Rab4A knockdown. Rab25 knockdown resulted in CaV1.2 degradation by a mechanism involving both lysosomal and proteasomal pathways and reduced whole cell CaV1.2 current density but did not alter voltage dependence of current activation or inactivation in isolated myocytes. Rab25 knockdown also inhibited depolarization (20–60 mM K+) and pressure-induced vasoconstriction (myogenic tone) in cerebral arteries. These data indicate that Rab25 is expressed in arterial myocytes where it promotes surface expression of CaV1.2 channels to control pressure- and depolarization-induced vasoconstriction.
Keywords: arterial smooth muscle cells, voltage-dependent calcium channel, smooth muscle, vasoconstriction, Rab GTPase
voltage-dependent calcium (Ca2+, CaV) channels are the primary plasma membrane Ca2+ entry pathway in many cell types, including smooth muscle cells (myocytes) of small resistance-size arteries (19). At least 10 different CaV channels subdivided into families CaV1 through 3 are expressed in mammals (9). Arterial myocytes express several CaV channels, principally CaV1.2, but also CaV3.2 and CaV3.3 (1, 11, 20, 21). CaV1.2 channels regulate multiple physiological functions in arterial myocytes, including gene expression and contractility, to control regional organ blood flow and systemic blood pressure (19, 26).
Intravascular pressure stimulates arterial myocyte depolarization, leading to CaV1.2 channel activation, Ca2+ influx, and vasoconstriction (23, 35). This reaction, termed the “myogenic response,” maintains blood flow during changes in intravascular pressure and provides a level of tone that can subsequently be modulated by both vasoconstrictors and vasodilators (23). To regulate extracellular Ca2+ influx and thus control arterial contractility, CaV1.2 channels must traffic from the endoplasmic reticulum/Golgi complex to the plasma membrane. Although this is an essential step in CaV1.2 functionality, the mechanisms that traffic and control the surface expression of CaV1.2 channels in arterial myocytes are poorly understood.
The plasma membrane abundance of proteins is determined by both anterograde and retrograde trafficking pathways (24, 37). Protein trafficking is regulated by a large family of ∼60 Rab GTPases that control the movement of vesicles between different cellular compartments (24, 33, 37). Rab GTPases have been demonstrated to promote surface expression of a variety of ion channel proteins including Kv1.5, human ether-a-go-go-related gene (hERG), epithelial sodium channel (ENaC), large-conductance Ca2+-activated K+ (BKCa), transient receptor potential (TRP)V5, and TRPC6 (6, 13, 28, 29, 31, 39). Many of these earlier studies were performed by examining the trafficking of recombinant proteins overexpressed in immortalized cell lines. Rab proteins that regulate CaV1.2 channel surface expression in native cell types are unclear, as are physiological functions of Rabs in arterial myocytes (4). Given that plasma membrane CaV1.2 channels are a primary Ca2+ influx pathway and regulate arterial contractility, we investigated Rab proteins that control functional CaV1.2 trafficking in arterial myocytes.
Here, we show that Rab25, a small GTPase previously associated with apical recycling endosomes, is expressed and locates in close spatial proximity to CaV1.2 channels in cerebral artery myocytes. Rab25 knockdown reduced surface CaV1.2 and whole cell CaV1.2 currents in isolated myocytes and depolarization and pressure-induced vasoconstriction in arteries. These data indicate that Rab25 controls arterial contractility by regulating the surface abundance of CaV1.2 channels in myocytes.
MATERIALS AND METHODS
Cell isolation and tissue preparation.
All animal protocols used were reviewed and approved by the Animal Care and Use Committee at the University of Tennessee Health Science Center. Male Sprague-Dawley rats (6–8 wk) were euthanized by intraperitoneal injection of an overdose of sodium pentobarbital (150 mg/kg body wt). The brain was removed and placed in oxygenated (21% O2-5% CO2) physiological saline solution (PSS) containing the following (in mM): 6 KCl, 112 NaCl, 24 NaHCO3, 1.2 MgSO4, 1.2 KH2PO4, 1.8 CaCl2, and 10 glucose. Middle cerebral, posterior cerebral, and cerebellar arteries (∼150- to 250-μm diameter) were dissected from the brain and cleaned of adventitia. Smooth muscle cells (myocytes) were dissociated from cerebral arteries as previously described (44). Experiments involving myocytes were completed on the same day of isolation.
Arterial transfection.
Isolated cerebral arteries were transfected with siRNA (Invitrogen) specific to either Rab4A, Rab11A, or Rab25 or control scrambled (scrm) siRNA (Ambion) using a CUY21Vivo-SQ electroporator (Bex) as previously described (34). Arteries were then allowed to equilibrate in sterile phosphate-buffered saline (PBS) containing control or active siRNA (10 ug/μl) for 10 min and subsequently transferred to PSS for 15 min at room temperature. Arteries were placed in serum-free DMEM F-12 media supplemented with 1% penicillin-streptomycin at 37°C (95% O2-5% CO2) for 3 days, after which they were used for experimentation.
Protein analysis and biochemistry.
Arteries were homogenized in 1× SDS-Laemmli buffer supplemented with 2% β-mercaptoethanol, and cellular debris was removed by centrifugation. Protein concentration was determined using the method of Henkel and Bieger (22). Proteins were separated on either 7.5 or 12% SDS-PAGE gels and analyzed by Western blotting. Blots were cut where appropriate to allow simultaneous probing of the same blot by multiple antibodies. For 12% gels, blots were cut between the 25- and 37-kDa markers (Bio-Rad Precision Plus) and between 75 and 100 kDa, yielding thre smaller blots. The upper portion was probed for CaV1.2, the middle for β-actin, and the lower for Rab proteins. For 7.5% gels, blots were cut between the 75- and 100-kDa markers, and the upper section was probed for CaV1.2 and the lower portion for β-actin. One exception is for data shown (see Fig. 3A) where blots from 7.5% gels were cut at both 150 and 100 kDa, with the upper part was probed for CaV1.2, the middle for KV2.1, and the lower for β-actin. For all experiments, 7.5% gels were used, except for the data shown in Fig. 1A, which was obtained using a 12% gel. Antibodies used were anti-CaV1.2, which recognizes an amino acid sequence located in the intracellular DII to DIII linker (Neuromab, UC Davis), anti-rab25 (AbCam), anti-rab4 (Sigma-Aldrich), anti-rab11A (AbCam), anti-KV2.1 (Neuromab, University of California, Davis, CA), and anti-actin (Millipore). Bands were visualized using horseradish peroxidase-conjugated secondary antibodies and a West Pico or Fempto Chemiluminescence kit (Pierce) and detected using a Kodak Image F-Pro system. Band intensities were determined using Quantity One (Bio-Rad) software. CaV1.2 protein was calculated as the sum of both the full-length 240-kDa and truncated 190-kDa subunits that are present in arterial myocytes and run as distinct bands on a 7.5% gel (2). For experiments where total protein was measured, band intensities were first normalized to actin before comparison to other samples.
Fig. 3.
Rab25 knockdown promotes CaV1.2 channel degradation. A: representative Western Blots illustrating effects of Rab25 knockdown on CaV1.2 and KV2.1 total protein. B: mean data, n = 4 for each. *P < 0.05 vs. scrm. C: representative Western blots illustrating effects of Rab25 knockdown on CaV1.2 total protein and regulation by bafilomycin (100 nM, 24 h) or MG132 (10 μM, 24 h). D: mean data, n = 3. *P < 0.05, compared with scrambled controls. #P < 0.05, compared with Rab25siRNA.
Fig. 1.
Rab25 regulates surface abundance of CaV1.2 channels. A: representative Western blots showing effect of scrambled, Rab4A, Rab11A, or Rab25siRNA on levels of indicated Rab proteins in cerebral arteries. B: mean data demonstrating the reduction in total Rab4, Rab11A and Rab25 proteins in arteries treated with Rab4A, Rab11A, or Rab25siRNAs, respectively compared with scrambled controls (n = 5 for each). C: representative Western blots illustrating effects of Rab4A and Rab11A siRNAs on surface and intracellular CaV1.2 protein. Blots illustrate both the full-length 240-kDa and truncated 190-kDa CaV1.2 subunits that are present in arterial myocytes (2). D: representative Western Blots showing effects of Rab25 siRNA on surface and intracellular CaV1.2 protein expression. E: mean data illustrating regulation of surface and intracellular CaV1.2 protein levels by Rab4A, Rab11A, and Rab25 knockdown normalized to scrambled controls (n = 6 for Rab4A knockdown, n = 3 for Rab11A knockdown, n = 4 for Rab25 knockdown). F: Rab4A, Rab11A, and Rab25 siRNAs do not alter CaV1.2 channel cellular distribution calculated as the percentage of total Ca2+ located at the cell surface (n = 6 for Rab4A knockdown, n = 3 for Rab11A knockdown, n = 4 for Rab25 knockdown). *P < 0.05, compared with scrm control.
Artery surface biotinylation.
Arteries were treated with biotin reagents as previously described (1). Briefly, arteries were incubated for 1 h in biotin (1 mg/ml each of EZ-Link Sulfo-NHS-LC-LC-Biotin and EZ-Link Maleimide-PEG2-Biotin; Pierce), and unbound biotin reagents were removed by quenching with glycine supplemented PBS (100 mM) and washed with PBS. Arteries were homogenized in RIPA buffer (Sigma), and cellular debris was removed by centrifugation. Protein concentration was determined using the method of Henkel and Bieger (22). Biotinylated proteins were then extracted from equivalent amounts of total protein lysate using avidin (Monomeric Avidin; Pierce). The supernatant (intracellular fraction) was removed and biotinylated surface proteins were eluted from avidin beads by boiling in 1× Laemmli buffer supplemented with 2% β-mercaptoethanol. Both intracellular and surface fractions were separated on SDS-PAGE gels and analyzed by Western blotting. Band intensities were determined using Quantity One software (Bio-Rad). Total protein was the sum of surface and intracellular band intensities. Surface and intracellular proteins were normalized to total protein.
Immunofluorescence resonance energy transfer and confocal imaging.
Immunofluorescence resonance energy transfer (ImmunoFRET) was performed on myocytes isolated from arteries that had been transfected with either scrm control or Rab25siRNA, as previously described (25). Briefly, freshly isolated myocytes were plated onto poly-l-lysine-coated coverslips, fixed with paraformaldehyde, and permeabilized with 0.1% Triton X-100 for 2 min at room temperature. After a 1-h incubation in PBS containing 5% bovine serum albumin (BSA), myocytes were treated overnight at 4°C with mouse monoclonal anti-CaV1.2 (Neuromab, University of California, Davis) and either rabbit polyclonal anti-Rab25 (Abcam), anti-rab4 (Sigma-Aldrich), or anti-rab11A (AbCam) at a dilution of 1:100 each in PBS containing 5% BSA. After being washed, cells were incubated for 1 h at 37°C with secondary antibodies: Alexa 546-conjugated donkey anti-mouse for CaV1.2 (1:100 dilution; Life Technologies) and Alexa 488-conjugated donkey anti-rabbit for Rab25, Rab4 or Rab11A (1:100 dilution; Life Technologies). Coverslips were then mounted onto glass slides for imaging.
Fluorescence images were acquired using a Zeiss LSM Pascal laser-scanning confocal microscope. Alexa 488 and Alexa 546 were excited at 488 and 543 nm and emission collected at 505–530 and >560 nm, respectively. The 512 × 512 pixel images were acquired using a z-resolution of ∼1 μm. Laser intensity and detector gain were maintained constant for each experiment and set below saturation. Images were background-subtracted and normalized FRET (N-FRET) was calculated on a pixel-by-pixel basis for the entire image using the Xia method (42) and Zeiss LSM FRET Macro tool version 2.5.
Patch-clamp electrophysiology.
CaV1.2 currents were recorded in myocytes isolated from arteries transfected with either scrm control or Rab25siRNA. The whole cell patch-clamp configuration was used with currents acquired using an Axopatch 200B amplifier and Digidata 1322A (Axon Instruments). Borosilicate glass electrodes (4–5 MΩ) were filled with pipette solution containing the following (in mM): 135 CsMeSO4, 5 CsCl, 5 EGTA, 4 MgATP, 0.25 Na2GTP, 10 HEPES, and 10 glucose (pH 7.2 adjusted using CsOH). Bath solution contained the following (in mM): 130 NMDG, 20 BaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.4 adjusted using l-aspartic acid). Cell capacitance was measured using a 5-mV test pulse and transients corrected using series resistance compensation.
CaV1.2 currents were stimulated by applying 1-s step depolarizations to between −80 and +60 mV in 10-mV increments before a 200-ms test pulse to 0 mV. Myocytes were maintained at a holding potential of −80 mV for 1 s before voltage steps. The peak current obtained during the 1-s pulse was used to generate current-voltage (I-V) relationships. The rate of current inactivation was calculated from the current decay during each 1-s conditioning pulse. The peak current generated during the 200-ms test pulse to 0 mV was used to derive steady-state inactivation. To measure steady-state activation, tail currents were elicited by repolarization to −80 mV from 20-ms test pulses to between −60 and +60 mV in 10-mV increments. Whole cell currents were filtered at 1 or 5 kHz and digitized at 5 or 20 kHz for the inactivation and activation protocols, respectively. P/−4 protocols were used to subtract leak and capacitance transients.
Steady-state inactivation and activation curves were fit with a single power Boltzmann function: I/Imax = Rin + (Rmax − Rin)/{1 + exp[(V − V1/2)/k]}, where I/Imax is the normalized peak current, V is the conditioning prepulse voltage, V1/2 is the voltage for half-inactivation or half-activation, k is the slope factor, Rin is the proportion of noninactivating current, and Rmax is the maximal current amplitude. Inactivation kinetic data were fit with a single exponential function: It = [A × e(−t/τ)] + I0, where It is the inward current at time t, A the amplitude, and I0 the residual current.
Pressurized artery myography.
Endothelium-denuded artery segments were cannulated at each end in a perfusion chamber (Living Systems Instrumentation), maintained at 37°C and continuously perfused with PSS gassed with 21% O2-5% CO2. Intravascular pressure was monitored using a pressure transducer and altered using an attached reservoir. Wall diameter was measured at 1 Hz using a charge-coupled device camera and the edge detection function of IonWizard (Ionoptix). Vasoconstriction to an elevation in extracellular K+ was calculated as 100 × (1 − D[K+]/D[6K+]), where D[K+] is diameter in the bath solution containing a higher K+ concentration and D[6K+] is diameter in 6K+ bath solution. Myogenic tone (%) was calculated as 100 × (1 − Dactive/Dpassive), where Dactive is active diameter and Dpassive is passive diameter determined by applying Ca2+-free PSS supplemented with 5 mM EGTA.
Statistical analysis.
OriginLab software (Microcal) was used for statistical analyses. Values are expressed as mean ± SE. Student's t-test was used for comparing unpaired data from two populations and ANOVA with Bonferroni post hoc test was used for multiple group comparisons. P < 0.05 was considered significant.
RESULTS
Rab25, but not Rab11A or Rab4A, stimulates surface trafficking of CaV1.2 channels.
Rab4A and Rab11, which regulate early and recycling endosome function, respectively, have been associated with trafficking of some ion channel subunits, including BKα and β1-subunits (24, 28, 29, 37). First, we examined regulation of CaV1.2 surface expression by Rab4A and Rab11A in cerebral artery myocytes. Rab4A or Rab11A siRNA reduced Rab4A and Rab11A total protein in cerebral arteries to 52.7 ± 3.3 and 50.0 ± 5.1% of scrambled controls, respectively (Fig. 1, A and B). In contrast, Rab4A and Rab11A knockdown did not reduce surface or intracellular CaV1.2 protein (Fig. 1, C and E).
Rab25 has been associated with apical recycling endosomes, although Rab25 expression in arterial myocytes and a link to ion channel trafficking in any cell type do not appear to have been established (37). Therefore, we investigated the regulation of CaV1.2 surface protein by Rab25 knockdown. Rab25 siRNA reduced Rab25 protein to 52.0 ± 8.0% of scrambled controls (Fig. 1, A and B). Rab25 knockdown also decreased surface and intracellular CaV1.2 protein to ∼65 and 64% of scrambled controls, respectively (Fig. 1, D and E). Rab4A, Rab11A, and Rab25 knockdown did not alter the relative cellular distribution of CaV1.2 channels, calculated as the percentage of total CaV1.2 that was located in the plasma membrane (Fig. 1F). These data suggest that Rab25 increases the abundance of CaV1.2 in the plasma membrane of arterial myocytes, whereas Rab4A and Rab11A do not control CaV1.2 surface levels.
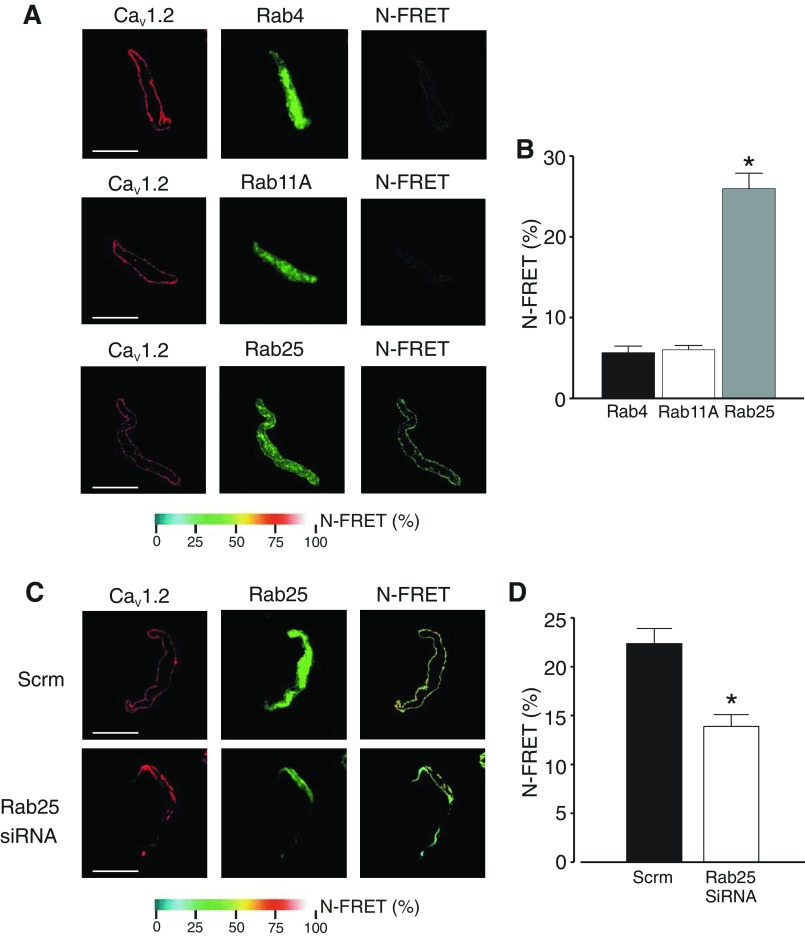
ImmunoFRET imaging was used to examine localization and spatial proximity of CaV1.2 with Rab4A, Rab11A, or Rab25 proteins in myocytes. Alexa 546- and Alex 488-tagged secondary antibodies bound to CaV1.2 and Rab25 primary antibodies, respectively, generated high N-FRET (∼26%) in arterial myocytes (Fig. 2, A and B). Similar experiments performed using primary antibodies raised to CaV1.2 and Rab4A or Rab11A did not generate significant N-FRET (Fig. 2, A and B). These data suggest that Rab25 and CaV1.2 are located in close spatial proximity, whereas CaV1.2 is not located nearby Rab11A or Rab4A in cerebral artery myocytes.
Fig. 2.
Rab25 colocalizes with CaV1.2 channels and stimulates CaV1.2 surface expression. A: representative images of myocytes labeled with fluorescent antibodies to either Rab4, Rab11A, or Rab25 (Alexa546) or CaV1.2 (Alex488) and respective normalized fluorescence resonance energy transfer (N-FRET) images. B: mean N-FRET data (n = 10). C: representative images of myocytes isolated from arteries treated with either scrm or Rab25siRNA labeled with fluorescent antibodies to either Rab25 (Alexa546) or CaV1.2 (Alex488) and respective N-FRET images. D: mean N-FRET data (n = 10). *P < 0.05, compared with scrm control. Scale bars = 10 μm.
Next, immunoFRET was performed on myocytes isolated from either scrm or Rab25-knockdown arteries. In scrambled controls, mean N-FRET was ∼22.4%, again indicating that CaV1.2 and Rab25 proteins are close (Fig. 2, C and D). Rab25 knockdown reduced N-FRET between CaV1.2- and Rab25-bound antibodies to ∼13.9% (Fig. 2, C and D). Taken together these data indicate that Rab25 is associated with CaV1.2 channels in arterial myocytes.
Rab25 knockdown promotes CaV1.2 channel protein degradation.
Rab25 knockdown reduced CaV1.2 total protein but did not alter total protein for KV2.1, a voltage-dependent K+ channel expressed in arterial myocytes, suggesting CaV1.2 protein loss was not nonspecific (27, 43) (Fig. 3, A and B). Rab25 knockdown reduced full-length (240 kDa) CaV1.2 subunits to 64.4 ± 10.1% of scrm controls and truncated (190 kDa) CaV1.2 subunits to 69.6 ± 10.7% of scrm controls (n = 4 for each, P > 0.05 when comparing the reduction in full-length vs. short CaV1.2). Thus Rab25 knockdown did not change the ratio of full-length to short CaV1.2 subunits.
Rab25 knockdown reduced surface CaV1.2 protein, but this protein did not appear in the intracellular fraction (Fig. 1, D–F). Rab25 knockdown also reduced CaV1.2 total protein (Fig. 3, A and B). To test the hypothesis that CaV1.2 total protein decreased due to degradation, involvement of proteasomal and lysosomal pathways was investigated. Bafilomycin, an inhibitor of lysosomal degradation, or MG132, a proteasomal degradation blocker, each prevented the reduction in CaV1.2 total protein that occurred in response to Rab25 knockdown (Fig. 3, C and D). These data suggest that Rab25 knockdown leads to CaV1.2 degradation via a mechanism involving both lysosomal and proteasomal pathways.
Rab25 knockdown reduces CaV1.2 currents in isolated cerebral artery myocytes.
To investigate the functional effect of Rab25 knockdown, CaV1.2 currents were recorded in myocytes isolated from either scrambled siRNA- or Rab25 siRNA-treated arteries. The mean peak CaV1.2 current density, using Ba2+ (20 mM) as the charge carrier, was 5.7 ± 0.4 pA/pF in scrambled control myocytes (Fig. 4, A and B). Rab25 knockdown decreased mean peak CaV1.2 current density to 3.1 ± 0.3 pA/pF or to ∼55% of controls (Fig. 4, A and B). Rab25 knockdown did not significantly alter the voltage dependence of half-maximal CaV1.2 current activation (V1/2act, mV: scrm, 26.4 ± 2.8; Rab25 siRNA, 27.8 ± 4.5; P > 0.05) or inactivation (V1/2inact, mV: scrm, −10.5 ± 2.1; Rab25 siRNA, −11.1 ± 4.0; P > 0.05; Fig. 4, C and D). The rate of CaV1.2 current inactivation (τ) was also similar between scrambled control and Rab25 siRNA myocytes (at +20 mV, ms: scrm, 321 ± 28; Rab25 siRNA, 328 ± 23; P > 0.05; Fig. 4E). Taken together these results indicate that Rab25 stimulates CaV1.2 channel surface expression, which elevates CaV1.2 currents in cerebral artery myocytes.
Fig. 4.
Rab25 knockdown reduces CaV1.2 currents in isolated cerebral artery myocytes. A: representative CaV1.2 currents recorded from myocytes isolated from scrambled control (black) or Rab25 siRNA (red) arteries. B: mean current density-voltage relationships (n = 21 for scrm and n = 16 for Rab25 siRNA). C: steady-state voltage-dependent activation fit with a single Boltzmann function (n = 10 for scrm and n = 6 for Rab25 siRNA). D: steady-state inactivation fit with Boltzmann functions (n = 21 for scrm and n = 16 for Rab25 siRNA). E: voltage dependence of rate of current inactivation illustrated as τ. Inactivation of currents elicited by voltage steps from −80 mV to between −10 and +60 mV were fit with a single exponential function (n = 21 for scrm and n = 16 for Rab25 siRNA). Inset: representative rates of current inactivation in a scrm- and Rab25-siRNA-treated myocyte during a voltage step to +20 mV. *P < 0.05, compared with scrambled controls.
Rab25 increases pressure- and depolarization-induced constriction in resistance-size cerebral arteries.
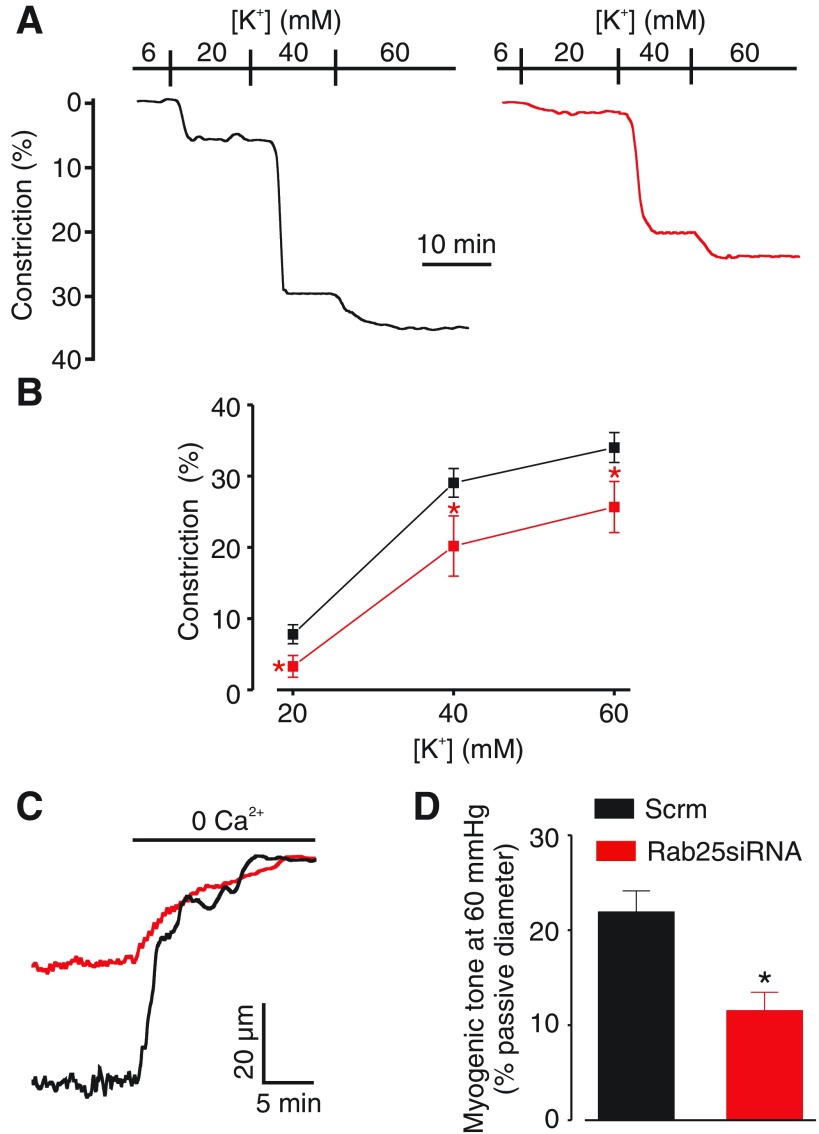
The functional significance of Rab25 in the vasculature is unclear. Our data indicate Rab25 controls CaV1.2 channel surface expression and currents in arterial myocytes. Therefore, we studied the regulation of arterial contractility by membrane potential and intravascular pressure in scrambled control and Rab25-knockdown resistance-size cerebral arteries. Intravascular pressure stimulates membrane depolarization, which activates CaV1.2 channels, leading to Ca2+ influx and vasoconstriction (35). To examine the regulation of arterial contractility by membrane potential, vasoconstriction to elevations in extracellular K+, which induce steady-state membrane potential depolarization, were measured in arteries pressurized to 10 mmHg. Increasing extracellular K+ from 6 mM to 20, 40, or 60 mM stimulated vasoconstriction in control arteries by 7.8, 29.1, and 34.0%, respectively (Fig. 5, A and B). In contrast, in Rab25 knockdown arteries the same elevations in extracellular K+ stimulated vasoconstriction of 3.3, 20.2 and 25.7%, which were 57.7, 30.6, and 24.4% of those in scrambled controls, respectively (Fig. 5B). Given that Rab25 knockdown reduced depolarization-induced vasoconstriction, the effects on pressure-induced constriction were studied. Rab25 knockdown reduced tone stimulated by an intravascular pressure of 60 mmHg from ∼21.9% of passive diameter in control arteries to ∼11.5% of passive diameter, or by ∼48% (Fig. 5, C and D). Taken together; these data indicate that Rab25 regulates arterial contractility by controlling surface expression of CaV1.2 channels in arterial myocytes.
Fig. 5.
Rab25 knockdown inhibits pressure- and depolarization-induced vasoconstriction in cerebral arteries. A: representative traces showing vasoconstriction to increasing extracellular K+ in scrm control (black) and Rab25 siRNA arteries (red). B: mean data illustrating depolarization-induced vasoconstriction in scrm control and RAB25 siRNA arteries (n = 12–16 for scrm; n = 7–8 for Rab25 siRNA). C: representative traces of pressure (60 mmHg)-induced vasoconstriction, illustrated as vasodilation to removal of bath Ca2+ with 5 mM EGTA, in a scrm control (black) and Rab25 siRNA (red) artery. D: mean data for scrm control and Rab25 siRNA arteries (n = 11 for scrm; n = 9 for Rab25 siRNA). *P < 0.05, compared with scrm control.
DISCUSSION
Plasma membrane-localized CaV1.2 channels regulate arterial contractility, but the trafficking pathways that control surface expression of these proteins are unclear. Similarly, expression and physiological functions of Rab25, a Rab GTPase associated with apical recycling endosomes, in the vasculature do not appear to have been investigated. Here, using Western blotting and immunoFRET we show that Rab25 is expressed in cerebral artery myocytes where it locates in close spatial proximity to CaV1.2 channels. Rab25 knockdown reduced surface and intracellular CaV1.2 abundance in arteries. In contrast, CaV1.2 was not located nearby Rab11A or Rab4 and CaV1.2 protein was unaltered by Rab11A or Rab4A knockdown. Rab25 knockdown led to CaV1.2 degradation by a mechanism involving both lysosomal and proteasomal pathways. Rab25 knockdown also reduced whole cell CaV1.2 current density in myocytes and depolarization and pressure-induced vasoconstriction in cerebral arteries. These results identify for the first time the expression and a physiological function of Rab25 in arterial myocytes. Data indicate that Rab25 stimulates CaV1.2 channel surface expression in cerebral artery myocytes to promote pressure- and depolarization-induced vasoconstriction.
Intravascular pressure and membrane potential regulate CaV1.2 channel activity, thereby modulating Ca2+ influx and contractility. To control plasma membrane Ca2+ influx, CaV1.2 proteins must traffic from the endoplasmic reticulum/Golgi complex to the surface, but the mechanisms by which this occurs remain poorly understood in all cell types, including arterial myocytes. Rab GTPases are a family of ∼60 proteins that regulate anterograde and retrograde trafficking of proteins between different cellular compartments (37). Rabs have been shown to traffic several different ion channels and auxiliary subunits (12, 14, 30, 40). Many of these previous studies have investigated the trafficking of recombinant proteins expressed in immortalized cell lines. Recently, we showed that large-conductance Ca2+-activated K+ (BK) channel pore-forming α-subunits are primarily plasma membrane localized, whereas BK channel auxiliary β1-subunits are intracellular, in rat and human cerebral and rat mesenteric arteries. Nitric oxide stimulated rapid (seconds) Rab11A-dependent surface trafficking of β1-subunits, which associated with BK channels to elevate Ca2+ sensitivity, leading to channel activation and vasodilation (28). In contrast, surface expression of BKα in arterial myocytes was dependent on Rab4A and occurred via a slower (hours) mechanism than for β1-subunits (29). These studies indicated that surface trafficking of BKα and auxiliary β1-subunits are regulated via distinct pathways. Rab11A knockdown did not alter myogenic tone in pressurized cerebral arteries (28). Here, Rab11A or Rab4A knockdown did not alter CaV1.2 surface or intracellular protein or CaV1.2 cellular distribution in arterial myocytes, suggesting a different trafficking mechanism to that for BK channel subunits. Rab11A and Rab4A are associated with recycling and early endosomes, respectively (24). Given that knockdown of these endosomal regulatory proteins did not alter CaV1.2 surface trafficking, we focused on Rab25, a Rab GTPase associated with trafficking of integrins via apical recycling endosomes (7, 8, 10, 17, 24). Rab25 contains a GTP-binding site (DTAGLE), which is different to that (DTAGQE) of other Rab proteins, making it constitutively active (18). Rab25 has been implicated in several cancers and proposed to regulate adhesion molecule trafficking, proliferation, migration, angiogenesis, and cell cycle (32). ImmunoFRET analysis indicated that CaV1.2 channels are located in close spatial proximity to Rab25 but not nearby Rab4 or Rab11A. Images revealed the FRET signal to be at the plasma membrane, suggesting that CaV1.2 and Rab25 remain localized after endosome fusion. Knockdown of Rab25, but not knockdown of Rab11A or Rab4A, reduced surface and intracellular CaV1.2 protein. In contrast, Rab25 knockdown did not alter KV2.1 protein, indicating that CaV1.2 loss was not nonspecific. A previous study described that Rab11b knockdown and inhibition increased recombinant CaV1.2 currents by interfering with protein degradation in HEK293 cells (4). In contrast, our data indicate that Rab25 increases the surface abundance of CaV1.2 channels in arterial myocytes. These data also suggest that surface expression of CaV1.2, BK, and β1-channel subunits is controlled by distinct Rab proteins in arterial myocytes.
Here, Rab25 knockdown did not result in intracellular CaV1.2 accumulation or alter the surface/intracellular distribution of CaV1.2, suggesting that internalized CaV1.2 was targeted for degradation. Bafilomycin and MG132 each completely inhibited CaV1.2 loss, suggesting that lysosomal and proteasomal pathways act in series rather than in parallel to degrade CaV1.2 in arterial myocytes. If each degradation pathway acted independently, bafilomycin or MG132 alone would partially prevent CaV1.2 protein loss. Arterial myocyte KV1.5 and recombinant KCa3.1 and ENaC also undergo degradation via both pathways (3, 16, 27). In arterial myocytes, KV1.5 channels are continuously recycled between the intracellular compartment and the plasma membrane. Intravascular pressure, by promoting membrane depolarization and CaV1.2 channel activation, stimulates KV1.5 surface expression (27). These data suggest that KV1.5 and CaV1.2 channels that are not trafficked to the plasma membrane are targeted for degradation in arterial myocytes. An alternative explanation is that Rab25 inhibits CaV1.2 channel degradation in addition to, or rather than, stimulating CaV1.2 surface expression in arterial myocytes. We consider this possibility less likely as 1) Rab25 is functionally associated with recycling endosomes, which deliver proteins to the surface, not phagosomes or late endosomes, which can deliver proteins to lysosomes for degradation (24, 37); 2) immunoFRET data indicate that Rab25 and CaV1.2 are located in close spatial proximity in the plasma membrane, which is consistent with Rab25 controlling surface expression; and 3) to our knowledge there are no studies showing Rab25 controls proteasomal degradation in any cell type.
Whole cell patch-clamp studies confirmed that Rab25 knockdown reduced CaV1.2 current density but did not alter current voltage dependence, consistent with a reduction in the number of surface CaV1.2 channels. Intravascular pressure stimulates arterial myocyte depolarization through the activation of several channels, including TRPM4 and TRPC6 and anoctamin 1, a Ca2+-activated chloride channel (5, 15, 41). Pressure-induced depolarization activates voltage-dependent Ca2+ channels, leading to Ca2+ influx and vasoconstriction. Here, functional studies indicated that Rab25 knockdown reduced both depolarization- and pressure-induced vasoconstriction in endothelium-denuded arteries. Thus we demonstrate that Rab25 regulates the cerebral artery myogenic response by controlling functional surface expression of CaV1.2 channels.
Arterial myocyte CaV1.2 currents are activated either directly or indirectly by a wide variety of physiological stimuli, including intravascular pressure; membrane depolarization; vasoconstrictors; including norepinephrine, endothelin, angiotensin II, serotonin, and histamine; cytokines; protein kinase C; tyrosine kinase c-Src; and cholesterol (19). Arterial myocyte CaV1.2 currents are inhibited by several mediators, including protein kinase G, cholesterol, acidosis, and hypoxia (19). Hypertension has also been associated with alterations in CaV1.2 currents in arterial myocytes, although differing results have been obtained. An increase in CaV1.2 current was described in cerebral, mesenteric, and renal artery myocytes of spontaneously hypertensive rats (36). The increase in CaV1.2 current density occurred due to an elevation in expression of α2δ-1, an auxiliary subunit that promotes surface trafficking of CaV1.2 channels and reduces current inactivation, producing vasoconstriction in cerebral artery myocytes. In contrast, in a murine genetic model of hypertension, CaV1.2 protein and current density were lower and Ca2+ sparklet activity was higher in mesenteric artery myocytes (38). The concept that these physiological stimuli and disease states modify Rab25-dependent CaV1.2 surface expression, trafficking, and degradation to regulate arterial contractility is beyond the scope of this study but worth future investigation.
In summary, our data indicate that Rab25 is expressed in cerebral artery myocytes and regulates the surface abundance of CaV1.2 channels to control arterial contractility.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-67061, HL-094378, and HL-110347 (to J. H. Jaggar) and an American Heart Association Scientist Development Grant (to M. D. Leo).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.P.B., S.B., M.D.L., and J.H.J. conception and design of research; J.P.B., S.B., M.D.L., and M.W.K. performed experiments; J.P.B., S.B., M.D.L., and M.W.K. analyzed data; J.P.B., S.B., M.D.L., M.W.K., and J.H.J. interpreted results of experiments; J.P.B., S.B., M.D.L., and M.W.K. prepared figures; J.P.B. drafted manuscript; J.P.B., S.B., M.D.L., and J.H.J. edited and revised manuscript; J.P.B., S.B., M.D.L., M.W.K., and J.H.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brent Williams for technical assistance.
REFERENCES
- 1.Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, Feng JY, Jaggar JH. Smooth muscle cell α-δ-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res 105: 948–955, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannister JP, Leo MD, Narayanan D, Jangsangthong W, Nair A, Evanson KW, Pachuau J, Gabrick KS, Boop FA, Jaggar JH. The voltage-dependent L-type Ca2+ (CaV1.2) channel C-terminus fragment is a bi-modal vasodilator. J Physiol 591: 2987–2998, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertuccio CA, Lee SL, Wu G, Butterworth MB, Hamilton KL, Devor DC. Anterograde trafficking of KCa3.1 in polarized epithelia is Rab1- and Rab8-dependent and recycling endosome-independent. PLoS One 9: e92013, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Best JM, Foell JD, Buss CR, Delisle BP, Balijepalli RC, January CT, Kamp TJ. Small GTPase Rab11b regulates degradation of surface membrane L-type Cav1.2 channels. Am J Physiol Cell Physiol 300: C1023–C1033, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulley S, Neeb ZP, Burris SK, Bannister JP, Thomas-Gatewood CM, Jangsangthong W, Jaggar JH. TMEM16A/ANO1 channels contribute to the myogenic response in cerebral arteries. Circ Res 111: 1027–1036, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butterworth MB, Edinger RS, Silvis MR, Gallo LI, Liang X, Apodaca G, Fizzell RA, Johnson JP. Rab11b regulates the trafficking and recycling of the epithelial sodium channel (ENaC). Am J Physiol Renal Physiol 302: F581–F590, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell 10: 47–61, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC. Rab25 associates with α5β1 integrin to promote invasive migration in 3D microenvironments. Dev Cell 13: 496–510, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D, Gray JW, Mills GB. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med 10: 1251–1256, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel CaV1.2 N-terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem 282: 29211–29221, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu YH, Alvarez-Baron C, Kim EY, Dryer SE. Dominant-negative regulation of cell surface expression by a pentapeptide motif at the extreme COOH terminus of an Slo1 calcium-activated potassium channel splice variant. Mol Pharmacol 77: 497–507, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui Z, Zhang S. Regulation of the human ether-a-go-go-related gene (hERG) channel by Rab4 protein through neural precursor cell-expressed developmentally down-regulated protein 4-2 (Nedd4-2). J Biol Chem 288: 21876–21886, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deutsch E, Weigel AV, Akin EJ, Fox P, Hansen G, Haberkorn CJ, Loftus R, Krapf D, Tamkun MM. KV2.1 cell surface clusters are insertion platforms for ion channel delivery to the plasma membrane. Mol Biol Cell 23: 2917–2929, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res 95: 922–929, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Eaton DC, Malik B, Bao HF, Yu L, Jain L. Regulation of epithelial sodium channel trafficking by ubiquitination. Proc Am Thorac Soc 7: 54–64, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda M, Kanno E, Ishibashi K, Itoh T. Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics 7: 1031–1042, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem 268: 18419–18422, 1993. [PubMed] [Google Scholar]
- 19.Gollasch M, Nelson MT. Voltage-dependent Ca2+ channels in arterial smooth muscle cells. Kidney Blood Press Res 20: 355–371, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Harraz OF, Brett SE, Zechariah A, Romero M, Puglisi JL, Wilson SM, Welsh DG. Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR-BKCa axis. Arterioscler Thromb Vasc Biol 35: 1843–1851, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harraz OF, Visser F, Brett SE, Goldman D, Zechariah A, Hashad AM, Menon BK, Watson T, Starreveld Y, Welsh DG. CaV1.2/CaV3x channels mediate divergent vasomotor responses in human cerebral arteries. J Gen Physiol 145: 405–418, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkel AW, Bieger SC. Quantification of proteins dissolved in an electrophoresis sample buffer. Anal Biochem 223: 329–331, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol 91: 973–983, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91: 119–149, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 281: C439–C448, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 279: C1528–C1539, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Kidd MW, Leo MD, Bannister JP, Jaggar JH. Intravascular pressure enhances the abundance of functional KV1.5 channels at the surface of arterial smooth muscle cells. Sci Signal 8: ra83, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leo MD, Bannister JP, Narayanan D, Nair A, Grubbs JE, Gabrick KS, Boop FA, Jaggar JH. Dynamic regulation of β1 subunit trafficking controls vascular contractility. Proc Natl Acad Sci USA 111: 2361–2366, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leo MD, Bulley S, Bannister JP, Kuruvilla KP, Narayanan D, Jaggar JH. Angiotensin II stimulates internalization and degradation of arterial myocyte plasma membrane BK channels to induce vasoconstriction. Am J Physiol Cell Physiol 309: C392–C402, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makhina EN, Nichols CG. Independent trafficking of KATP channel subunits to the plasma membrane. J Biol Chem 273: 3369–3374, 1998. [DOI] [PubMed] [Google Scholar]
- 31.McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem 282: 29612–29620, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Mitra S, Cheng KW, Mills GB. Rab25 in cancer: a brief update. Biochem Soc Trans 40: 1404–1408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem 81: 637–659, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayanan D, Bulley S, Leo MD, Burris SK, Gabrick KS, Boop FA, Jaggar JH. Smooth muscle cell transient receptor potential polycystin-2 (TRPP2) channels contribute to the myogenic response in cerebral arteries. J Physiol 591: 5031–5046, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol Cell Physiol 259: C3–C18, 1990. [DOI] [PubMed] [Google Scholar]
- 36.Sonkusare S, Palade PT, Marsh JD, Telemaque S, Pesic A, Rusch NJ. Vascular calcium channels and high blood pressure: pathophysiology and therapeutic implications. Vascul Pharmacol 44: 131–142, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Tajada S, Cidad P, Colinas O, Santana LF, Lopez-Lopez JR, Perez-Garcia MT. Down-regulation of CaV1.2 channels during hypertension: how fewer CaV12 channels allow more Ca2+ into hypertensive arterial smooth muscle. J Physiol 591: 6175–6191, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van de Graaf SF, Chang Q, Mensenkamp AR, Hoenderop JG, Bindels RJ. Direct interaction with Rab11a targets the epithelial Ca2+ channels TRPV5 and TRPV6 to the plasma membrane. Mol Cell Biol 26: 303–312, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vithlani M, Terunuma M, Moss SJ. The dynamic modulation of GABAA receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev 91: 1009–1022, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welsh DG, Morielli AD, Nelson MT, Brayden JE. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res 90: 248–250, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys J 81: 2395–2402, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu C, Lu Y, Tang G, Wang R. Expression of voltage-dependent K+ channel genes in mesenteric artery smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 277: G1055–G1063, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol 295: C1376–C1384, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]