Abstract
Pulmonary arterial hypertension (PAH) is a progressive, life-threatening disease for which there is currently no curative treatment available. Pathologic changes in this disease involve remodeling of the pulmonary vasculature, including marked proliferation of pulmonary artery smooth muscle cells (PASMCs). Recently, the bioactive lipid sphingosine-1-phosphate (S1P) and its activating kinase, sphingosine kinase 1 (SphK1), have been shown to be upregulated in PAH and promote PASMC proliferation. The mechanisms regulating the transcriptional upregulation of SphK1 in PASMCs are unknown. In this study, we investigated the role of platelet-derived growth factor (PDGF), a PAH-relevant stimuli associated with enhanced PASMC proliferation, on SphK1 expression regulation. In human PASMCs (hPASMCs), PDGF significantly increased SphK1 mRNA and protein expression and induced cell proliferation. Selective inhibition of SphK1 attenuated PDGF-induced hPASMC proliferation. In silico promoter analysis for SphK1 identified several binding sites for early growth response protein 1 (Egr-1), a PDGF-associated transcription factor. Luciferase assays demonstrated that PDGF activates the SphK1 promoter in hPASMCs, and truncation of the 5′-promoter reduced PDGF-induced SphK1 expression. Stimulation of hPASMCs with PDGF induced Egr-1 protein expression, and direct binding of Egr-1 to the SphK1 promoter was confirmed by chromatin immunoprecipitation analysis. Inhibition of ERK signaling prevented induction of Egr-1 by PDGF. Silencing of Egr-1 attenuated PDGF-induced SphK1 expression and hPASMC proliferation. These studies demonstrate that SphK1 is regulated by PDGF in hPASMCs via the transcription factor Egr-1, promoting cell proliferation. This novel mechanism of SphK1 regulation may be a therapeutic target in pulmonary vascular remodeling in PAH.
Keywords: gene expression, PDGF, PASMC, Egr1, SphK1, proliferation
pulmonary arterial hypertension (PAH) is a severe disease of multiple etiologies characterized by increases in pulmonary vascular resistance (PVR) primarily due to uncontrolled vascular remodeling, sustained vasoconstriction, or thrombosis in situ (12, 24). The precise mechanisms of these disease processes are poorly understood and few treatments are available (30). Remodeling of small pulmonary arteries is a hallmark of PAH, with human pulmonary artery smooth muscle cell (hPASMC) hyperproliferation and apoptosis resistance contributing to vessel obstruction and impaired blood flow (54). Understanding the mechanistic regulation of hPASMC proliferation in PAH is critical for developing novel therapeutics. With the discovery that some familial and sporadic cases of PAH arise from mutations in members of the transforming growth factor beta (TGF-beta) cell-signaling superfamily, including the bone morphogenetic protein type II receptor (BMPR2) gene (25), the role of different growth factors in disease pathogenesis has been explored. One of these growth factors, platelet-derived growth factor (PDGF), has been demonstrated to contribute to pulmonary vascular remodeling in human PAH and experimental models and is a potential therapeutic target (42, 47).
PDGF is a mitogenic and promigratory stimulus for hPASMCs with both autocrine and paracrine functions (6, 42, 53). Many cell types in the lung can synthesize PDGF, including vascular smooth muscle cells and endothelial cells, and it has been shown to be induced in alveolar hypoxia, causing vascular remodeling in lung parenchyma (5). Five ligand isoforms of PDGF are known (PDGF-AA, PDGF-BB, PDGF-AB, PDGF-CC, and PDGF-DD) along with two distinct receptor tyrosine kinase isotypes (PDGFR-α and PDGFR-β). The PDGF-BB isoform, which can bind to both PDGFR-α and PDGFR-β, is released at sites of vascular injury by endothelial cells and platelets and is a potent stimulus for hPASMC proliferation and migration (31, 42). Activation of PDGFRs leads to receptor dimerization, autophosphorylation, and subsequent signal transduction mainly via the mitogen-activated protein kinase/extracellular-signal-regulated kinase (MAPK/ERK) cascade, resulting in targeted gene transcription that promotes cell proliferation, migration, and differentiation, and the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, promoting cell survival (35). Recent studies have implicated PDGF and its receptors in both lung development and disease, including lung cancer, lung fibrosis, and PAH (3, 10, 23, 35). The circulating concentration of PDGF has been shown to be significantly elevated in patients with PAH (48), and concentrations within the pulmonary vasculature environment are likely to be much greater since small remodeled pulmonary arteries of PAH patients have increased PDGF and PDGFRs expression in the hPASMCs and pulmonary artery endothelial cells (PAECs) (42). In addition, inhibition of PDGFR-β using the specific tyrosine kinase inhibitor, imatinib (STI-571), has been shown to reverse vascular remodeling in severe experimental pulmonary hypertension (PH) (47). The therapeutic use of imatinib in refractory PAH has also been reported in several clinical case reports, leading to improvements in exercise capacity, hemodynamics, and functional class (17, 41). The precise mechanisms by which PDGF-BB stimulates hPASMC proliferation and vascular remodeling are not fully understood.
Crosstalk between PDGF/PDGFR signaling and sphingolipid signaling has been demonstrated in numerous cell types, including airway smooth muscle cells, where PDGFRs can be trans-activated by the bioactive lipid, sphingosine-1-phosphate (S1P), to elicit pro-proliferative and pro-migratory signaling (6a, 34a, 52a). Interestingly, PDGF has also been shown to stimulate activity of sphingosine kinase 1 (SphK1), the lipid kinase that generates S1P, and increase intracellular S1P in fibroblasts, while competitive inhibition of SphK1 prevents PDGF-induced cell proliferation (36, 43). PDGF has also been shown to increase expression of SphK1 in coronary artery smooth muscle cells (14). We recently reported that levels of S1P and SphK1 are elevated in patients with PAH and promote hPASMC proliferation (9). In addition, genetic deficiency or pharmacologic inhibition of SphK1 protects from the development of experimental PH in several rodent models (9). The mechanisms of SphK1/S1P upregulation and their role in pulmonary vascular remodeling in PAH are still largely unknown.
In this study, we investigated the mechanisms controlling SphK1 expression in PASMCs which may contribute to cell proliferation. We found that PDGF-induced SphK1 expression and PASMC proliferation is mediated in part by activation of the Egr-1 transcription factor.
MATERIALS AND METHODS
Materials.
Recombinant human PDGF-BB was purchased from Sigma Aldrich (St. Louis, MO). The primary antibodies for SPHK1, EGR1, Lamin B1, ERK1/2, phospho-ERK1/2, and HRP-conjugated β-Actin, and secondary anti-rabbit IgG HRP-linked antibody were purchased from Cell Signaling Technology (Danvers, MA). U0126 was purchased from Cell Signaling Technology. PF-543 was purchased from Cayman Chemical Company (Ann Arbor, MI).
Cell culture and treatments.
Primary human pulmonary artery smooth muscle cells (hPASMCs) were purchased from Lonza (Allendale, NJ) and used for all cell studies. Cells were cultured in a humidified atmosphere with 5% CO2 at 37°C in Medium-199 supplemented with 10% FBS and penicillin-streptomycin antibiotics. All studies were conducted from passages 4–8. For treatment studies, subconfluent hPASMCs plated in multi-well plates were subjected to serum deprivation and stimulation with PDGF-BB (20–100 ng/ml) before collection and analysis. The dose of PDGF-BB used was based on prior publications (20, 47). In some studies, pretreatment with the chemical inhibitor U0126 (10 μM) or PF-543 (100 nM) for 1 h was used before PDGF-BB stimulation. Further details of treatment timing are provided in the respective figure legends. The NE-PER kit from Thermo Fisher Scientific (Waltham, MA) was used for nuclear and cytoplasmic extractions.
Cell proliferation assays.
Cell proliferation was determined using a 5-bromo-2′-deoxyuridine (BrdU) incorporation assay from Calbiochem (San Diego, CA) per manufacturer's instructions in a 96-well format. Starting cell densities of 4,000 cells/well were used.
Chromatin immunoprecipitation (ChIP) assays.
ChIP studies were done using the SimpleChIP Plus Magnetic Bead ChIP kit purchased from Cell Signaling Technology (Danvers, MA) per manufacturer's instructions. In brief, cross-linking was completed after cell stimulation, followed by nuclei preparation and chromatin digestion. DNA gel electrophoresis was used to confirm adequate digestion. ChIP was then performed using the EGR1, positive control Histone H3, and negative control normal rabbit IgG antibodies. Elution of chromatin from antibody/beads and reversal of crosslinks was performed. DNA was purified and analyzed by both standard PCR and quantitative real-time PCR. Primers used to amplify the EGR1-B binding site were forward 5′-GCCTGTCGCCTGCTCTAC-3′ and reverse 5′-CCAGCTTCCCTCTTTCTTCC-3′.
Promoter analysis.
Putative EGR1 binding sites were identified within the proximal ∼2 kb promoter of SPHK1 using Genomatix software (similarity threshold > 0.95). The identified binding sites were confirmed in the public ENCODE ChIP-seq database (44).
Transfection and luciferase assays.
For promoter studies using the Gaussia Luciferase (GLuc) and Secreted Alkaline Phosphatase (SEAP) system, a human SphK1 promoter reporter clone was purchased from GeneCopoeia (Rockville, MD). Activities of GLuc/SEAP were analyzed with the Secrete-Pair Dual Luminescence Assay Kit (GeneCopoeia) per manufacturer's guidelines, using a GloMax luminometer (Promega). For promoter deletion studies, fragments of the SPHK1 promoter were amplified by PCR, purified, and ligated into pGL4.10[luc2] promoterless luciferase reporter vectors purchased from Promega (Madison, WI). The hRluc Renilla luciferase reporter vector pGL4.74[hRluc/TK] (Promega) was used as a transfection normalization control in these studies. Luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega). FuGENE HD transfection reagent (Promega) was used for all vector transfections in hPASMCs per manufacturer's guidelines. For siRNA experiments, ON-TARGETplus siRNAs specific for EGR1 and nontargeting control were purchased from GE Dharmacon (Lafayette, CO). Transfection of siRNAs (50 nM) was completed using Lipofectamine RNAiMAX reagent per manufacturer's instructions (Thermo Fisher Scientific). Results are representative of at least three independent experiments.
Western blotting analysis.
Solubilized protein lysates isolated from hPASMCs after stimulations were used for Western blotting. Cells were lysed using RIPA buffer (Sigma) supplemented with protease and phosphatase inhibitor cocktails (Calbiochem) and protein quantification and Western blot analysis were performed according to standard procedures.
RNA extraction and quantitative real-time PCR analysis.
Total RNA was isolated from hPASMCs using the RNeasy Mini kit from Qiagen (Valencia, CA) and reverse transcribed with the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Expression of SPHK1 mRNA was determined using a TaqMan primer assay with GAPDH used as an internal control (Thermo Fisher Scientific). Relative changes in mRNA expression were calculated using the comparative Ct method.
Statistical analysis.
Results are shown as means ± SE from at least three experiments and statistical significance was calculated with Student's t-test (*P < 0.05, **P < 0.01, ***P < 0.001 vs. controls) using GraphPad Prism software.
RESULTS
PDGF increases SphK1 expression in hPASMCs.
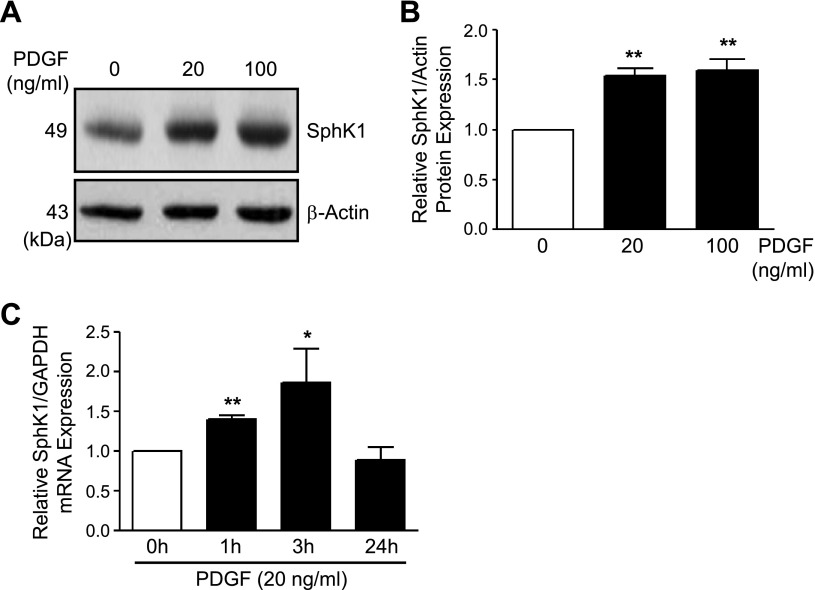
Given the upregulation of both SphK1 and PDGF in PAH and their association with pulmonary vascular remodeling, we first tested whether PDGF can induce SphK1 expression in hPASMCs. Following stimulation of hPASMCs with PDGF-BB (20–100 ng/ml, 0–6 h), SphK1 protein was increased by ∼1.5-fold at 6 h as measured by Western blotting and normalized to β-actin expression (Fig. 1, A and B). PDGF did not increase SphK2 expression in hPASMCs (data not shown). Next, quantitative real-time RT-PCR was used to measure SphK1 mRNA following stimulation of hPASMCs with PDGF-BB (20 ng/ml, 0–24 h). SphK1 mRNA normalized to GAPDH expression was significantly increased at 1 and 3 h, and expression returned to baseline levels by 24 h (Fig. 1C). These findings demonstrate the role of PDGF-BB in SphK1 upregulation in hPASMCs.
Fig. 1.
PDGF increases SphK1 expression in hPASMCs. A and B: representative Western blotting images and β-actin-normalized quantification of protein levels demonstrate increased SphK1 expression in hPASMC following stimulation with PDGF-BB (20–100 ng/ml, 6 h). C: SphK1 mRNA expression levels are also increased in hPASMC following PDGF-BB treatment (20 ng/ml, 0–24 h). Results are shown as means ± SE from at least 3 experiments. *P < 0.05, **P < 0.01 vs. untreated control.
PDGF activates the SphK1 promoter in hPASMCs.
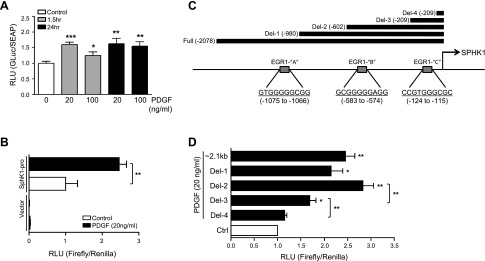
To investigate the mechanism(s) by which PDGF increases SphK1 expression in hPASMCs, we tested whether PDGF could induce activation of the SphK1 promoter using several approaches. First, a commercially available dual reporter construct was used to express and secrete Gaussia Luciferase (GLuc) under control of the ∼1.3-kb upstream human SphK1 promoter sequence, with Secreted Alkaline Phosphatase (SEAP) used as a control. PDGF-BB (20–100 ng/ml) stimulated SphK1 promoter activity at 1.5 and 24 h, as measured by quantification of relative luminescence of GLuc/SEAP in the culture media (Fig. 2A). To confirm these findings, the ∼2.1-kb human SphK1 promoter was isolated via PCR and cloned into the pGL4 luciferase reporter vector. Stimulation of hPASMCs transfected with this vector with PDGF-BB (20 ng/ml, 1.5 h) resulted in a 2.5-fold increase in transcriptional activity over basal levels (Fig. 2B). The empty control vector was not activated under basal or PDGF-BB stimulated conditions.
Fig. 2.
PDGF activates the SphK1 promoter in hPASMCs. A: relative luminescence of secreted Gaussia Luciferase (GLuc) and Secreted Alkaline Phosphatase (SEAP) in a SphK1 promoter dual-reporter system, demonstrating that PDGF (20–100 ng/ml, 1.5 h) stimulates transcriptional activity of the SphK1 promoter (1300 bp) in hPASMCs. B: relative luminescence of a full-length human Sphk1 promoter (∼2.1 kb) cloned into a pGL4 luciferase reporter vector (Firefly/Renilla) demonstrating induction of SphK1 promoter transcriptional activity with PDGF (20 ng/ml, 1.5 h) in hPASMCs. C: representation of the SphK1 promoter containing several putative EGR1 binding sites (EGR1-A, -B, and -C) identified in silico, with black bars depicting SphK1 promoter deletion regions cloned into pGL4 luciferase reporter vectors. D: relative luminescence of Sphk1 promoter deletion constructs demonstrating induction of SphK1 promoter transcriptional activity with PDGF (20 ng/ml, 1.5 h) in hPASMCs. Results are shown as means ± SE from at least 3 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control unless otherwise indicated.
Next, in silico analysis of the SphK1 promoter was conducted to identify transcription factor binding sites that may mediate PDGF-induced SphK1 expression. Three highly predicted binding sites for early growth response protein 1 (Egr-1), a transcription factor by which PDGF is known to signal intracellularly (45), were identified within the SphK1 promoter (labeled EGR1-“A”, “B”, and “C”) (Fig. 2C). These three binding sites were also observed in the public ENCODE ChIP-seq database (44). To determine whether the promoter regions containing these Egr-1 binding sites were functionally important in PDGF-mediated SphK1 activation, truncated promoter fragments containing the sites were amplified by PCR and cloned into the pGL4 luciferase reporter vector, as depicted in Fig. 2C. hPASMCs were transfected with these vector constructs and stimulated with PDGF (20 ng/ml, 1.5 h). Truncations that excluded binding sites for EGR1-“B” and EGR1-“C” demonstrated a significant loss in SphK1 promoter activity, with exclusion of the EGR1-“B” site fragment showing the largest decrease in activity (Fig. 2D). These results suggest that Egr-1 binding to the SphK1 promoter may be important for PDGF-induced transcriptional activation.
PDGF increases nuclear expression of Egr-1 in hPASMCs.
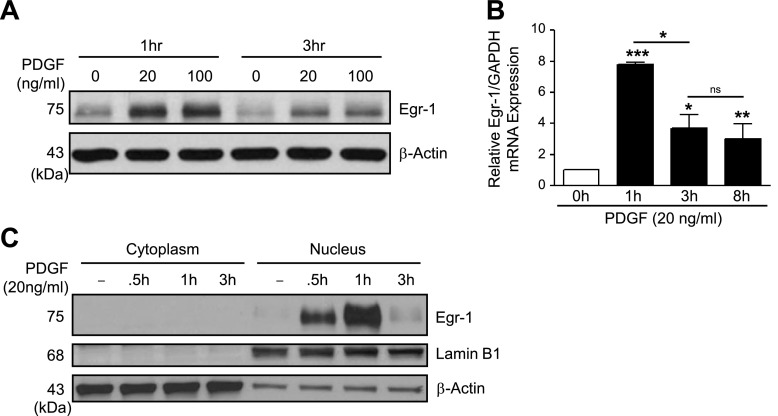
Since Egr-1 transcription factor binding sites may play a role in PDGF-induced SphK1 activation, we tested whether PDGF-BB could increase Egr-1 expression in hPASMCs. Stimulation with PDGF-BB (20–100 ng/ml, 1–3 h) resulted in a dramatic increase in Egr-1 protein expression by 1 h that subsided by 3 h (Fig. 3A). PDGF-BB also significantly increased Egr-1 mRNA levels, with maximum expression observed at 1 h (Fig. 3B). The basal expression of Egr-1 protein expression was also measured in three hPASMC lines derived from PAH patients, and no basal differences were observed compared with control PASMCs (data not shown). Although higher circulating PDGF concentrations within the pulmonary vascular microenvironment of PAH patients have been demonstrated (48) which may lead to elevations in Egr-1 expression, this elevation may not be retained following isolation of PASMCs and culturing in vitro.
Fig. 3.
PDGF increases EGR1 expression in hPASMCs. A: representative Western blotting images demonstrate increased EGR1 protein expression in human pulmonary artery smooth muscle cells (hPASMC) following stimulation with PDGF-BB (20–100 ng/ml, 1–3 h). B: EGR1 mRNA expression levels are increased in hPASMC following PDGF-BB treatment (20 ng/ml, 0–8 h) relative to GAPDH expression. C: Western blotting with cytoplasmic and nuclear hPASMC fractions demonstrates PDGF-BB treatment (20 ng/ml, 0–3 h) induces EGR1 expression limited to the nucleus, with Lamin B1 used as a nuclear loading control. Results are shown as means ± SE from at least 3 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control unless otherwise indicated.
Egr-1 has a bipartite nuclear localization domain allowing for nuclear expression that is pivotal for its functional role as a transcription factor (16, 39), although Egr-1 expression in the cytoplasm has also been reported in some cancer cell lines (32). We next tested whether PDGF-induced Egr-1 expression was localized in the nucleus. Stimulation with PDGF-BB (20 ng/ml, 0.5–3 h) resulted in abundant and transient nuclear Egr-1 protein expression that was maximal at 1 h (Fig. 3C), with Lamin B1 used as a nuclear loading control. These results show upregulation of Egr-1 by PDGF in hPASMCs.
PDGF induces Egr-1 binding to the SphK1 promoter in hPASMC.
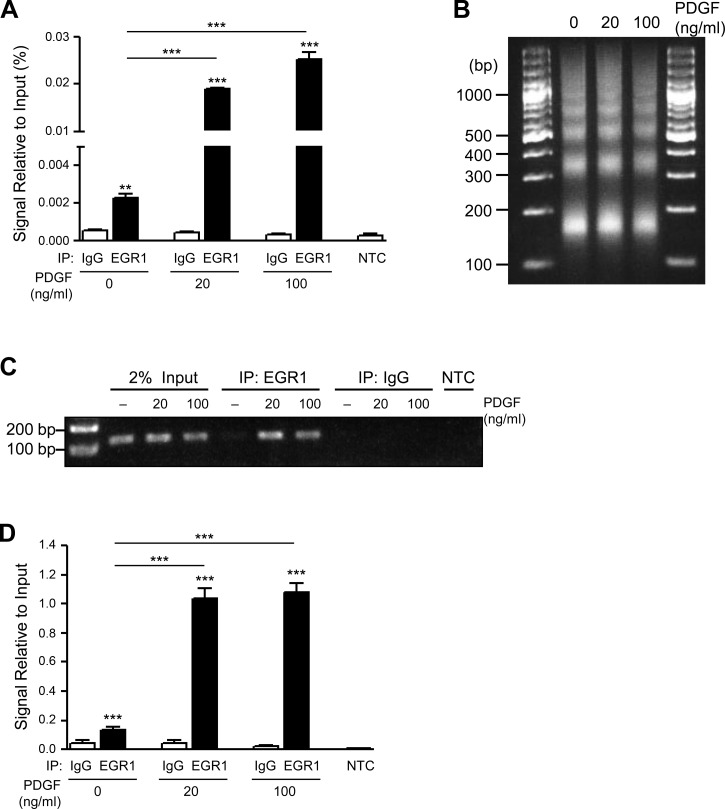
Regions of the SphK1 promoter containing both EGR1-“B” and EGR1-“C” transcription factor binding sites are important for PDGF-induced SphK1 expression, with loss of the EGR1-“B” resulting in the greatest reduction in expression (Fig. 2D). Given these results, we sought to determine whether PDGF could induce direct binding of Egr-1 to the EGR1-“B” predicted site within the SphK1 promoter. Following stimulation of hPASMCs with PDGF-BB (20–100 ng/ml, 1 h), chromatin immunoprecipitation (ChIP) studies coupled with quantitative real-time PCR demonstrated significantly increased binding of Egr-1 protein to the EGR1-“B” DNA binding site within the SphK1 promoter (Fig. 4A). Optimal chromatin shearing was achieved for these studies as demonstrated in Fig. 4B. To confirm these findings, standard PCR was used to amplify the EGR1-“B” binding site following immunoprecipitation with Egr-1 in hPASMCs treated with PDGF-BB (20–100 ng/ml, 1 h). DNA gel electrophoresis for these studies demonstrated increased Egr-1 protein binding to the EGR1-“B” site within the SphK1 promoter relative to IgG controls, with resulting quantification of signal relative to input (Fig. 4, C and D).
Fig. 4.
PDGF induces EGR1 binding to the SphK1 promoter in hPASMCs. A: ChIP real-time PCR analysis demonstrates EGR1 binding to the EGR1-“B” site within the SphK1 promoter following stimulation of hPASMC with PDGF (20–100 ng/ml, 1 h), with optimized chromatin digestion of ChIP samples (B). C and D: representative DNA gel electrophoresis of ChIP PCR products demonstrates enhanced EGR1 binding to the EGR1-“B” site within the SphK1 promoter following stimulation of hPASMC with PDGF (20–100 ng/ml, 1 h), with quantification of data signal relative to input. Results are shown as means ± SE from at least 3 experiments. **P < 0.01, ***P < 0.001 vs. IgG control unless otherwise indicated.
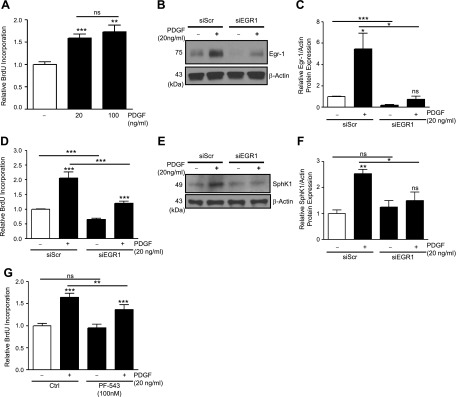
PDGF promotes SphK1 expression and hPASMC proliferation via EGR1.
PDGF-BB (20–100 ng/ml, 48 h) increases proliferation of hPASMCs (Fig. 5A). Since PDGF also enhances Egr-1 transcription factor expression, SphK1 expression, and binding of Egr-1 to the Sphk1 promoter, we aimed to determine whether silencing of Egr-1 could prevent PDGF-induced SphK1 expression. SiRNA-mediated silencing of Egr-1 in hPASMCs resulted in >80% reduction in basal Egr-1 protein expression after 48 h (Fig. 5, B and C). Egr-1 silencing also prevented induction of Egr-1 expression by PDGF-BB (20 ng/ml, 1 h) (Fig. 5, B and C).
Fig. 5.
PDGF promotes hPASMC proliferation and SphK1 expression via EGR1. A: BrdU-incorporation assays demonstrate enhanced proliferation of hPASMC following stimulation with PDGF-BB (20–100 ng/ml, 48 h). B and C: siRNA-mediated silencing of EGR1 reduces basal and PDGF-induced (20 ng/ml, 1 h) EGR1 expression, with data quantification. D: siRNA-mediated silencing of EGR1 reduces basal hPASMC proliferation and attenuates PDGF-induced (20 ng/ml, 48 h) proliferation. E and F: siRNA-mediated silencing of EGR1 in hPASMC prevents the induction of SphK1 protein expression by PDGF-BB (20 ng/ml, 6 h), with data quantification. G: selective SphK1 inhibition with PF-543 (100 nM, 1 h pretreatment) attenuates PDGF-induced (20 ng/ml, 48 h) proliferation but not basal proliferation. Results are shown as means ± SE from at least 3 experiments. **P < 0.01, ***P < 0.001 vs. untreated control unless otherwise indicated.
Uncontrolled proliferation of PASMCs contributes to pulmonary vascular remodeling in PAH, but the mechanisms regulating the proliferative phenotype of these cells are poorly understood. Therefore, we next tested whether silencing of Egr-1 could alter proliferation of hPASMCs. Compared with scrambled siRNA controls, silencing of Egr-1 decreased basal and PDGF-induced (20 ng/ml, 48 h) proliferation of hPASMCs as measured by relative BrdU incorporation (Fig. 5D). In addition, silencing of Egr-1 attenuated PDGF-induced (20 ng/ml, 6 h) expression of SphK1 in hPASMCs (Fig. 5, E and F). Next, we used the highly specific SphK1 inhibitor, PF-543, to assess the importance of SphK1 in mediating PDGF-induced proliferation. Treatment of hPASMCs with PF-543 (100 nM, 1 h pretreatment) did not alter basal hPASMC proliferation, but significantly reduced PDGF-induced (20 ng/ml, 48 h) proliferation (Fig. 5G). These results demonstrate the importance of the Egr-1/SphK1 signaling axis in the induction of hPASMC proliferation by PDGF.
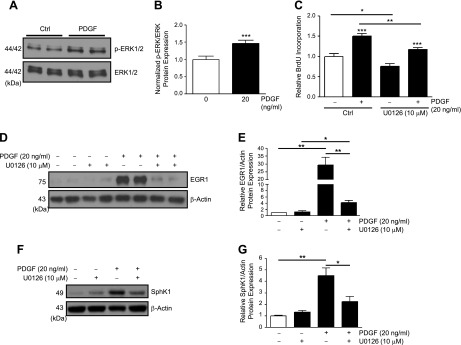
PDGF increases Egr-1 and SphK1 in hPASMCs via ERK.
Previous studies have identified the importance of the MAPK/ERK cascade in cell proliferation (9), Egr-1 activation (26), and PDGF-induced cell signaling (53). Here we explored whether MAPK/ERK signaling could be an upstream mediator of PDGF-induced EGR1 activation in hPASMCs. Treatment of hPASMCs with PDGF-BB (20 ng/ml) resulted in enhanced ERK1/2 phosphorylation at 15 min (Fig. 6, A and B). Inhibition of ERK phosphorylation with U0126 (10 μM, 1 h pretreatment), a highly selective inhibitor of MAPK/ERK kinases MEK1 and MEK2, resulted in reduced basal and PDGF-induced (20 ng/ml, 48 h) hPASMC proliferation (Fig. 6C) and significant attenuation of PDGF-induced (20 ng/ml, 1 h) Egr-1 protein expression in hPASMCs (Fig. 6, D and E). U0126 (10 μM) also inhibited induction of downstream SphK1 expression by PDGF (20 ng/ml, 6 h) (Fig. 6, F and G). These results demonstrate the critical importance of MAPK/ERK signaling in PDGF-induced proliferation and Egr-1 and SphK1 expression in hPASMCs.
Fig. 6.
ERK phosphorylation is required for PDGF-induced EGR1 and SphK1 expression in hPASMC. A and B: representative Western blotting images and ERK-normalized quantification of protein levels demonstrate induction of ERK phosphorylation by PDGF (20 ng/ml, 15 min) in hPASMC. C: U0126-mediated inhibition of ERK phosphorylation reduces basal hPASMC proliferation and attenuates PDGF-induced (20 ng/ml, 48 h) proliferation. D and E: representative Western blotting images and β-actin-normalized quantification of protein levels demonstrate U0126-mediated inhibition of ERK phosphorylation attenuates EGR1 induction by PDGF (20 ng/ml, 1 h) in hPASMC; and attenuates SphK1 induction by PDGF (20 ng/ml, 6 h) in hPASMC (F and G). Results are shown as means ± SE from at least 3 experiments. *P < 0.05, **P < 0.01, ***P < 0.001 vs. untreated control unless otherwise indicated.
DISCUSSION
The mechanistic processes contributing to PASMC proliferation and the development of pulmonary vascular remodeling in PAH are poorly understood. Given the important role of both PDGF and SphK1/S1P in the pathobiology of PAH (9, 42), we investigated whether PDGF-induced proliferation of hPASMCs involves induction of SphK1 expression and potential mechanisms by which this may occur. In this study, we demonstrate that 1) PDGF induces SphK1 and Egr-1 transcription factor expression in hPASMCs, 2) PDGF-mediated Sphk1 transcriptional activity is mediated through binding of Egr-1 to the proximal SphK1 promoter, 3) Egr-1 and SphK1 are important for PDGF-induced hPASMC proliferation, 4) downregulation of Egr-1 attenuates PDGF-induced expression of SphK1 in hPASMC, and 5) MAPK/ERK phosphorylation is critical for induction of Egr-1 and SphK1 by PDGF. Our data describe a novel mechanism contributing to PASMC proliferation in PAH and identify the PDGF/EGR1/SphK1 pathway as a potential therapeutic target.
PDGF-BB is released by endothelial cells and platelets during vascular injury and can stimulate hPASMC proliferation and migration (31, 42). The role of PDGF and its receptors in the pathobiology of PAH is well established, and the utility of pharmacologically targeting this pathway in PAH has been proposed (3, 42). One recent study found that in a rodent model of chronic hypoxia-mediated PH, mice genetically engineered with constitutively active PDGFR-β expression developed more severe pulmonary vascular remodeling than with chronic hypoxia alone (10). PDGF receptor antagonism using imatinib has also been shown to reverse advanced pulmonary vascular disease in several animal models of PH, including reversal of vascular remodeling and cor pulmonale (47). Clinically, the use of imatinib in several cases of refractory PAH has led to improvements in exercise capacity, hemodynamics, and functional class (17, 41). Here, we demonstrate that PDGF can stimulate SphK1 expression in hPASMCs and that regions of the proximal SphK1 promoter are important for this induction. In other cell types, PDGF-induced SphK1 activity has been shown to increase intracellular S1P levels and is important in mediating proliferation, due in part to the ability of S1P to mobilize calcium and enhance levels of mitogenic phosphatidic acid (36, 43, 55). In addition, silencing of SphK1 in mouse embryonic fibroblast cells reduces PDGF-induced migration (18). We recently reported that levels of both SphK1 and S1P are elevated in patients with PAH and in rodent models of experimental PH, and that SphK1/S1P promote hPASMC proliferation (9). Genetic deletion or pharmacologic inhibition of SphK1 also protects from the development of experimental PH in several rodent models (9). The present data indicate the importance of PDGF signaling in activating SphK1 in hPASMCs, mechanistically linking these important pathways in PAH which contribute to vascular remodeling.
The SphK1 promoter deletion and ChIP analyses identified the importance of Egr-1 in mediating PDGF-induced SphK1 expression via nuclear translocation and binding to the proximal promoter. Egr-1 is a highly conserved, Cys2His2 type zinc-finger transcription factor known to be induced by a variety of stimuli such as oxidative stress, shear stress, and growth factors, including PDGF (8, 22, 29, 38). In patients with both congenital heart disease–associated PAH and idiopathic PAH, Egr-1 expression is abundant in plexiform lesions and in smooth muscle cells in vessels with severe concentric intimal fibrosis (51). Enhanced expression of Egr-1 has also been shown in the pulmonary vascular smooth muscle cell layer in human PAH and in a severe MCT-induced PAH rat model, which directly correlated with the degree of pulmonary vascular remodeling (11). Expression of Egr-1 has also been shown to be increased in the lung and pulmonary vascular cells in response to hypoxia, where it can activate several downstream targets involved in vascular remodeling in PAH (4, 11). Interestingly, in a rat model of flow-associated PAH in rats, downregulation of Egr-1 in vivo led to reduced expression of vascular PDGF-BB, less vascular proliferation, and increased apoptosis (11).
Here, we report that Egr-1 expression is rapidly induced in the nucleus of hPASMCs following PDGF stimulation, and that activation of Egr-1 is important for PDGF-induced SphK1 expression and cell proliferation. Using a specific SphK1 inhibitor, PF-543, we also demonstrate that SphK1 is involved in mediating PDGF-induced hPASMC proliferation. PF-543 did not alter basal proliferation, confirming previous findings that this drug does not inhibit DNA synthesis in hPASMCs (7). Our studies are also consistent with reports in other cell types that induced expression of Egr-1 is important in the control of cell proliferation, survival, and arteriogenesis (40, 52). In addition to the novel activation of SphK1 by Egr-1 in hPASMCs presented here, the role of Egr-1 in activating other genes involved in regulation of vascular proliferation, inflammation, and apoptosis, including PDGF, TGF-β, IL-6, and p53, have been reported (11). These findings collectively highlight the importance of Egr-1 in key components of vascular remodeling, and suggest that targeting this pathway may be therapeutically beneficial in PAH. Several putative Egr-1 binding sites were identified within the SphK1 promoter, and this study explored the role of the EGR1-“B” site in detail due to the importance of the promoter region containing this site in SphK1 transcriptional activation. Future studies investigating the other potential Egr-1 binding sites may be important in determining the mechanistic regulation of SphK1 in other cell types or in response to other stimuli that activate Egr-1.
To further explore the mechanisms of PDGF-induced Egr-1 and downstream SphK1 expression in hPASMCs, we investigated the influence of the MAPK/ERK signaling pathway. We demonstrate that ERK1/2 phosphorylation is important for basal and PDGF-induced hPASMC proliferation and is critical for activation of Egr-1 and SphK1 expression by PDGF. The U0126 inhibitor used in these studies acts directly upon MEK1/2, so is highly specific in blocking ERK1/2 phosphorylation. The other MAPKs JNK and p38 are phosphorylated by MEK4/7 and MEK3/6, respectively (27). Our results demonstrate a nearly complete loss of PDGF-induced Egr-1 expression with U0126, suggesting that JNK and p38 are less involved in this signaling pathway in hPASMCs. Importantly, several supportive lines of evidence from the literature have demonstrated that inhibition of ERK1/2 phosphorylation can reduce hPASMC proliferation induced by a variety of stimuli in vitro. For example, pro-proliferative effects of hypoxia and brain-derived neurotrophic factor (BDNF) in hPASMCs have been attenuated using U0126 (28, 49). Another recent study demonstrated that both U0126 and inhibition of PDGF receptor signaling blocked peroxynitrite-induced proliferation and ERK1/2 phosphorylation in hPASMC (1). These studies highlight the multifaceted mechanisms by which ERK1/2 may regulate hPASMC proliferation and support our findings that downstream targets of ERK1/2 are involved in this process. Experiments to more precisely characterize the role of other downstream targets of ERK1/2 in PDGF-induced PASMC proliferation may be relevant to PAH pathobiology and could be explored in future studies.
The role of MAPK/ERK-dependent activation of Egr-1 by numerous stimuli, including PDGF, has been described in other vascular smooth muscle cell types (21, 22, 29). In mouse macrophages, inhibition of in ERK1/2 phosphorylation and activation resulted in reduced Egr-1-induced tissue factor (TF) expression. Lysophosphatidic acid-induced Egr-1 expression has also been shown to be dependent on the MEK/ERK and JNK cascades (26). Importantly, increased levels of ERK phosphorylation have been demonstrated in mouse and rat lung tissues and in the medial layer of vascular lesions in several models of experimental PH (2, 33, 47). Administration of imatinib in a monocrotaline-induced PH rat model strongly inhibited phosphorylation/activation of both PDGFR and ERK1/2 and reversed PH development (47), demonstrating an important relationship between PDGF and ERK signaling.
Notably, Egr-1 is also known to enhance expression of PDGFR (11), and S1P can induce mRNA and protein expression of PDGF-A and -B in vascular smooth muscle cells and neointimal cells from injured arteries (50). We therefore hypothesize that signaling of PDGF/ERK/Egr-1 in hPASMCs may create a positive feedback loop to generate additional PDGF/PDGFR. This would then lead to increased expression of SphK1 and other Egr-1 target genes involved in pulmonary vascular remodeling. Interestingly, the role of SphK1/S1P signaling as a positive regulator of Egr-1 has also been reported in several other cell types (15, 19, 46). Since our studies indicate that Egr-1 can activate expression of SphK1, which produces S1P, a positive feedback loop may be formed by the generation of additional Egr-1 expression. However, this loop may not be evident in cultured PASMCs since a biphasic induction of Egr-1 expression following PDGF stimulation has not been observed at time points as late as 48 h in our studies (data not shown). Future mechanistic studies to explore additional directions of the SphK1 activation pathway in PASMCs are warranted.
The process of pulmonary vascular remodeling in PAH also involves dysfunction and activation of other cell types besides smooth muscle, including endothelial cells and fibroblasts (34). During end-stage disease in a rat model of flow-associated PAH, Egr-1 expression was shown to be increased in both the medial smooth muscle and endothelial vessel layers (11). This study also demonstrated that downregulation of Egr-1 increased vascular cell apoptosis, predominantly in the endothelial layer (11). While the present study focused on the proliferative phenotype of PASMCs, the role of PDGF activation of SphK1 in these other cells types and their crosstalk with PASMCs warrants further investigation.
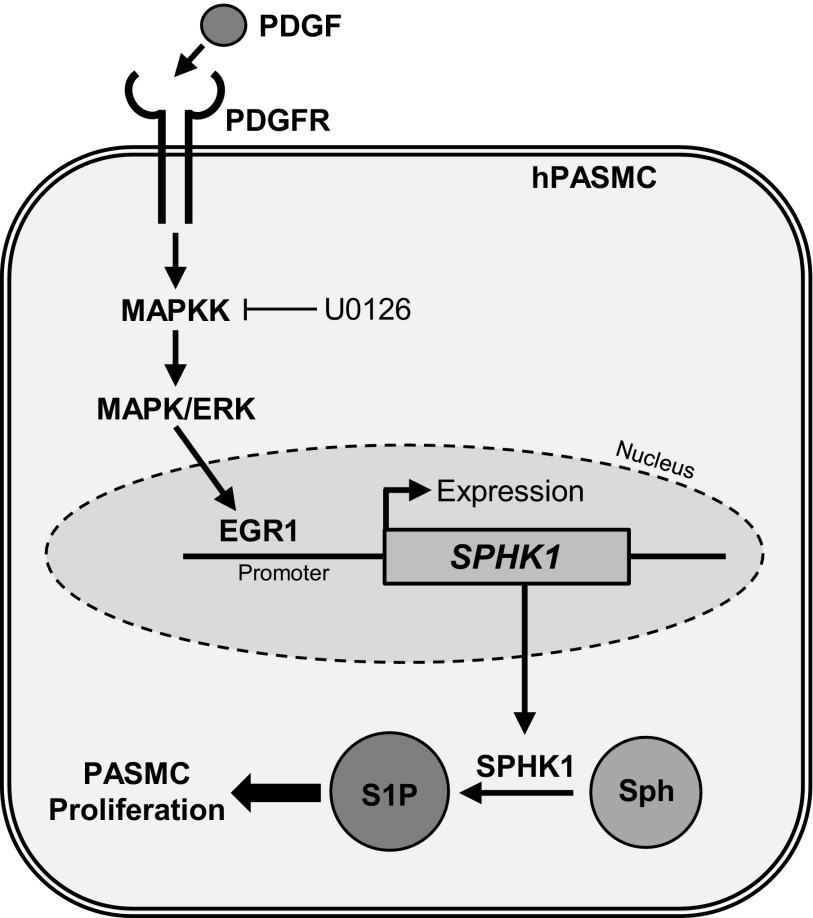
Vascular smooth muscle cells, including PASMCs, have high plasticity compared with terminally-differentiated skeletal or cardiac muscle cells (37). Various environmental stimuli, including growth factors and changes in oxygen tension, can cause PASMCs to undergo a transition from a quiescent to highly proliferative phenotype (37). These changes are apparent under pathological conditions in PAH, where PASMCs demonstrate an increased proliferation and migration rate due in part to increases in cytosolic calcium concentration, ultimately leading to vessel obstruction and enhanced pulmonary vascular resistance (13, 34). Here, we have demonstrated that PDGF-induced ERK/Egr-1 signaling is a novel pathway in hPASMCs that enhances SphK1 expression and cell proliferation, as outlined in Fig. 7. These studies advance our understanding of the ability of external stimuli in PAH to upregulate SphK1 expression and highlight the therapeutic potential of targeting this pathway in PAH to prevent or reverse pulmonary vascular remodeling.
Fig. 7.
Potential mechanism of PDGF-induced SphK1 expression and proliferation of hPASMCs in pulmonary arterial hypertension (PAH). The PDGF/PDGFR signaling pathway is upregulated in PAH. Activation of this pathway induces phosphorylation of MAPK/ERK, expression and nuclear translocation of the EGR1 transcription factor, and subsequent upregulation of SphK1 gene expression. Increased intracellular SphK1 may then lead to S1P production in hPASMCs, resulting in enhanced proliferation. Abnormal hPASMC proliferation leads to pulmonary vascular remodeling and increased pulmonary vascular resistance in PAH.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants R01-HL-127342 and R01-HL-111656 to R. F. Machado, P01-HL-98050 and R01-HL-127342 to V. Natarajan, NRSA-F30-HL-128034 to J. R. Sysol, American Heart Association Predoctoral Fellowship (15PRE2190004) to J. R. Sysol, UIC Center for Clinical and Translational Science (CCTS) Predoctoral Education for Clinical and Translational Scientists (PECTS) Award to J. R. Sysol, and UIC Biomedical Deiss Fund Award to J. R. Sysol.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.R.S., V.N., and R.F.M. conception and design of research; J.R.S. performed experiments; J.R.S. analyzed data; J.R.S. and R.F.M. interpreted results of experiments; J.R.S. prepared figures; J.R.S. drafted manuscript; J.R.S., V.N., and R.F.M. edited and revised manuscript; J.R.S., V.N., and R.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Jiwang Chen and Djanybek Adyshev for helpful discussions and input during the preparation of this article.
REFERENCES
- 1.Agbani EO, Coats P, Mills A, Wadsworth RM. Peroxynitrite stimulates pulmonary artery endothelial and smooth muscle cell proliferation: involvement of ERK and PKC. Pulm Pharmacol Ther 24: 100–109, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Al Husseini A, Bagnato G, Farkas L, Gomez-Arroyo J, Farkas D, Mizuno S, Kraskauskas D, Abbate A, Van Tassel B, Voelkel NF, Bogaard HJ. Thyroid hormone is highly permissive in angioproliferative pulmonary hypertension in rats. Eur Respir J 41: 104–114, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Antoniu SA. Targeting PDGF pathway in pulmonary arterial hypertension. Expert Opin Ther Targets 16: 1055–1063, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Banks MF, Gerasimovskaya EV, Tucker DA, Frid MG, Carpenter TC, Stenmark KR. Egr-1 antisense oligonucleotides inhibit hypoxia-induced proliferation of pulmonary artery adventitial fibroblasts. J Appl Physiol (1985) 98: 732–738, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Berg JT, Breen EC, Fu Z, Mathieu-Costello O, West JB. Alveolar hypoxia increases gene expression of extracellular matrix proteins and platelet-derived growth factor-B in lung parenchyma. Am J Respir Crit Care Med 158: 1920–1928, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev 81: 999–1030, 2001. [DOI] [PubMed] [Google Scholar]
- 6a.Brunati AM, Tibaldi E, Carraro A, Gringeri E, D'Amico F Jr, Toninello A, Massimino ML, Pagano MA, Nalesso G, Cillo U. Cross-talk between PDGF and S1P signalling elucidates the inhibitory effect and potential antifibrotic action of the immunomodulator FTY720 in activated HSC-cultures. Biochim Biophys Acta 1783: 347–359, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Byun HS, Pyne S, Macritchie N, Pyne NJ, Bittman R. Novel sphingosine-containing analogs selectively inhibit sphingosine kinase (SK) isozymes, induce SK1 proteasomal degradation and reduce DNA synthesis in human pulmonary arterial smooth muscle cells. Med Chem Comm 4: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao XM, Koski RA, Gashler A, McKiernan M, Morris CF, Gaffney R, Hay RV, Sukhatme VP. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Mol Cell Biol 10: 1931–1939, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, Gorshkova I, Wang L, Huang LS, Usatyuk PV, Sammani S, Zhou G, Raj JU, Garcia JG, Berdyshev E, Yuan JX, Natarajan V, Machado RF. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med 190: 1032–1043, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahal BK, Heuchel R, Pullamsetti SS, Wilhelm J, Ghofrani HA, Weissmann N, Seeger W, Grimminger F, Schermuly RT. Hypoxic pulmonary hypertension in mice with constitutively active platelet-derived growth factor receptor-beta. Pulm Circ 1: 259–268, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson MG, Kowalski PS, Bartelds B, Borgdorff MA, van der Feen D, Sietsma H, Molema G, Kamps JA, Berger RM. A critical role for Egr-1 during vascular remodelling in pulmonary arterial hypertension. Cardiovasc Res 103: 573–584, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 351: 1655–1665, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez RA, Wan J, Song S, Smith KA, Gu Y, Tauseef M, Tang H, Makino A, Mehta D, Yuan JX. Upregulated expression of STIM2, TRPC6, and Orai2 contributes to the transition of pulmonary arterial smooth muscle cells from a contractile to proliferative phenotype. Am J Physiol Cell Physiol 308: C581–C593, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francy JM, Nag A, Conroy EJ, Hengst JA, Yun JK. Sphingosine kinase 1 expression is regulated by signaling through PI3K, AKT2, and mTOR in human coronary artery smooth muscle cells. Biochim Biophys Acta 1769: 253–265, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Furuya H, Wada M, Shimizu Y, Yamada PM, Hannun YA, Obeid LM, Kawamori T. Effect of sphingosine kinase 1 inhibition on blood pressure. FASEB J 27: 656–664, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gashler AL, Swaminathan S, Sukhatme VP. A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor Egr-1. Mol Cell Biol 13: 4556–4571, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med 353: 1412–1413, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol Cell Biol 25: 4237–4249, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurgui M, Broere R, Kalff JC, van Echten-Deckert G. Dual action of sphingosine 1-phosphate in eliciting proinflammatory responses in primary cultured rat intestinal smooth muscle cells. Cell Signal 22: 1727–1733, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118: 1846–1857, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasan RN, Schafer AI. Hemin upregulates Egr-1 expression in vascular smooth muscle cells via reactive oxygen species ERK-1/2-Elk-1 and NF-kappaB. Circ Res 102: 42–50, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Hjoberg J, Le L, Imrich A, Subramaniam V, Mathew SI, Vallone J, Haley KJ, Green FH, Shore SA, Silverman ES. Induction of early growth-response factor 1 by platelet-derived growth factor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L817–L825, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, Capron F, Galanaud P, Duroux P, Simonneau G, Emilie D. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J 11: 554–559, 1998. [PubMed] [Google Scholar]
- 24.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425–1436, 2004. [DOI] [PubMed] [Google Scholar]
- 25.International PPHC, Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 26: 81–84, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Iyoda T, Zhang F, Sun L, Hao F, Schmitz-Peiffer C, Xu X, Cui MZ. Lysophosphatidic acid induces early growth response-1 (Egr-1) protein expression via protein kinase Cdelta-regulated extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) activation in vascular smooth muscle cells. J Biol Chem 287: 22635–22642, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannan Y, Wilson MS. TEC and MAPK kinase signalling pathways in T helper (T) cell development, T2 differentiation and allergic asthma. J Clin Cell Immunol Suppl 12: 11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwapiszewska G, Chwalek K, Marsh LM, Wygrecka M, Wilhelm J, Best J, Egemnazarov B, Weisel FC, Osswald SL, Schermuly RT, Olschewski A, Seeger W, Weissmann N, Eickelberg O, Fink L. BDNF/TrkB signaling augments smooth muscle cell proliferation in pulmonary hypertension. Am J Pathol 181: 2018–2029, 2012. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Zhang M, Zhang J, Zhang J, Chen C, Chen YE, Xiong JW, Zhu X. Platelet-derived growth factor induces Rad expression through Egr-1 in vascular smooth muscle cells. PLoS One 6: e19408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J; American College of Cardiology Foundation Task Force on Expert Consensus Documents, American Heart Association, American College of Chest Physicians, American Thoracic Society, and Pulmonary Hypertension Association. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc; and the Pulmonary Hypertension Association. J Am Coll Cardiol 53: 1573–1619, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Millette E, Rauch BH, Kenagy RD, Daum G, Clowes AW. Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc Med 16: 25–28, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Mora GR, Olivier KR, Cheville JC, Mitchell RF Jr, Lingle WL, Tindall DJ. The cytoskeleton differentially localizes the early growth response gene-1 protein in cancer and benign cells of the prostate. Mol Cancer Res 2: 115–128, 2004. [PubMed] [Google Scholar]
- 33.Moreno-Vinasco L, Gomberg-Maitland M, Maitland ML, Desai AA, Singleton PA, Sammani S, Sam L, Liu Y, Husain AN, Lang RM, Ratain MJ, Lussier YA, Garcia JG. Genomic assessment of a multikinase inhibitor, sorafenib, in a rodent model of pulmonary hypertension. Physiol Genomics 33: 278–291, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54: S20–S31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Mousseau Y, Mollard S, Richard L, Nizou A, Faucher-Durand K, Cook-Moreau J, Qiu H, Baaj Y, Funalot B, Fourcade L, Sturtz FG. Fingolimod inhibits PDGF-B-induced migration of vascular smooth muscle cell by down-regulating the S1PR1/S1PR3 pathway. Biochimie 94: 2523–2531, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Noskovicova N, Petrek M, Eickelberg O, Heinzelmann K. Platelet-derived growth factor signaling in the lung. From lung development and disease to clinical studies. Am J Respir Cell Mol Biol 52: 263–284, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 365: 557–560, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Pagel JI, Deindl E. Disease progression mediated by egr-1 associated signaling in response to oxidative stress. Int J Mol Sci 13: 13104–13117, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagel JI, Deindl E. Early growth response 1—a transcription factor in the crossfire of signal transduction cascades. Indian J Biochem Biophys 48: 226–235, 2011. [PubMed] [Google Scholar]
- 40.Pagel JI, Ziegelhoeffer T, Heil M, Fischer S, Fernandez B, Schaper W, Preissner KT, Deindl E. Role of early growth response 1 in arteriogenesis: impact on vascular cell proliferation and leukocyte recruitment in vivo. Thromb Haemost 107: 562–574, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Patterson KC, Weissmann A, Ahmadi T, Farber HW. Imatinib mesylate in the treatment of refractory idiopathic pulmonary arterial hypertension. Ann Intern Med 145: 152–153, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Perros F, Montani D, Dorfmuller P, Durand-Gasselin I, Tcherakian C, Le Pavec J, Mazmanian M, Fadel E, Mussot S, Mercier O, Herve P, Emilie D, Eddahibi S, Simonneau G, Souza R, Humbert M. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 178: 81–88, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Rani CS, Wang F, Fuior E, Berger A, Wu J, Sturgill TW, Beitner-Johnson D, LeRoith D, Varticovski L, Spiegel S. Divergence in signal transduction pathways of platelet-derived growth factor (PDGF) and epidermal growth factor (EGF) receptors. Involvement of sphingosine 1-phosphate in PDGF but not EGF signaling. J Biol Chem 272: 10777–10783, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Rosenbloom KR, Sloan CA, Malladi VS, Dreszer TR, Learned K, Kirkup VM, Wong MC, Maddren M, Fang R, Heitner SG, Lee BT, Barber GP, Harte RA, Diekhans M, Long JC, Wilder SP, Zweig AS, Karolchik D, Kuhn RM, Haussler D, Kent WJ. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res 41: D56–63, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rupprecht HD, Sukhatme VP, Lacy J, Sterzel RB, Coleman DL. PDGF-induced Egr-1 expression in rat mesangial cells is mediated through upstream serum response elements. Am J Physiol Renal Fluid Electrolyte Physiol 265: F351–F360, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Sato K, Ishikawa K, Ui M, Okajima F. Sphingosine 1-phosphate induces expression of early growth response-1 and fibroblast growth factor-2 through mechanism involving extracellular signal-regulated kinase in astroglial cells. Brain Res Mol Brain Res 74: 182–189, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115: 2811–2821, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Selimovic N, Bergh CH, Andersson B, Sakiniene E, Carlsten H, Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 34: 662–668, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Shan R, Chen L, Li X, Wu H, Liang Q, Tang X. Hypoxia promotes rabbit pulmonary artery smooth muscle cells proliferation through a 15-LOX-2 product 15(S)-hydroxyeicosatetraenoic acid. Prostaglandins Leukot Essent Fatty Acids 86: 85–90, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Usui S, Sugimoto N, Takuwa N, Sakagami S, Takata S, Kaneko S, Takuwa Y. Blood lipid mediator sphingosine 1-phosphate potently stimulates platelet-derived growth factor-A and -B chain expression through S1P1-Gi-Ras-MAPK-dependent induction of Kruppel-like factor 5. J Biol Chem 279: 12300–12311, 2004. [DOI] [PubMed] [Google Scholar]
- 51.van der Feen DE, Dickinson MG, Bartelds B, Borgdorff MA, Sietsma H, Levy M, Berger RM. Egr-1 identifies neointimal remodeling and relates to progression in human pulmonary arterial hypertension. J Heart Lung Transplant 35: 481–490, 2016. [DOI] [PubMed] [Google Scholar]
- 52.Virolle T, Krones-Herzig A, Baron V, De Gregorio G, Adamson ED, Mercola D. Egr1 promotes growth and survival of prostate cancer cells. Identification of novel Egr1 target genes. J Biol Chem 278: 11802–11810, 2003. [DOI] [PubMed] [Google Scholar]
- 52a.Waters C, Sambi B, Kong KC, Thompson D, Pitson SM, Pyne S, Pyne NJ. Sphingosine 1-phosphate and platelet-derived growth factor (PDGF) act via PDGF beta receptor-sphingosine 1-phosphate receptor complexes in airway smooth muscle cells. J Biol Chem 278: 6282–6290, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Yamboliev IA, Gerthoffer WT. Modulatory role of ERK MAPK-caldesmon pathway in PDGF-stimulated migration of cultured pulmonary artery SMCs. Am J Physiol Cell Physiol 280: C1680–C1688, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation 111: 534–538, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol 114: 155–167, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]