Abstract
The advent of cell reprogramming technologies has widely disclosed the possibility to have direct access to human neurons for experimental and biomedical applications. Human pluripotent stem cells can be instructed in vitro to generate specific neuronal cell types as well as different glial cells. Moreover, new approaches of direct neuronal cell reprogramming can strongly accelerate the generation of different neuronal lineages. However, genetic heterogeneity, reprogramming fidelity, and time in culture of the starting cells can still significantly bias their differentiation efficiency and quality of the neuronal progenies. In addition, reprogrammed human neurons exhibit a very slow pace in gaining a full spectrum of functional properties including physiological levels of membrane excitability, sustained and prolonged action potential firing, mature synaptic currents and synaptic plasticity. This delay poses serious limitations for their significance as biological experimental model and screening platform. We will discuss new approaches of neuronal cell differentiation and reprogramming as well as methods to accelerate the maturation and functional activity of the converted human neurons.
Keywords: Neurons, neuronal activity, synapses, induced pluripotent stem cells, embryonic stem cells, disease modeling
Introduction
Numerous methods are now available to obtain human neurons from a starting population of pluripotent stem cells (PSCs) or directly from reprogrammed fibroblasts, but the resulting neuronal cells often do not reach full functionality or required an extensive period of time to exhibit robust functional parameters. This long delay in acquiring functional properties has resulted into a severe hurdle for taking full advantage of human neurons as a biological model or a platform system. In fact the ability of neurons to fire action potentials as well as forming synapses in vitro is crucial to study the effects of mutations or drugs that can affect synaptic transmission and plasticity. Their capacity to functionally integrate in vivo when transplanted into a neuronal network already formed is also relevant for future cell therapies. Herein, we briefly discuss recent techniques to generate human neurons and in particular focus on new procedures that enhance functional maturation of the reprogrammed neuronal cells.
In vitro neuronal differentiation of human pluripotent stem cells (hPSCs)
Neuronal cells have been among the first lineages to be differentiated using hPSCs, a term describing both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs).1,2 Neuronal induction is traditionally obtained by promoting the differentiation of hPSCs in aggregate-like embryoid bodies. Subsequently, aggregates are placed in stringent serum-free culture conditions, which selectively facilitate the survival and growth of neural cells. This transition toward the neural lineage is readily manifested in hPSCs (but not in their murine counterparts) because of the appearance of rosette-like structures within the differentiating hPSC colonies.1,2 These structures develop from neural progenitor cells, which line up close together to form a round, columnar epithelium that is reminiscent of blooming rosettes when viewed under bright light.
Rosette neural progenitors can be expanded in vitro as a renewable cell population either as neurospheres in suspension or attached to a substrate. The generation of a multipotent intermediate is highly advantageous since they can maintain a stable cell growth in vitro and differentiate in both neurons and different glial cell types. In addition, various morphogens can be added to the culture medium to induce and guide terminal neuronal cell type specification modeling developmental signaling pathway acting during the central nervous system embryonic development.3–6
To accelerate neuronal differentiation, alternative protocols based on the dual inhibition of SMAD signaling by a combination of Noggin or LDN193189 together with SB431542 have been shown to efficiently neuralize hPSCs generally avoiding the step of EB formation or the use of feeder cells.4,7–13 Noggin represses the endogenous BMP signals and acts synergistically with SB431542 that inhibits the TGFβ pathway by blocking the phosphorylation of ALK4, ALK5 and ALK7 receptors. However, dual SMAD inhibition seemed not to be effective for all the iPSC lines due to their high intrinsic variability in response to extracellular signals.4
Using these methods, hPSCs have been successfully differentiated in human neurons of several different subtypes (Table 1). This is especially important since the diverse neuronal lineages that compose the nervous system own unique properties that may render them sensitive or resistant to particular acute or chronic insults. In particular, neurodegenerative diseases might lead to the loss of specific neuronal subtypes that can be reconstituted in vitro by cell reprogramming technologies as a way to properly understand the pathological mechanisms behind.
Table 1.
Comparison of neural differentiation methods from human PSCs
| Neuron subtype | Cell type | Days for MAP2+ or Tuj1+ | Efficiency | Subtype purity | Days for functional synapsis | SMAD inhibition | Reference |
|---|---|---|---|---|---|---|---|
| Neurons | iPSC | ≈21–28 | >90% | 15% VGLUT1 and 8% GABA | ≈28–35 | no | 5 |
| Neurons | ESC | 28 | ≈89% | N.D. | 56 | no | 6 |
| Neurons | ESC | 28 | 70–80% | mixed | ≈35 | Noggin in some cases | 14 |
| Glutamatergic | PSC | ≈30 | ≈70% | N.D. | ≈55–110 | Noggin and SB431542 | 13 |
| Glutamatergic | PSC | ≈7 | ≈100% | ≈100% | 15 | no | 15 |
| Glutamatergic | PSC | 44 | ≈65% | ≈40% | >38 | Noggin | 16 |
| GABAergic | ESC | 18 | ≈70% | ≈80% | >30 | LDN193189 and SB431542 | 12 |
| GABAergic | PSC | ≈20 | ≈80% | ≈70% | 56 | SB431542 and BMPRIA-Fc | 17 |
| Dopaminergic | iPSC | 16 | ≈40% | ≈20% | 70 | Noggin and SB431542 | 10 |
| Dopaminergic | PSC | 21–28 | N.D. | 60–80% | >49 | LDN193189 and SB431542 | 11 |
| Motor | PSC | 21 | N.D. | ≈50% | N.D. | LDN193189 and SB431542 | 7 |
| Motor | iPSC | >7–15 | N.D. | 20% | N.D. | no | 3 |
| Motor | iPSC | >21 | ≈16% | ≈4% | N.D. | no | 18 |
| Motor | PSC | 42 | N.D. | ≈40% | > 56 | Noggin and SB431542 for iPS | 4 |
| Sensory | PSC | 10 | 75% | >61% | N.D. | LDN193189 and SB431542 | 9 |
Note: Efficiency is the percentage of Tuj1 or MAP2 positive cells respect to total number of cells (DAPI nuclear staining). Subtype purity is the percentage of a specific subtype marker respect to the total number of cells (DAPI nuclear staining). ND, not determined. In case of PSCs, all data presented are from ESCs. For PSC, data are taken from ESC.
In this direction, different groups have described protocols to generate an enriched population of cortical excitatory neurons,13,16 GABAergic inhibitory interneurons12,17 or the dopaminergic midbrain neurons which are specifically lost during the progression of Parkinson's disease.8–11 For this last case, activation of the Wnt signaling by chemical GSK3β inhibitors combined with a strong Sonic Hedgehog stimulation directs the differentiation of hPSCs into dopamine-producing neurons passing through a LMX1A/FOXA2 positive midbrain floor plate intermediate cell stage.11
Spinal motor neurons, controlling body movements, can be severely damaged after an injury or a disease such as spinal muscular atrophy or amyotrophic lateral sclerosis. Due to its therapeutic implications, different groups have worked to develop a method to obtain motor neurons based on the synergic action of SHH and RA treatments.3,4,7,8,18
The major limitation shared by these protocols is that they are very laborious and highly time-consuming. In addition, ESC, but in particular iPSC lines, may change as a function of time in culture.19 A systematic comparison of the neural differentiation potential of different ESC and iPSC lines revealed a large variation in conversion efficiency, and it is likely that maturation stages and functional properties of the resulting neurons are also divergent.4
Direct reprogramming of fibroblasts to neurons
The successful conversion of fibroblasts all the way back to PSCs has wide disclosed the possibility to promote transdifferentiation toward other distantly related cell types using crucial cell-lineage-specific transcription factors. For neural cells, the deep knowledge of the molecular machinery directing the neuronal differentiation during embryonic development represented a privileged standpoint for designing cell transdifferentiation approaches the rationale being that the forced expression in fibroblasts of neural lineage specific transcription factors would be sufficient for a direct mesoderm-to-neuroectoderm cell conversion.
With this bold idea in mind, Vierbuchen and colleagues were successful for the first time to derive neuron-like cells from converting mouse fibroblasts, termed induced neuronal (iN) cells, by identifying a minimal combination of only three developmental transcription factors, Ascl1, Brn2 (also known as Pou3f2) and Myt1l (called together in short as BAM).20 Of interest, efficiency of the neuronal conversion results much higher than iPSC reprogramming in fibroblasts according to the relative ontogeny distance among these cell types. Ascl1 is the only pioneer factor among the three being able to bind its physiological neuronal targets also in a repressive chromatin configuration in fibroblasts.21 Consistent with this, Ascl1 is sufficient alone to reprogram fibroblasts and coax embryonic stem cell differentiation into iN cells, although with a reduced efficiency.22 In all these cases, iN cells result mostly glutamatergic and excitatory while only a minority express GABAergic neuronal markers. These results stand as counterintuitive since Ascl1 is exclusively expressed in the GABAergic inhibitory neuronal lineage during development of the forebrain and spinal cord.23 A way to interpret these data is that the GABAergic specific transcriptional program is under a tight epigenetic control, which antagonizes the Ascl1 reprogramming action. Alternatively, Ascl1 necessitates additional co-factors to activate GABAergic neuronal identity that are not present in the reprogramming cocktail. A year later, the Wernig's group succeeded in converting human fibroblasts into functional neurons by supplementing NeuroD1 as a fourth factor to the original reprogramming cocktail.24 Importantly, human iN cells presented convincing membrane excitability and about half of them exhibited synapse activity 4–5 weeks after reprogramming. However, overall iN cell efficiency conversion remained exceedingly low (less than 5%), thus, making a challenge to widely apply this procedure for human disease in vitro modeling.
Neurons of specific subtypes
The initial discovery of iN cells raised the question of whether a similar approach of direct neuronal reprogramming could be adapted to generate other neuronal sub-types by combining iN together with lineage specific transcription factors (TFs). The first evidence for this came with the generation of induced dopamine producing and motor neurons. In fact, combining Ascl1 with developmental dopaminergic factors such as Nurr1 and Lmx1a was sufficient to convert mouse fibroblasts into functional induced dopamine-producing (iDA) neurons.25 However, the same three factors were undoubtedly less efficient to transdifferentiate human fibroblasts. As for human iN cells, adding four additional dopaminergic factors to the BAM combination enabled Torper et al. to significantly enhance the conversion efficiency to dopaminergic neurons although their overall functionality was not assessed.26 If increasing the number of reprogramming TFs might be beneficial to enhance efficiency and gain functional properties, their practical use gets uneasy since they cannot be placed in a single vector and their stoichiometry is difficult to control. These results might suggest that conversion of mouse and human fibroblasts might necessitate different combinations of factors since human cells are intrinsically more resistant to initiate the cell conversion. While it remains a challenge to assess the proper fidelity of the conversion process in human cells. In fact, whether induced neuronal progenies might be directly compared to freshly isolated native neuronal counterparts, this remains prohibitive in the human setting. However, emerging technologies in genomics and the growing access to gene expression profiling datasets might offer unprecedented opportunities on this regard.
Conversion of mouse fibroblasts into spinal motor neurons (iMNs) has been shown by forced expression of seven factors (Ascl1, Ngn2, Brn2, Myt1l, Lhx3, Hb9 and Isl1).27 The iMNs expressed pan-neuronal and motor neuron-specific markers, as well as the receptors and channels that generate excitable membranes sensitive to transmitters, allowing them both to fire action potentials and receive synaptic inputs. Including also NeuroD1 to the reprogramming cocktail enabled to convert human fibroblasts as well into iMNs with an overall efficiency estimated in 5%.27
Assessing stability and in vivo performance of human-induced neurons
Multiple evidences have demonstrated that the direct conversion of mouse and human fibroblasts into neurons does not pass through a proliferative cell type.23,27–31 In fact, studies of BrdU incorporation, cell proliferation and reactivation of neural progenitor markers did not support any evidence for generation of neural precursor intermediates during the process of transdifferentiation.29 Thus, converted fibroblasts exit cell cycle during the reprogramming progress and do not revert to either a default pluripotent or a progenitor state suggesting that transdifferentiation does not follow the mechanisms of the embryonic development. Interestingly, because the cells do not pass through a stem cell state, it has been proposed that direct neural conversion can be performed in vivo without serious concerns of tumorigenesis or ectopic tissue dysplasia.26
Moreover, the described methods compared the gene expression profiling of the induced and native cells to determine how closely they resemble each other. Interestingly, induced neurons (iNs) obtained with direct reprogramming methods have acquired an expression pattern substantially similar to that of native cells but showed in some cases a relative delay in silencing the molecular signature of the cells of origin.25,27
Another critical issue is to determine whether the features of the reprogrammed cells remain stable and are maintained upon withdrawal of the inducing factors. For direct reprogramming, the use of doxycycline (dox)-inducible vectors has helped to address this issue since only the withdrawal of dox from the culture medium is enough to turn off the expression of the exogenous factors. The complete silencing of the reprogramming factors has been shown to leave unaltered the identity and functional properties of the reprogrammed cells over time in several protocols.24,25,29,32 These data indicate that long-term expression of exogenous factors is not required for the stability of the neural conversion. In fact, forced expression of transcriptional factors triggers the expression of their respective endogenous factors, therefore stabilizing the transcriptional network of the new differentiated state.24,29,32
Only few groups have tried to transplant human-induced neurons to see whether they can survive, integrate into neuronal networks and, most importantly, respond to physiological cues that could ultimately rescue a damage caused by a disease. Injected iDA neurons in the striatum of a PD rat model have shown to express dopaminergic markers after 16 weeks of the injection.30 Remarkably, after eight weeks, operated rats could ameliorate the rotational behavior induced by amphetamines and associated to PD. In another set of experiments, BAM-iNs have also been transplanted in the striatum of rats, and found integrated four weeks after transplantation but only few of them expressed TH.33 Interestingly, the authors observed that longer time in culture before transplantation reduced the efficiency of neuronal formation in the graft. Moreover, long-term survival of iMSNs has been reported when transplanted in the striatum of postnatal mice.32 Transplanted neurons integrated into host neuronal tissue, exhibiting functional characteristics similar to the native murine MSNs and forming axonal projections.
Promoting functional maturation of reprogrammed neurons
As mentioned earlier, the major limitation of all these approaches is the generation of neurons that only partially gained a full functional profile often lacking to develop appropriate membrane excitability, sustain long action potential firing, and display inhibitory and excitatory post-synaptic currents.
In general, the acquisition of reliable functional neuronal properties in reprogrammed neurons is a very slow process that requires many weeks independently of the culture medium composition (Tables 1 and 2). This hurdle poses serious shortcomings to the use of this system when investigating pathogenesis of neuropathological processes. However, recent technical advances promise to minimize these issues accelerating neuronal maturation and functional development.
Table 2.
Comparison of direct reprograming methods to generate neurons from human fibroblasts
| iN subtype | Human Fibroblasts | Factors | Days | Efficiency | Yield | Purity | Days for functional synapsis | Small molecules | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Neurons | Fetal | Ascl1, Brn2, Myt1l | 24 | ≈16% | N.D. | Glutamatergic and GABAergic | N.D. | no | 34 |
| Neurons | Neonatal | Ascl1, Myt1l, NeuroD2, miR-9/9*, miR-124 | 28 | ≈10% | ≈80% | Glutamatergic and GABAergic | 35--56 | VPA, dbc AMP | 31 |
| Neurons | Fetal | Ascl1 | 21 | N.D. | N.D. | N.D. | N.D. | no | 22 |
| Neurons | Adult | Ascl1, Sox2, Myt1l | >21 | ≈15% | N.D. | ≈2% Glutamatergic and 5% GABAergic | > 30 | no | 35 |
| Neurons | Fetal | Ascl1, Brn2, Myt1l | 15 | ≈120% | ≈20% | Glutamatergic and GABAergic | 90 | CHIR, SB, LDN, DBC AMP, AA, RA | 33 |
| Dopaminergic | Fetal | Ascl1, Nurr1, Lmx1a | 18 | ≈10% | N.D. | ≈60% | N.D. | no | 25 |
| Dopaminergic | Fetal | Ascl1, Brn2, Myt1l, Lmx1a, FoxA2 | N.D. | ≈7% | N.D. | ≈10% | N.D. | no | 34 |
| Dopaminergic | Fetal | Ascl1, Ngn2, Sox2, Nurr1, Pitx3 | 20 | 1–2% | N.D. | ≈100% | N.D. | SHH | 30 |
| Dopaminergic | Fetal | Ascl1, Brn2, Myt1l, Lmx1a, 1b, FoxA2, Otx2 | 12 | ≈150% | ≈20% | N.D. | N.D. | CHIR, SB, LDN, DBC AMP, AA, RA | 33 |
| Glutamatergic | Fetal | Brn2, Ascl1, Myt1l, Neurod1 | >21 | 2–4% | N.D. | ≈54% | 28--35 | no | 24 |
| Glutamatergic | Postnatal | miR-124, Brn2, Myt1L | >15 | 4–8% | N.D. | ≈44% Glutamatergic and 8% GABAergic | 30 | Noggin, Forskolin | 28 |
| Motor neurons | Fetal | Ascl1, Brn2, Myt1l, Lhx3, Hb9, Isl1, Ngn2, Neurod1 | 30 | N.D. | N.D. | N.D. | 30 | no | 27 |
| Motor neurons | Fetal | Ngn2, (Sox11 for postnatal and adult fibroblasts) | 14 | ≈56% | N.D. | ≈90% | > 50 | Forskolin, Dorsomorphin | 29 |
| Medium Spiny Neurons | Postnatal | Ctip2, Dlx1, Dlx2, Myt1l, miR-9/9*, miR-124 | 35 | N.D. | ≈90% | ≈90% | 84 | VPA, dbc AMP, RA | 32 |
Note: Efficiency is the percentage of Tuj1 or MAP2 positive cells respect to the number of plated cells. Yield is the percentage of Tuj1 or MAP2 positive cells respect to the total number of cells (DAPI nuclear staining). Subtype purity is the percentage of a specific subtype marker respect to Tuj1 or MAP2 or a reporter.
ND, not determined.
Transcription factor-induced neuronal differentiation and maturation
Zhang and colleagues have recently reported a fast and efficient protocol based on the ectopic expression of the transcription factor Ngn2.15 This approach entails a unique step of manipulation based on the infection of undifferentiated hPSCs with two viruses for the dox-inducible expression of Ngn2. In order to select only for the infected cells, a puromycin resistance gene was expressed together with Ngn2. Immature neuron-like cells were obtained with high purity (close to 100%) in less than 1 week and cells could progress to become functional after 2–3 weeks when co-cultured with murine glia or neurons. Gene expression analysis showed that Ngn2-derived neurons were mainly excitatory and expressed some critical markers of upper-layer cortical neurons although their molecular identity has not been thoroughly investigated. Of note, the conversion of iPSCs into neurons appeared to be direct, similar to the generation of iNs by direct reprogramming. Strikingly, the majority of Ngn2-iNs produced robust action potential firing, voltage-gated Na+ and K+ currents, massive spontaneous synaptic activity and short-term plasticity. Following the same experimental method,15 we were able to generate functional reprogrammed Ngn2-iNs using different hiPSC lines strongly indicating the robustness and reproducibility of this procedure. Figure 1 shows an example of the general morphology and basic electrophysiological properties recorded in whole-cell conditions in Ngn2–iNs after four weeks in co-cultures with murine neurons. These iNs displayed repetitive firing of action potentials (up to 40–50 Hz) in response to stimulation with suprathreshold current injection (60–100 pA), and robust spontaneous glutamatergic synaptic activity which was blocked by the selective AMPA-receptor antagonist NBQX (Figure 1(c) and (d)). About 50% of the recorded cells behaved in a similar way, whereas the remaining ones were less prone to respond to stimulation with little detectable synaptic activity (not shown). As predicted, neuronal feeder layers were indispensable in order to detect inhibitory synaptic inputs.15 To further improve this system, it would be valuable to generate human GABAergic interneurons to co-culture with Ngn2–iNs in order to generate a network of both excitatory and inhibitory neurons modeling the inherent complexity of the cerebral cortex circuitries.
Figure 1.
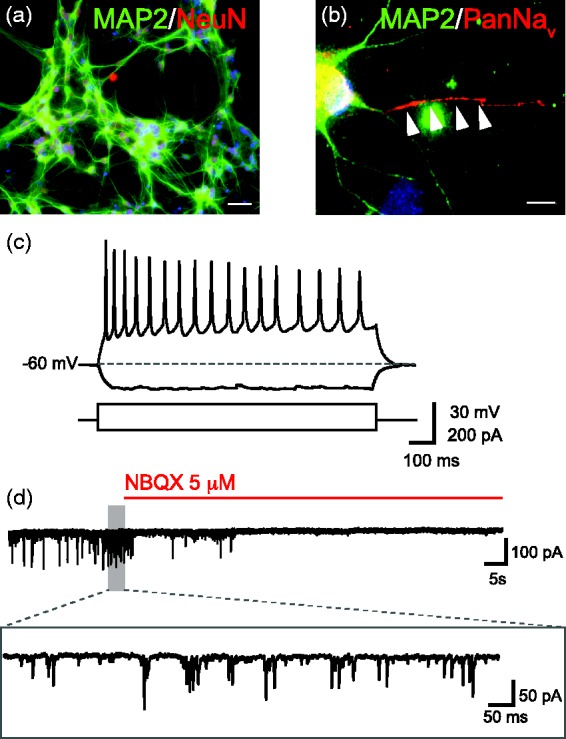
Morphology and electrophysiological properties of cultured Ngn2 induced neurons. (a) Representative image of MAP2/NeuN double immunostaining of Ngn2-infected iPSC-derived iNs. (b) High-magnification image of a pan-NaV antibody staining correctly localized at the axonal shaft of a Ngn2-iN. In (a) and (b), Hoechst is used as nuclear counterstaining (blue). Scale bar, 50 μm in (a) and 10 μm in (b). (c) Voltage change in response to injection of positive and negative current pulses (+60 and −40 pA, respectively). (d) Spontaneous synaptic activity before and after extracellular perfusion with the AMPA-receptor antagonist NBQX (5 μM). The portion of the trace included in the grey area is magnified below. (A color version of this figure is available in the online journal.)
An open question was whether such accelerated generation of iNs might be compatible with survival and integration after brain transplantation. Interestingly, iNs grafted into the striatum displayed a near physiological action-potential firing threshold and action potential amplitude and received highly active spontaneous inhibitory synaptic inputs.15
All together, these results indicate that Ngn2–iNs are a reliable human neuronal platform to investigate the physiological and pathological processes of neuronal functions. However, their use should be restricted to investigations where such neurons with an upper-cortical neuronal-like identity will result informative.
Reprogramming with microRNAs
A promising avenue to strengthen the functional properties of iNs is the co-expression of specific microRNAs (miRNAs) in the reprogramming cocktail together with the transcription factors.28,31,32 In fact, the use of miR-9/9* and miR-124 has been recently shown to impose a neuronal fate in fibroblasts by down-regulating genes implicated in the terminal differentiation.31 Both miRNAs are highly expressed in post-mitotic neurons and have been shown to play a crucial role in neuronal differentiation during brain development and in adult neurogenesis.36–38 miR-9/9* and miR-124 expression in fibroblasts is sufficient to generate MAP2 expressing cells, albeit with a very low rate.31 Noteworthy, the conversion process did not occur when miR-9/9* and miR-124 were expressed separately suggesting that the two miRNAs act synergically. In order to increase the conversion rate and the maturation process, NeuroD2, Ascl1 and Myt1l (DAM) were expressed together with miR-9/9*-124. Surprisingly, miR-9/9*-124-DAM generated neurons that expressed some crucial markers of the cerebral cortex identity but represented a mixed population of excitatory (VGLUT1 positive) and inhibitory (GAD67) cells. It remains unclear how these different neuronal cell types are generated with the same molecular cocktail and it might suggest that a dose-dependent mechanism is in place to specify different neuronal types depending by the relative expression of some of the reprogramming factors.
Further exploiting the use of miRNAs, Victor and colleagues recently established a protocol to convert human fibroblasts into functional striatal medium spiny neurons (MSNs), which specifically degenerate in Huntington's disease.32 Exogenous expression of Ctip2 (also known as Bcl11b), Dlx1, Dlx2 and Myt1l (CDM) together with miR-9/9*-124 was shown to convert postnatal human fibroblasts in a highly purified population of MSNs mainly expressing GABA and DARPP-32. Since miR-9/9*-124 overexpression resulted toxic to the cells, the authors included Bcl2 from protecting fibroblasts from cell death. Induced MSN-like neurons exhibited a well-developed morphology and mature electrophysiological properties. Importantly, gene expression profile in single converted neurons was analogous to human striatal cells microdissected from postmortem brain sections and, when transplanted in the mouse striatum, converted MSNs displayed functional properties closely similar to native murine MSNs. These results suggest that miR-9/9*-124 overexpression is particularly potent in promoting neuronal conversion, although its intrinsic cell toxicity has to be kept under control.
Supplementing small molecules and active peptides
Introduction of the dual-SMAD inhibitor system which blocks both BMP and activin/TGFβ signalings has enabled a strong and homogeneous neural induction of human ESCs/iPSCs avoiding the use of embryoid bodies and stromal feeders.8 Similarly, the concerted use of SMAD inhibitors together with Ascl1 and Ngn2 proved to substantially increase the yield of iNs.39 In addition, the concurrent induction of the Wnt signaling by pharmacological repression of GSK-3β kinase was synergic with SMAD inhibitors to promote iN reprogramming. Electrophysiological recordings showed that small molecule-treated iNs developed functional properties although the overall fraction of active neurons was not reported. With a similar approach, Liu et al. identified forskolin and dorsomorphin, respectively, a cAMP activator and a BMP inhibitor, as molecules able to strongly synergize with Ngn2 in converting fetal human fibroblasts into iNs.29 However, Sox11 and FGF2 needed also to be included in the reprogramming cocktail in order to obtain iNs with a robust efficiency. Thus, these data indicate that small molecules and active peptides are critical agents in promoting human iN reprogramming and maturation while reducing the number of transcription factors to be overexpressed.
Tumorigenic potential of reprogrammed neurons
The ultimate goal of producing iNs is devising cell replacement therapies as a clinical approach to treat neurological disorders. However, to meet these expectations, safety and oncogenic risks remain still a major concern for both iPSC and direct reprogramming approaches. Transplanted reprogrammed neurons might lead to tumor formation due either to incomplete differentiation, inappropriate reprogramming or mutagenic events during cell conversion. Reprogramming techniques are usually based on the integration of transgenes that can introduce mutations or activate oncogenes.40 To circumvent this issue, iPSCs have been successfully generated using alternative non-integrative methods such as the use of Sendai virus, episomal plasmids and mRNA expression.41–46 None of these procedures have been yet shown to be adequate for an efficient direct neuronal conversion. Thus, direct neuronal approaches are still relying on gene integrating methods that suffer from a high genotoxic burden.
Due to the possible presence of residual stem cells in the graft, the risk of tumor formation is higher for an iPSC-based approach and purifying lineage-restricted progenitors was shown to minimize this danger.47,48 In contrast, direct reprogramming might be safer since iN generation does not pass through a pluripotent or multipotent stem cell stage.25,28–31 Successful clinical applications of cell therapies based on neurons derived from iPSCs or directly from fibroblasts will depend on the development of good manufacturing practice (GMP) clinical-grade protocols for integration-free reprogramming methods, neuronal cell type specific differentiation procedures and stringent selection of post-mitotic neural precursors.
ACKNOWLEDGMENTS
All members of Broccoli's lab are acknowledged for helpful discussion and critical reading of the manuscript. Work in Broccoli's lab is supported by the Advanced ERC grant – ReproPARK.
Authors' contributions
AR generated and immunostained human Ngn2–iNs. LY and ST designed and performed the electrophysiological recordings of human Ngn2–iNs. All authors participated in writing and editing the manuscript.
References
- 1.Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, Ben-Hur T. Neural progenitors from human embryonic stem cells. Nat Biotechnol 2001; 19: 1134–40. [DOI] [PubMed] [Google Scholar]
- 2.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 2001; 19: 1129–33. [DOI] [PubMed] [Google Scholar]
- 3.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 2008; 321: 1218–21. [DOI] [PubMed] [Google Scholar]
- 4.Hu BY, Weick JP, Yu J, Ma LX, Zhang XQ, Thomson JA, Zhang SC. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A 2010; 107: 4335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature 2012; 482: 216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson MA, Weick JP, Pearce RA, Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci 2007; 27: 3069–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amoroso MW, Croft GF, Williams DJ, O'Keeffe S, Carrasco MA, Davis AR, Roybon L, Oakley DH, Maniatis T, Henderson CE, Wichterle H. Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J Neurosci 2013; 33: 574–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 2009; 27: 275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi SH, Studer L. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol 2012; 30: 715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartfield EM, Yamasaki-Mann M, Ribeiro Fernandes HJ, Vowles J, James WS, Cowley SA, Wade-Martins R. Physiological characterisation of human iPS-derived dopaminergic neurons. PLoS ONE 2014; 9: e87388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 2011; 480: 547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, Widmer HR, Eggan K, Goldstein PA, Anderson SA, Studer L. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 2013; 12: 559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci 2012; 15: 477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Xu J, Pang ZP, Ge W, Kim KJ, Blanchi B, Chen C, Sudhof TC, Sun YE. Integrative genomic and functional analyses reveal neuronal subtype differentiation bias in human embryonic stem cell lines. Proc Natl Acad Sci U S A 2007; 104: 13821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, Sudhof TC. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 2013; 78: 785–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espuny-Camacho I, Michelsen KA, Gall D, Linaro D, Hasche A, Bonnefont J, Bali C, Orduz D, Bilheu A, Herpoel A, Lambert N, Gaspard N, Peron S, Schiffmann SN, Giugliano M, Gaillard A, Vanderhaeghen P. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron 2013; 77: 440–56. [DOI] [PubMed] [Google Scholar]
- 17.Nicholas CR, Chen J, Tang Y, Southwell DG, Chalmers N, Vogt D, Arnold CM, Chen YJ, Stanley EG, Elefanty AG, Sasai Y, Alvarez-Buylla A, Rubenstein JL, Kriegstein AR. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell 2013; 12: 573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebert AD, Yu J, Rose FF, Jr., Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009; 457: 277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mekhoubad S, Bock C, de Boer AS, Kiskinis E, Meissner A, Eggan K. Erosion of dosage compensation impacts human iPSC disease modeling. Cell Stem Cell 2012; 10: 595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010; 463: 1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wapinski OL, Vierbuchen T, Qu K, Lee QY, Chanda S, Fuentes DR, Giresi PG, Ng YH, Marro S, Neff NF, Drechsel D, Martynoga B, Castro DS, Webb AE, Sudhof TC, Brunet A, Guillemot F, Chang HY, Wernig M. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 2013; 155: 621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chanda S, Ang CE, Davila J, Pak C, Mall M, Lee QY, Ahlenius H, Jung SW, Sudhof TC, Wernig M. Generation of induced neuronal cells by the single reprogramming factor ASCL1. Stem Cell Rep 2014; 3: 282–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development 2007; 134: 285–93. [DOI] [PubMed] [Google Scholar]
- 24.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature 2011; 476: 220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011; 476: 224–7. [DOI] [PubMed] [Google Scholar]
- 26.Torper O, Pfisterer U, Wolf DA, Pereira M, Lau S, Jakobsson J, Bjorklund A, Grealish S, Parmar M. Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A 2013; 110: 7038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 2011; 9: 205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 2011; 9: 113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu ML, Zang T, Zou Y, Chang JC, Gibson JR, Huber KM, Zhang CL. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat Commun 2013; 4: 2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Li F, Stubblefield EA, Blanchard B, Richards TL, Larson GA, He Y, Huang Q, Tan AC, Zhang D, Benke TA, Sladek JR, Zahniser NR, Li CY. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res 2012; 22: 321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 2011; 476: 228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Victor MB, Richner M, Hermanstyne TO, Ransdell JL, Sobieski C, Deng PY, Klyachko VA, Nerbonne JM, Yoo AS. Generation of Human Striatal Neurons by MicroRNA-Dependent Direct Conversion of Fibroblasts. Neuron 2014; 84: 311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira M, Pfisterer U, Rylander D, Torper O, Lau S, Lundblad M, Grealish S, Parmar M. Highly efficient generation of induced neurons from human fibroblasts that survive transplantation into the adult rat brain. Sci Rep 2014; 4: 6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A 2011; 108: 10343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang P, Zhang HL, Li W, Sha H, Xu C, Yao L, Tang Q, Tang H, Chen L, Zhu J. Generation of patient-specific induced neuronal cells using a direct reprogramming strategy. Stem Cells Dev 2014; 23: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci 2009; 12: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells 2006; 24: 857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 2007; 27: 435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, Herms S, Wernet P, Kogler G, Muller FJ, Koch P, Brustle O. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Meth 2012; 9: 575–8. [DOI] [PubMed] [Google Scholar]
- 40.Gore A, Li Z, Fung HL, Young JE, Agarwal S, Antosiewicz-Bourget J, Canto I, Giorgetti A, Israel MA, Kiskinis E, Lee JH, Loh YH, Manos PD, Montserrat N, Panopoulos AD, Ruiz S, Wilbert ML, Yu J, Kirkness EF, Izpisua Belmonte JC, Rossi DJ, Thomson JA, Eggan K, Daley GQ, Goldstein LS, Zhang K. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011; 471: 63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC. A nonviral minicircle vector for deriving human iPS cells. Nat Meth 2010; 7: 197–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 2009; 4: 472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandal PK, Rossi DJ. Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat Protoc 2013; 8: 568–82. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura K, Sano M, Ohtaka M, Furuta B, Umemura Y, Nakajima Y, Ikehara Y, Kobayashi T, Segawa H, Takayasu S, Sato H, Motomura K, Uchida E, Kanayasu-Toyoda T, Asashima M, Nakauchi H, Yamaguchi T, Nakanishi M. Development of defective and persistent Sendai virus vector: a unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem 2011; 286: 4760–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010; 7: 618–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009; 324: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-David U, Benvenisty N. Chemical ablation of tumor-initiating human pluripotent stem cells. Nat Protoc 2014; 9: 729–40. [DOI] [PubMed] [Google Scholar]
- 48.Doi D, Samata B, Katsukawa M, Kikuchi T, Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M, Takahashi J. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem Cell Rep 2014; 2: 337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


