Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has been shown to selectively induce apoptotic cell death in various tumor cells by engaging its death-inducing receptors (TRAIL-R1 and TRAIL-R2). This property has led to the development of a number of TRAIL–receptor agonists such as the soluble recombinant TRAIL and agonistic antibodies, which have shown promising anticancer activity in preclinical studies. However, besides activating caspase-dependent apoptosis in several cancer cells, TRAIL may also activate nonapoptotic signal transduction pathways such as nuclear factor-kappa B, mitogen-activated protein kinases, AKT, and signal transducers and activators of transcription 3, which may contribute to TRAIL resistance that is being now frequently encountered in various cancers. TRAIL resistance can be overcome by the application of efficient TRAIL-sensitizing pharmacological agents. Natural compounds have shown a great potential in sensitizing cells to TRAIL treatment through suppression of distinct survival pathways. In this review, we have summarized both apoptotic and nonapoptotic pathways activated by TRAIL, as well as recent advances in developing TRAIL–receptor agonists for cancer therapy. We also briefly discuss combination therapies that have shown great potential in overcoming TRAIL resistance in various tumors.
Keywords: TRAIL, cancer, NF-κB, apoptosis, natural products
Introduction: Discovery and structure of TRAIL
Generally, in normal tissues, a tight balance exists between self-renewal and cell death, and it aims to maintain the tissues’ integrity. Once this balance is broken, cells might grow out of control and exhibit resistance to cell death. Uncontrolled growth and apoptosis resistance are two critical hallmarks for cancer initiation as well as progression1; therefore, therapies targeting these two important aspects might be ideal modalities for cancer treatment. Furthermore, in comparison to proliferation inhibition, which only stops tumor growth without removing cancer cells, apoptosis induction might be a more potent therapy because it is also able to completely eliminate the cancer cells that have accumulated diverse mutations over a period of time.
There are two major pathways involved in the process of apoptosis: the intrinsic and extrinsic. The intrinsic pathway depends on mitochondria2; it can eliminate damaged cells via sensing cell damage such as oxidative stress and DNA damage.3 The tumor-suppressor protein p53 is critical in this pathway, as many intrinsic pathways are dependent on this molecule. Thus, p53 is considered as a potential target for cancer therapy. However, mutation or inactivation of p53 is commonly found in tumor cells, leading to the development of resistance to p53-dependent radio- and chemotherapy.4 The extrinsic apoptosis pathway is dependent on death ligands binding to the death receptors (DRs). With ligand engagement to the transmembrane receptors, a death signal is transmitted from the outside to the inside of cells. The first cell death ligand used for anticancer treatment was tumor necrosis factor (TNF), which was discovered in 1975.5 Although TNF showed apoptotic effect in some cancer types, its major function was later found to be involved in the pro-inflammatory process. Subsequently, the DR FAS/APO-1 (CD95) was found to be another anticancer target since antibodies targeting this receptor were able to induce apoptosis in a wide range of cancer cells.6,7 However, stimulation of CD95 also showed acute and lethal hepatic toxicity during its anticancer therapy.8 A few years later, TNF-related apoptosis-inducing ligand (TRAIL) was identified based on its sequence homology to TNF and CD95L.9,10 TRAIL has similar apoptotic effects as CD95L, but it does not affect normal cells,11,12 which makes TRAIL a promising therapeutic for cancer therapy.
There are five types of TRAIL receptors. They are four-membrane receptors TRAIL-R1 (DR4), TRAIL-R2 (DR5), TRAIL-R3 (DcR1), and TRAIL-R4 (DcR1), and one soluble receptor called osteoprotegerin. Among them, TRAIL-R1 (DR4) and TRAIL-R2 (DR5) mediate the apoptosis pathway, and hence are termed DRs, while the others protect cells from apoptosis, and are called decoy receptors (DcRs). With ligand binding to the DR, TRAIL apoptotic signaling is initiated and further induces caspases or mitochondrial-dependent death. Various agents such as recombinant human soluble TRAIL and selective agonistic antibodies targeting TRAIL-R have been developed. Their robust antitumor activities have been demonstrated in a number of preclinical studies. However, subsequent clinical trials revealed only limited therapeutic benefit. This sobering performance might be due to the resistance to TRAIL therapy in most primary cancer cells, since major cell survival signaling cascades including nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), and phosphatidylinositol-3-kinases (PI3K/AKT) could also be activated by TRAIL. Therefore, besides the TRAIL apoptotic signaling pathway, this review also describes the nonapoptotic signaling pathways that can be induced upon TRAIL treatment. An update on the potential anticancer effects of TRAIL in both preclinical and clinical studies is also summarized in this review. The role of few selected, natural compounds that can sensitize tumor cells to TRAIL treatment has been also highlighted briefly.
TRAIL-induced apoptotic signaling cascades
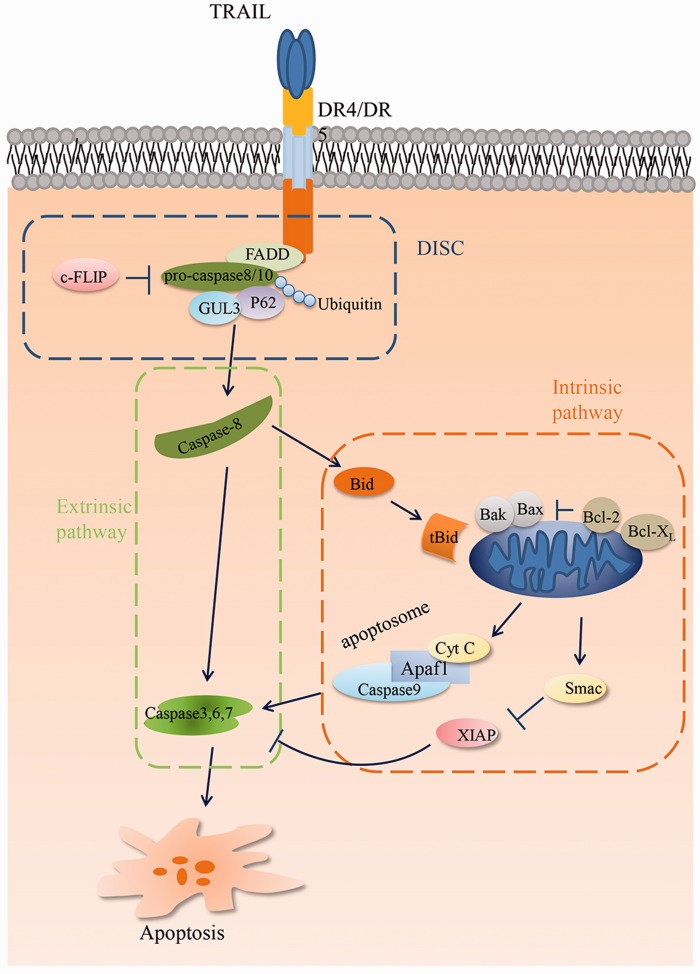
TRAIL interacts with five distinct receptors that are encoded by separate genes, but share high sequence homology in the extracellular domains. However, only DR4 and DR5, which contain an intracellular death domain, can produce apoptotic signals.13 The apoptotic signaling pathway of TRAIL is triggered by trimerized TRAIL binding to DR4 and DR5, which enables the receptors to homotrimerize, thereby driving formation of the death-inducing signaling complex (DISC).14 Upon ligand stimulation, DR4 and DR5 recruit Fas-associated death domain protein (FADD) through death domain interactions. FADD then recruits pro-caspase-8 and 10, and/or the cellular FLICE (caspase-8)-like inhibitory protein (c-FLIP) to the DISC (Figure 1). c-FLIP competes with caspase-8 for FADD binding in the DISC and inhibits the apoptosis signal.15 Following recruitment, procaspase-8 comes into contact with the ubiquitin E3 ligase subunit (CUL3), which catalyzes polyubiquitylation of caspase-8 on its C-terminal region. Polyubiquitylated caspase-8 binds with the ubiquitin-binding protein p62, which promotes the translocation of caspase-8 from the DISC into intracellular ubiquitin-rich foci and subsequently leads to the cleavage and activation of caspase-8.16 Activation of caspase-8 at the DISC transfers the apoptosis signal to executioners of apoptosis either directly via the extrinsic or indirectly via the intrinsic–mitochondrial pathway. In the extrinsic pathway, the DISC activates sufficient caspase-8 to stimulate the effector caspases 3, 6, and 7, and directly induce apoptosis. In the intrinsic–mitochondrial pathway, active caspase cleaves the BH3-interacting domain death agonist (Bid) to truncated Bid (tBid). tBid rapidly translocates to the mitochondria and drives permeabilization of the outer mitochondrial membrane by binding with Bax and Bak, releasing mitochondrial cytochrome c and mitochondria-derived activator of caspase (Smac).17,18 This process can be blocked by overexpression of X-linked inhibitor of apoptosis protein (XIAP), B-cell lymphoma 2 (Bcl-2), and B-cell lymphoma-extra-large (Bcl-xL).19,20 Once in the cytosol, cytochrome c conjugates with ATP and apoptotic peptidase-activating factor-1 (Apaf-1) to recruit the initiator caspase-9 into a signaling complex called the apoptosome. Activated caspase-9 then cleaves and activates the effector caspases-3, -6, and -7 to induce apoptosis.18
Figure 1.
A schematic diagram of TRAIL-induced apoptotic signaling cascades. Binding of TRAIL to TRAIL death receptors results in recruitment of the FADD and caspase-8 to form DISC. DISC leads to the cleavage and activation of caspase-8, which can activate caspase effectors and the BH3-only protein Bid. In the extrinsic pathway, activation of caspase-8 is sufficient to activate downstream caspases-3, -6, and -7, leading to cell death. However, in the intrinsic pathway, cleavage of Bid into its truncated form (tBid) is essential to induce cell death. tBid can rapidly translocate to the mitochondria and drive (through Bax and Bak) permeabilization of the outer mitochondrial membrane, releasing mitochondrial cytochrome c and Smac. This process can be blocked by overexpression of Bcl-2 and Bcl-xL. Once in the cytosol, cytochrome c binds to the adaptor Apaf-1 to recruit the initiator caspase-9 into the apoptosome, which can activate caspase-9 and the effector caspases. Release of Smac augments apoptosis by antagonizing the inhibitory effect of XIAP on the various effector caspases. (A color version of this figure is available in the online journal.)
Effect of TRAIL on other cell survival signaling cascades
Although TRAIL exerts a remarkable effect in apoptosis induction, it has been also reported to activate anti-apoptotic pathways such as NF-κB, MAPKs (c-Jun NH2 terminal kinases [JNK], p38, and extracellular signal-regulated kinases [ERK]1/2), PI3K/AKT, and signal transducers and activators of transcription (STATs), which may repair TRAIL-induced apoptosis. The mechanism(s) underlying stimulation of these anti-apoptosis pathways are still not well understood. For example, Eugene et al. have indicated that, subsequent to assembly of the DISC, a secondary complex is formed that may stimulate the nonapoptotic signaling pathways. The secondary complex contains FADD, caspase-8, receptor-interacting protein (RIP1), TNF receptor associated factor-2 (TRAF2), and NF-κB essential modulator (NEMO). The association of the secondary complex might be dependent on formation of the primary complex, but also requires its dissociation. The specific localization of the TRAIL receptor complex may be another mechanism involved in the TRAIL-induced anti-apoptotic signaling events. Moreover, the TRAIL receptor localized in membrane lipid rafts activates apoptosis signaling, while the TRAIL receptor complex outside the rafts enables activation of nonapoptotic pathways. Other possible early molecular events for nonapoptotic pathways include the DISC inhibitor cFLIP and modification of TRAIL RIPs. We briefly discuss below few important cell survival pathways that can be activated upon exposure of tumor cells to TRAIL.
NF-κB
NF-κB is a transcription factor that is involved in inflammation and cell survival. The NF-κB family has five members: p65, Rel B, cRel, p50, and p52. In the TRAIL/TRAIL-R system, NEMO/IKKγ in the secondary complex recruits IKKα/β, which phosphorylates IкBα and induces its ubiquitination and degradation. Degradation of IкBα activates NF-κB, and allows its nuclear translocation. NF-κB then binds to the DNA and induces transcription of anti-apoptotic genes such as Bcl-xl, Mcl-1, cFLIP, and cIAPs. Inhibition of NF-κB by using either an IкB dominant negative version or selective chemical factor was found to enhance TRAIL-induced apoptosis in several preclinical tumor models such as leukemia, neuroblastoma, pancreatic cancer, and nonsmall cell lung carcinoma (NSCLC).21–25 Interestingly, a pro-apoptotic effect was also reported in TRAIL-induced NF-κB activation. For example, deficiency of cRel resulted in resistance to TRAIL treatment in glioma cell lines,26 and a similar anti-apoptotic effect was also observed in human β islets cells.27 The mechanism(s) for the pro-apoptotic role of NF-κB are still elusive. However, it was found that NF-κB can help recruitment of FADD and caspase 8, and facilitate DISC formation. Few other evidences further indicates that a pro-apoptotic role of NF-κB might be related to the relative amount of RelA and cRel in activated NF-κB, as cRel upregulation was found to enhance the expression levels of TRAIL1 and TRAIL2 receptors,28 while Rel A overexpression had opposite effects.29 Besides above described apoptosis-related roles, NF-κB activation was also reported to be involved in the TRAIL-enhanced invasion of apoptosis-resistant pancreatic ductal adenocarcinoma cells.30
MAPKs
The MAPKs are kinases that control different cellular processes such as immunoregulation, inflammation, cell growth, cell differentiation, and cell death. This family consists of six members: the ERK1/2, ERK3/4, ERK5, ERK7/8, JNK1/2/3, and the p38-MAPK. Among them, TRAIL can significantly activate JNK, p38, and ERK1/2 in diverse tumor cell lines. For example, TRAIL-induced JNK activation requires both RIP and TRAF2 in the secondary complex,31–33 and JNK might be activated through the TRAF2–MEKK1–MKK4 signaling pathway.32 Different mechanisms are involved in JNK-mediated apoptosis induced by TRAIL. Bim is a pro-apoptotic Bcl-2 family member, which can mediate lysosome permeabilization and induce cell death through activating Bax. It was found that TRAIL can enhance Fas-induced cell death through activating JNK and its downstream substrate Bim in isolated murine hepatocytes34; and the pharmacological JNK inhibitor SP600125 can attenuate TRAIL-induced lysosomal permeabilization and cell death in cholangiocarcinoma.35 Stimulation of autophagic cell death might be another mechanism contributing to JNK-mediated cell death, because Beclin 1 (which is an important autophagy regulator), could be phosphorylated by TRAIL-induced JNK activation.36 In addition, JNK was found to have dual activity as inhibition of JNK-enhanced TRAIL-induced apoptosis in hepatocellular carcinoma cells,37 and this dual effect might be due to the magnitude of signal transduction and the isoforms involved. For example, prolonged JNK activation or long isoforms of JNK such as JNK1α2 and JNK1β2 induce cell apoptosis, while transient activation or short isoforms (JNK1α2 and JNK1β2) prevent apoptosis.38,39 Therefore, JNK may act as a pro- and/or anti-apoptotic molecule in different cell types and experimental systems.
TRAIL-induced p38 activation is RIP1 and TRAF2 dependent,31 and it has been reported that TRAIL-induced p38 activation through upstream kinases such as TGF-β activated kinase-1 (TAK1) and MKK4/MKK6.40 The roles of p38 in TRAIL-induced apoptosis are also controversial. In Hela cells, p38 activation was found to be responsible for TRAIL-induced apoptosis, because specific p38 kinase inhibitor SB203580 prevented apoptosis.41 TRAIL-induced reactive oxygen species (ROS) production may also contribute to p38 activation. Pretreatment with antioxidants such as glutathione attenuated p38 kinase activation as well as TRAIL-induced apoptosis. Meanwhile, TRAIL-induced p38 activation has also shown an anti-apoptotic effect. Son et al. reported that TRAIL can induce p38 activation in prostate cancer cells, and activated p38 can further upregulate the expression of Mcl-1 gene, which can suppress the intrinsic apoptosis pathway by inhibiting mitochondrial membrane permeabilization.40 In addition, p38 inhibition sensitized breast carcinoma cells to TRAIL treatment.42 However, in some other tumor cells such as the human colorectal cancer cell line DLD1, p38 did not play a major role in TRAIL-mediated apoptosis.43 For example, although p38 was activated in TRAIL-sensitive DLD1 cells but not in TRAIL-resistant DLD1 cells, p38 inhibition did not block TRAIL-mediated cell death. Therefore, the role of p38 in TRAIL-induced apoptosis might also be cell-type dependent.
On the other hand, ERK1/2 activation has been mainly implicated in cell survival and proliferation. The activation of ERK1/2 by TRAIL has been reported in a number of cell types,44,45 and the mechanism may be Mst1 (mammalian sterile 20-like kinase 1) dependent, as a caspase-3-generated 36 kDa form of Mst1 was found to activate ERK1/2.46 ERK1/2 protects cells from TRAIL-mediated apoptosis. Smac/direct IAP binding protein with low pI (DIABLO) release from mitochondria is an important pathway mediating TRAIL-induced apoptosis. In melanoma cells, it was shown that release of Smac/DIABLO was downregulated by EKR1/2 activation, thus attenuating TRAIL-induced apoptosis.45 Inhibition of ERK1/2 sensitized cells to TRAIL-induced apoptosis in breast cancer cells and HT-29 colon cancer cells, and further indicates that ERK1/2 is a critical proliferation mediator.47 In NSCLC, which lack caspase-8, TRAIL caused an increase in proliferation, and the induced proliferation was mediated by ERK1/2, as ERK inhibition attenuated the TRAIL-induced proliferation.48 A similar role of ERK1/2 was also observed in TRAIL-resistant human glioma cells, in which TRAIL-induced ERK1/2 increased cell proliferation via increasing cell cycle progression and inhibiting c-FLIP(L) (the long form of the caspase 8 inhibitor).49
PI3K/AKT
Akt is a PI3K-activated protein kinase, which is mainly involved in regulating cellular functions such as cell growth, apoptosis, and survival.50 TRAIL-induced Akt activation has been demonstrated in various cancer types. In the TRAIL-sensitive prostate cancer cell line DU145, TRAIL stimulated Akt activation via Rous sarcoma oncogene cellular homolog (Src) and c-Cbl, and suppression of Akt enhanced the TRAIL-induced apoptosis.51 Akt activation may also contribute to development of TRAIL resistance, as inhibition of TRAIL-induced Akt phosphorylation sensitized the TRAIL-resistant NSCLC cells for TRAIL treatment.52 A similar phenomenon was also observed in TRAIL-resistant ovarian and breast cancer cell lines.53
STAT3
STAT3 is a cytoplasmic transcription factor involved in cell proliferation, apoptosis, angiogenesis, and immune response. With the ligands (cytokines or growth factors such as epidermal growth factor [EGF]) binding to the receptors, monomeric STAT3 are phosphorylated by the receptor-associated tyrosine kinases such as JAK and Src, and then form dimers to migrate into the nucleus and activate gene transcription. In 2012, Azijli et al.52 found that TRAIL can enhance cell migration and invasion through activating the Src-STAT3 pathway in the TRAIL-resistant NSCLC cells. Inhibition of Src or STAT3 by either a chemical inhibitor or shRNA-attenuated TRAIL-induced migration and invasion. Activation of Src and STAT3 is mediated through RIP1 kinase. Silencing of RIP kinase suppressed TRAIL-induced Src and STAT3 phosphorylation as well as TRAIL-induced migration and invasion. TRAIL-R2 might mediate TRAIL-induced activation of Src and STAT3, as DHER (D269H/E195R, a selective TRAIL variant against TRAIL2) significantly enhanced cell migration and invasion. Src activation may also contribute to the apoptosis resistance, as it was found to impair DR/caspase 8-dependent apoptosis by phosphorylating caspase 8 at tyrosine 380.54 Src also can induce an autocrine or paracrine loop of TGF-α–EGFR activation.55 Figure 2 summarizes the various cell survival pathways activated upon exposure of tumor cells to TRAIL.
Figure 2.
A schematic overview of prosurvival signals elicited by the activation of TRAIL receptors. With agonists binding to TRAIL-R1/R2, a secondary complex can be formed after receptor activation, leading to the activation of various signaling pathways that are involved in induction of nonapoptotic responses as indicated. (A color version of this figure is available in the online journal.)
Anticancer effects of TRAIL
There is strong experimental evidence that the TRAIL pathway has an important role in the regulation of tumor initiation and development. As detailed below, TRAIL may contribute to host immune surveillance against tumors. Moreover, dysfunction of TRAIL-Rs through mutation or decreased expression may promote tumor progression and confer intrinsic resistance to TRAIL-induced apoptosis. TRAIL is expressed by effector lymphocytes, which are well known to contribute to host immune surveillance against primary tumor development and metastasis. TRAIL, along with perforin 1 and CD95L, is constitutively expressed on murine natural killer (NK) cells in the liver, but not NK T cells or ordinary T cells, and is responsible for spontaneous cytotoxicity against TRAIL-sensitive tumor cells in vitro and in vivo.56,57 Both the mouse and human Apo2L/TRAIL promoters are regulated by interferon-gamma (IFN-γ),58,59 and Apo2L/TRAIL expression and its contribution to preventing liver metastases depend on IFN-γ signaling.60 Furthermore, the IFN-γ-induced expression of TRAIL might change the tumor microenvironment to enable enhanced antigen presentation and tissue infiltration. The TRAIL-sensitive tumor cells interaction with TRAIL-expressing tumor infiltrating immune cells might be involved in tumor resistance and metastasis.61
In addition to Apo2L/TRAIL’s contribution to immune surveillance, Apo2L/TRAIL appears to play an important role in suppressing tumor progression and determining chemosensitivity. Both neutralization of TRAIL by monoclonal antibody and TRAIL knockout mice promoted tumor development in mice and supported a direct role for NK cells expressing TRAIL in the suppression of tumor metastasis, while no metastasis occurred with the TRAIL-resistant cells.60,62 A more recent study shows that syngeneic renal cell carcinomas grow faster and shows increased metastasis to the liver in Apo2L/TRAIL knockout mice as compared with wild-type controls.63 Furthermore, metastasis to lymph nodes was significantly enhanced in TRAIL-R-deficient mice, which indicates that TRAIL-R2 is a metastasis suppressor in the mouse multistage model of squamous cell carcinoma.64 Wang and El-Deiry65 showed that silencing of TRAIL-R2/DR5 in vivo promotes tumor growth and renders tumor cells resistant to the chemotherapeutic agent 5-fluorouracil. These findings provide the evidence for the physiological function of TRAIL as a tumor suppressor.
Ongoing clinical trials with TRAIL alone and in combination with other pharmacological agents
Since TRAIL was observed to have promising anticancer effects in preclinical research, agonists targeting the TRAIL receptor have been developed and have already undergone Phase I and Phase II clinical trials. There are two categories of clinically tested TRAIL receptor agonists (TRAs): recombinant forms of TRAIL and agonistic antibodies specific for TRAIL-R1 or TRAIL-R2. Recombinant forms of TRAIL have a stronger apoptotic effect compared to agonistic antibodies because they can target and trigger both apoptosis receptors (TRAIL-R1 and TRAIL-R2). However, since it has no selectivity, it also increases their chance to bind the DcRs such as TRAIL-R3 and TRAIL-R4, and thus attenuates its apoptotic activity. Recombinant forms of TRAIL were also found to be cleared by the body within hours, so repeated administration is required for systemic application.66
Considering the less selectivity of the recombinant forms of TRAIL, agonistic antibodies specific for TRAIL-R1 or TRAIL-R2 might be more effective for cancer treatment. The antibodies can selectively bind to specific apoptotic TRAIL-Rs, therefore they would not bind to nondeath-inducing TRAIL-Rs and activate the survival pathway. In addition, the half-life of agonistic antibodies is in the range of several days to weeks, which allows a more stable concentration within tissues and avoids the need for continuous application.66
Recombinant forms of TRAIL
Dulanermin, which contains the TNF homology domain within the extracellular domain of human soluble TRAIL, is the only recombinant TRAIL developed for clinical application.11 In several phase I clinical trials, dulanermin has proved to be a safe and well-tolerated drug in the treatment of different tumors such as colorectal cancer, lung cancer, and lymphoma, even when combined with other chemotherapies (Table 1).67–101 The antitumor activity of dulanermin has also been shown in patients for whom partial or complete clinical responses were observed. To further study the specific antitumor activity of dulanermin, Phase II b clinical trials (randomized control trials, RCTs) were performed in nonsmall cell lung cancer71 and non-Hodgkin’s lymphomas73 (Table 1). However, although these two trials further confirm the tolerability of dulanermin in cancer treatment, no significant anticancer activities were observed.
Table 1.
Results of recombinant TRAIL or agonistic antibodies targeting TRAIL-R in clinical trials
| Agents | Phase | Cancer type | Efficacy/n | References |
|---|---|---|---|---|
| Dulanermin | I | Advanced cancers | 2PR/71 | Herbst et al.67 |
| I | Colorectal | 13PR/23, 6PR/27, NA/30 | Wainberg et al.68 Kasubhai et al.69 Yee et al.70 | |
| I | Lung | (1CR+13 PR)/24 | Soria et al.139 | |
| I | Lymphoma | (2CR + 1PR)/7 | Yee et al.72 | |
| II (RCT) | Lung | No cancer activitya/213 | Soria et al.71 | |
| II (RCT) | Lymphoma | No cancer activitya/48 | Belada et al.73 | |
| Mapatumamab | I | Advanced cancers | No response/49, No response/41, 12PR/49, 5PR/27 | Tolcher et al.74 Hotte et al.75 Mom et al.76 Leong et al.77 |
| I/II | Lymphoma | (2CR + 1PR)/40 | Younes et al.78 | |
| II | Colorectal | No response/38 | Trarbach et al.79 | |
| II | Lung | No response/32 | Greco et al.80 | |
| II (RCT) | Multiple myeloma | No cancer activitya/104 | Belch et al.81 | |
| II (RCT) | Lung | No cancer activitya/109 | von Pawel et al.82 | |
| Conatumumab | I | Advanced cancers | 1PR/37, No response/18, No response/9 | Herbst et al.140 Doi et al.83 Chawla et al.84 |
| I | Soft tissue sarcoma | No response/6 | Demetri et al.85 | |
| I | Lung | (1CR + 3PR)/12 | Paz-Ares et al.141 | |
| I | Colorectal | 5PR/12 | Saltz et al.87 | |
| I | Pancreatic | 4PR/13 | Kindler et al.88 | |
| II (RCT) | Soft tissue sarcoma | No anticancer activitya/128 | Demetri et al.85 | |
| II (RCT) | Lung | No anticancer activitya/172 | Paz-Ares et al.86 | |
| II (RCT) | Pancreatic | No anticancer activitya/83 | Kindler et al.89 | |
| II (RCT) | Colorectal | No anticancer activitya/103, No anticancer activitya/190 | Cohn et al.90 Fuchs et al.91 | |
| Lexatumumab | I | Advanced cancers | No response/37, No response/31, PR/41 | Plummer et al.92 Wakelee et al.93 Sikic et al.94 |
| I | Pediatric cancers | No response/24 | Merchant et al.95 | |
| Tigatuzumab | I | Carcinoma–lymphoma | No response/17 | Forero-Torres et al.96 |
| II | Pancreatic | 8PR/61 | Forero-Torres et al.97 | |
| II (RCT) | Lung | No anticancer activitya/97 | Reck et al.98 | |
| Drozitumab | I | Colorectal | 2PR/9 | Rocha Lima et al.99 |
| I | Advanced cancers | No response/50 | Camidge et al.100 | |
| LBY-135 | I/II | Advanced cancers | 2PR/73 | Sharma et al.101 |
Note: TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; n, number of patients enrolled; CR, complete response; PR, partial response; NA, data about responses (efficacy) were not reported; RCT, randomized-controlled trials.
Anticancer activity was considered when the addition of the recombinant TRAIL or agonistic antibodies demonstrated statistically significant activity as compared to the standard therapy.
Agonistic antibodies
Mapatumumab is the only agonistic TRAIL-R1 specific antibody that has entered clinical trials (Table 1). Its safety and broad tolerability have been revealed in both Phase I and Phase II trials. In the Phase I trials, partial responses were observed in advanced cancers when used in combination with chemotherapy. Although Phase IIa and IIb trials have been conducted in cancers such as colorectal and lung cancers, an objective response was only observed in patients with lymphoma undergoing a Phase II a trial, while no anticancer activity was observed in other trials. There are several agonistic TRAIL-R2 specific antibodies such as conatumumab, lexatumumab, tigatuzumab, drozitumab, and LBY-135 (Table 1). So far, different clinical trials have been carried out either alone or in combination with chemotherapy. Some positive trends were observed in Phase I and Phase II a trials, while no significant anticancer activity was achieved in Phase II b trials (Table 1). These TRAs although have been found to be well tolerated, but they exhibited only minimal therapeutic activity in these clinical trials. Therefore, future work might be to focus on strategies that could achieve a significant anticancer effect with these pharmacological modulators.
Natural compounds that can sensitize tumor cells to TRAIL
Numerous natural compounds have shown great potential to enhance TRAIL-induced apoptosis through modulation of diverse nonapoptotic pathways such as NF-кB, STAT3, PI3K/AKT, MAPKs, and p53, which can be considered as a part of emerging treatments for unresponsive cancer (Table 2). For example, suppression of TRAIL-induced NF-кB activation is considered to be an important method to sensitize cancer cells to TRAIL by using natural compounds. Numerous natural compounds such as wogonin (derived from the popular Chinese herb Huang-Qin), sulforaphane (derived from enriched broccoli sprout extracts), and melittin (major component of bee venom) sensitize resistant malignant cells to TRAIL-induced apoptosis through the modulation of NF-кB signaling pathway.102–104 Upregulation of TRAIL receptors through NF-κB is also mediated by other natural compounds such as the ethanolic extract of Brazilian green propolis (EEP) and curcumin (a substance found in turmeric).105,106 However, kurarinone (a natural bioactive lavandulyl flavonoid),107 resveratrol (a type of natural phenol),108 artesunate (a derivative of the natural product artemisinin),109 and combertastatin A-4 (isolated from the bark of combretum caffrum)110 can also sensitize melanomas to TRAIL through abrogating TRAIL-induced NF-κB activation and modulation of expression of anti-apoptotic genes such as cFLIP, XIAP, and Bcl-xL.
Table 2.
A list of selected natural compounds that can sensitize tumor cells to TRAIL
| Compound | Tumor type(s) | Mechanism(s) of action | References | Compound | Tumor type(s) | Mechanism(s) of action | References |
|---|---|---|---|---|---|---|---|
| NF-кB dependent | ERK dependent | ||||||
| Wogonin | Leukemia | NF-кB ↓ | Fas et al.102 | Zerumbone | Human colorectal cancer | ERK/P38↑-DR4/5↑ | Yodkeeree et al.124 |
| Sulforaphane | Human prostate cancer | NF-кB ↓ | Labsch et al.103 | Azadirone | Human cancer cells | ERK↑-P53↑-DR4/5↑ | Gupta et al.122 |
| Melittin | Hepatocellular carcinoma | NF-кB↓ | Wang et al.104 | γ-tocotrienol | Human cancer cells | ERK↑-P53↑-DR4/5↑ | Kannappan et al.123 |
| Curcumin | Human bladder cancer | NF-кB↓-DR5↑ | Hussain et al.106 | Curcumin | Human breast cancer | ERK↑-DR5↑-Mcl-1↓ | Hussain et al.106 |
| Kurarinone | Human cervical carcinoma | NF-кB↓-cFLIP↓ | Seo et al.107 | Apigenin | Hepatocellular carcinoma | ERK↑-DR5↑ | Kim et al.120 |
| Resveratrol | Melanoma | NF-кB↓/STAT3↓-cFILP↓ | Ivanov et al.108 | Butein | Hepatocellular carcinoma | ERK↑-DR5↑ | Moon et al.118 |
| Artesunate | Human cervical carcinoma | NF-кB↓/PI3K↓-Bcl-XL↓ | Thanaketpaisarn et al.109 | ||||
| Combretastatin A-4 | Human colorectal cancer | NF-кB↓-XIAP↓ | Zhang et al.110 | ||||
| STAT3 mediated | JNK mediated | ||||||
| Chrysin | Human lung adenocarcinoma | STAT3↓-Mcl-1↓ | Lirdprapamongkol et al.111 | SVT | Human cancer cells | ROS↑-JNK↑-DR4/5↑ | Park et al.127 |
| 6BIO | Human breast cancer | STAT3↓- Mcl-1↓/cFLIP↓ | Braig et al.112 | Tricetin | Hepatocellular carcinoma | ROS↑-JNK↑-DR4/5↑ | Hsu et al.129 |
| Bufadienolide | Human breast cancer | STAT3↓-Mcl-1↓ | Dong et al.113 | Capsazepine | Human colorectal cancer | ROS↑-JNK↑ -DR4/5↑ | Sung et al.128 |
| Parthenolide | Hepatocellular carcinoma | STAT3↓-DR4/5↑ | Carlisi et al.114 | Ursolic acid | Human cancer cells | ROS↑-JNK↑-DR4/5↑ | Prasad et al.126 |
| Luteolin | Human renal carcinoma | STAT3↓/AKT↓-DR4/5↑ | Ou et al.115 | Cordycepin | Hepatocellular carcinoma | JNK↓-Bcl2↓ | Lee et al.125 |
| PI3K/AKT dependent | p53 dependent | ||||||
| Artesunate | Human cervical carcinoma | NF-кB↓/PI3K↓- Bcl-XL↓ | Thanaketpaisarn et al.109 | Triptolide | Human prostate cancer | p53↑-DR5↑ | Xiaowen et al.134 |
| Sanguinarine | Human gastric adenocarcinoma | PI3K↓/AKT↓-Bid↓ | Choi et al.117 | α-TOS | Human cancer cells | p53↑-DR4/5↑ | Tomasetti et al.135 |
| Eupatolide | Human breast cancer | AKT↓-cFLIP↓ | Lee et al.116 | Andrographolide | Hepatocellular carcinoma | P53↑-DR4↑ | Zhou et al.136 |
| Luteolin | Human renal carcinoma | STAT3↓/AKT↓- -DR4/DR5↑ | Ou et al.115 | 6-DHGD | Human hepatoblastoma | ROS/p53↑-DR5↑ | Chen et al.137 |
| p38 mediated | Nimbolide | Human colon cancer | ERK/p53↑-DR4/5↑-Bax↓ | Gupta et al.121 | |||
| Zerumbone | Human colorectal cancer | ERK/P38↑-DR4/5↑ | Yodkeeree et al.124 | γ-tocotrienol | Human cancer cells | ERK/P53↑-DR4/5↑ | Kannappan et al.123 |
| Diosgenin | Human colorectal cancer | P38↑-DR5↑ | Lepage et al.131 | Lupulone | Human colorectal cancer | P53/P38↑-DR4/5↑ | Lamy et al.130 |
| CAPE | Hepatocellular carcinoma | P38↑-DR4/5↑ | Kim et al.132 | Damnacanthal | Human cancer cells | P53/P38↑-DR5↑ | Lin et al.138 |
| Lupulone | Human colorectal cancer | P3↑8-P53↑-Mcl-1↓ | Lamy et al.130 | ||||
Note: NF-кB, nuclear factor-kappa B; ERK, extracellular signal-regulated kinases; PI3K, phosphatidylinositol-3-kinases; Brazilian EEP, Brazilian green propolis; 6BIO, 6-bromo-indirubin-3′-oxime; CAPE, caffeic acid phenethyl ester; SVT, snake venom toxin; α-TOS, alpha-tocopheryl succinate; 6-DHGD, 6-dehydrogingerdione; ↓, downregulated; ↑, upregulated.
Since suppression of the STAT3 pathway is linked to overcoming TRAIL resistance of tumor cells, numerous natural compounds have been investigated to determine whether they sensitize cancer cells via STAT3-dependent mechanism(s). Most of these natural compounds such as chrysin (a major constituent of Thai propolis), 6BIO (a derivative of indirubin), and bufadienolide (a major class of biologically active compounds isolated from ChanSu) can overcome TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation.111–113 Another natural agent, resveratrol, was found to increase sensitivity of melanomas to exogenous TRAIL through suppressed expression of cFLIP and Bcl-xL proteins and decreased STAT3 and NF-κB activation.108 Upregulation of DRs through the suppression of STAT3 activation by parthenolide (a sesquiterpene lactone found in European fever few)114 and luteolin (3′,4′,5,7-tetrahydroxyflavone, found in many pants)115 has also been found to be involved in enhancing the sensitivity of tumor cells to TRAIL.
Cellular resistance to TRAIL could also be developed through phosphorylation (activation) of the PI3K/AKT pathway. Eupatolide, the sesquiterpene lactone isolated from the medicinal plant Inula Britannica, could augment TRAIL-induced apoptosis in human breast cancer cells by downregulating c-FLIP expression through the inhibition of AKT phosphorylation.116 Besides this, there are various other natural compounds reported to activate TRAIL-induced apoptosis through inhibition of the PI3K/AKT pathway, such as sanguinarine (a benzophenanthridine alkaloid derived from the root of Sanguinaria Canadensis),117 artesunate,109 and luteolin.115
Several natural compounds can significantly increase the expression of ERK1/2, which further induces the expression of DRs. Gossypol (a polyphenol derived from cotton seed oil), curcumin (a natural compound derived from curcuma longa), apigenin (4′,5,7-trihydroxyflavone found in many plants), and butein (active component of the stems of Rhus verniciflua Stokes) induce DRs directly through activation of ERK1/2.106,118–120 Azadirone (a limonoid tetranortriterpene), γ-tocotrienol (an unsaturated vitamin E present predominantly in palm oil), and nimbolide (a terpenoid lactone derived from azadirachta indica)121–123 induce DRs through activation of ERK1/2 mediated by activation of p53 pathways. Zerumbone (a component of Asian ginger) has been shown to upregulate DR expression through induction of ERK and P38 activation.124 These studies indicate that ERK-dependent upregulation of TRAIL receptor DR4/5 can form the basis of an important strategy method to sensitize tumor cells to TRAIL.
Since inhibition of JNK can enhance TRAIL-induced apoptosis,37 numerous natural compounds have been investigated to determine whether they sensitize tumor cells to TRAIL via a JNK-dependent mechanism(s). One of these natural compounds, cordycepin, an active component of the caterpillar fungus cordyceps militaris, increases sensitivity of human hepatocellular carcinoma Hep3B cells to TRAIL-mediated apoptosis directly by inactivating the JNK signaling pathway.125 However, other natural compounds such as SVT (snake venom toxin from vipera lebetina turanica), ursolic acid (a pentacyclin triterpene), capsazepine (the active ingredient of chilli pepper), and tricetin (a flavonoid derivative found in Myrtaceae pollen and Eucalyptus Honey) induce DRs mediated by JNK1/2 activation through production of ROS.126–129
DRs can also be upregulated by diverse natural compounds through the activation of p38 signaling cascade. Another natural agent, lupulone, a β-acid largely present in hops (Humulus lupulus l), can significantly enhance the expression of p38, which plays a major role in the activation of p53 and the TRAIL-DR apoptotic pathway in SW620 human colon cancer-derived metastatic cells.130 Zerumbone (a sesquiterpene from the edible plant Zingiber zerumbet Smith), diosgenin (obtained from fenugreek), and caffeic acid phenethyl ester (CAPE; a phenolic compound derived from honeybee propolis) have also been shown to upregulate DR expression through induction of p38 activation.124,131,132 Our group has also recently reported that emodin, a naturally occurring anthraquinone present in the roots and barks of numerous plants, and an active ingredient of various Chinese medicinal herbs can downregulate the expression of various cell survival proteins, and induce the cell surface expression of both TRAIL receptors, DR 4 as well as 5 in hepatocellular carcinoma cells. In addition, emodin increased the expression of C/EBP homologous protein (CHOP) in a time-dependent manner.133 Knockdown of CHOP by small interfering RNA (siRNA) decreased the induction of emodin-induced DR5 expression and apoptosis. Emodin-induced induction of DR5 was mediated through the generation of ROS, as N-acetylcysteine blocked the induction of DR5 and the induction of apoptosis.
A critical factor for the TRAIL resistance of p53-mutant cell lines is the limited upregulation of the expression of DR4 and DR5 by mutant p53. Numerous natural compounds such as triptolide (isolated from the Chinese herb Tripterygium wilfordii Hook), alpha-tocopheryl succinate (α-TOS, an analogue of vitamin E), and andrographolide (a diterpenoid lactone isolated from a traditional herbal medicine Andrographis paniculata) upregulate the expression of DRs directly through the induction of p53.134–136 It has also been reported that ROS participates in the induction of DRs by 6-dehydrogingerdione (a compound isolated from the rhizomes of Zingiber officinale) mediated through expression of p53.137 However, nimbolide, azadirone, and γ-yocotrienol induce DR expression through p53 expression that is mediated by an ERK-p53 mechanism(s).121–123 Similarly, lupulone and damnacanthal (isolated from Morinda citrifolia) can upregulate DRs through induction of both p38–p53 mediated mechanism(s).130,138 These studies indicate that p53 plays an important role in induction of DRs by natural compounds, which can significantly sensitize tumor cells to TRAIL therapy. Table 2 summarizes the list of various natural compounds that can significantly summarize tumor cells to TRAIL.
Conclusions
This review briefly summarizes both the apoptotic and nonapoptotic pathways that can be activated upon TRAIL treatment as well as its physiological role in cancer. However, the molecular mechanism(s) contributing to TRAIL resistance in tumor cells still remain to be elucidated. Experimental preclinical as well as clinical evidences show that both TRAIL antibody and TRAIL used in combination with chemotherapeutics have a significant potential for anticancer treatment 139–141. From various reports, it is also clear that various pharmacological agents derived from natural sources can sensitize tumor cells to TRAIL through direct activation of intrinsic apoptotic pathway or modulation of diverse nonapoptotic pathways to upregulate DRs. However, as most of these studies have been conducted in cell lines or in preclinical mouse models of cancer; hence, additional clinical evidences are required to confirm whether these natural agents may also have synergistic therapeutic effects with TRAIL in cancer patients. Furthermore, since several recombinant TRAIL antibodies and agonistic antibodies against DRs are being used in the clinic, the combination of natural agents with these antibodies may greatly revolutionize cancer treatment. Therefore, in the coming years, we hope that such studies will be conducted and yield promising results.
Acknowledgments
This work was supported by NUHS Bench to Bedside to Product grant to GS. The Deanship of Scientific Research, College of Science Research Centre, King Saud University, Kingdom of Saudi Arabia also supported the work. KSA was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean Ministry of Education, Science and Technology (MoEST) (No. 2011-0006220). APK was supported by grants from the Singapore Ministry of Education Tier 2 [MOE2012-T2-2-139], NUHS Bench-to-Bedside-To-Product [R-184-000-243-515] and Cancer Science Institute of Singapore, Experimental Therapeutics I Program [R-713-001-011-271].
Authors’ contribution
XD and JZ reviewed the literature and compiled the review; FA, APK, AC, MZ, and SAA vetted the article; KSA and GS edited the final draft. Both XD and JZ contributed equally to this review article.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 2.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004; 305: 626–9. [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol 2012; 13: 780–8. [DOI] [PubMed] [Google Scholar]
- 4.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harbor Perspect Biol 2010; 2: a001008–a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proceedings of the National Academy of Sciences of the United States of America 1975; 72: 3666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 1989; 245: 301–5. [DOI] [PubMed] [Google Scholar]
- 7.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991; 66: 233–43. [DOI] [PubMed] [Google Scholar]
- 8.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature 1993; 364: 806–9. [DOI] [PubMed] [Google Scholar]
- 9.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 1996; 271: 12687–90. [DOI] [PubMed] [Google Scholar]
- 10.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995; 3: 673–82. [DOI] [PubMed] [Google Scholar]
- 11.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999; 104: 155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999; 5: 157–63. [DOI] [PubMed] [Google Scholar]
- 13.Gasparian ME, Chernyak BV, Dolgikh DA, Yagolovich AV, Popova EN, Sycheva AM, Moshkovskii SA, Kirpichnikov MP. Generation of new TRAIL mutants DR5-A and DR5-B with improved selectivity to death receptor 5. Apoptosis: Int J Program Cell Death 2009; 14: 778–87. [DOI] [PubMed] [Google Scholar]
- 14.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med 2007; 13: 1070–7. [DOI] [PubMed] [Google Scholar]
- 15.Scaffidi C, Schmitz I, Krammer PH, Peter ME. The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem 1999; 274: 1541–8. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 2010; 29: 4752–65. [DOI] [PubMed] [Google Scholar]
- 17.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94: 481–90. [DOI] [PubMed] [Google Scholar]
- 18.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102: 33–42. [DOI] [PubMed] [Google Scholar]
- 19.Hinz S, Trauzold A, Boenicke L, Sandberg C, Beckmann S, Bayer E, Walczak H, Kalthoff H, Ungefroren H. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene 2000; 19: 5477–86. [DOI] [PubMed] [Google Scholar]
- 20.Sun SY, Yue P, Zhou JY, Wang Y, Choi Kim HR, Lotan R, Wu GS. Overexpression of BCL2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. Biochem Biophys Res Commun 2001; 280: 788–97. [DOI] [PubMed] [Google Scholar]
- 21.Ehrhardt H, Fulda S, Schmid I, Hiscott J, Debatin KM, Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene 2003; 22: 3842–52. [DOI] [PubMed] [Google Scholar]
- 22.Karacay B, Sanlioglu S, Griffith TS, Sandler A, Bonthius DJ. Inhibition of the NF-kappaB pathway enhances TRAIL-mediated apoptosis in neuroblastoma cells. Cancer Gene Ther 2004; 11: 681–90. [DOI] [PubMed] [Google Scholar]
- 23.Braeuer SJ, Buneker C, Mohr A, Zwacka RM. Constitutively activated nuclear factor-kappaB, but not induced NF-kappaB, leads to TRAIL resistance by up-regulation of X-linked inhibitor of apoptosis protein in human cancer cells. Mol Cancer Res: MCR 2006; 4: 715–28. [DOI] [PubMed] [Google Scholar]
- 24.Roue G, Perez-Galan P, Lopez-Guerra M, Villamor N, Campo E, Colomer D. Selective inhibition of IkappaB kinase sensitizes mantle cell lymphoma B cells to TRAIL by decreasing cellular FLIP level. J Immunol 2007; 178: 1923–30. [DOI] [PubMed] [Google Scholar]
- 25.Voortman J, Resende TP, Abou El Hassan MA, Giaccone G, Kruyt FA. TRAIL therapy in non-small cell lung cancer cells: sensitization to death receptor-mediated apoptosis by proteasome inhibitor bortezomib. Mol Cancer Therapeut 2007; 6: 2103–12. [DOI] [PubMed] [Google Scholar]
- 26.Jennewein C, Karl S, Baumann B, Micheau O, Debatin KM, Fulda S. Identification of a novel pro-apoptotic role of NF-kappaB in the regulation of TRAIL- and CD95-mediated apoptosis of glioblastoma cells. Oncogene 2012; 31: 1468–74. [DOI] [PubMed] [Google Scholar]
- 27.Ou D, Wang X, Metzger DL, Robbins M, Huang J, Jobin C, Chantler JK, James RF, Pozzilli P, Tingle AJ. Regulation of TNF-related apoptosis-inducing ligand-mediated death-signal pathway in human beta cells by Fas-associated death domain and nuclear factor kappaB. Human Immunol 2005; 66: 799–809. [DOI] [PubMed] [Google Scholar]
- 28.Ravi R, Bedi GC, Engstrom LW, Zeng Q, Mookerjee B, Gelinas C, Fuchs EJ, Bedi A. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappaB. Nat Cell Biol 2001; 3: 409–16. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Kandasamy K, Srivastava RK. Differential roles of RelA (p65) and c-Rel subunits of nuclear factor kappa B in tumor necrosis factor-related apoptosis-inducing ligand signaling. Cancer Res 2003; 63: 1059–66. [PubMed] [Google Scholar]
- 30.Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, Emme D, Roder C, Kalthoff H, Wajant H. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene 2006; 25: 7434–9. [DOI] [PubMed] [Google Scholar]
- 31.Varfolomeev E, Maecker H, Sharp D, Lawrence D, Renz M, Vucic D, Ashkenazi A. Molecular determinants of kinase pathway activation by Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem 2005; 280: 40599–608. [DOI] [PubMed] [Google Scholar]
- 32.Hu WH, Johnson H, Shu HB. Tumor necrosis factor-related apoptosis-inducing ligand receptors signal NF-kappaB and JNK activation and apoptosis through distinct pathways. J Biol Chem 1999; 274: 30603–10. [DOI] [PubMed] [Google Scholar]
- 33.Lin Y, Devin A, Cook A, Keane MM, Kelliher M, Lipkowitz S, Liu ZG. The death domain kinase RIP is essential for TRAIL (Apo2L)-induced activation of IkappaB kinase and c-Jun N-terminal kinase. Mol Cell Biol 2000; 20: 6638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corazza N, Jakob S, Schaer C, Frese S, Keogh A, Stroka D, Kassahn D, Torgler R, Mueller C, Schneider P, Brunner T. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Investig 2006; 116: 2493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werneburg NW, Guicciardi ME, Bronk SF, Kaufmann SH, Gores GJ. Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. J Biol Chem 2007; 282: 28960–70. [DOI] [PubMed] [Google Scholar]
- 36.Park KJ, Lee SH, Lee CH, Jang JY, Chung J, Kwon MH, Kim YS. Upregulation of Beclin-1 expression and phosphorylation of Bcl-2 and p53 are involved in the JNK-mediated autophagic cell death. Biochem Biophys Res Commun 2009; 382: 726–9. [DOI] [PubMed] [Google Scholar]
- 37.Mucha SR, Rizzani A, Gerbes AL, Camaj P, Thasler WE, Bruns CJ, Eichhorst ST, Gallmeier E, Kolligs FT, Goke B, De Toni EN. JNK inhibition sensitises hepatocellular carcinoma cells but not normal hepatocytes to the TNF-related apoptosis-inducing ligand. Gut 2009; 58: 688–98. [DOI] [PubMed] [Google Scholar]
- 38.Ventura JJ, Hubner A, Zhang C, Flavell RA, Shokat KM, Davis RJ. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell 2006; 21: 701–10. [DOI] [PubMed] [Google Scholar]
- 39.Mahalingam D, Keane M, Pirianov G, Mehmet H, Samali A, Szegezdi E. Differential activation of JNK1 isoforms by TRAIL receptors modulate apoptosis of colon cancer cell lines. Br J Cancer 2009; 100: 1415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Son JK, Varadarajan S, Bratton SB. TRAIL-activated stress kinases suppress apoptosis through transcriptional upregulation of MCL-1. Cell Death Different 2010; 17: 1288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee MW, Park SC, Yang YG, Yim SO, Chae HS, Bach JH, Lee HJ, Kim KY, Lee WB, Kim SS. The involvement of reactive oxygen species (ROS) and p38 mitogen-activated protein (MAP) kinase in TRAIL/Apo2L-induced apoptosis. FEBS Lett 2002; 512: 313–8. [DOI] [PubMed] [Google Scholar]
- 42.Weldon CB, Parker AP, Patten D, Elliott S, Tang Y, Frigo DE, Dugan CM, Coakley EL, Butler NN, Clayton JL, Alam J, Curiel TJ, Beckman BS, Jaffe BM, Burow ME. Sensitization of apoptotically-resistant breast carcinoma cells to TNF and TRAIL by inhibition of p38 mitogen-activated protein kinase signaling. Int J Oncol 2004; 24: 1473–80. [PubMed] [Google Scholar]
- 43.Zhang L, Zhu H, Davis JJ, Jacob D, Wu S, Teraishi F, Gutierrez A, Wang Y, Fang B. Lack of p38 MAP kinase activation in TRAIL-resistant cells is not related to the resistance to TRAIL-mediated cell death. Cancer Biol Ther 2004; 3: 296–301. [DOI] [PubMed] [Google Scholar]
- 44.Milani D, Zauli G, Rimondi E, Celeghini C, Marmiroli S, Narducci P, Capitani S, Secchiero P. Tumour necrosis factor-related apoptosis-inducing ligand sequentially activates pro-survival and pro-apoptotic pathways in SK-N-MC neuronal cells. J Neurochem 2003; 86: 126–35. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XD, Borrow JM, Zhang XY, Nguyen T, Hersey P. Activation of ERK1/2 protects melanoma cells from TRAIL-induced apoptosis by inhibiting Smac/DIABLO release from mitochondria. Oncogene 2003; 22: 2869–81. [DOI] [PubMed] [Google Scholar]
- 46.Song JJ, Lee YJ. Differential cleavage of Mst1 by caspase-7/-3 is responsible for TRAIL-induced activation of the MAPK superfamily. Cell Signal 2008; 20: 892–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaculova A, Hofmanova J, Soucek K, Kozubik A. Different modulation of TRAIL-induced apoptosis by inhibition of pro-survival pathways in TRAIL-sensitive and TRAIL-resistant colon cancer cells. FEBS Lett 2006; 580: 6565–9. [DOI] [PubMed] [Google Scholar]
- 48.Belyanskaya LL, Ziogas A, Hopkins-Donaldson S, Kurtz S, Simon HU, Stahel R, Zangemeister-Wittke U. TRAIL-induced survival and proliferation of SCLC cells is mediated by ERK and dependent on TRAIL-R2/DR5 expression in the absence of caspase-8. Lung Cancer 2008; 60: 355–65. [DOI] [PubMed] [Google Scholar]
- 49.Vilimanovich U, Bumbasirevic V. TRAIL induces proliferation of human glioma cells by c-FLIPL-mediated activation of ERK1/2. Cell Mol Life Sci: CMLS 2008; 65: 814–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, Gonzalez-Baron M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev 2004; 30: 193–204. [DOI] [PubMed] [Google Scholar]
- 51.Song JJ, Kim JH, Sun BK, Alcala MA, Jr., Bartlett DL, Lee YJ. c-Cbl acts as a mediator of Src-induced activation of the PI3K-Akt signal transduction pathway during TRAIL treatment. Cell Signal 2010; 22: 377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azijli K, Yuvaraj S, Peppelenbosch MP, Wurdinger T, Dekker H, Joore J, van Dijk E, Quax WJ, Peters GJ, de Jong S, Kruyt FA. Kinome profiling of non-canonical TRAIL signaling reveals RIP1-Src-STAT3-dependent invasion in resistant non-small cell lung cancer cells. J Cell Sci 2012; 125: 4651–61. [DOI] [PubMed] [Google Scholar]
- 53.Xu J, Zhou JY, Wei WZ, Wu GS. Activation of the Akt survival pathway contributes to TRAIL resistance in cancer cells. PLoS ONE 2010; 5: e10226–e10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cursi S, Rufini A, Stagni V, Condo I, Matafora V, Bachi A, Bonifazi AP, Coppola L, Superti-Furga G, Testi R, Barila D. Src kinase phosphorylates Caspase-8 on Tyr380: a novel mechanism of apoptosis suppression. EMBO J 2006; 25: 1895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Schaeybroeck S, Kelly DM, Kyula J, Stokesberry S, Fennell DA, Johnston PG, Longley DB. Src and ADAM-17-mediated shedding of transforming growth factor-alpha is a mechanism of acute resistance to TRAIL. Cancer Res 2008; 68: 8312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 2001; 7: 94–100. [DOI] [PubMed] [Google Scholar]
- 57.Kayagaki N, Yamaguchi N, Nakayama M, Takeda K, Akiba H, Tsutsui H, Okamura H, Nakanishi K, Okumura K, Yagita H. Expression and function of TNF-related apoptosis-inducing ligand on murine activated NK cells. J Immunol 1999; 163: 1906–13. [PubMed] [Google Scholar]
- 58.Gong B, Almasan A. Genomic organization and transcriptional regulation of human Apo2/TRAIL gene. Biochem Biophys Res Commun 2000; 278: 747–52. [DOI] [PubMed] [Google Scholar]
- 59.Toomey NL, Deyev VV, Wood C, Boise LH, Scott D, Liu LH, Cabral L, Podack ER, Barber GN, Harrington WJ. Induction of a TRAIL-mediated suicide program by interferon alpha in primary effusion lymphoma. Oncogene 2001; 20: 7029–40. [DOI] [PubMed] [Google Scholar]
- 60.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumor necrosis factor-related apoptosis-inducing ligand in immune surveillance against tumor development. J Exp Med 2002; 195: 161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mace TA, Yamane N, Cheng J, Hylander BL, Repasky EA. The potential of the tumor microenvironment to influence Apo2L/TRAIL induced apoptosis. Immunol Investig 2006; 35: 279–96. [DOI] [PubMed] [Google Scholar]
- 62.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol 2002; 168: 1356–61. [DOI] [PubMed] [Google Scholar]
- 63.Seki N, Hayakawa Y, Brooks AD, Wine J, Wiltrout RH, Yagita H, Tanner JE, Smyth MJ, Sayer TJ. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res 2003; 63: 207–13. [PubMed] [Google Scholar]
- 64.Grosse-Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, Schutz G, Greiner EF, Kemp CJ, Walczak H. TRAIL-R deficiency in mice enhances lymph node metastasis without affecting primary tumor development. J Clin Investig 2008; 118: 100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang S, El-Deiry WS. Inducible silencing of KILLER/DR5 in vivo promotes bioluminescent colon tumor xenograft growth and confers resistance to chemotherapeutic agent 5-fluorouracil. Cancer Res 2004; 64: 6666–72. [DOI] [PubMed] [Google Scholar]
- 66.Lemke J, von Karstedt S, Zinngrebe J, Walczak H. Getting TRAIL back on track for cancer therapy. Cell Death Different 2014; 21: 1350–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O'Dwyer PJ, Gordon MS, Novotny W, Goldwasser MA, Tohnya TM, Lum BL, Ashkenazi A, Jubb AM, Mendelson DS. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol 2010; 28: 2839–46. [DOI] [PubMed] [Google Scholar]
- 68.Wainberg ZA, Messersmith WA, Peddi PF, Kapp AV, Ashkenazi A, Royer-Joo S, Portera CC, Kozloff MF. A phase 1B study of dulanermin in combination with modified FOLFOX6 plus bevacizumab in patients with metastatic colorectal cancer. Clin Colorect Cancer 2013; 12: 248–54. [DOI] [PubMed] [Google Scholar]
- 69.Kasubhai SM, Bendell JC, Kozloff M, Kapp AV, Ashkenazi A, Royer-Joo S, Portera CC. Phase Ib study of dulanermin combined with FOLFIRI (with or without bevacizumab [BV]) in previously treated patients (Pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2012; 30(15): 3543. [Google Scholar]
- 70.Yee L, Burris HA, Kozloff M, Wainberg Z, Pao M, Skettino S, Novotny W, Durbin B, Weston J, Hurwitz H. Phase Ib study of recombinant human Apo2L/TRAIL plus irinotecan and cetuximab or FOLFIRI in metastatic colorectal cancer (mCRC) patients (pts): preliminary results. J Clin Oncol 2009; 27(15): 4129. [Google Scholar]
- 71.Soria JC, Mark Z, Zatloukal P, Szima B, Albert I, Juhasz E, Pujol JL, Kozielski J, Baker N, Smethurst D, Hei YJ, Ashkenazi A, Stern H, Amler L, Pan Y, Blackhall F. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol 2011; 29: 4442–51. [DOI] [PubMed] [Google Scholar]
- 72.Yee L, Fanale M, Dimick K, Calvert S, Robin C, Ing J. A phase IB safety and pharmacokinetic (PK) study of recombinant human Apo2L/TRAIL in combination with rituximab in patients with low-grade non-Hodgkin lymphoma. J Clin Oncol 2007;25(abstract 8078).
- 73.Belada D, Mayer J, Czuczman MS, Flinn IW, Durbin-Johnson B, Bray GL. Phase II study of dulanermin plus rituximab in patients with relapsed follicular non-Hodgkin’s lymphoma (NHL). J Clin Oncol 2010; 28(15): 8104. [Google Scholar]
- 74.Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, Halpern W, Corey A, Cohen RB. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol 2007; 25: 1390–5. [DOI] [PubMed] [Google Scholar]
- 75.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, Iacobucci A, Maclean M, Lo L, Fox NL, Oza AM. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res: Off J Am Assoc Cancer Res 2008; 14: 3450–5. [DOI] [PubMed] [Google Scholar]
- 76.Mom CH, Verweij J, Oldenhuis CNAM. Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: a phase I study (vol 15, pg 5584, 2009). Clin Cancer Res 2009; 15: 6744–6744. [DOI] [PubMed] [Google Scholar]
- 77.Leong S, Cohen RB, Gustafson DL, Langer CJ, Camidge DR, Padavic K, Gore L, Smith M, Chow LQ, von Mehren M, O'Bryant C, Hariharan S, Diab S, Fox NL, Miceli R, Eckhardt SG. Mapatumumab, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study. J Clin Oncol 2009; 27: 4413–21. [DOI] [PubMed] [Google Scholar]
- 78.Younes A, Vose JM, Zelenetz AD, Smith MR, Burris HA, Ansell SM, Klein J, Halpern W, Miceli R, Kumm E, Fox NL, Czuczman MS. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin’s lymphoma. Br J Cancer 2010; 103: 1783–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trarbach T, Moehler M, Heinemann V, Kohne CH, Przyborek M, Schulz C, Sneller V, Gallant G, Kanzler S. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer 2010; 102: 506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, Lo L, Gallant G, Klein J. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer 2008; 61: 82–90. [DOI] [PubMed] [Google Scholar]
- 81.Belch A, Sharma A, Spencer A, Tarantolo S, Bahlis NJ, Doval D. A multicenter randomized phase ii trial of mapatumumab, a TRAIL-R1 agonist monoclonal antibody, in combination with bortezomib in patients with relapsed/refractory multiple myeloma (MM). Blood 2010; 116: abstracts 5031–abstracts 5031. [Google Scholar]
- 82.von Pawel J, Harvey JH, Spigel DR, Dediu M, Reck M, Cebotaru CL, Humphreys RC, Gribbin MJ, Fox NL, Camidge DR. Phase II trial of mapatumumab, a fully human agonist monoclonal antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1), in combination with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Clin Lung Cancer 2014; 15: 188–96 e2. [DOI] [PubMed] [Google Scholar]
- 83.Doi T, Murakami H, Ohtsu A, Fuse N, Yoshino T, Yamamoto N, Boku N, Onozawa Y, Hsu CP, Gorski KS, Fiberg G, Kawaguchi T, Sasaki T. Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2011; 68: 733–41. [DOI] [PubMed] [Google Scholar]
- 84.Chawla SP, Tabernero J, Kindler HL, Chiorean EG, LoRusso P, Hsu M, Haddad V, Bach BA, Baselga J. Phase I evaluation of the safety of conatumumab (AMG 655) in combination with ARM 479 in patients (pts) with advanced, refractory solid tumors. J Clin Oncol 2010; 28(15): 3102. [Google Scholar]
- 85.Demetri GD, Le Cesne A, Chawla SP, Brodowicz T, Maki RG, Bach BA, Smethurst DP, Bray S, Hei YJ, Blay JY. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study. Eur J Cancer 2012; 48: 547–63. [DOI] [PubMed] [Google Scholar]
- 86.Paz-Ares L, Balint B, de Boer RH, van Meerbeeck JP, Wierzbicki R, De Souza P, Galimi F, Haddad V, Sabin T, Hei YJ, Pan Y, Cottrell S, Hsu CP, RamLau R. A randomized phase 2 study of paclitaxel and carboplatin with or without conatumumab for first-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol 2013; 8: 329–37. [DOI] [PubMed] [Google Scholar]
- 87.Saltz L, Infante J, Schwartzberg L, Stephenson J, Rocha-Lima C, Galimi F, Dillingham K, Hsu M, Wiezorek J, Fuchs C. Safety and efficacy of AMG 655 plus modified FOLFOX6 (mFOLFOX6) and bevacizumab (B) for the first-line treatment of patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2009; 27(15): 4079. [Google Scholar]
- 88.Kindler HL, Garbo L, Stephenson J, Wiezorek J, Sabin T, Hsu M, Civoli F, Richards D. A phase Ib study to evaluate the safety and efficacy of AMG 655 in combination with gemcitabine (G) in patients (pts) with metastatic pancreatic cancer (PC). J Clin Oncol 2009; 27(15): 4501–4501. [Google Scholar]
- 89.Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ, Jr., Rocha-Lima CM, Safran H, Chan D, Kocs DM, Galimi F, McGreivy J, Bray SL, Feigal EG, Loh E, Fuchs CS. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 2012; 23: 2834–42. [DOI] [PubMed] [Google Scholar]
- 90.Cohn AL, Tabernero J, Maurel J, Nowara E, Sastre J, Chuah BY, Koop MV, Sakaeva DD, Mitchell EP, Dubeyy S, Suzuki S, Hei YJ, Galimi F, McCaffery I, Pan Y, Loberg R, Cottrel S, Choo SP. A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 2013; 24: 1777–85. [DOI] [PubMed] [Google Scholar]
- 91.Fuchs CS, Fakih M, Schwartzberg L, Cohn AL, Yee L, Dreisbach L, Kozloff MF, Hei YJ, Galimi F, Pan Y, Haddad V, Hsu CP, Sabin A, Saltz L. TRAIL receptor agonist conatumumab with modified FOLFOX6 plus bevacizumab for first-line treatment of metastatic colorectal cancer: a randomized phase 1b/2 trial. Cancer 2013; 119: 4290–8. [DOI] [PubMed] [Google Scholar]
- 92.Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey A, Calvert H, Bone J. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res: Off J Am Assoc Cancer Res 2007; 13: 6187–94. [DOI] [PubMed] [Google Scholar]
- 93.Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R, Ullrich SJ, Fisher GA, Tolcher AW. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 2010; 21: 376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sikic BI, Wakelee HA, Von Mehren M, Lewis N, Calvert AH, Plummer ER. A Phase 1b study to assess the safety of lexatumumab, a human monoclonal antibody that activates TRAIL-R2, in combination with gemcitabine, pemetrexed, doxorubicin or FOLFIRI. J Clin Oncol 2007; 25: 18S 14006–18S 14006. [Google Scholar]
- 95.Merchant MS, Geller JI, Baird K, Chou AJ, Galli S, Charles A, Amaoko M, Rhee EH, Price A, Wexler LH, Meyers PA, Widemann BC, Tsokos M, Mackall CL. Phase I trial and pharmacokinetic study of lexatumumab in pediatric patients with solid tumors. J Clin Oncol 2012; 30: 4141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Forero-Torres A, Shah J, Wood T, Posey J, Carlisle R, Copigneaux C, Luo F, Wojetowicz-Praga S, Percent I, Saleh M. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5). Cancer Biother Radiopharmaceut 2010; 25: 13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Forero-Torres A, Infante JR, Waterhouse D, Wong L, Vickers S, Arrowsmith E, He AR, Hart L, Trent D, Wade J, Jin X, Wang Q, Austin T, Rosen M, Beckman R, Roemeling RV, Greenberg Jonathan, Saleh Mansoor Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med 2013; 2: 925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reck M, Krzakowski M, Chmielowska E, Sebastian M, Hadler D, Fox T, Wang Q, Greenberg J, Beckman RA, Pawel JV. A randomized, double-blind, placebo-controlled phase 2 study of tigatuzumab (CS-1008) in combination with carboplatin/paclitaxel in patients with chemotherapy-naive metastatic/unresectable non-small cell lung cancer. Lung Cancer 2013; 82: 441–8. [DOI] [PubMed] [Google Scholar]
- 99.Rocha Lima CM, Bayraktar S, Flores AM, MacIntyre J, Montero A, Baranda JC, Wallmark J, Portera C, Raja R, Stern H, Royer-Joo S, Amler LC. Phase Ib study of drozitumab combined with first-line mFOLFOX6 plus bevacizumab in patients with metastatic colorectal cancer. Cancer Investig 2012; 30: 727–31. [DOI] [PubMed] [Google Scholar]
- 100.Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, Ing J, Tohnya TM, Jason S, Ashkenazi A, Bray G, Mendelson D. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res: Off J Am Assoc Cancer Res 2010; 16: 1256–63. [DOI] [PubMed] [Google Scholar]
- 101.Sharma S, de Vries EG, Infante JR, Oldenhuis CN, Gietema JA, Yang L, Sanela B, Katie P, Michael G, Jeffrey WS, Howard AB. Safety, pharmacokinetics, and pharmacodynamics of the DR5 antibody LBY135 alone and in combination with capecitabine in patients with advanced solid tumors. Investig New Drugs 2014; 32: 135–44. [DOI] [PubMed] [Google Scholar]
- 102.Fas SC, Baumann S, Zhu JY, Giaisi M, Treiber MK, Mahlknecht U, Krammer PH, Weber ML. Wogonin sensitizes resistant malignant cells to TNFalpha- and TRAIL-induced apoptosis. Blood 2006; 108: 3700–6. [DOI] [PubMed] [Google Scholar]
- 103.Labsch S, Liu L, Bauer N, Zhang Y, Aleksandrowicz E, Gladkich J, Shonsiegel F, Herr I. Sulforaphane and TRAIL induce a synergistic elimination of advanced prostate cancer stem-like cells. Int J Oncol 2014; 44: 1470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang C, Chen T, Zhang N, Yang M, Li B, Lu X, Cao X, Ling C. Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha kinase-NFkappaB. J Biol Chem 2009; 284: 3804–13. [DOI] [PubMed] [Google Scholar]
- 105.Szliszka E, Zydowicz G, Janoszka B, Dobosz C, Kowalczyk-Ziomek G, Krol W. Ethanolic extract of Brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. Int J Oncol 2011; 38: 941–53. [DOI] [PubMed] [Google Scholar]
- 106.Hussain AR, Ahmed M, Al-Jomah NA, Khan AS, Manogaran P, Sultana M, Abubaker J, Platanisa LC, Ai-Kuraya KS, Uddin S. Curcumin suppresses constitutive activation of nuclear factor-kappa B and requires functional Bax to induce apoptosis in Burkitt’s lymphoma cell lines. Mol Cancer Therapeut 2008; 7: 3318–29. [DOI] [PubMed] [Google Scholar]
- 107.Seo OW, Kim JH, Lee KS, Lee KS, Kim JH, Won MH, Ha KS, Kwon YG, Kim YM. Kurarinone promotes TRAIL-induced apoptosis by inhibiting NF-kappaB-dependent cFLIP expression in HeLa cells. Exp Mol Med 2012; 44: 653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK. Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp Cell Res 2008; 314: 1163–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thanaketpaisarn O, Waiwut P, Sakurai H, Saiki I. Artesunate enhances TRAIL-induced apoptosis in human cervical carcinoma cells through inhibition of the NF-kappaB and PI3K/Akt signaling pathways. Int J Oncol 2011; 39: 279–85. [DOI] [PubMed] [Google Scholar]
- 110.Zhang C, Wu R, Zhu H, Hu YZ, Jiang H, Lin NM, He QJ, Yang B. Enhanced anti-tumor activity by the combination of TRAIL/Apo-2L and combretastatin A-4 against human colon cancer cells via induction of apoptosis in vitro and in vivo. Cancer Lett 2011; 302: 11–9. [DOI] [PubMed] [Google Scholar]
- 111.Lirdprapamongkol K, Sakurai H, Abdelhamed S, Yokoyama S, Athikomkulchai S, Viriyaroj A, Awale S, Ruchirawat S, Svasti J, Saiki L. Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation. Int J Oncol 2013; 43: 329–37. [DOI] [PubMed] [Google Scholar]
- 112.Braig S, Bischoff F, Abhari BA, Meijer L, Fulda S, Skaltsounis L, Vollmar AM. The pleiotropic profile of the indirubin derivative 6BIO overcomes TRAIL resistance in cancer. Biochem Pharmacol 2014; 91: 157–67. [DOI] [PubMed] [Google Scholar]
- 113.Dong Y, Yin S, Li J, Jiang C, Ye M, Hu H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis: Int J Program Cell Death 2011; 16: 394–403. [DOI] [PubMed] [Google Scholar]
- 114.Carlisi D, D’Anneo A, Angileri L, Lauricella M, Emanuele S, Santulli A, Vento R, Tesoriere G. Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J Cell Physiol 2011; 226: 1632–41. [DOI] [PubMed] [Google Scholar]
- 115.Ou YC, Li JR, Kuan YH, Raung SL, Wang CC, Hung YY, Pan PH, Lu HC, Chen CJ. Luteolin sensitizes human 786-O renal cell carcinoma cells to TRAIL-induced apoptosis. Life Sci 2014; 100: 110–7. [DOI] [PubMed] [Google Scholar]
- 116.Lee J, Hwangbo C, Lee JJ, Seo J, Lee JH. The sesquiterpene lactone eupatolide sensitizes breast cancer cells to TRAIL through down-regulation of c-FLIP expression. Oncol Reports 2010; 23: 229–37. [PubMed] [Google Scholar]
- 117.Choi WY, Jin CY, Han MH, Kim GY, Kim ND, Lee WH, Kim SK, Choi YH. Sanguinarine sensitizes human gastric adenocarcinoma AGS cells to TRAIL-mediated apoptosis via down-regulation of AKT and activation of caspase-3. Anticancer Res 2009; 29: 4457–65. [PubMed] [Google Scholar]
- 118.Moon DO, Kim MO, Choi YH, Kim GY. Butein sensitizes human hepatoma cells to TRAIL-induced apoptosis via extracellular signal-regulated kinase/Sp1-dependent DR5 upregulation and NF-kappaB inactivation. Mol Cancer Therapeut 2010; 9: 1583–95. [DOI] [PubMed] [Google Scholar]
- 119.Sung B, Ravindran J, Prasad S, Pandey MK, Aggarwal BB. Gossypol induces death receptor-5 through activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer cells to TRAIL. J Biol Chem 2010; 285: 35418–27. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Kim EY, Yu JS, Yang M, Kim AK. Sub-toxic dose of apigenin sensitizes HepG2 cells to TRAIL through ERK-dependent up-regulation of TRAIL receptor DR5. Mol Cells 2013; 35: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gupta SC, Reuter S, Phromnoi K, Park B, Hema PS, Nair M, Aggarwal BB. Nimbolide sensitizes human colon cancer cells to TRAIL through reactive oxygen species- and ERK-dependent up-regulation of death receptors, p53, and Bax. J Biol Chem 2011; 286: 1134–46. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Gupta SC, Francis SK, Nair MS, Mo YY, Aggarwal BB. Azadirone, a limonoid tetranortriterpene, induces death receptors and sensitizes human cancer cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) through a p53 protein-independent mechanism: evidence for the role of the ROS-ERK-CHOP-death receptor pathway. J Biol Chem 2013; 288: 32343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kannappan R, Ravindran J, Prasad S, Sung B, Yadav VR, Reuter S, Madan MC, Aggarwal BB. Gamma-tocotrienol promotes TRAIL-induced apoptosis through reactive oxygen species/extracellular signal-regulated kinase/p53-mediated upregulation of death receptors. Mol Cancer Therapeut 2010; 9: 2196–207. [DOI] [PubMed] [Google Scholar]
- 124.Yodkeeree S, Sung B, Limtrakul P, Aggarwal BB. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: evidence for an essential role of reactive oxygen species. Cancer Res 2009; 69: 6581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee HH, Jeong JW, Lee JH, Kim GY, Cheong J, Jeong YK, Yoo YH, Choi YH. Cordycepin increases sensitivity of Hep3B human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by inactivating the JNK signaling pathway. Oncol Reports 2013; 30: 1257–64. [DOI] [PubMed] [Google Scholar]
- 126.Prasad S, Yadav VR, Kannappan R, Aggarwal BB. Ursolic acid, a pentacyclin triterpene, potentiates TRAIL-induced apoptosis through p53-independent up-regulation of death receptors: evidence for the role of reactive oxygen species and JNK. J Biol Chem 2011; 286: 5546–57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.Park MH, Jo M, Won D, Song HS, Song MJ, Hong JT. Snake venom toxin from Vipera lebetina turanica sensitizes cancer cells to TRAIL through ROS- and JNK-mediated upregulation of death receptors and downregulation of survival proteins. Apoptosis: Int J Program Cell Death 2012; 17: 1316–26. [DOI] [PubMed] [Google Scholar]
- 128.Sung B, Prasad S, Ravindran J, Yadav VR, Aggarwal BB. Capsazepine, a TRPV1 antagonist, sensitizes colorectal cancer cells to apoptosis by TRAIL through ROS-JNK-CHOP-mediated upregulation of death receptors. Free Rad Biol Med 2012; 53: 1977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hsu YL, Hou MF, Tsai EM, Kuo PL. Tricetin, a dietary flavonoid, induces apoptosis through the reactive oxygen species/c-Jun NH2-terminal kinase pathway in human liver cancer cells. J Agricul Food Chem 2010; 58: 12547–56. [DOI] [PubMed] [Google Scholar]
- 130.Lamy V, Bousserouel S, Gosse F, Minker C, Lobstein A, Raul F. Lupulone triggers p38 MAPK-controlled activation of p53 and of the TRAIL receptor apoptotic pathway in human colon cancer-derived metastatic cells. Oncol Reports 2011; 26: 109–14. [DOI] [PubMed] [Google Scholar]
- 131.Lepage C, Leger DY, Bertrand J, Martin F, Beneytout JL, Liagre B. Diosgenin induces death receptor-5 through activation of p38 pathway and promotes TRAIL-induced apoptosis in colon cancer cells. Cancer Lett 2011; 301: 193–202. [DOI] [PubMed] [Google Scholar]
- 132.Kim EY, Ryu JH, Kim AK. CAPE promotes TRAIL-induced apoptosis through the upregulation of TRAIL receptors via activation of p38 and suppression of JNK in SK-Hep1 hepatocellular carcinoma cells. Int J Oncol 2013; 43: 1291–300. [DOI] [PubMed] [Google Scholar]
- 133.Subramaniam A, Loo SY, Rajendran P, Manu KA, Perumal E, Li F, Shanmugam MK, Siveen KS, Park JI, Ahn KS, Hui KM, Kumar AP, Sethi G. An anthraquinone derivative, emodin sensitizes hepatocellular carcinoma cells to TRAIL induced apoptosis through the induction of death receptors and downregulation of cell survival proteins. Apoptosis: Int J Program Cell Death 2013; 18: 1175–87. [DOI] [PubMed] [Google Scholar]
- 134.Xiaowen H, Yi S. Triptolide sensitizes TRAIL-induced apoptosis in prostate cancer cells via p53-mediated DR5 up-regulation. Mol Biol Reports 2012; 39: 8763–70. [DOI] [PubMed] [Google Scholar]
- 135.Tomasetti M, Andera L, Alleva R, Borghi B, Neuzil J, Procopio A. Alpha-tocopheryl succinate induces DR4 and DR5 expression by a p53-dependent route: implication for sensitisation of resistant cancer cells to TRAIL apoptosis. FEBS Lett 2006; 580: 1925–31. [DOI] [PubMed] [Google Scholar]
- 136.Zhou J, Lu GD, Ong CS, Ong CN, Shen HM. Andrographolide sensitizes cancer cells to TRAIL-induced apoptosis via p53-mediated death receptor 4 up-regulation. Mol Cancer Therapeut 2008; 7: 2170–80. [DOI] [PubMed] [Google Scholar]
- 137.Chen CY, Tai CJ, Cheng JT, Zheng JJ, Chen YZ, Liu TZ, Yiin S, Chern CL. 6-Dehydrogingerdione sensitizes human hepatoblastoma Hep G2 cells to TRAIL-induced apoptosis via reactive oxygen species-mediated increase of DR5. J Agric Food Chem 2010; 58: 5604–11. [DOI] [PubMed] [Google Scholar]
- 138.Lin FL, Hsu JL, Chou CH, Wu WJ, Chang CI, Liu HJ. Activation of p38 MAPK by damnacanthal mediates apoptosis in SKHep 1 cells through the DR5/TRAIL and TNFR1/TNF-alpha and p53 pathways. Eur J Pharmacol 2011; 650: 120–9. [DOI] [PubMed] [Google Scholar]
- 139.Soria JC, Smit E, Khayat D, Besse B, Yang X, Hsu CP, Reese D, Wiezorek J, Blackhall F. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol 2010; 28: 1527–33. [DOI] [PubMed] [Google Scholar]
- 140.Herbst RS, Kurzrock R, Hong DS, Valdivieso M, Hsu CP, Goyal L, Gloria J, Yuying CH, Susan W, John SH, Greg F, Patricia ML. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res: Off J Am Assoc Cancer Res 2010; 16: 5883–91. [DOI] [PubMed] [Google Scholar]
- 141.Paz-Ares L, Torres JMS, Diaz-Padilla I, Links M, Reguart N, Boyer M, Wiezorek J, Sabin T, Pan Y, Meerbeeck JV. Safety and efficacy of AMG 655 in combination with paclitaxel and carboplatin (PC) in patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2009; 27(15): e19048. [Google Scholar]