Abstract
Cardiac ischemia associated with acute coronary syndrome and myocardial infarction is a leading cause of mortality and morbidity in the world. A rapid detection of the ischemic events is critically important for achieving timely diagnosis, treatment and improving the patient's survival and functional recovery. This minireview provides an overview on the current biomarker research for detection of acute cardiac ischemia. We primarily focus on inosine and hypoxanthine, two by-products of ATP catabolism. Based on our published findings of elevated plasma concentrations of inosine/hypoxanthine in animal laboratory and clinical settings, since 2006 we have originally proposed that these two purine molecules can be used as rapid and sensitive biomarkers for acute cardiac ischemia at its very early onset (within 15 min), hours prior to the release of heart tissue necrosis biomarkers such as cardiac troponins. We further developed a chemiluminescence technology, one of the most affordable and sensitive analytical techniques, and we were able to reproducibly quantify and differentiate total hypoxanthine concentrations in the plasma samples from healthy individuals versus patients suffering from ischemic heart disease. Additional rigorous clinical studies are needed to validate the plasma inosine/hypoxanthine concentrations, in conjunction with other current cardiac biomarkers, for a better revelation of their diagnostic potentials for early detection of acute cardiac ischemia.
Keywords: Biomarker, diagnostic methods, cardiac ischemia, chemiluminescence, nucleoside, ATP breakdown by-products
Introduction
Based on the recent epidemiologic data reported by the American Heart Association and the World Health Organization, cardiovascular diseases represent the leading cause of death worldwide.1–4 Approximately 24 individuals die every minute in the world from heart attacks, with many individuals waiting more than two hours before choosing to seek emergency medical services. The American Heart Association cites that approximately 75–80% of heart attacks occur at home, with a mortality rate of over 92% for those individuals experiencing a heart attack outside of the hospital setting and this rate has remained relatively unchanged over the last three decades.5 In addition, of the 7–8 million individuals who do present to the hospital emergency room with non-traumatic chest pain, approximately 500,000 are discharged after being evaluated only to subsequently experience a heart attack.6,7 These sobering heart attack statistics both within the USA and worldwide clearly demonstrate the critical need for endogenous biomarkers aiming at early detection of acute cardiac ischemia. Therefore, the importance of addressing improved diagnostic capabilities during the early onset of acute cardiac ischemia for life saving and improvement of clinical outcomes in patients with acute myocardial infarction remains as an unmet global need.
The clinical symptoms of patients during the onset of acute cardiac ischemia may include non-traumatic chest pain, which is one of the leading causes for visits to the hospital emergency room (ER). Non-traumatic chest pain can be caused by more than a dozen of medical conditions, e.g. gastroesophageal reflux, musculoskeletal pain, nerve impingement, pulmonary embolus, acute pericarditis, peptic ulcer, and anxiety attack. This complex nature of chest pain often makes it difficult for ER personnel to start necessary life-saving treatments (such as thrombolysis and percutaneous coronary angioplasty) for the patients suffering acute myocardial infarction (AMI). Current emergency medical evaluation on chest pain patients with suspected AMI includes obtaining the patient history, signs and symptoms, vitals, electrocardiogram (ECG), and blood testing for the biomarkers of AMI, such as myoglobin, creatine kinase (CK), and more cardiac specific biomarkers – myocardial muscle creatine kinase (CK-MB) and cardiac troponins (cTn).8 However, the accuracy for diagnosing acute MI is still relatively low. When using patients' signs and symptoms, ECG and cardiac-specific troponin together, the diagnostic accuracy is approximately 50%. With the addition of the recently FDA approved ischemia-modified albumin assay, the diagnostic accuracy has increased to approximately 70%. Therefore, there is still an urgent need for identifying additional biomarkers for a more rapid detection of acute cardiac ischemia, in order to substantially improve the diagnostic accuracy and timeliness for initiating medical treatments.
Biochemical pathways and theoretic basis for cardiac hypoxia/ischemia-induced ATP breakdown and the resultant purine products – inosine and hypoxanthine as potential biomarkers for acute cardiac ischemia
As the vital engine for blood circulation, heart muscle utilizes adenosine triphosphate (ATP), a high-energy phosphate molecule, as its primary fuel to perform contraction–relaxation for pumping blood. Vast majority of ATP (∼80%) in cardiac cells is produced by the powerhouse cellular organelle – mitochondria – via aerobic oxidative phosphorylation in the electron transport chain. In order to produce large quantities of ATP in supporting the functional demand of heart, human cardiac cells have an abundance of mitochondria, which comprise approximately 40–50% of the cardiac cellular mass. Since this crucial aerobic process is heavily oxygen dependent, there are major metabolic consequences caused by disruption or reduction of blood flow in coronary circulation and the resultant decrease in oxygen supply to the affected portion of myocardium (i.e. ischemia) and/or other types of lack of oxygen (i.e. hypoxia) in myocardial tissues caused by environmental factors or demand/supply imbalance, etc.
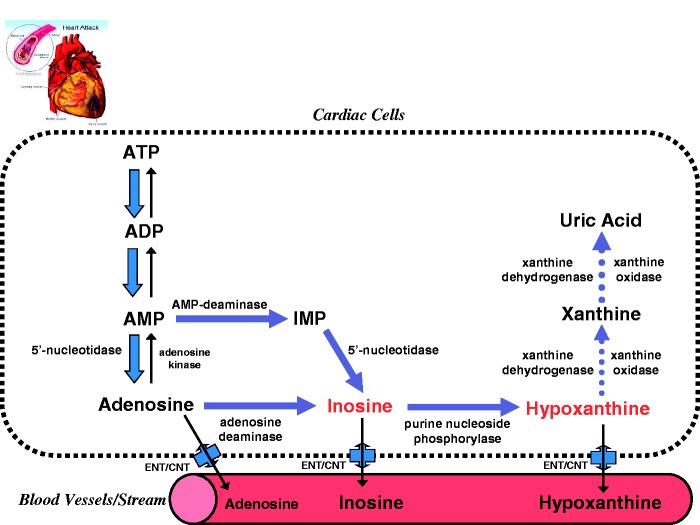
As illustrated in Figure 1, at the onset of cardiac ischemia/hypoxia, the high energy phosphates (creatine phosphate and ATP) are rapidly depleted and heart tissues would lose 65% of their ATP contents within 15 min of total ischemia.9 Such ischemic events in turn mobilize a ATP breakdown cascade10 that leads to cellular accumulation of ATP catabolic by-products, including adenosine diphosphate (ADP), adenosine monophosphate (AMP), and activates normally dormant enzymes, such as 5′-nucleotidase, adenosine deaminase, purine nucleoside phosphorylase, and xanthine oxidase, which sequentially catabolize AMP into adenosine, inosine, hypoxanthine, xanthine, and uric acid. Upon reperfusion of the heart with oxygenated blood or perfusate, xanthine oxidase and xanthine dehydrogenase convert hypoxanthine to xanthine and uric acid.
Figure 1.
Illustrative summary of major metabolic pathways of ATP degradation during myocardial ischemia. Abbreviations: ATP: adenosine triphosphate; ADP: adenosine diphosphate; AMP: adenosine monophosphate; IMP: inosine monophosphate; ENT: equilibrative nucleoside transporters; CNT: concentrative nucleoside transporters. (A color version of this figure is available in the online journal.)
It should be emphasized that inosine and hypoxanthine are small polar substances with low molecular weight of 268 Dalton (Da) for inosine and 136 Da for hypoxanthine. Therefore, inosine and hypoxanthine can be instantly transported by passive diffusion from affected heart tissue into the bloodstream, a process that is also facilitated by equilibrative nucleoside transporters (ENT) and/or concentrative nucleoside transporters (CNT)11 (Figure 1). Since an acute cardiac ischemic event occurs at the onset of myocardial infarction, it is conceivable that the concentrations of inosine and/or hypoxanthine should theoretically appear elevated in the bloodstream prior to any elevated concentrations of larger protein biomarkers indicative of tissue necrosis of AMI, usually detected several hours after the AMI event.
Basic laboratory evidence in animal models of acute cardiac ischemia suggesting inosine and hypoxanthine as highly prominent and sensitive biomarkers for myocardial ischemic events
To the best of our knowledge, the first report of a release of significant amounts of inosine and hypoxanthine from the heart during ischemia/hypoxia can be found in the seminal work published a half of century ago by a legendary cardiovascular physiologist – Robert M. Berne in 1963.12 However, in his study Dr. Berne focused only on his newly discovered vasodilatory effects of adenosine, which could serve as a feedback mechanism for regulating coronary flow under cardiac hypoxia or ischemia. There was no suggestion for these ATP breakdown by-products as potential candidate biomarkers for cardiac ischemia.
This interesting phenomenon of cardiac efflux of adenosine/inosine/hypoxanthine triggered by hypoxia/ischemia has been subsequently confirmed by other American and European investigators since 1980s in various animal models of cardiac ischemia.10,13 For example, in 1981 Jennings et al. used healthy mongrel dogs with both ex vivo (excised heart) and in vivo cardiac ischemia.10 The eluted nucleotides and their by-products (e.g. inosine, hypoxanthine) were evaluated. They reported that during both ex vivo global cardiac ischemia and in vivo regional ischemia (via ligation of coronary artery), a significant elevation of inosine and hypoxanthine concentrations were detected in blood or coronary effluent as early as 15 min after the in vivo ischemia and 60 min after the ex vivo global ischemia. Similar findings were also reported in other studies using isolated rat or rabbit hearts.14,15 It is noteworthy that de Jong and co-workers demonstrated in isolated adult or neonatal rat hearts that there were large age-related differences in purine release.16,17 In the early phase of reperfusion, adult hearts released inosine (58%) and adenosine (18%), whereas newborns released inosine (53%) and hypoxanthine (38%). The authors interpreted the data as a lower activity of xanthine oxidoreductase (XO) in newborn hearts, which was confirmed by enzymatic assay. ATP-catabolite release during reperfusion was less in newborn than in the adults' hearts, and this coincided with lower xanthine oxidase activity.16,17
In another elegant study published in 1996, Mei and colleagues introduced a fast and sensitive method for a simultaneous determination of adenosine, inosine, hypoxanthine, xanthine, and uric acid in the interstitial fluid of canine myocardium.18 They used microdialysis, microbore column high-performance liquid chromatography (HPLC), and a photo diode array detector, which allowed the simultaneous detection of UV absorbance at multiple wavelengths, allowing the detection of each compound at their maximal UV absorbance. Such use of a microbore HPLC column and detection of UV absorbance could improve the measurement sensitivity. These authors thoroughly demonstrated the temporal effect of myocardial ischemia and reperfusion upon interstitial adenosine, inosine, hypoxanthine, xanthine, and uric acid concentrations in an in vivo canine model.18 Similarly, Backstrom et al. employed a pig model of cardiac ischemia via coronary artery occlusion (for 0, 10, 15, or 60 min) using microdialysis catheterization at several different blood vessel sites including great cardiac vein and pulmonary artery.13 They reported a significant increase in dialysate concentrations of inosine and hypoxanthine at the time points of 15 and 60 min after ischemia, as compared with the pre-ischemic baseline concentration, suggesting a graded outflow of purine metabolites in response to the ischemic conditions.
In order to test our original hypothesis that inosine and/or hypoxanthine could serve as potential biomarker(s) of acute cardiac ischemia, we performed an ex vivo study using Langendorff isolated buffer-perfused mouse hearts.19 A well-established protocol of cardiac ischemia-reperfusion consisted of 30 min of stabilization, 20 min of zero-flow global ischemia, and 30 min of reperfusion, as per our previous publications in the field of cardioprotection against ischemia-reperfusion injury.20–23 Time-matched normoxic perfusion was carried out as the control group. Upon heart reperfusion, samples of coronary effluent from the isolated hearts were collected at predetermined time-points (0, 1, 3, 5, 10, and 20 min) for HPLC analysis. Our key findings were the significant efflux amount of inosine (22 to 69 fold), hypoxanthine (>7 fold), xanthine (∼3 fold), and uric acid (∼3 fold) found in the perfusate from the mouse hearts following 20 min of global ischemia, as compared with the non-ischemic normoxic control hearts.19
In a subsequent study, we determined the effects of salicylic acid on inosine efflux,24 since acetyl salicylic acid (aspirin) is one of the most widely prescribed drugs in the patients with high risk for heart attack and it may have inhibitory effects on oxidative phosphorylation and ATP synthesis in cardiac mitochondria. Interestingly, we observed that inosine efflux was approximately nine times higher in the ischemic hearts perfused with Krebs buffer fortified with 1.0 mM salicylic acid than those without salicylic acid (P < 0.01).24 These results indicate a significant potentiation of ATP nucleotide catabolism into its metabolites, and inosine and hypoxanthine can be resulted from salicylic acid at 1.0 mM, a common anti-arthritic or anti-inflammatory concentration in current clinical practice. Salicylic acid (0.1 or 1.0 mM) did not appreciably inhibit purine nucleoside phosphorylase (the enzyme converts inosine to hypoxanthine) suggesting that the augmented inosine efflux was due to the salicylic acid effect on upstream elements of cellular respiration. While post-ischemic cardiac function was further depressed by 1.0 mM salicylic acid, perfusion with 0.1 mM salicylic acid led to a remarkable functional improvement despite moderately increased inosine efflux (2.7-fold). We concluded that inosine was a sensitive biomarker for detecting cardiac ischemia and salicylic acid-induced effects on cellular respiration. However, the inosine efflux concentration appears to be a poor predictor of the individual post-ischemic cardiac functional recovery in this ex vivo model.
Clinical evidence from plasma samples of ER chest pain patients for supporting inosine and hypoxanthine as biomarkers for acute cardiac ischemia in humans
The post-ischemia efflux of inosine and hypoxanthine identified in the aforementioned animal studies has also been examined in several clinical studies since 1980s.25–27 Notably, in 1981, Harmsen et al. used an isocratic HPLC system to measure purine nucleosides and oxypurines in blood samples of six healthy volunteers and 13 patients with angiographically documented ischemic heart disease, undergoing an atrial pacing stress test.26 They reported no significant release of adenosine, inosine or xanthine was detectable in the blood stream. However, another study from Russia (1989) reported that a rise in intermediate and end products of purine metabolism (inosine, hypoxanthine, xanthine, uric acid) was found in venous blood of patients with AMI and angina pectoris.25 In 1994, Kock et al. showed that no significant differences were observed for the serum concentration of hypoxanthine and xanthine, both the sum (hypoxanthine+xanthine) and ratio (xanthine/hypoxanthine), between the healthy males, healthy females, the patients suffering from angina pectoris, and the patients suffering from cerebral insult, although an increase of serum xanthine concentration was found in the patients with AMI.27 However, in our opinion, the potential artifacts caused by blood sampling methods with SST vacutainer gel tubes may have contributed to these negative findings on inosine/hypoxanthine in AMI patients.27 It is possible that using serum (SST) tubes could result in red blood cell (RBC) hemolysis during the blood sample preparation and since RBC also functions as an ATP storage vesicle, ATP released from the RBC into the blood sample matrix would enzymatically be converted to form artificially high concentrations of hypoxanthine.
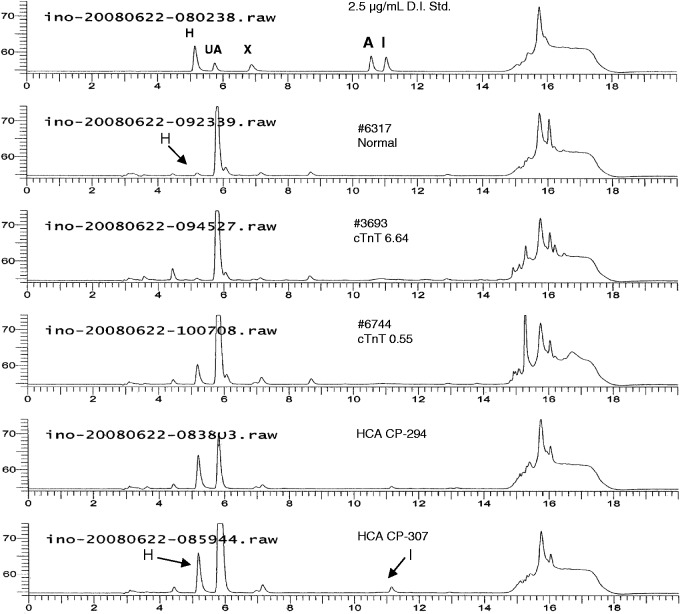
On the other hand, the HPLC-UV results from our human plasma evaluations support inosine and hypoxanthine as potential candidate biomarkers for indicating acute cardiac ischemia.28 In this study, residual plasma samples (heparinized) from ER non-traumatic chest pain patients were obtained and evaluated using a validated quantitative HPLC-UV method. Briefly, the plasma samples were prepared using 10 K MWCO filters, with 15 µL of the filtrate injected for HPLC-UV analysis. Figure 2 depicts an overlay of HPLC-UV chromatograms representing a calibration standard, a healthy individual (Control), two positive cardiac troponin T (cTnT) samples (cTnT 6.64 and cTnT 0.55 µg/L), and two chest pain patient samples (CP-294 and CP-307).28 The representative chromatogram of a control subject had a typical quantifiable concentration of hypoxanthine, with no detectable amount of inosine. The hypoxanthine concentrations for normal subjects were consistent with those previously reported in literature.29 The plasma samples with elevated cTnT concentrations were purchased from a FDA-approved biospecimen repository with validated cTnT values (6.64 and 0.55 µg/L). There was an inverse relationship between the concentrations of cTnT and hypoxanthine, which will be discussed in the next section of this article.
Figure 2.
Overlay of HPLC–UV chromatograms representing (top to bottom) a calibration standard, healthy normal individual plasma sample, cardiac troponin T plasma samples (cTnT 6.64 and cTnT 0.55 µg/L), and non-traumatic chest pain patient samples (CP 294 and CP 307). Calibration standards included the components hypoxanthine (H, RT ∼5.2 min), uric acid (UA, RT ∼5.9 min), xanthine (X, RT ∼6.9 min), adenosine (A, RT ∼10.6 min), and inosine (I, RT ∼11.1 min)
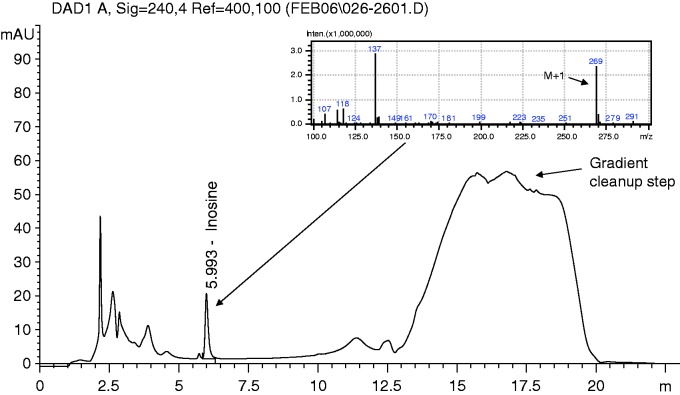
As seen in the CP-294 and CP-307 samples, hypoxanthine concentrations were found significantly elevated as compared with the control subjects, whereas CP-307 also had an increased inosine concentration. Inosine was typically found at low or non-detectable concentrations, most likely depending on how long the whole blood sample sat prior to separate the heparinized plasma from the blood cells. This is because in human whole blood, RBC contains purine nucleoside phosphorylase (PNP), an enzyme that converts inosine to hypoxanthine. On the other hand, in our previous study using ex vivo Langendorff isolated mouse hearts perfused with a RBC-free Krebs buffer solution,19 and we detected high concentrations of inosine and very low to non-detectable concentrations of hypoxanthine following 20 min of global cardiac ischemia (see Figure 3 for original LC-ESI-MS data confirming the presence of elevated inosine). The presence or lack of PNP (to convert inosine to hypoxanthine) would determine the relative increases in inosine and/or hypoxanthine among these ex vivo19 and in vivo28 studies.
Figure 3.
HPLC-DAD (diode array detector) chromatogram representing a typical Krebs buffer eluent sample obtained and evaluated from a mouse heart undergoing ex vivo acute cardiac ischemia in Langendorff model. The samples were also evaluated using LC-ESI-MS, for confirmation that inosine (MW 268 Da) was the elevated component in the mouse-induced acute cardiac ischemia samples. Adapted with permission from Farthing et al. Biomarkers 2006.19. (A color version of this figure is available in the online journal.)
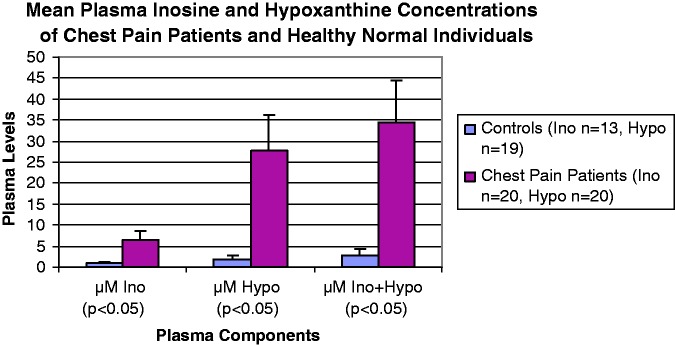
Figure 4 is a bar chart depicting results of plasma samples from healthy individuals (Ino n = 13, Hypo n = 19) and first blood sample draws from ER non-traumatic chest pain patients (n = 20). The plasma concentrations of inosine and hypoxanthine were significantly elevated in the ER patients, as compared to the normal subjects (Figure 4). The HPLC-UV method for plasma evaluation was quantitative and fit-for-purpose validated, with an analytical run time of ∼20 min. The method demonstrated linearity from 1.8 to 184 µM (R>0.99) for both inosine and hypoxanthine, with a limit of detection at ∼0.7 µM. The simple sample preparation using MWCO filters yielded ∼98% recovery of these analytes, with 15 µL of the filtrate being injected for HPLC analysis. Nevertheless, HPLC technology cannot be utilized for a point-of-care analysis for several obvious reasons, such as high cost, large equipment size and a strict requirement of technical expertise for performing the sample measurements.
Figure 4.
Bar chart representing inosine (Ino) and hypoxanthine (Hypo) plasma concentrations from healthy individuals and ER non-traumatic chest pain patients. The ER patients had significantly elevated concentrations of inosine and hypoxanthine, as compared to the healthy normal individuals. As inosine is metabolized to hypoxanthine in the blood stream, the cumulative amounts (mole to mole) for these biomarkers in plasma are depicted in the bar chart as µM Ino + Hypo. (A color version of this figure is available in the online journal.)
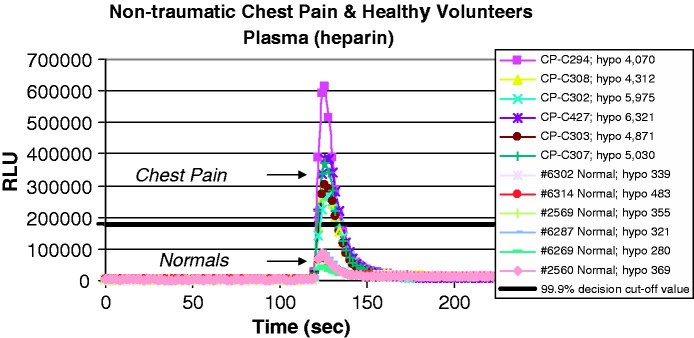
To further develop a cost-effective point-of-care technology for rapid measurement of plasma inosine and hypoxanthine, several important criteria have to be satisfied, including portable size, ease of use, speed of analysis, specificity, sensitivity and reproducibility. We have developed a chemiluminescence method using a microplate luminometer to address the issues of equipment size, ease of use, speed of analysis, specificity, sensitivity and reproducibility.30 Our simple method utilizes a small quantity of plasma and employs enzymes to convert inosine and hypoxanthine to their metabolic products. As these substrate-specific enzymes turn over, free radicals are generated and excite a sensitive luminescent material, which generates a quantifiable blue-green light. The light intensity is positively correlated with the concentrations of inosine and hypoxanthine presented in the plasma samples. Furthermore, the chemiluminescence method is rapid (∼30 sec), specific (utilizes enzymes), sensitive (∼10 µL plasma), and reproducible. As demonstrated in Figure 5, the chemiluminescence (RLU) lights generated by the chest pain patients were significantly higher than those generated by healthy subjects. The RLU peak maximum signal rapidly occurs within 5 s after the enzyme XO is injected into the sample.
Figure 5.
Overlay of chemiluminescence scans representing plasma analysis from healthy individuals and non-traumatic chest pain patients. Sample equilibrium time is 120 s, followed by the xanthine oxidase (enzyme) injection. Time to RLU peak maximum signal was ∼5 s after XO enzyme injection, with ∼30 s required for completion of the luminescence reaction. (A color version of this figure is available in the online journal.)
Comparative analysis of inosine and hypoxanthine with other current and potential biomarkers for acute cardiac ischemia: pros and cons
In order to provide a head-to-head comparison between inosine/hypoxanthine and other current or potential biomarkers for cardiac ischemia, in Table 1 we summarize the key characteristics, i.e. molecular weight (MW), detectable time, sensitivity, specificity, etc. The advantages versus disadvantages between each of the biomarkers are discussed in more detail in the following paragraphs.
Table 1.
Comparative summary of current and potential biomarkers for the detection of cardiac ischemia.
| Names of biomarker | Molecular weight (Daltons) | Cellular events detected | Detectable time after onset of ischemia | Sensitivity | Specificity | Detection methods | Representative references |
|---|---|---|---|---|---|---|---|
| Inosine | 268 | ATP breakdown | 15–20 min | High | Low | HPLC luminescence | Farthing et al.19,24,28 |
| Hypoxanthine | 136 | ATP breakdown | 15–20 min | High | Low | LC-MS/MS HPLC-UV luminescence | Kock et al.,27 Lewis et al.,33 Farthing et al.19,24,28 |
| Myoglobin | ∼17,500 | Myocyte necrosis | 1–4 h | High | Medium | ELISA fluorescence immunoassay | Nikulina et al. 199934 |
| CK-MB | ∼82,000, exist as dimer | Myocyte necrosis | 3–6 h | Medium | Medium | Fluorescence immunoassay | Zabel et al. 199335 |
| Cardiac Troponins (cTnI, cTnT) | cTnI ∼24,000; cTnT ∼34,000 | Myocyte necrosis | >2–4 h | High | High | Chemiluminescence immunoassay fluorescence immunoassay | Collinson et al. 200136 Chenevier-Gobeaux et al. 201137 |
| Albumin | ∼66,437 | Hypothesized free radical modification of plasma albumin | <3 h | High | Low | Colorimeter | Apple et al.,8 Christenson et al. 2001 |
| Choline | 104 | Hypothesized cleavage of membrane phospholipid phosphatidylcholine | <3 h | High | Low | LC-MS | Apple et al.,8 Danne et al.27 |
| Unbound free fatty acids (FFAu) | Variable, less than 500 | Hypothesized as being released from serum albumin | <3 h | High | Low | Fluorimeter | Apple et al.,8 Azzazy et al. 200638 |
HPLC: high-pressure liquid chromatography; LC-MS: liquid chromatography–mass spectrometry; ELISA: enzyme-linked immunosorbent assay.
Among the biomarkers listed in Table 1, several protein and peptide biomarkers are currently used to detect heart disease, including myoglobin, CK-MB, lactate dehydrogenase (LDH), and cardiac troponins (cTnI and cTnT) for indicating cardiac tissue necrosis, whereas B-type natriuretic peptide (BNP) and N-terminal pro-BNP are used as indicators of heart failure. These protein biomarkers can be used individually or in a panel to improve their diagnostic accuracy. In particular, the cardiac troponins have demonstrated higher specificity and sensitivity for indicating AMI, because they are structural components of the thin filament of myocardium, which are leaked from the necrotic cardiomyocytes into the bloodstream following a heart attack. A recently reported improvement to the cardiac troponin luminescence assay, termed high sensitivity cardiac troponin (hscTn), allows for earlier detection of the cardiac troponins in plasma. cTn and hscTn have become the gold standard biomarkers for AMI. However, these protein biomarkers (MW > 24000 Da) indicating AMI are typically detectable several hours after the cardiac ischemia event. Therefore, an unmet critical care need still exists for a biomarker to rapidly detect the early onset of acute cardiac ischemia, prior to AMI.
Another FDA-approved diagnostic approach (2003) is the ischemia-modified albumin (IMA) – a serum protein (MW ∼66,437 Da) that detects albumin in the bloodstream that has been modified by ischemia-generated free radicals (e.g. hydroxyl radicals) at the amino end terminus of the albumin moiety. The FDA clearance was based on the use of IMA in conjunction with two other diagnostic tests (e.g. electrocardiogram and cTn). This test utilizes an aqueous cobalt chloride solution and under conditions of acute cardiac ischemia, the cobalt cannot bind to the free radical-modified amino terminus of albumin and in turn affects the final color of the solution, which is measurable using a spectrophotometer. It is notable that several recent clinical studies have reported significant false positive results of the IMA test, specifically for patients having cancer, liver disease, infections, brain ischemia, and end-stage renal disease.8,31
In addition, several non-protein biomarkers are currently under evaluation for acute cardiac ischemia; however, none has been approved by the FDA for clinical use. These small molecule biomarkers include choline32 and unbound free fatty acids (FFAu).8 During cardiac ischemia, phospholipase D cleaves membrane phosphatidylcholine into phosphatidic acid and choline (MW 104 Da), which can be released into the bloodstream. Indeed, elevated whole blood concentrations of choline from patients undergoing acute cardiac ischemia were reported using LC-MS technology.32 As the chemical structure of choline can be defined as a small organic molecule with a charged quaternary ammonium group, it may be difficult to develop an antibody to bind to it for use in an immunoassay clinical test method. Hence, due to such methodological limitations, choline may not be suitable for use in a simple point-of-care test method. Another small molecule – free fatty acids (FFA; MW < 500 Da) – is found in the bloodstream bound to albumin, with a small amount of FFAs found in the unbound form (FFAu). Under conditions of cardiac ischemia, the concentrations of FFAu are found significantly elevated. The determination of FFAu concentrations consists of their binding to a protein labeled with a fluorescent tag and subsequent measurement using a fluorimeter. It was recommended that additional clinical evaluations should be conducted to further evaluate the potential of these small molecules as biomarkers of acute cardiac ischemia.8
To this context, inosine and hypoxanthine have some unique features as biomarkers for cardiac ischemia (see Table 1). Inosine and hypoxanthine are small stable organic substances typically found in human plasma at low concentrations (inosine 0.75 to 1.49 μM, hypoxanthine 1.47 to 2.94 μM), resulting from purine metabolism.29 In 1994, Kock et al. studied the role of xanthine oxidase in purine metabolism in patients with myocardial ischemia.27 They evaluated components such as hypoxanthine, xanthine, and uric acid, and reported insignificant differences in hypoxanthine concentrations between their healthy males and patients with AMI and other ischemic diseases.27 However, they did not measure inosine. An earlier study by Harmsen et al. (1981) reported elevated blood concentrations of hypoxanthine in the patients with documented ischemic heart disease undergoing atrial pacing stress testing as compared with healthy volunteers, whereas the concentrations of adenosine, inosine and xanthine did not significantly change.26 In 1989, another group also reported a rise in purine metabolic end-products in patients with angina and MI.25 They suggested that plasma concentrations of xanthine and uric acid were better indicators of the severity of cardiac ischemia than inosine and hypoxanthine concentrations. However, since both xanthine and uric acid can be increased by other medical conditions, such as xanthine oxidase deficiency (for xanthine) and gout (for uric acid), these two substances may produce false positive results in their use as biomarkers for acute cardiac ischemia.
A more recent metabolomic study applied a novel LC-MS/MS technique in blood samples from patients who underwent heart surgery.33 The metabolomics profile identified several small organic substances (e.g. threonine, aconitric acid, and, to our primary interest, hypoxanthine), which were significantly elevated as early as 10 minutes after beginning the surgical intervention (i.e. alcohol septal ablation for hypertrophic obstructive cardiomyopathy). This interesting small molecule metabolomics approach may represent a shift from the current paradigm of primarily focusing on the discovery of proteins as disease biomarkers. Our research group utilized blood samples acquired from non-traumatic chest pain patients admitted by a local hospital ER.28 These patients had chest pain with potential acute cardiac ischemia for several hours and we found elevated plasma concentrations of inosine and/or hypoxanthine in all of the samples.
It is noteworthy that there are also some potential sources of errors in the use of inosine and/or hypoxanthine as plasma biomarkers for cardiac ischemia. First, individuals born with enzyme deficiencies (e.g. adenosine deaminase or purine nucleoside phosphorylase) can cause erroneous results, either false negative or false positive. However, these individuals should have a medical history of immune system problems, which are due to these enzyme deficiencies. Similarly, the patients with xanthine oxidase deficiency or taking xanthine oxidase inhibitor – allopurinol for treatment of gout – may have elevated concentrations of hypoxanthine and/or xanthine in their blood, causing false positive results. In addition, kidney disease and renal failure are two medical conditions which may alter inosine and hypoxanthine test results. This is because the elimination of substances from blood is severely compromised in these patients and even small polar components such as inosine and hypoxanthine may be retained in the bloodstream and cause false positive results. Finally, several food sources may also cause false positive test results. Elevated concentrations of plasma inosine and hypoxanthine can result from high consumption of purine containing food sources, such as organ meats, spinach, and beer, or from the use of nutritional dietary supplements, e.g. inosine for enhancing athletic performance.
Summary and concluding perspectives
Myocardial ischemia associated with AMI is a leading cause of mortality and morbidity in the world and a rapid detection and diagnosis of cardiac ischemia are critically important for achieving timely treatment and improving clinical outcomes of patient survival and functional recovery. This minireview provides a brief overview in evaluating the past and current biomarker research for detection of acute cardiac ischemia, especially those biomarkers that may be translatable for use in a point-of-care environment outside of a hospital ER. We hereby propose that the consistent observance, in either animal laboratory or clinical settings, of elevated plasma concentrations of inosine and hypoxanthine, two well-known by-products of ATP catabolism (Figure 1), can be used as rapid and sensitive biomarkers for acute cardiac ischemia at an early stage (within 15 min of the ischemia onset), hours prior to the heart tissue necrosis. Inosine/hypoxanthine can also differentiate themselves from other endogenous small organic substances, such as choline and unbound free fatty acids, in which the exact mechanism for their elevated concentrations in the bloodstream remains poorly understood.
We should emphasize with caution that total inosine and hypoxanthine concentrations are found endogenously in the body from normal purine metabolism in virtually all cell types and organs, thus making the specificity of these two biomarker candidates of acute cardiac ischemia relatively low (Table 1), unlike those of highly specific cTn. However, this shortcoming of inosine/hypoxanthine in low cardiac specificity can be superseded by the extremely high sensitivity for detecting the early onset of cardiac ischemia. In particular, our published results using chemiluminescence technology, one of the most affordable and sensitive analytical techniques currently available, demonstrated a rapid, sensitive, and highly reproducible method for measuring plasma total hypoxanthine concentrations in healthy individuals versus cardiac patients with confirmed cTnT elevation.30 Therefore, our proposed candidate biomarkers (inosine/hypoxanthine) may follow the similar path as IMA biomarker, which also has low specificity and high sensitivity and was approved by FDA for the use in conjunction with other FDA-approved cardiac tests (e.g. ECG and cTn) to improve patient diagnostic accuracy for cardiac ischemia. Nevertheless, additional rigorous clinical studies are needed to monitor in serial the post-AMI plasma inosine and hypoxanthine concentrations, in conjunction with hscTn concentrations, for a better understanding of their tremendous diagnostic potential for early detection of acute cardiac ischemia, prior to heart tissue necrosis.
ACKNOWLEDGEMENTS
We acknowledge our colleagues for their substantial contributions and useful comments related to the original laboratory and clinical studies discussed in this review article, including Drs. L. Gehr, T. Gehr, D. Sica, H.T. Karnes, M. Hindle, L. Edinboro, J. Knight, and Ms. T. Larus, Ms. I. Fakhry, and Mr. B. Wilson. This review work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors participated in designing this review, in which each author wrote selected sections of the review, which were assembled by LX. The final manuscript was proof-read, edited, and approved by all authors.
Conflict of interest
DEF and LX are co-inventors of the United States patents #8,343,731 and #8,609,360, which are directly related to the topics of this minireview. DEF is owner of the Bioanalytical Services and Technologies, LLC (Rockville, MD).
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin AA, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De LD, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD, III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd HK, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA, III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van DR, van DA, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J 2004; 148: 7–15. [DOI] [PubMed] [Google Scholar]
- 4.Naudziunas A, Jankauskiene L, Kalinauskiene E, Pilvinis V. Implementation of the patient education about cardiovascular risk factors into a daily routine of the Cardiology Unit of the hospital. Prev Med 2005; 41: 570–4. [DOI] [PubMed] [Google Scholar]
- 5.McNally B, Robb R, Mehta M, Vellano K, Valderrama AL, Yoon PW, Sasson C, Crouch A, Perez AB, Merritt R, Kellermann A. Out-of-hospital cardiac arrest surveillance – Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005–December 31, 2010. MMWR Surveill Summ 2011; 60: 1–19. [PubMed] [Google Scholar]
- 6.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH, Apple FS, Francis G, Tang W. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem 2007; 53: 552–74. [DOI] [PubMed] [Google Scholar]
- 7.Chang AM, Shofer FS, Weiner MG, Synnestvedt MB, Litt HI, Baxt WG, Hollander JE. Actual financial comparison of four strategies to evaluate patients with potential acute coronary syndromes. Acad Emerg Med 2008; 15: 649–55. [DOI] [PubMed] [Google Scholar]
- 8.Apple FS, Wu AH, Mair J, Ravkilde J, Panteghini M, Tate J, Pagani F, Christenson RH, Mockel M, Danne O, Jaffe AS. Future biomarkers for detection of ischemia and risk stratification in acute coronary syndrome. Clin Chem 2005; 51: 810–24. [DOI] [PubMed] [Google Scholar]
- 9.Jennings RB, Hawkins HK, Lowe JE, Hill ML, Klotman S, Reimer KA. Relation between high energy phosphate and lethal injury in myocardial ischemia in the dog. Am J Pathol 1978; 92: 187–214. [PMC free article] [PubMed] [Google Scholar]
- 10.Jennings RB, Reimer KA, Hill ML, Mayer SE. Total ischemia in dog hearts, in vitro. 1. Comparison of high energy phosphate production, utilization, and depletion, and of adenine nucleotide catabolism in total ischemia in vitro vs. severe ischemia in vivo. Circ Res 1981; 49: 892–900. [DOI] [PubMed] [Google Scholar]
- 11.Molina-Arcas M, Casado FJ, Pastor-Anglada M. Nucleoside transporter proteins. Curr Vasc Pharmacol 2009; 7: 426–34. [DOI] [PubMed] [Google Scholar]
- 12.Berne RM. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol 1963; 204: 317–22. [DOI] [PubMed] [Google Scholar]
- 13.Backstrom T, Goiny M, Lockowandt U, Liska J, Franco-Cereceda A. Cardiac outflow of amino acids and purines during myocardial ischemia and reperfusion. J Appl Physiol 2003; 94: 1122–8. [DOI] [PubMed] [Google Scholar]
- 14.Ronca-Testoni S, Borghini F. Degradation of perfused adenine compounds up to uric acid in isolated rat heart. J Mol Cell Cardiol 1982; 14: 177–80. [DOI] [PubMed] [Google Scholar]
- 15.Cargnoni A, Ceconi C, Curello S, Benigno M, de Jong JW, Ferrari R. Relation between energy metabolism, glycolysis, noradrenaline release and duration of ischemia. Mol Cell Biochem 1996; 160–161: 187–94. [DOI] [PubMed] [Google Scholar]
- 16.de Jong JW, Achterberg PW. Developmental differences in myocardial ATP metabolism. Basic Res Cardiol 1987; 82: 121–6. [DOI] [PubMed] [Google Scholar]
- 17.Achterberg PW, Nieukoop AS, Schoutsen B, de Jong JW. Different ATP-catabolism in reperfused adult and newborn rat hearts. Am J Physiol 1988; 254: H1091–H1098. [DOI] [PubMed] [Google Scholar]
- 18.Mei DA, Gross GJ, Nithipatikom K. Simultaneous determination of adenosine, inosine, hypoxanthine, xanthine, and uric acid in microdialysis samples using microbore column high-performance liquid chromatography with a diode array detector. Anal Biochem 1996; 238: 34–9. [DOI] [PubMed] [Google Scholar]
- 19.Farthing D, Xi L, Gehr L, Sica D, Larus T, Karnes HT. High-performance liquid chromatography (HPLC) determination of inosine, a potential biomarker for initial cardiac ischaemia, using isolated mouse hearts. Biomarkers 2006; 11: 449–59. [DOI] [PubMed] [Google Scholar]
- 20.Xi L, Hess ML, Kukreja RC. Ischemic preconditioning in isolated perfused mouse heart: reduction in infarct size without improvement of post-ischemic ventricular function. Mol Cell Biochem 1998; 186: 69–77. [PubMed] [Google Scholar]
- 21.Xi L, Jarrett NC, Hess ML, Kukreja RC. Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation 1999; 99: 2157–63. [DOI] [PubMed] [Google Scholar]
- 22.Xi L, Jarrett NC, Hess ML, Kukreja RC. Myocardial ischemia/reperfusion injury in the inducible nitric oxide synthase knockout mice. Life Sci 1999; 65: 935–45. [DOI] [PubMed] [Google Scholar]
- 23.Xi L, Das A, Zhao ZQ, Merino VF, Bader M, Kukreja RC. Loss of myocardial ischemic postconditioning in adenosine A1 and bradykinin B2 receptors gene knockout mice. Circulation 2008; 118: S32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farthing D, Gehr L, Karnes HT, Sica D, Gehr T, Larus T, Farthing C, Xi L. Effects of salicylic acid on post-ischaemic ventricular function and purine efflux in isolated mouse hearts. Biomarkers 2007; 12: 623–34. [DOI] [PubMed] [Google Scholar]
- 25.Toguzov RT, Korochkin IM, Tikhonov I, Novikova TE, Pimenov AM. Metabolic pool of purine and pyrimidine compounds in the venous blood of patients with myocardial infarction and stenocardia. Kardiologiia 1989; 29: 57–60. [PubMed] [Google Scholar]
- 26.Harmsen E, de Jong JW, Serruys PW. Hypoxanthine production by ischemic heart demonstrated by high pressure liquid chromatography of blood purine nucleosides and oxypurines. Clin Chim Acta 1981; 115: 73–84. [DOI] [PubMed] [Google Scholar]
- 27.Kock R, Delvoux B, Sigmund M, Greiling H. A comparative study of the concentrations of hypoxanthine, xanthine, uric acid and allantoin in the peripheral blood of normals and patients with acute myocardial infarction and other ischaemic diseases. Eur J Clin Chem Clin Biochem 1994; 32: 837–42. [DOI] [PubMed] [Google Scholar]
- 28.Farthing D, Sica D, Gehr T, Wilson B, Fakhry I, Larus T, Farthing C, Karnes HT. An HPLC method for determination of inosine and hypoxanthine in human plasma from healthy volunteers and patients presenting with potential acute cardiac ischemia. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 854: 158–64. [DOI] [PubMed] [Google Scholar]
- 29.Feng JD, Yeung PK. A simple high-performance liquid chromatography assay for simultaneous measurement of adenosine, guanosine, and the oxypurine metabolites in plasma. Ther Drug Monit 2000; 22: 177–83. [DOI] [PubMed] [Google Scholar]
- 30.Farthing DE, Sica D, Hindle M, Edinboro L, Xi L, Gehr TW, Gehr L, Farthing CA, Larus TL, Fakhry I, Karnes HT. A rapid and simple chemiluminescence method for screening concentrations of inosine and hypoxanthine in non-traumatic chest pain patients. Luminescence 2011; 26: 65–75. [DOI] [PubMed] [Google Scholar]
- 31.Wu AH. The ischemia-modified albumin biomarker for myocardial ischemia. MLO Med Lab Obs 2003; 35: 36–8. 40. [PubMed] [Google Scholar]
- 32.Danne O, Lueders C, Storm C, Frei U, Mockel M. Whole blood choline and plasma choline in acute coronary syndromes: prognostic and pathophysiological implications. Clin Chim Acta 2007; 383: 103–9. [DOI] [PubMed] [Google Scholar]
- 33.Lewis GD, Wei R, Liu E, Yang E, Shi X, Martinovic M, Farrell L, Asnani A, Cyrille M, Ramanathan A, Shaham O, Berriz G, Lowry PA, Palacios IF, Tasan M, Roth FP, Min J, Baumgartner C, Keshishian H, Addona T, Mootha VK, Rosenzweig A, Carr SA, Fifer MA, Sabatine MS, Gerszten RE. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Invest 2008; 118: 3503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikulina VA, Kizim EA, Massino I, Segal OL, Smirnova MB, Avilov VV, Saprygin DB, Smotrov SP, Koliaskina GI, Dmitriev AD. One-stage solid phase immunoenzyme sandwich technique for the determination of myoglobin in serum using three types of monoclonal antibodies to various epitopes. Biull Eksp Biol Med 1999; 127: 597–600. [PubMed] [Google Scholar]
- 35.Zabel M, Hohnloser SH, Koster W, Prinz M, Kasper W, Just H. Analysis of creatine kinase, CK-MB, myoglobin, and troponin T time-activity curves for early assessment of coronary artery reperfusion after intravenous thrombolysis. Circulation 1993; 87: 1542–50. [DOI] [PubMed] [Google Scholar]
- 36.Collinson PO, Boa FG, Gaze DC. Measurement of cardiac troponins. Ann Clin Biochem 2001; 38: 423–49. [DOI] [PubMed] [Google Scholar]
- 37.Chenevier-Gobeaux C, Meune C, Blanc MC, Cynober L, Jaffray P, Lefevre G. Analytical evaluation of a high-sensitivity troponin T assay and its clinical assessment in acute coronary syndrome. Ann Clin Biochem 2011; 48: 452–8. [DOI] [PubMed] [Google Scholar]
- 38.Azzazy HM, Pelsers MM, Christenson RH. Unbound free fatty acids and heart-type fatty acid-binding protein: diagnostic assays and clinical applications. Clin Chem 2006; 52: 19–29. [DOI] [PubMed] [Google Scholar]