Abstract
Breast cancer is one of the most common malignancies, often with complicated etiology and poor clinical outcome. In recent years, a critical role has emerged for the WW domain-containing oxidoreductase (WWOX) in breast cancer. WWOX is a tumor suppressor; it is deleted or attenuated in 29–63.2% of breast cancer tissues and is associated with a poor prognosis of breast cancer patients. WWOX heterozygous knockout mice show a higher incidence of mammary tumors and impaired branching morphogenesis. At the molecular level, WWOX interacts with AP2γ, ErbB4, SMAD3, and WBP2 suppressing their transcription activities in breast cancer cell lines. This review provides comprehensive insights into the current knowledge of WWOX activities in the pathogenesis and endocrine therapy of breast cancer.
Keywords: WW domain-containing oxidoreductase, breast cancer, tumorigenesis, endocrine therapy
Introduction
WW domain-containing oxidoreductase (WWOX) is cloned and mapped to the chromosome region 16q23. The Wwox gene spans a genomic locus of more than 1 Mb but encodes an open reading frame of 1.2 kb. The Wwox gene overlaps the second chromosomal fragile site FRA16D, which exhibits an increased incidence of gaps or breaks when exposed to extrinsic or intrinsic factors such as UV radiation, ionizing radiation, and hypoxia.1–4
The full-length WWOX is a 414-amino acid protein possessing two typical N-terminal WW domains and a short-chain dehydrogenase reductase (SDR) domain. The WW domain is needed for the classical WW–PPxY interaction. WWOX interacts with the proteins that possess PPxY motifs, including ErbB4, AP-2γ, Run2, p73, and C-Jun. WWOX sequesters these proteins in the cytoplasm and suppresses their transcriptional functions.5–9 Proteins with the SDR domain are involved in oxidation and reduction of various substrates, such as lipid hormones, sugars, alcohols, and retinoids.10 The SDR domain-containing protein WWOX is found to be highly expressed in hormone-dependent tissues, such as the mammary gland, prostate, and ovary. Thus, WWOX may be involved in steroid metabolism.11,12
The subcellular localization and function of WWOX are regulated by its phosphorylation. Tyr33 phosphorylation of the WWOX protein can be activated by steroid hormone 17β-estradiol (E2) independent of the estrogen receptor (ER).13 Tyr33 phosphorylation of WWOX enhances the recognition of the PPxY motif and promotes the WW–PPxY interaction.5,6 WWOX polyubiquitination and degradation are associated with its phosphorylation at Tyr287 by CDC42-associated tyrosine kinase 1 (ACK1).14
WWOX is an important tumor suppressor and loss or deregulation of WWOX contributes to development of various tumors. Numerous studies have been conducted on the roles of WWOX in bone homeostasis and osteosarcoma and their findings have been reviewed previously.9,15,16 In this minireview, we focus on the roles of WWOX in the pathogenesis and endocrine therapy of breast cancer.
WWOX is involved in mammary gland development and breast tumorigenesis
WWOX has been found to be highly expressed in hormone-dependent tissues, such as the mammary gland, prostate, and ovary.12 Aldaz et al. conditionally knocked out the Wwox gene in the mouse mammary epithelium utilizing two transgenic lines expressing Cre recombinase, BK5-Cre, and MMTV-Cre. They found that Wwox knockout (KO) mice showed a significant impairment in mammary gland branching morphogenesis, with rudimentary mammary epithelium and little or no evidence of ductal structures.17 These results indicate that WWOX may be involved in mammary gland development.
The Wwox KO mouse is a useful tool to study the roles of WWOX in vivo. The single and double allelic targeted ablations result in two different phenotypes of Wwox KO mice. It has been found that Wwox−/− mice suffer metabolic disorders and eventually postnatal lethality, while Wwox+/− mice show higher incidence of mammary and lung tumors than wild-type mice. When Wwox+/− mice are given a single dose of ethyl-nitrosourea (a powerful chemical mutagen), 80% of Wwox+/− mice develop mammary, lymphoblastic, or lung tumors.12,18 Molecular analysis of global gene expression in Wwox ablated mammary epithelium has revealed that Wwox deletion results in the up-regulation of cancer-related genes, such as the Wnt signaling pathway genes, the tissue remodeling related genes, and the cell migration- and adhesion-related genes.17 These results suggest that WWOX may be involved in breast tumorigenesis.
Taken together, WWOX is necessary for mammary gland development and loss of WWOX contributes to breast tumorigenesis.
WWOX expression is associated with breast cancer progression and prognosis
Loss or reduction of WWOX expression is associated with breast cancer. Immunohistochemical staining shows strong WWOX expression in the normal human breast tissue.19 WWOX expression is reduced in 55–63.2% of breast cancer tissues and lost in 29% of breast cancer tissues.20–22 However, breast tumor tissues have increased expressions of short WWOX variants, when compared to normal breast tissues. The FORIII transcript, which lacks the majority of the WWOX SDR domain, is expressed in 50% of the breast tumors and 90% of the breast cancer cell lines.23 These results suggest that the WWOX gene, especially the SDR domain of WWOX, may be involved in breast cancer progression. The above studies show that the SDR domain may play a crucial role in the function of WWOX proteins as a breast tumor suppressor.
Further analysis shows that the WWOX expression level is highly positively related with hormone receptor status, but negatively correlated with clinical stages of breast cancer. ER or progestogen receptor (PR) positive cancer has a higher level of WWOX expression than ER or PR negative cancer.24 Stage III breast cancer has higher WWOX expression than stage III breast cancer.22 Ovarian cancer, another hormone-dependent tumor, is associated with the expression of WWOX in the same manner as breast cancer. Normal ovarian tissue samples show consistently strong WWOX expressions while 37% ovarian carcinomas show reduced or undetectable WWOX protein expression levels. Reduced WWOX expressions have been found to be significantly associated with clinical stage IV, negative PR status, and negative ER in ovarian cancer tissues.25,26 The studies on breast and ovarian cancers indicate that reduced or lost WWOX expression may be involved in the progression of hormone-dependent tumors.
As shown in Table 1, reduced WWOX expression has been found to be associated with basal-like, triple-negative, and invasive breast cancer subtypes.27–29 Basal-like and triple-negative subtypes are associated with high local recurrence rates, distant metastases, lack of effective targeted therapies, and poor disease-free survival in young women. Distant metastases occur in 28% of triple-negative breast cancers within five and 10 years and the most characteristic sites of metastases include the brain and lungs. Ninety percent of metaplastic breast carcinomas, as well as the majority of medullary carcinomas, consistently show a basal-like phenotype.30–32 These results indicate that reduced WWOX expression is associated with a poor prognosis of breast cancer patients.
Table 1.
Dysregulation of WWOX expression in breast cancer tissues
| Tissues | Technique | Frequency | Ref |
|---|---|---|---|
| Breast cancer | Immunohistochemistry | 29% (negative) | 22 |
| Breast cancer | Immunohistochemistry | 63.2% (reduced) | 28 |
| Breast cancer | RT-PCR | 55% (reduced) | 33 |
| Ductal carcinoma in situ | Immunohistochemistry | 68% (reduced) | 20 |
| Invasive breast cancer | Immunohistochemistry | 77.1% (negative or reduced) | 29 |
| Triple negative breast cancer | Immunohistochemistry | 90% (negative or reduced) | 29 |
WWOX is associated with cancer metastasis and invasion. WWOX protein expression is lost or reduced in nearly 100% of metastatic breast cancer tissues.28 Reduced protein level of WWOX increases migration of cancer cells through the basal membrane. Integrin α3 is a transmembrane receptor that mediates the attachment between cells and extracellular matrix (ECM) and plays a vital role in migration and tumor invasion. Loss of WWOX expression induces the membranous integrin α3 protein expression and modulates the interaction between cancer cells and ECM, resulting in migration of cancer cells through the basal membrane.34
Taken together, WWOX is lost or reduced in breast cancer tissues, and the expression of WWOX is highly positively related with hormone receptor status, but negatively correlated with clinical stages of breast cancer. Loss or reduction of WWOX expression has significant correlation with breast cancer progression and prognosis.
Molecular function of WWOX in breast cancer
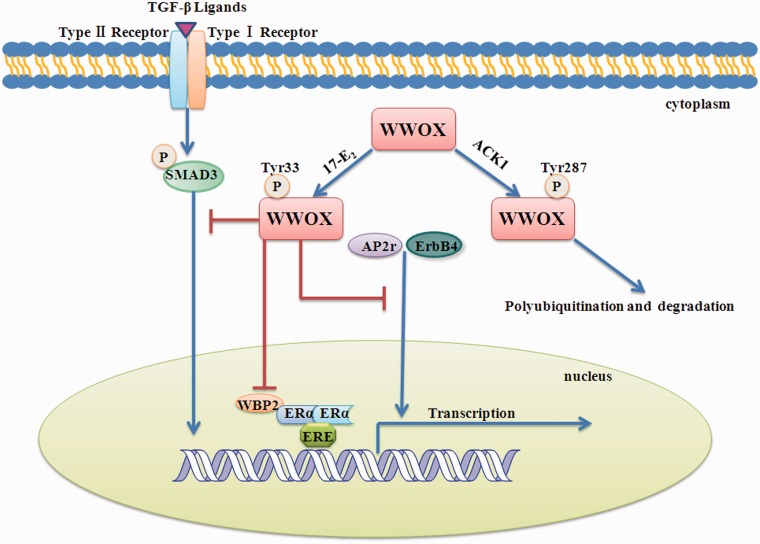
Decreased WWOX expression in breast cancer suggests that loss of WWOX may contribute to the pathogenesis of breast cancer. WWOX has been shown to partner with several proteins involved in the pathogenesis of breast cancer, including activator protein 2γ (AP2γ), erythroblastic leukemia viral oncogene homolog 4 (ErbB4), and mothers against DPP homolog 3 (SMAD3). Figure 1 shows the regulatory effects of WWOX on proteins involved in the pathogenesis of breast cancer. AP2γ is an essential regulator of breast cancer-related genes in vitro and associates with poor prognosis of breast cancer.35,36 Transiently overexpressed WWOX physically interacts with the PPPY motif of AP2γ via the first WW domain and inhibits the oncogenic activity of AP2γ by sequestering AP2γ in the cytoplasm.6 ErbB4 plays an important role in cellular proliferation and differentiation and is involved in the pathogenesis and progression of breast cancer.37 Recent results have suggested that ectopic WWOX is associated with the expression of ErbB4 and inhibits the transactivation function of ErbB4.5 SMAD3 is a transcription factor directly phosphorylated and activated by TGF-β type 1 receptor kinase. After phosphorylation, SMAD3 is translocated into the nucleus, where it induces the transcription of cell cycle inhibitors such as CDKN2B (p15) and CDKN1A (p21).38 Transiently overexpressed WWOX can inhibit TGF-β signaling by interacting with SMAD3 and sequestering it in the cytoplasm of breast cancer cells.39 Thus, WWOX can suppress the pathogenesis of breast cancer through down-regulation of some transcription factors. However, TGF-β1 induces re-location of endogenous WOX1 to the nuclei by binding to cell surface hyaluronidase Hyal-2 in murine L929 fibroblasts. The Hyal-2·WOX1 complexes relocate in the nuclei and further recruits Smad4, enhancing the Smad promoter activity.40
Figure 1.
Regulatory mechanisms of WWOX in breast cancer. WWOX binds PPxY domain-containing proteins and sequesters them in the cytoplasm, suppressing their transcriptional functions, including ErbB4, AP-2γ, SMAD3, and WBP2. Tyr33 phosphorylation of WWOX promotes the WW–PPxY interaction. Tyr287 phosphorylation process is associated with WWOX polyubiquitination and degradation. (A color version of this figure is available in the online journal.)
As is mentioned earlier, ectopic WWOX suppresses the transcriptional functions of AP2γ, ErbB4, and SMAD3 by sequestering them in the cytoplasm of cultured cell lines. However, endogenous WWOX suppresses the transcriptional functions of CREB by binding to CREB in rats. Thus, multiple modes may be involved in WWOX regulation of transcription factors. Sequestering transcription factors in the cytoplasm may be specific for WWOX regulation of breast cancer-related transcription factors. Further studies are needed to prove this assumption.
Loss of WWOX correlates with resistance to endocrine therapy for breast cancer
Endocrine therapy for breast cancer has been used for more than a century. Endocrine therapy inhibits the accelerating effect of ER signaling pathway on cell proliferation by suppressing the estrogen production and restraining the ER activity.41,42 Tamoxifen is one of the most widely used endocrine therapy drugs for breast cancer. It functions as a selective estrogen-receptor modulator by blocking estrogen from binding to its receptor in breast cancer cells. Tamoxifen reduces the risk of recurrence of early stage breast cancers in premenopausal and postmenopausal women.43,44 However, five years of tamoxifen is presently established as the standard duration for endocrine therapy for breast cancer. Due to the development of drug resistance, prolongation of the therapy cannot enhance the treatment efficacy. Thus, it is important to investigate the mechanism underlying tamoxifen resistance in breast cancer.
Gothlin et al. first discovered the relationship between WWOX expression and tamoxifen treatment. They examined 912 paraffin-embedded breast cancer tissues by immunohistochemistry. The results indicated that high expression of WWOX was associated with better outcome of tamoxifen treatment.45 In breast cancer cell lines, loss of WWOX expression induces the release of Ap2γ from the cytoplasma to the nucleus and the up-regulation of human epidermal growth factor receptor 2 (Her2). Increased Her2 promotes the growth of cancer cells against endocrine therapy for breast cancer and leads to tamoxifen resistance.6,46,47 The WW domain binding protein-2 (WBP2), a co-activator of ERα, may be another key regulatory protein for tamoxifen resistance. WBP2 can activate the ER transactivation pathway and promote cell proliferation. WWOX physically interacts with WBP2 and suppresses ER transactivation pathways48,49 (Figure 1). Thus, loss of WWOX expression can result in tamoxifen resistance by up-regulating ER and Her2 transcriptional activities.
Conclusion and future directions
Fragile site gene Wwox has a critical role in the pathogenesis and endocrine therapy of breast cancer. Wwox is consistently lost or reduced in the majority of breast cancers, and Wwox KO mice show rudimentary mammary epithelium, defects in ductal structure, and breast cancer.17,22,24 Loss of WWOX expression leads to tumorigenesis, cancer progression, and resistance to endocrine therapy for breast cancer. WWOX may affect tumorigenesis and drug resistance by inhibiting activities of some transcription factors or transcription activators, including ErbB4, AP-2γ, SMAD3, and WBP2. Reduced WWOX expression is also significantly correlated with clinical stages and poor survival. Therefore, WWOX can be used as a biological marker for progression and prognosis of breast cancer.
The function of WWOX SDR domain is still obscure. Is the dehydrogenase/reductase activity of WWOX associated with tumorigenesis? Is WWOX SDR domain related to the functions of hormone receptors and endocrine therapy resistance? Exploration of the functions of WWOX SDR domain may provide more clues to the roles of WWOX in progression of breast cancer and resistance to endocrine therapy.
ACKNOWLEDGEMENT
This work was supported by the Science and Technology Development Foundation of Shaanxi Province (No. 2014K11-01-01-17).
Authors’ contributions
All authors participated in the writing, review, and editing of this manuscript.
References
- 1.Dillon LW, Burrow AA, Wang YH. DNA instability at chromosomal fragile sites in cancer. Curr Genomics 2010; 11: 326–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lukusa T, Fryns JP. Human chromosome fragility. Biochim Biophys Acta 2008; 1779: 3–16. [DOI] [PubMed] [Google Scholar]
- 3.Durkin SG, Glover TW. Chromosome fragile sites. Annu Rev Genet 2007; 41: 169–92. [DOI] [PubMed] [Google Scholar]
- 4.Popescu NC. Genetic alterations in cancer as a result of breakage at fragile sites. Cancer Lett 2003; 192: 1–17. [DOI] [PubMed] [Google Scholar]
- 5.Aqeilan RI, Donati V, Gaudio E, Nicoloso MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H, Croce CM, Elenius K. Association of Wwox with ErbB4 in breast cancer. Cancer Res 2007; 67: 9330–6. [DOI] [PubMed] [Google Scholar]
- 6.Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2gamma transcription factor. Cancer Res 2004; 64: 8256–61. [DOI] [PubMed] [Google Scholar]
- 7.Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G, Huebner K, Croce CM. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci USA 2004; 101: 4401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM, Aqeilan RI. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res 2006; 66: 11585–9. [DOI] [PubMed] [Google Scholar]
- 9.Kurek KC, Del Mare S, Salah Z, Abdeen S, Sadiq H, Lee SH, Gaudio E, Zanesi N, Jones KB, DeYoung B, Amir G, Gebhardt M, Warman M, Stein GS, Stein JL, Lian JB, Aqeilan RI. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res 2010; 70: 5577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 2007; 13: 12–22. [DOI] [PubMed] [Google Scholar]
- 11.Aqeilan RI, Hagan JP, de Bruin A, Rawahneh M, Salah Z, Gaudio E, Siddiqui H, Volinia S, Alder H, Lian JB, Stein GS, Croce CM. Targeted ablation of the WW domain-containing oxidoreductase tumor suppressor leads to impaired steroidogenesis. Endocrinology 2009; 150: 1530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y, Hagan JP, Zanesi N, Kaou M, Stein GS, Lian JB, Croce CM. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci USA 2007; 104: 3949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang NS, Schultz L, Hsu LJ, Lewis J, Su M, Sze CI. 17beta-Estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene 2005; 24: 714–23. [DOI] [PubMed] [Google Scholar]
- 14.Mahajan NP, Whang YE, Mohler JL, Earp HS. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res 2005; 65: 10514–23. [DOI] [PubMed] [Google Scholar]
- 15.Aqeilan RI, Hassan MQ, de Bruin A, Hagan JP, Volinia S, Palumbo T, Hussain S, Lee SH, Gaur T, Stein GS, Lian JB, Croce CM. The WWOX tumor suppressor is essential for postnatal survival and normal bone metabolism. J Biol Chem 2008; 283: 21629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res 2002; 8: 2085–90. [PubMed] [Google Scholar]
- 17.Ludes-Meyers JH, Kil H, Parker-Thornburg J, Kusewitt DF, Bedford MT, Aldaz CM. Generation and characterization of mice carrying a conditional allele of the Wwox tumor suppressor gene. PLoS One 2009; 4: e7775–e7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdeen SK, Del Mare S, Hussain S, Abu-Remaileh M, Salah Z, Hagan J, Rawahneh M, Pu XA, Russell S, Stein JL, Stein GS, Lian JB, Aqeilan RI. Conditional inactivation of the mouse Wwox tumor suppressor gene recapitulates the null phenotype. J Cell Physiol 2013; 228: 1377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunez MI, Ludes-Meyers J, Aldaz CM. WWOX protein expression in normal human tissues. J Mol Histol 2006; 37: 115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, McCue P, Huebner K. Concordant loss of fragile gene expression early in breast cancer development. Pathol Int 2005; 55: 471–8. [DOI] [PubMed] [Google Scholar]
- 21.Ekizoglu S, Muslumanoglu M, Dalay N, Buyru N. Genetic alterations of the WWOX gene in breast cancer. Med Oncol 2012; 29: 1529–35. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Chao L, Ma G, Chen L, Zang Y, Sun J. The prognostic significance of WWOX expression in patients with breast cancer and its association with the basal-like phenotype. J Cancer Res Clin Oncol 2011; 137: 271–8. [DOI] [PubMed] [Google Scholar]
- 23.Driouch K, Prydz H, Monese R, Johansen H, Lidereau R, Frengen E. Alternative transcripts of the candidate tumor suppressor gene, WWOX, are expressed at high levels in human breast tumors. Oncogene 2002; 21: 8–8. [DOI] [PubMed] [Google Scholar]
- 24.Pluciennik E, Kusinska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX—the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur J Surg Oncol 2006; 32: 153–7. [DOI] [PubMed] [Google Scholar]
- 25.Nunez MI, Rosen DG, Ludes-Meyers JH, Abba MC, Kil H, Page R, Klein-Szanto AJ, Godwin AK, Liu J, Mills GB, Aldaz CM. WWOX protein expression varies among ovarian carcinoma histotypes and correlates with less favorable outcome. BMC Cancer 2005; 5: 64–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lan C, Chenggang W, Yulan B, Xiaohui D, Junhui Z, Xiao W. Aberrant expression of WWOX protein in epithelial ovarian cancer: a clinicopathologic and immunohistochemical study. Int J Gynecol Pathol 2012; 31: 125–32. [DOI] [PubMed] [Google Scholar]
- 27.Guler G, Himmetoglu C, Jimenez RE, Geyer SM, Wang WP, Costinean S, Pilarski RT, Morrison C, Suren D, Liu J, Chen J, Kamal J, Shapiro CL, Huebner K. Aberrant expression of DNA damage response proteins is associated with breast cancer subtype and clinical features. Breast Cancer Res Treat 2011; 129: 421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, McCue P, Huebner K. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer 2004; 100: 1605–14. [DOI] [PubMed] [Google Scholar]
- 29.Guler G, Huebner K, Himmetoglu C, Jimenez RE, Costinean S, Volinia S, Pilarski RT, Hayran M, Shapiro CL. Fragile histidine triad protein, WW domain-containing oxidoreductase protein Wwox, and activator protein 2gamma expression levels correlate with basal phenotype in breast cancer. Cancer 2009; 115: 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology 2008; 52: 108–18. [DOI] [PubMed] [Google Scholar]
- 31.Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer—current status and future directions. Ann Oncol 2009; 20: 1913–27. [DOI] [PubMed] [Google Scholar]
- 32.Krishnamurthy S, Poornima R, Challa VR, Goud YG. Triple negative breast cancer—our experience and review. Indian J Surg Oncol 2012; 3: 12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Driouch K, Prydz H, Monese R, Johansen H, Lidereau R, Frengen E. Alternative transcripts of the candidate tumor suppressor gene, WWOX, are expressed at high levels in human breast tumors. Oncogene 2002; 21: 1832–40. [DOI] [PubMed] [Google Scholar]
- 34.Gourley C, Paige AJ, Taylor KJ, Ward C, Kuske B, Zhang J, Sun M, Janczar S, Harrison DJ, Muir M, Smyth JF, Gabra H. WWOX gene expression abolishes ovarian cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin alpha3. Cancer Res 2009; 69: 4835–42. [DOI] [PubMed] [Google Scholar]
- 35.Bosher JM, Totty NF, Hsuan JJ, Williams T, Hurst HC. A family of AP-2 proteins regulates c-erbB-2 expression in mammary carcinoma. Oncogene 1996; 13: 1701–7. [PubMed] [Google Scholar]
- 36.deConinck EC, McPherson LA, Weigel RJ. Transcriptional regulation of estrogen receptor in breast carcinomas. Mol Cell Biol 1995; 15: 2191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundvall M, Iljin K, Kilpinen S, Sara H, Kallioniemi OP, Elenius K. Role of ErbB4 in breast cancer. J Mammary Gland Biol Neoplasia 2008; 13: 259–68. [DOI] [PubMed] [Google Scholar]
- 38.Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med 2003; 13: 301–7. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson BW, Gao X, Zelazowski MJ, Lee J, Jeter CR, Abba MC, Aldaz CM. The cancer gene WWOX behaves as an inhibitor of SMAD3 transcriptional activity via direct binding. BMC Cancer 2013; 13: 12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu LJ, Schultz L, Hong Q, Van Moer K, Heath J, Li MY, Lai FJ, Lin SR, Lee MH, Lo CP, Lin YS, Chen ST, Chang NS. Transforming growth factor beta1 signaling via interaction with cell surface Hyal-2 and recruitment of WWOX/WOX1. J Biol Chem 2009; 284: 16049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lumachi F, Luisetto G, Basso SM, Basso U, Brunello A, Camozzi V. Endocrine therapy of breast cancer. Curr Med Chem 2011; 18: 513–22. [DOI] [PubMed] [Google Scholar]
- 42.Orlandoa L, Schiavonea P, Fedelea P, Calvania N, Naccia A, Rizzoa P, Marinoa A, D’Amicoa M, Sponzielloa F, Mazzoni E, Cinefraa M, Faziob N, Maielloc E, Silvestrisd N, Coluccid G, Cinieria S. Molecularly targeted endocrine therapies for breast cancer. Cancer Treat Rev 2010; 36S3: 64–71. [DOI] [PubMed] [Google Scholar]
- 43.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998; 351: 1451–67. [PubMed] [Google Scholar]
- 44.Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, Greene GL. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 1998; 95: 927–37. [DOI] [PubMed] [Google Scholar]
- 45.Gothlin Eremo A, Wegman P, Stal O, Nordenskjold B, Fornander T, Wingren S. Wwox expression may predict benefit from adjuvant tamoxifen in randomized breast cancer patients. Oncol Rep 2013; 29: 1467–74. [DOI] [PubMed] [Google Scholar]
- 46.Guler G, Iliopoulos D, Guler N, Himmetoglu C, Hayran M, Huebner K. Wwox and Ap2gamma expression levels predict tamoxifen response. Clin Cancer Res 2007; 13: 6115–21. [DOI] [PubMed] [Google Scholar]
- 47.Salah Z, Aqeilan R, Huebner K. WWOX gene and gene product: tumor suppression through specific protein interactions. Future Oncol 2010; 6: 249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McDonald CB, Buffa L, Bar-Mag T, Salah Z, Bhat V, Mikles DC, Deegan BJ, Seldeen KL, Malhotra A, Sudol M, Aqeilan RI, Nawaz Z, Farooq A. Biophysical basis of the binding of WWOX tumor suppressor to WBP1 and WBP2 adaptors. J Mol Biol 2012; 422: 58–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhananjayan SC, Ramamoorthy S, Khan OY, Ismail A, Sun J, Slingerland J, O’Malley BW, Nawaz Z. WW domain binding protein-2, an E6-associated protein interacting protein, acts as a coactivator of estrogen and progesterone receptors. Mol Endocrinol 2006; 20: 2343–54. [DOI] [PubMed] [Google Scholar]