Abstract
The WW domain-containing oxidoreductase (WWOX) encodes a tumor suppressor that is frequently altered in cancer. WWOX binds several proteins and thus is postulated to be involved in a variety of cellular processes. Interestingly, Wwox-knockout mice develop normally in utero but succumb to hypoglycemia and other metabolic defects early in life resulting in their death by 3–4 weeks of age. Cumulative evidence has linked WWOX with cellular metabolism including steroid metabolism, high-density lipoprotein cholesterol (HDL-C) metabolism, bone metabolism and, more recently, glucose metabolism. In this review, we discuss these evolving functions for WWOX and how its deletion affects cellular metabolism and neoplastic progression.
Keywords: WW domain-containing oxidoreductase, hypoxia-inducible transcription factor 1, tumor suppressor, metabolism, aerobic glycolysis
Introduction
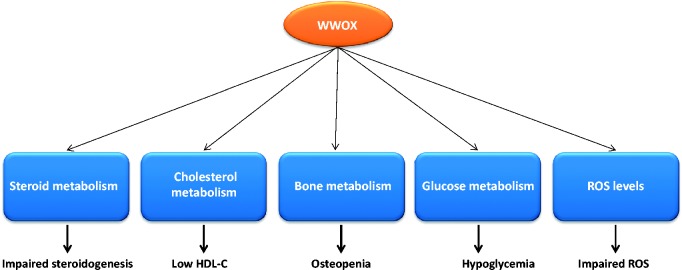
Cancer cells display several hallmarks and features that make them tumorigenic.1 These cells exhibit not only uncontrolled cell proliferation but also adjust their metabolism to ensure enough energy to maintain cell growth and division.2 This metabolic change is obligatory to support the anabolic demands accompanying cell growth and proliferation and is usually controlled by many tumor suppressors and oncogenes. Recently, the tumor suppressor WW domain-containing oxidoreductase (WWOX) was reported to modulate lipids, steroids and glucose metabolism (Figure 1), emerging and novel functions that WWOX controls thus contributing to the neoplastic process.
Figure 1.
WWOX modulates cellular metabolism. Using animal models and cell culture it was demonstrated that WWOX alteration is associated with impaired steroidogenesis, low HDL-C levels, osteopenia and impaired osteoblast differentiation, hypoglycemia, and impaired ROS levels. (A color version of this figure is available in the online journal.)
WWOX spans a large genomic region, FRA16D, which is considered an active chromosomal fragile site that is involved in cancer. WWOX encodes a relatively small protein3 that comprises two WW domains, known to facilitate protein–protein interaction,4 and a short-chain dehydrogenase/reductase (SDR) domain, which is believed to play a role in oxidation–reduction reactions involving steroids. WWOX is ubiquitously expressed, but its expression is most prominent in hormonally-regulated tissues and secretory epithelial cells such as those of testes, prostate, ovary, and breast.5,6 Altered WWOX expression has been reported in a variety of malignancies and has been widely associated with aggressive course of those diseases.7,8 Characterization of animal models with targeted Wwox gene knockout revealed important roles of WWOX in homeostasis and tumorigenesis. At birth, Wwox-null pups appear normal however these pups display growth retardation9 and postnatal lethality by 3–4 weeks due to severe metabolic defects.10
Better understanding of WWOX function came from protein–protein interaction studies that uncovered WWOX partners and shed light on its requirement for signaling pathways in cancer cells. WWOX utilizes its first WW (WW1) domain to physically interact with numerous proteins containing the canonical PPxY or non-canonical LPxY motifs.11,12 Other proteins were also reported to interact via the SDR domain.13 Several lines of evidence have shown that WWOX functions as an adapter protein that regulates localization of transcription factors in different cellular compartments. Generally, WWOX sequester transcription factors in the cytoplasm hence regulating their transactivation function.11,14,15 Nevertheless, subtle amount of WWOX was also detected in the nucleus regulating transactivation function of, for example, RUNX2.10 Recent proteomic studies utilizing mass spectrometry have suggested that WWOX participates in several signaling pathways involved in mRNA processing, adhesion, and metabolism.12 In this review, we discuss the recent evidence that links WWOX with cell metabolism and how does it contribute to its tumor suppressor function.
Wwox-knockout mice display severe metabolic disorders
Trying to model WWOX loss in human cancer (reviewed in Gardenswartz and Aqeilan7), mouse models of Wwox deletion have been generated. In 2007, we reported the generation of the first Wwox-knockout mice.9 These mice are born with no obvious malformations, however by age of one week, impaired ratio of organ to body mass in several tissues including brain and spleen was observed.10 Prior to their death, Wwox-knockout mice became weak and display signs of wasting; likely due to severe metabolic defects. This included low levels of glucose (hypoglycemia) and impaired levels of lipids, including hypotriglyceremia and hypocholesteremia.10 These mice also display severe bone metabolic phenotypes and impaired steroidogenesis (Figure 1). Furthermore, mutant Wwox mice have increased incidence of tumor formation.9,16–18 Due to early lethality, we and others have set to generate a conditional knockout (cKO) mouse model, using the cre-loxp technology, which could be studied at adult stages. We recently reported the generation of cKO mice and showed that using a general deleter of transgenic mouse (EIIA-cre) results in similar phenotypes resembling those of Wwox conventional knockout mice.16 Another mouse model for the Wwox gene was also generated by the Aldaz group and demonstrated similar phenotypes.19 These models shall be instrumental in future research to dissect the molecular function of WWOX in regulating cellular metabolism.
WWOX and steroid metabolism
The WWOX protein with a classical SDR domain has been predicted to be associated with oxidation and reduction of steroids. Meanwhile, the generation of the Wwox-knockout mice suggested possible functions of WWOX in steroid metabolism.20 As mentioned above, analysis of WWOX expression pattern in mouse tissues reveals its predominant expression in hormonally-regulated tissues thus a possible physiological role of WWOX in the physiology of these organs has been postulated. Indeed, WWOX-mammary-specific cKO mice, using MMTV-cre and K5-cre transgenic lines, displayed impaired mammary branching morphogenesis.21,22 Of note, WWOX loss is also associated with reduced estrogen receptor (ER) expression and ER-negativity in breast cancer patients.23–25 Importantly, WWOX expression is induced at three weeks of mammary gland development21 suggesting that WWOX and ER might be tightly regulated during mammary gland development and likely in ER-dependent cancers and suggesting a functional link between WWOX and ER, a major steroid hormone receptor.
Our observations also demonstrate that Wwox-knockout mice display defects in the mouse reproductive system including hypogonadism and impaired levels of steroids and expression of steroidogenic enzymes.20 Thus, WWOX is essential for gonadal development and function. Moreover, transcript levels of both the follicle-stimulating hormone and luteinizing hormone are attenuated in pituitary gland of Wwox-knockout mice.20 Similarly, testes from Wwox hypomorphic males have a high numbers of atrophic seminiferous tubules and reduced fertility when compared with their wild-type counterparts.18 Since WWOX contains an SDR domain, it is speculated that WWOX might indeed play a critical role in steroid metabolism. Indeed, a recent report by Bednarek and co-workers has suggested that the SDR domain of WWOX has dehydrogenase activity implying a possible function in steroid metabolism.26 This activity was observed in crude bacterial extracts using WWOX recombinant protein and thus further physiological validation is required. To date, no authenticated substrates of WWOX’s SDR domain are known so far therefore further delineation of WWOX oxidoreductase activity is required. Overall, cumulative evidence links WWOX with steroidogenesis that yet needs to be further explored.
Bone metabolism disorders in Wwox-deficient mice
Mice with a homozygous deletion of WWOX also exhibit a metabolic bone disease.10,16,19 Wwox-deficient mice suffer a disorder of skeleton development characterized by osteopenia, characterized by thinner cortical bones and decreased mineral density and bone volume.10 Ex vivo analysis of osteoblast culture isolated from Wwox-deficient mice revealed an impaired osteoblast differentiation phenotype suggesting an important role of WWOX during this process.10 In the same venue, Wwox-deficient mice display increased osteoclast activity, characterized by increased tartrate-resistant acidic phosphatase (TRAP) staining, which might also contribute to the observed osteopenic phenotype.27 Specific deletion of WWOX in the osteoblast compartment (using Osterix-cre and Osteocalcin-cre) has been generated and is being currently investigated (Aqeilan et al., unpublished data). At the molecular level, WWOX was shown to physically interact with RUNX2, the principal transcriptional regulator of osteoblast differentiation.10 WWOX represses RUNX2 transcriptional function and RUNX2 level is elevated in femoral bones of Wwox-deficient mice.28 Whether lack of functional crosstalk between WWOX and RUNX2 contribute to the metabolic bone abnormalities in Wwox-deficient mice is under investigation. Nevertheless, WWOX expression is significantly altered in osteosarcoma. Several research groups recently reported deletion or reduced levels of WWOX in osteosarcoma.28–30 RUNX2 levels are also altered in osteosarcoma.28,31 However, a significant statistical association between WWOX and RUNX2 levels in osteosarcoma was not established, so far, likely due to a complex relationship, in part due to multiple additional aberrations that occur in the tumors and/or because of tight regulation of RUNX2 in cancer cells. The fact that WWOX regulates turn over and cancer of bony tissues should stimulate future research in this direction.
WWOX regulates circulating HDL-C levels
A major risk factor for coronary artery disease is low levels of serum high-density lipoprotein cholesterol (HDL-C). Recent evidence has demonstrated a genetic association between the WWOX gene and HDL-C levels.32 A SNP (rs2548861) was identified in WWOX that associates with region-wide significance for low HDL-C in dyslipidemic families of Mexican and European descent and in low-HDL-C cases and controls of European descent. Moreover, use of next-generation sequencing of the WWOX locus eight variants were identified to be significantly associated and perfectly segregating with the low-HDL trait in two multi-generational French Canadian dyslipidemic families.33 Furthermore, using mouse genetics, it has been found that Wwox-deficient and liver specific Wwox cKO mice display reduction in ApoA-I and ABCA1 levels,33 critical components in reverse cholesterol transport and generation of nascent HDL particles. These observations raise the possibility that WWOX is involved in the complex network of cellular cholesterol homeostasis. Indeed, several reports have linked WWOX with impaired lipid metabolism.34–36 Altogether, these genetic and molecular results indicate a significant role for WWOX in cellular lipogenesis and metabolism. Disruption of lipogenesis involves modulation of multiple lipogenic enzymes that is linked with cancer-associated metabolic changes and thus could contribute to oncogenic transformation. Whether changes in WWOX expression modulate lipogenesis observed in tumor cells is not yet established.
WWOX modulates glucose metabolism
During malignant transformation cancer cells undergo significant metabolic changes. One of the best known examples of metabolic reprogramming in cancer cells is changes in glucose metabolism. In presence of oxygen (aerobic conditions), normal cells oxidize glucose, first to pyruvic acid via glycolysis in the cytosol and thereafter to carbon dioxide in the mitochondria; in absence of oxygen (anaerobic conditions), little pyruvate is dispatched to mitochondria and glycolysis is favored. In cancer cells, the main source of cellular energy is glycolysis, even under aerobic conditions. This shift to aerobic glycolysis is a hallmark of malignant cells known as Warburg effect.2,37,38 The reliance of cancer cells on aerobic glycolysis to produce ATP results in high glucose consumption (to compensate for the low efficiency of glycolysis). Identifying the molecular mechanisms responsible for the shift to aerobic glycolysis is important for understanding the basic biology of malignant transformation and for designing targeted therapies.
Recent work by Richards and co-workers using Drosophila has demonstrated that WWOX might interact with a number of proteins involved in metabolism like malate dehydrogenase and isocitrate dehydrogenase.39 Intriguingly, it has been also noted that changes in metabolism from glycolysis to oxidative phosphorylation affect WWOX transcripts.40 We recently reported an unforeseen role of WWOX in controlling glucose metabolism in primary normal cells.41 Under aerobic conditions, loss of WWOX enhances expression of key glycolytic genes hence diverting pyruvate away from the mitochondrial tricarboxylic acid (TCA) cycle. In other words, WWOX is plausibly functioning to inhibit aerobic glycolysis and to enhance the mitochondrial TCA cycle for efficient ATP production.
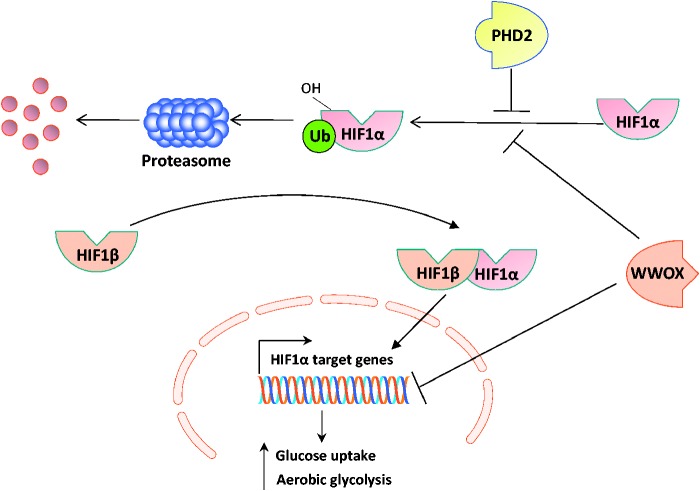
How does WWOX affect glycolysis? Our data demonstrate that WWOX interacts with the hypoxia-inducible transcription factor (HIF1α), the master regulator of glycolytic genes. This interaction results in (1) destabilization of HIF1α and (2) repression of HIF1α transcriptional activity41 (Figure 2). Cancer cells upregulate HIF1α, one of the key cellular regulators in response to oxygen stress42, which at one end enhances glucose uptake by upregulating glucose transporters, notably GLUT1 that act independently of insulin. Furthermore, HIF1α upregulates the expression of key glycolytic enzymes including HK2, PKM2, LDH-A, and others.43 At the other end, HIF1α directly inhibits TCA cycle by upregulating expression of pyruvate dehydrogenase kinase 1 (PDK1), which inactivates the rate-limiting enzyme pyruvate dehydrogenase (PDH) that catalyzes the conversion of pyruvate into acetyl-CoA to fuel the TCA cycle. Consequently, HIF1α accumulation results in increased rates of glycolysis and production of lactic acid and decreased rate of TCA cycle, a phenotype that is observed in Wwox-knockout tissues and cells.41
Figure 2.
Model: WWOX modulates HIF1α activity through multiple pathways. Under hypoxic conditions, HIF1α is stabilized and binds HIF1β to transactivate many target genes resulting in increase rate of glycolysis and glucose uptake and inhibiting TCA cycle. Under these conditions, HIF1α is less hydroxylated by PHD2 and thus is not targeted for degradation mediated by Von Hippel–Lindau (VHL) E3 ligase complex. Our recent studies demonstrate that loss of tumor suppressor WWOX enhances HIF1α accumulation and its transcriptional function. WWOX physically interacts with HIF1α and induces its hydroxylation by PHD2 and so leads it to proteasomal degradation. WWOX also, inhibits HIF1α transactivation function. (A color version of this figure is available in the online journal.)
How does WWOX affect HIF1α function and glucose metabolism? WWOX could control HIF1α function by suppressing its transactivation function,41 likely by sequestering HIF1α from its target sequences. WWOX could also enhance hydroxylation of HIF1α by modulating the function of prolyl hydroxylase 2 (PHD2) leading to HIF1α degradation thereby maintaining proper glucose uptake, glycolysis, and TCA cycle (Figure 2). Interestingly, Wwox-knockout mice exhibit higher levels of serum lactic acid relative to wild type mice. Moreover, Wwox-deficient cells exhibit higher glucose uptake while lower ATP production and oxygen consumption. These cells also display upregulation of glycolytic genes level when compared to wild-type cells.41 Overall, Wwox-deficient cells present a phenotype of enhanced glycolysis, even under normoxic conditions (Warburg effect).
As lack of WWOX mimics an oxygen stress response, we hypothesized that WWOX levels change upon hypoxic conditions. Indeed, WWOX expression is downregulated under hypoxic conditions,40,41 indicating that these circumstances, by unknown mechanism, hamper WWOX levels which in turn contribute to increase the levels of HIF1α. These observations suggest that WWOX deficiency elicits a metabolic switch that is HIF1α dependent. Given these observations, we hypothesized that depleting HIF1α in Wwox-deficient cells would increase glucose uptake. Indeed, inhibiting HIF1α both genetically and pharmacologically reversed the glucose uptake phenotype both in vitro and in vivo.
At the molecular level, WWOX, through its WW1 domain, interacts with HIF1α and functionally decreases HIF1α levels and hence attenuates glucose uptake.41 By contrast, mutated WWOX (WWOX-WFPA), which displays impaired WWOX interaction ability with HIF1α, does not affect HIF1α levels nor it decreases glucose uptake and hence does not affect HIF1α target genes. Furthermore, WWOX-deficient cells display lower levels of HIF1α hydroxylation cells as compared with WWOX-sufficient cells. After all, WWOX inhibits glycolysis through inhibition of HIF1α function.
To determine whether WWOX-HIF1α association has functional relevance on tumorigenesis, we investigated the effect of HIF1α expression on WWOX-mediating tumor suppression. Targeted deletion of WWOX in transformed mouse embryonic fibroblasts is associated with enhanced tumorigenesis in immunocompromised mice compared to wild-type cells.41 Notably, depletion of HIF1α inhibited tumorigenicity of Wwox-deficient cells to a better extent than of WWOX-sufficient cells.41 Using a human breast cancer tissue array, it was shown that WWOX expression is inversely correlated with that of glucose transporter GLUT1, a target gene of HIF1α.41 It is thus likely that WWOX inhibits HIF1α activity under aerobic conditions, to ensure glucose flux into mitochondria thereby inhibiting aerobic glycolysis. Altogether, these data indicate that WWOX loss activates aerobic glycolysis (Warburg effect), a hallmark of cancer cells. Future research will examine the significance of using WWOX as a biomarker for impaired metabolism in cancer cells and in clinical samples.
WWOX affects ROS levels
It is known that the main source of endogenous reactive oxygen species (ROS) in normal cells is mitochondrial respiration.44 Consistent with the emerging role of WWOX in metabolism, WWOX was suggested to act as a regulator of ROS; once WWOX is overexpressed in Drosophila larvae, ROS levels were elevated whereas overexpression of mutated WWOX did not show this elevation.39 Since WWOX loss is associated with reduced rates of TCA cycle, this could affect ROS production. Whether the SDR domain of WWOX plays a role in regulating ROS levels is being addressed.
Concluding remarks
In this review, we described the emerging role of WWOX in cellular metabolism. Emerging evidence has linked WWOX with steroid metabolism, HDL-C metabolism, bone metabolism, and glucose metabolism (Figure 1). Much still remains to be learned on the physiological role of WWOX in this context and how it contributes to the neoplastic process. The WWOX gene spans the fragile site FRA16D, one of the most active common fragile sites, a genomic region that is involved both in chromosome translocation in multiple myeloma and also in homo and hemizygous deletions in cancer and cancer-derived cell lines.8,45 Since WWOX is frequently lost in cancer, it is thus likely that this loss contributes to alter metabolism and cancer progression.
Several indications support WWOX function as a tumor suppressor. First, WWOX is frequently altered in numerous cancer types.7,8 Both hemizygous and homozygous deletions are common but WWOX alteration due to other mechanisms including epigenetics and posttranslational modifications was also suggested. It is thus likely to propose that one allele of WWOX might be lost due to genomic modification while the other is lost by other mechanisms fulfilling the Knudson’s two-hits hypothesis. Second, WWOX ectopic expression in multiple WWOX-deficient cancer cell lines results in apoptosis and reduced tumorigenicity (reviewed in Chang et al.13 and Del Mare et al.46). Third, Wwox mutant mice display increased incidence and multiplicity of tumor development suggesting WWOX as a bone fide tumor suppressor.9,17,47 Fourth, loss of WWOX facilitates a selective advantage of neoplastic growth. For example, Ras-mediated transformation of Wwox-deficient cells displays increased tumorigenicity as compared to wild-type cells.41 Finally, the WWOX interactome supports a direct role of WWOX as an oncosuppressor. The mechanisms of tumor suppression of WWOX involve apoptosis,13 modulation of the extracellular matrix,48 and, as discussed here, modulation of cell bioenergetics.41 WWOX interacts and modulates the function of different proteins involved in tumor progression. When WWOX is lost, many of these proteins lose their checks which alters signaling that feeds into the neoplastic process. Future work shall further define the relevance of these interactions in vivo and test whether WWOX can be used as a biomarker for impaired metabolism in cancer cells and in clinical samples. Loss of tumor suppressor WWOX could therefore affect different levels of cellular metabolism and thus feed tumorigenesis.
Acknowledgment
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
The Aqeilan lab is funded, in part, by the Association for International Cancer Research (AICR).
Author contributions
Both authors MA and RIA wrote and designed this review article.
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 3.Bednarek AK, Keck-Waggoner CL, Daniel RL, Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ, Aldaz CM. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res 2001; 61: 8068–73. [PubMed] [Google Scholar]
- 4.Salah Z, Alian A, Aqeilan RI. WW domain-containing proteins: retrospectives and the future. Front Biosci 2012; 17: 331–48. [DOI] [PubMed] [Google Scholar]
- 5.Aqeilan RI, Croce CM. WWOX in biological control and tumorigenesis. J Cell Physiol 2007; 212: 307–10. [DOI] [PubMed] [Google Scholar]
- 6.Nunez MI, Ludes-Meyers J, Aldaz CM. WWOX protein expression in normal human tissues. J Mol Histol 2006; 37: 115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardenswartz A, Aqeilan RI. WW domain-containing oxidoreductase's role in myriad cancers: clinical significance and future implications. Exp Biol Med 2014; 239: 253–63. [DOI] [PubMed] [Google Scholar]
- 8.Paige AJ, Taylor KJ, Taylor C, Hillier SG, Farrington S, Scott D, Porteous DJ, Smyth JF, Gabra H, Watson JE. WWOX: a candidate tumor suppressor gene involved in multiple tumor types. Proc Natl Acad Sci U S A 2001; 98: 11417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y, Hagan JP, Zanesi N, Kaou M, Stein GS, Lian JB, Croce CM. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci U S A 2007; 104: 3949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aqeilan RI, Hassan MQ, de Bruin A, Hagan JP, Volinia S, Palumbo T, Hussain S, Lee SH, Gaur T, Stein GS, Lian JB, Croce CM. The WWOX tumor suppressor is essential for post-natal survival and normal bone metabolism. J Biol Chem 2008; 283: 21629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salah Z, Aqeilan R, Huebner K. WWOX gene and gene product: tumor suppression through specific protein interactions. Future Oncol 2010; 6: 249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abu-Odeh M, Bar-Mag T, Huang H, Kim T, Salah Z, Abdeen SK, Sudol M, Reichmann D, Sidhu S, Kim PM, Aqeilan RI. Characterizing WW domain interactions of tumor suppressor WWOX reveals its association with multiprotein networks. J Biol Chem 2014; 289: 8865–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 2007; 13: 12–22. [DOI] [PubMed] [Google Scholar]
- 14.Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2 gamma transcription factor. Cancer Res 2004; 64: 8256–61. [DOI] [PubMed] [Google Scholar]
- 15.Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G, Huebner K, Croce CM. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci U S A 2004; 101: 4401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdeen SK, Del Mare S, Hussain S, Abu-Remaileh M, Salah Z, Hagan J, Rawahneh M, Pu XA, Russell S, Stein JL, Stein GS, Lian JB, Aqeilan RI. Conditional inactivation of the mouse Wwox tumor suppressor gene recapitulates the null phenotype. J Cell Physiol 2013; 228: 1377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aqeilan RI, Hagan JP, Aqeilan HA, Pichiorri F, Fong LY, Croce CM. Inactivation of the Wwox Gene accelerates fore stomach tumor progression in vivo. Cancer Res 2007; 67: 5606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludes-Meyers JH, Kil H, Nunez MI, Conti CJ, Parker-Thornburg J, Bedford MT, Aldaz CM. WWOX hypomorphic mice display a higher incidence of B-cell lymphomas and develop testicular atrophy. Genes Chromosomes Cancer 2007; 46: 1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludes-Meyers JH, Kil H, Parker-Thornburg J, Kusewitt DF, Bedford MT, Aldaz CM. Generation and characterization of mice carrying a conditional allele of the Wwox tumor suppressor gene. PLoS One 2009; 4: e7775–e7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aqeilan RI, Hagan JP, de Bruin A, Rawahneh M, Salah Z, Gaudio E, Siddiqui H, Volinia S, Alder H, Lian JB, Stein GS, Croce CM. Targeted ablation of the WW domain-containing oxidoreductase tumor suppressor leads to impaired steroidogenesis. Endocrinology 2009; 150: 1530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdeen SK, Salah Z, Khawaled S, Aqeilan RI. Characterization of WWOX inactivation in murine mammary gland development. J Cell Physiol 2013; 228: 1391–6. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson BW, Gao X, Kil H, Lee J, Benavides F, Abba MC, Aldaz CM. Conditional Wwox deletion in mouse mammary gland by means of two Cre recombinase approaches. PLoS One 2012; 7: e36618–e36618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aqeilan RI, Donati V, Gaudio E, Nicoloso MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H, Croce CM, Elenius K. Association of Wwox with ErbB4 in breast cancer. Cancer Res 2007; 67: 9330–6. [DOI] [PubMed] [Google Scholar]
- 24.Nunez MI, Ludes-Meyers J, Abba MC, Kil H, Abbey NW, Page RE, Sahin A, Klein-Szanto AJ, Aldaz CM. Frequent loss of WWOX expression in breast cancer: correlation with estrogen receptor status. Breast Cancer Res Treat 2005; 89: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, McCue P, Huebner K. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer 2004; 100: 1605–14. [DOI] [PubMed] [Google Scholar]
- 26.Saluda-Gorgul A, Seta K, Nowakowska M, Bednarek AK. WWOX oxidoreductase–substrate and enzymatic characterization. Z Naturforsch C 2011; 66: 73–82. [PubMed] [Google Scholar]
- 27.Del Mare S, Kurek KC, Stein GS, Lian JB, Aqeilan RI. Role of the WWOX tumor suppressor gene in bone homeostasis and the pathogenesis of osteosarcoma. Am J Cancer Res 2011; 1: 585–94. [PMC free article] [PubMed] [Google Scholar]
- 28.Kurek KC, Del Mare S, Salah Z, Abdeen S, Sadiq H, Lee SH, Gaudio E, Zanesi N, Jones KB, DeYoung B, Amir G, Gebhardt M, Warman M, Stein GS, Stein JL, Lian JB, Aqeilan RI. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res 2010; 70: 5577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Cogdell D, Yang D, Hu L, Li H, Zheng H, Du X, Pang Y, Trent J, Chen K, Zhang W. Deletion of the WWOX gene and frequent loss of its protein expression in human osteosarcoma. Cancer Lett 2010; 291: 31–8. [DOI] [PubMed] [Google Scholar]
- 30.Yang J, Zhao L, Tian W, Liao Z, Zheng H, Wang G, Chen K. Correlation of WWOX, RUNX2 and VEGFA protein expression in human osteosarcoma. BMC Med Genomics 2013; 6: 56–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin JW, Zielenska M, Stein GS, van Wijnen AJ, Squire JA. The role of RUNX2 in osteosarcoma oncogenesis. Sarcoma 2011; 2011: 282745–282745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JC, Weissglas-Volkov D, Kyttala M, Dastani Z, Cantor RM, Sobel EM, Plaisier CL, Engert JC, van Greevenbroek MM, Kane JP, Malloy MJ, Pullinger CR, Huertas-Vazquez A, Aguilar-Salinas CA, Tusie-Luna T, de Bruin TW, Aouizerat BE, van der Kallen CC, Croce CM, Aqeilan RI, Marcil M, Viikari JS, Lehtimaki T, Raitakari OT, Kuusisto J, Laakso M, Taskinen MR, Genest J, Pajukanta P. WW-domain-containing oxidoreductase is associated with low plasma HDL-C levels. Am J Hum Genet 2008; 83: 180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iatan I, Choi HY, Ruel I, Reddy MV, Kil H, Lee J, Abu Odeh M, Salah Z, Abu-Remaileh M, Weissglas-Volkov D, Nikkola E, Civelek M, Awan Z, Croce CM, Aqeilan RI, Pajukanta P, Aldaz CM, Genest J. The WWOX gene modulates HDL and lipid metabolism. Circ Cardiovasc Genet 2014;7:491–504. [DOI] [PMC free article] [PubMed]
- 34.Saez ME, Gonzalez-Perez A, Martinez-Larrad MT, Gayan J, Real LM, Serrano-Rios M, Ruiz A. WWOX gene is associated with HDL cholesterol and triglyceride levels. BMC Med Genet 2010; 11: 148–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leduc MS, Lyons M, Darvishi K, Walsh K, Sheehan S, Amend S, Cox A, Orho-Melander M, Kathiresan S, Paigen B, Korstanje R. The mouse QTL map helps interpret human genome-wide association studies for HDL cholesterol. J Lipid Res 2011; 52: 1139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadzadeh A, Azizi F. Genes associated with low serum high-density lipoprotein cholesterol. Arch Iran Med 2014; 17: 444–50. [PubMed] [Google Scholar]
- 37.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011; 27: 441–64. [DOI] [PubMed] [Google Scholar]
- 38.Warburg O. On the origin of cancer cells. Science 1956; 123: 309–14. [DOI] [PubMed] [Google Scholar]
- 39.O'Keefe LV, Colella A, Dayan S, Chen Q, Choo A, Jacob R, Price G, Venter D, Richards RI. Drosophila orthologue of WWOX, the chromosomal fragile site FRA16D tumour suppressor gene, functions in aerobic metabolism and regulates reactive oxygen species. Hum Mol Genet 2011; 20: 497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dayan S, O'Keefe LV, Choo A, Richards RI. Common chromosomal fragile site FRA16D tumor suppressor WWOX gene expression and metabolic reprograming in cells. Genes Chromosomes Cancer 2013; 52: 823–31. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Remaileh M, Aqeilan RI. Tumor suppressor WWOX regulates glucose metabolism via HIF1alpha modulation. Cell Death Differ 2014;21:1805–14. [DOI] [PMC free article] [PubMed]
- 42.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008; 8: 705–13. [DOI] [PubMed] [Google Scholar]
- 43.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ 2008; 15: 621–7. [DOI] [PubMed] [Google Scholar]
- 44.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 2003; 278: 36027–31. [DOI] [PubMed] [Google Scholar]
- 45.Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E, Richards RI. Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet 2000; 9: 1651–63. [DOI] [PubMed] [Google Scholar]
- 46.Del Mare S, Salah Z, Aqeilan RI. WWOX: its genomics, partners, and functions. J Cell Biochem 2009; 108: 737–45. [DOI] [PubMed] [Google Scholar]
- 47.Abdeen SK, Salah Z, Maly B, Smith Y, Tufail R, Abu-Odeh M, Zanesi N, Croce CM, Nawaz Z, Aqeilan RI. Wwox inactivation enhances mammary tumorigenesis. Oncogene 2011; 30: 3900–6. [DOI] [PubMed] [Google Scholar]
- 48.Gourley C, Paige AJ, Taylor KJ, Ward C, Kuske B, Zhang J, Sun M, Janczar S, Harrison DJ, Muir M, Smyth JF, Gabra H. WWOX gene expression abolishes ovarian cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin alpha3. Cancer Res 2009; 69: 4835–42. [DOI] [PubMed] [Google Scholar]