Abstract
WW domain-containing oxidoreductase (WWOX) is a well-documented tumor suppressor protein that controls growth, survival, and metastasis of malignant cells. To counteract WWOX’s suppressive effects, cancer cells have developed many strategies either to downregulate WWOX expression or to functionally inactivate WWOX. Relatively unknown is, in the context of those cancers associated with certain viruses or bacteria, how the oncogenic pathogens deal with WWOX. Here we review recent studies showing different strategies utilized by three cancer-associated pathogens. Helicobactor pylori reduces WWOX expression through promoter hypermethylation, an epigenetic mechanism also occurring in many other cancer cells. WWOX has a potential to block canonical NF-κB activation and tumorigenesis induced by Tax, an oncoprotein of human T-cell leukemia virus. Tax successfully overcomes the blockage by inhibiting WWOX expression through activation of the non-canonical NF-κB pathway. On the other hand, latent membrane protein 2A of Epstein–Barr virus physically interacts with WWOX and redirects its function to trigger a signaling pathway that upregulates matrix metalloproteinase 9 and cancer cell invasion. These reports may be just “the tip of the iceberg” regarding multiple interactions between WWOX and oncogenic microbes. Further studies in this direction should expand our understanding of infection-driven oncogenesis.
Keywords: WW domain-containing oxidoreductase, infection-associated cancers, Helicobactor pylori, human T-cell leukemia virus, Epstein–Barr virus
Introduction
Human WW domain-containing oxidoreductase, namely WWOX, WOX1 or FOR, participates in a broad spectrum of biologic functions, including control of neoplasia, stress response, metabolism, hematopoiesis, and cell differentiation.1–5 WWOX is expressed in many tissues/organs (e.g. skin, bones, nerve systems, reproductive/endocrine systems, immune systems, and various cancers) and has been involved in homeostatic or pathogenic states therein. Its versatile nature is attributed to the broad subcellular localizations of WWOX and a wide variety of WWOX-interacting proteins.2,3 Depending on cell types and assay conditions, WWOX has been detected in nuclei, perinuclear areas, mitochondria, Golgi, endoplasmic reticulum, and plasma membrane.1,3 WWOX’s interactions with partner proteins, as well as most of its known functions, predominantly depend on the first WW domain at the N-terminus of WWOX.1–3 This domain, categorized as the group I WW domain, preferentially binds to a consensus PPXY motif present in many WWOX-targeting proteins.6 A recent study reveals another consensus LPXY motif as a novel WW domain/WWOX-binding site, extending the spectrum of potential WWOX-interacting partners.7 In addition, the C-terminus of WWOX contains a short-chain alcohol dehydrogenase–reductase domain, which is not only a putative steroid hormone-binding domain but also involved in some critical cases of WWOX’s protein–protein interactions.8,9
Tumor suppression is a well-documented function of WWOX, and this function is majorly achieved by multiple interactions between WWOX and its specific partners.1,2 Unsurprisingly, accumulating studies indicate that cancer cells utilize many approaches to alter WWOX expression or to withstand the anticancer effects of WWOX. On the other hand, relatively unknown is what happens to WWOX in those cancers associated with certain viruses or bacteria. Being effective initiators or promoters of malignancy, these oncogenic microbes should have evolved several ways to deal with WWOX and incorporated them into their cancer-promoting mechanisms. Three recent studies support this idea, showing that some cancer-associated microbes may either adapt anti-WWOX approaches similar with those used by tumor cells, or even develop a novel mechanism to redirect WWOX’s role in cancer progression.10–12 Here we start from an overview of WWOX-mediated anticancer effects and their underlying mechanisms, followed by a summary of the anti-WWOX approaches used by cancer cells. Subsequently, we will focus on current knowledge about how oncogenic microbes affect WWOX’s expression or function, and discuss potential directions to explore more interactions between WWOX and infectious pathogens.
WWOX as a tumor suppressor
The association between WWOX and cancers has been noticed since the gene encoding WWOX was initially identified within a common fragile site FRA16D, a chromosomal region frequently affected in many human cancers.13,14 Although homozygous deletion or mutation of this gene is rare, downregulation of WWOX at the mRNA and protein levels is frequently observed in clinical specimens and cell lines of many human cancers. For example, compared with control normal tissues, tumor tissues showing complete loss or reduced expression of WWOX protein are detected in about 30–60% of breast cancers, 30% of ovarian carcinomas, 60% of osteosarcomas, 70% of cutaneous squamous cell carcinomas, and 80% of prostate cancers.15–21 In addition, the clinical impacts of WWOX reduction in tumor tissues have been noticed. For breast cancers, absent or decreased WWOX expression is associated with clinical markers of poor prognosis, with poor overall survival, with high risk of recurrence, and with the poor response to tamoxifen treatment.16,17,22–24 For ovarian carcinomas and renal cell carcinomas, reduced WWOX expression in tumor tissues is detected only in some specific histotypes but the expression reduction is also correlated with the unfavorable clinical outcome.18,25 For breast cancers and osteosarcomas, WWOX expression in metastatic tumors is further reduced or even completely lost, suggesting that WWOX potentially hampers tumor metastasis.19,23,26 Collectively these clinical observations shed light on WWOX’s roles in tumor control.
The tumor-suppressive activity of WWOX is further supported by several experiments manipulating WWOX expression in cancer cell lines. For WWOX-null cell lines of lung cancers, prostate cancers, pancreatic cancers, breast cancers, osteosarcomas, and glioblastomas, ectopically forced expression of WWOX induces apoptosis and inhibits cell growth, thus suppressing the tumorigenicity of the cancer cells in immunocompromised mice.19,21,27–30 Overexpression of WWOX sensitizes, while knockdown of WWOX attenuates, the cell death triggered by various apoptosis inducers including tumor necrosis factor α (TNFα), UV light, anticancer reagents, complement C1q, and transforming growth factor β1 (TGF-β1), further substantiating the critical function of WWOX in regulation of cell death.20,31–35 For cell lines of hetapocellular carcinomas, gastric signet-ring cell carcinomas, osteosarcomas, and ovarian cancers, ectopic expression of WWOX suppresses cell adhesion to or cell invasion through extracellular matrix, while knockdown of WWOX enhances the matrigel invasion activity, suggesting that WWOX executes a function in control of cancer metastasis.19,36–38
The murine Wwox protein is 93.2% identical to human WWOX in the amino acid sequence, implying their functional conservation.32 To get more insight into the biologic roles of WWOX, several mouse models with genetic manipulations of the Wwox gene have been generated, and these models corroborate the anticancer functions of murine Wwox. Although the homozygous Wwox-deficient (Wwox–/–) mice with targeted disruption of exons 2–4 of the Wwox gene show severe metabolic disorders and die postnatally within 2–3 weeks, spontaneous occurrence of osteosarcomas in the juvenile is detected.39,40 The mice with heterozygous Wwox deficiency (Wwox+/–) develop lung papillary carcinomas spontaneously in adult, and upon treatment with a chemical mutagen, Wwox+/– mice develop more lung tumors and lymphomas than the wild-type mice.39 In the Wwox-hypomorphic mice generated by a gene-trap strategy, the insufficient Wwox expression results in a higher incidence of spontaneous B-cell lymphomas in female.41 In addition, the female Wwox+/– mice with the C3H mammary tumor-susceptible genetic background develop dramatically more mammary carcinomas than the control Wwox+/+ mice with the same genetic background.42 As is an exception, the Wwox-knockout mice with targeted disruption of the first exon of the Wwox gene show severe metabolic and hematopoietic defects without evidence of spontaneous neoplasia.43 Generally these animal studies provide additional evidence of Wwox-dependent tumor suppression.
Molecular mechanisms underlying WWOX-mediated anticancer effects
As is mentioned, functions of WWOX, including those involved in tumor suppression, are majorly determined by protein–protein interactions between WWOX and specific partners. These interactions regulate downstream signaling pathways and transcriptional programs, thus affecting multiple biologic events. Here we summarize the WWOX–partner interactions and some downstream molecular mechanisms, with focus on those contributing to anticancer effects of WWOX (Figure 1).
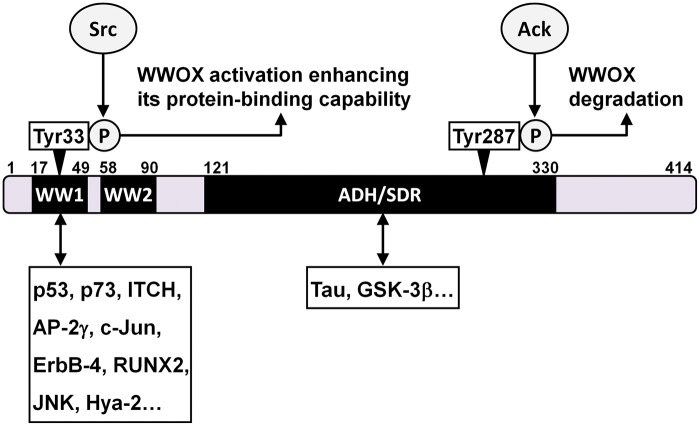
Figure 1.
WWOX contains domains for protein–protein interaction. WWOX is a 414-amino-acid protein with two N-terminal WW (WW1 and WW2) domains and a C-terminal short-chain alcohol dehydrogenase–reductase (ADH/SDR) domain. A tyrosine kinase Src phosphorylates WWOX at Tyr33, which activates WWOX and enhances its interaction with partner proteins. Another tyrosine kinase Ack1 phosphorylates WWOX at Tyr287, which accelerates WWOX degradation. The first WW domain of WWOX is responsible for interaction with most of known WWOX-binding proteins, while the ADH/SDR domain mediates the interaction with Tau and GSK-3β
Cellular tumor suppressor proteins are considered as the first group of WWOX-binding partners involved in the anticancer activity. Two well-recognized examples of this group are p53 and p73. Although p53 has no consensus PPXY motif, its N-terminal proline-rich domain (amino acid residuals 66–100) along with phosphorylation at the adjacent serine 46 is responsible for the physical interaction with the first WW domain of WWOX.32 Phosphorylation of tyrosine 33 (Tyr33) in the first WW domain of WWOX activates WWOX and enhances its association with p53, resulting in stabilization of serine 46-phosphorylated p53.35 The WWOX–p53 complex is translocated into the nucleus and mediates the cell death triggered by TNF, UV light, and other apoptosis inducers.32,35 Steroid hormone 17β-estradiol induces WWOX activation and nuclear translocation of the WWOX–p53 complex in the cells expressing no estrogen receptor, but fails to do so in estrogen receptor-positive cells, suggesting that certain factors regulate the WWOX–p53 pathway.26 On the other hand, p73 contains a PPPPY motif serving as a binding site for the Tyr33-phosphorylated WW domain of WWOX.44 The Src kinase is responsible for the Tyr33 phosphorylation, and similar to that observed in WWOX–p53 interaction, phosphorylation at this tyrosine residual enhances WWOX binding to p73.44 The WWOX–p73 interaction drives the nucleus-to-cytoplasm redistribution of p73, and the cytoplasmic p73 mediates the apoptosis-inducing effect of WWOX. A recent study reveals that WWOX interacts with ITCH, an E3 ubiquitin ligase promoting polyubiquitination and degradation of p73.7 WWOX reduces ITCH-mediated p73 polyubiquitination/degradation and enhances proapoptotic activity of p73, showing an additional effect of WWOX on p73.
The second group of WWOX-binding partners involved in tumor suppression includes oncogenic transcription factors that are functionally antagonized by WWOX. AP-2γ, c-Jun, ErbB-4, and RUNX2 belong to this category.40,45–47 The proline-rich motif of c-Jun, as well as the PPXY motifs of other three proteins, interacts with WWOX, and the Tyr33 in the first WW domain of WWOX is required for the protein–protein interaction. Overexpressed WWOX binds to AP-2γ, c-Jun, and RUNX2 and inhibits their functions in promoter transactivation mainly through sequestering these transcription factors in the cytoplasm.40,45,46 Overexpressed WWOX also physically associates with full-length ErbB-4 and keeps it in the cytoplasm, thus blocking nuclear translocation of the C-terminal fragment of ErbB-4 and inhibiting YAP-mediated transcriptional coactivation with this fragment.47 On the other hand, endogenous WWOX physically interacts with CREB and co-translocates with it to the nucleus in transected sciatic nerves in rat, and this event may affect promoter activation and neuronal survival.48
The third group of WWOX-binding partners for tumor suppression includes various proteins regulating signal transduction. For example, WWOX binds to Dvl-2 and sequesters it in the cytoplasm, thus blocking activation of the Wnt/β-catenin signaling pathway.49 Binding of TGF-β1 to membrane Hyal-2 facilitates interaction between the catalytic domain of Hyal-2 and the Tyr33-phosphorylated WW domain of WWOX.33 The Hyal-2-WWOX complex is relocated into the nucleus, thereby enhancing Smad-mediated transcription activity and sensitizing TGF-β1-induced cell apoptosis.33 In T-cell leukemia cells, WWOX physically interacts with mitogen-activated protein kinase kinase 1 (MEK1) as an inactive complex; treatment with phorbol ester releases WWOX from the complex and triggers cell apoptosis.50
Certain molecular mechanisms underlying WWOX-mediated inhibition of cancer metastasis have been uncovered. For the cells of breast cancers and osteosarcomas, ectopic WWOX suppresses cell invasion through functional inactivation or expressional downregulation of RUNX2. WWOX restoration reduces expression of a panel of RUNX2-regulated genes linked to metastasis.19,40 For ovarian cancer cells, WWOX decreases membrane expression of intergrin-α3, thus reducing cell adhesion to fibronectin in vitro and tumorigenesis in vivo.38
How cancer cells dodge or overcome WWOX-mediated tumor suppression
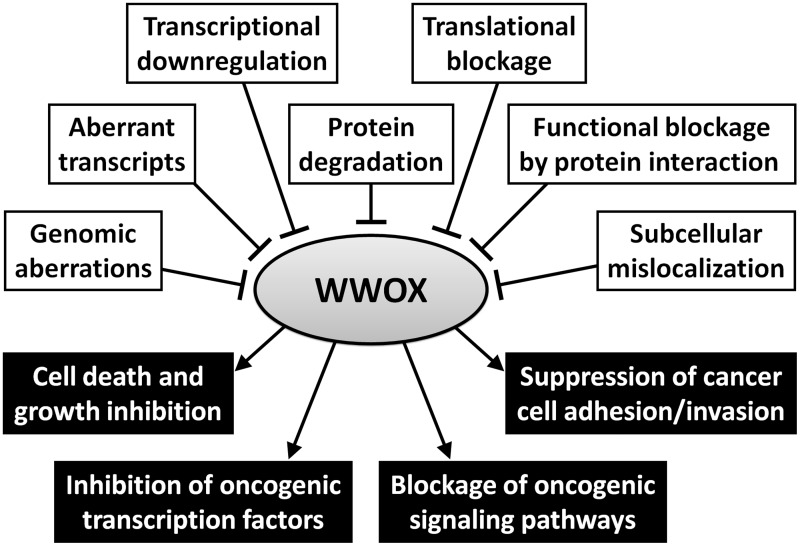
As is summarized in Figure 2, WWOX can be counteracted by cancer cells in multiple ways. WWOX expression can be abrogated at genomic, transcriptional, post-transcriptional, translational, and post-translational levels. Previous studies have also revealed some cellular proteins that physically interact with WWOX and inhibit its anticancer effects.
Figure 2.
WWOX plays multiple roles in tumor suppression but it can be downregulated or functionally inhibited in many ways. Black boxes in the lower panel present the anticancer mechanisms of WWOX, while white boxes in the upper panel indicate the potential mechanisms of cancer cells to overcome WWOX
Although the WWOX gene spans the FRA16D common chromosomal fragile site, the cases with absence of WWOX owing truly to gene deletion or missense mutation in cancer cells are rare.51–53 One exception is observed in primary effusion lymphoma cell lines, of which 85% (11/13) show WWOX gene deletion.54 Aberrant WWOX transcripts with exon deletions have been detected in many tumor specimens and cell lines, including those of breast cancers, ovarian cancers, hepatocellular carcinomas, non-small cell lung carcinomas, and esophageal squamous cell carcinomas.51–53,55,56 These transcripts have been associated with low expression of full-length WWOX mRNA, abnormal subcellular localization of WWOX protein, or advanced cancer stages.30,51,56 Therefore, though it remains unclear how the aberrant transcripts are generated, they should contribute to the dysregulation of WWOX in cancer cells. Downregulation of WWOX in cancer cells can also be attributed to transcriptional silencing by epigenetic modification. For example, reduced WWOX expression has been associated with promoter hypermethylation in various cancer cells.29,57 Treatment with a DNA demethylating reagent or a histone deacetylase inhibitor restores WWOX expression and WWOX-mediated tumor-suppressive effects on prostate cancer cells.21 On the other hand, absence of WWOX protein in the presence of full-length WWOX mRNA has been observed in breast cancer cell lines and tumor tissues of cutaneous squamous cell carcinomas, suggesting a blockage of WWOX expression at the translational/post-transcriptional level.20,51 In addition, loss of WWOX in cancer cells can be due to reduced protein stability. For prostate tumorigenesis, an activated tyrosine kinase Ack1 phosphorylates WWOX at tyrosine 287, resulting in WWOX polyubiquitination followed by accelerated WWOX degradation.58
While WWOX expression is frequently downregulated in cancer cells, there are still substantial cases showing the presence of wild-type WWOX protein in malignancies. A normal or even elevated level of WWOX expression has been reported in certain tumor tissues or cell lines of gastric carcinomas, prostate cancers, and breast cancers.13,26,59 These observations raise a possibility that there are other strategies to antagonize WWOX’s tumor suppressive functions. Some cellular proteins functionally inhibiting WWOX have been identified. Phosphorylated JNK interacts with Tyr33-phosphorylated WWOX and suppresses WWOX-mediated apoptosis.60 Two PPXY-containing proteins, TMEM207 and COTE1, interact with WWOX and counteract WWOX-mediated inhibition of cell invasion.36,37 Alteration of subcellular localization may be another way leading to dysfunction of WWOX. For example, overexpressed Zfra sequesters WWOX in the cytoplasm and blocks TNFα/UV light-induced nuclear translocation of WWOX, thus disturbing the proapoptotic functions of WWOX and p53.61
How oncogenic microbes deal with WWOX
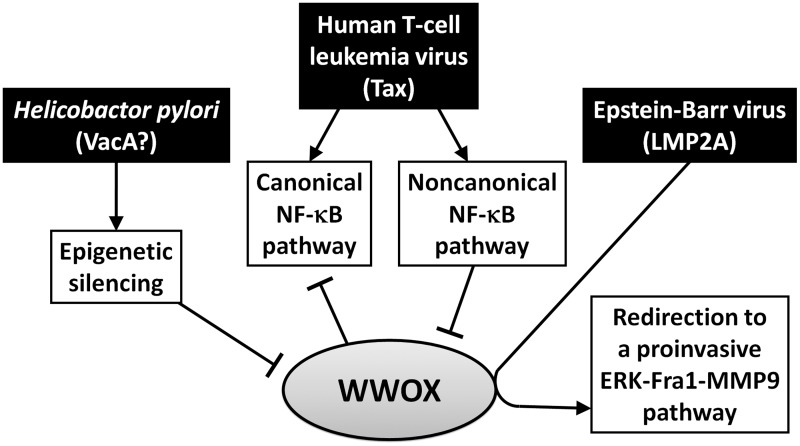
The roles of WWOX in the cancers associated with infectious pathogens are less explored, but it is reasonably expected that the oncogenic microbes must deal with the tumor-suppressive functions of WWOX in the context of their infection-driven oncogenesis. As is illustrated in Figure 3, distinct mechanisms utilized by three cancer-associated microbes have been reported recently.
Figure 3.
Oncogenic microbes use different strategies to deal with WWOX. H. pylori suppresses WWOX expression through epigenetic silencing. The HTLV-1 Tax-activated canonical NF-κB pathway is blocked by WWOX, while the Tax-activated non-canonical NF-κB pathway suppresses WWOX expression. EBV LMP2A interacts with WWOX and redirects WWOX’s function to activation of a proinvasive ERK-Fra-1-MMP9 pathway
Epigenetic silencing of WWOX expression by Helicobactor pylori10
H. pylori infection is an etiologic factor for chronic gastritis, peptic ulcers, gastric adenocarcinomas, and gastric mucosa-associated lymphoid tissue lymphomas.62 Gastric cancer cells infected with H. pylori or co-cultured with the bacterium-conditioned medium show alteration of cell morphology and reduced apoptosis.63 These effects depend on VacA, a vacuolating toxin of H. pylori, as an isogenic strain losing the vacA gene exerts a mild influence on the cells. A cDNA microarray analysis reveals that H. pylori infection causes significant changes of expression of cellular genes involved in cytoskeleton, cell cycle, cell death, and proliferation. Among these genes, WWOX expression is dramatically decreased at 24 h postinfection.63 Following a precedent that H. pylori induces promoter methylation and silencing of a tumor suppressor trefoil factor 2, the H. pylori-triggered downregulation of WWOX is subsequently found to be mediated by the similar epigenetic regulation.64 H. pylori infection induces expression of DNA methyltransferases, DNMT1 and DNMT3A, concurrently with hypermethylation of the WWOX promoter and reduction of WWOX transcription.10 In addition, WWOX gene hypermethylation is associated with H. pylori infection in primary gastric tumor tissues. Therefore, downregulation of WWOX by epigenetic silencing is a convergent anti-WWOX mechanism adapted by both H. pylori and microbe-free cancer cells.
Reciprocal counteraction between WWOX and Tax of human T-cell leukemia virus type 1 (HTLV-1)11
A relatively complex interaction is recognized between WWOX and HTLV-1, a retrovirus causing adult T-cell leukemia. HTLV-1 encodes an oncoprotein Tax, which triggers multiple signaling pathways, regulates expression of viral and cellular genes, and induces tumorigenesis in transgenic mice.65 Tax activates both canonical and non-canonical NF-κB pathways, which are pivotal to Tax’s oncogenic functions.66–68 Notably, a recent study shows that WWOX is a negative regulator of Tax.11 Knockdown of WWOX enhances, while overexpression of WWOX suppresses, the effects of Tax on cell transformation and tumorigenesis. It is further revealed that WWOX interacts with Tax, thus blocking the Tax-activated canonical NF-κB pathway without affecting the non-canonical NF-κB pathway. The underlying mechanism involves WWOX-mediated interference with Tax-induced recruitment of IKKα to the canonical NF-κB subunit RelA, thus inhibiting the phosphorylation of RelA at serine 536 and its activation. Interestingly, Tyr33-mutated WWOX retains the ability to bind to Tax but fails to block Tax-induced IKKα-RelA interaction, suggesting that the first WW domain may not mediate WWOX–Tax protein interaction but it contributes to the functional inhibition of Tax. Another note is that WWOX-mediated NF-κB inhibition is Tax specific, as WWOX does not affect the canonical NF-κB pathway activated by other stimuli such as TNFα. Further studies are required to identify the protein domains involved in WWOX–Tax interaction and the exact mechanisms through which WWOX interferes with the Tax-specific canonical NF-κB pathway.
To overcome WWOX’s negative effect, Tax downregulates WWOX expression through activation of the non-canonical NF-κB pathway.11 The Tax-driven, non-canonical NF-κB-mediated reduction of WWOX expression occurs at a transcriptional level, while WWOX protein is reduced by Tax more prominently. Tax fails to reduce WWOX expression in the cells with deficiency of the non-canonical NF-κB subunit p100/p52. Of note, the repression of WWOX expression by the non-canonical NF-κB pathway is not Tax specific, since the pathway activated by either p52 overexpression or CD40 signaling can also decrease WWOX expression.11,69 The p52-mediated gene silencing is probably attributed to the fact that p52 is a DNA-binding protein lacking intrinsic transcriptional activity.11 Considering that hyperactivation of the non-canonical NF-κB pathway frequently occurs in various tumors, it should be a general mechanism to downregulate WWOX expression in cancer cells.70
Functional redirection of WWOX by latent membrane protein 2 A (LMP2A) of Epstein–Barr virus (EBV)12
EBV is a herpesvirus associated with nasopharyngeal carcinomas and various B-cell lymphomas. LMP2A is an EBV oncoprotein contributing to growth, survival, and metastasis of tumor cells.71,72 This membrane protein exerts oncogenic functions through triggering multiple signaling pathways.73 The N-terminal intracellular domain of LMP2A, which is responsible for the signal-triggering events, contains two PPPPY (PY) motifs. We have found that, through the PY motifs, LMP2A activates extracelluar signal-regulated kinase (ERK) and a downstream AP-1 transcription factor Fra-1, thus upregulating matrix metalloproteinase 9 (MMP9) and MMP9-mediated cancer cell invasion.74 As is somewhat unexpected, our recent study reveals that WWOX–LMP2A interaction is actually essential for the LMP2A-triggered ERK-Fra-1-MMP9 pathway.12 Firstly, WWOX physically interacts with LMP2A, which requires Tyr33 in the first WW domain of WWOX and the PY motifs of LMP2A. Knockdown of endogenous WWOX by siRNA significantly inhibits the LMP2A-triggered ERK activation, Fra-1 induction, MMP9 production, and cell invasion. When endogenous WWOX is knocked down, the LMP2A-induced ERK-Fra-1-MMP9 event can be restored by exogenous wild-type WWOX but not by Tyr33-mutated WWOX. Moreover, the ERK-Fra-1-MMP9 pathway cannot be induced by WWOX overexpression in the absence of LMP2A, suggesting that WWOX positively mediates the signaling pathway only when it interacts with LMP2A.
Our data reflect that WWOX may be redirected by LMP2A to a novel role in activation of a proinvasive signaling pathway potentially linked to cancer metastasis, though the underlying mechanisms remain to be explored. In previous studies, under conditions without EBV LMP2A, WWOX exerts negative effects on the MEK1–ERK signaling pathway and cancer cell invasion.9,19,36,50 As LMP2A serves as a signaling adaptor that recruits not only WWOX but also many other factors including protein kinases and ubiquitin ligases, it is likely that WWOX–LMP2A interaction leads WWOX to certain new partners and brings out new functions of WWOX.75–78
After literature reviewing, we notice more examples showing potentially opposite functions of WWOX and EBV LMP2A (Table 1). While WWOX is a proapoptotic tumor suppressor, LMP2A promotes cell survival and cell transformation.21,27,29,35,71,79–81 Expression and activation of WWOX are positively correlated with normal keratinocyte differentiation, but LMP2A inhibits keratinocyte differentiation and contributes to an undifferentiated feature of nasopharyngeal carcinoma.20,71,82 At a molecular level, both WWOX and LMP2A can bind to and stabilize ΔNp63α, a cellular oncoprotein driving cell proliferation and suppressing epithelial cell differentiation, but they cause opposite effects on ΔNp63α activity: inhibition by WWOX and activation by LMP2A.34,83 As for oncogenic signaling transduction, WWOX blocks the Wnt/β-catenin pathway and the canonical NF-κB pathway, while both pathways can be activated by LMP2A.11,49,80,81,84 Therefore, we expect that WWOX–LMP2A interaction may cause antagonism or redirection of WWOX’s functions in many ways, potentially resulting in other impacts on cancer cells.
Table 1.
Opposite biologic or molecular effects of WWOX and EBV LMP2A
| WWOX’s effects | LMP2A’s effects | |
|---|---|---|
| Cell viability | Proapoptosis21,27,29,35 | Prosurvival71,79–81 |
| Cell invasion | Suppression19,36 | Promotion72,74 |
| Keratinocyte differentiation | Positive correlation20 | Inhibition71,82 |
| ΔNp63 activity | Inhibition34 | Activation83 |
| Wnt/β-catenin pathway | Inhibition49 | Activation84 |
| NF-κB pathway | Inhibition11 | Activation80,81 |
Concluding remarks and perspectives
Clinical, biological, and molecular studies conclude the critical roles of WWOX in tumor suppression. Malignant cells must have strategies to withstand this cancer blocker. These strategies include downregulation of WWOX expression at multiple levels and inhibition of WWOX’s functions through protein–protein interaction and/or subcellular mislocalization. An emerging study topic is how cancer-associated microbes deal with WWOX. Some oncogenic microbes may adapt the similar WWOX-targeting strategies used by microbe-free cancer cells. For example, H. pylori infection downregulates WWOX through promoter hypermethylation, an epigenetic mechanism known to suppress WWOX expression in many cancer cells. On the other hand, though WWOX has a potential to inhibit HTLV-1 Tax-triggered canonical NF-κB activation and tumorigenesis, Tax activates the non-canonical NF-κB pathway to successfully suppress WWOX expression. The non-canonical NF-κB-mediated WWOX downregulation is not only induced by this viral oncoprotein but may also be an anti-WWOX strategy shared with other cancers. In addition, EBV LMP2A evolves a novel strategy to redirect WWOX to a proinvasive signaling pathway. Since the N-terminal PPXY-containing domain of LMP2A mimics a cellular signaling adaptor, certain cellular oncoproteins may also twist WWOX’s roles in a LMP2A-like manner.
We believe that the current reports about WWOX–microbe interaction are just “the tip of the iceberg.” PPXY, LPXY, and other potential WWOX-binding motifs may exist in other microbial proteins, especially for those intracellular pathogens that closely interact with host cells. Dysregulation of WWOX at expressional or functional levels should be achieved by many cancer-associated pathogens in various ways, and further studies in this direction should expand our understanding of infection-driven oncogenesis. Meanwhile, the WWOX–microbe interaction may not be restricted to cancer-related pathogens; the broad biologic functions of WWOX can be linked to host–microbe crosstalk in other ways. For example, PPXY-containing motifs also exist in two EBV proteins, BdRF1 (the internal scaffold protein) and BVRF2 (the maturational protease), which are essential for viral capsid assembly.85 Whether WWOX binds to these viral proteins and is thus involved in the replicative stage of EBV is intriguing. We hope this review can trigger more study interests in the potential interactions between WWOX and microbial infection.
ACKNOWLEDGEMENTS
Accomplishment of this review was supported by the National Science Council, Taiwan (NSC99-2628-B-400-001-MY3) and the National Health Research Institutes, Taiwan (IV-102-PP-19 and IV-103-PP-19).
Authors’ contributions
All authors participated in literature reviewing, idea building, and logic designing for this article. YC wrote the manuscript and NSC edited it.
References
- 1.Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 2007; 13: 12–22. [DOI] [PubMed] [Google Scholar]
- 2.Del Mare S, Salah Z, Aqeilan RI. WWOX: its genomics, partners, and functions. J Cell Biochem 2009; 108: 737–45. [DOI] [PubMed] [Google Scholar]
- 3.Chang JY, He RY, Lin HP, Hsu LJ, Lai FJ, Hong Q, Chen SJ, Chang NS. Signaling from membrane receptors to tumor suppressor WW domain-containing oxidoreductase. Exp Biol Med (Maywood) 2010; 235: 796–804. [DOI] [PubMed] [Google Scholar]
- 4.Del Mare S, Kurek KC, Stein GS, Lian JB, Aqeilan RI. Role of the WWOX tumor suppressor gene in bone homeostasis and the pathogenesis of osteosarcoma. Am J Cancer Res 2011; 1: 585–94. [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Liu J, Ren Y, Yang J, Liu P. Common chromosomal fragile site gene WWOX in metabolic disorders and tumors. Int J Biol Sci 2014; 10: 142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludes-Meyers JH, Kil H, Bednarek AK, Drake J, Bedford MT, Aldaz CM. WWOX binds the specific proline-rich ligand PPXY: identification of candidate interacting proteins. Oncogene 2004; 23: 5049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu-Odeh M, Bar-Mag T, Huang H, Kim T, Salah Z, Abdeen SK, Sudol M, Reichmann D, Sidhu S, Kim PM, Aqeilan RI. Characterizing WW domain interactions of tumor suppressor WWOX reveals its association with multiprotein networks. J Biol Chem 2014; 289: 8865–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HY, Juo LI, Lin YT, Hsiao M, Lin JT, Tsai CH, Tzeng YH, Chuang YC, Chang NS, Yang CN, Lu PJ. WW domain-containing oxidoreductase promotes neuronal differentiation via negative regulation of glycogen synthase kinase 3β. Cell Death Differ 2012; 19: 1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sze CI, Su M, Pugazhenthi S, Jambal P, Hsu LJ, Heath J, Schultz L, Chang NS. Down-regulation of WW domain-containing oxidoreductase induces Tau phosphorylation in vitro. A potential role in Alzheimer’s disease. J Biol Chem 2004; 279: 30498–506. [DOI] [PubMed] [Google Scholar]
- 10.Yan J, Zhang M, Zhang J, Chen X, Zhang X. Helicobacter pylori infection promotes methylation of WWOX gene in human gastric cancer. Biochem Biophys Res Commun 2011; 408: 99–102. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Qu Z, Yan P, Ishikawa C, Aqeilan RI, Rabson AB, Xiao G. The tumor suppressor gene WWOX links the canonical and noncanonical NF-κB pathways in HTLV-I Tax-mediated tumorigenesis. Blood 2011; 117: 1652–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan YY, Wu SY, Lai HC, Chang NS, Chang FH, Tsai MH, Su IJ, Chang Y. WW domain-containing oxidoreductase is involved in upregulation of matrix metalloproteinase 9 by Epstein-Barr virus latent membrane protein 2A. Biochem Biophys Res Commun 2013; 436: 672–6. [DOI] [PubMed] [Google Scholar]
- 13.Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3–24.1, a region frequently affected in breast cancer. Cancer Res 2000; 60: 2140–5. [PubMed] [Google Scholar]
- 14.Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E, Richards RI. Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet 2000; 9: 1651–63. [DOI] [PubMed] [Google Scholar]
- 15.Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, McCue P, Huebner K. The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer 2004; 100: 1605–14. [DOI] [PubMed] [Google Scholar]
- 16.Aqeilan RI, Donati V, Gaudio E, Nicoloso MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H, Croce CM, Elenius K. Association of Wwox with ErbB4 in breast cancer. Cancer Res 2007; 67: 9330–6. [DOI] [PubMed] [Google Scholar]
- 17.Nunez MI, Ludes-Meyers J, Abba MC, Kil H, Abbey NW, Page RE, Sahin A, Klein-Szanto AJ, Aldaz CM. Frequent loss of WWOX expression in breast cancer: correlation with estrogen receptor status. Breast Cancer Res Treat 2005; 89: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunez MI, Rosen DG, Ludes-Meyers JH, Abba MC, Kil H, Page R, Klein-Szanto AJ, Godwin AK, Liu J, Mills GB, Aldaz CM. WWOX protein expression varies among ovarian carcinoma histotypes and correlates with less favorable outcome. BMC Cancer 2005; 5: 64–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurek KC, Del Mare S, Salah Z, Abdeen S, Sadiq H, Lee SH, Gaudio E, Zanesi N, Jones KB, DeYoung B, Amir G, Gebhardt M, Warman M, Stein GS, Stein JL, Lian JB, Aqeilan RI. Frequent attenuation of the WWOX tumor suppressor in osteosarcoma is associated with increased tumorigenicity and aberrant RUNX2 expression. Cancer Res 2010; 70: 5577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai FJ, Cheng CL, Chen ST, Wu CH, Hsu LJ, Lee JY, Chao SC, Sheen MC, Shen CL, Chang NS, Sheu HM. WOX1 is essential for UVB irradiation-induced apoptosis and down-regulated via translational blockade in UVB-induced cutaneous squamous cell carcinoma in vivo. Clin Cancer Res 2005; 11: 5769–77. [DOI] [PubMed] [Google Scholar]
- 21.Qin HR, Iliopoulos D, Semba S, Fabbri M, Druck T, Volinia S, Croce CM, Morrison CD, Klein RD, Huebner K. A role for the WWOX gene in prostate cancer. Cancer Res 2006; 66: 6477–81. [DOI] [PubMed] [Google Scholar]
- 22.Pluciennik E, Kusinska R, Potemski P, Kubiak R, Kordek R, Bednarek AK. WWOX—the FRA16D cancer gene: expression correlation with breast cancer progression and prognosis. Eur J Surg Oncol 2006; 32: 153–7. [DOI] [PubMed] [Google Scholar]
- 23.Guler G, Himmetoglu C, Jimenez RE, Geyer SM, Wang WP, Costinean S, Pilarski RT, Morrison C, Suren D, Liu J, Chen J, Kamal J, Shapiro CL, Huebner K. Aberrant expression of DNA damage response proteins is associated with breast cancer subtype and clinical features. Breast Cancer Res Treat 2011; 129: 421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gothlin Eremo A, Wegman P, Stal O, Nordenskjold B, Fornander T, Wingren S. Wwox expression may predict benefit from adjuvant tamoxifen in randomized breast cancer patients. Oncol Rep 2013; 29: 1467–74. [DOI] [PubMed] [Google Scholar]
- 25.Lin JT, Tzai TS, Liao CY, Wang JS, Wu TT, Wang HY, Wu CH, Yu CC, Lu PJ. WWOX protein expression varies among RCC histotypes and downregulation of WWOX protein correlates with less-favorable prognosis in clear RCC. Ann Surg Oncol 2013; 20: 193–9. [DOI] [PubMed] [Google Scholar]
- 26.Chang NS, Schultz L, Hsu LJ, Lewis J, Su M, Sze CI. 17β-Estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene 2005; 24: 714–23. [DOI] [PubMed] [Google Scholar]
- 27.Fabbri M, Iliopoulos D, Trapasso F, Aqeilan RI, Cimmino A, Zanesi N, Yendamuri S, Han SY, Amadori D, Huebner K, Croce CM. WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc Natl Acad Sci USA 2005; 102: 15611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang MF, Yeh ST, Liao HF, Chang NS, Chen YJ. Overexpression of WW domain-containing oxidoreductase WOX1 preferentially induces apoptosis in human glioblastoma cells harboring mutant p53. Biomed Pharmacother 2012; 66: 433–8. [DOI] [PubMed] [Google Scholar]
- 29.Kuroki T, Yendamuri S, Trapasso F, Matsuyama A, Aqeilan RI, Alder H, Rattan S, Cesari R, Nolli ML, Williams NN, Mori M, Kanematsu T, Croce CM. The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenesis. Clin Cancer Res 2004; 10: 2459–65. [DOI] [PubMed] [Google Scholar]
- 30.Bednarek AK, Keck-Waggoner CL, Daniel RL, Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ, Aldaz CM. WWOX, the FRA16D gene, behaves as a suppressor of tumor growth. Cancer Res 2001; 61: 8068–73. [PubMed] [Google Scholar]
- 31.Hong Q, Sze CI, Lin SR, Lee MH, He RY, Schultz L, Chang JY, Chen SJ, Boackle RJ, Hsu LJ, Chang NS. Complement C1q activates tumor suppressor WWOX to induce apoptosis in prostate cancer cells. PLoS One 2009; 4: e5755–e5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang NS, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J Biol Chem 2001; 276: 3361–70. [DOI] [PubMed] [Google Scholar]
- 33.Hsu LJ, Schultz L, Hong Q, Van Moer K, Heath J, Li MY, Lai FJ, Lin SR, Lee MH, Lo CP, Lin YS, Chen ST, Chang NS. Transforming growth factor β1 signaling via interaction with cell surface Hyal-2 and recruitment of WWOX/WOX1. J Biol Chem 2009; 284: 16049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salah Z, Bar-mag T, Kohn Y, Pichiorri F, Palumbo T, Melino G, Aqeilan RI. Tumor suppressor WWOX binds to ΔNp63α and sensitizes cancer cells to chemotherapy. Cell Death Dis 2013; 4: e480–e480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang NS, Doherty J, Ensign A, Schultz L, Hsu LJ, Hong Q. WOX1 is essential for tumor necrosis factor-, UV light-, staurosporine-, and p53-mediated cell death, and its tyrosine 33-phosphorylated form binds and stabilizes serine 46-phosphorylated p53. J Biol Chem 2005; 280: 43100–8. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi T, Adachi Y, Nagayama T. A WWOX-binding molecule, transmembrane protein 207, is related to the invasiveness of gastric signet-ring cell carcinoma. Carcinogenesis 2012; 33: 548–54. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Huang CJ, Tian Y, Wang YP, Han ZG, Li XC. Ectopic overexpression of COTE1 promotes cellular invasion of hepatocellular carcinoma. Asian Pac J Cancer Prev 2012; 13: 5799–804. [DOI] [PubMed] [Google Scholar]
- 38.Gourley C, Paige AJ, Taylor KJ, Ward C, Kuske B, Zhang J, Sun M, Janczar S, Harrison DJ, Muir M, Smyth JF, Gabra H. WWOX gene expression abolishes ovarian cancer tumorigenicity in vivo and decreases attachment to fibronectin via integrin α3. Cancer Res 2009; 69: 4835–42. [DOI] [PubMed] [Google Scholar]
- 39.Aqeilan RI, Trapasso F, Hussain S, Costinean S, Marshall D, Pekarsky Y, Hagan JP, Zanesi N, Kaou M, Stein GS, Lian JB, Croce CM. Targeted deletion of Wwox reveals a tumor suppressor function. Proc Natl Acad Sci USA 2007; 104: 3949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aqeilan RI, Hassan MQ, de Bruin A, Hagan JP, Volinia S, Palumbo T, Hussain S, Lee SH, Gaur T, Stein GS, Lian JB, Croce CM. The WWOX tumor suppressor is essential for postnatal survival and normal bone metabolism. J Biol Chem 2008; 283: 21629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludes-Meyers JH, Kil H, Nunez MI, Conti CJ, Parker-Thornburg J, Bedford MT, Aldaz CM. WWOX hypomorphic mice display a higher incidence of B-cell lymphomas and develop testicular atrophy. Genes Chromosomes Cancer 2007; 46: 1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdeen SK, Salah Z, Maly B, Smith Y, Tufail R, Abu-Odeh M, Zanesi N, Croce CM, Nawaz Z, Aqeilan RI. Wwox inactivation enhances mammary tumorigenesis. Oncogene 2011; 30: 3900–6. [DOI] [PubMed] [Google Scholar]
- 43.Ludes-Meyers JH, Kil H, Parker-Thornburg J, Kusewitt DF, Bedford MT, Aldaz CM. Generation and characterization of mice carrying a conditional allele of the Wwox tumor suppressor gene. PLoS One 2009; 4: e7775–e7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aqeilan RI, Pekarsky Y, Herrero JJ, Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G, Huebner K, Croce CM. Functional association between Wwox tumor suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci USA 2004; 101: 4401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aqeilan RI, Palamarchuk A, Weigel RJ, Herrero JJ, Pekarsky Y, Croce CM. Physical and functional interactions between the Wwox tumor suppressor protein and the AP-2γ transcription factor. Cancer Res 2004; 64: 8256–61. [DOI] [PubMed] [Google Scholar]
- 46.Gaudio E, Palamarchuk A, Palumbo T, Trapasso F, Pekarsky Y, Croce CM, Aqeilan RI. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res 2006; 66: 11585–9. [DOI] [PubMed] [Google Scholar]
- 47.Aqeilan RI, Donati V, Palamarchuk A, Trapasso F, Kaou M, Pekarsky Y, Sudol M, Croce CM. WW domain-containing proteins, WWOX and YAP, compete for interaction with ErbB-4 and modulate its transcriptional function. Cancer Res 2005; 65: 6764–72. [DOI] [PubMed] [Google Scholar]
- 48.Li MY, Lai FJ, Hsu LJ, Lo CP, Cheng CL, Lin SR, Lee MH, Chang JY, Subhan D, Tsai MS, Sze CI, Pugazhenthi S, Chang NS, Chen ST. Dramatic co-activation of WWOX/WOX1 with CREB and NF-κB in delayed loss of small dorsal root ganglion neurons upon sciatic nerve transection in rats. PLoS One 2009; 4: e7820–e7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, Lidereau R, Lallemand F. Inhibition of the Wnt/β-catenin pathway by the WWOX tumor suppressor protein. Oncogene 2009; 28: 2569–80. [DOI] [PubMed] [Google Scholar]
- 50.Lin HP, Chang JY, Lin SR, Lee MH, Huang SS, Hsu LJ, Chang NS. Identification of an in vivo MEK/WOX1 complex as a master switch for apoptosis in T cell leukemia. Genes Cancer 2011; 2: 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Driouch K, Prydz H, Monese R, Johansen H, Lidereau R, Frengen E. Alternative transcripts of the candidate tumor suppressor gene, WWOX, are expressed at high levels in human breast tumors. Oncogene 2002; 21: 1832–40. [DOI] [PubMed] [Google Scholar]
- 52.Park SW, Ludes-Meyers J, Zimonjic DB, Durkin ME, Popescu NC, Aldaz CM. Frequent downregulation and loss of WWOX gene expression in human hepatocellular carcinoma. Br J Cancer 2004; 91: 753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuroki T, Trapasso F, Shiraishi T, Alder H, Mimori K, Mori M, Croce CM. Genetic alterations of the tumor suppressor gene WWOX in esophageal squamous cell carcinoma. Cancer Res 2002; 62: 2258–60. [PubMed] [Google Scholar]
- 54.Roy D, Sin SH, Damania B, Dittmer DP. Tumor suppressor genes FHIT and WWOX are deleted in primary effusion lymphoma (PEL) cell lines. Blood 2011; 118: e32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yendamuri S, Kuroki T, Trapasso F, Henry AC, Dumon KR, Huebner K, Williams NN, Kaiser LR, Croce CM. WW domain containing oxidoreductase gene expression is altered in non-small cell lung cancer. Cancer Res 2003; 63: 878–81. [PubMed] [Google Scholar]
- 56.Gourley C, Paige AJ, Taylor KJ, Scott D, Francis NJ, Rush R, Aldaz CM, Smyth JF, Gabra H. WWOX mRNA expression profile in epithelial ovarian cancer supports the role of WWOX variant 1 as a tumour suppressor, although the role of variant 4 remains unclear. Int J Oncol 2005; 26: 1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iliopoulos D, Guler G, Han SY, Johnston D, Druck T, McCorkell KA, Palazzo J, McCue PA, Baffa R, Huebner K. Fragile genes as biomarkers: epigenetic control of WWOX and FHIT in lung, breast and bladder cancer. Oncogene 2005; 24: 1625–33. [DOI] [PubMed] [Google Scholar]
- 58.Mahajan NP, Whang YE, Mohler JL, Earp HS. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res 2005; 65: 10514–23. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe A, Hippo Y, Taniguchi H, Iwanari H, Yashiro M, Hirakawa K, Kodama T, Aburatani H. An opposing view on WWOX protein function as a tumor suppressor. Cancer Res 2003; 63: 8629–33. [PubMed] [Google Scholar]
- 60.Chang NS, Doherty J, Ensign A. JNK1 physically interacts with WW domain-containing oxidoreductase (WOX1) and inhibits WOX1-mediated apoptosis. J Biol Chem 2003; 278: 9195–202. [DOI] [PubMed] [Google Scholar]
- 61.Hong Q, Hsu LJ, Schultz L, Pratt N, Mattison J, Chang NS. Zfra affects TNF-mediated cell death by interacting with death domain protein TRADD and negatively regulates the activation of NF-κB, JNK1, p53 and WOX1 during stress response. BMC Mol Biol 2007; 8: 50–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991; 325: 1127–31. [DOI] [PubMed] [Google Scholar]
- 63.Yuan JP, Li T, Chen HB, Li ZH, Yang GZ, Hu BY, Shi XD, Tong SQ, Li YX, Guo XK. Analysis of gene expression profile in gastric cancer cells stimulated with Helicobacter pylori isogenic strains. J Med Microbiol 2004; 53: 965–74. [DOI] [PubMed] [Google Scholar]
- 64.Peterson AJ, Menheniott TR, O'Connor L, Walduck AK, Fox JG, Kawakami K, Minamoto T, Ong EK, Wang TC, Judd LM, Giraud AS. Helicobacter pylori infection promotes methylation and silencing of trefoil factor 2, leading to gastric tumor development in mice and humans. Gastroenterology 2010; 139: 2005–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nerenberg M, Hinrichs SH, Reynolds RK, Khoury G, Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science 1987; 237: 1324–9. [DOI] [PubMed] [Google Scholar]
- 66.Chu ZL, Shin YA, Yang JM, DiDonato JA, Ballard DW. IKKγ mediates the interaction of cellular IκB kinases with the tax transforming protein of human T cell leukemia virus type 1. J Biol Chem 1999; 274: 15297–300. [DOI] [PubMed] [Google Scholar]
- 67.O'Mahony AM, Montano M, Van Beneden K, Chen LF, Greene WC. Human T-cell lymphotropic virus type 1 tax induction of biologically active NF-κB requires IκB kinase-1-mediated phosphorylation of RelA/p65. J Biol Chem 2004; 279: 18137–45. [DOI] [PubMed] [Google Scholar]
- 68.Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, Sun SC. Retroviral oncoprotein Tax induces processing of NF-κB2/p100 in T cells: evidence for the involvement of IKKα. EMBO J 2001; 20: 6805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qing G, Qu Z, Xiao G. Endoproteolytic processing of C-terminally truncated NF-κB2 precursors at κB-containing promoters. Proc Natl Acad Sci USA 2007; 104: 5324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun SC, Xiao G. Deregulation of NF-κB and its upstream kinases in cancer. Cancer Metastasis Rev 2003; 22: 405–22. [DOI] [PubMed] [Google Scholar]
- 71.Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol 2000; 74: 10681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu J, Lin WH, Chen SY, Longnecker R, Tsai SC, Chen CL, Tsai CH. Syk tyrosine kinase mediates Epstein-Barr virus latent membrane protein 2A-induced cell migration in epithelial cells. J Biol Chem 2006; 281: 8806–14. [DOI] [PubMed] [Google Scholar]
- 73.Pang MF, Lin KW, Peh SC. The signaling pathways of Epstein-Barr virus-encoded latent membrane protein 2A (LMP2A) in latency and cancer. Cell Mol Biol Lett 2009; 14: 222–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lan YY, Hsiao JR, Chang KC, Chang JS, Chen CW, Lai HC, Wu SY, Yeh TH, Chang FH, Lin WH, Su IJ, Chang Y. Epstein-Barr virus latent membrane protein 2A promotes invasion of nasopharyngeal carcinoma cells through ERK/Fra-1-mediated induction of matrix metalloproteinase 9. J Virol 2012; 86: 6656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ikeda M, Ikeda A, Longnecker R. PY motifs of Epstein-Barr virus LMP2A regulate protein stability and phosphorylation of LMP2A-associated proteins. J Virol 2001; 75: 5711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swart R, Ruf IK, Sample J, Longnecker R. Latent membrane protein 2A-mediated effects on the phosphatidylinositol 3-kinase/Akt pathway. J Virol 2000; 74: 10838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Panousis CG, Rowe DT. Epstein-Barr virus latent membrane protein 2 associates with and is a substrate for mitogen-activated protein kinase. J Virol 1997; 71: 4752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winberg G, Matskova L, Chen F, Plant P, Rotin D, Gish G, Ingham R, Ernberg I, Pawson T. Latent membrane protein 2A of Epstein-Barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases. Mol Cell Biol 2000; 20: 8526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fukuda M, Longnecker R. Latent membrane protein 2A inhibits transforming growth factor-β1-induced apoptosis through the phosphatidylinositol 3-kinase/Akt pathway. J Virol 2004; 78: 1697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hino R, Uozaki H, Inoue Y, Shintani Y, Ushiku T, Sakatani T, Takada K, Fukayama M. Survival advantage of EBV-associated gastric carcinoma: survivin up-regulation by viral latent membrane protein 2A. Cancer Res 2008; 68: 1427–35. [DOI] [PubMed] [Google Scholar]
- 81.Swanson-Mungerson M, Bultema R, Longnecker R. Epstein-Barr virus LMP2A imposes sensitivity to apoptosis. J Gen Virol 2010; 91: 2197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morrison JA, Raab-Traub N. Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of β-catenin signaling. J Virol 2005; 79: 2375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fotheringham JA, Mazzucca S, Raab-Traub N. Epstein-Barr virus latent membrane protein-2A-induced ΔNp63α expression is associated with impaired epithelial-cell differentiation. Oncogene 2010; 29: 4287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morrison JA, Klingelhutz AJ, Raab-Traub N. Epstein-Barr virus latent membrane protein 2A activates β-catenin signaling in epithelial cells. J Virol 2003; 77: 12276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Henson BW, Perkins EM, Cothran JE, Desai P. Self-assembly of Epstein-Barr virus capsids. J Virol 2009; 83: 3877–90. [DOI] [PMC free article] [PubMed] [Google Scholar]