Abstract
WW domain containing oxidoreductase, designated WWOX, FOR or WOX1, is a known pro-apoptotic factor when ectopically expressed in various types of cancer cells, including glioblastoma multiforme (GBM). The activation of sonic hedgehog (Shh) signaling, especially paracrine Shh secretion in response to radiation, is associated with impairing the effective irradiation of cancer cells. Here, we examined the role of Shh signaling and WOX1 overexpression in the radiosensitivity of human GBM cells. Our results showed that ionizing irradiation (IR) increased the cytoplasmic Shh and nuclear Gli-1 content in GBM U373MG and U87MG cells. GBM cells with exogenous Shh treatment exhibited similar results. Pretreatment with Shh peptides protected U373MG and U87MG cells against IR in a dose-dependent manner. Cyclopamine, a Hedgehog/Smoothened (SMO) inhibitor, reversed the protective effect of Shh in U87MG cells. Cyclopamine increased Shh plus IR-induced H2AX, a marker of DNA double-strand breaks, in these cells. To verify the role of Shh signaling in the radiosensitivity of GBM cells, we tested the effect of the Gli family zinc finger 1 (Gli-1) inhibitor zerumbone and found that it could sensitize GBM cells to IR. We next examined the role of WOX1 in radiosensitivity. Overexpression of WOX1 enhanced the radiosensitivity of U87MG (possessing wild type p53 or WTp53) but not U373MG (harboring mutant p53 or MTp53) cells. Pretreatment with Shh peptides protected both WOX1-overexpressed U373MG and U87MG cells against IR and increased the cytoplasmic Shh and nuclear Gli-1 content. Zerumbone enhanced the radiosensitivity of WOX1-overexpressed U373MG and U87MG cells. In conclusion, overexpression of WOX1 preferentially sensitized human GBM cells possessing wild type p53 to radiation therapy. Blocking of Shh signaling may enhance radiosensitivity independently of the expression of p53 and WOX1. The crosstalk between Shh signaling and WOX1 expression in human glioblastoma warrants further investigation.
Keywords: Sonic hedgehog, WOX1, p53, glioblastoma, radiosensitivity
Introduction
Glioblastoma multiforme (GBM), also known as a WHO grade IV astrocytoma, is one of the most lethal brain malignancies in adults.1 Despite aggressive surgical resection and adjuvant intensified radiation therapy, even concurrent with chemotherapy, the five-year overall survival prognosis for GBM patients remains poor at less than 10%.2 Treatment resistance and a high recurrence rate are the main causes of death in GBM patients. It is important to search for novel targets and develop molecular-targeted therapeutic regimens for further treatment of GBM.
Sonic hedgehog (Shh) is a signaling protein discovered in Drosophila that plays a critical role during embryogenesis. The Shh signaling pathway regulates the proliferation and differentiation of various types of stem cells.3,4 It mediates the activation of the transcription factors of the Gli family. Upon activation, Gli proteins translocate into the nucleus from the cytosol and activate target gene transcription to control the cell cycle, cell adhesion, signal transduction, angiogenesis, and apoptosis.5 Shh signaling and the release of paracrine in response to IR have been demonstrated to be protective against IR in hepatocellular carcinoma cells.6 Nuclear Gli-1 overexpression correlated with primary tumor size, lymphatic metastasis, and tumor recurrence in patients with oral cavity squamous cell carcinoma that received surgery and radiotherapy.7
The WW domain containing oxidoreductase gene (WOX1) has been studied in various kinds of cancer cells.8–10 The WOX1 protein has been shown to be a tumor suppressor with pro-apoptotic properties, and it can work synergistically to induce apoptosis with p53.8,11 The expression of WOX1 is known to be altered in multiple malignancies, such as non-small cell lung carcinoma,12 gastric carcinoma,13 pancreatic carcinoma,14 and invasive breast carcinoma.15 The restoration of the WOX1 gene could prevent the growth of multiple cancers, such as lung cancer16 and pancreatic cancer.17 In treatment evaluation, the overexpression of WOX1 preferentially inhibited cell viability and induced apoptosis in human glioblastoma U373 MG cells expressing mutant p53 via a mechanism independent of the intrinsic apoptotic pathway.18
p53 is a well known tumor suppressor. The N-terminal proline-rich region and the C-terminal basic region are essential for p53 to mediate apoptosis.19 It has been previously reported that p53 can interact with WOX1 on the WW domain via its proline-rich region,20 and the stabilization of phosphorylated p53 by WOX1 is essential for p53-mediated cell death.21 For radiotherapy effectiveness, the presence of mutant p53 has been reported to be an unfavorable prognostic factor in glioma cells.22 Collectively, the status of WOX1 and p53 may have a role in modulating treatment susceptibility in glioma cells.
In clinical practice, clarifying the role of each therapeutic factor may help in the development of biomarkers and therapeutic targets for patients. Given that WOX1 and Shh signaling could modulate the IR sensitivity of glioma cells for treatment, the functional interactions of WOX1 with the component(s) of the Shh signaling may have a significant clinical potential for the development of new strategies to treat GBM.
In this study, we examined the role of Shh signaling and WOX1 overexpression in the radiosensitivity of human GBM cell lines that have different p53 statuses.
Materials and methods
Cell lines and transfection
Human glioblastoma cell lines, U87MG and U373MG, were cultured in a DMEM medium supplemented with 10% fetal bovine serum at 37℃ and humidified with 5% CO2. Cells were transfected with pEGFPC1 (Clontech Laboratories, Inc., Palo Alto, California, USA) and human WOX1-pEGFPC1 using a jetPEI™ transfection reagent (Polyplus Transfection, Illkrich, France). The cells were sorted by GFP fluorescence expression using flow cytometry before performing further experiments.
Immunofluorescence staining
Cells were seeded on cover slips in a 24-well plate. For immunofluorescence staining, the cells were fixed by cold methanol and blocked by 5% bovine serum albumin. The cells on the cover slips were incubated with a specific antibody against Shh and Gli-1 (Santa Cruz Biothechnology, CA, USA) for 1 h at room temperature. After washing, the cells were then incubated with anti-mouse FITC-conjugated secondary antibodies (1:100; Molecular Probes, Eugene, OR, USA). The cover slips were mounted with VECTASHIELD Mounting Medium containing DAPI (Vecta Laboratories, Burlington, CA, USA).
Shh treatment and radiation delivery
Cells were pretreated with various doses (10 pg/mL–1 ng/mL) of Shh for 24 h. After washing, the cells were irradiated with graded doses (sham RT, 1, 2 and 4 Gy) of 6 MeV of electron beam energy delivered by a linear accelerator (Clinac® 1800, Varian Associates, Inc., CA.; dose rate 2.4 Gy/min) in a single fraction. Full electron equilibrium was ensured for each fraction by using a parallel plate PR-60C ionization chamber (CAPINTEL, Inc., Ramsey, NJ, USA). After radiation, cells were plated for the colony formation assay.
Colony formation assay
Viable tumor cells (103) were plated into separate 35-mm culture dishes to grow in McCoy’s 5 A medium containing 20% heat-inactivated fetal bovine serum and 0.24% agarose at 37℃ in a humidified 5% CO2 incubator. After 10–14 days, the dishes were stained with crystal violet, and colonies containing more than 50 cells were counted. The surviving fraction was calculated as mean colonies (cells inoculated × plating efficiency). The control plating efficiency for U87MG and U373MG cells was approximately 63% and 55%, respectively. Survival curves were plotted by a linear-quadratic model as previously described.6
Immunofluorescence for Shh, Gli-1, and gamma-H2AX expression
For the immunofluorescence assay, cells were harvested, washed, blocked, and stained with primary rabbit anti-human polyclonal antibodies against Shh and Gli-1 (Santa Cruz Biothechnology, CA, USA), followed by the secondary antibody rhodamine red-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). For the detection of DNA double-strand breaks with gamma-H2AX antibody, we used primary mouse anti-human monoclonal Ab against gamma-H2AX (Abcam, Cambridge, MA, USA), followed by the secondary CytTM-2-conjugated donkey anti-mouse IgG (Jackson Immuno-Research Laboratories, West Grove, PA, USA). Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA) was used for nuclear counterstaining before mounting. Cell images were obtained using an Olympus fluorescence microscope.
Calculation for sensitizer enhancement ratios
The sensitizer enhancement ratio (SER) was calculated as the radiation dose needed for radiation alone divided by the dose needed for various treatments plus radiation at a survival fraction of 37% (D0 in radiobiology).
Results
Shh signaling and radiosensitivity in human glioblastoma cells
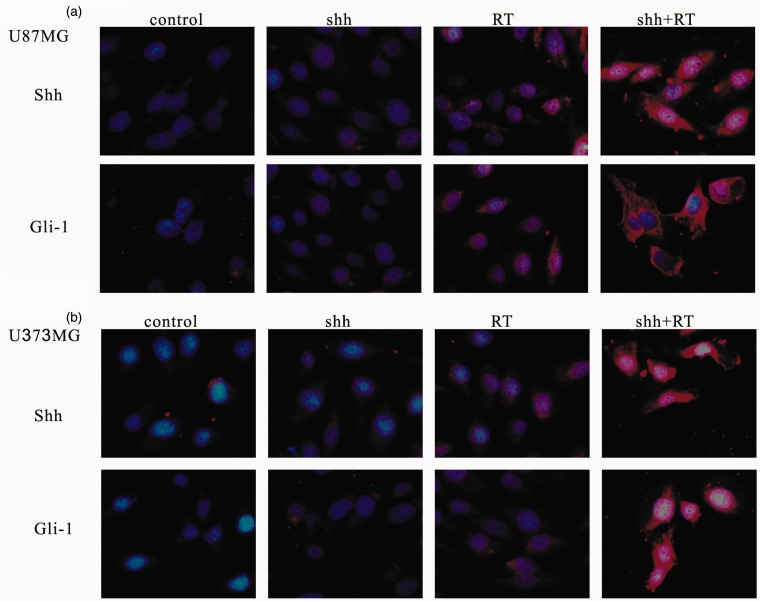
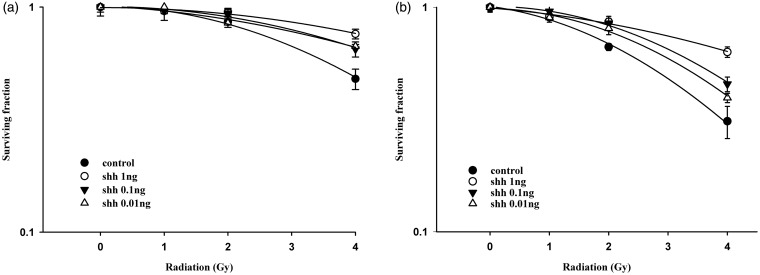
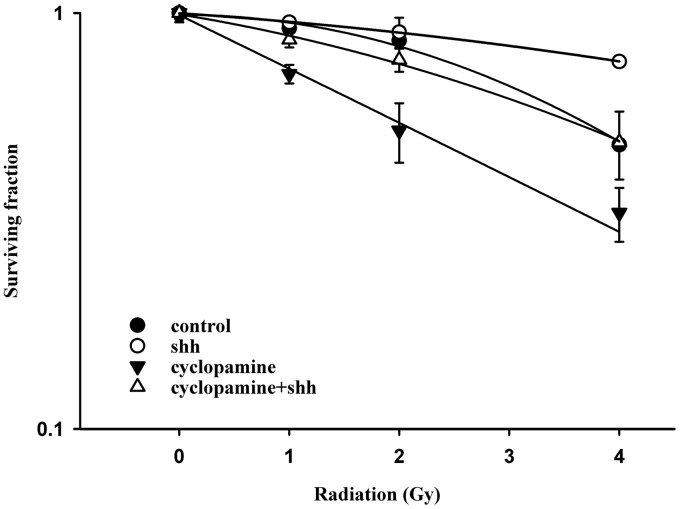
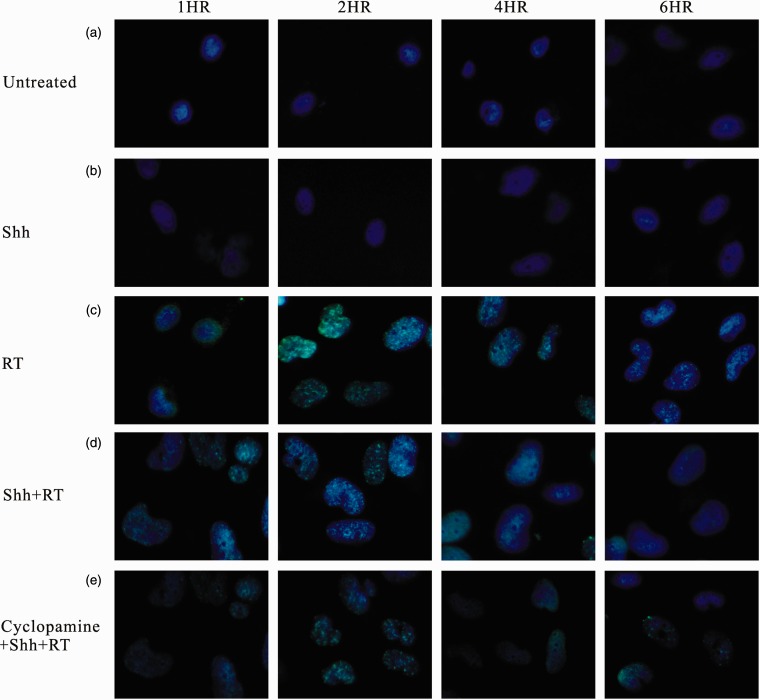
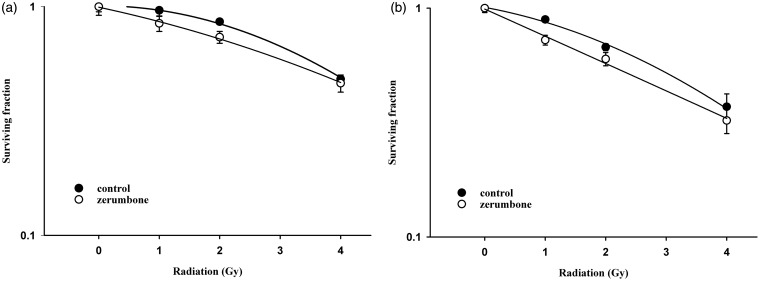
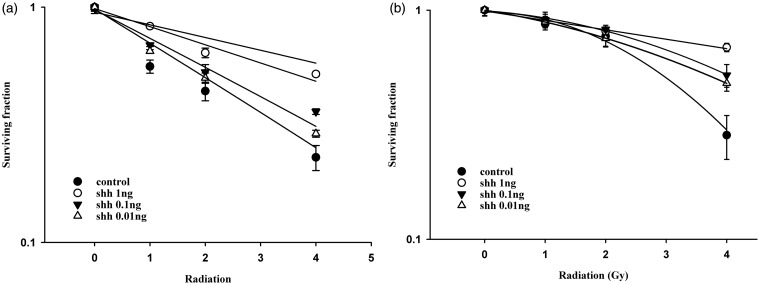
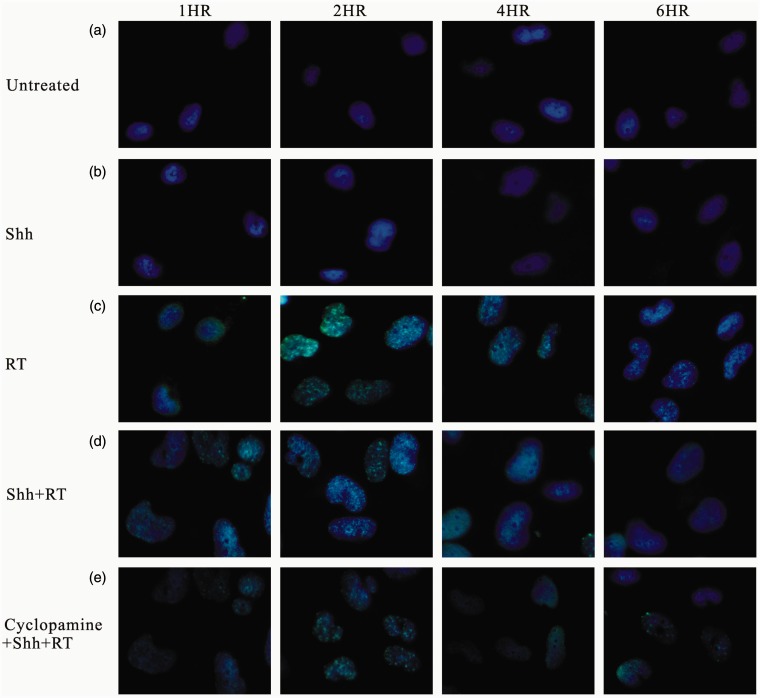
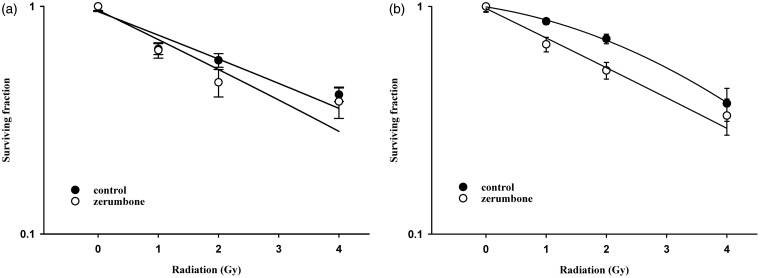
Ionizing irradiation (IR) increased the cytoplasmic Shh and nuclear Gli-1 content in GBM U373MG and U87MG cells (Figure 1), with an immunofluorescence assay used to obtain a semi-quantiation estimation. Exogenous Shh treatment exhibited similar results on the expression of Shh and Gli-1 (Figure 1). Pretreatment with the N-terminal Shh peptide protected U373MG and U87MG cells against IR with increased survival in a dose-dependent manner (Figure 2). A mechanistic assay showed that cyclopamine, an SMO inhibitor, reversed the protective effect of Shh in U87MG cells (Figure 3). Cyclopamine increased the expression of Shh plus IR-induced γ-H2AX, a marker of DNA double-strand break, in these cells (Figure 4) according to the immunofluorescence assay. To further assess the role of Shh signaling in the radiosensitivity of GBM cells, we tested the radiosensitizing effect of the Gli-1 inhibitor zerumbone. The results showed that zerumbone sensitized U373MG and U87MG cells to IR (Figure 5).
Figure 1.
Immunofluorescence analysis for expression of Shh and Gli-1 in human glioblastoma cells. (a) U87MG cells. (b) U373MG cells. For immunofluorescence staining, cells were harvested after radiation, stained with Shh or Gli-1 antibodies and secondary Rhodamine Red-conjugated antibody (red), and nuclei were counterstained with DAPI (blue). Original magnification 400×
Figure 2.
Exogenous Shh N-terminal peptide protected human glioblastoma cells against radiation cytotoxicity. (a) U87MG cell radiation survival curves. D0 values (radiation dose with 37% survival) of U87MG cells for control, Shh 0.01 ng/mL, Shh 0.1 ng/mL and Shh 1.0 ng/mL were 4.7 Gy, 6.6 Gy, 6.2 Gy and 7.7 Gy, respectively. (b) U373MG cell radiation survival curves. D0 values (radiation dose with 37% survival) of U373MG cells for control, Shh 0.01 ng/mL, Shh 0.1 ng/mL and Shh 1.0 ng/mL were 3.6 Gy, 4.2 Gy, 4.5 Gy and 6.5 Gy, respectively. Cells were plated for colony formation assay after radiation. After 10–14 days, the dishes were stained with crystal violet, and colonies containing ≥50 cells were counted (in materials and methods section). Data from three separate experiments were expressed as mean ± standard error of mean. Symbol: control (closed circle), Shh 0.01 ng/mL (black down-pointing triangle), Shh 0.1 ng/mL (up down-pointing triangle), Shh 1.0 ng/mL (open circle)
Figure 3.
SMO inhibitor cyclopamine reduced the radioprotective effect of Shh ligand. The U87MG cells were treated with cyclopamine for 24 h before radiation. D0 values (radiation dose with 37% survival) for control, Shh, cyclopamine and cyclopamine plus Shh were 4.7 Gy, 9.6 Gy, 4.7 Gy and 5.1 Gy, respectively. Cells were plated for colony formation assay after radiation. After 10–14 days, the dishes were stained with crystal violet, and colonies containing ≥50 cells were counted. Data from three separate experiments were expressed as mean ± standard error of mean. D0 values (radiation dose with 37% survival) for control, Shh, cyclopamine and cyclopamine plus Shh were 4.7 Gy, 9.6 Gy, 4.7 Gy and 5.1 Gy, respectively
Figure 4.
Immunofluorescent staining for expression of gamma-H2AX in glioblastoma cells. Cells that were (a) untreated (control) or treated with (b) Shh ligand, (c) radiation of 2 Gy, (d) Shh ligand plus radiation, (e) cyclopamine plus Shh ligand and radiation. For immunofluorescent staining, cells were harvested after radiation, stained with gamma-H2AX antibody, secondary Cyt-2-conjugated donkey anti-mouse IgG (green), and nuclei were counterstained with Hoechst 33342 (blue). Original magnification 400×
Figure 5.
Gli-1 inhibitor zerumbone enhanced the radiosensitivity of human glioblastoma cells. (a) U87MG cell radiation survival curves. D0 values (radiation dose with 37% survival) of U87MG cells for control and zerumbone were 4.6 Gy and 4.9 Gy, respectively. (b) U373MG cell radiation survival curves. D0 values (radiation dose with 37% survival) of U373MG cells for control and zerumbone were 4.0 Gy and 3.6 Gy, respectively. Cells were plated for colony formation assay after radiation. After 10–14 days, the dishes were stained with crystal violet, and colonies containing ≥50 cells were counted. Data from three separate experiments were expressed as mean ± standard error of mean
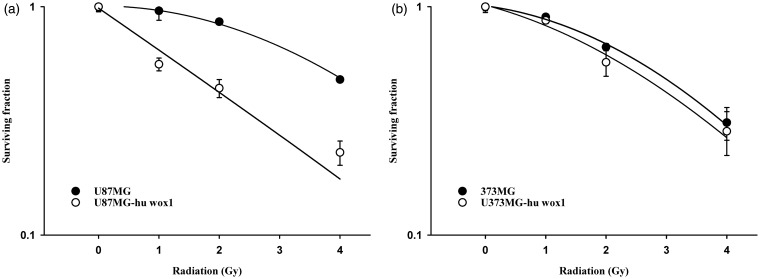
WOX1 overexpression enhanced radiosensitivity in U87MG cells
The overexpression of WOX1 enhanced the radiosensitivity of U87MG (possessing wild type p53) in comparison with parental U87MG cells (Figure 6a). In U373MG cells harboring mutant p53, the overexpression of WOX1 had no significant effect on radiosensitivity (Figure 6b).
Figure 6.
Effect of WOX1 overexpression on radiosensitivity of human glioblastoma cells. (a) Radiation survival curves of U87MG cells with (open circle) and without (closed circle) WOX1 overexpression. D0 values (radiation dose with 37% survival) of U87MG cells with and without WOX1 overexpression were 2.2 Gy and 4.7 Gy, respectively. (b) Radiation survival curves of U373MG cells with (open circle) and without (closed circle) WOX1 overexpression. D0 values (radiation dose with 37% survival) of U373MG cells with and without WOX1 overexpression were 3.6 Gy and 3.7 Gy, respectively. Cells were plated for colony formation assay after radiation. After 10–14 days, the dishes were stained with crystal violet, and colonies containing ≥50 cells were counted. Data from three separate experiments were expressed as mean ± standard error of mean
Shh signaling affected the radiosensitivity of glioblastom cells independent of WOX1 expression
Treatment with an Shh peptide prior to IR protected both WOX1-overexpressed U373MG and U87MG cells against IR (Figure 7) and decreased the expression of γ-H2AX (Figure 8). Zerumbone enhanced the radiosensitivity of WOX1-overexpressed U373MG and U87MG cells (Figure 9).
Figure 7.
Exogenous Shh N-terminal peptide protected WOX1-overexpressed human glioblastoma cells against radiation cytotoxicity. (a) WOX1-overexpressed U87MG cell radiation survival curves. D0 values (radiation dose with 37% survival) of U87MG cells for control, Shh 0.01 ng/mL, Shh 0.1 ng/mL and Shh 1.0 ng/mL were 1.1 Gy, 3.3 Gy, 4.0 Gy and 4.4 Gy, respectively. (b) WOX1-overexpressed U373MG cell radiation survival curves. D0 values (radiation dose with 37% survival) of U373MG cells for control, Shh 0.01 ng/mL, Shh 0.1 ng/mL, and Shh 1.0 ng/mL were 3.6 Gy, 4.9 Gy, 5.1 Gy, and 7.7 Gy, respectively. Cells were plated for colony formation assay after radiation. After 10–14 days, the dishes were stained with crystal violet, and colonies containing ≥50 cells were counted. Data from three separate experiments were expressed as mean ± standard error of mean. Symbol: control (open circle), Shh 0.01 ng/mL (black down-pointing triangle), Shh 0.1 ng/mL (white down-pointing triangle), and Shh 1.0 ng/mL (closed circle)
Figure 8.
Immunofluorescent staining for expression of gamma-H2AX in glioblastoma cells with WOX1 overexpression. Cells that were (a) untreated (control) or treated with (b) Shh ligand, (c) radiation of 2 Gy, (d) Shh ligand plus radiation, and (e) cyclopamine plus Shh ligand and radiation. For immunofluorescent staining, cells were harvested after radiation, stained with gamma-H2AX antibody, secondary Cyt-2-conjugated donkey anti-mouse IgG (green), and nuclei were counterstained with Hoechst 33342 (blue). Original magnification 400×
Figure 9.
Gli-1 inhibitor zerumbone enhanced the radiosensitivity of WOX1-overexpressed human glioblastoma cells. (a) WOX1-overexpressed U87MG cell radiation survival curves. D0 values (radiation dose with 37% survival) of U87MG cells for control and zerumbone were 4.3 Gy and 3.4 Gy, respectively. (b) WOX1-overexpressed U373MG cell radiation survival curves. D0 values (radiation dose with 37% survival) of U373MG cells for control and zerumbone were 4.1 Gy and 2.6 Gy, respectively. Cells were plated for colony formation assay after radiation. After 10–14 days, the dishes were stained with crystal violet, and colonies containing ≥50 cells were counted. Data from three separate experiments were expressed as mean ± standard error of mean. Symbol: control (closed circle), zerumbone (open circle)
Discussion
During skin tumorigenesis, transient overexpression of WOX1 occurs in the initial stage, followed by downregulation as the tumor grows.23,24 Up to date, whether the transiently overexpressed WOX1 induces apoptosis of cancer cells is largely unknown.18,25 Shh signaling involves carcinogenesis, drug resistance, and poor prognosis of various types of cancers.26–28 In this study, we determined that IR might activate Shh signaling to protect cancer cells. Accordingly, synthetic Shh peptides protected human glioblastoma cells from IR-mediated death. The radioprotective effect of the Shh peptides was noted in both parental and WOX1-overexpressed glioblastoma cells and could be reversed by an Shh signaling inhibitor. WOX1 overexpression preferentially sensitized glioblastoma cells possessing wild type p53.
Given that p53 expression enhances intrinsic radiosensitivity,29,30 the differential radiosensitizing effect of WOX1 overexpression in wild type p53 U87MG cells, but not p53 mutant U373MG cells, may involve interactions between the p53 and WOX1 in human glioblastoma cells. We have determined that U373MG cells are more sensitive to apoptosis induced by temozolomide.18 It is interesting that the correlation between WOX1 expression and sensitivity to temozolomide or IR is different and this issue warrants further investigations.
The crosstalk between WOX1 expression and Shh signaling has not been reported. In this study, we have demonstrated for the first time that Shh peptides counteracted the ectopic WOX1-mediated enhancement of radiosensitivity. Furthermore, the inhibitor of Shh signaling zerumbone sensitized glioblastoma cells in both parental and ectopic WOX1-expressing glioblastoma cells. The observation implies that Shh signaling may counteract the radiosensitizing effect of WOX1 overexpression.
Radiation could induce DNA double-strand break and the capacity of cells to repair lethal DNA damages is regarded as a significant factor of radiosensitivity. In this study, we found that radiation-induced DNA double-strand break, with γ©-H2AX as a marker, could be repaired within 6 h after radiation. Exogenous addition of shh peptide shortened the repair time and inhibition of shh signaling reversed this effect in both parental and WOX1-expressing glioblastoma cells. Enhancement of repair for DNA double-strand break might be an important event induced by shh signaling of glioblastoma cells.
The Shh signaling inhibitors used in this study included the SMO inhibitor cyclopamine and the Gli-1 inhibitor zerumbone. Because of their radiosensitizing effects on glioblastoma cells noted in this study, the potential application of these inhibitors in radiotherapy for glioblastoma could be further investigated.
In conclusion, transient overexpression of WOX1 may preferentially sensitize human wild type p53-positive glioblastoma cells to radiation therapy. Blocking the Shh signaling enhances the radiosensitivity of GBM cells independent of the levels of p53 and WOX1. However, WOX1 interaction with a single or multiple components in the Shh signaling in human glioblastoma cells remains to be established.
Acknowledgements
This study was supported by grant NSC 98-2314-B-195-004-MY3 from the National Science Council of Republic of China, Taipei, Taiwan and MMH-E-103-13, MMH-E-102 from Mackay Memorial Hospital, Taipei, Taiwan.
Author contributions
This study was carried out in collaboration between all authors. M-FC and Y-JC designed the research scheme. H-HC, C-WC, M-LH, and H-RS carried out the laboratory experiments and analyzed the data. M-FC wrote the manuscript and discussed issues with the coauthors. C-IS and J-TT organized the revision work and analyzed the revised data to improve the content as well as the quality of the revised manuscript.
References
- 1.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol 2003; 30: 10–4. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009; 10: 459–66. [DOI] [PubMed] [Google Scholar]
- 3.Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet 2000; 67: 1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287: 795–801. [DOI] [PubMed] [Google Scholar]
- 5.Platt KA, Michaud J, Joyner AL. Expression of the mouse Gli and Ptc genes is adjacent to embryonic sources of hedgehog signals suggesting a conservation of pathways between flies and mice. Mech Dev 1997; 62: 121–35. [DOI] [PubMed] [Google Scholar]
- 6.Chen YJ, Lin CP, Hsu ML, Shieh HR, Chao NK, Chao KS. Sonic hedgehog signaling protects human hepatocellular carcinoma cells against ionizing radiation in an autocrine manner. Int J Radiat Oncol Biol Phys 2011; 80: 851–9. [DOI] [PubMed] [Google Scholar]
- 7.Wang YF, Chang CJ, Lin CP, Chang SY, Chu PY, Tai SK, Li WY, Chao KS, Chen YJ. Expression of hedgehog signaling molecules as a prognostic indicator of oral squamous cell carcinoma. Head Neck 2012; 34: 1556–61. [DOI] [PubMed] [Google Scholar]
- 8.Chang NS, Hsu LJ, Lin YS, Lai FJ, Sheu HM. WW domain-containing oxidoreductase: a candidate tumor suppressor. Trends Mol Med 2007; 13: 12–22. [DOI] [PubMed] [Google Scholar]
- 9.Ried K, Finnis M, Hobson L, Mangelsdorf M, Dayan S, Nancarrow JK, Woollatt E, Kremmidiotis G, Gardner A, Venter D, Baker E, Richards RI. Common chromosomal fragile site FRA16D sequence: identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum Mol Genet 2000; 9: 1651–63. [DOI] [PubMed] [Google Scholar]
- 10.Del Mare S, Salah Z, Aqeilan RI. WWOX: its genomics, partners, and functions. J Cell Biochem 2009; 108: 737–45. [DOI] [PubMed] [Google Scholar]
- 11.Chang NS, Doherty J, Ensign A, Lewis J, Heath J, Schultz L, Chen ST, Oppermann U. Molecular mechanisms underlying WOX1 activation during apoptotic and stress responses. Biochem Pharmacol 2003; 66: 1347–54. [DOI] [PubMed] [Google Scholar]
- 12.Donati V, Fontanini G, Dell'Omodarme M, Prati MC, Nuti S, Lucchi M, Mussi A, Fabbri M, Basolo F, Croce CM, Aqeilan RI. WWOX expression in different histologic types and subtypes of non-small cell lung cancer. Clin Cancer Res 2007; 13: 884–91. [DOI] [PubMed] [Google Scholar]
- 13.Aqeilan RI, Kuroki T, Pekarsky Y, Albagha O, Trapasso F, Baffa R, Huebner K, Edmonds P, Croce CM. Loss of WWOX expression in gastric carcinoma. Clin Cancer Res 2004; 10: 3053–8. [DOI] [PubMed] [Google Scholar]
- 14.Kuroki T, Yendamuri S, Trapasso F, Matsuyama A, Aqeilan RI, Alder H, Rattan S, Cesari R, Nolli ML, Williams NN, Mori M, Kanematsu T, Croce CM. The tumor suppressor gene WWOX at FRA16D is involved in pancreatic carcinogenesis. Clin Cancer Res 2004; 10: 2459–65. [DOI] [PubMed] [Google Scholar]
- 15.Aqeilan RI, Donati V, Gaudio E, Nicoloso MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H, Croce CM, Elenius K. Association of Wwox with ErbB4 in breast cancer. Cancer Res 2007; 67: 9330–6. [DOI] [PubMed] [Google Scholar]
- 16.Fabbri M, Iliopoulos D, Trapasso F, Aqeilan RI, Cimmino A, Zanesi N, Yendamuri S, Han SY, Amadori D, Huebner K, Croce CM. WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc Natl Acad Sci U S A 2005; 102: 15611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakayama S, Semba S, Maeda N, Aqeilan RI, Huebner K, Yokozaki H. Role of the WWOX gene, encompassing fragile region FRA16D, in suppression of pancreatic carcinoma cells. Cancer Sci 2008; 99: 1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang MF, Yeh ST, Liao HF, Chang NS, Chen YJ. Overexpression of WW domain-containing oxidoreductase WOX1 preferentially induces apoptosis in human glioblastoma cells harboring mutant p53. Biomed Pharmacother 2012; 66: 433–8. [DOI] [PubMed] [Google Scholar]
- 19.Walker KK, Levine AJ. Identification of a novel p53 functional domain that is necessary for efficient growth suppression. Proc Natl Acad Sci U S A 1996; 93: 15335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang NS, Pratt N, Heath J, Schultz L, Sleve D, Carey GB, Zevotek N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J Biol Chem 2001; 276: 3361–70. [DOI] [PubMed] [Google Scholar]
- 21.Chang NS, Doherty J, Ensign A, Schultz L, Hsu LJ, Hong Q. WOX1 is essential for tumor necrosis factor-, UV light-, staurosporine-, and p53-mediated cell death, and its tyrosine 33-phosphorylated form binds and stabilizes serine 46-phosphorylated p53. J Biol Chem 2005; 280: 43100–8. [DOI] [PubMed] [Google Scholar]
- 22.Squatrito M, Brennan CW, Helmy K, Huse JT, Petrini JH, Holland EC. Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell 2010; 18: 619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai FJ, Cheng CL, Chen ST, Wu CH, Hsu LJ, Lee JY, Chao SC, Sheen MC, Shen CL, Chang NS, Sheu HM. WOX1 is essential for UVB irradiation-induced apoptosis and down-regulated via translational blockade in UVB-induced cutaneous squamous cell carcinoma in vivo. Clin Cancer Res 2005; 11: 5769–77. [DOI] [PubMed] [Google Scholar]
- 24.Chiang MF, Chen ST, Lo CP, Sze CI, Chang NS, Chen YJ. Expression of WW domain-containing oxidoreductase WOX1 in human nervous system tumors. Anal Cell Pathol (Amst) 2013; 36: 133–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang NS, Doherty J, Ensign A. JNK1 physically interacts with WW domain-containing oxidoreductase (WOX1) and inhibits WOX1-mediated apoptosis. J Biol Chem 2003; 278: 9195–202. [DOI] [PubMed] [Google Scholar]
- 26.Clemons N, Phillips W, Lord RV. Signaling pathways in the molecular pathogenesis of adenocarcinomas of the esophagus and gastresophageal junction. Cancer Biol Ther 2013;14: 782--95. [DOI] [PMC free article] [PubMed]
- 27.Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell 2014; 25: 139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keysar SB, Le PN, Anderson RT, Morton JJ, Bowles DW, Paylor JJ, Vogler BW, Thorburn J, Fernandez P, Glogowska MJ, Takimoto SM, Sehrt DB, Gan GN, Eagles-Soukup JR, Serracino H, Hirsch FR, Lucia MS, Thorburn A, Song JI, Wang XJ, Jimeno A. Hedgehog signaling alters reliance on EGF receptor signaling and mediates anti-EGFR therapeutic resistance in head and neck cancer. Cancer Res 2013; 73: 3381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huerta S, Gao X, Dineen S, Kapur P, Saha D, Meyer J. Role of p53, Bax, p21, and DNA-PKcs in radiation sensitivity of HCT-116 cells and xenografts. Surgery 2013; 154: 143–51. [DOI] [PubMed] [Google Scholar]
- 30.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, Peet CR, Lorenz LD, Nickel KP, Klingelhutz AJ, Lambert PF, Harari PM. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res 2013; 73: 4791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]