Abstract
This study was performed to evaluate and compare the antioxidant substance content and antioxidant activities of white (Superior) and colored (Hongyoung, Jayoung, Jasim, Seohong, and Jaseo) potatoes. The potatoes were extracted with 80% ethanol and were evaluated for the total polyphenol, flavonoid, and anthocyanin contents and for 1,1-diphenyl- 2-picrylhydrazyl (DPPH)/2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical scavenging activity, reducing power, and ferrous metal ion chelating effect. The total polyphenol, flavonoid, and anthocyanin contents of Hongyoung and Jayoung were higher than white and other colored potatoes. All colored potato extracts, except for Jaseo and Seohong, showed higher ABTS radical scavenging activities than the general white potato extract. Hongyoung and Jayoung had the highest ABTS and DPPH radical scavenging activities. Optical density values for the reducing power of Jayoung and Jaseo at concentration of 2 mg/mL were 0.148 and 0.090, respectively. All colored potato extracts had lower ferrous metal ion chelating effect than the white potato. A significant (P<0.05) positive correlation was observed between total polyphenol content and total flavonoid content (r=0.919), anthocyanin content (r=0.992), and ABTS radical scavenging activity (r=0.897). Based on these results, this research may be useful in developing the Hongyoung and Jayoung cultivars with high antioxidant activities.
Keywords: colored potato, polyphenol, flavonoid, anthocyanin, antioxidant activities
INTRODUCTION
Potatoes (Solanum tuberosum L.) have short growth duration and high yield per local union. Additionally, potatoes easily adapt to various environments and are now cultivated in approximately 120 nations, making them internationally important foods along with rice, wheat, soybean, and corn. Currently, most potatoes cultivated around the world have white or yellow-white flesh color, but potatoes have a variety of colors due to anthocyanins caused by the diversity of DNA involved in generating color (1). Food color is critical for determining quality. In some cases, synthesized pigments are added to food because of color deterioration caused by processing conditions or storage during food processing (2). However, with recent findings regarding the hazards of synthesized pigments, regulations have been imposed and the use of synthesized pigments has been restricted. Thus, natural color has again come to the forefront as a replacement for synthesized pigments. Anthocyanins occur in fruits, flowers, stems, leaves, and roots of plants and are soluble flavonoid pigments colored red, purple, and blue. Anthocyanins have anticancer activities, and acted as anti-inflammatory agents for cardiovascular disease in a clinical trial (3). For this reason, plants such as sweet potato, blueberry, red cabbage, and purple potato have received attention as critical functional foods with antioxidant activity (4). Phenolic compounds in purple sweet potatoes occur at levels 2.5 times higher than those in yellow and sweet potatoes (5). Furthermore, blueberry has antioxidants found to promote enhanced sight (6). Moreover, colored rice contains higher polyphenol content than white-rice, a feature that is expected to promote consumption of colored rice (7).
In Korea, a potato named “Jasim” has been listed as a species since 2,000 and has attracted consumer attention because of its purple color. This potato has been quantitatively analysed for antioxidant, anti-mutagenic, and anti-cancer characteristics (1,3), as well as its anthocyanin characteristics and stability (2). However, other studies are limited in this Korean purple potato. The objective of this study was to compare the antioxidant activities and anthocyanin content of different varieties of white (Superior) and colored potato cultivars of Hongyoung, Jayoung, Jasim, Seohong, and Jaseo in Korea.
MATERIALS AND METHODS
Potatoes and reagents
The white (Superior), purple (Hongyoung, Jayoung, and Jasim), and yellow (Seohong and Jaseo) potato cultivars were provided from Goesan, Korea. They were freeze-dried using a FD-5508 (Ilshin Lab Co., Seoul, Korea) and grounded to fine powder. The flour was stored at 4°C in a dark room until use. 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), iron (II) chloride tetrahydrate, potassium ferricyanide (III), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4′,4″-disulfonic acid sodium salt (ferrozine), ethylenediaminetetraacetic acid (EDTA), gallic acid, (+)-catechin hydrate, L-ascorbic acid, and Trolox were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Reagents used were of grade quality.
Color evaluation
The samples were transferred into a glass cell (CM A-98, 10 mm in width), and color was measured with a Color Difference Meter (Spectrophotometer CM-3500d, Minolta, Osaka, Japan). The instrument was calibrated to standard black and white tiles before analysis. A large size aperture was used, and the measurement was performed in triplicate through the computerized system using Spectra Magic Software (Version 2.11, Minolta, Osaka, Japan). ΔE which is the difference of color in the samples was calculated using the following equation:
where ΔL, Δa, and Δb means difference of color between Superior (control sample) and other samples.
Preparation of ethanol extract
Five grams of the freeze-dried potato flour was mixed with 300 mL of 80% ethanol and extracted with sonicator (SD-350H, Seong Dong, Seoul, Korea) for 1 h. The suspension was filtered through filter paper (Whatman No. 1, Whatman International Ltd., Maidstone, England), and the residue was re-extracted with 80% ethanol for 1 h. The filtrate was dried using a vacuum evaporator and nitrogen gas. Sample solutions were stored at 4°C and served as the stock solution (100 mg/mL in dimethyl sulfoxide) for subsequent analyses.
Determination of total polyphenol content
A modified method by Kwon et al. (8) was used to determine the total phenolic content of the samples. Briefly, 100 μL of the sample was mixed with 2 mL of 2% Na2CO3. After at least 3 min, 100 mL of 50% Folin-Ciocalteu reagent was added. The absorbance was measured at 750 nm using a spectrophotometer (UV-1650, Shimadzu Corporation, Kyoto, Japan). Standard gallic acid solutions (0.1~0.5 mg/mL) were prepared similarly for the construction of the calibration curve.
Determination of total flavonoid content
A modified method by Kim et al. (9) was used to determine the total flavonoid content of the samples. One mL of distilled water was added to 250 μL of the sample, and was mixed with 75 μL of 5% NaNO2. After 5 min, 150 μL of 10% AlCl3 was added. After 6 min, 500 μL of 1 M NaOH was added. The absorbance was measured at 510 nm using a spectrophotometer. (+)-Catechin hydrate solution (0.1~0.5 mg/mL) was prepared similarly for the construction of the calibration curve.
Determination of anthocyanin content
A modified method by Türker and Erdogdu (10) was used to determine the total anthocyanin content of the samples. The sample was mixed with 10 μL of methanol with 0.1% HCl. After 2 h, 100 mL of Folin-Ciocalteu’s reagent was added. The resulting mixture was centrifuged at 4,500 rpm for 20 min. One milliliter of 0.025 M potassium chloride buffer (pH 1.0) was added to 1 mL of the supernatant. The absorbance was measured at 510 nm and 700 nm using a spectrophotometer. The total anthocyanin content of the sample is calculated as the molar absorbance (ɛ=26,900 M−1·cm−1) of cyaniding-3-glucoside:
where A (absorbance)=(A510 −A700)pH 1.0 −(A510 −A700) pH 4.5, MW (molecular weight of cyanidine-3-glucoside) is 449.2, and ɛ is 26,900 M−1·cm−1.
ABTS radical scavenging activity
The procedure followed the method of Nho et al. (11) with some modifications. The stock solutions included 7.4 mM ABTS•+ solution and 2.6 mM potassium persulfate solution. The working solution was then prepared by mixing the two stock solutions in equal quantities and allowing them to react for 12 h at room temperature in the dark. The solution was then diluted by mixing 1 mL ABTS•+ solution with distilled water to obtain an absorbance of 1.1±0.02 units at 735 nm using a spectrophotometer. Fresh ABTS•+ solution was prepared for each assay. 50 μL of potato extracts were allowed to react with 1 mL of the ABTS•+ solution for 30 min. Then the absorbance was measured at 734 nm using a spectrophotometer. L-ascorbic acid was added as the standard, and the total antioxidant activity was expressed as L-ascorbic acid equivalent (AAE mg/100 g).
DPPH radical scavenging activity
DPPH radical scavenging activity of potato extracts was determined using the method outlined by Hwang et al. (12). Sample solutions with concentrations of 10, 20, 30, 40, and 50 mg/mL were prepared from the stock solution. A 800 μL of sample solution was mixed with 800 μL of freshly prepared DPPH in methanol. The mixture was kept in the dark for 30 min. The absorbance was measured at 517 nm using a spectrophotometer.
Reducing power
Reducing power of the extracts was determined using the method described by Mau at al. (13). Sample solutions with concentration of 20, 40, 60, 80, and 100 mg/mL were prepared from the stock solution. One mL of 0.2 M phosphate buffer pH 6.6 and 1 mL of 1% (w/v) K3Fe(CN)6 were added to a 1 mL aliquot of the extract. The mixture was incubated at 50°C for 20 min. Ten percent (w/v) trichloroacetic acid (1 mL) was added to the mixture. The resulting mixture was centrifuged at 3,000 rpm for 10 min. One mL of distilled water and 0.2 mL of 0.1% (w/v) FeCl3 solution were added to 1 mL of the supernatant. The absorbance was measured at 700 nm using a spectrophotometer. The reducing power of Trolox (2 mg/mL) was also determined.
Ferrous metal ions chelating effect
The ferrous ion-chelating potential of the extract was investigated according to the method of Decker et al. (14), where the Fe2+-chelating ability of the extract was monitored by measuring the ferrous ironferrozine complex [Fe2+-tripyridyltriazine (TPTZ) complex] at 562 nm. Briefly, the reaction mixture, containing the sample (1.25 ~5.0 mg), FeCl2 (2 mM), and ferrozine (5 mM), was adjusted to a total volume of 0.8 mL with methanol, shaken well and incubated for 10 min at room temperature. The absorbance of the mixture was measured at 562 nm against a blank and EDTA (0.05~0.2 mg) was used as positive control.
Statistical analysis
The results were reported as mean±standard deviation (n=9). The significance of differences among the treatment means was determined using one-way analysis of variance (ANOVA), calculated by SPSS version 12 (SPSS Inc., Chicago, IL, USA), with a significance level of P< 0.05 by Duncan’s multiple range test.
RESULTS AND DISCUSSION
Color
The color of white and colored potato varieties were evaluated by lightness (L*), redness (a*), yellowness (b*) and color difference (ΔE) (Table 1). White potatoes (Superior) and yellow-colored potatoes (Jaseo and Seohong) showed higher lightness and yellowness than purple-colored potatoes (Hongyoung, Jayoung, and Jasim). However, redness of purple-colored potatoes was higher than white and yellow-colored potatoes. Among the potato varieties, Hongyoung had the highest redness followed by Jayoung, Jasim, Jaseo, Seohong, and Superior. Jeon et al. (3) reported that the lightness and yellowness of yellow- colored potatoes was higher than purple-colored potatoes, but redness was lower. The color difference (ΔE) value of Hongyoung and Jayoung was higher than yellow- colored potatoes.
Table 1.
Hunter Lab values of white and colored potato varieties
| L* (lightness) | a* (redness) | b* (yellowness) | ΔE (color difference) | |
|---|---|---|---|---|
| Superior | 82.7±0.2a | −0.5±0.0f | 17.5±0.1a | 0f |
| Hongyoung | 69.9±0.4e | 8.9±0.3a | 4.8±0.0e | 20.53±0.16b |
| Jayoung | 61.0±0.2f | 7.9±0.0b | −3.2±0.1f | 31.35±0.29a |
| Jasim | 73.7±0.1d | 3.1±0.1c | 8.2±0.2d | 13.64±0.25c |
| Jaseo | 76.9±0.0c | 1.1±0.0d | 12.1±0.2c | 8.30±0.19d |
| Seohong | 81.5±0.1b | 0.7±0.0e | 14.4±0.5b | 3.70±0.67e |
Values with different letters (a–f) with a column are significantly different (P<0.05) by Duncan’s multiple range test.
Total polyphenol content
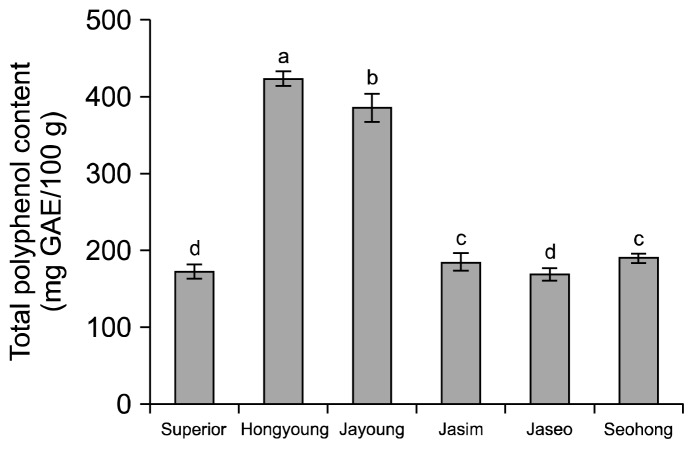
The total polyphenol contents of Hongyoung and Jayoung were 423.92 and 385.94 mg gallic acid equivalent (GAE)/100 g dried samples, respectively. Total polyphenol contents in Superior and Jaseo were 172.60 and 168.44 mg GAE/100 g, respectively (Fig. 1). The purple-colored potatoes Hongyoung and Jayoung had significantly higher total polyphenol contents than the white (Superior) and yellow-colored (Seohong and Jaseo) potatoes. In particular, Hongyoung and Jayoung had total polyphenol contents twice as high as those of Superior. Cha et al. (15) reported that typical potato contains a total polyphenol content of 43 mg/100 g fresh weight. Al-Saikhan et al. (16) reported that potato contains 11.41~27.47 mg GAE/100 g fresh weight, while, Karadenüz et al. (17) reported a phenolic content of 32.44~6.07 mg GAE/100 g fresh weight. Higher values for phenolic content of potato were 231.46 mg GAE/100 g fresh weight obtained by Kaur and Kapoor (18). Jeon et al. (3) indicated that the high phenolic content of purple-fleshed potato differs in cultures, cropping systems, and fertilizer controlled environments. These results showed that the purple-colored potatoes (Hongyoung, Jayoung, and Jasim) used in this experiment were similar or higher in polyphenol content compared to white (Superior) and yellow-colored (Seohong and Jaseo) potato varieties.
Fig. 1.
Total polyphenol content of ethanolic extracts on the white and colored potato varieties. Means on bars with different letters (a–d) are significantly different (P<0.05) by Duncan’s multiple range test. GAE: gallic acid equivalent.
Total flavonoid content
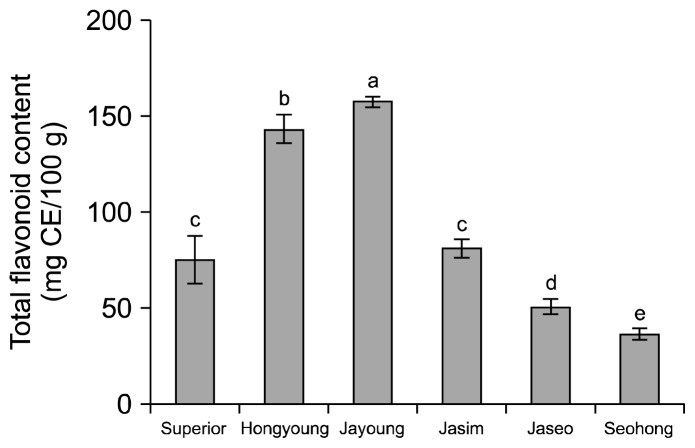
The total flavonoid content of the purple-colored potato varieties are shown in Fig. 2. The values ranged from 36.47 to 157.33 mg (+)-catechin equivalent (CE)/100 g dried samples, with Jayoung having the highest flavonoid content (157.33 mg CE/100 g), followed by Hongyoung (143.44 mg CE/100 g). Jaseo (50.80 mg CE/100 g) and Seohong (36.47 mg CE/100 g) had lower flavonoid contents than Superior (75.35 mg CE/100 g). Lewis et al. (19) reported that the flavonoids content of skin higher than flesh (48.4~148.3 mg CE/100 g dried samples).
Fig. 2.
Total flavonoid contents of ethanolic extracts on the white and colored potato varieties. Means on bars with different letters (a–e) are significantly different (P<0.05) by Duncan’s multiple range test. CE: (+)-catechin equivalent.
Anthocyanin content
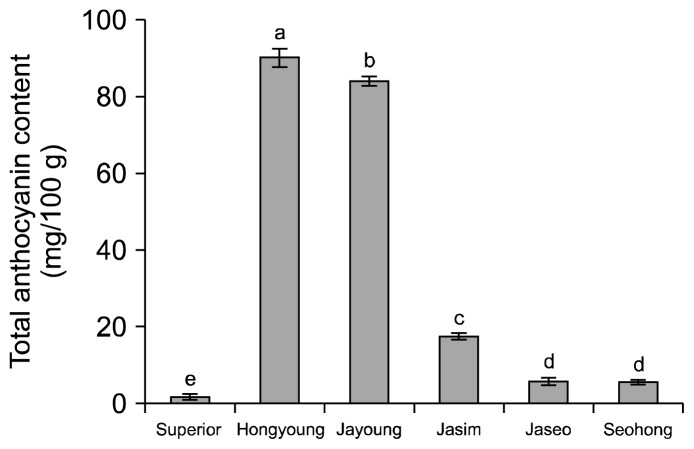
The anthocyanin contents of the purple-colored potato varieties are shown in Fig. 3. Hongyoung and Jayoung had the highest anthocyanin contents of 89.95 and 83.90 mg/100 g dried samples, respectively. Superior had the lowest anthocyanin contents of 1.56 mg/100 g. Significance differences were observed between varieties (P< 0.05). Howard et al. (20) reported that while purple-colored potato has many kinds of anthocyanins, the main anthocyanin is pelagonidin-3-glucoside. Park et al. (21, 22) found that the anthocyanin contents of Hongyoung, Jayoung, and Jasim were 58.1, 51.4, and 13.4 mg/100 g dried samples, respectively. However, the anthocyanin contents varied from 30.0 mg to 215.3 mg/100 g dried samples depending on the harvest times and growing regions. Reyes et al. (23) demonstrated that the light intensity, temperature and amount of sunlight played major roles in anthocyanin biosynthesis in potatoes. Furthermore, Park et al. (1) reported that Jayoung, among purple-colored potatoes, has the highest anticancer activity and that purple-colored potato extracts could be used in combination with anticancer drugs for securing stability.
Fig. 3.
Total anthocyanin content of ethanolic extracts on the white and colored potato varieties. Means on bars with different letters (a–e) are significantly different (P<0.05) by Duncan’s multiple range test.
ABTS and DPPH radical scavenging activity
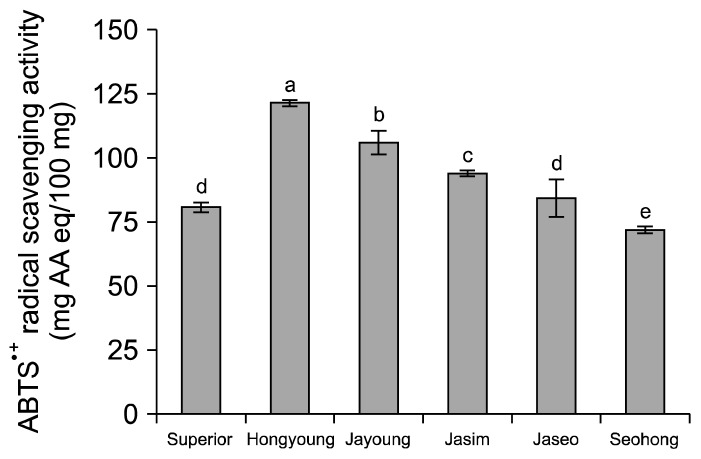
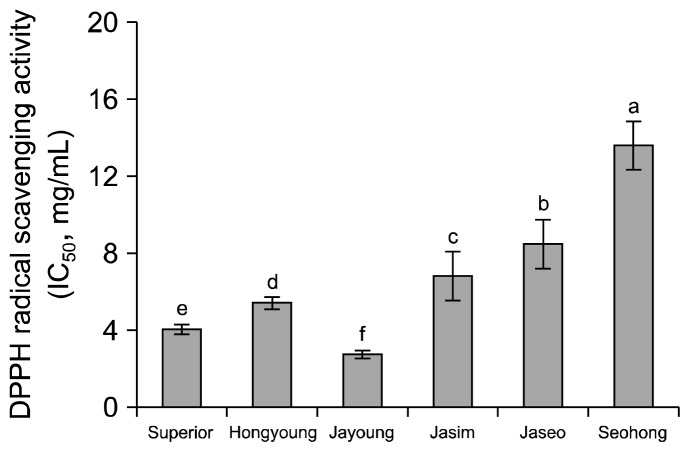
The ABTS radical scavenging activities of the purple-colored potato varieties are shown in Fig. 4. Hongyoung had the highest ABTS radical scavenging activity of 121.39 mg AAE/100 g dried samples, whereas Superior and Seohong had lower ABTS radical scavenging activities of 80.64 and 71.88 mg AAE/100 g, respectively. Lachman et al. (24) reported that the antioxidant activity of red/purple-fleshed potatoes was 72.51~144 mg AAE/100 g fresh weight. DPPH radical scavenging activities (IC50) of the purple-colored potato varieties are shown in Fig. 5. Jayoung had an IC50 value of 2.76 mg/mL, whereas values for Hongyoung, Jaseo, and Seohong were 5.44, 8.49, and 13.64 mg/mL, respectively. All samples except for Jayoung had lower radical scavenging activity than the 4.07 mg/mL of Superior. Hongyoung had the highest ABTS radical scavenging activity, whereas Jayoung had the highest DPPH radical scavenging activity. Rumbaoa et al. (25) reported that the range of IC50 values of the sweet potato varieties was 0.7~6.4 mg/mL dried sample and Reyes et al. (26) also reported that the range of IC50 values was 49~5.23 mg/mL methanolic extract from sweet potato flours. Lachman et al. (24) showed that the antioxidant activity of red/purple-fleshed potatoes was 20.8~123 mg AAE/100 g fresh weight. The present results showed that the purple-colored potatoes had similar or higher radical scavenging activities compared to those reported in previous studies.
Fig. 4.
ABTS radical scavenging activity of ethanolic extracts on the white and colored potato varieties. Means on bars with different letters (a–e) are significantly different (P<0.05) by Duncan’ s multiple range test. AAE: L-ascorbic acid equivalent.
Fig. 5.
DPPH radical scavenging activity of ethanolic extracts on the white and colored potato varieties. Means on bars with different letters (a–f) are significantly different (P<0.05) by Duncan’s multiple range test.
Reducing power
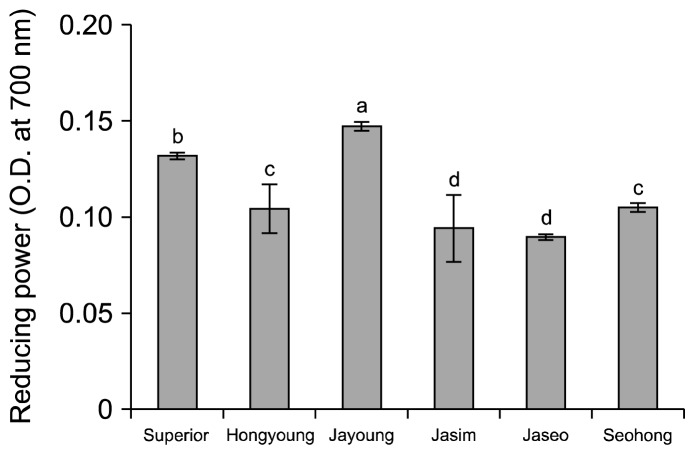
The optical density values for the reducing power of the purple-colored potato varieties are shown in Fig. 6. With a sample concentration of 2 mg/mL, Jayoung had the highest reducing power of 0.1476. Jasim and Jaseo had less reducing power of 0.0946 and 0.0901, compared to 0.1321 in Superior. No significant differences were found between Hongyoung and Seohong. Rumbaoa et al. (25) reported that the reducing power (IC50) of sweet potatoes was 2.5~13.9 mg/mL, whereas values for typical potatoes ranged from 66.2 to 78.2 mg/mL (dry weight basis). The IC50 value is the concentration at which the absorbance is 0.500. Our results showed higher IC50 values in the purple-colored potatoes varieties than in the others varieties.
Fig. 6.
Reducing power of ethanolic extracts on the white and colored potato varieties. Means on bars with different letters (a–d) are significantly different (P<0.05) by Duncan’s multiple range test.
Ferrous metal ions chelating activity
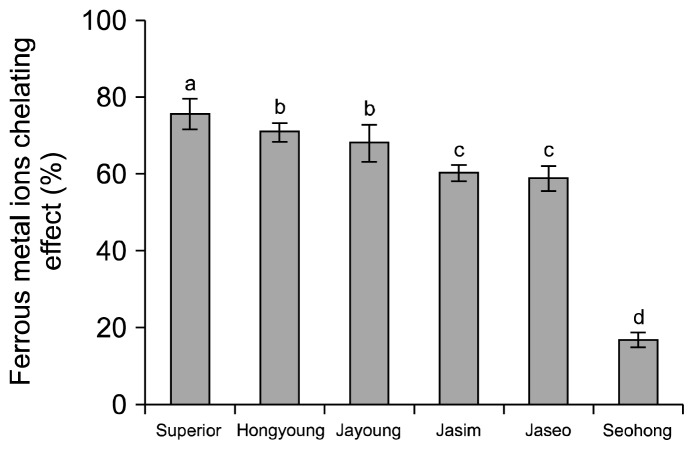
The chelating effect of the purple-colored potato varieties is shown in Fig. 7. With a sample concentration of 2 mg/mL, Superior had the highest chelating effect at 75.58%. Chelating effects in Hongyoung and Jayoung were 70.75 and 67.94%, respectively but the difference was not significant. Seohong had the lowest chelating effect at 16.48%. All sampled varieties had lower chelating effects than those of the Superior control. Rumbaoa et al. (25) reported that the chelating effect (IC50) was 2.4 ~4.1 mg/mL for sweet potatoes and 11.0~14.7 mg/mL (dry weight basis) for typical potatoes. Our results showed that purple-colored potatoes had a higher chelating effect than those of other varieties.
Fig. 7.
Iron chelating effect of ethanolic extracts on the white and colored potato varieties. Means on bars with different letters (a–d) are significantly different (P<0.05) by Duncan’s multiple range test.
Correlation between functional components and antioxidant activity
The ABTS radical scavenging activity of the purple-colored potato varieties was highly positively correlated with the total polyphenol content (r=0.897), flavonoid content (r=0.910), anthocyanin content (r=0.923), and the a* value of color (r=0.941) (P<0.05, Table 2). According to the results obtained by Brown (4), anthocyanins, as well as phenolic acids, contribute to higher total polyphenol content and antioxidant activity. The a* value of color was correlated with the anthocyanin content (r=0.985; P<0.05) because anthocyanins are related to red color. DPPH radical scavenging activity was negatively correlated with the chelating effect (r=−0.933). Antioxidant compounds in purple-colored potato are related to radical scavenging activity as well as chelating activity of iron. DPPH radical scavenging activity was not correlated with anthocyanin contents (r=−0.537); these results are attributed to the underestimation of antioxidant activity of anthocyanin because of similar absorption wavelengths for DPPH and anthocyanins (27). DPPH radical scavenging activity was not correlated with ABTS radical scavenging activity (r=−0.627) because the effect of antioxidants on DPPH radical scavenging was due to their hydrogen donating ability but the effect of antioxidants on ABTS radical scavenging activity was the relative ability of antioxidants to scavenge the radical of ABTS•+ (28). Anthocyanin content was not correlated with reducing power or the chelating effect. Rumbaoa et al. (25) reported that phenolic compounds were not correlated with reducing power, which supports our results.
Table 2.
Correlation coefficients among functional substances, antioxidant activity, and colors of colored potato varieties
| TPP | TF | ABTS | DPPH | RP | FC | AC | L* | a* | b* | |
|---|---|---|---|---|---|---|---|---|---|---|
| TPP | 1.000 | 0.919** | 0.897* | −0.494 | 0.400 | 0.357 | 0.992** | −0.807 | 0.962** | −0.815* |
| TF | 1.000 | 0.910* | −0.774 | 0.554 | 0.619 | 0.944** | −0.875* | 0.914* | −0.860* | |
| ABTS | 1.000 | −0.627 | 0.171 | 0.597 | 0.923** | −0.784 | 0.941** | −0.774 | ||
| DPPH | 1.000 | −0.620 | −0.933** | −0.537 | 0.578 | −0.482 | 0.514 | |||
| RP | 1.000 | 0.344 | 0.386 | −0.403 | 0.253 | −0.382 | ||||
| FC | 1.000 | 0.396 | −0.392 | 0.361 | −0.315 | |||||
| AC | 1.000 | −0.869* | 0.985** | −0.875* | ||||||
| L* | 1.000 | −0.888* | 0.995** | |||||||
| a* | 1.000 | −0.899* | ||||||||
| b* | 1.000 |
TPP, total polyphenol contents; TF, total flavonoid contents; ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) radical scavenging activity; DPPH, 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity; RP, reducing power; FC, Fe iron chelating effect; AC, anthocyanin content.
P<0.05 and
P<0.01.
CONCLUSION
Antioxidant activity was significantly higher in the purple-colored potato varieties than in the white and yellow-colored potatoes. Differences among the samples in terms of polyphenol content, DPPH radical scavenging activity, reducing power, and iron chelating capacity may be due to the genotype and potato variety. Hongyoung and Jayoung had the highest total polyphenol content and higher antioxidant activity among the studied varieties. Reducing power and chelating effect were generally lower in the purple-colored potatoes than in Superior, indicating that the antioxidant compounds in purple-colored potatoes are not correlated with reducing power or the chelating effect. Furthermore, the antioxidant effect was related to polyphenol content. Generally, the purple-colored potato varieties used in this study had higher antioxidant activity than reported for other varieties grown in other countries. Additional analyses are required to investigate polyphenol compound structures and their mechanisms of action. The use of natural and functional cosmetics has increased in worldwide, and purple-colored potatoes varieties could be a color source for the cosmetics industry. To satisfy consumers’ demands for natural colors, the light and heat stability as well as the pH and oxidation activity of purple-colored potatoes must be improved. As a result, this research may be useful in developing the Hongyoung and Jayoung cultivars which have highly antioxidants and can be used as functional food agents.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Park YE, Jeong JC, Cho HM, Hwang YS, Lee HJ, Choi SSN, Lee SJ, Park ES, Ko EA, Kim NA, Lim JD, Choung MG. Antimutagenic effect and cytotoxicity to human cancer cell lines colored potato extracts. Korean J Crop Sci. 2008;53:75–84. [Google Scholar]
- 2.Rhim JW, Kim SJ. Characterictics and stability of anthocyanin pigment extracted from purple-fleshed potato. Korean J Food Sci Technol. 1999;31:348–355. [Google Scholar]
- 3.Jeon TW, Cho YS, Lee SH, Cho SM, Cho HM, Chang KS, Park HJ. Studies on the biological activities and physicochemical characteristics of pigments extracted from Korean purple-fleshed potato. Korean J Food Sci Technol. 2005;37:247–254. [Google Scholar]
- 4.Brown CR. Antioxidants in potato. Am J Potato Res. 2005;82:163–172. doi: 10.1007/BF02853654. [DOI] [Google Scholar]
- 5.Song J, Chung MN, Kim JT, Chi HY, Son JR. Quality characteristics and antioxidative activities in various cultivars of sweet potato. Korean J Crop Sci. 2005;50:141–146. [Google Scholar]
- 6.Schmidt BM, Howell AB, McEniry B, Knight CT, Seigler D, Erdman JW, Jr, Lila MA. Effective separation of potent antiproliferation and antiadhesion components from wild blueberry (Vaccinium angustifolium Ait.) fruits. J Agric Food Chem. 2004;52:6433–6442. doi: 10.1021/jf049238n. [DOI] [PubMed] [Google Scholar]
- 7.Seo SJ, Choi Y, Lee SM, Kim KJ, Son JR, Lee J. Determination of selected antioxidant compounds in specialty rice. J Korean Soc Food Sci Nutr. 2007;36:499–502. doi: 10.3746/jkfn.2007.36.4.499. [DOI] [Google Scholar]
- 8.Kwon OC, Woo KS, Kim TM, Kim DJ, Hong JT, Jeong HS. Physicochemical characteristics of garlic (Allium sativum L.) on the high temperature and pressure treatment. Korean J Food Sci Technol. 2006;38:331–336. [Google Scholar]
- 9.Kim HY, Woo KS, Hwang IG, Lee YR, Jeong HS. Effects of heat treatments on the antioxidant activities of fruits and vegetables. Korean J Food Sci Technol. 2008;40:166–170. [Google Scholar]
- 10.Türker N, Erdogdu F. Effects of pH and temperature of extraction medium on effective diffusion coefficient of anthocynanin pigments of black carrot (Daucus carota var. L.) J Food Eng. 2006;76:579–583. doi: 10.1016/j.jfoodeng.2005.06.005. [DOI] [Google Scholar]
- 11.Nho JW, Hwang IG, Joung EM, Kim HY, Chang SJ, Jeong HS. Biological activities of Magnolia denudata Desr. flower extracts. J Korean Soc Food Sci Nutr. 2009;38:1478–1484. doi: 10.3746/jkfn.2009.38.11.1478. [DOI] [Google Scholar]
- 12.Hwang IG, Woo GS, Kim TM, Kim DJ, Yang MH, Jeong HS. Change of physicochemical characteristics of Korean pear (Pyrus pyrifolia Nakai) juice with heat treatment conditions. Korean J Food Sci Technol. 2006;38:342–347. [Google Scholar]
- 13.Mau JL, Lin HC, Song SF. Antioxidant properties of several specialty mushrooms. Food Res Int. 2002;35:519–526. doi: 10.1016/S0963-9969(01)00150-8. [DOI] [Google Scholar]
- 14.Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- 15.Cha JY, Cho YS. Effect of potato polyphenolics on the hyperlipidemia in rats. J Korean Soc Food Sci Nutr. 2000;29:274–279. [Google Scholar]
- 16.Al-Saikhan MS, Howard LR, Miller JC., Jr Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum L.) J Food Sci. 1995;60:341–343. doi: 10.1111/j.1365-2621.1995.tb05668.x. [DOI] [Google Scholar]
- 17.Karadenüz F, Burdurlu HS, Koca N, Soyer Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk J Agric For. 2005;89:297–303. [Google Scholar]
- 18.Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37:153–161. doi: 10.1046/j.1365-2621.2002.00552.x. [DOI] [Google Scholar]
- 19.Lewis CE, Walker JRL, Lancaster JE, Sutton KH. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. J Sci Food Agric. 1998;77:45–57. doi: 10.1002/(SICI)1097-0010(199805)77:1<45::AID-JSFA1>3.0.CO;2-S. [DOI] [Google Scholar]
- 20.Howard HW, Kukimura H, Whitmore ET. The anthocyanin pigments of the tubers and sprouts of Tuberosum potatoes. Potato Res. 1970;13:142–145. doi: 10.1007/BF02355924. [DOI] [Google Scholar]
- 21.Park YE, Cho JH, Cho HM, Yi JY, Seo HW, Chung MG. A new potato cultivar “Jayoung”, with high concentration of anthocyanin. Korean J Breed Sci. 2009;41:51–55. [Google Scholar]
- 22.Park YE, Cho JH, Cho HM, Yi JY, Seo HW, Chung MG. A new potato cultivar “Hongyoung”, with red skin and flesh color, and high concentrations of anthocyanins. Korean J Breed Sci. 2009;41:502–506. [Google Scholar]
- 23.Reyes LF, Miller JC, Cisneros-Zevallos L. Environmental conditions influence the content and yield of anthocyanins and total phenolics in purple- and red-flesh potatoes during tuber development. Am J Potato Res. 2004;81:187–193. doi: 10.1007/BF02871748. [DOI] [Google Scholar]
- 24.Lachman J, Hamouz K, Šulc M, Orsák M, Pivec V, Hejtmánková A, Dvořák P, Čepl J. Cultivar differences of total anthocyanins and anthocyanidins in red and purple-fleshed potatoes and their relation to antioxidant activity. Food Chem. 2009;114:836–843. doi: 10.1016/j.foodchem.2008.10.029. [DOI] [Google Scholar]
- 25.Rumbaoa RGO, Cornago DF, Geronimo IM. Phenolic content and antioxidant capacity of Philippine potato (Solanum tuberosum) tubers. J Food Compos Anal. 2009;22:546–550. doi: 10.1016/j.jfca.2008.11.004. [DOI] [Google Scholar]
- 26.Reyes LF, Miller JC, Cisneros-Zevallos L. Antioxidant capacity, anthocyanins and total phenolics in purple-and red-fleshed potato (Solanum tuberosum L.) genotypes. Am J Potato Res. 2005;82:271–277. doi: 10.1007/BF02871956. [DOI] [Google Scholar]
- 27.Arnao MB, Cano A, Acosta M. Methods to measure the antioxidant activity in plant material. A comparative discussion. Free Radic Res. 1999;31:S89–S96. doi: 10.1080/10715769900301371. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Li J, Rangarajan M, Shao Y, LaVoie EJ, Huang TC, Ho CT. Antioxidative phenolic compounds from sage (Salvia officinalis) J Agric Food Chem. 1998;46:4869–4873. doi: 10.1021/jf980614b. [DOI] [Google Scholar]