Abstract
Bioconversion of aglycone-formed isoflavones from glycoside-formed isoflavones by commercial lactic acid bacteria in fermented soybean paste was evaluated. Enterococcus faecium KCTC 13410 showed the most resistant capacity and Lactobacillus acidophilus KCTC 3925 had a sensitive susceptibility at a high NaCl concentration (13.2%) in fermented soybean paste. Among the 5 strains tested, Lac. acidophilus KCTC 3925 showed the highest relative ratio of aglycone-formed isoflavones to total isoflavones in fermented soybean paste. Production of exopolysaccarides (EPS) by lactic acid bacteria was compared using de Man, Rogosa, and Sharpe medium containing 1% sucrose at 37°C for 48 h. Among the 5 lactic acid bacteria, Lac. acidophilus KCTC 3925 and Lactobacillus rhamnosus KCTC 3929 were investigated to produce EPS. Based on the results concerning growing susceptibility and conversion of aglycone-formed isoflavones/EPS production, it is anticipated that Lac. acidophilus KCTC 3925 may be used for preparation of Cheonggukjang, which contains relative low NaCl content.
Keywords: Cheonggukjang, fermented soybean paste, exopolysaccharide, isoflavone, lactic acid bacteria
INTRODUCTION
Generally, Korean traditional soybean paste contains soybeans, table salts, and water. Soybean paste is prepared by cleaning, soaking, steaming of soybeans, fermentation of soybean lump, addition of brine, fermentation, and aging, which takes approximately 2~3 years (1,2). Many chemical changes occur in the ingredients and components for the long fermentation and aging process of soybean paste. Glycoside-formed isoflavones in soybeans are converted to more absorbable forms, aglycon isoflavones, the antioxidant activity is increased, and carbohydrates and proteins in soybean are degraded in the fermentation and aging process of soybean paste. On the other hand, the undesirable color and ethyl carbamate may be produced during aging of fermented soybean paste (1,2). Cheonggukjang is prepared with soybeans and water using Bacillus subtilis as a starter for the relatively short period fermentation, and it is stored at low or freezing temperatures due to its low NaCl concentration.
Lactic acid bacteria are gram-positive that grow well in facultative anaerobic and micro-aerophilic conditions, and they are found abundantly in vegetables, grains, meats, and fermented dairy products. Lactic acid or a mixture of lactic acid/acetic acid was produced during the metabolism of carbon sources by lactic acid bacteria. Organic acids produced by lactic acid bacteria increase the level of hydrogen ions, and this causes the pH change toward the acid in which the weak pathogens against acidic condition are suppressed and acidophilic yeasts and molds are stimulated to grow (3).
Isoflavones, which are phytoestrogens found in plants such as soybeans and kudzu root, have preventive activity for breast cancer, colorectal cancer, uterine cancer, and osteoporosis. Long term intake of isoflavones relieves menopausal symptoms. Soybeans have diverse isoflavones, including 3 aglycones (daidzein, glycitein, and genistein) and 9 glycosides, which are formed by binding aglycones and some sugars, such as glucose, acetylglucoside, and malonylglucoside (4). Generally, isoflavones in raw soybeans exist as glycoside-formed isoflavones. During food processing, such as enzymatic degradation by β-glucosidase and acid-degradation, the glycoside-formed isoflavones are changed to more absorbable aglycone-forms in the body (5,6).
Some lactic acid bacteria produce viscous exopolysaccharide (EPS), which are composed of homopolymers consisting of single monosaccharides, and heteropolymers consisting of different monosaccharides (7–9). Texture, immunity, and anti-cancer activity of foods were improved by EPS (8). Interest on EPS function in foods is currently increasing.
In this study, the viability and EPS production of commercial lactic acid bacteria were investigated in fermented soybean paste with a high NaCl concentration. In a low NaCl concentration and a short period of fermentation, the suitable lactic acid bacterium was isolated for production of aglycone-formed isoflavones and EPS.
MATERIALS AND METHODS
Chemicals and reagents
Daidzein, daidzin, genistein, genistin, glycitein, and glycitin were purchased from Fujicco Co., Ltd. (Kobe, Japan), dissolved in dimethyl sulfoxide (Sigma-Aldrich Co., St. Louis, MO, USA), and used as a reference for high-performance liquid chromatography (HPLC) analysis. HCl (35%), NaOH, and ethyl alcohol (EtOH, 99.9% ACS grade) were purchased from Sigma-Aldrich Co..
Strains and cultivation
Five lactic acid bacteria, such as Lactobacillus acidophilus KCTC 3925, Leuconostoc mesenteroides (Gene Bank: AY675249), Lactobacillus rhamnosus KCTC 3929, Pediococcus pentosaceus KCTC 10297BP, and Enterococcus faecium KCTC 13410, were used for production of aglycone-formed isoflavones and exopolysaccharides. Lactic acid bacteria were cultured in de Man, Rogosa, and Sharpe (MRS) medium (Difco, St. Louis, MO, USA) for 2~3 days at 37°C without agitation.
Analysis of general compositions and dietary fiber
For analysis of water content, the sample (2 g) was dried by heating under normal pressure using an air drier (OF-12, Jeio Tech, Gimpo, Korea) at 105°C for 12 h to obtain a constant weight of the sample. The crude ash content of the sample (4 g) was measured with a direct ashing method using a Maffle’s furnace (MF31G, Jeio Tech) at 600°C for 22 h. The content of crude protein was measured using the continuous micro-Kjeldahl method, composed of a Kjeldahl digestion system (Digestion unit K-424, Büchi Labortechnik AG, Flawil, Switzerland), a distillation system (Kjelflex K-360, Büchi Labortechnik AG), and a titration system (702 SM Titrino Metrohm, Büchi Labortechnik AG). The protein content was calculated using the nitrogen coefficient, 6.25. The content of crude lipid was analyzed by extraction of the sample (5 g) using Soxlet apparatus (E-816, Büchi Labortechnik AG) with diethyl ether, after preliminary drying of sample in dryer at 105°C. The content of carbohydrates was calculated using the following equation:
The dietary fiber content in the sample was analyzed by an Association of Official Analytical Chemists method (Enzymatic method 985.29). Briefly, the sample was treated using the following steps: enzyme digestion step of amylase, protease, and amyloglucosidase, precipitation step by EtOH, and filtration step using a Fibertec™ E1023 Filtration Module (Foss, Hilleroed, Denmark). Then the sample was dried in an air drier at 105°C at normal pressure to obtain a constant weight. The ash content of the dried sample was analyzed as mentioned above, and a content of non-digestable protein by enzyme treatment of the sample was measured by the micro-Kjeldahl method. The content of dietary fiber in the sample was calculated by subtracting the content of ash and protein from the total dried weight of the sample. All assays were performed in triplicate and expressed as a mean value.
Fermentation of soybean paste by lactic acid bacteria
Fermented soybean paste was prepared by harvesting of soybeans, steaming process, fermentation of boiled soybean lumps, addition of brine, fermentation, and the isolation of soybean lumps. Fermentation and aging of soybean paste lasted approximately 3 months at room temperature (fermented soybean paste sample A). Lactic acid bacteria (0.43 g, 0.5×1010 CFU/g~1.0×1010 CFU/g) was inoculated in unsterile fermented soybean paste (25 g) at 25°C without agitation.
Determination of pH and salinity
Fermented soybean paste sample (10 g) was mixed with distilled water (9 mL) and the supernatant was separated. The pH of the supernatant was measured using a pH meter (model 725P, istek, Inc., Seoul, Korea). For determination of salinity, a fermented soybean paste sample (2 g) was mixed in sterile distilled water (25 mL) and was spun using a centrifuge (5810R, Eppendorf, Hamburg, Germany) at 2,465 g for 10 min. The supernatant was filtered using Whatman No. 5 paper (Whatman International Ltd., Maidstone, UK) and titrated with 0.05 N AgNO3. All samples were analyzed in triplicate, and the data were expressed as mean±standard deviation.
Determination for viability of commercial lactic acid bacteria in fermented soybean paste containing various NaCl concentrations
Fermented soybean paste sample (1 g) was mixed with 0, 1, 4, and 9 mL of distilled water in 20-mL volume bottle and was sterilized at 121°C for 15 min. The 16 h-cultured lactic acid bacteria (A600=1.1~2.0) were inoculated to the fermented soybean paste suspension at 1% (v/v) concentration and were cultured at 37°C for 24 h without shaking. The number of viable lactic acid bacteria in the fermented soybean paste was counted by the serial dilution method using MRS agar plates at 37°C for 24 h, anaerobically.
Analysis of isoflavones contents
The composition and contents of isoflavones in the samples were measured by the procedure of Kim et al. (10) with slight modifications. Lactic acid bacteria (1%, v/v) were inoculated in a fermentation medium containing sterile fermented soybean paste sample A (2.5 g), isoflavones (0.43 g, Soyavone EX 30, Ingredion, Westchester, IL, USA), and sterile distilled water (10 mL), and were cultured at 37°C for 120 h. Contents of aglycone-formed isoflavones were extracted for 24 h using 9 volumes of 80% EtOH for the fermented soybean paste sample and mixed vigorously for 30 s. Total isoflavones content (aglycones and glycosides) was measured by the refluxing extraction of fermented soybean paste sample with 10 mL of 2 N HCl for 1 h.
Fermented soybean paste sample was hydrolyzed by acid and neutralized with 10 N NaOH to pH 6.5~7.5, and then it was extracted with 80% EtOH. The supernatant of the extracts was filtered with 0.45 μm filter and was injected into an HPLC (Agilent 1200, Agilent Technologies, Santa Clara, CA, USA), equipped with Eclipse XDB-C18 column (4.6×250 mm ID, Agilent Technologies). Mobile phases were used as a gradient solution with solution A (0.1% acetic acid in H2O) and solution B (0.1% acetic acid in acetonitrile) with the following ratio: 93:7, 93:7, 85:15, 80:20, 75:25, 75:25, 65:30, and 65:30 at 0, 25, 50, 55, 70, 75, 80, and 90 min of running time, respectively, at a flow rate of 1.2 mL/min. Each isoflavone was detected at 254 nm by an UV detector. The content of isoflavones in the sample was calculated by a linear plot of standard isoflavones and the dilution factor of the sample.
The relative ratio of aglycone-formed isoflavones to total isoflavones (aglycon-formed isoflavones and glycoside-formed isoflavones) in the sample was calculated, as follow:
Determination of EPS
Among 5 lactic acid bacteria, the EPS producing-lactic acid bacteria were screened by formation of a viscous substance on the colony and were anaerobically incubated in 1% sucrose-MRS medium at 37°C. After the mass culture, the viscous substance (supernatant, 6 mL) produced by lactic acid bacteria was collected by centrifugation (1,000 rpm for 2 min at 4°C) and mixed vigorously with 2 volumes of 100% EtOH. The precipitate was collected by centrifugation at 4,000 rpm for 10 min at 4°C.
For analysis of sugar composition, a precipitate was hydrated with sterile distilled water (6 mL) and was separated by HPLC without enzymatic treatment and with fructanase- and glucanase-treatment. For treatment of fructanase, the hydrated sample (2 mL) was incubated with invertase (10 μL, Novozymes, Bagsværd, Denmark) at 30°C for 16 h. In case of glucanase, the hydrated sample (2 mL) was incubated with liquefying Termamyl® 120 L (17 μL, Novozymes) at 80°C for 2 h, and then was incubated further with Neutrase 0.8 L (50 μL, Novozymes) at 37°C for 30 min, and β-amylase (17 μL, Amg-300L, Novozymes) at 60°C for 2 h.
Analysis of sugar composition by HPLC
For analysis of sugar composition, the sample was treated with 0.45 μm filter (DISMIC-13HP, Toyo Roshi Kaisha, Ltd., Tokyo, Japan) and was separated with an Agilent 1200 HPLC (Agilent Technologies), equipped with Kromasil 100-10 NH2 column (Eka Chemicals AB, Bohus, Sweden) and RI detector (Agilent Technologies). Injection volume was 20 μL, and mobile phase was 75% acetonitrile and 25% water at a flow rate of 1 mL/min at 30°C.
Statistical analysis
All samples were analyzed in triplicate, and results were expressed as mean±standard deviation. Data was analyzed statistically by one-way analysis of variance (ANOVA) (11) (Microsoft Excel Statpro, Microsoft Corporation, Chevy Chase, MD, USA).
RESULTS AND DISCUSSION
General composition and dietary fiber content of fermented soybean paste
General compositions and dietary fiber in 3 months-aged fermented soybean paste are shown in Table 1. Contents of moisture, protein, ash, fat, carbohydrate, and dietary fiber of 3 months-aged fermented soybean paste were 57.9, 12.1, 14.9, 3.9, 11.6, and 6.8%, respectively, whereas a traditional fermented soybean paste had 54.0% of moisture, 13.6% of protein, 12.5% of ash, 8.2% of fat, 8.1% of carbohydrate, and 3.6% of dietary fiber (12). In this study, 3 months-aged fermented soybean paste had higher moisture, ash, carbohydrate, and dietary fiber, and less content of protein and fat compared with a previous report (12).
Table 1.
Contents of general nutrients in fermented soybean paste samples (unit: %)
| Sample | Moisture | Protein | Ash | Fat | Carbohydrate | Fiber | References |
|---|---|---|---|---|---|---|---|
| Fermented soybean paste | 57.9±0.7 | 12.1±0.4 | 14.9±0.9 | 3.9±0.1 | 11.6±0.7 | 6.8±0.2 | – |
| Doenjang (ref) | 54.0 | 13.6 | 12.5 | 8.2 | 8.1 | 3.6 | 12 |
Growth of lactic acid bacteria in fermented soybean paste with NaCl concentrations
To investigate the growth of lactic acid bacteria in fermented soybean paste with various NaCl concentrations, fermented soybean paste sample A was diluted to 0, 2, 5, and 10 times with sterile distilled water. Five lactic acid bacteria were inoculated in diluted fermented soybean paste samples, and the viable cells were counted on MRS medium after incubation (Table 2). The number of viable lactic acid bacteria was inversely related to NaCl concentration, and each lactic acid bacteria showed a different inhibitory pattern of growth by NaCl.
Table 2.
Number of lactic acid bacteria in fermented soybean paste samples (unit: CFU/g fermented soybean paste)
| Starter | Number of viable cells | |||
|---|---|---|---|---|
|
| ||||
| 1.3% NaCl | 2.6% NaCl | 6.6% NaCl | 13.2% NaCl | |
| Lactobacillus acidophilus KCTC 3925 | 2.3×108a1) | 7.9×107b | 3.6×105b | >100c |
| Leuconostoc mesenteroides AY675249 | 6.8×108a | 2.4×108ab | 6.5×107b | 1.1×107b |
| Lactobacillus rhamnosus KCTC 3929 | 4.5×108a | 2.3×108ab | 2.3×107b | 8.4×105b |
| Pediococcus pentosaceus KCTC 10297BP | 5.9×108a | 3.6×108ab | 9.3×107bc | 1.0×106c |
| Enterococcus faecium KCTC 13410 | 9.5×108 | 5.4×108 | 1.2×108 | 9.3×107 |
Different letters (a–c) in the same row are significantly different at P<0.05.
Five lactic acid bacteria showed resistance for growth in the undiluted fermented soybean paste sample (13.2% of NaCl) in the following order: Ent. faecium KCTC 13410, Leu. mesenteroides strain, Ped. pentosaceus KCTC 10297BR, Lac. rhamnosus KCTC 3929, and Lac. acidophilus KCTC 3925. Lac. acidophilus KCTC 3925 showed the most growth susceptibility in NaCl of fermented soybean paste. The viable number of Lac. acidophilus KCTC 3925 were 2.3× 108, 7.9×107, and 3.6×105 (CFU/g fermented soybean paste) at 1.32, 2.64, and 6.6% NaCl concentration, respectively, and was suppressed to 0.16% in 6.6% NaCl concentration. Based on these results, it is expected that Lac. acidophilus KCTC 3925 is suitable to use for production of Cheonggukjang, which contains comparatively low NaCl content, rather than fermented soybean paste and soy sources with high NaCl content.
The pH of fermented soybean paste
Soybean paste was fermented with lactic acid bacteria and glycoside-formed isoflavones at 30°C for 120 h, and the pH was measured, as shown in Table 3. Fermented soybean paste sample with or without lactic acid bacteria had pH 4.01~4.33 and pH 5.04, respectively. A decrease in pH means that some organic acids were produced by lactic acid bacteria in fermented soybean paste sample.
Table 3.
Change of pH in fermented soybean paste containing lactic acid bacteria
| Strains | pH | |
|---|---|---|
| Fermented soybean paste containing | No lactic acid bacteria | 5.04±0.01a1) |
| Lactobacillus acidophilus KCTC 3925 | 4.29±0.06b | |
| Leuconostoc mesenteroides (Gene Bank: AY675249) | 4.17±0.14b | |
| Lactobacillus rhamnosus KCTC 3929 | 4.09±0.08b | |
| Pediococcus pentosaceus KCTC 10297BP | 4.01±0.05b | |
| Enterococcus faecium KCTC 13410 | 4.33±0.03b |
Different letters (a, b) are significantly different at P<0.05.
Fermented soybean paste containing lactic acid bacteria was incubated at 30°C for 120 h.
Metabolism of isoflavones by lactic acid bacteria in fermented soybean paste
Isoflavones exist mostly as aglycone-forms in well fermented soybean paste. Change of isoflavones structure by β-glucosidase of lactic acid bacteria was investigated by addition of commercial isoflavones in all fermented soybean paste samples (samples B). Commercial isoflavones contained more than 95% of glycoside-formed isoflavones. Without lactic acid bacteria, the content of aglycone-formed isoflavones in soybean paste before and after fermentation was approximately 341 and 348 mg/kg, respectively, and the difference of this content was insignificant (Table 4). On the other hand, the content of the aglycone-formed isoflavones (daizein, glycitein, and genistein) was increased time-dependently in fermented soybean paste sample B, containing lactic acid bacteria Lac. acidophilus KCTC 3925, Leu. mesenteroides (Gene Bank: AY 675249), and Lac. rhamnosus KCTC 3929 (P<0.01). However, Ped. pentosaceus KCTC 10297BP and Ent. faecium KCTC 13410 did not change the content of aglycone-formed isoflavones significantly.
Table 4.
Conversion of glycoside-formed isoflavones to aglycone-formed isoflavones from by lactic acid bacteria in fermented soybean paste (unit: mg/kg)
| Starter | Aglyconeisoflavone | Amount of aglyconeisoflavone | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| 0 h | 60 h | 120 h | |||
| No lactic acid bacteria | |||||
| Daizein | 152.7±4.7 | 147.7±9.6 | 156.7±13.3 | 0.5660 | |
| Glycitein | 90.0±3.6 | 85.0±4.0 | 86.7±0.6 | 0.2175 | |
| Genistein | 98.7±4.7 | 93.0±7.8 | 104.3±15.4 | 0.4537 | |
| Lactobacillus acidophilus KCTC 3925 | |||||
| Daizein | 152.7±4.7b1) | 767.7±76.7b | 3,017.7±586.5a | 0.0001 | |
| Glycitein | 90.0±3.6b | 156.3±20.5b | 576.7±93.9a | 0.0001 | |
| Genistein | 98.7±4.7c | 289.3±21.1b | 663.7±34.3a | 0.0000 | |
| Leuconostoc mesenteroides (Gene Bank: AY675249) | |||||
| Daizein | 152.7±4.7c | 237.0±13.8b | 343.3±28.9a | 0.0001 | |
| Glycitein | 90.0±3.6b | 101.3±3.8ab | 107.3±5.5a | 0.0079 | |
| Genistein | 98.7±4.7c | 137.3±4.0b | 219.7±19.9a | 0.0000 | |
| Lactobacillus rhamnosus KCTC 3929 | |||||
| Daizein | 152.7±4.7c | 474.0±71.1b | 742.3±9.0a | 0.0000 | |
| Glycitein | 90.0±3.6c | 110.3±8.4b | 148.3±1.5a | 0.0000 | |
| Genistein | 98.7±4.7c | 185.0±24.0b | 354.7±7.2a | 0.0000 | |
| Pediococcus pentosaceus KCTC 10297BP | |||||
| Daizein | 152.7±4.7 | 261.0±3.0 | 273.3±111.9 | 0.1156 | |
| Glycitein | 90.0±3.6 | 97.7±2.9 | 86.0±33.3 | 0.7651 | |
| Genistein | 98.7±4.7 | 137.7±2.9 | 145.3±56.5 | 0.2516 | |
| Enterococcus faecium KCTC 13410 | |||||
| Daizein | 152.7±4.7 | 151.3±5.1 | 185.0±58.4 | 0.4394 | |
| Glycitein | 90.0±3.6 | 91.3±4.6 | 91.0±10.4 | 0.9704 | |
| Genistein | 98.7±4.7 | 100.3±4.2 | 112.7±22.1 | 0.4217 | |
Different letters in the same row (a–c) are significantly different.
Fermented soybean paste supplemented with lactic acid bacteria and commercial glycoside-formed isoflavones was incubated at 37°C.
Contents of daizein, glycitein, and genistein, which are aglycone-formed isoflavones, were approximately 152.7, 90.9, and 98.7 mg/kg, respectively, prior to fermentation by Lac. acidophilus KCTC 3925. Content of daizein, glycitein, and genistein were changed to 767.7, 156.3, and 289.3 mg/kg at 60 h fermentation and 3,017.7, 576.7, and 663.7 mg/kg at 120 h fermentation, respectively. Relative ratios by no lactic acid bacteria, Lac. acidophilus KCTC 3925, Leu. mesenteroides (Gene Bank: AY675249), Lac. rhamnosus KCTC 3929, Ped. pentosaceus KCTC 10297 BP, and Ent. faecium KCTC 13410 were 4.9, 60.2, 9.5, 17.6, 7.1, and 5.5% at 120 h fermentation, respectively. Lac. acidophilus KCTC 3925 showed the highest relative ratio for aglycone-formed isoflavones from glycoside-formed isoflavones in fermented soybean paste (Table 5). Bioconversion of glycoside-formed isoflavones to aglycone-formed isoflavones by lactic acid bacteria, including Lactobacillus plantarum and Lac. acidophilus, were reported (13,14). Donkor and Shah (14) reported that Lac. acidophilus converted the most amount of aglycone-formed isoflavones at 36 h of fermentation in soymilk, compared to Bifidobacterium lactis and Lactobacillus casei. In the present study, the fermented soybean paste was used as a growth medium for lactic acid bacteria, whereas the previous reports used soybean extract (13) and soymilk (14).
Table 5.
Relative ratio of aglycone-formed isoflavones to total isoflavones by lactic acid bacteria in fermented soybean paste (unit: %)
| Starter | Relative ratio1) | ||
|---|---|---|---|
|
| |||
| Daizein | Glycitein | Genistein | |
| No lactic acid bacteria | 3.6±0.3b2) | 4.1±0.2b | 16.1±2.4d |
| Lactobacillus acidophilus KCTC 3925 | 70.0±13.6a | 27.3±1.3a | 102.5±5.3a |
| Leuconostoc mesenteroides AY675249 | 8.0±0.7b | 5.1±0.2b | 33.9±3.0c |
| Lactobacillus rhamnosus KCTC 3929 | 17.2±0.2b | 7.0±0.3b | 54.8±1.1b |
| Pediococcus pentosaceus KCTC 10297BP | 6.3±2.6b | 4.1±0.2b | 22.4±8.7cd |
| Enterococcus faecium KCTC 13410 | 4.3±1.3b | 4.3±0.2b | 17.4±3.4d |
| P-value | 0.0000 | 0.0000 | 0.0000 |
Values represent relative ratios calculated from the amount of aglycone-formed isoflavones and total isoflavones measured in acid hydrolysis. Total isoflavones (glucoside- and aglycone-forms) of daidzein, glycitein, and genistein were 4,313, 2,113, and 647.5 mg/kg, respectively.
Different letters (a–d) in the same column are significantly different.
Fermented soybean paste supplemented with lactic acid bacteria and commercial glycoside-formed isoflavones was incubated at 37°C for 120 h.
Generally, it takes 1~3 days and a few months/years for fermentation of Cheonggukjang and soybean paste/soy sources, respectively. Therefore, a relatively short fermentation period (60 and 120 h) using Lac. acidophilus KCTC 3925 may increase the content of aglycone-formed isoflavones in fermented soybean foods.
EPS production by lactic acid bacteria
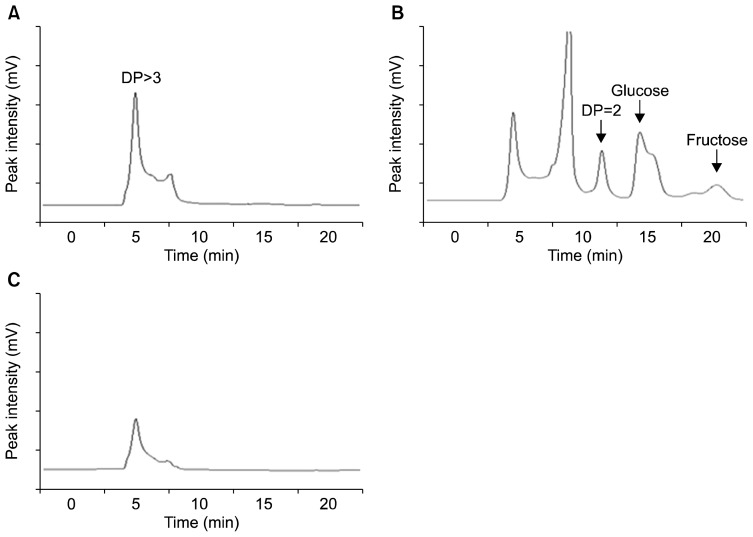
EPS produced by lactic acid bacteria was investigated by detection of a viscous substance on a MRS agar plate. Among 5 lactic acid bacteria, Leu. mesenteroides (Gene Bank: AY675249), Lac. acidophilus KCTC 3925, and Lac. rhamnosus KCTC 3929 synthesized a viscous substance from colonies. The viscous substance by lactic acid bacteria was obtained by EtOH precipitation after collection from MRS agar plates. Leu. mesenteroides (Gene Bank: AY675249), Lac. acidophilus KCTC 3925, and Lac. rhamnosus KCTC 3929 produced approximately 88, 77, and 119 mg of dry weight precipitate, respectively. After a precipitate was hydrated with 6 mL of sterile distilled water, the composition of sugar was analyzed quantitatively by HPLC with no enzymatic treatment, glucanase-, and fructanase- treatment. Samples with no enzymatic and fructanase- treatment did not show the additional increase of monosaccharide (glucose and fructose) content in 3 precipitates (Fig. 1). However, the glucanase-treated 3 samples had increased peaks for maltose and glucose at 11.7 and 14.2 min, respectively, and showed no additional increased in fructose content in 3 samples (Fig. 1). EPS produced by Leu. mesenteroides (Gene Bank: AY675249), Lac. acidophilus KCTC 3925, and Lac. rhamnosus KCTC 3929 is anticipated to be a glucan, composed of glucose (Fig. 1). Cheonggukjang has specific viscous substances, which contain poly-glutamic acid and the fructose polymer levan. After the conventional Cheonggukjang fermentation and the low-NaCl soybean fermentation, the additional fermentation by Lac. acidophilus KCTC 3925 and sucrose may produce glucans in these soybean fermented foods. Glucan is produced by a dextransucrase in Leu. mesenteroides (15). Because gene encoding dextransucrase in Lac. acidophilus KCTC 3925 was not studied in this study, a further investigation on a gene (or genes) for glucan formation will need to be performed.
Fig. 1.
HPLC chromatogram of EPS produced by Lactobacillus acidophilus KCTC 3925 in MRS agar containing 1% sucrose. (A) no enzyme treatment, (B) glucanases (Termamyl® 120L, Neutrase, and AMG 300L) treatment, and (C) fructanse treatment. DP: degree of polymerization.
Glycoside-formed isoflavones were metabolized slowly in conventional soybean paste fermentation. Lac. acidophilus KCTC 3925, Lac. rhamnosus KCTC 3929 with β-glucosidase may control soybean paste fermentation, in which the contents of aglycone-formed isoflavones may be increased for a short period.
ACKNOWLEDGEMENTS
This study is supported by 2015 Research Grant from Kangwon National University (No. 201510105).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Park SK, Seo KI, Choi SH, Moon JS, Lee YH. Quality assessment of commercial doenjang prepared by traditional method. J Korean Soc Food Sci Nutr. 2000;29:211–217. [Google Scholar]
- 2.Jo SJ, Hong CO, Yang SY, Choi KK, Kim HK, Yang H, Lee KW. Changes in contents of γ-aminobutyric acid (GABA) and isoflavones in traditional Korean Doenjang by ripening periods. J Korean Soc Food Sci Nutr. 2011;40:557–564. doi: 10.3746/jkfn.2011.40.4.557. [DOI] [Google Scholar]
- 3.Arena ME, Saguir FM, Manca de Nadra MC. Arginine, citrulline and ornithine metabolism by lactic acid bacteria from wine. Int J Food Microbiol. 1999;52:155–161. doi: 10.1016/S0168-1605(99)00133-6. [DOI] [PubMed] [Google Scholar]
- 4.Kudou S, Shimoyamada M, Imura T, Uchida T, Okubo K. A new isoflavone glycoside in soybean seeds (Glycine max MERRILL), glycitein 7-O-β-D-(6″-O-acetyl) glucopyranoside. Agric Biol Chem. 1991;55:859–860. [Google Scholar]
- 5.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, Kataoka S, Kubota Y, Kikuchi M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000;130:1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KDR, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 7.Jeanes A, Wilham CA, Tsuchiya HM, Haynes WC. Properties of dextrans isolated from whole cultures at various stages of incubation. Arch Biochem Biophys. 1957;71:293–302. doi: 10.1016/0003-9861(57)90038-3. [DOI] [PubMed] [Google Scholar]
- 8.Kimmel SA, Roberts RF, Ziegler GR. Optimization of exopolysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus RR grown in a semidefined medium. Appl Environ Microbiol. 1998;64:659–664. doi: 10.1128/aem.64.2.659-664.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yun HJ, Lee YJ, Yeo SH, Park HY, Park HD, Baek SY. Isolation and characterization of exopolysaccharide producing lactic acid bacteria from Korean soy sauce and soybean paste. Korean J Microbiol Biotechnol. 2013;41:190–197. doi: 10.4014/kjmb.1302.02004. [DOI] [Google Scholar]
- 10.Kim MJ, Koh EM, Surh JH, Kim Lee YK, Kwon HJ. Distribution of isoflavones and coumestrol in legumes and their products consumed in Korea. Food Sci Biotechnol. 2003;12:278–284. [Google Scholar]
- 11.Albright SC, Winston WL, Zappe C. Data analysis & decision making with microsoft excel. South-Western Cengage Learning; Mason, OH, USA: 1999. pp. 601–668. [Google Scholar]
- 12.NAAS. Food components table. National Academy of Agricultural Science, Rural Development Administration; Jeonbuk, Korea: 2001. p. 361. [Google Scholar]
- 13.Kim IB, Shin S, Lim BL, Seong GS, Lee YE. Bioconversion of soybean isoflavone by Lactobacillus plantarum and Bifidobacterium longum. Korean J Food Cook Sci. 2010;26:214–219. [Google Scholar]
- 14.Donkor ON, Shah NP. Production of β-glucosidase and hydrolysis of isoflavone phytoestrogens by Lactobacillus acidophilus, Bifidobacterium lactis, and Lactobacillus casei in soymilk. J Food Sci. 2008;73:M15–M20. doi: 10.1111/j.1750-3841.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- 15.Kang HK, Ko EA, Kim JH, Kim DM. Molecular cloning and characterization of active truncated dextransucrase from Leuconostoc mesenteroides B-1299CB4. Bioprocess Biosyst Eng. 2013;36:857–865. doi: 10.1007/s00449-013-0933-3. [DOI] [PubMed] [Google Scholar]