Abstract
Enhanced production of individual phenolic compounds by subcritical water hydrolysis (SWH) of pumpkin leaves was investigated at various temperatures ranging from 100 to 220°C at 20 min and at various reaction times ranging from 10 to 50 min at 160°C. Caffeic acid, p-coumaric acid, ferulic acid, and gentisic acid were the major phenolic compounds in the hydrolysate of pumpkin leaves. All phenolic compounds except gentisic acid showed the highest yield at 160°C, but gentisic acid showed the highest yield at 180°C. The cumulative amount of individual phenolic compounds gradually increased by 48.1, 52.2, and 78.4 μg/g dry matter at 100°C, 120°C, and 140°C, respectively, and then greatly increased by 1,477.1 μg/g dry matter at 160°C. The yields of caffeic acid and ferulic acid showed peaks at 20 min, while those of cinnamic acid, p-coumaric acid, p-hydroxybenzoic acid, and procatechuic acid showed peaks at 30 min. Antioxidant activities such as 2,2-diphenyl-1-picrylhydrazyl and ferric reducing antioxidant power values gradually increased with hydrolysis temperature and ranged from 6.77 to 12.42 mg ascorbic acid equivalents/g dry matter and from 4.25 to 8.92 mmol Fe2+/100 g dry matter, respectively. Color L* and b* values gradually decreased as hydrolysis temperature increased from 100°C to 140°C. At high temperatures (160°C to 220°C), L* and b* values decreased suddenly. The a* value peaked at 160°C and then decreased as temperature increased from 160°C to 220°C. These results suggest that SWH of pumpkin leaves was strongly influenced by hydrolysis temperature and may enhanced the production of phenolic compounds and antioxidant activities.
Keywords: pumpkin leaves, subcritical water hydrolysis, phenolic compounds, antioxidant activity
INTRODUCTION
Phenolic compounds are secondary plant metabolites ubiquitous in the plant kingdom, and their chemical structures vary from simple phenolics to complex polymers (1). They have various functionalities such as antioxidant, antimicrobial, anticancer, anti-obesity, antidiabetic, anti-hypertensive, and anti-mutagenic properties (2,3). Most naturally occurring phenolic compounds are present as conjugates with mono- and polysaccharides and linked to one or more of the phenolic groups (4). Once phenolic glycosides convert into their corresponding aglycones, the biological activity may be increased (5). Flavonoids bound to sugars as glycosides in foods cannot be absorbed from the intestine, while only free flavonoids without a sugar molecule are able to pass through the gut wall (6).
The major technologies for hydrolysis of phenolic glycosides include acid or base treatments, which are unacceptable due to environmental concerns. Subcritical water may be an attractive reaction medium for hydrolyzing glycosides to aglycones because it is non-toxic, inexpensive, and environmentally friendly. Subcritical water has two unique properties, such as a lower relative dielectric constant and a higher ion product than ambient water. At room temperature and pressure, the dielectric constant is 80. However, when heating water to 150°C, 200°C, 225°C, and 250°C, the dielectric constant is decreased to 45, 35, 31, and 27 which are similar to those of dimethyl sulfoxide, acetonitrile, methanol, and acetone at ambient temperature, respectively. Thus, the ability of water to elute non-polar compounds is increased (7,8). In addition, the ion product of water substantially increases with temperature. The dissociation constant of water at room temperature is 1.0×10−14. However, when heating water to 100°C, 250°C, and 350°C, the dissociation constant of water is increased to 5.6×10−13, 4.9×10−12, and 1.2×10−12, respectively (9). This indicates that the pH of water is decreased from about 7.0 to 5.5, and it provides the ability of acidic hydrolysis to water (8).
Pumpkins are widely used in Korea as healthy and functional vegetables, because they are rich in phenolics, flavonoids, vitamin A and C, β-carotene, α-tocopherol, amino acids, carbohydrates, minerals, and fibers. Pumpkins are known to have physiological activities such as anticancer, antioxidant, and anti-obesity (10–13). Pumpkin leaves have distinct taste and texture and are used as Korean cuisine “ssam” to wrap a piece of meat such as pork or other filling accompanied by a condiment. Cha (14) reported that 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of the water extract from pumpkin leaves was 44.6% at the concentration of 20 mg/mL. Kim et al. (15) reported that the ethanol extract of pumpkin leaves had the highest total phenolic contents (29.62 mg gallic acid equivalents/g dry matter), followed by skin (12.08), flesh (7.08), and seed (1.52), and had stronger DPPH-, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)-radical scavenging activities, and ferric reducing antioxidant power (FRAP) value than any other parts.
To date, there has been no report on the study of subcritical water hydrolysis of pumpkin leaves. Therefore, the objective of this study was to investigate the possibility of phenolic compounds production from pumpkin leaves by subcritical water hydrolysis. The influences of subcritical water temperature and reaction time as main parameters on individual phenolic compounds, antioxidant activity, and color were measured.
MATERIALS AND METHODS
Materials and chemicals
Pumpkin (Cucurbita moschata Duch.) leaves were purchased from a local market, and were washed, dried, ground (−30 mesh), and stored in a refrigerator at 4°C until needed. DPPH and 2,4,6-tripyridyl-S-triazine (TPTZ) were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), respectively. Potassium persulfate, ferric chloride hexahydrate, and ferrous sulfate heptahydrate were purchased from Daejung Chemicals, Ltd. (Gyeonggi, Korea), Junsei Chemical Co., Ltd. (Tokyo, Japan), and Wako Pure Chemicals Industries, Ltd. (Osaka, Japan), respectively. N,O-bis(trimethylsilyl) trifluoroacetamide (BSTFA) was purchased from Supelco (Bellefonte, PA, USA). All phenolic standards were purchased from Fluka (Steinheim, Switzerland).
Subcritical water hydrolysis
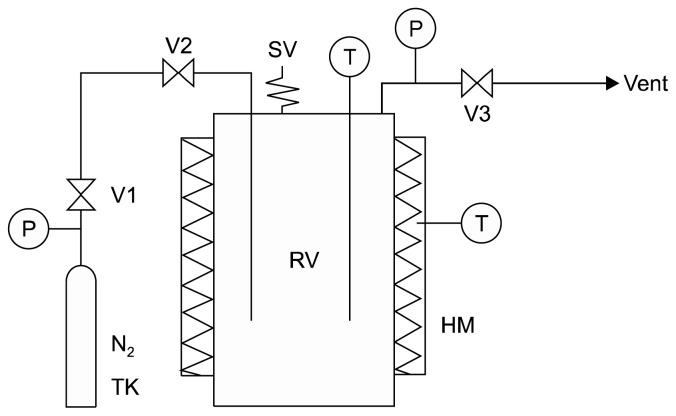
The lab-scale subcritical water hydrolysis (SWH) system was self-built in our laboratory (Fig. 1). The system consists of a reaction vessel, heating mantle, nitrogen gas tank, and auxiliary valve and piping systems. SWH of pumpkin leaves was carried out in a 500 mL high-temperature and high-pressure stainless steel batch type reactor (Autoclave Engineers, Erie, PA, USA) designed for a maximum operating temperature of 238°C at 413 bar. Powdered pumpkin leaves (2.0 g) suspended in 100 mL distilled water were loaded into the reactor with a heating mantle. After being tightly capped, the reactor was purged with inert nitrogen gas to remove dissolved atmospheric oxygen. The reactor was heated electrically at the desired temperature. After treatment for the desired reaction time, the reactor was quickly cooled by soaking in a water bath at room temperature. The hydrolyzed sample was collected and filtered through a filter paper (Toyo No. 5A, Advantec Toyo Kaisha, Ltd., Tokyo, Japan). The filtered solution was brought to a total volume of 100 mL with distilled water (hereafter referred to as the “hydrolysate”). The hydrolysis of pumpkin leaves was performed at different temperatures ranging from 100 to 220°C for 20 min. The effect of hydrolysis time was also investigated ranging from 10 to 50 min at 160°C.
Fig. 1.
Schematic diagram of subritical water hydrolysis system. HM, heating mantle; N2 TK, nitrogen gas tank; P, pressure gauge; RV, reaction vessel; SV, safety valve; T, temperature gauge; V, on/off valve.
Determination of phenolic compounds by gas chromatography/mass spectrometry
The hydrolysate (10 mL) was mixed with 15 mL ethyl acetate in a conical tube and vortexed. After centrifugation (10,000 g) for 10 min, the supernatant was recovered, and the residue was further re-extracted twice. Anhydrous sodium sulfate was added to the mixed supernatants to remove moisture. The ethyl acetate layer was evaporated to remove the solvent using a rotary vacuum evaporator (Rotavapro R-124, Büchi Labortechnik AG, Flawil, Switzerland) at 40°C. The dried residue was dissolved in 0.25 mL BSTFA and incubated for 40 min at 75°C for derivatization.
Individual phenolic compounds were qualified and quantified using an Agilent series GC 6890N coupled with an HP 5973 MS detector and an HP 7683 autosampler (Agilent Technologies, Santa Clara, CA, USA) (16). An HP-5 MS capillary column (30 m×0.25 mm, 0.25 μm film thickness) was used. The injector and transfer line temperatures were 280 and 300°C, respectively. The oven temperature was held at 120°C for 1 min, increased to 220°C at 5°C/min, then to 300°C at 10°C/min, and held for 10 min. Helium was used as a carrier gas at a flow rate of 0.6 mL/min. Injection volume was 1 μL at a split ratio of 1:5. Chromatographic peaks were identified by comparing the retention times and 3 fragment ions of each phenolic compound with those of reference compounds. Target and qualifier ions for 7 phenolic compounds were set as follows: cinnamic acid, 205, 220, and 161; p-hydroxybenzoic acid, 267, 223, and 193; gentisic acid, 355, 356, and 357; protocatechuic acid, 193, 355, and 370; p-coumaric acid; 293, 308, and 219; ferulic acid, 338, 323, and 308; and caffeic acid, 396, 381, and 219.
Determination of antioxidant activity
Antioxidant activities of the hydrolysates were measured in terms of DPPH free radical scavenging activity and FRAP. The DPPH value was measured according to the method of Kamiloglu et al. (17). Briefly, a 0.1 mL of the hydrolysate was added to 1.5 mL of 0.1 mM DPPH in methanol. After vortexing, the mixed solution was incubated for 30 min in the dark at room temperature. The absorbance was measured at 517 nm using a Spectronic Genesys 2 spectrophotometer (Spectronic Instruments, Rochester, NY, USA). Ascorbic acid was used as the standard, and the DPPH value was expressed as mg ascorbic acid equivalents/g dry matter.
FRAP of the hydrolysate was measured according to the method of Thaipong et al. (18) with minor modifications. The FRAP solution consists of 300 mM acetate buffer, 10 mM TPTZ solution in 40 mM HCl, and 20 mM FeCl3·6H2O solution at the ratio of 10:1:1 (v/v), respectively. The hydrolysate (50 μL) was mixed with 150 μL distilled water and 1.5 mL FRAP solution, and incubated at 37°C in a water bath for 10 min. The absorbance was measured at 595 nm using a Multiskan EX microplate reader (Thermo Electron Corp., Beverly, MA, USA). Ferrous sulfate (FeSO4·7H2O) was used as the standard, and the FRAP value was expressed as mmol Fe2+/100 g dry matter.
Determination of color
Color of the hydrolysate was determined using an Ultra-ScanVIS color spectrophotometer (Hunter Associates Lab., Inc., Reston, VA, USA) in terms of L* (lightness), a* (redness to greenness), and b* (yellowness to blueness) values.
Statistical analysis
All measurements were performed in triplicate. Results were presented as mean±standard deviation. Statistical analysis was made by the SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). The difference was considered to be significant when the P-value was less than 0.05 among the treatment means determined by Duncan’s multiple range tests.
RESULTS ANS DISCUSSION
Yields of phenolic compounds
In order to apply subcritical water for the production of phenolic compounds from pumpkin leaves, experiments were performed over a temperature range of 100~220°C at a reaction time of 20 min and over a reaction time range of 10~50 min at a hydrolysis temperature of 160°C.
Table 1 shows the effect of hydrolysis temperature on the yield of individual phenolic compounds from pumpkin leaves at a reaction time of 20 min. Seven phenolic compounds were detected in the hydrolysate of pumpkin leaves: cinnamic acid, p-hydroxybenzoic acid, gentisic acid, protocatechuic acid, p-coumaric acid, ferulic acid, and caffeic acid. Caffeic acid, p-coumaric acid, ferulic acid, and gentisic acid were high in quantities among the others and were considered as the major phenolics in pumpkin leaves. All phenolic compounds except gentisic acid showed the highest yields at 160°C, but gentisic acid showed the highest yield at 180°C. The cumulative amount of individual phenolic compounds gradually increased by 48.1, 52.2, and 78.4 μg/g dry matter at 100°C, 120°C, and 140°C, respectively, and then greatly increased by 1,477.1 μg/g dry matter at 160°C. Total phenolics were more than 30-fold higher at 160°C compared to those at 100°C. Ko et al. (19) also reported that quercetin yields by subcritical water extraction from onion skin was over 6-, 8- and 4-fold greater than those obtained using ethanol, methanol, and water-at-boiling-point extractions.
Table 1.
Yield of phenolic compounds in pumpkin leaves hydrolyzed by subcritical water at various temperatures for 20 min (unit: μg/g dry matter)
| Phenolic compounds | Yields | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 100°C | 120°C | 140°C | 160°C | 180°C | 200°C | 220°C | |
| Cinnamic acid | ND1) | 4.0±0.8d | 4.0±0.8d | 16.2±0.8b | 8.7±0.8c | 19.8±1.3a | 18.6±0.9a |
| p-Hydroxybenzoic acid | 2.5±0.6e | 4.4±1.1e | 8.1±1.7d | 24.0±1.2b | 8.7±1.4d | 30.1±1.7a | 14.8±0.5c |
| Gentisic acid | 7.9±2.3d | 6.2±0.1d | 17.8±1.7d | 237.1±16.3b | 263.9±18.5a | 61.7±3.8c | 23.6±1.0d |
| Protocatechuic acid | 1.1±0.0cd | 1.0±0.1d | 1.7±0.4c | 11.4±0.3a | 3.9±0.6b | 11.6±0.5a | 4.4±0.1b |
| p-Coumaric acid | 7.9±2.3d | 5.9±0.0d | 6.2±0.2d | 363.2±13.9a | 100.3±11.0b | 36.4±1.3c | 26.2±1.3c |
| Ferulic acid | 11.1±1.1d | 13.7±1.2d | 19.1±2.9c | 237.5±5.0a | 24.9±2.6b | ND | ND |
| Caffeic acid | 17.5±2.3b | 16.9±0.4b | 21.3±2.9b | 587.8±24.2a | 20.1±1.1b | 19.9±0.6b | 16.5±0.3b |
| Total | 48.1±9.5d | 52.2±3.5d | 78.4±7.7d | 1,477.2±57.6a | 430.6±31.5b | 179.5±8.5c | 104.1±2.9c |
Means±SD (n=3).
The means in each row followed by common letters (a–e) are not significantly different by Duncan’s multiple range test at P<0.05.
ND: not detected.
Total phenolics were drastically decreased at higher hydrolysis temperatures than 160°C due to the decomposition reactions. Singh and Saldaña (20) also found that higher concentrations of phenolic compounds were recovered at temperatures from 140 to 180°C, and further increases in temperatures from 180 to 240°C lowered the yield of phenolic compounds in subcritical water extraction of potato peel. This could be attributed to sample pyrolysis above 180°C which resulted in degradation of phenolic compounds.
Table 2 shows the effect of reaction time on the yield of individual phenolic compounds in subcritical water hydrolysates of pumpkin leaves at hydrolysis temperature of 160°C. The hydrolysis temperature was set at 160°C due to the highest yield of phenolic compounds as shown in Table 1. The yields of caffeic acid and ferulic acid showed peaks at 20 min, while those of cinnamic acid, p-coumaric acid, p-hydroxybenzoic acid, and procatechuic acid showed peaks at 30 min. Specially the yield of gentisic acid consistently increased with reaction time. The cumulative amount of individual phenolic compounds gradually increased with reaction time up to 30 min and then decreased gradually at longer reaction times, which may be due to the decomposition of phenolic compounds. Longer reaction times as well as higher temperatures had destructive effects on the yields of phenolic compounds due to decomposition reactions, which may occur under subcritical water condition (20).
Table 2.
Yield of phenolic compounds in pumpkin leaves hydrolyzed by subcritical water at various reaction times at 160°C (unit: μg/g dry matter)
| Phenolic compounds | Yields | ||||
|---|---|---|---|---|---|
|
| |||||
| 10 min | 20 min | 30 min | 40 min | 50 min | |
| Cinnamic acid | 13.2±0.2b | 16.2±0.8a | 16.7±0.7a | 15.5±0.8a | 16.2±0.3a |
| p-Hydroxybenzoic acid | 17.4±0.7c | 24.0±1.2b | 30.9±1.5a | 28.8±2.4a | 30.6±1.3a |
| Gentisic acid | 76.5±1.2d | 237.1±16.3c | 342.5±22.9b | 354.8±21.1ab | 373.5±7.2a |
| Protocatechuic acid | 8.1±0.5d | 11.4±0.3c | 15.6±0.8ab | 14.0±1.5b | 15.9±0.8a |
| p-Coumaric acid | 258.4±11.4d | 363.2±13.9b | 399.3±18.1a | 348.1±25.3bc | 330.4±10.3c |
| Ferulic acid | 184.3±12.2c | 237.5±5.0a | 217.8±8.8b | 161.8±15.5d | 130.1±6.8e |
| Caffeic acid | 592.7±41.7a | 587.8±24.2a | 503.0±21.0b | 308.8±29.2c | 203.8±10.0d |
| Total | 1,150.6±67.4bc | 1,477.2±57.6a | 1,525.7±67.1a | 1,231.9±94.8b | 1,100.4±35.0c |
Means±SD (n=3).
The means in each row followed by common letters (a–f) are not significantly different by Duncan’s multiple range test at P<0.05.
Cheigh et al. (21) reported that the yields of hesperidin and narirutin increased for 10 min and then decreased with increasing extraction time at 160°C and 170°C in subcritical water extraction of Citrus unshiu peel. Ko et al. (19) also reported that total amount of quercetin increased for 5~15 min, and then decreased for 20~30 min at 165°C in subcritical water extraction of onion skin.
Antioxidant activity
Table 3 shows the effect of hydrolysis temperature on antioxidant activities, such as DPPH radical scavenging activity and FRAP value of subcritical water hydrolysates from pumpkin leaves at 20 min. The antioxidant activities of DPPH and FRAP gradually increased with hydrolysis temperature and ranged from 6.77 to 12.42 mg ascorbic acid equivalents/g dry matter and from 4.25 to 8.92 mmol Fe2+/100 g dry matter, respectively. Even though the cumulative yields of individual phenolic compounds were low at higher hydrolysis temperatures (>160°C), the DPPH and FRAP values were high. This suggested the possibility that degradation compounds from phenolic compounds with high antioxidant activity could be formed or extracted at such higher hydrolysis temperatures (22).
Table 3.
DPPH free radical scavenging activity and FRAP value of pumpkin leaves hydrolyzed by subcritical water at various temperatures for 20 min
| Hydrolysis temperature (°C) | DPPH scavenging activity (mg ascorbic acid equivalents/g dry matter) | FRAP value (mmol Fe2+/100 g dry matter) |
|---|---|---|
| 100 | 6.77±0.24f | 4.25±0.08e |
| 120 | 7.05±0.26f | 4.44±0.15e |
| 140 | 8.03±0.19e | 6.02±0.27d |
| 160 | 9.71±0.21c | 7.36±0.46b |
| 180 | 9.16±0.03d | 6.55±0.05c |
| 200 | 12.42±0.08a | 8.67±0.30a |
| 220 | 12.03±0.14b | 8.92±0.12a |
Means±SD (n=3).
The means in each column followed by common letters (a–f) are not significantly different by Duncan’s multiple range test at P<0.05.
Jose et al. (23) reported that increasing the hydrolysis temperature above 160°C decreased polyphenol contents but increased antioxidant activity because high temperatures favor the formation of derived antioxidant compounds from phenolic compounds in grape pomace. Rangsriwong et al. (24) also reported that despite the lowest total phenolic contents in the subcritical water extract from Terminalia chebula obtained at 220°C, the antioxidant activity was comparable to that of those obtained at lower temperatures because at high temperature, other highly antioxidative nonphenolic compounds including triterpenes, coumarins, steroids, and benzenoids could be extracted.
Table 4 shows the effect of reaction time on antioxidant activities of DPPH free radical scavenging activity and FRAP of subcritical water hydrolysates from pumpkin leaves at 160°C. The DPPH and FRAP values increased up to 20 min of reaction time and then stayed almost the same value. Khuwijitjaru et al. (25) reported that DPPH free radical scavenging activity of subcritical water extract from cinnamon bark didn’t increase even though the extraction time was longer than 30 min.
Table 4.
DPPH free radical activity and FRAP value of pumpkin leaves hydrolyzed by subcritical water at various reaction times at 160°C
| Reaction time (min) | DPPH scavenging activity (mg ascorbic acid equivalents/g dry matter) | FRAP value (mmol Fe2+/100 g dry matter) |
|---|---|---|
| 10 | 7.39±0.02d | 5.74±0.01c |
| 20 | 9.71±0.21b | 7.36±0.46a |
| 30 | 9.35±0.07c | 6.71±0.04b |
| 40 | 9.40±0.16c | 7.12±0.16ab |
| 50 | 10.16±0.06a | 7.16±0.14a |
Means±SD (n=3).
The means in each column followed by common letters (a–d) are not significantly different by Duncan’s multiple range test at P<0.05.
Color values
Color values of subcritical water hydrolysates from pumpkin leaves are shown in Table 5 at various temperatures for 20 min and in Table 6 at various reaction times at 160°C. As the hydrolysis temperature increased from 100°C to 140°C, the color values L* and b* of the hydrolysates gradually decreased. At high temperatures (160°C to 220°C), color values L* and b* decreased suddenly and were leveled off. Color value a* showed a peak at 160°C and then decreased as the temperature increased from 160°C to 220°C. All color values L*, a*, and b* decreased gradually with reaction time.
Table 5.
Color values of pumpkin leaves hydrolyzed by subcritical water at various temperatures for 20 min
| Hydrolysis temperature (°C) | L* | a* | b* |
|---|---|---|---|
| 100 | 48.42±0.19a | 3.47±0.07d | 11.57±0.31a |
| 120 | 48.35±0.09a | 3.76±0.02c | 11.67±0.10a |
| 140 | 47.62±0.37b | 4.26±0.03b | 11.04±0.52b |
| 160 | 43.97±0.09c | 5.49±0.07a | 6.14±0.14c |
| 180 | 41.34±0.02d | 3.14±0.02e | 1.85±0.03d |
| 200 | 40.19±0.01e | 1.98±0.03f | 0.07±0.02e |
| 220 | 40.00±0.04e | 1.62±0.02g | −0.29±0.03e |
Means±SD (n=3).
The means in each column followed by common letters (a–g) are not significantly different by Duncan’s multiple range test at P<0.05.
Table 6.
Color values of pumpkin leaves hydrolyzed by subcritical water at various reaction times at 160°C
| Reaction time (min) | L* | a* | b* |
|---|---|---|---|
| 10 | 46.36±0.13a | 5.49±0.08a | 9.72±0.23a |
| 20 | 43.97±0.09b | 5.49±0.07a | 6.14±0.14b |
| 30 | 43.30±0.05c | 5.22±0.02b | 4.90±0.02c |
| 40 | 43.34±0.04c | 5.17±0.05b | 4.92±0.06c |
| 50 | 42.65±0.01d | 4.60±0.02c | 3.75±0.04d |
Means±SD (n=3).
The means in each column followed by common letters (a–d) are not significantly different by Duncan’s multiple range test at P<0.05.
Singh and Saldaña (20) reported that at higher temperatures (140°C~180°C), the color of potato peel extract was darker than at lower temperatures (100°C~120°C). But, at temperatures of 180°C to 240°C, the color of the extract became very dark due to sample pyrolysis. Watchararuji et al. (26) also reported that L* values of subcritical water hydrolysates from rice bran and soybean meal decreased when the hydrolysis time increased from 10 to 30 min at 200°C, 210°C, and 220°C because of Maillard reaction.
In conclusion, production of phenolic compounds from pumpkin leaves was strongly influenced by hydrolysis temperature instead of reaction time. At 160°C for 20 min, the highest yield of phenolic compounds and favorable color of the hydrolysates from pumpkin leaves was obtained. The antioxidant activity of the hydrolysates increased as hydrolysis temperature increased, but it was not proportional to the content of phenolic compounds in the hydrolysates at higher temperatures. Further studies are needed for clarifying the degradation products from phenolic compounds with high antioxidant activity.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Mukhopadhyay S, Luthria DL, Robbins RJ. Optimization of extraction process for phenolic acids from black cohosh (Cimicifuga racemosa) by pressurized liquid extraction. J Sci Food Agric. 2006;86:156–162. doi: 10.1002/jsfa.2326. [DOI] [Google Scholar]
- 2.Chiou A, Karathanos VT, Mylona A, Salta FN, Preventi F, Andrikopoulos NK. Currants (Vitis vinifera L.) content of simple phenolics and antioxidant activity. Food Chem. 2007;102:516–522. doi: 10.1016/j.foodchem.2006.06.009. [DOI] [Google Scholar]
- 3.Kunyanga CN, Imungi JK, Okoth MW, Biesalski HK, Vadivel V. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT-Food Sci Technol. 2012;45:269–276. doi: 10.1016/j.lwt.2011.08.006. [DOI] [Google Scholar]
- 4.Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 5.Wiczkowski W, Romaszko J, Bucinski A, Szawara-Nowak D, Honke J, Zielinski H, Piskula MK. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J Nutr. 2008;138:885–888. doi: 10.1093/jn/138.5.885. [DOI] [PubMed] [Google Scholar]
- 6.Kühnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191. doi: 10.1159/000399407. [DOI] [PubMed] [Google Scholar]
- 7.Amashukeli X, Pelletier CC, Kirby JP, Grunthaner FJ. Subcritical water extraction of amino acids from Atacama Desert soils. J Geophys Res. 2007;112 doi: 10.1029/2006JG000308. [DOI] [Google Scholar]
- 8.Shitu A, Izhar S, Tahir TM. Sub-critical water as a green solvent for production of valuable materials from agricultural waste biomass: a review of recent work. Global J Environ Sci Manage. 2015;1:255–264. [Google Scholar]
- 9.Plaza M, Turner C. Pressurized hot water extraction of bioactives. Trends Anal Chem. 2015;71:39–54. doi: 10.1016/j.trac.2015.02.022. [DOI] [Google Scholar]
- 10.Kim SR, Ha TY, Song HN, Kim YS, Park YK. Comparison of nutritional composition and antioxidative activity for Kabocha squash and pumpkin. Korean J Food Sci Technol. 2005;37:171–177. [Google Scholar]
- 11.Que F, Mao L, Fang X, Wu T. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. Int J Food Sci Technol. 2008;43:1195–1201. doi: 10.1111/j.1365-2621.2007.01590.x. [DOI] [Google Scholar]
- 12.Lee HJ, Do JR, Kwon JH, Kim HK. Physiological activities of Cucurbita moschata Duch. extracts with different extraction conditions. J Korean Soc Food Sci Nutr. 2010;39:165–171. doi: 10.3746/jkfn.2010.39.2.165. [DOI] [Google Scholar]
- 13.Valenzuela GM, Soro AS, Tauguinas AL, Gruszycki MR, Cravzov AL, Gimenez MC, Wirth A. Evaluation polyphenol content and antioxidant activity in extracts of Cucurbita spp. Open Access Library J. 2014;1:1–6. [Google Scholar]
- 14.Cha YY. Experimental study on effects of Cucurbita moschata Duch. on antioxidation. J Korean Med Obes Res. 2009;9:57–63. [Google Scholar]
- 15.Kim MJ, Hong CO, Nam MH, Lee KW. Antioxidant effects and physiological activities of pumpkin (Cucurbita moschata Duch.) extract from different aerial parts. Korean J Food Sci Technol. 2011;43:195–199. doi: 10.9721/KJFST.2011.43.2.195. [DOI] [Google Scholar]
- 16.Kim MB, Park JS, Lim SB. Antioxidant activity and cell toxicity of pressurised liquid extracts from 20 selected plant species in Jeju, Korea. Food Chem. 2010;122:546–552. doi: 10.1016/j.foodchem.2010.03.007. [DOI] [Google Scholar]
- 17.Kamiloglu S, Pasli AA, Ozcelik B, Van Camp J, Capanoglu E. Colour retention, anthocyanin stability and antioxidant capacity in black carrot (Daucus carota) jams and marmalades: Effect of processing, storage conditions and in vitro gastrointestinal digestion. J Funct Foods. 2015;13:1–10. doi: 10.1016/j.jff.2014.12.021. [DOI] [Google Scholar]
- 18.Kriengsak T, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 19.Ko MJ, Cheigh CI, Cho SW, Chung MS. Subcritical water extraction of flavonol quercetin from onion skin. J Food Eng. 2011;102:327–333. doi: 10.1016/j.jfoodeng.2010.09.008. [DOI] [Google Scholar]
- 20.Singh PP, Saldaña MDA. Subcritical water extraction of phenolic compounds from potato peel. Food Res Int. 2011;44:2452–2458. doi: 10.1016/j.foodres.2011.02.006. [DOI] [Google Scholar]
- 21.Cheigh CI, Chung EY, Chung MY. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J Food Eng. 2012;110:472–477. doi: 10.1016/j.jfoodeng.2011.12.019. [DOI] [Google Scholar]
- 22.Khuwijitjaru P, Plernjit J, Suaylam B, Samuhaseneetoo S, Pongsawatmanit R, Adachi S. Degradation kinetics of some phenolic compounds in subcritical water and radical scavenging activity of their degradation products. Can J Chem Eng. 2014;92:810–815. doi: 10.1002/cjce.21898. [DOI] [Google Scholar]
- 23.Vergara-Salinas JR, Bulnes P, Zúñiga MC, Pérez-Jiménez J, Torres JL, Mateos-Martín ML, Agosin E, Pérez-Correa JR. Effect of pressurized hot water extraction on antioxidants from grape pomace before and after enological fermentation. J Agric Food Chem. 2013;61:6929–6936. doi: 10.1021/jf4010143. [DOI] [PubMed] [Google Scholar]
- 24.Rangsriwong P, Rangkadilok N, Satayavivad J, Goto M, Shotipruk A. Subcritical water extraction of polyphenolic compounds from Terminalia chebula Retz. fruits. Sep Purif Technol. 2009;66:51–56. doi: 10.1016/j.seppur.2008.11.023. [DOI] [Google Scholar]
- 25.Khuwijitjaru P, Sayputikasikorn N, Samuhasaneetoo S, Penroj P, Siriwongwilaichat P, Adachi S. Subcritical water extraction of flavoring and phenolic compounds from cinnamon bark (Cinnamomum zeylanicum) J Oleo Sci. 2012;61:349–355. doi: 10.5650/jos.61.349. [DOI] [PubMed] [Google Scholar]
- 26.Watchararuji K, Goto M, Sasaki M, Shotipruk A. Value-added subcritical water hydrolysate from rice bran and soybean meal. Bioresour Technol. 2008;99:6207–6213. doi: 10.1016/j.biortech.2007.12.021. [DOI] [PubMed] [Google Scholar]