Abstract
In this study, we report the optimal extraction conditions for obtaining organosulfur compounds, such as cycloalliin, from garlic by using principal component analysis (PCA). Extraction variables including temperature (40~80°C), time (0.5~12 h), and pH (4~12) were investigated for the highest cycloalliin yields. The cycloalliin yield (5.5 mmol/mL) at pH 10 was enhanced by ~40% relative to those (~3.9 mmol/mL) at pH 4 and pH 6. The cycloalliin level at 80°C showed the highest yield among the tested temperatures (5.05 mmol/mL). Prolonged extraction times also increased cycloalliin yield; the yield after 12 h was enhanced ~2-fold (4 mmol/mL) compared to the control. Isoalliin and cycloalliin levels were inversely correlated, whereas a direct correlation between polyphenol and cycloalliin levels was observed. In storage for 30 days, garlic stored at 60°C (11 mmol/mL) showed higher levels of cycloalliin and polyphenols than those at 40°C, with the maximum cycloalliin level (13 mmol/mL) on day 15. Based on the PCA analysis, the isoalliin level depended on the extraction time, while cycloalliin amounts were influenced not only by extraction time, but also by pH and temperature. Taken together, extraction of garlic at 80°C, with an incubation time of 12 h, at pH 10 afforded the maximum yield of cycloalliin.
Keywords: Allium sativum L., garlic, organosulfur compound, cycloalliin, PCA
INTRODUCTION
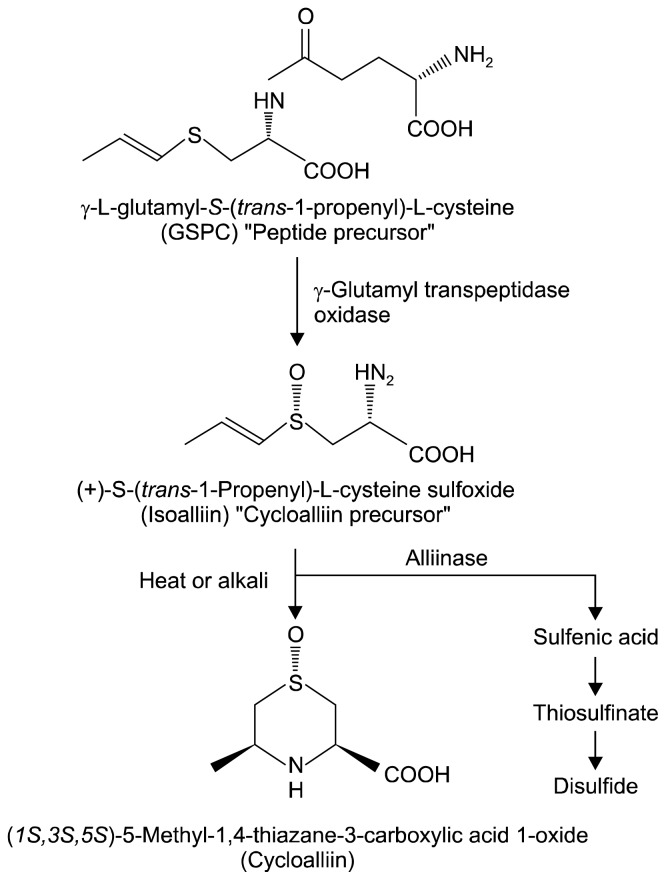
Garlic is a bulbous plant of the genus Allium. Relatives of garlic include, but are not limited to, onions, shallots, leeks, and chives. The cultivation of garlic has been in practice since the early periods of human history, and it has been used for both culinary and medicinal purposes. In addition to its hard bulbs, which make it resistant to pests and animals such as rabbits and moles, garlic contains an abundance of sulfur-containing compounds, which are responsible for its pungent flavour and beneficial health properties (1). Sulfoxides (alliin), thiosulfinates (alliicin), and dithiin (ajoene) are the major sulfur-based compounds found in garlic (2). The synthesis of these organosulfur compounds involves the transformation of γ-glutamyl peptides (GSPCs) into sulfoxides (3, 4). GSPCs are converted into alk(en)yl-L-cysteine sulfoxides such as alliin, isoalliin, and methiin by γ-glutamyl transpeptidase and oxidase (3,4). When garlic is cut or crushed, alliinase is released, and it promotes the production of thiosulfinates from sulfoxides (4,5). Upon heating, a condition under which alliinase is deactivated, isoalliin, an S-propenyl-cystein-sulfoxide, is converted to cycloalliin [(1S,3R,5S)-5-methyl-1,4-thiazane-3-carboxylic acid 1-oxide], a stable cyclic compound (6,7) (Fig. 1). Unwilling flavours unique to garlic almost disappear in this compound (7). The synthesis of cycloalliin is usually achieved during the storage of raw garlic at high temperatures, during which its cycloalliin content increases (2). In 2003, Nagao et al. (8) reported that during the cooking process, cycloalliin accounted for ~50% of all sulfur-containing compounds in garlic.
Fig. 1.
Biosynthetic pathway of organosulfur compounds in garlic.
Like other sulfur-containing compounds in garlic, cycloalliin is known to be associated with many health benefits of garlic. Previous studies have shown that cycloalliin is responsible for reducing the risk of both cancer and cardiovascular disease, as well as playing an important role in the reduction of serum triglyceride in rats (9, 10). Xiao and Parkin (11) have reported that cycloalliin promotes the reduction of quinone reductase activity, which is elevated in several tumors (12) in vitro. In addition, cycloalliin has been reported to increase the fibrinolytic activity of blood in humans, while also inhibiting platelet aggregation (13–15). All of these studies suggest that cycloalliin can be useful as a chemical and/or biological marker for the evaluation of garlic, onion, or garlic-derived foods.
Cycloalliin is much more stable and flavourless compared to the other organosulfur compounds derived from garlic, yet it has similar health promoting properties. The maximization of cycloalliin levels found in garlic is very important in the application of garlic in both food and medications. In this study, we have investigated the optimal conditions for the extraction of cycloalliin from garlic and the optimal steaming time for the pre-processing of garlic. The changes in the cycloalliin levels according to storage temperature were also determined. Moreover, the extraction variables that affected garlic components were examined via principal component analysis (PCA) analysis.
MATERIALS AND METHODS
Chemical and plant materials
Cycloalliin was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). GSPC and isoalliin were isolated from garlic and onion bulbs, respectively, according to the methods of Lawson et al. (16) and Shen and Parkin (17) with slight modifications. Acetonitrile, methanol, and water for high-performance liquid chromatography (HPLC) were purchased from Honeywell Burdick & Jackson® (Muskegon, MI, USA). All other chemicals were of reagent grade and obtained from local suppliers.
Extraction of organosulfur compounds
Bulbs of a garlic plant that had been harvested in the Uiseong, Korea and stored at room temperature for either 1 or 6 months were obtained from a local market. The extraction of garlic was carried out as follows: the de-hulled garlic was first steamed (5, 10, 20, 30, or 60 min) in order to deactivate the native alliinase. This was followed by homogenizing for 10 min to obtain an uniform slurry. Extraction temperature (40, 50, 60, and 80°C), extraction pH (pH 4.0, 6.0, 8.0, 10.0, and 12.0), and extraction time (0.5, 1, 2, 4, 8, and 12 h) were investigated in order to elucidate the optimal extraction conditions. Solids were separated by centrifugation at 2,000 g for 10 min under 5°C, and the resulting supernatant was analysed.
Analysis of isoalliin and cycloalliin
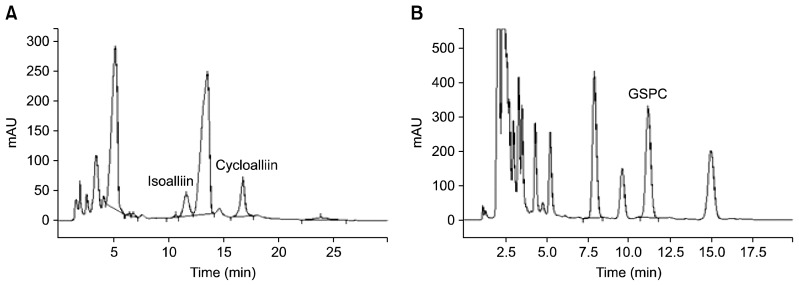
The HPLC analysis of isoalliin and cycloalliin was performed by the method described by Ichikawa et al. (18), with some modifications (Fig. 2A). The HPLC system employed was a Varian Prostar 200 (Varian Medical Systems Inc., Palo Alto, CA, USA) equipped with a quaternary solvent delivery system, an autosampler, and a UV detector, which measured the absorbance at 210 nm. The column was a Shodex Asahipak NH2P-50 4E column (250×4.6 mm, Showa Donko Co., Tokyo, Japan). Gradient elution was employed using solvent A (0.2%, v/v, phosphoric acid in water) and solvent B (0.2%, v/v, phosphoric acid in acetonitrile) at 30°C: 0→5 min, 84% B (isocratic); 5→15 min, 84→80% B; 15→20 min, 80→84% B; 20→30 min, 84% B (isocratic). The flow rate was kept at 1.5 mL/min and the sample injection volume was 10 μL.
Fig. 2.
Typical (A) normal-phase, (B) reversed-phase HPLC chromatograms of garlic extracts.
Analysis of GSPC
GSPC was analysed by reversed-phase HPLC, a Varian Prostar 200 system (Varian Medical Systems Inc.), of the same construction as described above, and used as described above (Fig. 2B). The HPLC conditions were as follows: the column used was an SP column C18 type UG120 (150×4.6 mm, 5 μm, Macherey-Nagel GmbH & Co. KG, Düren, Germany); the column temperature was 30°C, the flow rate was 0.8 mL/min, the mobile phase was 50 mM phosphate buffer (pH 2.6) in methanol (85: 15, v/v) under isocratic condition, the wavelength employed was 205 nm, and the injection volume was 10 μL.
Analysis of polyphenols
The polyphenol content was determined using the Folin-Ciocalteu method (19), adapted to a micro scale. In a 1.5-mL Eppendorf tube, 0.79 mL of distilled water, 0.01 mL of sample, and 0.05 mL of 0.9 N Folin-Ciocalteu reagent were added and mixed. After exactly 1 min, 0.15 mL of 20% sodium carbonate was added, and the mixture was allowed to stand at room temperature for 120 min. The absorbance was read at 750 nm, and the polyphenol concentration was calculated from a calibration curve using gallic acid as a standard.
Changes in the amount of organosulfur compounds present in garlic cloves during storage
The garlic extract was stored at 40°C and 60°C for 0, 1, 2, 3, 5, 10, 15, or 30 days. The organosulfur compound composition and polyphenol content in the garlic extract were determined according to the quantitative method described for the analysis of polyphenol and organosulfur compounds. The pH was measured with a Orion 3-Star pH meter (Thermo Scientific Orion, Beverly, MA, USA) with a glass electrode.
The degree of browning was also measured spectrophotometrically (NanoQuant Infinite M200, Tecan Group Ltd., Männedorf, Switzerland) at 420 nm. The colour determinations of the garlic extracts were performed using a colour measurement spectrophotometer (Minolta Camera, Tokyo, Japan) set for Hunter L (lightness), a (redness), b (yellowness), and ΔE (total colour difference) values. The results of the Hunter L, a, and b values were averaged from 10 replications, and analyses were performed with the garlic samples from each storage condition in triplicate.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences version 12.0 (SPSS Inc., Chicago, IL, USA). The statistical significance of differences was determined using unpaired Student’s t-tests and one-way ANOVA at a significance level of P<0.05 followed by Duncan’s multiple range test. All data are reported as mean±standard deviations (SD). PCA was performed on a set of data derived from extraction and storage experiments by transforming the original variables into a smaller set of linear combinations [principal components (PCs)]. We selected two PCs. The best condition vector was extracted from the combination plot of the two PCs.
RESULTS AND DISCUSSION
Effect of steaming time
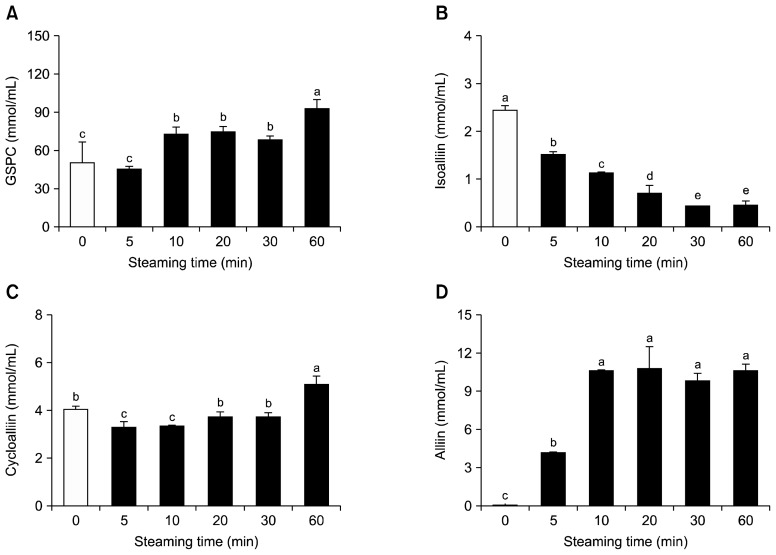
To induce the production of cycloalliin from isoalliin, alliinase (which can also produce other sulfur-containing compounds such as thiosulfinates) should be deactivated. Thus, we performed a steaming treatment as a means of deactivating alliinase, and determined its effects on the levels of cycloalliin and related sulfur compounds (i.e. its precursors). The yield of each garlic extract showed no significant change (data not shown). The levels of cycloalliin, GSPC, and alliin were enhanced as steaming time increased, whereas the level of isoalliin decreased in a time-dependent manner (Fig. 3). Steaming for 60 min resulted in the highest observed levels of GSPC and cycloalliin, whereas the level of isoalliin was the lowest at 30 min, and the level of alliin greatly increased until 10 min and then reached a plateau (Fig. 3). Consequently, 10 min seems to be the optimal steaming time for the inhibition of alliinase activity during the processing of garlic. However, this study did not provide an exact correlation between enzyme activity and steaming time. Our data shows that alliinase was active after 10 min considering the level of cycloalliin. Therefore, a detailed steaming time should be established through the examination of alliinase enzymatic activity. Our results demonstrate that the deactivation of alliinase through steaming allows for the synthesis of cycloalliin by blocking alliinase-catalysed pathways, which produce thiosulfinates such as allicin. The increase in the level of GSPC by steaming appears to be due to the deactivation of γ-glutamyl transpeptidase oxidase, which catalyses the synthesis of isoalliin (a precursor of cycloalliin) from GSPC. We also believe that the deactivation of alliinase and γ-glutamyl transpeptidase oxidase by steaming led to a significant drop in the level of isoalliin content.
Fig. 3.
Effect of steaming time on the organosulfur compounds and polyphenol content in garlic. Each bar represents mean±SD (n=3). (A) γ-glutamyl peptide (GSPC), (B) isoalliin, (C) cycloalliin, and (D) alliin. Different letters (a–e) are significantly different at P<0.05 using Duncan’s multiple range test.
Both garlic and onion contain pungent sulfur compounds, which can generate undesirable breath odours. Previous studies have reported that boiling or homogenizing garlic bulbs in alcohol mixed with water removed the pungent odour and deactivated alliinase (20,21). Anno et al. (22) reported that at 70°C, the endogenous alliinase could be deactivated in 5~10 min, and that at 80°C or 100°C, the enzyme could be deactivated in 1 min. Meanwhile, despite the fact that blanching treatment with water at 90°C or 100°C for 15 min appears to achieve the complete deactivation of alliinase (23,24), the optimum blanching conditions have not yet been established for garlic. Over-blanching, apart from exhibiting high energy consumption, may cause the loss of nutrients and texture. Our steaming treatment, at 100°C for 10 min, was considered to be sufficient for the deactivation of alliinase.
Effect of pH
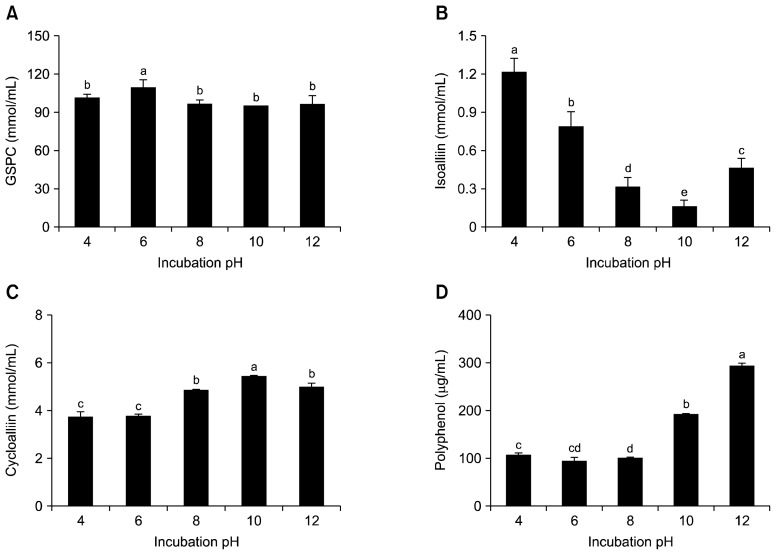
In order to determine the optimal pH for the extraction of organosulfur compounds from garlic, the levels of GSPC, isoalliin, cycloalliin, and polyphenols were analysed after extraction over a range of pH values (Fig. 4). To investigate the effects of pH on the levels of organosulfur compounds, the pH values of the garlic extract were varied from 4.0 to 12.0, while keeping the incubation temperature at 40°C and the incubation time at 12 h (Fig. 4). The GSPC level did not show any significant change at any pH value, and only showed a slight increase at pH 6. The levels of cycloalliin and polyphenols increased under alkaline conditions, while the level of isoalliin considerably decreased at high pH values. Cycloalliin was at its maximum level (5.43 mmol/mL) at pH 10, but the level of isoalliin was lowest at pH 10. Meanwhile, the polyphenol level was maximum at pH 12.
Fig. 4.
Effect of pH on the organosulfur compounds and polyphenol content of garlic extracts. Each bar represents mean±SD (n=3). (A) γ-glutamyl peptide (GSPC), (B) isoalliin, (C) cycloalliin, and (D) polyphenol. Different letters (a–e) are significantly different at P<0.05 using Duncan’s multiple range test.
These results indicate that the conversion of isoalliin to cycloalliin is promoted under alkaline conditions. Ueda et al. (7) reported that the degradation of isoalliin is accelerated at high pH levels, which is in agreement with our data. In addition, alkaline conditions could partially contribute to the suppression of the remaining activity of alliinase, the optimal pH of which is around 6. Our results showed that the polyphenol level was enhanced at pH 9 and 10 as opposed to at lower pH values. Wissam et al. (25) reported that the level of proanthocyanidins, the polyphenols found in pomegranate peels, was increased at pH 9 and 10 compared to that at pH 5.5, but the polyphenol level was not altered. However, Cheng et al. (26) showed that the levels of polyphenols extracted from blueberry leaves at pH 2 were higher than those extracted at pH 6. The dependence of the level of extracted polyphenol on pH values seems to be affected by both the type and source of the polyphenols. However, polyphenols have been known to undergo oxidation to form quinones under alkaline conditions (27), which could impact our analysis of the polyphenol content. Ultimately, our data showed that the optimal pH for the effective extraction of cycloalliin is 10.
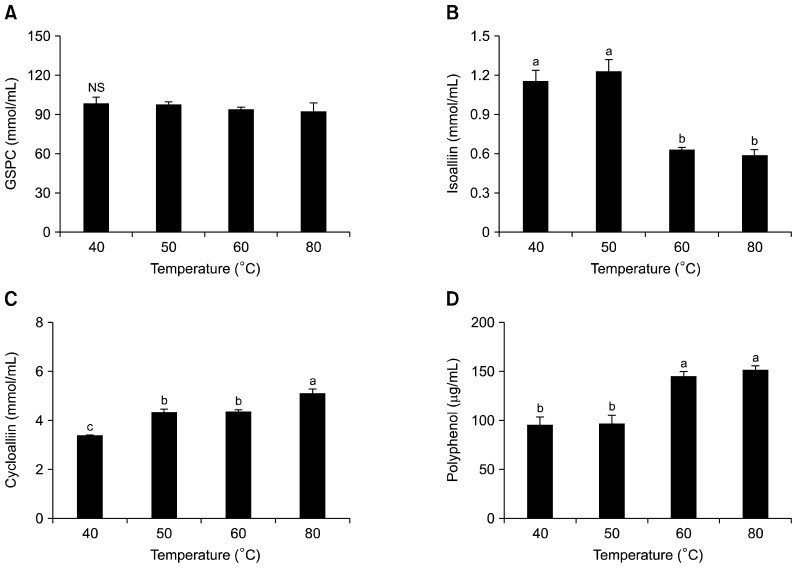
Effect of extraction temperature
We next sought to determine the effect of temperature on the extraction of the aforementioned organosulfur compounds (Fig. 5). After 12 h of incubation, the cycloalliin level was found to increase when the temperature was increased, with cycloalliin content at 80°C enhanced by 45% compared with that at 40°C (Fig. 5C). Additionally, the isoalliin level was decreased 2-fold at 80°C compared with that at 40°C (Fig. 5B), which demonstrates that the conversion of isoalliin to cycloalliin is more effectively promoted at higher temperatures, and that 80°C is the optimum extraction temperature. This temperature increase also induced higher amounts of polyphenols (Fig. 5D), although the GSPC content was not altered within the tested temperature range (Fig. 5A). It should be noted that Ichikawa et al. (2) observed the conversion of isoalliin to cycloalliin at 35°C, and that Anno et al. (22) found that extraction temperatures needed to be increased in order to promote the conversion of isoalliin to cycloalliin.
Fig. 5.
Effect of temperature on the organosulfur compounds and polyphenol content of garlic extracts. Each bar represents mean± SD (n=3). (A) γ-glutamyl peptide (GSPC), (B) isoalliin, (C) cycloalliin, and (D) polyphenol. Different letters (a–c) are significantly different at P<0.05 using Duncan’s multiple range test. NS: not significant.
A recent study reported that a significant increase in the levels of phenolic compounds found in garlic was observed upon heating (28). Polyphenols contain a phenolic hydroxyl group, which provides an antioxidative effect via interactions with the phenol ring and a resonance stabilization effect (29). Stewart et al. (30) reported that this heat-induced increase in polyphenol levels is due to the fact that phenolic compounds contain bound cellular constituents that can be released upon heating. Elevated temperatures could also cause a breakdown of the phenol-protein and phenol-polysaccharide interactions in the garlic tissues, thus releasing more phenolic compounds.
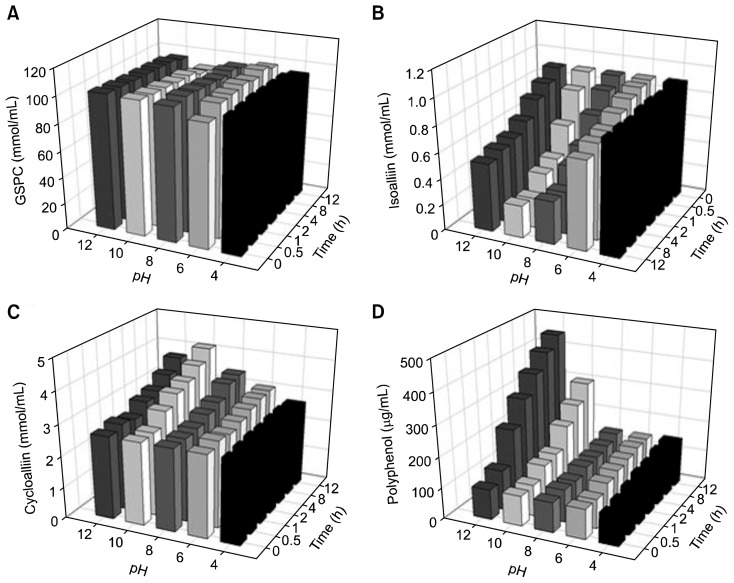
Effect of extraction time
The effect of incubation time on the extraction of organosulfur compounds was also examined (Fig. 6). Homogenized samples were incubated for various periods of time at different pH levels, and the contents analysed in order to determine the levels of organosulfur compounds present. The GSPC content did not show any significant change during the incubations, except for a slight decrease after 8 and 12 h (Fig. 6A). The cycloalliin level was increased with increased incubation time (Fig. 6C), a trend that was most obvious at pH 10 (Fig. 6C). In contrast, the isoalliin content showed a marked decrease when the incubation time was extended, particularly at pH 8 and 10 (Fig. 6B). The polyphenol content increased with longer incubation times, with an increase in the polyphenol levels also observed under alkaline conditions (Fig. 6D). These results indicate that increasing the extraction time promoted the conversion of isoalliin to cycloalliin, with a concomitant increase in the level of polyphenols present. Anno et al. (22) reported that the cycloalliin content increased more than 2-fold when the extraction time was increased from 1 min to 30 min in onion juice. Likewise, Kim et al. (31) reported that thiosulfinate content slightly increased (from 177.9 μmol/g of garlic to 195.8 μmol/g) when the ethanol extraction time was increased from 1 to 3 h in garlic oleoresin. Our data also showed that an incubation period of 12 h led to the maximum cycloalliin and the minimum isoalliin yields, meaning that the conversion between both compounds reached a peak. Additionally, our results showed that the amount of cycloalliin was further enhanced when the pH was optimized at 10, thus demonstrating that combining the optimized conditions maximized the conversion of isoalliin to cycloalliin.
Fig. 6.
Effect of pH and time on the organosulfur compounds and polyphenol content of garlic extracts. Each bar represents mean± SD (n=3). (A) γ-glutamyl peptide (GSPC), (B) isoalliin, (C) cycloalliin, and (D) polyphenol.
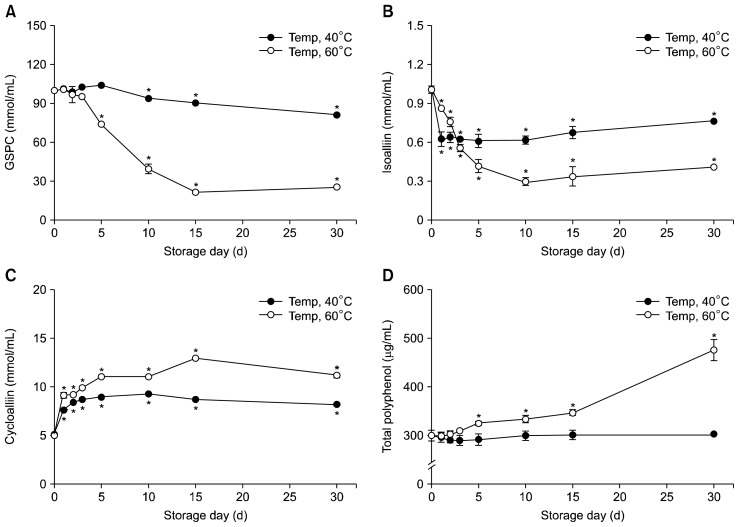
Changes in the amounts of organosulfur compounds present during storage
We observed changes in the levels of organosulfur compounds present in garlic extracts after prolonged storage at different temperatures. Levels of organosulfur compounds were monitored during storage at 40°C or 60°C for 30 days (Fig. 7). The GSPC content did not change until day 5, after which time the level of GSPC stored at 60°C dramatically decreased (eventually going from 100 mmol/mL to less than 30 mmol/mL by day 30), and the level of GSPC in the solution stored at 40°C slightly decreased (from 100 mmol/mL to 80 mmol/mL). The isoalliin level was also reduced until day 10, after which the level reached a plateau (Fig. 7B). In the solution stored at 60°C, the isoalliin level was reduced by ~70% compared to the initial levels, while the level was stabilized at 0.6 mmol/mL after day 1; this indicated a significant decrease in isoalliin compared to that on day 0 (Fig. 7B). The level of cycloalliin increased until day 5 in solutions stored at both temperatures. However, the cycloalliin level stored at 40°C was maintained at less than 9 mmol/mL, while the cycloalliin level in the solution stored at 60°C showed an increase until day 15, eventually stabilizing at higher than 11 mmol/mL (Fig. 7C). The polyphenol levels in the solution stored at 60°C also showed a significant increase during prolonged storage, with a great increase after day 15, thus, showing an increase by 1.5-fold at day 30 compared to the initial levels (Fig. 7D). However, the polyphenol level in the 40°C solution was not significantly changed during storage (Fig. 7D). These results demonstrate that the storage of garlic extract at 60°C effectively promotes the conversion of isoalliin to cycloalliin, with a concomitant increase in the level of polyphenols present. Based on the above data (Fig. 5), a storage temperature of 80°C was expected to show an increased conversion of isoalliin to cycloalliin, given that a higher cycloalliin level was observed at 80°C than at 60°C. However, the levels of isoalliin and polyphenols were virtually identical at both 80°C and 60°C (Fig. 5C). The analysis at 80°C was not recognized to be valuable due to the small difference of cycloalliin content between 80°C and 60°C. In addition to this consideration, the relative economy of storing extracts at 60°C is also an important factor.
Fig. 7.
Changes in the organosulfur compounds found in garlic extracts during storage at 40°C and at 60°C for 30 days. Each bar represents mean±SD (n=3). (A) γ-glutamyl peptide (GSPC), (B) isoalliin, (C) cycloalliin, and (D) polyphenol of garlic extract. *Significant differences as compared with day 0 by Student’s t-test (P<0.05).
Because our results indicate that the maximum level of cycloalliin and corresponding minimum level of isoalliin occurred on day 15, storage for 15 d at 60°C is thought to be optimal for the desirable conversion of organosulfur compounds.
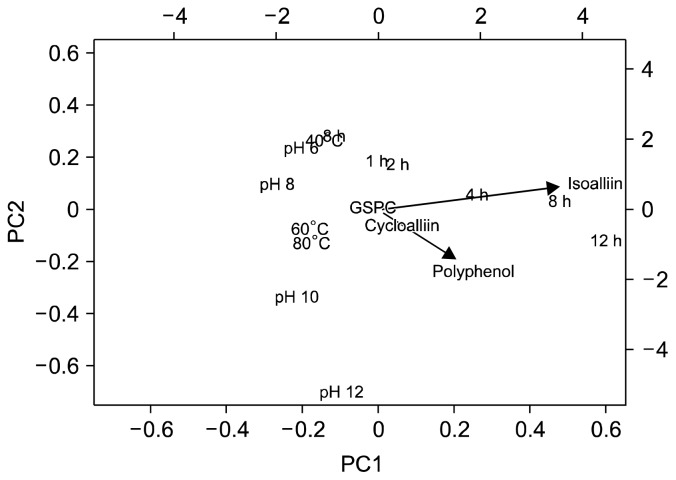
PCA analysis
Principal component analysis (PCA) is a non-parametric method to induce relevant information from complex data (32). It is useful in identifying meaningful variables from complex data. We adopted the PCA analysis to determine which extraction variables affected the levels of garlic components more importantly. Our PCA analysis data showed that the level of isoalliin in the extract was significantly affected by the extraction time rather than the other variables on principal component 1 (PC1), while GSPC levels did not undergo crucial changes by the combination of PC1 and PC2. In particular, cycloalliin levels were easily separated from isoalliin by PC1 and PC2 combinational analysis (Fig. 8), which presents that cycloalliin levels were influenced not only by extraction time but also by pH and temperatures. The condition for the polyphenol extraction also followed the same pattern as cycloalliin (Fig. 8). This data indicated that various extraction variables should be considered to optimize the levels of cycloalliin from garlic. In addition, since isoalliin levels were inversely correlated to cycloalliin production, enough time for extraction and storage should be considered more importantly.
Fig. 8.
Score plots of principal component analysis (PCA) of various garlic extracts by the combination of principal component (PC) 1 and PC2. PCA was performed on a set of data derived from extraction and storage experiments by transforming the original variables into a smaller set of linear combinations (PCs).
CONCLUSION
We have investigated various extraction conditions for the production of organosulfur compounds, particularly cycloalliin, from garlic. The extraction of garlic at pH 10.0, after heating at 80°C for 12 h, was found to provide the highest amounts of cycloalliin and polyphenols. The storage of garlic extract at 60°C significantly increased the amounts of cycloalliin and polyphenol content by factors of 1.5 and 2.6, respectively, compared to storage at 40°C. Overall, our results indicate that isoalliin produced enzymatically from GSPC is chemically converted to cycloalliin by heat and alkaline treatment, and that cycloalliin content is increased during storage at higher temperatures. This information could be useful as a chemical marker for the non-pungent preparation of garlic containing a high level of cycloalliin, and in the quality control of garlic extract.
ACKNOWLEDGEMENTS
This work was supported by a research grant from Seoul Women’s University (2015).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Block E, Dane AJ, Thomas S, Cody RB. Applications of direct analysis in real time mass spectrometry (DART-MS) in Allium chemistry. 2-Propenesulfenic and 2-propenesulfinic acids, diallyl trisulfane S-oxide, and other reactive sulfur compounds from crushed garlic and other Alliums. J Agric Food Chem. 2010;58:4617–4625. doi: 10.1021/jf1000106. [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa M, Ide N, Ono K. Changes in organosulfur compounds in garlic cloves during storage. J Agric Food Chem. 2006;54:4849–4854. doi: 10.1021/jf060083o. [DOI] [PubMed] [Google Scholar]
- 3.Lancaster JE, Shaw ML. γ-Glutamyl peptides in the bio-synthesis of S-alk(en)yl-L-cysteine sulphoxides (flavour precursors) in Allium. Phytochemistry. 1989;28:455–460. doi: 10.1016/0031-9422(89)80031-7. [DOI] [Google Scholar]
- 4.Lawson LD, Gardner CD. Composition, stability, and bioavailability of garlic products used in a clinical trial. J Agric Food Chem. 2005;53:6254–6261. doi: 10.1021/jf050536+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 6.Ide N, Ichikawa M, Ryu K, Yoshida J, Sasaoka T, Sumi SI, Sumiyoshi H. Antioxidants in processed garlic: tetrahydro-β-carboline derivatives in aged garlic extract. In: Shahidi F, Ho CT, Watanabe S, Osawa T, editors. Food Factors in Health Promotion and Disease Prevention. Vol. 851. ACS Publications; Washington, DC, USA: 2003. pp. 250–263. [DOI] [Google Scholar]
- 7.Ueda Y, Tsubuku T, Miyajima R. Composition of sulfur-containing components in onion and their flavor characters. Biosci Biotechnol Biochem. 1994;58:108–110. doi: 10.1271/bbb.58.108. [DOI] [PubMed] [Google Scholar]
- 8.Nagao T, Yamauchi-Sato Y, Sugihara A, Iwata T, Nagao K, Yanagita T, Adachi S, Shimada Y. Purification of conjugated linoleic acid isomers through a process including lipase-catalyzed selective esterification. Biosci Biotechnol Biochem. 2003;67:1429–1433. doi: 10.1271/bbb.67.1429. [DOI] [PubMed] [Google Scholar]
- 9.Kendler BS. Garlic (Allium sativum) and onion (Allium cepa): a review of their relationship to cardiovascular disease. Prev Med. 1987;16:670–685. doi: 10.1016/0091-7435(87)90050-8. [DOI] [PubMed] [Google Scholar]
- 10.Rahman K. Garlic and aging: new insights into an old remedy. Ageing Res Rev. 2003;2:39–56. doi: 10.1016/S1568-1637(02)00049-1. [DOI] [PubMed] [Google Scholar]
- 11.Xiao H, Parkin KL. Antioxidant functions of selected Allium thiosulfinates and S-alk(en)yl-L-cysteine sulfoxides. J Agric Food Chem. 2002;50:2488–2493. doi: 10.1021/jf011137r. [DOI] [PubMed] [Google Scholar]
- 12.Bianchet MA, Erdemli SB, Amzel LM. Structure, function, and mechanism of cytosolic quinone reductases. Vitam Horm. 2008;78:63–84. doi: 10.1016/S0083-6729(07)00004-0. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal RK, Dewar HA, Newell DJ, Das B. Controlled trial of the effect of cycloalliin on the fibrinolytic activity of venous blood. Atherosclerosis. 1977;27:347–351. doi: 10.1016/0021-9150(77)90044-2. [DOI] [PubMed] [Google Scholar]
- 14.Allison GL, Lowe GM, Rahman K. Aged garlic extract and its constituents inhibit platelet aggregation through multiple mechanisms. J Nutr. 2006;136:782S–788S. doi: 10.1093/jn/136.3.782S. [DOI] [PubMed] [Google Scholar]
- 15.Harenberg J, Giese C, Zimmermann R. Effect of dried garlic on blood coagulation, fibrinolysis, platelet aggregation and serum cholesterol levels in patients with hyperlipoproteinemia. Atherosclerosis. 1988;74:247–249. doi: 10.1016/0021-9150(88)90244-4. [DOI] [PubMed] [Google Scholar]
- 16.Lawson LD, Wang ZJ, Hughes BG. γ-Glutamyl-S-alkylcysteines in garlic and other Allium spp.: precursors of age-dependent trans-1-propenyl thiosulfinates. J Nat Prod. 1991;54:436–444. doi: 10.1021/np50074a014. [DOI] [Google Scholar]
- 17.Shen C, Parkin KL. In vitro biogeneration of pure thiosulfinates and propanethial-S-oxide. J Agric Food Chem. 2000;48:6254–6260. doi: 10.1021/jf000711g. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa M, Ide N, Yoshida J, Yamaguchi H, Ono K. Determination of seven organosulfur compounds in garlic by high-performance liquid chromatography. J Agric Food Chem. 2006;54:1535–1540. doi: 10.1021/jf051742k. [DOI] [PubMed] [Google Scholar]
- 19.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 20.Stoll A, Seebeck E. Chemical investigations on alliin, the specific principle of garlic. In: Nord FF, editor. Advances in Enzymology and Related Areas of Molecular Biology. Vol. 11. Interscience Publishers, Inc.; New York, NY, USA: 2006. pp. 377–400. [DOI] [PubMed] [Google Scholar]
- 21.Ueda Y, Sakaguchi M, Hirayama K, Miyajima R, Kimizuka A. Characteristic flavor constituents in water extract of garlic. Agric Biol Chem. 1990;54:163–169. [Google Scholar]
- 22.Anno T, Fujino M, Sawada H. Onion extract rich in sulfurized cyclic amino acid and process for producing the same. US Patent. 2000;6:468, 565. [Google Scholar]
- 23.Mochizuki E, Nakayama A, Kitada Y, Saito K, Nakazawa H, Suzuki S, Fujita M. Liquid chromatographic determination of alliin in garlic and garlic products. J Chromatogr. 1988;455:271–277. doi: 10.1016/S0021-9673(01)82125-7. [DOI] [PubMed] [Google Scholar]
- 24.Rejano L, Sanchez AH, de Castro A, Montano A. Chemical characteristics and storage stability of pickled garlic prepared using different processes. J Food Sci. 1997;62:1120–1123. doi: 10.1111/j.1365-2621.1997.tb12226.x. [DOI] [Google Scholar]
- 25.Wissam Z, Ghada B, Wassim AW, Warid K. Effective extraction of polyphenols and proanthocyanidins from pomegranate’s peel. Int J Pharm Pharm Sci. 2010;4:675–682. [Google Scholar]
- 26.Cheng A, Chen X, Wang W, Gong Z, Liu L. Contents of extractable and non-extractable polyphenols in the leaves of blueberry. Czech J Food Sci. 2013;31:275–282. [Google Scholar]
- 27.Sondheimer E. Chlorogenic acids and related depsides. Bot Rev. 1964;30:667–712. doi: 10.1007/BF02858654. [DOI] [Google Scholar]
- 28.Lee YR, Lee YK, Hwang IG, Woo KS, Han CS, Jeong HS. Evaluation of heat processing temperature and time on functional properties of garlic juice. J Food Sci Nutr. 2008;13:327–333. doi: 10.3746/jfn.2008.13.4.327. [DOI] [Google Scholar]
- 29.Lim HK, Yoo ES, Moon JY, Jeon YJ, Cho SMK. Antioxidant activity of extracts from Dangyuja (Citrus grandis Osbeck) fruits produced in Jeju island. Food Sci Biotechnol. 2006;15:312–316. [Google Scholar]
- 30.Stewart AJ, Bozonnet S, Mullen W, Jenkins GI, Lean ME, Crozier A. Occurrence of flavonols in tomatoes and tomato-based products. J Agric Food Chem. 2000;48:2663–2669. doi: 10.1021/jf000070p. [DOI] [PubMed] [Google Scholar]
- 31.Kim YP, Lee GW, Oh HI. Optimization of extraction conditions for garlic oleoresin and changes in the quality characteristics of oleoresin during storage. Korean J Food Nutr. 2006;19:219–226. [Google Scholar]
- 32.Fischer SL, Hampton RH, Albert WJ. A simple approach to guide factor retention decisions when applying principal component analysis to biomechanical data. Comput Methods Biomech Biomed Engin. 2014;17:199–203. doi: 10.1080/10255842.2012.673594. [DOI] [PubMed] [Google Scholar]