Abstract
Our studies and others recently demonstrate that polydatin, a resveratrol glucoside, has antioxidative and cardioprotective effects. This study aims to investigate the direct effects of polydatin on Ang II-induced cardiac hypertrophy to explore the potential role of polydatin in cardioprotection. Our results showed that in primary cultured cardiomyocytes, polydatin blocked Ang II-induced cardiac hypertrophy in a dose-dependent manner, which were associated with reduction in the cell surface area and [3H]leucine incorporation, as well as attenuation of the mRNA expressions of atrial natriuretic factor and β-myosin heavy chain. Furthermore, polydatin prevented rat cardiac hypertrophy induced by Ang II infusion, as assessed by heart weight-to-body weight ratio, cross-sectional area of cardiomyocyte, and gene expression of hypertrophic markers. Further investigation demonstrated that polydatin attenuated the Ang II-induced increase in the reactive oxygen species levels and NADPH oxidase activity in vivo and in vitro. Polydatin also blocked the Ang II-stimulated increases of Nox4 and Nox2 expression in cultured cardiomyocytes and the hearts of Ang II-infused rats. Our results indicate that polydatin has the potential to protect against Ang II-mediated cardiac hypertrophy through suppression of NADPH oxidase activity and superoxide production. These observations may shed new light on the understanding of the cardioprotective effect of polydatin.
Keywords: Polydatin, cardiac hypertrophy, angiotensin II, reactive oxygen species, NADPH oxidase
Introduction
Cardiac hypertrophy is an adaptive remodeling response to increased cardiac wall stress caused by pressure and/or volume overload as well as neurohormonal activation. It is recognized as a risk factor for the development of heart failure and a very strong predictor of cardiovascular mortality.1,2 Angiotensin II (Ang II) plays an important role in the pathogenesis and progression of cardiac hypertrophy and heart failure.3,4 Numerous studies have shown that continuous infusion of Ang II contributes to alterations in cardiac functions and increases in cardiac mass, which is independent of its pressor action.5,6 Additionally, inhibition of Ang II by angiotensin converting enzyme inhibitors or angiotensin type 1 receptor antagonists prevents or reverses cardiac hypertrophy after pressure overload.6,7 Molecular mechanisms by which Ang II evokes cardiac hypertrophy are related to activation of protein kinase cascades, initiation of a fetal-like gene program, impairment of calcium handling, and increased production of reactive oxygen species (ROS).8 ROS have emerged as important triggers of the hypertrophic responses, both in vitro and in vivo, in response to stretch or other hypertrophy stimuli, such as Ang II.9–11 Several studies have shown that Ang II treatment increases ROS generation in cardiomyocytes and that inhibition of ROS generation by antioxidants abolishes Ang II-induced cardiomyocytes enlargement.12,13 Therefore, developing new compounds and strategies to manipulate Ang II-mediated effects and reduce myocardial superoxide are highly valued for treatment of cardiac remolding.
Polydatin, a resveratrol glucoside with a 3,4',5-trihydroxystilbene-3 -β-D-glucopyranoside molecular structure, is a natural component extracted from the perennial herb Polygonum cuspidatum Sieb. et Zucc. This plant has been used clinically in the treatment of cardiovascular disorders and ischemia/reperfusion injury in traditional Chinese medicine.14,15 Polydatin is one of the major stilbenoid glucosides in grape juice and red wine.16 Similar to its analog resveratrol, polydatin has multiple pharmacological effects, including anti-inflammatory, neuroprotective, lipid-lowering actions, and especially strong antioxidative effects.17–19 Recently, it was suggested that polydatin has strong protective effects against cardiac ischemia–reperfusion injury and pressure-overload-induced ventricular remodeling.20–23 There are also studies showing that polydatin ameliorates burn-generated cardiac dysfunction by reducing oxidative modification of ryanodine receptors.24 Despite these cardioprotective and antioxidant activities, the extent and mechanism to which polydatin improves cardiac remodeling remains poorly understood. Therefore, the present study was performed to determine whether polydatin attenuates Ang II-induced cardiac hypertrophy in vitro and in vivo by regulating NADPH oxidase activity and ROS production.
Materials and methods
Cell culture
All animal procedures were approved by the Animal Research Committee at Shaanxi University of Chinese Medicine, and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Primary cardiomyocyte cultures were prepared from 1-day-old Sprague–Dawley rats as described previously.25 Briefly, the ventricles of neonatal rats were enzymatically dissociated and the resulting cell suspension was enriched. Cells were suspended in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin. After pre-plating twice (45 min at 37℃) to minimize fibroblast, the cells were plated at 2.0–3.0 × 104 cells mL−1 onto poly-D-lysine-coated coverslips (Sigma, St Louis, MO), well plates, or dishes. The culture medium was replaced after 48 h with serum-free DMEM consisting of bromodeoxyuridine (0.1 mmol/L), transferrin (5 µg/mL), and insulin (1 µg/mL), and cells were then incubated at 37℃ for 48 h prior to treatment.
Following removal of cardiomyocyte-enriched culture medium at the end of the two pre-plating steps, the remaining attached cells (cardiac fibroblasts, assessed by microscopic evaluation) were cultured in DMEM supplemented with 10% FBS and grown to confluence. Cardiac fibroblasts were then plated to confluence in 96-well plates for determination of cardiac fibroblast superoxide generation. Cell cultures were incubated at 37℃ in a humidified atmosphere of 5% CO2 and 95% air. Cardiac fibroblasts up to passage level 3–4 were used for experiments with 24 h serum starvation prior to study.
Polydatin (3,4',5-trihydroxystilbene-3 -β-D-glucopyranoside; purity, >97%; wt. 390.38; PubChem ID 24880655) was obtained from Sigma-Aldrich. For the in vitro study, polydatin was dissolved in dimethyl sulfoxide (DMSO) medium. Cardiomyocytes were pretreated with DMSO, polydatin (5–50 µmol/L) for 60 min, and subsequently treated with or without 100 nmol/L Ang II for 24 h. Each treatment was performed in triplicate wells. DMSO alone, without polydatin, served as a control.
[3H]leucine incorporation
[3H]Leucine incorporation was measured as described previously.26 Briefly, cardiomyocytes were pretreated with polydatin for 60 min and subsequently stimulated with Ang II (100 nmol/L) and coincubated with [3H]leucine (2 μCi/mL) for 24 h. At the end of the experiment, the cells were washed twice with ice-cold phosphate-buffered saline (PBS), scraped off the dish, and then treated with trichloroacetic acid (10%) for 1 h at 4℃. The precipitates were washed twice with ice-cold water and dissolved in 1 mL NaOH (100 mmol/L), and subsequently counted with a scintillation counter.
Immunocytochemistry and measurement of cell surface area
Cultured myocytes on coverslips were fixed with 3.7% formaldehyde and permeabilized with 0.3% Triton X-100 in PBS. After blocking with PBS containing 0.3% bovine serum albumin (BSA) and 1% horse serum for 1 h, the cells were sequentially incubated overnight at 4℃ with monoclonal anti-α-sarcomeric actin antibody (Sigma, 1:1000) and Alexa fluorescent dye-conjugated specific-secondary antibodies (Invitrogen, 1:2000) for 1 h. After washing three times with PBS, the fluorescence staining was visualized using an Olympus fluorescence microscope as in our previous publication.25 The surface area of positive-staining cardiomyocytes was analyzed using image analysis software (Image J). At least 50 cells were randomly selected and analyzed for surface area on each slip.
Real-time PCR
Atrial natriuretic factor (ANF) and β-myosin heavy chain (β-MHC) mRNA were analyzed using quantitative real-time polymerase chain reaction (PCR) as detailed by us previously.27 The total RNA was isolated from cultured cardiomyocytes or left ventricular lysates using an RNeasy kits (Qiagen, Valencia, CA) according to the manufacturer's instructions. TaqMan PCR probes for rat ANF, β-MHC, and internal standard gene GAPDH were obtained from Applied Biosystems, Inc. (Foster City, CA). Amplification was performed in an Applied Biosystems PRISM 7000 sequence detection system. A comparative cycle of threshold (CT) fluorescence method was applied with GAPDH as the internal control. The final data of real-time PCR were presented as the ratio of the mRNA of interest to GAPDH.
Determination of intracellular ROS production
The fluorescent indicator 2′,7′-dichlorofluorescein diacetate (DCF-DA; Sigma) was used to determine intracellular ROS in cardiomyocytes as described previously.25 Myocytes were plated into 96-well plates, grown to 50–60% confluence, and then exposed to the appropriate treatment. At the end of treatment, myocytes were washed twice with PBS and incubated with DCF-DA at a concentration of 5 µmol/L for 30 min at 37℃ in dark. The DCF fluorescence was detected at 485 nm for excitation and 530 nm for emission using a fluorescent plate reader (SpectraMAX GeminiXPS, Molecular Devices). Temperature was kept at 37℃ throughout the experiment. The change in fluorescence intensity was expressed as a percentage of respective control value. Each treatment condition was performed in triplicate within experiments and three separate culture dishes were used in each set of experiments.
Measurement of NADPH oxidase activity
The NADPH oxidase activity was measured using lucigenin chemiluminescence’s assay as previously described.28 Cultured myocytes or left ventricular lysates were prepared as described above and homogenized in lysis buffer (1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L EDTA, 20 mmol/L KH2PO4, pH 7.0). After centrifugation at 3000 g for 10 min, an aliquot of the supernatant was distributed (100 µg/well) onto a 96-well microplate and incubated with 5 µmol/L lucigenin in modified 4 -(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer for 2 min followed by adding 100 µmol/L NADPH as substrate. The chemiluminescent signal was measured every 15 s for 3 min using a Wallac 1450 Micro-Beta JET Luminometer (PerkinElmer Life and Analytical Sciences, Waltham, MA). The NADPH oxidase activity was determined by the amount of superoxide anion generated in the reaction mixture. Protein concentrations were measured by a Bio-Rad protein assay kit. Data are expressed as µmol/mg of protein per minute.
Animal experiments
All animal experimental protocols were approved by the Shaanxi University of Chinese Medicine Institutional Animal Care and Use Committee. Male Sprague–Dawley rats (within 180–220 g) were kept in a 12-h light/12-h dark cycle at a controlled room temperature and had free access to standard chow and tap water. Rats were divided into four experimental groups: one group received sham surgery, the second group with Ang II infusion (300 ng/kg/min for 28 days), the third group with Ang II and polydatin (oral gavage, 50 mg/kg/d, 7 days before surgery), and the fourth group with only polydatin treatment. For Ang II infusion, rats were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally), and osmotic pumps (model 2004; Alzet, Cupertino, CA) were implanted subcutaneously in the flank region. Ang II was delivered by the pumps at a rate of 300 ng/kg/min for 28 days. Polydatin was dissolved in 0.5% carboxy methylcellulose solution for animal experiments and administered by oral gavage at a volume of 1 mL/100 g body weight. The control animals were given the same volume of vehicle solution (0.5% carboxy methylcellulose). Tail-cuff plethysmography (Harvard Apparatus Ltd) was used to measure the systolic blood pressure (BP) before and every other day during the treatment. At the end of the experiment, the rats were euthanized, and hearts were removed, blotted, and weighed to determine the ratio of heart weight to body weight (HW/BW).
Histological analysis
Hearts were fixed in 10% formalin and embedded in paraffin. The middle segments of the hearts were cut into five sub-serial sections with a thickness of 10 µm at intervals of 0.3 mm. The transverse sections were stained with hematoxylin and eosin to determine myocyte cross-sectional area, and with picrosirius red staining for assessment of interstitial and perivascular fibrosis as previously described.27 Micrographs were detected using a microscope equipped with digital camera (Infinity 2). The histology and morphology were examined and analyzed by Image Analysis Software (Image Pro plus 6.0, Media Cybernetics, Bethesda, MD).
Western blot analysis
Left ventricular homogenates were prepared in radioimmunoprecipitation assay (RIPA) lysis buffer as described previously.27 Total proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred electrophoretically to nitrocellulose membranes (Millipore, Billerica, MA). After blocking with 5% skim milk, the membranes then were hybridized at 4℃ overnight with the primary antibody, polyclonal rabbit anti-Nox2 (1:500; Santa Cruz Biotechnology, CA), polyclonal rabbit anti-Nox4 (1:500; Santa Cruz Biotechnology), polyclonal rabbit anti-Nox1 (1:500; Santa Cruz Biotechnology), and polyclonal goat anti-GAPDH (1:500; Santa Cruz Biotechnology). The membranes were washed with PBS-Tween and further incubated with secondary antibody, horseradish peroxidase-conjugated IgG (Santa Cruz Biotechnology), at 37℃ for 60 min. Immunoreactivity was detected using chemiluminescence reagent kit (Millipore). The blots in the film were visualized and analyzed using Quantity One Software (Bio-Rad).
Plasma transforming growth factor-β (TGF-β) analysis
Plasma was collected from the abdominal aorta at the end of in vivo experiment, and then used to measure TGF-β level. Total plasma TGF-β was determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN; catalog No. MB100B). To be able to measure total TGF-β1, samples were first acid-activated (with 1 N HCl) before assaying to isolate free TGF-β1 molecules from latent complex. The ELISA assay was performed according to the manufacturer’s protocol. All samples were run in duplicate. The lowest level of detection for total TGF-β1 was 4.3 ± 2.6 pg/mL.
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). Statistical analysis was tested by one-way analysis of variance (ANOVA) followed by Dunnett post hoc test, or Student’s t-test. P < 0.05 was viewed as statistically significant.
Results
Pretreatment with polydatin inhibits Ang II-induced cardiac hypertrophy in vitro
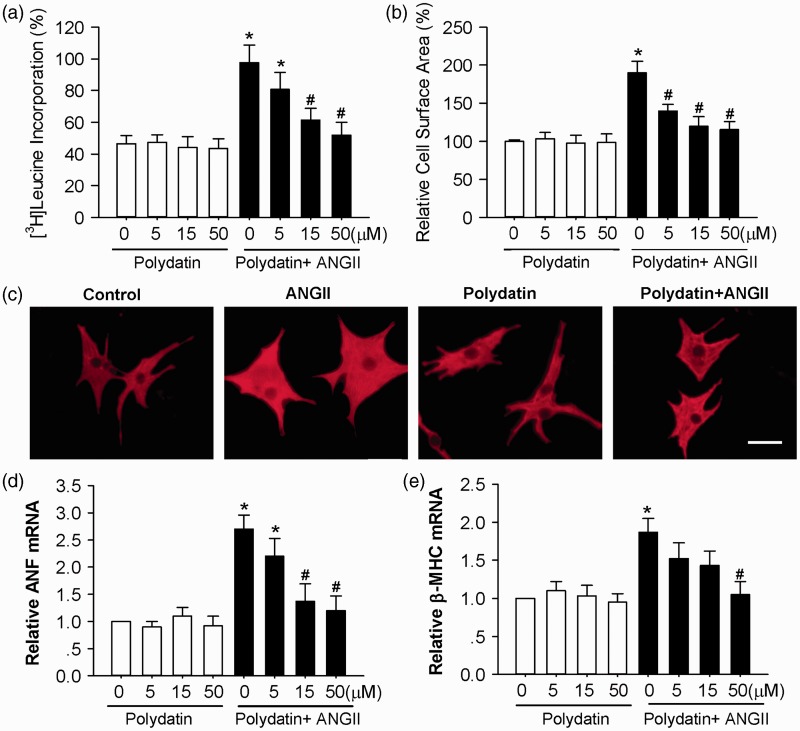
Cardiac hypertrophy can be monitored by enlargement of myocyte cross-sectional area, increased protein synthesis, and induction of fetal gene expression. To investigate the direct effect of polydatin on Ang II-induced cardiomyocyte hypertrophy, cultured neonatal rat myocytes were pretreated with polydatin (5–50 µmol/L) for 60 min and subsequently exposed to 100 nmol/L Ang II for 24 h. As shown in Figure 1a, pretreatment with polydatin decreased the Ang II-induced increases in [3H]leucine incorporation in a dose-dependent manner, with the maximal effects at 50 µmol/L. In addition, the increase in cardiomyocyte size in the presence of Ang II for 24 h was also significantly reduced by polydatin (Figure 1b and c). Polydatin markedly attenuated the increase of ANF and β-MHC mRNA expression induced by Ang II (Figure 1d and e). However, polydatin alone had no effect on [3H]leucine incorporation, cardiomyocyte size, or expression of ANF and β-MHC.
Figure 1.
Pretreatment with polydatin inhibits Ang II-induced cardiac hypertrophy in rat cardiomyocytes. (a) Polydatin inhibited Ang II-induced [3H]leucine incorporation. (b) Quantification of cell cross-sectional area from experiments shown in C by measuring 50 random cells. (c) Representative fields of cardiomyocytes stained with α-actin after the following treatments: control (DMSO, 24 h), Ang II (100 nmol/L, 24 h), polydatin (50 µmol/L, 25 h), and Ang II (100 nmol/L, 24 h) plus polydatin (50 µmol/L, 25 h). The scale is 50 µm. (d) Polydatin blunted Ang II-induced ANF mRNA expression levels by real-time PCR. (e) Polydatin blunted Ang II-induced β-MHC mRNA expression levels by real-time PCR. Data are means ± SE and derived from three experiments and at least triplicate wells in each experiment. *P < 0.05 versus control. #P < 0.05 versus the treatment with Ang II alone. (A color version of this figure is available in the online journal.)
Pretreatment with polydatin suppresses Ang II-induced ROS generation
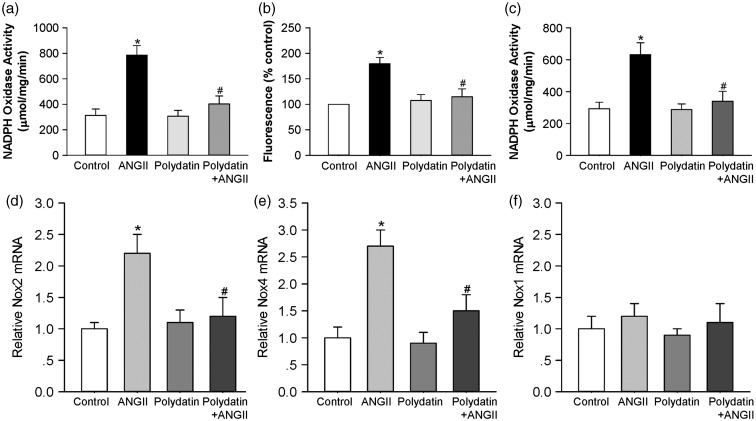
To explore the mechanism for the inhibitory effect of polydatin on cardiomyocyte hypertrophy, we analyzed the effect of polydatin on NADPH oxidase activation induced by Ang II. Neonatal rat cardiomyocytes were exposed to 100 nmol/L Ang II with or without polydatin. As shown in Figure 2a, NADPH-driven superoxide generation in the presence of Ang II was significantly increased to 252 ± 16% of control (n = 9, P < 0.05), which was inhibited by pretreatment with polydatin to only 129 ± 7% of control (n = 9, P < 0.05 versus Ang II alone). Polydatin alone had no effect on superoxide generation in control myocytes. The data obtained with lucigenin detection of superoxide generation were confirmed using DCF-DA fluorescence, to examine a range of ROS in cardiomyocytes. As expected, polydatin blocked the increase in Ang II-induced cardiomyocyte ROS generation by 72 ± 7% (P < 0.05 versus Ang II, Figure 2b).
Figure 2.
Pretreatment with polydatin suppresses Ang II-induced NADPH oxidase activity. Cultured cardiomyocytes or fibroblasts were treated with control (DMSO, 24 h), Ang II (100 nmol/L, 24 h), polydatin (50 µmol/L, 25 h), or Ang II (100 nmol/L, 24 h) plus polydatin (50 µmol/L, 25 h). (a) NADPH oxidase activity was measured in rat primary cardiomyocytes by lucigenin chemiluminescence’s assay. (b) Intracellular superoxide anion levels were detected in rat primary cardiomyocytes using fluorogenic probe DCFH-DA. (c) NADPH oxidase activity was measured in rat primary fibroblasts by lucigenin chemiluminescence’s assay. (d–f) Summary of real-time PCR data showing levels of Nox1, Nox2, and Nox4 mRNA in rat primary cardiomyocytes. Results were expressed as means ± SE (n = 6–8). *P < 0.05 versus control values. #P < 0.05 versus Ang II alone
In neonatal rat cardiac fibroblasts, Ang II (100 nmol/L) also induced a significant increase in superoxide generation to 213 ± 18% of control at 24 h (n = 7, P < 0.05). Similar to the effect in cardiomyocytes, this was reduced by polydatin treatment in cultured fibroblasts, to 116 ± 9% of control (P < 0.05 versus Ang II, Figure 2c).
We further assessed the effect of polydatin on the mRNA levels of NADPH oxidase subunits. As shown in Figure 2d–f, Ang II (100 nmol/L) increased Nox4 and Nox2 mRNA expression to 2.7 ± 0.4 and 2.2 ± 0.3-fold of control cardiomyocytes after 24 h of incubation (P < 0.05, n = 8). Polydatin (50 µmol/L) completely prevented this Ang II-stimulated increases of Nox4 mRNA (1.5 ± 0.3-fold) and Nox2 mRNA (1.2 ± 0.3-fold) expression. Polydatin alone had no effect on Nox4 and Nox2 expression in neonatal cardiomyocytes.
Pretreatment with polydatin inhibits Ang II-induced cardiac hypertrophy in vivo
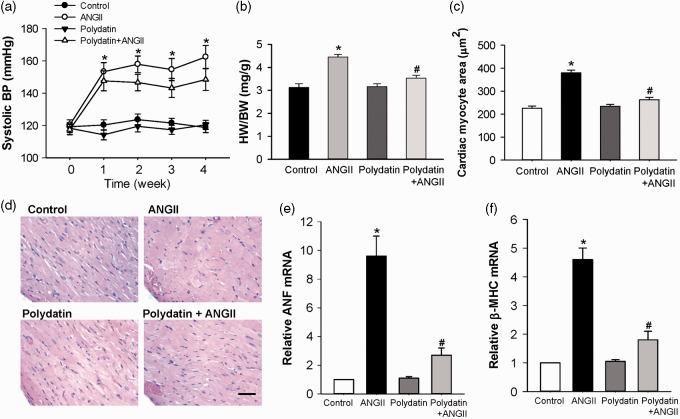
To examine the antihypertrophic effect of polydatin in vivo, the effect of oral administration of polydatin on cardiac hypertrophy induced by chronic Ang II infusion in rats was assessed. The systolic blood pressure (SBP) recorded in the rats received treatment with control, Ang II, polydatin, and Ang II + polydatin were presented in Figure 3a. Chronic subcutaneous infusion of Ang II (300 ng/kg/min) significantly increased SBP from 120.3 ± 3.2 mmHg to 162.4 ± 7.1 mmHg (n = 6, P < 0.01) in Ang II group. The time-dependent pressor effect of Ang II reached its peak in seven days and lasted at least four weeks (Figure 3a). However, daily oral administration of polydatin (50 mg/kg/d) for five weeks did not significantly alter this pressor effect of Ang II. Neither Ang II subcutaneous infusion nor polydatin oral administration altered the heart rate (HR) compared with control group (data not shown).
Figure 3.
Pretreatment with polydatin attenuates Ang II-induced cardiac hypertrophy in Sprague–Dawley rats. Sprague–Dawley rats (180–220 g, male) received polydatin (50 mg/kg/d) by oral gavage for 32 days. Seven days after beginning polydatin administration, a minipump was implanted in the dorsal region to deliver Ang II (300 ng/kg/min) for 28 days. (a) Time course of changes in systolic blood pressure in rats treated with control vehicle, Ang II infusion, polydatin, or Ang II infusion plus polydatin. (b) Heart weight-to-body weight ratio (HW/BW) in rats treated with control vehicle, Ang II infusion, polydatin, or Ang II infusion plus polydatin. (c) Bar graph shows quantitative analysis of cardiomyocyte cross-sectional areas. (d) Representative cross-sections of rat left ventricles histochemically stained with H&E staining in each group of rats. The scale is 50 µm. (e and f) Real-time PCR analysis of ANF and β-MHC mRNA level in each group of rats. Results were expressed as means ± SE (n = 6). *P < 0.05 versus control values. #P < 0.05 versus AngII alone. (A color version of this figure is available in the online journal.)
HW/BW and the cross-sectional area of cardiomyocytes were also examined in all groups of rats. The results demonstrated that subcutaneous Ang II infusion (300 ng/kg/min, 4 weeks) significantly increased HW/BW, and cardiomyocyte size as compared with control rats (Figure 3b, c, d). Oral administration of polydatin (50 mg/kg/d, 5 weeks) significantly attenuated Ang II-induced increases in HW/BW by 69.7 ± 2.6% (Figure 3b), and the size of cardiomyocytes by 75.7 ± 3.1% (Figure 3c). Additionally, polydatin markedly reduced the observed increase in hypertrophic marker (ANF and β-MHC mRNA) expression caused by Ang II infusion (Figure 3e and f).
Pretreatment with polydatin blunts cardiac fibrosis
We also observed the effects of polydatin on the perivascular and interstitial fibrosis in the heart of rats that received subcutaneous infusion of Ang II. Compared to control rats, perivascular fibrosis (24.3 ± 2.2% in control and 37.6 ± 3.1% in Ang II treatment rats, respectively, n = 5, P < 0.05) and interstitial fibrosis (6.3 ± 0.7% in control and 12.2 ± 1.5% in Ang II treatment rats, respectively, n = 5, P < 0.05) were significantly increased in the heart of Ang II-infused rats. Oral administration of polydatin (50 mg/kg/d, 5 weeks) significantly attenuated the increases in perivascular fibrosis by 82.6% and interstitial fibrosis by 84.7% caused by Ang II in heart (Figure 4). Oral administration of polydatin alone did not show any effect on either interstitial fibrosis or perivascular fibrosis in control (non-Ang-II infusion) rats.
Figure 4.
Pretreatment with polydatin inhibits left ventricles fibrosis induced by Ang II in rats. Left ventricles fibrosis was measured in the rats treated with control vehicle, Ang II infusion, polydatin, or Ang II infusion plus polydatin. (a) Representative images of intramuscular arteries with perivascular fibrosis stained with picric-sirius red. (b) Bar graph shows quantified perivascular fibrotic areas (%). (c) Representative images of myocardium with interstitial fibrosis stained with picric-sirius red. (d) Bar graph shows quantified interstitial fibrotic areas (%). The scale in the images is 50 µm. Results were expressed as means ± SE (n = 6). *P < 0.05 versus control values. #P < 0.05 versus Ang II alone. (A color version of this figure is available in the online journal.)
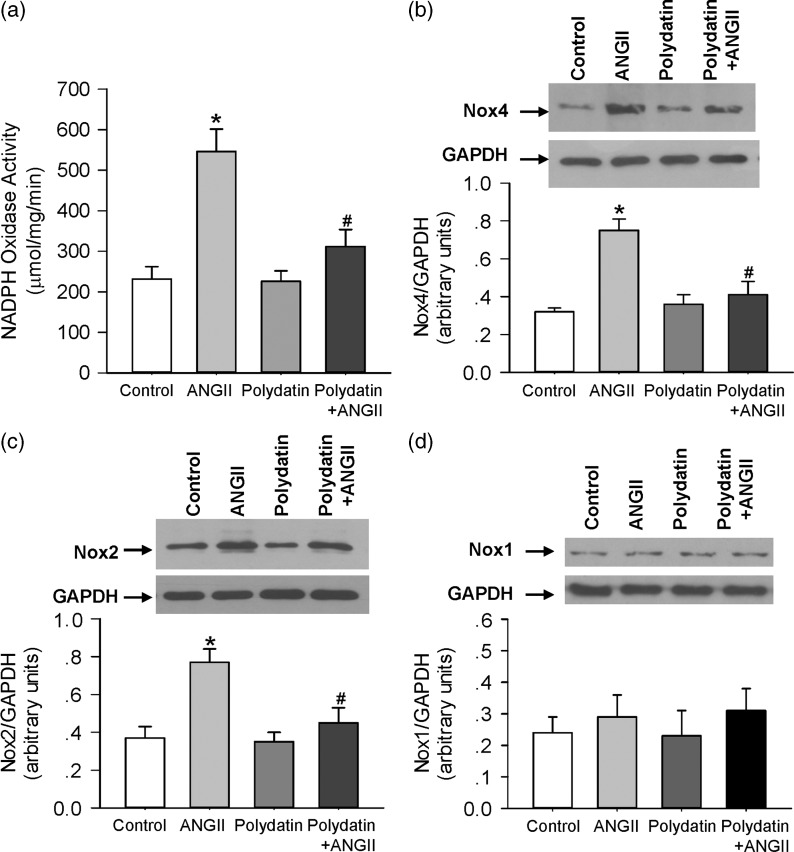
Pretreatment with polydatin prevented Ang II-induced NADPH oxidase activity in vivo
We determined the effect of polydatin on NADPH oxidase activity in the heart of rats which received subcutaneous infusion of Ang II. As shown in Figure 5a, the activity of NADPH oxidase was significantly elevated in heart of Ang II-infused rats, and oral administration of polydatin significantly reduced the increase in NADPH oxidase activity in heart of Ang II-infused rats. Further analysis revealed that the expression of NADPH oxidase subunits, Nox4 and Nox2, were significantly elevated in the same heart tissue (Figure 5b and c). Polydatin pretreatment effectively reduced the protein level of Nox4 and Nox2 in the heart of Ang II-infused rats. Neither Ang II infusion nor polydatin treatment altered the Nox1 level compared with control group (Figure 5d).
Figure 5.
Pretreatment with polydatin prevents Ang II-induced NADPH oxidase activity in rats. (a) NADPH oxidase activity was measured in heart of rats treated with control vehicle, Ang II infusion, polydatin, or Ang II infusion plus polydatin. (b–d) The protein expression of NOX2, NOX4, and NOX1 were detected by Western blotting. Results were expressed as means ± SE (n = 6). *P < 0.05 versus control values. #P < 0.05 versus Ang II alone
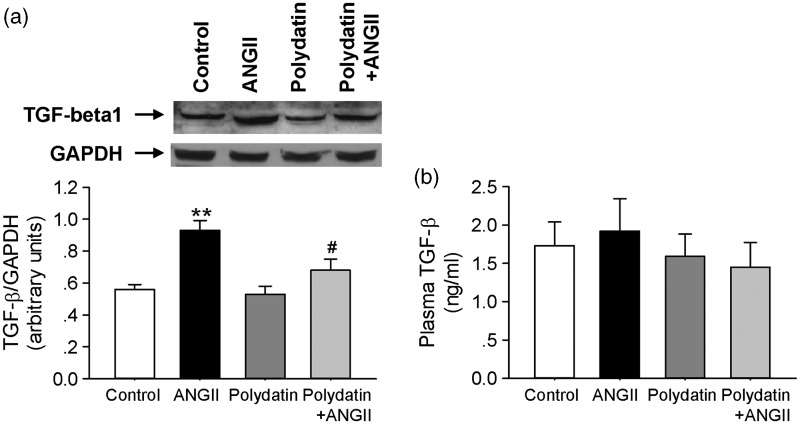
Polydatin attenuate Ang II-induced TGF-β1 expression in vivo
We determined the effect of polydatin on the TGF-β1 expression in the heart of rats that received chronic infusion of Ang II. As shown in Figure 6a, TGF-β1 level was significantly elevated in heart of Ang II-infused rats, and oral administration of polydatin reduced the increase in TGF-β1 expression in heart of Ang II-infused rats. In addition, circulating levels of the TGF-β in plasma were analyzed by ELISA. Chronic infusion of Ang II had no effect on plasma TGF-β levels. Pretreatment with polydatin in Ang II-infused rats also did not show significant change in plasma TGF-β level (P > 0.05, Figure 6b).
Figure 6.
Polydatin attenuates Ang II-induced TGF-β1 expression in vivo. (a) The protein expression of TGF-β1 was detected in heart of rats treated with control vehicle, Ang II infusion, polydatin, or Ang II infusion plus polydatin by Western blotting. (b) The plasma TGF-β level was measured in rats treated with control vehicle, Ang II infusion, polydatin, or Ang II infusion plus polydatin by ELISA. Results were expressed as means ± SE (n = 6). **P < 0.01 versus control values. #P < 0.05 versus Ang II alone
Discussion
The current study presents the first evidence that polydatin protects against Ang II-induced cardiac hypertrophy both in vitro and in vivo. Our data showed that pretreatment with polydatin significantly inhibited the increase of Ang II-induced cardiomyocyte size and hypertrophic gene expression, attenuated interstitial and perivascular fibrosis. Importantly, this study has identified that reduction of ROS generation and decreased activation of NADPH oxidase were associated with the antihypertrophic activity of polydatin.
It is well known that Ang II plays an important role in the pathogenesis and progression of cardiac remolding and heart failure.3 Evidence in cultured cardiomyocytes indicates that Ang II directly increases myocytes size and hypertrophic gene expression.29 Chronic Ang II infusion in rats also leads to alteration of cardiac function and increases left ventricular mass independent of its pressure-raising effects.26 Therefore, the cardiac hypertrophy model induced by Ang II was used to examine the effect of polydatin on cardiac protection. In the current study, we demonstrate that pretreatment with polydatin prevents Ang II-induced cardiac hypertrophy both in vitro and in vivo. It should be emphasized that despite the fact that BP was raised significantly in Ang II-infused rats, polydatin treatment did not reduce BP. These observations, combined with direct effects seen in cardiomyocytes, indicate that polydatin exerts a direct reversal of the hypertrophic program in the myocardium.
Accumulated studies demonstrate that over production of ROS and inflammatory factors generated by Ang II stimulation contributes to the development of hypertrophy and remodeling in the heart.10,30 This conclusion is also supported by the current observation that Ang II promotes excess amounts of intracellular ROS production via stimulation of NADPH oxidase, in parallel to hypertrophic responses in cardiomyocytes. Our results also show that polydatin treatment significantly attenuated Ang II-induced ROS production. Interestingly, in neonatal cardiac fibroblasts, polydatin exerted similar antioxidant actions, implying that ROS generated from both cardiomyocytes and cardiac fibroblasts in vivo will likely be inhibited by polydatin. This is supported by our in vivo observation showing that polydatin treatment significantly attenuated Ang II-induced perivascular and interstitial fibrosis. These observations support the hypothesis that the antioxidative properties of polydatin might play an important part in its beneficial protective cardiovascular effects.
Polydatin is an active stilbene compound which has a strong antioxidant property.15,24 In this study, the basal level of ROS generation in cardiomyocytes was not changed by polydatin treatment alone, probably because of the basal oxidative activity was maintained successfully at a minimum in the normal physiological situation by superoxide dismutases and other endogenous scavengers of ROS. Therefore, the observations that pretreatment with polydatin completely prevented Ang II-induced superoxide generation while simultaneously blocking cardiac hypertrophy, strongly supports the hypothesis that cardiac remolding stimulated by chronic Ang II treatment is mediated mainly by oxidative stress.
To further investigate the mechanisms underlying the inhibitory effect of polydatin on cardiac hypertrophy, its potential effect on NADPH oxidases was studied. NADPH oxidase is an enzyme that catalyzes superoxide production from molecular oxygen and NADPH. It is a multimeric enzyme consisting of two membrane-bound components including a catalytic Nox subunit and the p22phox subunit, and four components in the cytosol, p40phox, p47phox, p67phox, plus the small GTP-binding protein Rac.30,31 To date, the Nox family consists of five isoforms (Nox1-5), expressed in a tissue-specific manner. Among these, Nox2 and Nox4 are the main isoforms expressed in the myocardium and play an important role in mediating oxidant production at baseline and under various stimuli.30 Evidence from Nox2 knockout mice shows that cardiac hypertrophy induced by Ang II infusion was inhibited.10,32 Genetic deletion of Rac1, an essential component of the Nox2 NADPH oxidase complex, blocks Ang II-induced superoxide generation and cardiac hypertrophy.33 These studies suggest that endogenous Nox2 mediates Ang II-induced cardiac hypertrophy. In addition, studies using cardiac specific Nox4 knockout mice indicate that Nox4 was involved in the development of cardiac hypertrophy in response to pressure overload.34 Several lines of evidence also showed that expression of Nox2 and Nox4 was upregulated by hypertrophic stimuli including Ang II, phenylephrine, and pressure overload.35–37 In the current study, polydatin specifically blunted the increase of Nox2 and Nox4 expression induced by Ang II, thus suggesting that polydatin attenuates superoxide generation by specific inhibition of NADPH oxidase. Antioxidant mechanism of polydatin has been attributed to the induction of antioxidant enzymes, and the direct scavenging and/or chelation of redox-active metal ions, as well as to inhibition of redox-sensitive transcription factors.19,24 The present findings highlight that the direct inhibition of NADPH oxidase is an additional antioxidant property of polydatin.
It has been well documented that TGF-β is a key cytokine that promotes accumulation of collagen and other major components of the extracellular matrix in many fibrotic disorders, including cardiac and pulmonary fibrosis, glomerulonephritis, and vascular restenosis.38 Several studies indicate that Ang II upregulates TGF-β1 expression in cardiac myocytes and fibroblasts; TGF-β, in turn, promotes cardiac hypertrophy by activation of Smad proteins and TGF-β-activated kinase-1.39 Furthermore, absence of the Tgfb1 gene in transgenic mice completely prevented the development of cardiac hypertrophy induced by Ang II.40 Studies have also shown that TGF-β was involved in NAD(P)H oxidase activation in cardiac remolding.41,42 In our study, polydatin reduced cardiac hypertrophy and inhibited Ang II-induced TGF-β1 expression, thus suggesting that the protective effect of polydatin may be at least partial because of its effect on regulating TGF-β1 signaling. The development of cardiac hypertrophy is a complex process involving signal integration of multiple pathways. Therefore, it may be that the observed antihypertrophic effect of polydatin is mediated by the modulation of not one but several of these pathways. To further elucidate the mechanisms involved in polydatin’s antihypertrophic effects, it will be necessary to identify the essential signaling molecules involved in the development of cardiac hypertrophy, and the cross talk between them.
In conclusion, the present study demonstrates that polydatin prevents cardiac hypertrophy in response to Ang II both in vitro and in vivo. Polydatin inhibited the formation of cardiac hypertrophy by blocking activation of NADPH oxidase. Our data confirm that NADPH oxidase is a main target of polydatin’s pharmacological actions, although we cannot conclude whether NADPH oxidase is its only target. These observations are relevant to the understanding of the protective effect of polydatin against cardiac remolding, which are characterized by an increase in oxidative stress or by the activation of rennin–angiotensin system.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 31171101 and No. 81100175) and the Key Project of Chinese Ministry of Education (212173).
Author contributions
QZ, YT, NZ and FY performed the experiments and collected the data. QZ and FY wrote the manuscript. QZ and YT have contributed equally to this manuscript.
References
- 1.Koitabashi N, Kass DA. Reverse remodeling in heart failure – mechanisms and therapeutic opportunities. Nat Rev Cardiol 2011; 9: 147–57. [DOI] [PubMed] [Google Scholar]
- 2.Maytin M, Colucci WS. Molecular and cellular mechanisms of myocardial remodeling. J Nucl Cardiol 2002; 9: 319–27. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev 2000; 52: 11–34. [PubMed] [Google Scholar]
- 4.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 2007; 292: C82–97. [DOI] [PubMed] [Google Scholar]
- 5.Ainscough JF, Drinkhill MJ, Sedo A, Turner NA, Brooke DA, Balmforth AJ, Ball SG. Angiotensin II type-1 receptor activation in the adult heart causes blood pressure-independent hypertrophy and cardiac dysfunction. Cardiovasc Res 2009; 81: 592–600. [DOI] [PubMed] [Google Scholar]
- 6.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 2004; 63: 423–32. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta C, Zhang L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov Today 2011; 16: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurdi M, Booz GW. New take on the role of angiotensin II in cardiac hypertrophy and fibrosis. Hypertension 2011; 57: 1034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maejima Y, Kuroda J, Matsushima S, Ago T, Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol 2011; 50: 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 2002; 105: 293–6. [DOI] [PubMed] [Google Scholar]
- 11.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res 2001; 89: 453–60. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi D, Kudoh S, Shiojiama I, Zou Y, Harada K, Shimoyama M, Imai Y, Monzen K, Yamazaki T, Yazaki Y, Nagai R, Komuro I. Atrial natriuretic peptide inhibits cardiomyocyte hypertrophy through mito-geneactivated protein kinase phosphatase-1. Biochem Biophys Res Commun 2004; 322: 310–9. [DOI] [PubMed] [Google Scholar]
- 13.Papparella I, Ceolotto G, Montemurro D, Antonello M, Garbisa S, Rossi G, Semplicini A. Green tea attenuates angiotensin II-induced cardiac hypertrophy in rats by modulating reactive oxygen species production and the Src/epidermal growth factor receptor/Akt signaling pathway. J Nutr 2008; 138: 1596–601. [DOI] [PubMed] [Google Scholar]
- 14.Dong J, Wang H, Wan L, Hashi Y, Chen S. Identification and determination of major constituents in Polygonum cuspidatum Sieb. et Zucc. by high performance liquid chromatography/electrospray ionization-ion trap-time-of-flight mass spectrometry. Se Pu 2009; 27: 425–30. [PubMed] [Google Scholar]
- 15.Liu LT, Guo G, Wu M, Zhang WG. The progress of the research on cardio-vascular effects and acting mechanism of polydatin. Chin J Integr Med 2012; 18: 714–9. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg DM, Eric Ng AK, Diamandis EP, Soleas GJ. Resveratrol glucosides are important components of commercial wines. Am J Enol Vitic 1996; 47: 415–20. [Google Scholar]
- 17.Lanzilli G, Cottarelli A, Nicotera G, Guida S, Ravagnan G, Fuggetta MP. Anti-inflammatory effect of resveratrol and polydatin by in vitro IL-17 modulation. Inflammation 2012; 35: 240–8. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Tan Y, Yao F, Zhang Q. Polydatin alleviates non-alcoholic fatty liver disease in rats by inhibiting the expression of TNF-α and SREBP-1c. Mol Med Rep 2012; 6: 815–20. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Lan Z, Lin Q, Mi X, He Y, Wei L, Lin Y, Zhang Y, Deng X. Polydatin ameliorates renal injury by attenuating oxidative stress-related inflammatory responses in fructose-induced urate nephropathic mice. Food Chem Toxicol 2013; 52: 28–35. [DOI] [PubMed] [Google Scholar]
- 20.Miao Q, Wang S, Miao S, Wang J, Xie Y, Yang Q. Cardioprotective effect of polydatin against ischemia/reperfusion injury: roles of protein kinase C and mito KATP activation. Phytomedicine 2011; 19: 8–12. [DOI] [PubMed] [Google Scholar]
- 21.Gao JP, Chen CX, Gu WL, Wu Q, Wang Y, Lu J. Effects of polydatin on attenuating ventricular remodeling in isoproterenol-induced mouse and pressure-overload rat models. Fitoterapia 2010; 81: 953–60. [DOI] [PubMed] [Google Scholar]
- 22.Zhang LP, Yang CY, Wang YP, Cui F, Zhang Y. Protective effect of polydatin against ischemia/reperfusion injury in rat heart. Sheng Li Xue Bao 2008; 60: 161–8. [PubMed] [Google Scholar]
- 23.Deng J, Liu W, Wang Y, Dong M, Zheng M, Liu J. Polydatin modulates Ca(2+) handling, excitation-contraction coupling and β-adrenergic signaling in rat ventricular myocytes. J Mol Cell Cardiol 2012; 53: 646–56. [DOI] [PubMed] [Google Scholar]
- 24.Jiang X, Liu W, Deng J, Lan L, Xue X, Zhang C, Cai G, Luo X, Liu J. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic Biol Med 2013; 60: 292–9. [DOI] [PubMed] [Google Scholar]
- 25.Xuan CL, Yao FR, Guo LR, Liu Q, Chang SK, Liu KX, Sun CW. Comparison of extracts from cooked and raw lentil in antagonizing angiotensin II-induced hypertension and cardiac hypertrophy. Eur Rev Med Pharmacol Sci 2013; 17: 2644–53. [PubMed] [Google Scholar]
- 26.Hu CM, Chen YH, Chiang MT, Chau LY. Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo. Circulation 2004; 110: 309–16. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Yao F, Raizada MK, O'Rourke ST, Sun C. Apelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ Res 2009; 104: 1421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang SY, Siow YL, Au-Yeung KK, House J, O K. Folic acid supplementation inhibits NADPH oxidase-mediated superoxide anion production in the kidney. Am J Physiol Renal Physiol 2011; 300: F189–98. [DOI] [PubMed] [Google Scholar]
- 29.Schlüter KD, Wenzel S. Angiotensin II: a hormone involved in and contributing to pro-hypertrophic cardiac networks and target of anti-hypertrophic cross-talks. Pharmacol Ther 2008; 119: 311–25. [DOI] [PubMed] [Google Scholar]
- 30.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol 2009; 302: 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirker A, Zhang M, Murdoch C, Shah AM. Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am J Nephrol 2007; 27: 649–60. [DOI] [PubMed] [Google Scholar]
- 32.Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 2003; 93: 802–5. [DOI] [PubMed] [Google Scholar]
- 33.Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Sci U S A 2006; 103: 7432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A 2010; 107: 15565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 2010; 106: 1253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maejima Y, Kuroda J, Matsushima S, Ago T, Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol 2011; 50: 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics 2006; 26: 180–91. [DOI] [PubMed] [Google Scholar]
- 38.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 2011; 51: 600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 2004; 63: 423–32. [DOI] [PubMed] [Google Scholar]
- 40.Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest 2002; 109: 787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 2005; 97: 900–7. [DOI] [PubMed] [Google Scholar]
- 42.Jiang F, Liu GS, Dusting GJ, Chan EC. NADPH oxidase-dependent redox signaling in TGF-β-mediated fibrotic responses. Redox Biol 2014; 2: 267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]