Abstract
The physiology of vascular cells depends on stimulating mechanical forces caused by pulsatile flow. Thus, mechano-transduction processes and responses of primary human endothelial cells (ECs) and smooth muscle cells (SMCs) have been studied to reveal cell-type specific differences which may contribute to vascular tissue integrity. Here, we investigate the dynamic reorientation response of ECs and SMCs cultured on elastic membranes over a range of stretch frequencies from 0.01 to 1 Hz. ECs and SMCs show different cell shape adaptation responses (reorientation) dependent on the frequency. ECs reveal a specific threshold frequency (0.01 Hz) below which no responses is detectable while the threshold frequency for SMCs could not be determined and is speculated to be above 1 Hz. Interestingly, the reorganization of the actin cytoskeleton and focal adhesions system, as well as changes in the focal adhesion area, can be observed for both cell types and is dependent on the frequency. RhoA and Rac1 activities are increased for ECs but not for SMCs upon application of a uniaxial cyclic tensile strain. Analysis of membrane protrusions revealed that the spatial protrusion activity of ECs and SMCs is independent of the application of a uniaxial cyclic tensile strain of 1 Hz while the total number of protrusions is increased for ECs only. Our study indicates differences in the reorientation response and the reaction times of the two cell types in dependence of the stretching frequency, with matching data for actin cytoskeleton, focal adhesion realignment, RhoA/Rac1 activities, and membrane protrusion activity. These are promising results which may allow cell-type specific activation of vascular cells by frequency-selective mechanical stretching. This specific activation of different vascular cell types might be helpful in improving strategies in regenerative medicine.
Keywords: Smooth muscle cell, endothelial cell, uniaxial cyclic tensile strain, actin, focal adhesion, Rho GTPases
Introduction
The structural integrity and function of many tissues and cell types are regulated by mechanical forces. In this manner, gravitational compressive forces control the homeostasis of bones, just as isotonic tension causes muscles to grow.1,2 In the vascular system, hemodynamic stresses such as tension and shear are required to maintain the structural organization and functional activity of vascular endothelial cells (ECs) and vascular smooth muscle cells (SMCs) which form the vessel wall.3–5 Simply spoken, a shear stress is generated by the pulsatile blood flow and the circumferential stress is caused by the pulsatile blood pressure.4,6,7
When exposed to mechanical loading, ECs act like mechanical sensors. ECs, exposed to a cyclic tensile strain, hydrostatic pressure, or fluid shear stress, orient along the vascular axis in vivo.8,9 Not only their morphology and structure but also their functions change with the application of tensile or shear loadings.4,10–13 However, the elevation of the circumferential tensile strain in an arterial wall may result in endothelial damage, plaque formation, and atherosclerosis.11,14 The orientation and structural network of the SMC layers that build up the arterial wall are very important for maintaining its mechanical strength and function and also for providing the mechanical compliance required for pulsatile blood flow.15–17 SMCs are primarily aligned in the circumferential direction in the arterial wall, which is a result of the complicated mechanical environment in vivo.
In the context of tissue engineering, applied forces are important for creating functional vessels that can withstand the mechanical stresses present in vivo.18–20 Cellular responses to mechanical stimuli have been studied extensively through exposure of cultured cells or arterial segments to cyclic loading.7,21–26 The responses depend on external stresses or on intracellular processes like the contractile cytoskeleton–motor protein complex.27–29
One common method of examining cell responses to mechanical stress is the use of flexible membranes as culture substrates.30,31 Cells adherent on an expandable elastomeric substrate coated with extracellular matrix molecules like fibronectin or collagen can be exposed to a mechanical tensile strain.32 Several studies have shown that cells on uniaxial cyclically stretched substrates reorient themselves nearly perpendicular to the direction of strain32–41 and that the actin cytoskeleton is reorganized perpendicular to the strain direction.33–35,42 Cultured SMCs in vitro can be induced to reorient to a uniform perpendicular alignment into the direction of principal uniaxial mechanical strain.16,41,43,44 The SMC orientation response is consistent with the response that has been found for many different cell types such as ECs, osteoblasts, fibroblasts, and melanocytes.23,40,41,45,46 The results for the cell orientation support the common hypothesis that cell alignment is an avoidance reaction of the cells exposed to tension. It is believed that the sensing of cells is mediated by cell-matrix adhesions that mechanically link the extracellular matrix with the cytoskeleton.47 Despite many experimental studies, only limited information about the dynamics of cell reorientation is available to this date. Neidlinger-Wilke et al. and Goldyn et al. showed, for example, that fibroblasts tend to reorient within the first 3 h during application of uniaxial cyclic tensile strain.34,35,39 Liu et al. demonstrated a frequency dependence for the alignment of arterial SMCs observed in time steps of three hours.48 Jungbauer et al. systematically investigated the influence of dynamic frequency and amplitude changes on fibroblasts.46 Overall, a detailed quantitative examination of the temporal behavior of cells under cyclic tensile strain will be helpful for understanding the molecular mechanisms involved and is crucial for theoretical modeling.49
In this study, the effects of the stretch frequency on the temporal kinetics of primary human EC and SMC reorientation are compared. For the two cell types a characteristic difference in the behavior of cell reorganization with frequencies of 0.01, 0.1, and 1 Hz is demonstrated. Furthermore, this observation is supported by the investigation of the intracellular actin stress fiber, the cell-matrix adhesion system, and the activities of the small Rho GTPases RhoA and Rac1.
Materials and methods
Primary human endothelial and SMC culture
Primary human coronary artery endothelial cells (HCAECs) and primary human coronary artery smooth muscle cells (HCASMCs) from PromoCell (Heidelberg, Germany) of different donors were used. In total, we used three different donors for ECs and four different donors for SMCs. The cells from the different donors were not pooled for the experiments. However, the data of the experiments with cells of the same cell type but from different donors were pooled. The cells were grown to confluence using EC growth medium and SMC growth medium, respectively, both with low serum concentration (2.5%). All cells had passage numbers less than six. Cells were washed with HepesBSS, and then trypsinized with a trypsin/EDTA (0.25% trypsin/1 mmol/L ethylenediaminotetracetic acid) solution. When the cells were completely detached from the cell culture container, trypsin neutralizing solution based on soy bean extract (like all cell media and solutions by PromoCell) was added. The resulting cell suspension was spun down and resuspended in fresh media. Cells were counted with the help of a Neubauer counting chamber. An initial cell density of 50 cells/(mm2 sample surface) was used for the experiments which resulted in non-confluent cell layers with reduced cell–cell contact. To avoid bacterial contamination, 100 units/mL of penicillin-streptomycin (Gibco, Darmstadt, Germany) was added to the medium. After cell seeding, the culture dishes were placed in a cell culture incubator (37℃, 5% CO2, high humidity) for 24 h before they were mounted on the stretching setup.
Cell stretching device and experiment
A customized stretching device was developed in our laboratory which applied periodical mechanical strain to an elastomeric membrane which served as cell culture substrate.46 The stretching device is mounted on a motorized inverted light microscope (Axiovert 200M, 10×/0.25Ph1 objective, Zeiss, Jena, Germany) which is equipped with a CCD-camera (PCO Sensicam, Kelheim, Germany). The microscope is equipped with an environmental chamber to control the temperature and the CO2 concentration of the atmosphere. The cells were plated on a 20 × 20 mm2 poly(dimethylsiloxane) (PDMS, Corning Sylgard 184, Midland, USA) membrane with an elastic modulus of approximately 1 MPa. The suitability of PDMS dishes for cell culture was shown for example by Neidlinger-Wilke et al.,32 Lee et al.,31 Jungbauer et al.,46 and Goldyn et al.34,35 Before cell seeding, the PDMS dishes were sterilized with ethanol and rinsed several times with phosphate-buffered saline (PBS) (Gibco, Germany), and incubated overnight (12–14 h) with a 20 µg/mL fibronectin solution (Sigma-Aldrich, Munich, Germany), then again washed with PBS.
In each experiment a stretching frequency of 0.01, 0.1, or 1 Hz was selected. The linear strain amplitude was 8% for all experiments. The stretching of the substrate created a uniaxial strain at the center of the PDMS substrate described by a longitudinal elongation and a transverse contraction.46 The strain field in the center region of the PDMS substrate was relatively homogenous and for an applied strain of 8% the transverse contraction was about 1.55%.46 Phase contrast images were acquired in the non-strained position at the center of the PDMS substrate at intervals of 100 s over a time course of 300 min. Each image was focused before acquisition by a software-based auto focusing routine.
Fluorescence staining and imaging
After stretching for 300 min the cells were fixed in 3.7% paraformaldehyde (Serva, Heidelberg, Germany) for 10 min at room temperature. Then, the cells were permeabilized in 0.1% Triton X-100 (Sigma) in PBS for 5 min. Before staining, cells were treated with bovine serum albumin (BSA) (Sigma) for 5 min, to block unspecific antibody binding. The cells were incubated with first antibody (focal adhesion marker anti-paxillin [Y113], monoclonal, rabbit [Abcam, Cambridge, UK], 1:400 in PBS) for 60 min, then they were washed with PBS and incubated with the secondary antibody AlexaFluor568 anti-rabbit (Invitrogen, Darmstadt, Germany), 1:200 and with 6.7 µmol/L AlexaFluor488 phalloidin (Invitrogen) for 30 min to stain for F-actin.
Images were acquired by an AxioImager microscope (Zeiss, Germany) with a 63x water-immersion objective. Images of each z-stack were merged to a single image (extended depth focus, Zeiss).
Image analysis
Cell orientation was measured via the image processing software ImageJ (http://rsb.info.nih.gov/ij/). Phase contrast images were taken from the time-lapse movies and the outline of each cell was marked. Small tethers of the cells were neglected in this process; only the coarse cell body was marked. An image processing macro was used to fit an ellipse to each elongated cell outline. The macro gives results for the orientation angle ϕ of the long axis of a cell with respect to the strain direction, the minor and major axes of the ellipse, and the total cell area. These data are used to calculate the mean values with corresponding standard error for each point of time chosen.
Quantification of data
The cellular orientation response was quantified by determining the density distribution g(ϕ) of the cell orientation angle and the order parameter S calculated as:37,46
| (1) |
Between 20 and 30 cells from an image at a particular time point were used for calculating S. It follows from the above definition that S = 0 if the cells are in average randomly orientated, S = 1 if they are parallel oriented, and S = −1 if they are perpendicularly oriented with respect to the strain direction (further details about the order parameter are given in Kemkemer et al.).37 Following a procedure based on a stochastic differential equation and the definition of this order parameter, the following exponential expression can be derived which enables us to evaluate the time dependence of the order parameter:37,46
| (2) |
Starting at a steady state order parameter a new steady state order parameter is reached with a characteristic time τ upon application of a uniaxial cyclic tensile strain. In our experiments, the characteristic time τ was determined through matching equation (2) to the experimental data via a least-square fit. Basically, the characteristic time constant τ indicates the needed time for the stretch-induced realignment of cells and describes how fast the cells reorient upon cyclic stretch application. More precisely, it gives the time until the orientation parameter <cos(2ϕ)> reached 1/e (approximately 63%) of the maximum (final) orientation.
The elongation of a cell, E, was calculated using the lengths of the major (Amaj) and minor axes (Amin) of an ellipse fitted to the main cell body (excluding highly branched cell areas) as defined below (equation 3).
| (3) |
For the cell shape is a perfect circle and for the cell shape is a thin line.
Analysis of actin stress fibers and cell-matrix adhesions (focal adhesions)
For analysis of actin stress fiber orientation, a self-developed software macro embedded in ImageJ was used. Each cell was divided in several square-shaped subareas. Within each square, the mean actin orientation was analyzed by texture analysis via a fast Fourier transformation (FFT) in analogy to Kemkemer et al.50 The orientation of focal adhesions (expressed as the order parameter S ≤ cos(2ϕ) >), the focal adhesion area (µm2), and the focal adhesion circularity (where zero equals a perfect circle and one equals a line) were measured with ImageJ using a background subtraction followed by the application of a threshold and subsequent particle analysis. In total 15–30 cells from three independent experiments were evaluated.
ELISA measurements of Rac1 and RhoA activity
The Rac1- or RhoA-activity assay was performed with a commercially available ELISA Kit (Cytoskeleton, Denver, CO) and experiments were performed as described in the manufacturer’s manual. Luminescence readout for evaluation of Rho GTPase activities was detected with a fluorescence spectrophotometer (Tecan, Crailsheim, Germany). The obtained values were normalized to one for the non-treated non-stretched condition.
Protrusion activity
The analyzed cells were divided in “end” and “side” corresponding to the cells’ part being either penpendicular or parallel to the direction of applied uniaxial cyclic tensile strain. Membrane protrusions of SMCs were counted manually. Membrane protrusive activty of ECs was quantified by kymograph analysis as described previously.34 Membrane protrusions were identified by their characteristic centripetal movement, beginning at the lamella edge. The number of membrane protrusions over time yielded in a frequency of membrane protrusions per hour.
Statistics
We performed the experiments with primary cells and the advantage is the direct relevance to translational aspects. However, disadvantage might be donor-dependency of cellular reaction. We used at least three different donors for this study (see “Materials and methods” section in the paragraphs about cell culture routine and statistics) revealing identical cell-specific but donor-independent cellular reaction. We tried to account for inter-donor variation by pooling the data from several donors.
At least four independent experiments per data set have been performed and in experimental data sets where single cells were analyzed in total at least 36 cells were evaluated (N > 4, n > 36) if not indicated differently.
All data were expressed as means ± standard error of the mean (SEM). OriginPro 8G software (OriginLab Cooperation, Northampton, USA) was used for statistical analysis (ANOVA or Student’s t-test or ANOVA followed by a t-test modified for multiple comparisons). Differences were considered as statistically significant when the calculated P value was less than 0.05.
Results
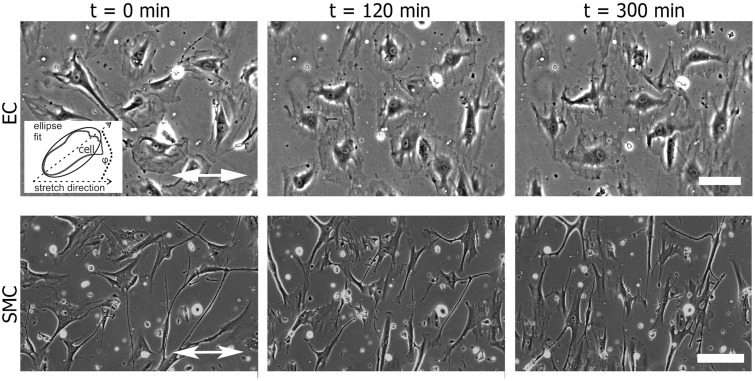
In Figure 1, a series of phase contrast images for the two cell types is depicted. Before the application of a uniaxial cyclic tensile strain, adherent elongated non-confluent ECs and SMCs were randomly oriented on the fibronectin-coated elastomeric substrates. Upon application of uniaxial cyclic tensile strain, cell elongation and orientation perpendicular to the strain direction was observed (Figure 1 and Supplemental movies M1 and M2). Averages of cell orientation of at least three time lapses for each measured frequency are shown in Figure 2 and Figure 3, where the mean cell elongation E and the mean order parameter S are plotted as a function of time to highlight the dynamic evolution of the shape and the orientation of the cells.
Figure 1.
The alignment of vascular cells with respect to the direction of a uniaxial cyclic tensile strain application. Human primary endothelial cells (ECs) and smooth muscle cells (SMCs) adherent on fibronectin-coated silicone surfaces were exposed to uniaxial cyclic tensile strain. Still images of ECs and SMCs from phase contrast movies show the cells before (t = 0 min) and after 120 and 300 min of uniaxial cyclic strain with a frequency of 1 Hz. The direction of strain is indicated by the white double-headed arrow. The scale bar represents 200 µm. Inset: Scheme of ellipse fitting for analysis of cell orientation; the angle ϕ is between the ellipse main axis and the stretch direction
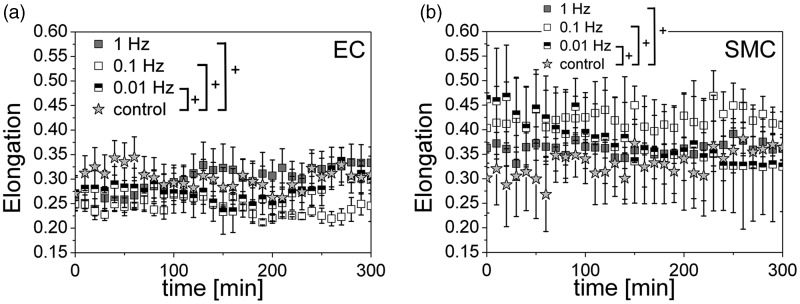
Figure 2.
The cell elongation is independent of a uniaxial cyclic tensile strain application. Evolution of the cell elongation E over time for human primary endothelial cells (ECs) (a) and smooth muscle cells (SMCs) (b) for non-stretched control conditions (grey star) and three different stretching frequencies: 1 Hz (grey), 0.1 Hz (white), 0.01 Hz (black-white). For the cell shape is a perfect circle and for the cell shape is a line. ECs slightly change (not significant) their shape measurable only at 1 Hz. SMCs show nearly no (significant) change in their elongation and typically display great variations for all frequencies. (ANOVA; +P > 0.05)
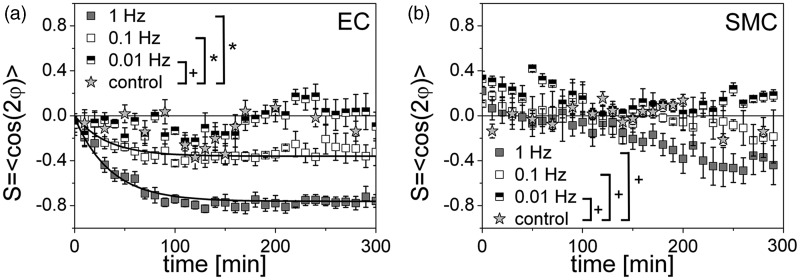
Figure 3.
The kinetics of the cell reorientation upon exposure to a uniaxial cyclic tensile strain is time- and frequency-dependent. Time development of the cell orientation (order parameter S = <cos2ϕ>) for human primary endothelial cells (ECs) (a) and smooth muscle cells (SMCs) (b) for three different frequencies: 1 Hz (grey), 0.1 Hz (white), 0.01 Hz (black-white); control: non-stretched (grey star). S = 0 if the cells are in average randomly oriented, S = 1 if they are parallel oriented, and S = −1 if they are perpendicularly oriented with respect to the stretch axis. In the plot for the ECs are fitting lines (least square fit, see result section) for 1 Hz and 0.1 Hz included (black curves). No fit is possible for the orientation data of the SMCs. The cell reorientation is cell-type-, time-, and frequency-dependent. ECs show a initiation of a reorientation response already after 25 min for 0.1 Hz and 1 Hz, while SMCs initiated their reorientation only for 1 Hz after 200 min. (ANOVA; *P < 0.05; +P > 0.05)
The cell elongation is independent of a uniaxial cyclic tensile strain application
After the cyclic strain was switched on (f = 0.01, 0.1 or 1 Hz), the mean elongation of the cells changed only for ECs at a stretching frequency of 1 Hz. While the mean elongation was E ≈ 0.25 without strain, which corresponds to rather round-shaped cells, ECs became slightly more elongated (E ≈ 0.35, not significant) upon the application of strain with a frequency of 1 Hz. No changes were detected for the cell elongation for any other experimental condition (Figure 2a; ANOVA, +, P > 0.05). For SMCs the average elongation under control condition is similar (E ≈ 0.32) compared to ECs but seemed to be slightly increased upon application of strain of different frequencies (E ≈ 0.37–0.40, not significant). SMC elongation showed typical broad variations. This is due to their irregular cell shape, even though for analysis only the main cell body of the SMCs, excluding highly branched cells parts, was considered (Figure 2b; ANOVA, +P > 0.05).
The cell orientation is significantly influenced by a uniaxial cyclic tensile strain
ECs changed their orientation towards a perpendicular alignment upon the applied strain at 0.1 and 1 Hz. Correspondingly, the orientation parameter S changed exponentially from an initial value of S ≈ 0 (random cell orientation) to a new steady state order parameter between S ≈ −0.4 (for 0.1 Hz) and S ≈ −0.8 (for 1 Hz) (Figure 3a; ANOVA, *P < 0.05; +P > 0.05). SMCs showed no clear reorientation for the measured frequencies and time period. Only after about 200 min at a frequency of 1 Hz perpendicular alignment of SMCs seemed to be initiated even though the differences in orientation are not significant different compared to the control and the other stretching frequencies (Figure 3b; +P > 0.05).
The kinetics of the cell reorientation upon application of a uniaxial cyclic tensile strain is time- and frequency-dependent
To analyze the kinetics of cell orientation, the characteristic time tau (τ) was determined. τ is defined as the time until the cell orientation level reached 1/e (∼63%) of its maximum value. The dynamic cell reorganization at uniaxial cyclic tensile strain was quantified by the characteristic time τ for the strain frequencies from 0.01 Hz to 1 Hz for ECs (Figure 3a). The orientation of SMCs was not significantly altered until 200 min and thus τ could not be evaluated with certainty (Figure 3b). At 0.01 Hz τ was not measurable for ECs, since there was no significant deviation from the control situation (S ≈ 0). At 0.1 Hz the mean value for three experiments was τ = 37.60 ± 4.79 min and at 1 Hz τ = 91.68 ± 50.24 min (mean value of three movies with SEM). This demonstrates the dependence of cell reorientation dynamics on the stretching frequency. The big variation of τ at 0.1 Hz (±50.24 min) was due to the divergent values between each data set. In summary, the reorientation reaction of ECs was faster with increased frequency of cyclic tensile strain.
The cell reorientation upon exposure to a uniaxial cyclic tensile strain depends on a threshold frequency
Below a certain threshold frequency f depending on the cell type, cell reorientation perpendicular to strain direction could no longer be observed and the mean cell orientation fluctuated around S = 0. Only at frequencies higher than the threshold value (f > ft) did cells change to a perpendicular orientation with time. The observed threshold frequency was cell-type dependent: for ECs the threshold frequency was between 0.01 Hz and 0.1 Hz, as ECs showed significant differences in orientation for the two frequencies (Figure 3a). For SMCs the threshold frequency could not be determined with certainty as frequencies higher than 1 Hz were not assessed. However, the threshold frequency of SMCs is assumed to be above 1 Hz, as the SMCs revealed for 1 Hz after 200 min an initiation of a cell reorientation response (Figure 3b).
Actin stress fibers and focal adhesions orientation are dependent on a threshold frequency of stretching while focal adhesion area is only changed cell-type dependent and focal adhesion shape is not altered upon stretching
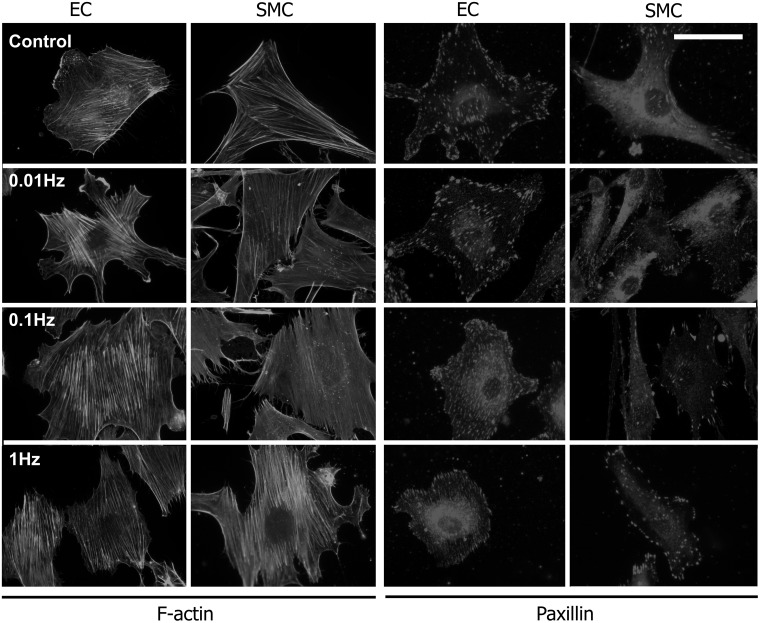
The actin cytoskeleton and focal adhesions are important for mechano-transduction.34,35 For further analysis of the cells’ orientation response to uniaxial cyclic strain, the actin network and paxillin as focal adhesion marker were stained and compared with those of non-stretched cells. For both cell types, actin stress fibers and focal adhesions were not oriented at a frequency of 0.01 Hz and under control conditions (Figure 4). For 0.1 Hz and 1 Hz, actin stress fibers and focal adhesions were oriented. The quantification of the mean orientation demonstrates generally stronger perpendicular alignment of the actin network and focal adhesions for ECs than for SMCs (Figure 5a, b; ANOVA followed by a t test modified for multiple comparisons; *P < 0.05; +P > 0.05).
Figure 4.
Actin stress fibers and focal adhesions orientation upon application of a uniaxial cyclic tensile strain. Representative fluorescence microscopy images of phalloidin-stained actin (a) and paxillin-stained focal adhesions (b) in human primary endothelial cells (ECs) and a smooth muscle cells (SMCs) at non-stretched control conditions and after 300 min of uniaxial cyclic tensile strain application at frequencies of 0.01 Hz, 0.1 Hz, and 1 Hz (the direction of strain is indicated by white double-headed arrow); scale bar represents 30 µm for (a) and 20 µm for (b).
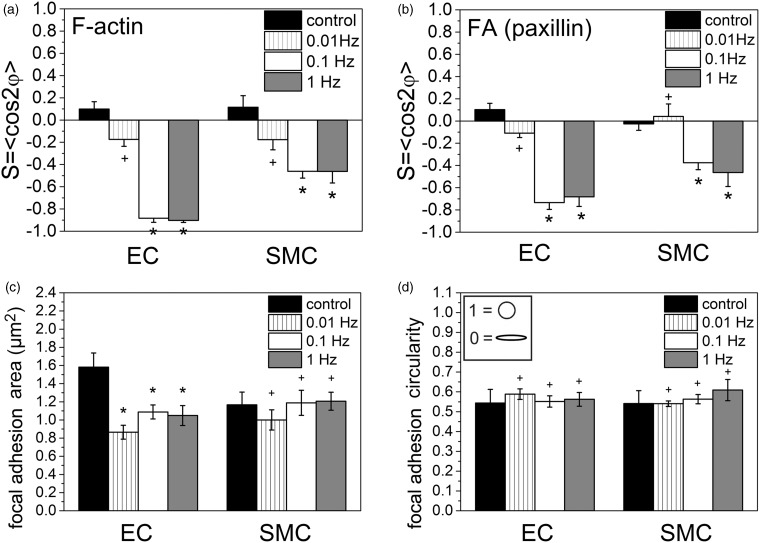
Figure 5.
Actin stress fibers and focal adhesions orientation are dependent on a threshold frequency of stretching while focal adhesion area is changed cell type-dependent and focal adhesion shape is not altered upon stretching. Analysis of F-actin orientation (a), paxillin-marked focal adhesion orientation (b), focal adhesion area (c) and focal adhesion circularity after 300 min at indicated stretching frequencies (1 Hz, 0.1 Hz, 0.01 Hz, and non-stretched control) for human primary endothelial cells (ECs) and smooth muscle cells (SMCs). (ANOVA followed by a t test modified for multiple comparisons; *P < 0.05; +P > 0.05)
Actin stress fibers oriented almost perpendicular in ECs at frequencies of 0.1 Hz and 1 Hz (S ≈ −0.9). In SMCs, the actin stress fiber orientation was less pronounced, but around the same value for 0.1 Hz and 1 Hz (S ≈ −0.5) (Figure 5a). Focal adhesions showed a similar alignment behavior. In SMCs, the overall focal adhesion orientation was weaker than in ECs (Figure 5b). For SMCs, the maximum orientation of focal adhesions was observed for 0.1 Hz and 1 Hz (S ≈ −0.45), whereas the focal adhesions oriented in a not significant manner for below 0.1 Hz. For ECs, the focal adhesion orientation was at its maximum at 0.1 Hz (S ≈ −0.7) and is slightly smaller at 1 Hz (S ≈ −0.65) (Figure 5b).
The focal adhesion area was determined for ECs and SMCs under control conditions and subjected to uniaxial cyclic tensile strain (Figure 5c). Although, the focal adhesions of SMCs showed an alignment response for stretching frequencies above 0.1 Hz, the focal adhesion area was not significantly changed compared to non-stretched control conditions (∼1.15 µm2 for all conditions; Figure 5c; ANOVA followed by a t test modified for multiple comparisons, +P > 0.05). In contrast, the focal adhesion area was significantly decreased (about 37%) for ECs for all stretching conditions (control: ∼1.6 µm2; 0.01 Hz–1 Hz: ∼1 µm2; Figure 5c; ANOVA followed by a t test modified for multiple comparisons, *P < 0.05) even though focal adhesion realignment could only be detected for a stretching frequency of at least 0.1 Hz. The circularity of the focal adhesion (spherical vs. elongated) for both cell types not significantly changed upon application of uniaxial cyclic tensile strain (Figure 5d; ANOVA followed by a t test modified for multiple comparisons; *P < 0.05; +P > 0.05).
The activity of RhoA and Rac1 is time- and cell type-dependent while the localization of membrane protrusions is spatially not changed upon stretching
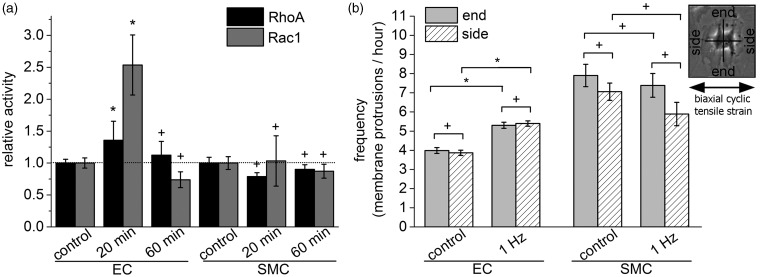
Rho and Rac GTPases have a key role in cell polarization and cell migration.51 To explore the regulation of GTPases in relation to the observed significant changes in cell polarity for ECs, we tested the GTPase activities under uniaxial cyclic stretching conditions (at 1 Hz) and normalized it to non-stretched control conditions. Using an enzyme linked immunosorbent assay (ELISA), we found no changes for RhoA and Rac1 activities in SMCs after 20 and 60 min of uniaxial cyclic strain. In contrast, we detected for ECs after 20 min stretching a 1.7-fold increase in RhoA and an 2.5-fold increase in Rac1 activity after 20 min while RhoA and Rac1 activities were not altered after 60 min (Figure 6a; Student’s t-test; *P < 0.05; +P > 0.05).
Figure 6.
The activity of RhoA and Rac1 is time- and cell type-dependent while the localization of membrane protrusions is spatially not changed upon stretching. (a) Analysis of the relative activity of the two small Rho GTPases RhoA and Rac1 in human primary endothelial cells (ECs) and smooth muscle cells (SMCs) using an enzyme linked immunosorbent assay (ELISA). The data were normalized to non-stretched control conditions for each cell type (dotted black line). The cells were exposed for 20 or 60 min to a uniaxial cyclic tensile strain at a frequency of 1 Hz. (b) Analysis of directional protrusion activity over a time course of 300 min. Cell protrusions occurring perpendicular to the direction of uniaxial cyclic tensile strain were determined as “end”; cell protrusions parallel to the axis of uniaxial cyclic tensile strain were called “side”; the direction of cyclic tensile strain is indicated by a black double-headed arrow (see inset). (Student’s t-test; *P < 0.05; +P > 0.05)
Rac1 activity is involved in the regulation of cell membrane protrusions51 and it has been shown that uniaxial cyclic tensile strain affects spatially the protrusion activity in fibroblasts.34 Thus, we explored whether membrane protrusions were changed either perpendicular (“end”) or parallel (“side”) with respect to the direction of a uniaxial cyclic tensile strain. We analyzed the directional protrusion activity of ECs and SMCs under non-stretched control conditions and upon the application of a cyclic tensile strain of 1 Hz over a time course of 300 min. The protrusions were counted to yield in a frequency of membrane protrusions per hour. The protrusion activity of ECs and SMCs was, regardless of the application of a uniaxial cyclic tensile strain, not changed at the ends relative to the sides. However, the total membrane protrusive activity per hour was significantly increased for ends and sides for ECs upon application of cyclic tensile strain whereas no changes were observed for SMCs. Overall, SMCs formed more membrane protrusions over time than ECs (Figure 6b; Student’s t-test; *P < 0.05; +P > 0.05).
Together, the temporal RhoA and Rac1 activity and the total numbers of protrusion per hour were only changed in ECs upon application of a uniaxial cyclic tensile strain.
Discussion
SMCs form the vessel wall and they reveal a circumferential orientation along the arterial wall and are subjected to mechanical stimulation derived from the periodic strain of the artery.3 Thus, the behavior of vascular cells such as ECs and SMCs cultured on stretchable substrates which were periodically stretched and relaxed is of interest and was analyzed by various investigators.23,25,33,36,38,42,43,52,53 These studies have shown that vascular cells tend to align perpendicular to the direction of strain independently of their animal or tissue origin. This suggests that, irrespective of cellular species and tissue and irrespective of the usage of primary cells or non-vascular cell lines, mechanical stress-induced cellular alignment is a common behavior of adhesive cells in vitro, which may operate to induce cellular orientation in tissues in vivo.
In this study, human primary aortic ECs and SMCs were subjected to uniaxial cyclic tensile strain up to 300 min at different stretching frequencies but with constant linear amplitude. In contrast to other studies,32,39,41 we achieve in our experiments a very high temporal resolution (100 s) which allows us to study in detail the cellular kinetics upon application of uniaxial cyclic tensile strain. Additionally, we studied the behavior of primary human ECs and SMCs with the advantage that there is a direct relevance to translational aspects in humans. The potential disadvantage of donor-dependency of the cellular reactions could be minimized in our study, since at least three different donors for each cell type were used, all revealing similar cell type-specific but donor-independent cellular reaction.
Our work shows that non-confluent ECs reorient significantly quicker and more pronounced than non-confluent SMCs. We demonstrate that the dynamic orientation of ECs perpendicular to the strain direction depends on the stretch frequency. ECs react faster and stronger for 1 Hz stretching frequency than for 0.1 Hz. Thus, it can be stated that the reorientation reaction of ECs is faster with increased frequency of cyclic tensile strain. In the frequency regime used in our study, SMCs’ reorientation does not show distinct frequency dependence even though for a frequency of 1 Hz a slight, but not significant, reorientation response after 200 min was observed. Thus, the lower threshold frequency for SMC is speculated to be above 1 Hz. In contrast, Kanda et al. showed differences in the time scale of cell orientation responses of ECs, SMCs, and fibroblasts where SMC and fibroblast orientation advanced the reorientation of ECs (all from bovine arterial wall).36,43 Similarly to our study, Kanda et al. demonstrate that the level of realignment of cells increased with higher frequency of the applied cyclic tensile strain.36,43 Other studies showed that SMCs of the rat thoracic aorta and of the bovine pulmonary artery need up to 10 h (which was beyond the time scale of our study) to react to cyclic tensile strain of 0.5 Hz and higher and that the cell response was frequency-dependent.48,52 Overall, it seems that the cellular reorientation response with respect to an applied frequency is cell-type dependent. However, the perpendicular reorientation response of vascular cells of various origins itself seems to be independent of the cell density. Sparsely seeded cells (our study) and confluent cell layers reacted similarly—they both orient perpendicular to the direction—to the direction of applied cyclic tensile strain even though the time scales of their orientation response are different (confluent cells align more slowly).23,25,33,36,38,43,48,52–55 This is also confirmed by a study where the threshold frequency leading to a reorientation response was different for a rat embryonic fibroblasts cell line (0.01 Hz) and primary human dermal fibroblasts (0.1 Hz).46 However, it needs to be considered that in vivo SMCs exist in a three dimensionally (3D) matrix and are circumferentially organized56 which contributes to an alignment parallel to the direction of (vessel) stretch rather than the perpendicular alignment observed in our and other studies.36,43,48,52 Accordingly, the alignment of SMCs subjected to directional tension observed in vitro is in marked contrast with that observed in vivo. Thus, the detected perpendicular alignment of SMC in vitro might be an avoidance reaction in in vitro experiments due to the non-physiological experimental conditions in 2D culture. It also has to be recognized that SMCs dedifferentiate quickly in 2D culture and thus the cell passage number (“cell age”) should always be taken into account when performing in vitro experiments. In our experiments, the passage number of the primary ECs and SMCs was always below six, however in many other studies the passage number is not indicated.
The essential features of reorientation are the same for all ECs and SMCs independent of animal and tissue origin. However, the threshold frequency ft1 and the characteristic time of reorientation τ are significantly different for the presented data of the EC realignment. The reorientation of ECs at 0.1 Hz and 1 Hz of stretching frequency starts already after 30–50 min and reaches its maximum after about 60 min (at 0.1 Hz) and 120 min (at 1 Hz), whereas SMCs start to reorient after more than 200 min of applied strain (only for 1 Hz). Thus, it might be speculated that ECs have at least a 10 times lower frequency threshold (0.1 Hz) of reorientation than the SMCs (1 Hz) even though the threshold frequency of SMC realignment could not be determined as the behavior of SMCs to frequencies higher than 1 Hz has not been studied. One possible explanation of the different reaction time of ECs and SMCs could be the different structure or amount of the two cell types’ actin (stress) fiber system. Measurements of F- and G-actin content revealed that the cells differ in their cytoskeleton constitution.48,57 Alternatively, a similar approach was performed by Na et al.58 with SMCs of the arterioles of a rat muscle and Wu et al.59 with cardiomyocytes, vascular ECs, and SMCs. These authors could clarify the differences in initial cell mechanical properties (e.g. stiffening) and actin filament turnover at cyclic strain by atomic force microscopy (AFM) indention measurements.
Cell reorientation requires a continuous remodeling of focal adhesions and the actin cytoskeleton.34,58,60 The actin stress fibers need to be depolymerized and polymerized or turned in a new direction to establish a new equilibrium after an adaptive process.61 A continuous remodeling of focal adhesion sites is needed for cell migration, reorientation, and cell shape changes.34,62,63 However, our data show that focal adhesion shape is not influenced by uniaxial cyclic tensile strain. Nevertheless, our data reveal that the focal adhesion alignment in ECs is dependent on a threshold frequency of 0.1 Hz. Interestingly, also focal adhesion alignment in SMCs above a stretching frequency of 0.1 Hz is observed even though these cells do not show significant cell body realignment. However, this is in agreement with a previous study where it has been demonstrated for a fibroblast and an osteosarcoma cell line as well as for primary bovine artery ECs, that the reorientation of internal cell structures (actin stress fibers and focal adhesions) is more rapid than the whole cell body orientation and precedes cell body realignment.35,64 SMC actin stress fibers show an orientation after 300 min already for 0.1 Hz and do not increase their perpendicular alignment for 1 Hz, whereas the cell body of SMCs show only weak orientation after 300 min at 1 Hz. This is consistent with the study of Liu et al., who show cell body and actin stress fibers reorientation of rat thoracic aorta SMC after more than 10 h.48 The initiation of actin stress fiber orientation and cell body alignment of SMCs thus takes longer compared to ECs even though the temporal realignment response of focal adhesions is similar between ECs and SMCs. Thus, it might be speculated that SMCs are less sensitive to mechanical perturbations than ECs. Yamada and Ando analyzed pork thoracic aorta EC actin stress fibers for a frequency of 0.5 Hz and found a dependence on cell density and cell–cell contacts.65 Additionally, Lee et al. revealed for primary bovine artery ECs and an osteosarcoma cell line that actin stress fiber reorientation perpendicular to the direction of stretching at 1 Hz and that this process did not involve disassembly of the stress fiber distal ends located at focal adhesion sites.64 The authors also observed a threshold frequency (0.01 Hz) at which actin stress fiber disassembly and reorientation were not induced.64 These results indicate that the actin filamentous system is one critical point in the chain of cyclic strain-induced signal transduction of cells. Furthermore, Yano et al. showed that β1 integrin was organized in a pattern along the axis of elongated primary human umbilical vein ECs, creating a fusion of focal adhesions in ECs after 240 min of strain exposure66 indicating that focal adhesions increase in size upon exposure to strain. However, we observed in our studies for ECs a decrease in single focal adhesion area upon the application of cyclic stretching. In contrast, the focal adhesion size was unchanged for SMCs even though the reorientation response of focal adhesions in SMCs allows assuming that SMCs are able to detect and to react to mechanical perturbations. Finally, it needs to be considered that the distinct organization of the cytoskeleton and focal adhesions in ECs and SMCs at the initial time point impact on the realignment process and thus the reorientation response of pre-align, “synchronized” cells should be investigated.
Additionally, the small RhoGTPases RhoA and Rac1 are involved in cell polarization and cell migration and are key elements in controlling the actomyosin-focal adhesion system and cell protrusions.51 Different activities of RhoA and Rac1 in uniaxial cyclic tensile strain experiments have been reported and the results are conflicting: e.g. RhoA activity has been reported to both increase and remain unchanged in various vascular and non-vascular cell types;34,52,67–69 similarly, decreases as well as increases of Rac1 activity have been reported in cells of different origin.51,67,69 In our study, RhoA and Rac1 activities were increased only in ECs after 20 min of stretching while they were constant in SMCs. This coincides with the fact that ECs reorientation is stronger and quicker than SMCs, which show almost no reorientation response upon exposure to a uniaxial cyclic tensile strain after 60 min. However, cell-type specific differences of RhoGTPase activation upon stretching might be an explanation of the deviation of the results present in the literature. Furthermore, we observed that the number of membrane protrusions per hour is increased only for ECs upon application of a uniaxial cyclic tensile strain of 1 Hz. This rise in the number of total membrane protrusions might be initiated by the increased Rac1 activity observed in ECs after 20 min of stretching. We did not observe differences in the spatial arrangement of membrane protrusions (perpendicular or parallel to the stretch axis) for ECs and SMCs when subjected to a uniaxial cyclic tensile strain. At least for ECs, which show a pronounced cell reorientation response at 1 Hz. Different activities of protrusions perpendicular (“end”) vs. parallel (“sides”) to the stretch direction would have been expected, since a previous study showed that the membrane protrusive activity of a fibroblast cell line is increased perpendicular to the stretch direction.34 However, cell-type specific responses (fibroblast vs. ECs vs. SMCs) of protrusion activities upon stretching might eventually explain this observation which is also supported by the fact that the absolute number of membrane protrusions in highest in SMCs, medium in ECs and lowest in fibroblasts (this study and Goldyn et al.34). However, concerning SMCs, these cells show an initiation of a reorientation response at 1 Hz after 200 min and thus RhoA and Rac1 activity as well as membrane protrusion activity should be checked in future studies for later time points. In summary, an increased Rac1 and RhoA activity and a rise in the number of cell membrane protrusion seems to play an important role for the reorientation response of ECs upon application of uniaxial cyclic tensile strain. This leads to a hypothesis which would predict that a RhoA or Rac1 inhibitor, respectively, should block the alignment of ECs without any or low effect on the alignment of SMCs. Experiments testing this need to be performed in future and are pending. Additionally, further studies will include the investigation of protein concentration of known pathways for mechano-transduction in ECs and SMCs from the same donor, to understand cellular differences on a molecular level.3 Speculating about the mechanisms that are involved in the interaction between the two cell types, cyclic strain studies in co-culture of the two cell types seem to be promising.3,70 Smith et al. demonstrated e.g. that vascular SMCs exposed to physiological levels of strain increase their vascular endothelial growth factor (VEGF) secretion and may provide an arterial stimulus for maintenance of steady state levels of VEGF essential for EC survival.71
Conclusion
In summary, ECs vs. SMCs respond differently to a uniaxial cyclic tensile strain with respect to their alignment and the reorganization of their actin cytoskeleton and cell-matrix adhesion system. We detected a threshold stretch frequency for ECs that obviously differed compared to SMCs and this information can be used to differentially modulate cellular function in both cell types independently. However, it needs to be taken into account that the intima of a vessel and the media layer containing the SMCs cannot be subjected to different stretching forces simultaneously. The varying levels of activity of the small GTPases RhoA and Rac1 together with the varying changes in membrane protrusion activity, as a response to mechanical stimulation in ECs and SMCs, indicate the different cell-type specific activation of these both pathways upon application of stretching. Overall, the specific activation of different vascular cell types might be helpful in improving strategies in regenerative medicine. Generally, understanding in more detail the reactions of vascular cells to mechanical perturbations can be helpful elucidating physiological and pathological aspects such as vessel biology or atherosclerosis. This might then be helpful for medical applications such as the design of biomedical implants or tissue engineering.
Acknowledgments
We acknowledge Dr Simon Jungbauer for his help with the stretching setup and Dr Melih Kalafat for his help with the self-written ImageJ-Makro. AMG, CH, and RK thank the Baden-Wuerttemberg-Stiftung for financial support within the Kompetenznetz Funktionelle Nanostrukturen.
The authors declare that there is no conflict of interest.
Author contributions
All authors participated in the design, interpretation of the studies, and the review of the manuscript; AMG and SAB conducted the experiments and analyzed the data; CH analyzed the focal adhesion data; AMG, SAB, and RK wrote the manuscript.
References
- 1.Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng 2004; 6: 275–302. [DOI] [PubMed] [Google Scholar]
- 2.Kontulainen SA, Hughes JM, Macdonald HM, Johnston JD. The biomechanical basis of bone strength development during growth. Med Sport Sci 2007; 51: 13–32. [DOI] [PubMed] [Google Scholar]
- 3.Anwar MA, Shalhoub J, Lim CS, Gohel MS, Davies AH. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res 2012; 49: 463–78. [DOI] [PubMed] [Google Scholar]
- 4.Chen LJ, Wei SY, Chiu JJ. Mechanical regulation of epigenetics in vascular biology and pathobiology. J Cell Mol Med 2013; 17: 437–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 1995; 75: 519–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla A, Dunn AR, Moses MA, Van Vliet KJ. Endothelial cells as mechanical transducers: enzymatic activity and network formation under cyclic strain. Mech Chem Biosyst 2004; 1: 279–90. [PubMed] [Google Scholar]
- 7.Stamenovic D, Wang N. Invited review: engineering approaches to cytoskeletal mechanics. J Appl Physiol 2000; 89: 2085–90. [DOI] [PubMed] [Google Scholar]
- 8.Yoshigi M, Clark EB, Yost HJ. Quantification of stretch-induced cytoskeletal remodeling in vascular endothelial cells by image processing. Cytometry A 2003; 55: 109–18. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty JT, Pierce JE, Ferrans VJ, Patel DJ, Tucker WK, Fry DL. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ Res 197; 230, 23–33. [DOI] [PubMed]
- 10.Benbrahim A, L'Italien GJ, Kwolek CJ, Petersen MJ, Milinazzo B, Gertler JP, Abbott WM, Orkin RW. Characteristics of vascular wall cells subjected to dynamic cyclic strain and fluid shear conditions in vitro. J Surg Res 1996; 65: 119–27. [DOI] [PubMed] [Google Scholar]
- 11.Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, Wofovitz E. Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol 2003; 81: 177–99. [DOI] [PubMed] [Google Scholar]
- 12.Sotoudeh M, Jalali S, Usami S, Shyy JY, Chien S. A strain device imposing dynamic and uniform equi-biaxial strain to cultured cells. Ann Biomed Eng 1998; 26: 181–9. [DOI] [PubMed] [Google Scholar]
- 13.Takemasa T, Sugimoto K, Yamashita K. Amplitude-dependent stress fiber reorientation in early response to cyclic strain. Exp Cell Res 1997; 230: 407–10. [DOI] [PubMed] [Google Scholar]
- 14.Lee T, Sumpio BE. Cell signalling in vascular cells exposed to cyclic strain: the emerging role of protein phosphatases. Biotechnol Appl Biochem 2004; 39: 129–39. [DOI] [PubMed] [Google Scholar]
- 15.Finlay HM, Whittaker P, Canham PB. Collagen organization in the branching region of human brain arteries. Stroke 1998; 29: 1595–601. [DOI] [PubMed] [Google Scholar]
- 16.Standley PR, Cammarata A, Nolan BP, Purgason CT, Stanley MA. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am J Physiol Heart Circ Physiol 2002; 283: H1907–14. [DOI] [PubMed] [Google Scholar]
- 17.Tranquillo RT, Girton TS, Bromberek BA, Triebes TG, Mooradian DL. Magnetically orientated tissue-equivalent tubes: application to a circumferentially orientated media-equivalent. Biomaterials 1996; 17: 349–57. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Sengupta D, Chien S. Vascular tissue engineering: from in vitro to in situ. Wiley Interdiscip Rev Syst Biol Med 2014; 6: 61–76. [DOI] [PubMed] [Google Scholar]
- 19.Qiu J, Zheng Y, Hu J, Liao D, Gregersen H, Deng X, Fan Y, Wang G. Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. J R Soc Interface 2014; 11: 20130852–20130852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol 2005; 98: 2321–7. [DOI] [PubMed] [Google Scholar]
- 21.Manolopoulos VG, Lelkes PI. Cyclic strain and forskolin differentially induce cAMP production in phenotypically diverse endothelial cells. Biochem Biophys Res Commun 1993; 191: 1379–85. [DOI] [PubMed] [Google Scholar]
- 22.Moore MM, Goldman J, Patel AR, Chien S, Liu SQ. Role of tensile stress and strain in the induction of cell death in experimental vein grafts. J Biomech 2001; 34: 289–97. [DOI] [PubMed] [Google Scholar]
- 23.Shoajei S, Tafazzoli-Shahdpour M, Shokrgozar MA, Haghighipour N. Alteration of human umbilical vein endothelial cell gene expression in different biomechanical environments. Cell Biol Int 2014; 38: 577–81. [DOI] [PubMed] [Google Scholar]
- 24.Silverman MD, Waters CR, Hayman GT, Wigboldus J, Samet MM, Lelkes PI. Tissue factor activity is increased in human endothelial cells cultured under elevated static pressure. Am J Physiol 1999; 277: C233–42. [DOI] [PubMed] [Google Scholar]
- 25.Sipkema P, van der Linden PJ, Westerhof N, Yin FC. Effect of cyclic axial stretch of rat arteries on endothelial cytoskeletal morphology and vascular reactivity. J Biomech 2003; 36: 653–9. [DOI] [PubMed] [Google Scholar]
- 26.Von Offenberg Sweeney N, Cummins PM, Cotter EJ, Fitzpatrick PA, Birney YA, Redmond EM, Cahill PA Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochem Biophys Res Commun 2005; 329: 573–82. [DOI] [PubMed] [Google Scholar]
- 27.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310: 1139–43. [DOI] [PubMed] [Google Scholar]
- 28.Huang H, Kamm RD, Lee RT. Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am J Physiol Cell Physiol 2004; 287: C1–11. [DOI] [PubMed] [Google Scholar]
- 29.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Annu Rev Biomed Eng 2007; 9: 1–34. [DOI] [PubMed] [Google Scholar]
- 30.Banes AJ, Gilbert J, Taylor D, Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J Cell Sci 1985; 75: 35–42. [DOI] [PubMed] [Google Scholar]
- 31.Lee AA, Delhaas T, Waldman LK, MacKenna DA, Villarreal FJ, McCulloch AD. An equibiaxial strain system for cultured cells. Am J Physiol 1996; 271: C1400–8. [DOI] [PubMed] [Google Scholar]
- 32.Neidlinger-Wilke C, Wilke HJ, Claes L. Cyclic stretching of human osteoblasts affects proliferation and metabolism: a new experimental method and its application. J Orthop Res 1994; 12: 70–8. [DOI] [PubMed] [Google Scholar]
- 33.Dartsch PC, Hammerle H, Betz E. Orientation of cultured arterial smooth muscle cells growing on cyclically stretched substrates. Acta Anat 1986; 125: 108–13. [DOI] [PubMed] [Google Scholar]
- 34.Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J Cell Sci 2009; 122: 3644–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldyn AM, Kaiser P, Spatz JP, Ballestrem C, Kemkemer R. The kinetics of force-induced cell reorganization depend on microtubules and actin. Cytoskeleton 2010; 67: 241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanda K, Matsuda T. Behavior of arterial wall cells cultured on periodically stretched substrates. Cell Transplant 1993; 2: 475–84. [DOI] [PubMed] [Google Scholar]
- 37.Kemkemer R, Neidlinger-Wilke C, Claes L, Gruler H. Cell orientation induced by extracellular signals. Cell Biochem Biophys 1999; 30: 167–92. [DOI] [PubMed] [Google Scholar]
- 38.Moretti M, Prina-Mello A, Reid AJ, Barron V, Prendergast PJ. Endothelial cell alignment on cyclically-stretched silicone surfaces. J Mater Sci Mater Med 2004; 15: 1159–64. [DOI] [PubMed] [Google Scholar]
- 39.Neidlinger-Wilke C, Grood E, Claes L, Brand R. Fibroblast orientation to stretch begins within three hours. J Orthop Res 2002; 20: 953–6. [DOI] [PubMed] [Google Scholar]
- 40.Wang JH, Goldschmidt-Clermont P, Wille J, Yin FC. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech 2001; 34: 1563–72. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Ip W, Boissy R, Grood ES. Cell orientation response to cyclically deformed substrates: experimental validation of a cell model. J Biomech 1995; 28: 1543–52. [DOI] [PubMed] [Google Scholar]
- 42.Iba T, Sumpio BE. Morphological response of human endothelial cells subjected to cyclic strain in vitro. Microvasc Res 1991; 42: 245–54. [DOI] [PubMed] [Google Scholar]
- 43.Kanda K, Matsuda T, Oka T. Two-dimensional orientational response of smooth muscle cells to cyclic stretching. ASAIO J 1992; 38: M382–5. [DOI] [PubMed] [Google Scholar]
- 44.Zhao S, Suciu A, Ziegler T, Moore JE, Jr, Bürki E, Meister JJ, Brunner HR. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler Thromb Vasc Biol 1995; 15: 1781–6. [DOI] [PubMed] [Google Scholar]
- 45.Wang JH, Goldschmidt-Clermont P, Moldovan N, Yin FC. Leukotrienes and tyrosine phosphorylation mediate stretching-induced actin cytoskeletal remodeling in endothelial cells. Cell Motil Cytoskeleton 2000; 46: 137–45. [DOI] [PubMed] [Google Scholar]
- 46.Jungbauer S, Gao H, Spatz JP, Kemkemer R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys J 2008; 95: 3470–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol 2006; 85: 165–73. [DOI] [PubMed] [Google Scholar]
- 48.Liu B, Qu MJ, Qin KR, Li H, Li ZK, Shen BR, Jiang ZL. Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro. Biophys J 2008; 94: 1497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De R, Zemel A, Safran SA. Dynamics of cell orientation. Nature Phys 2007; 3: 655–9. [Google Scholar]
- 50.Kemkemer R, Kling D, Kaufmann D, Gruler H. Elastic properties of nematoid arrangements formed by amoeboid cells. Eur Phys J 2000; 1: 215–25. [Google Scholar]
- 51.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420: 629–35. [DOI] [PubMed] [Google Scholar]
- 52.Liu WF, Nelson CM, Tan JL, Chen CS. Cadherins, RhoA, and Rac1 are differentially required for stretch-mediated proliferation in endothelial versus smooth muscle cells. Circ Res 2007; 101: e44–52. [DOI] [PubMed] [Google Scholar]
- 53.Moore JE, Jr, Bürki E, Suciu A, Zhao S, Burnier M, Brunner HR, Meister JJ A device for subjecting vascular endothelial cells to both fluid shear stress and circumferential cyclic stretch. Ann Biomed Eng 1994; 22: 416–22. [DOI] [PubMed] [Google Scholar]
- 54.Kaunas R, Usami S, Chien S. Regulation of stretch-induced JNK activation by stress fiber orientation. Cell Signal 2006; 18: 1924–31. [DOI] [PubMed] [Google Scholar]
- 55.Hsu HJ, Lee CF, Kaunas R. A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PloS one 2009; 4: e4853–e4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhein SMFW, Delmar M (eds) Practical methods in cardiovascular research. Heidelberg, NY: Springer, 2005.
- 57.Zahn JT, Louban I, Jungbauer S, Bissinger M, Kaufmann D, Kemkemer R, Spatz JP. Age-dependent changes in microscale stiffness and mechanoresponses of cells. Small 2011; 7: 1480–7. [DOI] [PubMed] [Google Scholar]
- 58.Na S, Meininger GA, Humphrey JD. A theoretical model for F-actin remodeling in vascular smooth muscle cells subjected to cyclic stretch. J Theor Biol 2007; 246: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu X, Sun Z, Meininger GA, Muthuchamy M. Application of atomic force microscopy measurements on cardiovascular cells. Methods Mol Biol (Clifton, NJ) 2012; 843: 229–44. [DOI] [PubMed] [Google Scholar]
- 60.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc Natl Acad Sci U S A 2005; 102: 15895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deibler M, Spatz JP, Kemkemer R. Actin fusion proteins alter the dynamics of mechanically induced cytoskeleton rearrangement. PloS one 2011; 6: e22941–e22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol 2010; 26: 315–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wehrle-Haller B. Structure and function of focal adhesions. Curr Opin Cell Biol 2012; 24: 116–24. [DOI] [PubMed] [Google Scholar]
- 64.Lee CF, Haase C, Deguchi S, Kaunas R. Cyclic stretch-induced stress fiber dynamics - dependence on strain rate, Rho-kinase and MLCK. Biochem Biophys Res Commun 2010; 401: 344–9. [DOI] [PubMed] [Google Scholar]
- 65.Yamada H, Ando H. Orientation of apical and basal actin stress fibers in isolated and subconfluent endothelial cells as an early response to cyclic stretching. Mol Cell Biomech 2007; 4: 1–12. [PubMed] [Google Scholar]
- 66.Yano Y, Geibel J, Sumpio BE. Cyclic strain induces reorganization of integrin alpha 5 beta 1 and alpha 2 beta 1 in human umbilical vein endothelial cells. J Cell Biochem 1997; 64: 505–13. [DOI] [PubMed] [Google Scholar]
- 67.Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA Effects of cell tension on the small GTPase Rac. J Cell Biol 2002; 158: 153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith PG, Roy C, Zhang YN, Chauduri S. Mechanical stress increases RhoA activation in airway smooth muscle cells. Am J Respir Cell Mol Biol 2003; 28: 436–42. [DOI] [PubMed] [Google Scholar]
- 69.Yamane M, Matsuda T, Ito T, Fujio Y, Takahashi K, Azuma J Rac1 activity is required for cardiac myocyte alignment in response to mechanical stress. Biochem Biophys Res Commun 2007; 353: 1023–7. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y, Rayatpisheh S, Chew SY, Chan-Park MB. Impact of endothelial cells on 3D cultured smooth muscle cells in a biomimetic hydrogel. ACS Appl Mater Interfaces 2012; 4: 1378–87. [DOI] [PubMed] [Google Scholar]
- 71.Smith JD, Davies N, Willis AI, Sumpio BE, Zilla P. Cyclic stretch induces the expression of vascular endothelial growth factor in vascular smooth muscle cells. Endothelium 2001; 8: 41–8. [DOI] [PubMed] [Google Scholar]