Abstract
Induced pluripotent stem cells (iPSCs) hold great promise for basic research and regenerative medicine. They offer the same advantages as embryonic stem cells (ESCs) and moreover new perspectives for personalized medicine. iPSCs can be generated from adult somatic tissues by over-expression of a few defined transcription factors, including Oct4, Sox2, Klf4, and c-myc. For regenerative medicine in particular, the technology provides great hope for patients with incurable diseases or potentially fatal disorders such as heart failure. The endogenous regenerative potentials of adult hearts are extremely limited and insufficient to compensate for myocardial loss occurring after myocardial infarction. Recent discoveries have demonstrated that iPSCs have the potential to significantly advance future cardiovascular regenerative therapies. Moreover, iPSCs can be generated from somatic cells of patients with genetic basis for their disease. This human iPSC derivates offer tremendous potential for new disease models. This paper reviews current applications of iPSCs in cardiovascular regenerative medicine and discusses progress in modeling cardiovascular diseases using iPSCs-derived cardiac cells.
Keywords: Induced pluripotent stem cells, regenerative medicine, cardiovascular disease, diseases modeling
Introduction
In 2006, Yamanaka’s group generated induced pluripotent stem cells (iPSCs) by reprogramming of mouse embryonic fibroblasts. These cells closely resemble embryonic stem cells (ESCs) in terms of pluripotency and epigenetic status.1 Reprogramming evolves the over-expression in a somatic cell of a set of core pluripotency-related transcription factors (in most cases Oct4, Sox2, Klf4).2 In 2007, Dr Yamanaka showed that the same four genes, when over-expressed in human fibroblasts, could also generate iPSCs. Independently successful reprogramming reported Dr Thomson based on different combination of four factors (Oct4, Sox2, Nanog, and Lin28).3 These iPSCs are relevant to a range of applications, including: autologus cell therapy; the modeling of monogenic and multigenic diseases; the study of complex genetic features and allelic variation; and as a substrate for drug, toxicity, differentiation, and therapeutic screening.4
Heart failure increases mortality risk and decreases quality of life. Optimized medical therapies have drastically elongated life expectancy in patients, however the only radical therapy and possible cure for heart failure is cardiac transplantation. Organ transplantations, including cardiac transplantation, demand immunosuppressive therapies for a patient’s whole life that might cause life-threatening problems such as malignancy and infection.5,6 Cell therapy for cardiac regeneration has evolved considerably during the last decade from skeletal myoblasts in the early stages to the use of mesenchymal and cardiac progenitor cells (CPCs) and ESCs.7–9 In comparison, ESCs have many advantages due to the highly proliferating and cardiomyogenic potentials, however the further application of ESCs in cardiac tissue engineering is still hampered due to the ethical problem and immune response. However, Chong et al.10 reported the intra-myocardial delivery of human embryonic-stem-cell-derived cardiomyocytes into primate model of myocardial ischemia to promote regeneration. They showed extensive re-muscularization of the infarcted heart and progressive but incomplete maturation over a three-month period. Other recent preclinical and clinical studies indicate that some common sources of stem cells (hematopoietic stem cells, endothelial progenitor cells, cardiac stem cells, mesenchymal stem cells, or adipose stem cells) may reduce infarct size and improve cardiac contractile function in patients with myocardial infarction. Nevertheless, the beneficial effects are poor in humans and thought to be due largely to paracrine effects of secreted factors from adult stem cells rather than the incorporation of the cells into the affected tissue.11 Under suitable conditions, iPSCs could long-term propagate in undifferentiated state or differentiate into many other cell types, including cardiomyocytes.12 Technologies involving iPSCs could potentially eliminate the need for immunosuppression because differentiated cells for transplantation would ideally be differentiated into specific cell types as need from patient’s own tissue. This scenario present the perfect therapy to restore lost organ function, however there are still several steps that need to be optimized to realize successful clinical application.5
The main goal of this paper is to review current applications of iPSCs in cardiovascular regenerative medicine and discuss progress in modeling cardiovascular diseases (CVDs) using iPSCs-derived cardiac cells.
Reprogramming methods
Several methods, which involve the transfer of genes into the target cells, have been employed to reprogram terminally differentiated somatic cells into iPSCs. They can be divided into integrating and non-integrating methods.13,14
Integrative delivery system
Most of the iPSC lines established to date have been generated using integrating retroviral and lentiviral vectors to deliver reprogramming factors.15 The first sets on iPSCs were generated through infection of fibroblasts with four separate retroviral vectors. While retroviral vectors are most commonly used because of ease of handling, retroviral reprogramming has some shortcomings. It is relatively inefficient and generates iPSCs with multiple viral transgenes that randomly integrate in the genome, thus increasing the risk of insertional mutagenesis. In addition, epigenetic silencing of the retroviral transgenes is sometimes incomplete giving rise to partially reprogrammed cells.16 Residual transgene expression might suppress the differentiation of iPSCs, resulting in a higher tumorigenic potential when these cells are transplanted into patients.15,17 Tumors result either from basal expression levels of the c-myc transgenes or other oncogene-related factors or tissue-specific reactivation of these transgenes owing the promoter- or enhancer-trapping events.4,18 For these reasons many efforts have been made to identify safer strategies. Emphasis has been placed on the use of peptides and recombinant proteins or non-integrating vectors.19
Other strategies to generate transgene-free iPSCs involve transient expression of reprogramming factors followed by faithful removal of transgenes. One such method makes use of the Cre/loxP excision technology.20 A different excision approach uses piggyBac (PB) transposons. The PB system is usually composed of a donor plasmid containing the transposon, co-transfected with a helper plasmid expressing the transposase. PB iPSCs could represent another source of xeno-free cell in clinical application.4
Integrative-free delivery system
Recently, several approaches have been developed to generate safer transgene-free or integration-free iPSCs. These methods completely eliminate the use of integrating retroviral vectors. Adenoviral vectors do not insert into the genome and are diluted out as the cells divide, preventing genomic modification of reprogrammed cells, however they suffer from a low efficiency.21,18
Virus-free reprogramming can be achieved by plasmid transfection, episomal vectors or minicircles, protein delivery, transfection of synthetic modified mRNAs, or the bacteriophage φC31 integrase, which reduce the risk of genomic integration.17,20
However, reprogramming remains largely an inefficient and non-specific process, with efficiencies of transduced cells becoming fully reprogrammed iPSCs lower than 0.01%. Moreover, safety concerns still represent major challenges for clinical translation. An improved understanding of the complex regulation of stem cells through cell-intrinsic and -extrinsic signals is essential to rationally suggest appropriate conditions for controlling stem cell fate, state, and function. Small molecules provide an attractive approach because they offer a number of unique advantages. Their biological effects are typically rapid, reversible, and dose-dependent, allowing precise control over specific outcomes by fine-tuning their concentrations and combinations. Moreover, the structural diversity that can be provided by synthetic chemistry allows the functional optimization of small molecules and compared with genetic interventions, their relative ease of the handling and administration make them more practical for in vitro and in vivo applications, and for further therapeutic development.22,23 To these small molecules belong inhibitors of histone deacetylases (HDACs) – valproic acid, suberoylanilide hydroxamic acid, trichostatin A, butyrate; histone demethylases (HDMs) – tranylcypromine; lysine, and arginine methyltransferases (HMTs) – BIX-01294, SUV39h1, DOT1L, AMI-5; DNA methyltransferases (DNMTs) – 5-azacytidine, RG108, and MEK (mitogen activated protein kinase) and GSK3 (glycogen synthase kinase-3) – PD0325901 and CHIR99021. They regulate chromatin remodeling and act as major players in building up the epigenetic landscape.1,24 Their major disadvantages are that they may have more than one target, their unexpected toxicity, or other side effects in vivo.22 Until last year the main goal of researchers was to find chemical-only cocktails to reprogram somatic cells without use of any reprogramming factor. This success was achieved in 2013 by Hou et al., who demonstrated that chemically iPSCs could be generated from mouse somatic cells using a combination of seven small molecule compounds. This strategy has great potential in generating functional suitable cell types for clinical applications.25
Cell sources for generation of iPSCs
Up to now, iPSCs have been generated from plenty of cell types. Fibroblasts and keratinocytes are easy to obtain and might be suitable source for future large-scale screening based on iPSCs technology. Fibroblasts are the mostly used somatic cells, but they are suboptimal for large-scale derivation because local anesthesia, an incision, and suturing are needed to obtain a skin biopsy.17,26 The goal is to find most successful, efficient and safer cells to reprogram.27 For example, reprogramming of juvenile human primary keratinocytes by retroviral transduction was shown to be 100-fold more efficient and two-fold faster compared with human fibroblast.28 An attractive source of cells is also cord blood. Immature cells, abundant in cord blood, may be suitable source because of their availability and moreover because they may have acquired fewer genetic mutations than adult-derived cells.26 Hematopoietic stem and progenitor cells give rise to iPSCs up to 300 times more efficiently than terminally differentiated B and T cell, yielding reprogramming efficiencies of up 28%.13 Human adipose tissue is also rich source of multipotent stem cells, which can be obtained by liposuction. These human adipose stem cells can differentiate into adipogenic, osteogenic, chondrogenic, and myogenic cell lineages and can be reprogrammed into iPSCs with 20-fold greater efficiency and two-fold faster as compared to adult fibroblast reprogramming.29 In the case of cardiomyocytes, recent study reports that CPC-iPSCs demonstrate a greater efficiency of differentiation into cardiomyocytes as compared with fibroblast progenitor cell-iPSCs at early passage.30 Moreover, iPS-derived fetal liver kinase-1 (Flk-1)+ progenitor cells have been produced in vitro and they were shown to improve cardiac function in vivo in mouse model of acute myocardial infarction. Also, a population of iPS cell-derived progenitor cells enriched for the LIM-homeobox transcription factor islet-1 (Isl1) was shown to survive after transplantation into infracted hearts and differentiated into cardiomyocytes, endothelial cells, and smooth muscle cells.31 Critical issue is the need to generate chamber-specific cardiomyocytes. Pacemaker, atrial, and ventricular myocytes have distinct functional and electrophysiological properties that may contribute to cardiac arrhythmias in the wrong environment. Methods for purifying chamber-specific cardiomyocytes from a population of stem-cell-derived cardiomyocytes remain to be established.32
Cardiomyocytes differentiation and purification
iPSC colonies can be differentiated into functional cardiomyocytes using a variety of methods, which are very similar to those traditionally employed to produce cardiomyocytes from human ESCs since both iPSCs and human ESCs share very similar characteristics and differentiation potential.13 There are several protocols, which are available for cardiac differentiation from human pluripotent stem cells (hPSCs): the embryoid body (EB) culture system, the monolayer culture system, and the inductive co-culture system.5,33
The differentiation of iPSCs into cardiomyocytes in vitro was firstly reported in murine iPSCs lines in 2008.34 The resulted cardiac cells showed typical features of ESC-derived cardiomyocytes, including spontaneous rhythmical beating, expression of cardiac genes, including Nkx2.5, cTnT (troponin T type 2), αMHC, α-actin, myosin light chain 2 atrial isoform, myosin light chain 2 ventricular isoform, ANF, and phospholamban, expression of cardiomyocyte-typical proteins, spontaneous rhythmic intracellular Ca2+ fluctuation and presence of β-adrenergic and muscarinic signaling cascade. Moreover, iPSC-derived cardiomyocytes contained atrial- and ventricular-like cells and responded to β-adrenergic signaling, a canonical CM signaling pathway. However, iPSCs showed a delayed and less efficient differentiation of beating EBs compared with ESCs.12,35 Cardiac differentiation of human iPSCs was firstly reported in 2009. The study showed that both human iPSCs and ESCs have similar capacity for differentiation into nodal-, atrial-, and ventricular-like phenotypes. Cardiomyocytes derived from human iPSCs and ESCs share similar cardiac genes expression patterns, proliferation, and sarcomeric organizations.36
In vitro differentiation of stem cells to cardiomyocytes mimics the sequential stages of embryonic cardiac development. Three families of protein growth factors are thought to control these early stages of mesoderm formation and cardiogenesis: bone morphogenic proteins (BMPs), which are members of the transforming growth factor β superfamily; the Wingless/INT proteins (WNTs); and the fibroblast growth factors (FGFs). These factors, or their inhibitors, are expressed in the endoderm.33,37 At first, human PSCs should be developed into endomesodermal lineage cells that express T-box factor Brachyury (T) and several factors form mesodermal differentiation. After the differentiation into early CPCs, Mesp-1-positive cells and small chemical Wnt inhibitors (IWR, IWP, XAV) are also useful for the next stage of development. The first widely-used method for developing PSCs-derived cardiomyocytes involved co-culturing human ESCs with the mouse endodermal cell line END-2, which stimulates differentiation toward a cardiomyocyte-like phenotype. However, this technique proved relatively inefficient and remains infrequently used in practice. Most commonly are cardiomyocytes generated by either EBs.38 Pluripotent stem cells are cultured in suspension for about eight days in differentiation medium, which induces EB formation and then EBs are further cultured on gelatin-coated dishes for another 8–10 days. The EBs contain cell types derived from mesoderm, ectoderm, and endoderm. Within these mixed population, contracting cardiomyocytes can be detected.17 However, due to challenges in low differentiation efficiency and purity of derived cardiomyocytes using EB system, techniques have been developed to enhance their differentiation process, namely, directing the differentiation of cardiomyocytes using various factors on monolayer cultures. Combination of growth factors such as activin A, bone morphogenic protein 4 (Bmp4), FGF 2, wingless-type mouse mammary tumor virus integration site family members 3A, and vascular endothelial growth factor (VEGF) have been shown to induce cardiomyocyte differentiation with increased efficiencies.20 Transit inhibition of BMP signaling very early during differentiation is crucial for cardiogenesis in murine ES cells. Egashira's group suggests that administration of noggin, a BMP antagonist, before the EB formation stage would mimic its transient and strong expression during early gastrulation. Indeed, noggin administration around EB formation day led to a marked increase in cardiogenesis from murine ES cells. Other BMP antagonists also act to increase cardiomyocyte differentiation efficiency, indicating that transient relief from the intrinsic BMP signal is critical for cardiomyocyte induction. Growth factor granulocyte colony-stimulating factor (G-CSF) is critical for cardiomyocyte proliferation and could be used to boost the yield of cardiomyocytes from iPSCs for their potential application in regenerative medicine.37 Growth factors, however, can be expensive and they can degrade quickly. More recently, small molecule-based approaches are indicating to be highly efficient in cardiomyocyte generation from iPSCs.20 For example, Lian et al. demonstrated an efficient and robust generation of cardiomyocytes from multiple hPSC lines solely via small molecule modulation of regulatory elements of Wnt/β-catenin signaling. Investigators suggest that β-catenin is essential for cardiogenesis upon hPSC treatment with activin A and BMP4. They showed that small molecules are sufficient to convert hPSCs to cardiomyocytes efficiently when applied at the appropriate developmental stages.39
Many other inducers used in cardiac differentiation of ESCs were also confirmed effective to induce cardiac differentiation from iPSCs, including 5-azacytidine, ascorbic acid, cyclosporine-A, sulfonyl hydrazones, DMSO, isoxazolyl-serines and so on. The use of small molecules instead of growth factors ultimately could allow inexpensive and reproducible generation of human cardiomyocytes or multipotent tissue-specific stem cells in completely chemically defined conditions, facilitating translation of these cells to high through put screening applications or regenerative therapies.17,29
Recently it was reported that chromatin modification also has a great impact on cardiac differentiation, and it might be necessary to have a deep knowledge of epigenetic mechanisms to achieve better cardiomyocytes differentiation protocols.5
Establishment of an excellent purification system for iPSC-derived cardiomyocytes is necessary for clinical application. Micro-dissection and Percoll density centrifugation have been widely used, but these methods are labor intensive and still do not provide high purity. Transgenic selection techniques have been employed for cardiomyocytes enrichment by insertion of a reporter gene and antibiotic resistant gene under the transcriptional control of a cardiac specific promoter. However, the genetic modification may provide a hurdle for further clinical applications.40 The identification of markers expressed specifically on cardiomyocytes, including EMILIN2, SIRPA, and VCAM has made it possible to isolate highly enriched population of these cells from pluripotent stem cells by fluorescent activated cell sorting (FACS) or magnetic beat sorting. FACS is the most prominent method reported for selecting specific cell types, although it requires antibodies, a long processing time, and can process only small amounts of cells at one time. However, human heart is a large organ, thus current protocols still need further optimization to control in vitro maturation as well towards the desired subtype.5,41
Applications of iPSC-derived cardiomyocytes
Cardiac repair
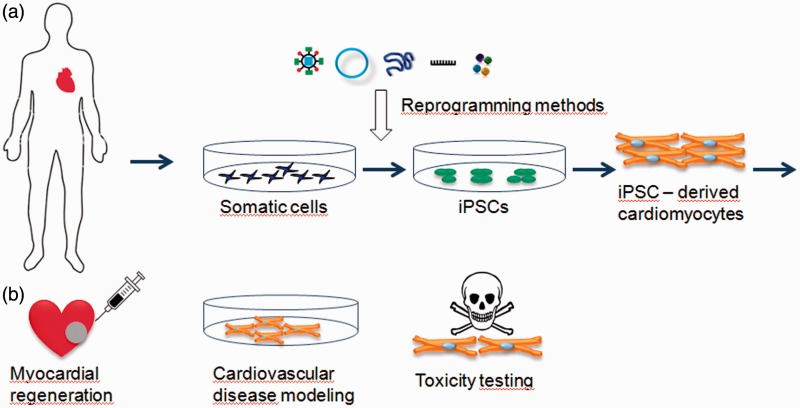
Human iPSCs have the potential to revolutionize regenerative medicine, human disease modeling, as well as drug safety and discovery studies (Figure 1). Since human iPSCs can differentiate into any cell type and the supply is virtually unlimited, they are an ideal source of cells for transplantation to treat end stage organ failure.42
Figure 1.
Schematic picture of iPSCs generation and their potential application. (a) Generation of patient-specific iPSCs and differentiation into cardiomyocytes; (b) potential applications of iPSC-derived cardiomyocytes. (A color version of this figure is available in the online journal.)
CVD is a leading cause of mortality and morbidity and there are few if any options, to reverse or repair damage after a myocardial infarction. Adult human cardiomyocytes have a very limited proliferation capacity that is not sufficient to repair larger areas of damage heart. To prevent scar formation caused by death of cardiomyocytes, it would be highly desirable to either induce resident progenitor cells to differentiate into cardiomyocytes or to replace dead cardiomyocytes with stem cell-derived cardiomyocytes. Once derived, human iPSCs can serve as an inexhaustible source of more differentiated cardiovascular cells, including the cardiomyocyte, endothelial cell, and smooth muscle lineages, therefore the advent of human iPSCs has been tremendously exciting for the field of cardiovascular regeneration.17,43
To date, a wide variety of different stem cell types have been investigated for their ability to repair infarcted heart in both animal studies44,45 and human clinical trials.46,47 Recent studies have begun to focus on the application of iPSCs in rodent models of cardiac injury. Proof-of-concept experiments with murine iPSC – cardiomyocytes have shown the ability of these cells to survive and form nascent myocardium.48 Nelson et al. showed that intra-myocardial delivery of iPSCs into the infracted hearts of mice following ligation of the left anterior descending artery yielded progeny that properly engrafted without disrupting cytoarchitecture.49 Mauritz et al. showed that injection of iPSCs-derived Flk1-positive progenitor cells into the ischemic myocardium of left anterior descending artery-ligated mice improved cardiac function following myocardial infarction.50 Zhang et al. have derived cardiomyocytes from the EBs of human iPSC lentivirally transduced with Oct4, Sox2, Nanog, and Lin28. These cells were comparable to cardiomyocytes derived from the EBs of human ESCs. Human iPSCs proliferated robustly, generated atrial, nodal, and ventricular action potentials, and responded to electrical and chemical stimulation of the β-adrenergic signaling pathway. There were quite fewer human iPSCs-cardiomyocytes that exhibited contractile ability than human ESCs, and ineffective silencing of Oct4 and Nanog transgenes was also present.36 Tulloch et al. reported three-dimensional (3D) human cardiac tissue patches, which were generated by combining collagen type 1 and human ESC- and hiPSC-derived cardiomyocytes. Cardiomyocytes showed alignment and proliferation within the collagen 3D matrix when subjected to mechanical stress.51 Kawamoto et al. successfully differentiated cardiovascular blast populations (CBPs) to form vascular networks both in vivo and in the ex vivo organ culture systems by recapitulating the process of vasculogenesis from human iPSCs.52
The preceding experience in rodent infarct models set the stage for transplantation studies with human iPSC-cardiomyocytes in more relevant large animal preclinical models.48 Kawamura's group incorporated human iPSCs-cardiomyocytes into bioengineered sheets that were applied to the epicardial surface of infracted pig hearts. At eight weeks post-transplantation, the recipient hearts had very few surviving graft cells, but they showed better preserved ejection and left ventricular dimensions than untreated controls. Interestingly, the therapeutic benefit of hiPSC-derived cardiomyocytes sheets in porcine model of myocardial infarction was more recently reported by same researchers. They developed another strategy for improved engraftment and survival using human iPSCs-cardiomyocytes sheets by transplanting the cell sheets with an omentum flap, resulting in significantly improved cardiac function and attenuated left ventricular remodeling following ischemic damage. Importantly, few surviving cells were found when human iPSC-derived cardiomyocytes grafts were monitored eight weeks after transplantation, and no teratoma formation was observed.53,54 Templin et al. injected human iPSCs (hiPSCs) in pig models of myocardial infarction and found that hiPSCS could be detected for up to 15 weeks in pig myocardium. More importantly, they observed hiPSC-derived endothelial cells contributed to vascularization of infracted myocardium.55
Even though this progress in animal models gives reason for hope, a number of major hurdles must still be overcome. Enthusiasm for use of iPSC technology in regenerative medicine has been hampered by the increasingly recognized problems of the unpredictable nature and behavior of transplanted iPSCs and the potential for these cells to cause harm.13,48 For cardiovascular regeneration, more robust selection markers and refined experimental protocols are required to reproducibly guide iPSCs to cardiovascular lineage. Moreover, effective negative selection against pluripotent cells is necessary to avoid teratoma formation by contaminating pluripotent stem cells.11 Of a great concern is also that gene expression pattern of hiPSC–cardiomyocytes more closely resemble fetal cardiac tissue than adult cells and that they act mechanically and electro-physiologically like immature cardiomyocytes.56 Developing new techniques to make hiPSC-CMs more “mature” will be critical for the technology to reach its full potential.38
CVD modeling
In addition to their regenerative capacity, iPSCs constitute an important tool for modeling CVD, allowing to study the molecular mechanisms involved in cardiac syndromes and to test specific drug targets. The advantage of using iPSCs is that it would enable modeling disease as closely to the patient’s physiology and genetic as possible to isolating patient-specific fibroblasts,20,31 unlike human ESC-cardiomyocytes-based disease models which can only be created from known mutations that are introduced into the cells. Several cardiac disease models have been established with hiPSC-cardiomyocytes and demonstrate the ability to recapitulate the cellular pathogenic hallmarks of the diseases.57 These models are useful for understanding mechanism of physiology and pathology of disease, validating therapeutic targets, and drug screening or discovery.58
To date, iPSC models have been used to model a large number of genetic arrhythmias including Long-QT syndrome (LQTS), catecholaminergic polymorphic ventricular tachycardia, arrhythmogenic right ventricular cardiomyopathy, Overlap syndrome, LEOPARD syndrome, Timothy syndrome (TS), etc.59 While there is a wide array of CVDs, we chose to focus on several with well defined clinical presentation, strong genetic component, and significant research progress.
LEOPARD syndrome
Investigators created the first hiPSC model of a CVD using skin fibroblasts from a patient with lentigines, electrocardiogram conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormal genitalia, retardation of growth, and sensorineural deafness (LEOPARD) syndrome, an autosomal dominant disorder caused in 90% of cases by a mutation in the PTPN11 gene encoding the protein-tyrosine phosphatase Shp2 that also results in myocardial hypertrophy. The hiPSC-CMs that were generated exhibited increased cell size and sarcomeric organization, suggestive of the cardiac hypertrophic response, as well as aberrant RAS-MAPK signaling. When cardiomyocytes generated from the diseased iPSCs were compared with cardiomyocytes derived from hESCs or non-diseased iPSCs generated from a healthy brother, a significant enlargement in cell surface area, a higher degree of sarcomeric organization, and nuclear translocation of the NFATC4 transcription factor could be observed, all of which correlate with the hypertrophic phenotype observed in patients.59 One limitation of the model is that cardiac differentiation from human iPSCs was performed by standard EB culture, resulting in a heterogeneous population of cells.60
Long QT syndrome
The other reported disease-specific iPSC line mimics congenital LQTSs. LQTS is characterized by prolonged cardiac repolarization that can result in fatal ventricular arrhythmias. More than a dozen different types of inherited LQTS have been described, and human iPSCs have been used to study LQTS types 1,2,3, and 8.37,58 In long-QT syndrome type 1 (LQT-1), mutations occur in the KCNQ1 gene, which encodes the repolarizing potassium channel mediating the delayed rectifier IKs current. Moretti et al.61 provided an early example, taking skin biopsies from patients with LQT-1, reprogramming their cells into iPSCs, and then differentiating those iPSCs into cardiac cells. These patient-specific cardiomyocytes recapitulated the clinical presentation of long-QT phenotype (increased susceptibility to catecholamine-induced tachyarrhythmia, phenotype attenuated by beta-blockade).62 Using a similar approach, successfully modeled long QT syndrome type 2. These studies clearly establish iPSC-derived cardiomyocytes as a powerful tool for drug discovery and personalized medicine.58
Timothy syndrome
Yazawa et al. derived human iPSCs from patient with TS, a disorder in which patients suffer from LQTS, autism, immune deficiency, and syndactyly due to a mutation in the CACNA1C gene encoding Cav1.2 L-type channel. Electrophysiological recording and calcium Ca(2+) imaging studies of these cells revealed irregular contraction, excess Ca(2+) influx, prolonged action potentials, irregular electrical activity, and abnormal calcium transients in ventricular-like cells. In later study, the same group of investigators tested candidate drugs in TS cardiomyocytes and found that roscovitine could successfully rescue these cellular phenotypes.63,64
Dilated cardiomyopathy (DCM) is characterized by ventricular dilatation, systolic dysfunction, and progressive heart failure, which is one of the leading causes of heart failure and is associated with substantial mortality.65,66 Sun et al. generated cardiomyocytes from iPSCs derived from patients in a DCM family carrying a point mutation (R173W) in the gene encoding sarcomeric protein cardiac troponin T. Compared to control healthy individuals in the same family cohort, cardiomyocytes derived from iPSCs from DCM patients exhibited altered regulation of calcium ion Ca(2+), decreased contractility, and abnormal distribution of sarcomeric α-actinin. The over-expression of Serca2a, a gene therapy treatment for heart failure currently in clinical trials, significantly improved the contractility force generated by iPSC-cardiomyocytes derived from DCM patients.66
These studies into patient-specific iPSCs indicate a tremendous potential for our increased understanding of pathogenesis. However, significant hurdles still exist in modeling the more complex CVD using iPSCs technology. There are difficulties in ensuring a purified cardiomyocytes population from iPSCs through standard cardiomyocytes differentiation protocols; the complexities of reproducing a heterogeneous disease phenotype which may involve other systematic factors in vitro using only cardiomyocytes; and limitations of modeling essentially adult-onset diseases using iPSC-cardiomyocytes with a predominantly fetal-like phenotype.13 Resolving these obstacles will also have great impact on facilitating in vivo studies and widespread applications in drug discovery and development.67
Conclusion
As CVDs continue to be a leading cause of morbidity and mortality in the developing world, it is essential to develop strategies to combat these diseases. iPSCs technology has been heralded as the key for therapeutic regenerative strategies due to its potential of differentiating into any cell type of interest. iPSCs and iPSC-derived cardiomyocytes recently emerged a powerful tool to model cardiac development and congenital cardiac disorders. Importantly, the use of specific growth factors, chemically synthesized molecules, epigenetic modifiers, miRNAs, or cardiac-specific transcription factors has significantly improved the yield of cardiac differentiation. Moreover, hESC/iPSC-cardiomyocytes are beginning to be used in 3D cultures that are likely to more accurately mimic the physiological state of cardiac muscle. Transplantation studies of cardiomyocytes in animal models reveal many hurdles and challenges that must be overcome before any human iPSC products can be safely brought to the clinic, including advances in isolation and purification techniques. With better strategies to circumvent immune rejection and better understanding in long-term assessment of cell engraftment after transplantation in large animal models, the prospect of employing human iPSC-CMs as an unlimited source for cell replacement therapy to treat heart failure and other conditions have to be realized.
Acknowledgments
The present work was supported by the grants UK/55/2014 and APVV-0434-12.
Author contributions
All authors participated in writing and reviewing of the manuscript.
References
- 1.Lu J, Kong X, Luo Ch, Li KK. Application of epigenome-modifying small molecules in induced pluripotent stem cells. Med Res Rev 2013; 33: 790–822. [DOI] [PubMed] [Google Scholar]
- 2.Drews K, Jozefczuk J, Prigione A, Adjaye J. Human induced pluripotent stem cells – from mechanisms to clinical applications. J Mol Med 2012; 90: 735–45. [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318: 1917–20. [DOI] [PubMed] [Google Scholar]
- 4.González F, Boué S, Belmonte JCI. Methods for making induced pluripotent stem cells: reprogramming á la carte. Nat Rev 2011; 12: 231–42. [DOI] [PubMed] [Google Scholar]
- 5.Fujita J, Fukuda K. Future prospects for regenerated heart using induced pluripotent stem cells. J Pharmacol Sci 2014; 125: 1–5. [DOI] [PubMed] [Google Scholar]
- 6.Suh CY, Wang Z, Bartulos O, Qyang Y. Advancements in induced pluripotent stem cell technology for cardiac regenerative medicine. J Cardiovasc Pharmacol Ther 2014; 19: 330–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brickwedel J, Gulbins H, Reichenspurner H. Long-term follow-up after autologous skeletal myoblast transplantation in ischaemic heart disease. Interact Cardiovasc Thorac Surg 2014; 18: 61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Liu XC, Yang L, Zhu DL, Zhang YD, Chen Y, Zhang HY. Wharton's jelly-derived mesenchymal stem cells promote myocardial regeneration and cardiac repair after miniswine acute myocardial infarction. Coron Artery Dis 2013; 24: 549–58. [DOI] [PubMed] [Google Scholar]
- 9.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, Sussman MA. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol 2012; 60: 1278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014; 510: 273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong WT, Sayed N, Cooke J.P. Induced pluripotent stem cells: how they will change the practice of cardiovascular medicine. Methodist Debakey Cardiovasc J 2013; 9: 206–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Zhou J, Zhao M, Wang Ch. Current status of induced pluripotent stem cells in cardiac tissue regeneration and engineering. Regen Med Res 2013; 1: 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh Y, Wei H, Ma D, Sun X, Liew R. Clinical application of patient-specific induced pluripotent stem cells in cardiovascular medicine. Heart 2012; 98: 443–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng W. Induced pluripotent stem cells: path to new medicine. EMBRO Rep 2012; 11: 161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seifinejad A, Tabebordbar M, Baharvand H, Boyer L, Salakdeh GH. Progress and promise towards safe induced pluripotent stem cells for therapy. Stem Cell Rev Rep 2010; 6: 297–306. [DOI] [PubMed] [Google Scholar]
- 16.Apostolou E, Hochedlinger K. iPS cells under attack. Nature 2011; 474: 165–6. [DOI] [PubMed] [Google Scholar]
- 17.Thorrez L, Sampaolesi M. The future of induced pluripotent stem cells for cardiac therapy and drug development. Curr Pharm Des 2011; 17: 3258–70. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Zeng F. Integration-free methods for generating induced pluripotent stem cells. Genomics Proteomics Bioinformatics 2013; 11: 284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Chen M, Han W, Fu X. How far are induced pluripotent stem cells from the clinic? Ageing Res Rev 2010; 9: 257–64. [DOI] [PubMed] [Google Scholar]
- 20.Suh CY, Wang Z, Bártulos O, Qyang Y. Advancement in induced pluripotent stem cell technology for cardiac regenerative medicine. J Cardiovasc Pharmacol Ther 2014; 19: 330–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science 2008; 322: 945–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Li W, Laurent T, Ding S. Small molecules, big roles – the chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci 2012; 125: 5609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Zhang L, Xie X. iPSCs and small molecules: a reciprocal effort towards better approaches for drug discovery. Acta Pharmacol Sin 2013; 34: 765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Federation AJ, Bradner JE, Meissner A. The use of small molecules in somatic-cell reprogramming. Trends Cell Biol 2014; 24: 179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou P, Li Y, Zhang X, Liu CH, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013; 341: 651–4. [DOI] [PubMed] [Google Scholar]
- 26.Takhashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development 2013; 140: 2457–61. [DOI] [PubMed] [Google Scholar]
- 27.Vitale AM, Wolvetang E, Mackay-Sim A. Induced pluripotent stem cells: a new technology to study human disease. Int J Biochem Cell Biol 2011; 43: 843–6. [DOI] [PubMed] [Google Scholar]
- 28.Aasent T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzales F, Vassena R, Bilic J, Pekarik V, Tiscornia G, Edel M, Boué S, Belmonte J. Efficient and rapid generation of induces pluripotent stem cells from human keratinocytes. Nat Biotechnol 2008; 26: 1276–84. [DOI] [PubMed] [Google Scholar]
- 29.Sun N, Panetta N, Gupta DM, Wilson KD, Lee A, Jia F, Hu S, Cherryc AM, Robbinsd RC, Longakerb MT, Wu JC. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. PNAS 2009; 106: 15720–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Freire V, Lee AS, Hu S, Abilez OJ, Liang P, Lan F, Huber BC, Ong SG, Hong WX, Huang M, Wu JC. Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J Am Coll Cardiol 2014; 64: 436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iglesias-García O, Pelacho B, Prósper F. Induced pluripotent stem cells as a new strategy for cardiac regeneration and disease modeling. J Mol Cell Cardiol 2013; 62: 43–50. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Yi BA, Wu H, Bock C, Gu H, Lui KO, Park JH, Shao Y, Riley AK, Domian IJ, Hu E, Willette R, Lepore J, Meissner A, Wang Z, Chien KR. Highly efficient derivation of ventricular cardiomyocytes from induced pluripotent stem cells with a distinct epigenetic signature. Cell Res 2011; 22: 142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res 2012; 111: 344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 2008; 118: 507–17. [DOI] [PubMed] [Google Scholar]
- 35.Cho GS, Fernandez L, Kwon C. Regenerative medicine for the heart: perspectives on stem-cell therapy. Antioxid Redox Signal. In press. [DOI] [PMC free article] [PubMed]
- 36.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res 2009; 104: e30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egashira T, Yuasa Sh, Fukuda K. Induced pluripotent stem cells in cardiovascular medicine. Stem Cell Int 2011, pp. 348960–348960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savla JJ, Nelson BC, Perry CN, Adler ED. Induced pluripotent stem cells for the study of cardiovascular disease. J Am Coll Cardiol 2014; 64: 512–9. [DOI] [PubMed] [Google Scholar]
- 39.Lian X, Hsiao Ch, Wilson G, Zhu K, Hazeltine LB, Azarina SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. PNAS 2012; 109: E1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vidarsson H, Hyller J, Sartipy P. Differentiation of human embryonic stem cells to cardiomyocytes for in vitro and in vivo applications. Stem Cell Rev 2010; 6: 108–20. [DOI] [PubMed] [Google Scholar]
- 41.Sinnecker D, Dirschinger RJ, Goedel A, Moretti A, Lipp P, Laugwitz KL. Induced pluripotent stem cells in cardiovascular research. Rev Physiol Biochem Pharmacol 2012; 163: 1–26. [DOI] [PubMed] [Google Scholar]
- 42.Nsair A, MacLellan WR. Induced pluripotent stem cell for regenerative cardiovascular therapies and biomedical discovery. Adv Drug Deliv Rev 2011; 63: 324–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narsinh K, Narsinh KH, Wu JC. Derivation of induced pluripotent stem cells for cardiovascular disease modeling. Circ Res 2011; 108: 1146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hughey CC, Ma L, James FD, Bracy DP, Wang Z, Wasserman DH, Rottman JN, Hittel DS, Shearer J. Mesenchymal stem cell transplantation for the infarcted heart: therapeutic potential for insulin resistance beyond the heart. Cardiovasc Diabetol 2013; 12: 128–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carvalho JL, Braga VB, Melo MB, Campos AC, Oliveira MS, Gomes DA, Ferreira AJ, Santos RA, Goes AM. Priming mesenchymal stem cells boosts stem cell therapy to treat myocardial infarction. J Cell Mol Med 2013; 17: 617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marbán E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol 2014; 63: 110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robbers LF, Nijveldt R, Beek AM, Hirsch A, van der Laan AM, Delewi R, van der Vleuten PA, Tio RA, Tijssen JG, Hofman MB, Piek JJ, Zijlstra F, van Rossum AC. HEBE Investigators. Cell therapy in reperfused acute myocardial infarction does not improve the recovery of perfusion in the infarcted myocardium: a cardiac MR imaging study. Radiology 2014; 272: 113–22. [DOI] [PubMed] [Google Scholar]
- 48.Lundy SD, Gantz JA, Pagan ChM, Filicine D, Laflamme MA. Pluripotent stem cell derived cardiomyocytes for cardiac repair. Curr Treat Options Cardiovasc Med 2014; 16: 319–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 2010; 120: 408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mauritz C, Martens A, Rojas SV, Schnick T, Rathert C, Schecker N, Menke S, Glage S, Zweigerdt R, Haverich A, Martin U, Kutschka I. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J 2011; 32: 2634–41. [DOI] [PubMed] [Google Scholar]
- 51.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res 2011; 109: 47–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawamoto T, Kobayashi Y, Nakajima H, Yamagishi Y. Generation of robust vascular networks from cardiovascular blast populations derived from human induced pluripotent stem cells in vivo and ex vivo organ culture system. Biochem Biophys Res Commun 2013; 441: 180–85. [DOI] [PubMed] [Google Scholar]
- 53.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T, Sawa Y. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012; 126: S29–37. [DOI] [PubMed] [Google Scholar]
- 54.Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Ito E, Sougawa N, Kawamura T, Daimon T, Shimizu T, Okano T, Toda K, Sawa Y. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation 2013; 128: S87–94. [DOI] [PubMed] [Google Scholar]
- 55.Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, Müller E, Küest SM, Cohrs S, Schibli R, Kronen P, Hilbe M, Reinisch A, Strunk D, Haverich A, Hoerstrup S, Lüscher TF, Kaufmann PA, Landmesser U, Martin U. Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation 2012; 126: 430–39. [DOI] [PubMed] [Google Scholar]
- 56.Yang X, Pabon L, Murry CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res 2014; 114: 511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blazeski A, Zhu R, Hunter DW, Weinberg SH, Zambidis ET, Tung L. Cardiomyocytes derived from human induced pluripotent stem cells as models for normal and diseased cardiac electrophysiology and contractility. Prog Biophys Mol Biol 2012; 110: 166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liang P, Du J. Human induced pluripotent stem cell for modeling cardiovascular diseases. Regenerative Med Res 2014; 2: 4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chow MZ, Boheler KR, Li RA. Human pluripotent stem cell-derived cardiomyocytes for heart regeneration, drug discovery and disease modeling: from the genetic, epigenetic, and tissue modeling perspectives. Stem Cell Res Ther 2013; 4: 97–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carvajal-Vergara X, Sevilla A, D'Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell derived models of LEOPARD syndrome. Nature 2010; 465: 808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, Dorn T, Goedel A, Höhnke C, Hofmann F, Seyfarth M, Sinnecker D, Schömig A, Laugwitz KL. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 2010; 363: 1397–409. [DOI] [PubMed] [Google Scholar]
- 62.Vincent GM, Schwartz PJ, Denjoy I, Swan H, Bithell C, Spazzolini C, Crotti L, Piippo K, Lupoglazoff J-M, Villain E, Priori SG, Napolitano C, Zhang L. High efficacy of β-blockers in long-QT syndrome type 1. Circulation 2009; 119: 215–21. [DOI] [PubMed] [Google Scholar]
- 63.Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 2011; 471: 230–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yazawa M, Dolmetsch RE. Modeling Timothy syndrome with iPS cells. J Cardiovasc Transl Res 2013; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Refaat MM, Lubitz SA, Makino S, Islam Z, Frangiskakis JM, Mehdi H, Gutmann R, Zhang ML, Bloom HL, MacRae CA, Dudley SC, Shalaby AA, Weiss R, McNamara DM, London B, Ellinor PT. Genetic variation in the alternative splicing regulator RBM20 is associated with dilated cardiomyopathy. Heart Rhythm 2012; 9: 390–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med 2012; 4: 130ra47–130ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Josowitz R, Carvajal-Vergara X, Lemischka IR, Gelb BD. Induced pluripotent stem cell-derived cardiomyocytes as models for genetic cardiovascular disorders. Curr Opin Cardiol 2011; 26: 223–9. [DOI] [PubMed] [Google Scholar]