Abstract
This study was conducted to determine if correlations exist between the numbers of microscopic follicles comprising ovarian follicular reserve (OFR) and antral follicle counts (AFCs), and to assess the usefulness of computerized analyses of ovarian ultrasonograms and magnetic resonance (MR) images for estimating OFR in excised porcine, ovine and bovine ovaries. As a pre-requisite to these analyses, we characterized and compared ovarian cortical histomorhpology and follicle populations in the three species varying in prolificacy and overall reproductive longevity, and hence the total number of microscopic and antral follicles. Ultrasonographic and MR images were obtained at the scanner settings optimized to provide opposing contrasts between antral follicles and the ovarian stroma. Commercially available ImageProPlus® analytical software was used to calculate numerical pixel values (NPVs) and pixel heterogeneity (standard deviation of the pixel values) along the computer-generated lines (4–6) placed in the area corresponding to the ovarian cortex. The numbers of primordial (r = 0.38, P < 0.01) and intermediate follicles (r = 0.37, P < 0.01) were correlated with the numbers of antral follicles in bovine ovarian sections. The numbers of primordial (r = 0.28, P < 0.05), intermediate (r = 0.31, P < 0.01) and primary follicles (r = 0.27, P < 0.05) correlated directly with mean NPVs of the ultrasonographic ovarian images in cattle. There was a negative correlation between primary follicle numbers and NPVs of MR images (3D FAST-SPOILED GRADIENT ECHO) of the porcine ovarian cortex (r = −0.31, P < 0.05). To summarize, the numbers of primordial and intermediate follicles could only be estimated from AFCs in cows. Using ultrasound NPVs, the numbers of primordial, intermediate and primary follicles could be directly estimated in bovine ovaries and the quantitative image attributes of MR images were useful for quantifying porcine primary follicles. The bovine ovarian model is compatible with human situation and hence future studies should be undertaken to ascertain the usefulness of AFCs and ultrasonographic image analyses for estimating OFR in women.
Keywords: Ovarian follicle reserve, ovarian histology, ultrasonography, magnetic resonance imaging, digital image analysis
Introduction
The females of mammalian species are born with a set amount of oocytes, which become activated to complete oogenesis after the onset of puberty. The proper and coordinated progression of this process results in the release of viable oocytes throughout the reproductive life.1–3 According to a recent Scottish study analyzing the female egg supply from conception to menopause in 350 women of various ages, in Europe and the USA, women lose 88% of their eggs by the age of 30, and by the age of 40 only 3% of their reserve of oocytes remain.2,3 Premature ovarian failure (a.k.a. premature ovarian insufficiency, primary ovarian insufficiency, premature menopause, or hypergonadotropic hypogonadism) is the loss of cyclic ovarian function before the age of 40; it affects 1% of the population.4
The ovarian follicular reserve (OFR) comprises microscopic primordial, transitory (intermediate) and primary follicles – the oocytes surrounded by a single layer of squamous or cuboidal follicular cells – residing and developing in the cortex of the ovary.1,3 This reserve represents the ability of the ovaries to produce functional growing follicles, which become responsive to stimulation with both endogenous and exogenous gonadotropins. Various methods can be employed to estimate the OFR, but clinical assessment of ovarian health status relies primarily on the evaluation of the pool of mature (Graafian) follicles, using hormonal profiles or follicle counts.1,5–7 In this approach, the proper functioning of the ovary is assessed through the normal development of antral follicles and the release of hormones involved with their recruitment and maturation. It has generally been assumed that hormonal tests serving as indicators of the endocrine capacity of large antral follicles provide an indirect measurement of the follicular reserve pool. Among the static tests, the most common are basal serum follicle-stimulating hormone (FSH), basal serum estradiol, and basal serum inhibin-B level measurements.8–10 Due to considerable individual variation, determining appropriate cut-off points for hormonal tests is very difficult and hence the tests may have compromised sensitivity. Dynamic testing involves initial estimation of basal hormone levels followed by ovarian suppression/stimulation, and then another collection and testing of serum samples. One of the most widely used dynamic clinical tests is the clomiphene citrate (estradiol antagonist) challenge performed in conjunction with serum FSH measurements.8 However, determining the cut-off point for FSH, as mentioned previously, is a limiting factor. In addition, the clomiphene citrate challenge test is often associated with side effects such as pelvic pain, bloating, nausea, and breast discomfort.8 Hence, even though a few hormonal tests are routinely employed to evaluate OFR, they appear to fall short of what is considered an ideal sensitivity. Most importantly, however, no studies to date have shown a direct association between the results of the aforementioned hormonal tests and the population of microscopic ovarian follicles. Measuring serum levels of anti-Mullerian hormone (AMH) is considered the most reliable marker of OFR as the granulosa cells of microscopic ovarian follicles produce the hormone.9,11 Circulating AMH concentrations correlate with the number and activity of the reserve-pool follicles, but although the test tends to be more sensitive than measurements of FSH, estradiol, and inhibin-B levels, it still has a wide range of the cut-off concentrations.12
At present, the only option for direct estimation of OFR is through an increasingly criticized ovarian biopsy.5–7 Ovarian biopsy involves taking a scalpel biopsy from one of the ovaries coupled with an ultrasonographic measurement of the size of the ovary. The biopsy is then serially sectioned and microscopically analyzed to enumerate the primordial, intermediate, and primary follicles. There are major drawbacks to this method, in that the distribution of follicles is not uniform across the cortex in addition to the obvious invasiveness and possible surgical complications including adhesions and scarring. Non-invasive techniques for determining the number of microscopic ovarian follicles within the ovaries would therefore be a desirable alternative to currently used methods.
Depending on the species, antral follicle count (AFC) can be accomplished using transrectal, transvaginal, or transabdominal ultrasonography,13–15 or magnetic resonance imaging.16,17 Recently, Ireland et al.18 have reported the existence of a positive correlation between the antral follicle numbers at the peak of the follicular wave (a cohort of antral follicles stimulated to grow by a transient increase in serum FSH concentrations), the concentration of circulating AMH, and the number of healthy primordial, transitory, and primary ovarian follicles in heifers. This study solidified the use of ultrasonography to enumerate antral follicles as a non-invasive estimation of OFR in cows. Cattle are considered an appropriate model for ovarian processes in women, as the ovaries and intraovarian structures are of a similar size, and both species exhibit similar endocrine control of folliculogenesis and ovarian pathological conditions.19,20 However, similar studies in women and other mammalian species do not exist.
It has now been established that changes that occur at the cellular and macromolecular level and affect large proportions of cells may change ovarian morphology sufficiently to be detected by computerized analysis of ultrasonographic and magnetic resonance (MR) images. Previous research has shown a correlation between the ultrasonographic image characteristics and histophysiological properties of antral follicles in domestic ruminants and women.14,21 Changes in the intensity and heterogeneity of ovarian ultrasonograms have been found to be related to cell density, thickness, and proliferating cell indices of the granulosa and theca layer.21 The analysis of MR image attributes has also allowed for detecting the microscopic changes occurring in ovarian structures ex situ.16,17
Hence, the main objectives of this study were to assess the suitability of AFCs and computer-assisted analysis of ultrasonographic and magnetic resonance images for quantifying the reserve pool of ovarian follicles in three animal species ex situ. Additionally, we described and compared various histomorphological characteristics of the ovarian cortex and follicle populations in the three species under study. Swine, sheep, and cattle were used due to differences in prolificacy and reproductive longevity, and hence the total numbers of ovarian follicles present. Our major null hypotheses were that: (i) microscopic follicle counts would be correlated with the numbers of ovarian antral follicles on ovarian ultrasonograms and sequential MR images; and (ii) changes in the quantitative image attributes of the ovarian cortex would be correlated with the number of microscopic follicles determined through histological examinations of the ovarian tissue.
Materials and methods
Experimental design and procedures
Slaughterhouse ovaries were obtained from gilts (Yorkshire/Landrace × Duroc/Hampshire, aged ∼6 months), ewe lambs (Suffolk, aged ∼7 months), and heifers (Hereford, aged 15–18 months); five pairs of ovaries were collected from each species. Corpora lutea (CL) were present in two ovaries of one gilt (four on one ovary and five on the contralateral ovary); in two ewes (each containing one CL); and in two heifers (one heifer had one CL, one animal had two CLs on one ovary, and one animal had a CL on each ovary). Immediately after collection, the ovaries were placed in re-sealable plastic bags filled with saline. The temperature was maintained at 37℃. The bags were placed in a warm thermos and transported to the laboratory; the travel time was restricted to 1 h.
Upon arrival to the laboratory, the ovaries were scanned in a transverse plane in a degassed water bath using a 7.5-MHz linear-array small parts transducer connected to the Aloka 900-SSD echo camera (Aloka Ltd., Tokyo, Japan). The transducer was kept at a distance of about 0.5 cm from the surface of the ovary. Each scan was recorded on a hard drive of a Pioneer DVD recorder (Pioneer Electronics of Canada Inc.; Markham, ON, Canada) at the constant settings for an overall gain (60% of maximum value) and time-gain compensation (0%). All ovaries were then prepared for MRI by removing any excess tissue, put on a plastic wrap in a row of pairs, and placed onto a custom-made phased array surface coil. The plastic wrap functioned not only to keep the ovaries in place but also to prevent moisture loss during examination. The ovaries were scanned using a 1.5 Tesla GE Sigma scanner (General Electric Medical Systems; Milwaukee, WI, USA) to produce T1-weighted SPIN ECHO (T1 SE), T2-weighted FAST SPIN ECHO (T2 FSE), and T1-weighted 3D FAST-SPOILED GRADIENT ECHO (3D FSPGRE) images. The T1 SE sequence was acquired in a coronal plane using the following settings: echo time (TE) = 15 ms, repetition time (TR) = 480 ms, 12 × 9 cm field of view (FOV), 2 mm/0 mm (slice/gap), 224 × 224 matrix, number of excitations (NEX) = 8, bandwidth (BW) = 31.2, and Imaging Option ZIP 512 (Zero Fill Interpolation). The T2 FSE sequence was acquired at TE = 50 ms, TR = 2000 ms, 12 × 9 cm FOV, 2 mm/0 mm (slice/gap), 224 × 224 pixel matrix, NEX = 10, BW = 31.2, and Imaging Option ZIP 512. Lastly, the 3D FSPGR images were acquired at TE = 4.2 ms, TR = 93 ms, flip angle (FA) = 15, 16 × 14 cm FOV, 1 mm/0 mm (slice/gap), 192 × 192 pixel matrix, NEX = 18, and BW = 15.63. Such protocols have been found to provide the optimal, opposing contrasts for antral follicles and the ovarian stroma.16 The MR image acquisition was attained using the Efilm Light Software (version 3.1.2. General Electric Medical Systems; Milwaukee, WI, USA); the images were saved as MIP images at 10.4× magnification.
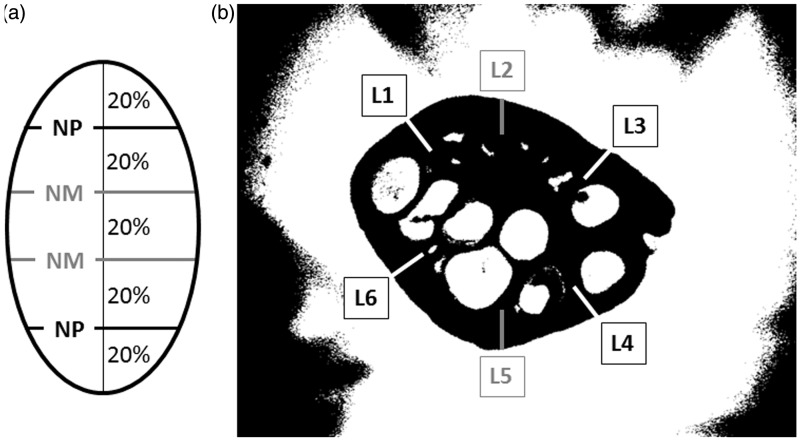
Immediately following an MR scan, the ovaries were transferred to a labelled tube containing Bouin’s fixative, and then placed in plastic cassettes and put in 70% ethanol until further tissue processing for histology. Ovarian tissue sections were subsequently processed in a graded ethanol and paraffin system, placed into paraffin blocks, and then sectioned using a standard rotary microtome (Leica CM3050 S; Leica Microsystems, Nußloch, Germany) at a thickness of 5 µm; the sections were taken to represent four evenly spaced regions of each ovary (Figure 1a). The slides were air-dried and stained with hematoxylin and eosin as per routine staining techniques.
Figure 1.
(a) A diagram illustrating the topographic location of cross-sections (two from near-middle (NM) and two from near-pole (NP) regions of each ovary) used for comparative histological assessment and computer-assisted image analyses of the ovarian cortex; (b) demonstration of line placement (4–6 lines per cross-section) for image analysis using the Image ProPlus® analytical software. Computer-generated lines with the length corresponding to the area occupied by ovarian cortex (on the basis of earlier histological examinations of ovarian histograms) were drawn at approximately 10, 12, 2, 4, 6, and 8 o’clock positions. A special care was taken to avoid visible antral follicles and, in the ultrasonographic images, the lines at 12 and 6 o’clock positions were frequently omitted due to the presence of reflection artifacts
The slides of the cross-sections were photographed using a Zeiss Stemi 2000-C dissection microscope with a Leica Opti-tech OT6246 microscope/video inspection and measurement system and the attached QImaging Q1cam1294 (Leica CM3050 S; Leica Microsystems, Nußloch, Germany). The images were saved as JPEG files using the QCapture 2.73.0 software (Quorum Technologies Inc., Guelph, ON, Canada) at 10×, 40× and 100× image magnification. The ultrasonographic and MR ultrasound images were digitized using Adobe PremierePro™ (version 1.5; Adobe Systems Inc., San Jose, CA, USA) and stored as BITMAP images. The entire cross-sectional images (10× image magnification) were used to identify the matching ultrasonographic and MRI images. Microscopic analyses of the histological sections were done to enumerate primordial, primary, intermediate, secondary, and pre-antral follicles (characterized in Figure 2) based on previously defined microscopic criteria.12 To ensure that all follicles were accounted for, a mark was made randomly on each side of the slide and follicles were counted in a clockwise motion until the mark was in view again. For each slide, the diameter of the largest and smallest follicle in each category was measured, at 40× or 100× image magnification, to calculate the mean follicular size. At the same time, the diameters of the cross section of the ovary as well as the percentage of the diameter represented by the cortex (defined as a region containing microscopic ovarian follicles) were determined. Antral follicle numbers were taken directly from the ultrasonograms and MR images.
Figure 2.
Histomorphological classification and photographic reproductions of porcine, ovine and bovine follicles at 100× (dark gray background) or 40× image magnification (light gray background).
Image analysis was completed on all of the ultrasound, T1 SE, T2 FSE, and 3D FSPGRE images using a line technique in the Image ProPlus® analytical software (Media Cybernetics Inc., San Diego, CA, USA). A computer-generated line was drawn in the area corresponding to the ovarian cortex; the length of the line was determined using the previously calculated percentage of the diameter corresponding to the cortex. At least four lines were used for each image (Figure 1b). The line analysis was used to calculate the average pixel intensity (NPVs) and pixel heterogeneity (standard deviation (SD) of the pixel values) along the lines.
The numbers and sizes of follicles in different categories were compared between the three species studied by one-way analysis of variance, and the numbers of follicles in the near-middle (NM) sections of the ovary were compared to those in the two near-pole (NP) sections by a Student t-test (SigmaPlot® version 11.0; Systat Software Inc., Richmond, CA, USA). Pearson Product Moment correlations were determined among echotextural and MRI parameters, and the numbers of follicles in each category except for AFCs. Significance was set as P < 0.05. All values are expressed as mean ± SEM.
Results
Histomorphology and ovarian follicle numbers
The mean diameter and percentage of the diameter spanning the ovarian cortex did not vary (P > 0.05) between the near-middle (NM) and near-pole (NP) transverse ovarian sections in the three species under study. The diameter of porcine ovarian cross-sections averaged 15.7 ± 1.2 mm, approximately 16% of which corresponded to the cortex (16.4 ± 1.6%). Ovine cross-sections were the smallest (P < 0.05), with an average diameter of 9.1 ± 0.6 mm, and the percentage of the diameter spanning the cortex approximating 25% (24.7 ± 1.2%). Bovine ovaries had the largest cross-sectional diameters, averaging at 26.4 ± 1.2 mm, with the percentage of diameter occupied by the cortex equal to 9.8 ± 1.0%. The percentage of the ovarian diameter spanning the ovarian cortex was greater (P < 0.05) in sheep than in pigs, and they both were greater (P < 0.05) compared to that in cattle.
With regards to the distribution of ovarian follicles within the cortex in the three species under study, the porcine microscopic follicles tended to be clustered together while bovine and ovine follicles were scattered unevenly. The total number of follicles in all size classes as well as the numbers of primordial and secondary follicles per cross-section were greatest in the porcine ovaries, followed by the bovine and ovine sections (P < 0.05; Table 1). The number of intermediate ovarian follicles was greatest in bovine ovarian sections, followed by porcine and ovine ovaries (P < 0.05). The number of pre-antral follicles did not differ (P > 0.05) between ovine and bovine ovarian sections but they were greater (P < 0.05) in porcine ovaries. The number of antral follicles did not differ (P > 0.05) between porcine and bovine ovarian sections, but there were significantly less antral follicles in ovine ovaries. There were no differences (P > 0.05) in mean numbers of primary follicles per cross-section among the three species under study.
Table 1.
Mean (±SEM) follicular counts in each size category (number of follicles/cross section) from histological slides of the porcine, ovine, and bovine ovaries (each species: n = 10 ovaries)
| Follicle category/ Species | Porcine | Ovine | Bovine |
|---|---|---|---|
| Primordial | 91.8 ± 7.9a | 17.6 ± 2.5c | 32.6 ± 3.3b |
| Intermediate | 29.9 ± 4.2b | 8.7 ± 1.3c | 34.9 ± 4.1a |
| Primary | 8.7 ± 1.1 | 6.8 ± 0.8 | 5.6 ± 1.6 |
| Secondary | 3.2 ± 0.6a | 1.1 ± 0.2c | 1.6 ± 0.2b |
| Pre-antral | 0.7 ± 0.1a | 0.2 ± 0.09b | 0.2 ± 0.06b |
| Antral | 10.4 ± 0.8a | 3.5 ± 0.3b | 8.7 ± 0.6ab |
| Total | 144.8 ± 10.8a | 37.5 ± 4.2c | 83.9 ± 8.0b |
Note: Within rows, mean values denoted by different letters vary significantly (P < 0.05).
In the porcine ovaries, there was a significant difference in follicle distribution between NM and NP ovarian sections (Table 2), with the NP sections containing fewer follicles than NM sections; the difference in follicle counts was significant for primary, secondary, and pre-antral follicles. In ovine ovarian sections, there was no overall difference in follicle numbers between the NM and NP sections (P > 0.05); however, no pre-antral follicles were detected in the NP ovarian sections. In cattle, the overall difference between NM and NP sections was approaching to significance (P = 0.07), but post-ANOVA tests did not reveal significant differences in any size category. The average follicular diameter for primordial, intermediate, primary, secondary, and pre-antral follicles in each species studied is shown in Table 3. There were numerical differences in follicle sizes but only the secondary and pre-antral ovarian follicles were significantly larger in pigs compared with sheep.
Table 2.
Differences in follicle numbers (mean ± SEM) between the near-middle (NM) and near-pole (NP) cross sections of the porcine, ovine, and bovine ovaries
| Species | Follicle category | Average no. of follicles per cross-section (NM sections) | Average no. of follicles per cross-section (NP sections) |
|---|---|---|---|
| Porcine | Primordial | 95.1 ± 9.8 | 88.6 ± 12.3 |
| Intermediate | 35.4 ± 6.9 | 24.5 ± 4.4 | |
| Primary | 10.9 ± 2.1a | 6.4 ± 0.7b | |
| Secondary | 3.4 ± 0.9a | 0.2 ± 0.7b | |
| Pre-antral | 0.9 ± 0.2a | 0.4 ± 0.1b | |
| Antral | 11.4 ± 1.3 | 9.7 ± 0.9 | |
| Ovine | Primordial | 17.1 ± 3.1 | 18.0 ± 4.0 |
| Intermediate | 7.9 ± 1.5 | 9.8 ± 6.6 | |
| Primary | 6.5 ± 1.0 | 6.8 ± 0.8 | |
| Secondary | 1.4 ± 0.3 | 0.8 ± 0.3 | |
| Pre-antral | 0.5 ± 0.1 | ND | |
| Antral | 3.9 ± 0.5 | 3.0 ± 0.5 | |
| Bovine | Primordial | 36.4 ± 6.9 | 29.4 ± 6.0 |
| Intermediate | 35.6 ± 21.0 | 34.9 ± 8.3 | |
| Primary | 6.8 ± 3.4 | 5.0 ± 1.5 | |
| Secondary | 1.7 ± 0.4 | 1.3 ± 0.4 | |
| Pre-antral | 0.2 ± 0.1 | 0.3 ± 0.1 | |
| Antral | 8.7 ± 0.7 | 7.8 ± 1.2 |
Note: Within rows, means denoted by different letters vary significantly (P < 0.05).
ND: not detected.
Table 3.
Mean (±SEM) diameter (µm) of microscopic ovarian follicles in swine, sheep, and cattle
| Follicle category/ Species | Porcine | Ovine | Bovine |
|---|---|---|---|
| Primordial | 25.0 ± 8.2 | 36.9 ± 9.9 | 30.0 ± 7.6 |
| Intermediate | 34.6 ± 19.6 | 40.3 ± 13.1 | 42.2 ± 14.8 |
| Primary | 67.4 ± 13.2 | 66.0 ± 10.0 | 68.4 ± 8.4 |
| Secondary | 141.3 ± 18.8a | 98.6 ± 16.0b | 112.0 ± 19.9ab |
| Pre-antral | 386.6 ± 78.8a | 201.4 ± 58.9b | 247.4 ± 52.6ab |
Note: Within rows, mean values denoted by different letters vary significantly (P < 0.05).
Correlations among the numbers of follicles in different categories
Correlations were found among follicle numbers in different size categories (Table 4). Significant albeit moderate correlations existed between the number of primordial and intermediate as well as between the number of primordial and primary follicles in each species studied. A significant positive correlation between primordial and secondary follicles was only recorded in bovine ovaries. In pigs, the numbers of pre-antral ovarian follicles correlated directly with the numbers of intermediate, primary, secondary, and antral follicles. Correlations between the numbers of antral and primary follicles, and between antral and secondary porcine follicles approached to significance (P < 0.08). Lastly, significant correlations detected in bovine ovarian cross-sections but not in porcine or ovine ovaries included the correlations between the numbers of: primordial and secondary follicles, primordial and antral follicles, intermediate and secondary follicles, intermediate and antral follicles, and between primary and secondary follicles.
Table 4.
Summary of correlations among the numbers of follicles in different size categories
| Follicle category/Species | Porcine |
Ovine |
Bovine |
|||
|---|---|---|---|---|---|---|
| r | P-value | r | P-value | R | P-value | |
| Primordial and intermediate | 0.71 | <0.001 | 0.73 | <0.001 | 0.72 | <0.001 |
| Primordial and primary | 0.37 | <0.05 | 0.64 | <0.001 | 0.52 | <0.001 |
| Primordial and secondary | NS | NS | 0.57 | <0.001 | ||
| Primordial and pre-antral | NS | NS | NS | |||
| Primordial and antral | NS | NS | 0.38 | <0.01 | ||
| Intermediate and primary | NS | NS | NS | |||
| Intermediate and secondary | NS | NS | 0.61 | <0.001 | ||
| Intermediate and pre-antral | 0.36 | <0.05 | NS | NS | ||
| Intermediate and antral | NS | NS | 0.37 | <0.01 | ||
| Primary and secondary | NS | NS | 0.29 | <0.05 | ||
| Primary and pre-antral | 0.33 | <0.05 | NS | NS | ||
| Primary and antral | 0.26 | 0.08 | NS | NS | ||
| Secondary and pre-antral | 0.34 | <0.05 | NS | NS | ||
| Secondary and antral | 0.29 | 0.06 | NS | NS | ||
| Pre-antral and antral | 0.39 | <0.01 | NS | NS | ||
Note: Correlations approaching to significance (P ≤ 0.10) are italicized.
NS: not significant; r: coefficient of correlation.
Correlations between quantitative attributes of ultrasonographic/MR images and microscopic follicle numbers
Correlations between image characteristics determined by computer-assisted analyses of ovarian cortex and follicle numbers are summarized in Table 5. Significant albeit moderate correlations existed between ultrasound pixel intensity (NPVs) and the numbers of primordial, intermediate, and primary ovarian follicles determined in bovine ovaries. A significant negative correlation was recorded between the pixel intensity of 3D FSGRE images and the numbers of primary follicles in pigs. There was a significant positive correlation between the numbers of secondary follicles and pixel heterogeneity values of 3D FSGRE of ovine ovaries. Additionally, the correlations between ultrasound pixel intensity and primary follicle counts as well as between 3D FSGRE pixel intensity and the numbers of primordial follicles approached to significance. For ovine ovaries, the two negative correlations approaching significance (P = 0.07) were recorded between the numbers of primordial follicles and 3D FSGRE pixel heterogeneity, and between the numbers of intermediate ovarian follicles and ultrasound pixel heterogeneity values.
Table 5.
Summary of correlations among the numbers of follicles in different size categories and image attributes of ovarian ultrasonographic, T1-weighted SPIN ECHO (T1 SE), T2-weighted FAST SPIN ECHO (T2 FSE), and T1-weighted 3D FAST-SPOILED GRADIENT ECHO (3D FSPGRE) images
| Species/Quantitative image attribute and follicle category | Porcine |
Ovine |
Bovine |
||||||
|---|---|---|---|---|---|---|---|---|---|
| r | P-value | r | P-value | r | P-value | ||||
| Pixel Intensity | Primordial and U/S (7.5 MHz) | NS | NS | 0.28 | <0.05 | ||||
| Primordial and T1 SE | NS | NS | NS | ||||||
| Primordial and T2 FSE | NS | NS | NS | ||||||
| Primordial and 3D FSGRE | −0.25 | 0.10 | NS | NS | |||||
| Intermediate and U/S (7.5 MHz) | NS | NS | 0.31 | <0.01 | |||||
| Intermediate and T1 SE | NS | NS | NS | ||||||
| Intermediate and T2 FSE | NS | NS | NS | ||||||
| Intermediate and 3D FSGRE | NS | NS | NS | ||||||
| Primary and U/S (7.5 MHz) | −0.25 | 0.09 | NS | 0.27 | <0.05 | ||||
| Primary and T1 SE | NS | NS | NS | ||||||
| Primary and T2 FSE | NS | NS | NS | ||||||
| Primary and 3D FSGRE | −0.31 | <0.05 | NS | NS | |||||
| Secondary and U/S (7.5 MHz) | NS | NS | NS | ||||||
| Secondary and T1 SE | NS | NS | NS | ||||||
| Secondary and T2 FSE | NS | NS | NS | ||||||
| Secondary and 3D FSGRE | NS | NS | NS | ||||||
| Pixel Heterogeneity | Primordial and U/S (7.5 MHz) | NS | NS | NS | |||||
| Primordial and T1 SE | NS | NS | NS | ||||||
| Primordial and T2 FSE | NS | NS | NS | ||||||
| Primordial and 3D FSGRE | NS | −0.29 | 0.07 | NS | |||||
| Intermediate and U/S (7.5 MHz) | NS | −0.29 | 0.07 | NS | |||||
| Intermediate and T1 SE | NS | NS | NS | ||||||
| Intermediate and T2 FSE | NS | NS | NS | ||||||
| Intermediate and 3D FSGRE | NS | NS | NS | ||||||
| Primary and U/S (7.5 MHz) | NS | NS | NS | ||||||
| Primary and T1 SE | NS | NS | NS | ||||||
| Primary and T2 FSE | NS | NS | NS | ||||||
| Primary and 3D FSGRE | NS | NS | NS | ||||||
| Secondary and U/S (7.5 MHz) | NS | NS | NS | ||||||
| Secondary and T1 SE | NS | NS | NS | ||||||
| Secondary and T2 FSE | NS | NS | NS | ||||||
| Secondary and 3D FSGRE | NS | 0.32 | <0.05 | NS | |||||
Note: Correlations approaching to significance (P ≤ 0.10) were italicized.
NS: not significant; r: coefficient of correlation.
Discussion
Documenting the differences in ovarian macro- and microscopic morphology among the three species varying in body size, prolificacy, and reproductive longevity is important as it can facilitate tailoring the optimal technique(s) for determining the OFR. The average diameter and area of ovarian cross-sections were largest in cattle, followed by porcine and ovine ovarian tissue samples. Variations in the percentage of the ovary occupied by the cortex between the three species were also observed; it was greatest in the sheep, followed by that in pigs and then cows, which seems to indicate an inverse relationship with ovarian dimensions.
There were differences in follicle distribution patterns in the three species under study. In porcine ovaries, the microscopic follicles were distributed in clusters while in cows and sheep they were scattered unevenly. It has been demonstrated that in litter-bearing species, the follicles are typically distributed in clusters.22 Porcine follicles (intermediate, secondary, and pre-antral) were located primarily in the inner sections of the ovary. In sheep, the microscopic follicles were evenly distributed between the inner and outer ovarian sections; however, no pre-antral follicles were found in the outer ovarian sections. A likely explanation for this phenomenon could be that pre-antral follicles in the near-pole (NP) regions of the ewe’s ovary do not develop possibly due to unfavourable conditions such as a lower blood supply and hypoxia. The antral follicles are significantly larger than pre-antral follicles, so when they enlarge they could “re-colonize” the NP ovarian segments located farther from the ovarian hilus. Similarly, more favorable conditions for the development of porcine primary, secondary, and pre-antral follicles can exist in the near middle (NM) as compared with the NP segments of the ovary. This particular aspect of folliculogenesis requires more research.
The pig consistently had the greatest number of ovarian follicles, with the exception of intermediate and primary follicles, while the sheep generally had the least follicles. Of the follicles comprising the follicular reserve, the pigs and sheep had the most primordial follicles, whereas cows had the greatest number of intermediate follicles. A study by Ireland et al.18 also showed that intermediate follicle counts were higher than those of primordial ovarian follicles in cows, which suggests that the intermediate follicles are the major component of the bovine follicular reserve. A plausible reason for higher numbers of intermediate than primordial follicles in the cow could be a high apoptotic rate of primordial follicles or a prolonged lifespan of the intermediate follicles. Interestingly, there was no difference in the number of primary follicles among the three species, suggesting the existence of the species-specific rates of follicular growth and apoptosis between the primordial and primary stages. Similarly, the pattern of follicular development after recruitment from the reserve pool (i.e., secondary stage onwards) appears to differ between pigs and ruminant species. If the rates of follicular growth and apoptosis were constant for ovarian follicles throughout their lifespan, the numbers of follicles in every size class should be positively correlated with the numbers of follicles in a previous stage. However, such a linear relationship was not consistently seen in all three species studied. For all stages, the changes in follicle numbers do not appear to be regular indicating that there exists tremendous interspecies and perhaps even individual variability in follicle populations especially during the early stages of folliculogenesis. This is then reflected in variations in antral/vesicular follicle numbers.23–25
The sizes of microscopic ovarian follicles determined in the present study were similar to those previously reported by Kerong et al.,26 Muruvi et al.,27 and Aearts28 for porcine, ovine, and bovine ovaries, respectively. Microscopic follicle size does not appear to be related to the size of the ovary; secondary and pre-antral follicles were significantly larger in pigs compared with sheep, but all types of microscopic follicles in sheep and cows had similar diameters. Ovarian dimensions were consistently smallest in sheep followed by pigs and cows.
Both intermediate and primary follicle numbers were found to be significantly correlated with AFCs in the cow. As two of the three categories of the follicular reserve can be directly estimated from the numbers of antral follicles, it can be concluded that AFC is a decent predictor of the OFR in cows. In the pig, the correlation between the numbers of primary and antral follicles only approached to significance, and hence using AFCs to estimate the numbers of this subset of follicular reserve in porcine ovaries may be associated with a significantly higher degree of error. Since there were no correlations between the reserve pool follicles and the number of antral follicles in ovine ovaries, enumerating antral follicles cannot be used to determine the follicular reserve in sheep.
Significant correlations were recorded between the numbers of primordial, intermediate, and primary follicles, and the pixel intensity (NPVs) of bovine ultrasonographic ovarian images. Conversely, there were no significant correlations between the quantitative attributes of MR images techniques and microscopic follicle counts in cows. Further studies using a three-dimensional (3D) ultrasound29,30 combined with computerized image analyzes could potentially find a strong, direct correlation between the ovarian follicular reserve and AFCs. Intermediate follicle numbers and ultrasonographic NPVs exhibited a slightly higher correlation coefficient compared to that for primordial and primary follicle numbers; this could be due to a greater abundance of intermediate follicles in bovine ovaries. Nevertheless, ultrasound imaging appears to be a useful method for estimtaing OFR in cows. This is of importance because reproductive ultrasonography and computerized image analysis of ovarian ultrasonograms are also applicable to human clinical practice.31 The bovine experimental model is accessible, malleable, lends itself to quantitative assessments, and is appropriate for the development of new ovarian imaging strategies in women,19,20,31 including the estimation of OFR.
A significant negative correlation has been found between the numbers of primary follicles pixel intensity of the 3D FSGRE ovarian images in pigs. There were no other significant correlations between the follicles of the reserve pool and ultrasonographic or MR image attributes in all three species under study. Almost a complete lack of quantitative correlations between MR image characteristics and ovarian follicular populations is difficult to explain. Future studies aimed to include a larger portion of the ovarian cortex into analyses may be necessary to ameliorate the use of both imaging techniques for estimating OFR in different mammalian species.
To summarize, one of the objectives of this study was to examine porcine, ovine, and bovine ovaries for correlations between the OFR and antral follicles counts. However, the present results do not completely support the hypothesis that antral follicle numbers reflect the size of ovarian follicle reserve. The best results were obtained in the cow where both primary and intermediate follicle numbers were correlated with AFCs. Another purpose of this study was to find a non-invasive, precise technique to estimate the ovarian follicle reserve. Other techniques that are used include an ovarian biopsy, which is highly invasive and can actually cause a decrease in the ovarian follicular reserve due mainly to the development of adhesions.5–7 Also, because of the uneven distribution of the follicles, it may not be an accurate estimator of OFR in all species albeit in cows there were no apparent differences in the numbers of follicles at different regions of the ovary. The primordial, primary, and intermediate follicle numbers could be estimated from ultrasonographic pixel intensity of bovine ovarian cortex, and these were the most promising correlations found in the present experiment. Although these correlations were rather moderate, it can be anticipated that they might become more pronounced with the ensuing advances in ovarian imaging techniques and computerized image analysis. Since the ultrasound technology is less expensive and more accessible, this procedure would be preferred both in humans and animal species of veterinary interest. Future studies should be conducted to see if the results observed in cows would be reproduced in women. Ultrasound imaging combined with computer-assisted analysis of ovarian ultrasonograms can be easily accomplished in women and, based on the similarities between bovine and human ovarian morphology, may prove to be very useful non-invasive techniques in monitoring female fertility.
Authors’ contributions
PMB, MM, DAZ, and LW conceived this study. LW and KW completed all image and statistical analyses and, jointly with PMB, performed ultrasonographic examinations and finalized the preparation of the final version of the present manuscript.
Acknowledgements
This study was funded by the Department of Biomedical Sciences, University of Guelph. Thanks are extended to Ms Alice Daw (OVC HSC Clinical Support) for help with MR imaging. The present results were presented, in the preliminary form, at the Annual Meeting of the Polish Society for Biology of Reproduction (27 February to 1 March 2013) in Gdańsk, Poland.
References
- 1.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004; 127: 569–80. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey TW, Anderson RA, Wright P, Nelson SM, Wallace WHB. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod 2011; 18: 79–87. [DOI] [PubMed] [Google Scholar]
- 3.Wallace WHB, Kelsey TW. Human ovarian reserve from conception to menopause. PLoS ONE 2011; 5: e8772–e8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee S, Modi D, Maitra A, Kadam S, Patel Z, Gokrall J, Meherji P. Screening for FOXL2 gene mutations in women with premature ovarian failure: an Indian experience. Reprod Biomed Online 2007; 15: 554–60. [DOI] [PubMed] [Google Scholar]
- 5.Lass A. Assessment of ovarian reserve: Is there still a role for ovarian biopsy in the light of new data? Hum Reprod 2004; 19: 467–9. [DOI] [PubMed] [Google Scholar]
- 6.Lambalk CB, de Koning CH, Flett A, Van Kasteren Y, Gosden R, Homburg R. Assessment of ovarian reserve. Ovarian biopsy is not a valid method for the prediction of ovarian reserve. Hum Reprod 2004; 19: 1055–9. [DOI] [PubMed] [Google Scholar]
- 7.Kwok R, Johnson NP. Ovarian biopsy has no role as a routine diagnostic test of ovarian reserve: a systematic review. Reprod Biomed Online 2012; 24: 492–4. [DOI] [PubMed] [Google Scholar]
- 8.Jain T, Soules MR, Collins JA. Comparison of basal follicle stimulating hormone versus the clomiphene citrate challenge test for ovarian reserve screening. Fertil Steril 2004; 82: 180–5. [DOI] [PubMed] [Google Scholar]
- 9.Jirge PR. Ovarian reserve tests. J Hum Reprod Sci 2011; 4: 108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwee J, Schats R, McDonnell J, Schoemaker J, Lambalk CB. The clomiphene citrate challenge test versus the exogenous follicle-stimulating hormone ovarian reserve test as a single test for identification of low responders and hyperresponders to in vitro fertilization. Fertil Steril 2006; 85: 1714–22. [DOI] [PubMed] [Google Scholar]
- 11.Ficicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimullerian hormone as an indicator of ovarian reserve. Fertil Steril 2006; 85: 592–6. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho BR, Sobrinho DBG, Vieira ADD, Resende MPS, Barbosa ACP, Silva AA, Nakagawa HM. Ovarian reserve assessment for infertility investigation. ISRN Obstet Gynecol, http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3302183/ (Published online 26 January 2012). DOI: 10.5402/2012/576385. [DOI] [PMC free article] [PubMed]
- 13.Waberski D, Kunz-Schmidt A, Borchard Neto G, Richter L, Weitze K. Real-time ultrasound diagnosis of ovulation and ovarian cysts in sows and its impact on artificial insemination efficiency. Proc Am Soc Anim Sci, www.asas.org/jas/symposia/proceedings/0944.pdf (1999) (accessed August 2014).
- 14.Birtch RL, Baerwald AR, Olatunbosun OA, Pierson RA. Ultrasound image attributes of human ovarian dominant follicles during natural and oral contraceptive cycles. Reprod Biol Endocrinol 2005; 3: 12–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginther OJ, Gastal EL, Gastal MO, Bergfelt DR, Baerwald AR, Pierson R. Comparative study of the dynamics of follicular waves in mares and women. Biol Reprod 2004; 71: 1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarty GE, Adams GP, Pierson RA. Three-dimensional magnetic resonance imaging for the study of ovarian function in a bovine in vitro model. J Reprod Fertil 2000; 119: 69–75. [PubMed] [Google Scholar]
- 17.Hilton JL, Sarty GE, Adams GP, Pierson RA. Magnetic Resonance Image attributes of the ovarian follicle wall during development and regression. Biol Reprod 2001; 65: 1067–73. [DOI] [PubMed] [Google Scholar]
- 18.Ireland JL, Scheetz D, Jimenez-Krassel F, Themmen AP, Ward F, Lonergan P, Smith GW, Perez GI, Evans AC, Ireland JJ. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol Reprod 2008; 79: 1219–25. [DOI] [PubMed] [Google Scholar]
- 19.Adams GP, Pierson RA. Bovine model for study of ovarian follicular dynamics in humans. Theriogenology 1995; 43: 113–20. [Google Scholar]
- 20.Baerwald AR, Adams GP, Pierson RA. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril 2003; 80: 116–22. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Hart EJ, Petrik JJ, Nykamp SG, Bartlewski PM. Relationships between ultrasonographic image attributes, histomorphology and proliferating cell nuclear antigen expression of bovine antral follicles and corpora lutea ex situ. Reprod Dom Anim 2008; 43: 27–34. [DOI] [PubMed] [Google Scholar]
- 22.Payan-Carreira R, Pires MA. Multioocyte follicles in domestic dogs: A survey of frequency of occurrence. Theriogenology 2008; 69: 977–82. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal RS, Singh J, Adams GP. Developmental pattern of small antral follicles in the bovine ovary. Biol Reprod 2004; 71: 1244–51. [DOI] [PubMed] [Google Scholar]
- 24.Mossa F, Jimenez-Krassel F, Folger JK, Ireland JLH, Smith GW, Lonergan P, Evans ACO, Ireland JJ. Evidence that high variation in the antral follicle count during follicular waves is linked to alterations in ovarian androgen production in cattle. Reproduction 2010; 140: 713–20. [DOI] [PubMed] [Google Scholar]
- 25.Silva-Santos KC, Siloto LS, Santos GMG, Morotti F, Marcantonio TN, Seneda MM. Comparison of antral and preantral ovarian follicle populations between Bos indicus and Bos indicus-taurus cows with high or low antral follicles counts. Reprod Dom Anim 2014; 49: 48–51. [DOI] [PubMed] [Google Scholar]
- 26.Kerong S, Xuefeng Y, Liying D, Dengke P, Yunhai Z, Yonghui Z, Xuemei D, Xiaoxiang H, Changxin W, Ning L. Advanced methods of isolation and identification of porcine primordial follicles. Anim Reprod Sci 2007; 101: 163–71. [DOI] [PubMed] [Google Scholar]
- 27.Muruvi W, Picton HM, Rodway RG, Joyce IM. In vitro growth of oocytes from primordial follicles isolated from frozen-thawed lamb ovaries. Theriogenology 2005; 64: 1357–70. [DOI] [PubMed] [Google Scholar]
- 28.Aerts JM, Bols PE. Ovarian follicular dynamics: a review with emphasis on the bovine species. Part I: Folliculogenesis and pre-antral follicle development. Reprod Dom Anim 2010; 45: 171–9. [DOI] [PubMed] [Google Scholar]
- 29.Singh J, Adams GP, Pierson R. Promise of new imaging technologies for assessing ovarian function. Anim Reprod Sci 2003; 78: 371–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pierson RA. Three-dimensional ultrasonography. J Obstet Gynecol 2007; 29: 205–6. [DOI] [PubMed] [Google Scholar]
- 31.Pierson RA, Adams GP. Computer-assisted image analysis, diagnostic ultrasonography and ovulation induction: Strange bedfellows. Theriogenology 1998; 43: 105–12. [Google Scholar]