Abstract
Glaucoma is one of the leading eye diseases resulting in blindness due to the death of retinal ganglion cells. This study aimed to develop novel protocol to promote the differentiation of retinal Müller cells into ganglion cells in vivo in a rat model of glaucoma. The stem cells dedifferentiated from rat retinal Müller cells were randomized to receive transfection with empty lentivirus PGC-FU-GFP or lentivirus PGC-FU-Atoh7-GFP, or no transfection. The stem cells were induced further to differentiate. Ocular hypertension was induced using laser photocoagulation. The eyes were injected with Atoh7 expression vector lentivirus PGC-FU-Atoh7-GFP. Eyeball frozen sections, immunohistochemistry, RT-PCR, Western bolt, and apoptosis assay were performed. We found that the proportion of ganglion cells differentiated from Atoh7-tranfected stem cells was significantly higher than that of the other two groups. The mean intraocular pressure of glaucomatous eyes was elevated significantly compared with those of contralateral eyes. Some retinal Müller cells in the inner nuclear layer entered the mitotic cell cycle in rat chronic ocular hypertension glaucoma model. Atoh7 contributes to the differentiation of retinal Müller cells into retinal ganglion cells in rat model of glaucoma. In conclusion, Atoh7 promotes the differentiation of Müller cells-derived retinal stem cells into retinal ganglion cells in a rat model of glaucoma, thus opening up a new avenue for gene therapy and optic nerve regeneration in glaucoma.
Keywords: Glaucoma, Müller cells, retinal ganglion cells, Atoh7, stem cells, transplantation
Introduction
Glaucoma is a complex multivariate irreversible blinding eye disease which is initiated by several risk factors, including high intraocular pressure (HIOP), increased oxidative stress and free radicals, the release of neurotransmitters such as NO and glutamate, increased calcium concentration, the depletion of neurotrophins and growth factors, and the initiation of apoptotic signals.1–6 These factors create a hostile microenvironment that contributes to secondary damage and results in massive death of retinal ganglion cells. Blockade of the signaling pathway of retinal ganglion cell apoptosis, reduction of intraocular pressure, and nourishment of the optic nerve are known to be somewhat effective in prolonging the life of ganglion cells and retarding disease progression for patients with early glaucoma or progressing glaucoma. However, such treatment strategies are ineffective for patients with advanced and absolute glaucoma. In the late stage of glaucoma, about 90% of retinal ganglion cells can be damaged. This discrepancy lies in the fact that most or all retinal ganglion cells have already undergone apoptosis in patients with advanced and absolute glaucoma, and that the number of surviving ganglion cells is too few to reverse the pathological changes resulting from glaucoma. Therefore, there is a definite need to develop new approach to regenerate retinal ganglion cells to prevent disease progression or even restore vision.
The emergence of cell engineering, particularly stem cell engineering, makes it possible to fulfill this need.7 By incorporating stem cells into the retina and inducing their proliferation and differentiation into target cells, it is possible to replenish retinal neurons and restore retinal function. However, retinal stem cells exist only in the pigmented ciliary epithelium and are too few to meet the clinical need. On the other hand, the use of other stem cells, such as embryonic stem cells and neural stem cells, is greatly restricted due to ethical issues and graft rejection. Retinal Müller cells are glial cells in the retina, and they retain proliferation potential and offer an abundant source for cell engineering.8–10 Müller cells span the entire thickness of the retina and are widely distributed among ganglion cells. This makes it likely to better integrate the cells converted from retinal Müller cells into the ganglion cell layer. All these features suggest that retinal Müller cells may be the most promising source of stem cells for the treatment of glaucoma. However, up to now no studies have examined their differentiation into ganglion cells in a rat model of glaucoma.
The induction and differentiation of retinal stem cells are largely regulated by the joint action of extracellular and intracellular factors. Atoh7 is an essential transcription factor for ganglion cell differentiation.11,12 Our preliminary study showed that Atoh7 overexpression significantly increased the number of retinal ganglion cells differentiated from in vitro cultured retinal stem cells.13 Accordingly, we hypothesize that Atoh7 may promote the differentiation of stem cells dedifferentiated from retinal Müller cells into ganglion cells in rat chronic ocular hypertension glaucoma model.
In this study, we cultured rat retinal Müller cells in vitro and the cells in the 3rd–4th passage were induced to dedifferentiate into stem cells with a stem cell-conditioned medium. Next, the purified neurospheres were collected and dissociated with Accutase. The stem cells were transfected with Atoh7 expression vector and injected into vitreous cavity of rat glaucoma model to explore the signaling mechanisms that regulate the re-differentiation of stem cells derived from Müller cells into ganglion cells.
Methods
Ethics statement
The use of animals in this study was in accordance with the Guidelines for Animal Experiments of Central South University, Changsha, China. All animal experiments in this study were conducted with the approval of the Animal Research Committee, Xiangya School of Medicine, Central South University, Changsha, China (Permit No. SCXK 2006-0002).
Müller cell culture and dedifferentiation
The enrichment of Müller cells was performed as previously described.11 Briefly, the eyes from day 21 Sprague–Dawley (SD) rats were enucleated and washed several times with a phosphate buffer solution (PBS) (GIBCO). The retinae were dissected carefully to avoid contamination from the lens, the retinal pigment epithelium, and the ciliary epithelium. The retina was mechanically dissociated into small aggregates and trypsinized with 0.25% trypsin–EDTA (Sigma) in a 37℃ incubator for 20 min. The digested retina was suspended in DMEM containing 20% FBS and 1:100 penicillin/streptomycin (Sigma), and inoculated in a 25 cm2 culture flask (Corning) for 5–7 days, until the Müller cells attached to the bottom of the flask. The cells were trypsinized and cultured in DMEM containing 20% FBS for six days to further purify the Müller cell population. Cells of the third passage were dissociated with 0.25% trypsin–EDTA and cultured in a serum-free dedifferentiation media containing DMEM/F12 (GIBCO), 1 ×N2 supplement (GIBCO), 2 ×B27 supplement (GIBCO), 20 ng/mL EGF (Peprotech), 10 ng/mL bFGF (Peprotech), 2 mM l-glutamine (HyClone), 100 U/mL penicillin, and 100 µg/mL streptomycin at a density of 1 × 105 cells/cm2 for 5–7 days to generate neurospheres. The dedifferentiation media was half changed every other day. The suspended and semi-suspended neurospheres were collected and dissociated with Accutase and then cultured in serum-free dedifferentiation media to obtain a purified generation.
Establishment of chronic ocular hypertension glaucoma model of rats
Ocular hypertension was induced using a method developed by Chiu et al.14 Briefly, rats were anesthetized with 10% chloral hydrate (0.4 mL/100 g; Sigma-Aldrich Inc., St. Louis, MO, USA) injected intraperitoneally and placed in front of a slit lamp equipped with a 532-nm diode laser that delivered 0.7 W pulses for 0.6 s (Carl Zeiss, Germany). One drop of 1% proparacaine (Alcon-Pharm Inc., Texas, USA) was applied to the right eye (experimental eye) as a topical anesthetic before laser photocoagulation. Then, 50–60 laser pulses were directed to the trabecular meshwork 270° around the circumference of the aqueous outflow area and 15–20 laser spots on each episcleral aqueous humor drainage vein of the right eye. The left eye was control eye without any treatment. IOP was measured bilaterally using a digital tonometer (Tonopen XL, Reichert, USA) at day 3, day 7, day 14, day 28, day 60 after laser photocoagulation.
Apoptosis assay
The apoptosis of RGCs was quantified by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) on retinal tissue sections. TUNEL staining was performed using the DeadEnd™ Fluorometric TUNEL System (Beyotime, Institute of Biotechnology, Wuhan, China). Frozen tissue sections were rinsed in PBS and treated with 1% Triton X-100 in PBS for 2 mins on ice. Slides were equilibrated with equilibration buffer and then incubated for 60 min at 37℃ with recombinant terminal deoxynucleotidyl transferase (rTdT) incubation buffer. The negative control sections were incubated with control incubation buffer without the rTdT enzyme. The number of apoptotic cells was counted from three sections in the ganglion cell layer (GCL) (from three different rats) and from six microscopic fields in each section including two optic disc areas, two peripapillary areas, and two peripheral areas.15 The slides were analyzed using confocal microscope.
Lentivirus PGC-FU-Atoh7-GFP constructs, infection, and intraocular injections
Atoh7 expression vector lentivirus PGC-FU-Atoh7-IRES-GFP was constructed by GENECHEM (Shanghai, China). The neurosphere cells were transfected with the lentivirus at the multiplicity of infection of 10 and the efficiency of transfection was detected by fluorescence activated cell sorting (FACS). Neurospheres dedifferentiated from Müller cells were divided into three groups. Group A: neurospheres transfected by PGC-FU-Atoh7-GFP; group B: neurospheres transfected by empty vector PGC-FU-GFP; and group C: neurospheres without transfection. After 24 h of transfection, the neurospheres were dissociated into single stem cells with Accutase. The stem cells of each group were collected at a concentration of 1 × 104 cells/µL. The rats were anesthetized by inhalation of diethyl ether and i.p. injection of pentobarbital. The eyes were injected with 5 µL of retinal stem cells, 5 µL brain-derived neurotrophic factor (BDNF) (1 ng/mL) (Peprotech), 30 nmol BrdUrd, 100 ng RA (1 μM) (Sigma). At days 7 and 14, eyeball frozen sections and immunohistochemistry were performed for immunocytochemical analysis.
Immunohistochemistry analysis
Eyeball frozen sections and immunohistochemistry were performed as described.16,17 Briefly, rat retinal tissue sections or retinal cells were incubated in PBS containing 3% bovine serum albumin (BSA), 5% goat serum, and 0.3% TritonX-100 at 37℃ for 1 h, followed by incubation at 4℃ overnight with the primary antibodies. The primary antibodies and working dilutions in this study were as follows: mouse anti-BrdUrd (1:50, Roche Applied Science), rabbit anti-GS (1:200, Sigma), mouse anti-Nestin (1:100, Abcam). Rat retinal tissue sections were then incubated in the dark at room temperature for 1 h with anti-rabbit IgG conjugated with FITC (1:100, Sigma), anti-mouse IgG conjugated with FITC (1:100, Sigma), anti-rabbit IgG conjugated with TRITC (1:100, Sigma), and anti-mouse IgG conjugated with TRITC (1:100, Sigma). Finally, the retinal tissue sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime, Institute of Biotechnology, Wuhan, China) for 5 min and images were captured using fluorescent inverse microscopy (Leica DMI4000B).
RT-PCR analysis
Total RNA was isolated from rat retinal tissue or retinal cells using Trizol reagent according to the manufacturer’s protocol and reverse transcribed to cDNA. PCR was performed in a 20 µL volume containing the following: 10 µL 2 ×SYBR Green mix, 1 µL 10 µM forward and reverse primers, 2 µL diluted cDNA and 6 µL double-distilled H2O. The primers were as follows: Glutamine synthetase (GS) Forward: 5′TCACAGGGACAAATGCCGAG3′, Reverse: 5′GTTGATGTTGGAGGTTTCGTGG3′; Vimentin Forward: 5′AAGGCACTAATGAGTCCCTGGAG3′ Reverse: 5′GTTTGGAAGAGGCAGAGAAATCC3′; Clusterin Forward: 5′CCTCCAGTCCAAGATGCTCAAC3′ Reverse: 5′TTTCCTGCGGTATTCCTGTAGC3′; Brn-3 b Forward: 5′GGCTGGAGGAAGCAGAGAAATC 3′ Reverse: 5′TTGGCTGGATGGCGAAGTAG 3′; Nestin Forward: 5′TGGAGCAGGAGAAGCAAGGTCTAC3′ Reverse: 5′TCAAGGGTATTAGGCAAGGGGG3′; Ki-67 Forward: 5′CCATCTTTGCTTGGGAAATCC3′ Reverse: 5′TCATCCGAGTCTTCTCCATTGG3′; Atoh7 Forward: 5′ATGAAGTCGGCCTGCAAAC3′ Reverse: 5′GGGTCTACCTGGAGCCTAGC3′; β-Actin Forward: 5′GTGGGGCGCCCCAGGCACCA 3′ Reverse: 5′ CTCCTTAATGTCACGCACGATTTC 3′. Amplification conditions were as follows: 15 s at 95℃ (one cycle), 5 s at 95℃, 5 s at annealing temperature, and 30 s at 72℃ (45 cycles). The results were analyzed by ABIViia7 (USA).
Western blot analysis
Proteins were extracted from the rat retinal tissue by using radioimmuno precipitation assay buffer containing protease inhibitor and phosphatase inhibitor. Protein concentration was determined by using microplate reader. Lysates were separated on SDS-PAGE and transferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% non-fat milk in Tris Buffer Solution (TBS) plus 0.1% Tween (TBS-T) for 1 h and then incubated with primary antibodies for 1 h at room temperature. After several washes, the membranes were incubated with HRP-conjugated secondary antibodies for 1 h. Blots were visualized with a electro-chemi-luminescence (ECL) kit.
FACS analysis
The purity of the enriched Müller cells was examined by FACS analysis using antibodies specific to Müller cells. Briefly, the cells in the monolayer culture were dissociated into single cells by the method of passage and centrifugation. The cells were fixed with 4% paraformaldehyde and blocked with a PBS containing 1% BSA and 0.1% TritonX-100 for 30 min at 4℃ and then incubated at 4℃ for 1 h with the primary antibodies. After that, the cells were incubated in a PBS–BSA solution containing the appropriate secondary antibodies linked to FITC at 4℃ for 1 h in the dark. The cells were washed with PBS and re-suspended in PBS for FACS analysis.
Statistical analysis
Statistical analysis was performed with one-way ANOVA using SPSS 13.0. P < 0.05 was considered significant.
Results
Purification of Müller cells and dedifferentiation into retinal stem cells
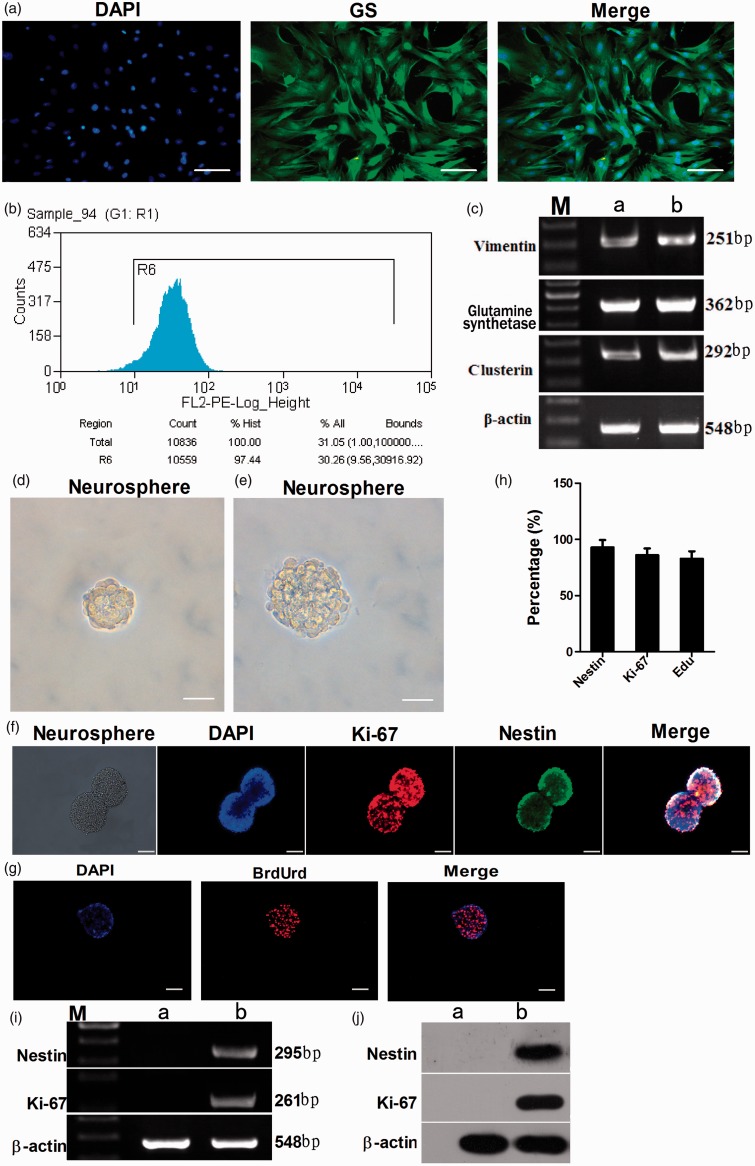
After the dissociated and trypsinized retinal tissues were filtrated by a 200 mesh filter, retinal cells were inoculated in a 25 cm2 culture flask. After 5–7 days of culture, numerous bipolar spindle-shaped cells were observed on the surface of the flask. At 8–10 days, the cells culture medium became light yellow and the culture medium was replaced every 2–3 days, both aggregated and loosely attached materials were discarded, leaving only tightly adherent cells on the flask surface. When the proliferated cells formed a complete confluent monolayer of epithelioid cells, the cells were trypsinized and subcultured in DMEM containing 20% FBS for another 5–6 days to obtain a further purified Müller cell population. After the fourth passage, the cells were examined for immunoreactivity for Müller cell-specific marker GS. The results showed that 98.10 ± 2.18% of the cells in the monolayer culture were immunoreactive for GS (Figure 1(a)). To further determine the purity of Müller cell culture, we carried out FACS and RT-PCR analysis. FACS showed that 97.44% of purified cells were immunoreactive for GS (Figure 1(b)). RT-PCR analysis revealed that cells in the culture expressed a battery of transcripts characteristic of Müller cells, such as GS, vimentin, clusterin9 (Figure 1(a)).
Figure 1.
Characterization of enriched Müller cells. Immunocytochemical analysis showed that more than 98.10% of retinal Müller cells were immunoreactive to Müller cell marker GS (a). Bar = 200 µm. FACS showed that 97.44% of purified cells were immunoreactive for GS (b). The purity of enriched cells was evaluated by RT-PCR analysis to detect the expression of Müller cell-specific transcripts (c). Enriched Müller cells exposed to the stem cell-conditioned medium formed neurospheres (d). Bar = 200 µm. The neurospheres were passaged to get new clonal neurospheres (e). Bar = 200 µm. Immunofluorescence staining showed that the stem cells within the cell spheres had positive expression of retinal stem cell-specific markers Nestin (92.94 ± 6.48%) and Ki-67 (85.96 ± 6.04%) (f, h). Bar = 100 µm. Immunocytochemical analysis of BrdUrd showed that newborn cell spheres had the capacity of effective proliferation (82.80 ± 6.65%) (g, h). Bar = 100 µm. RT-PCR and Western blot analysis to detect the expression of stem cell markers Nestin and Ki-67 (i, h). c: Lane M: DNA marker; lane a: PN21 retina; lane b: purified Müller cells. i,j: lane M: DNA marker; lane a: Müller cells; lane b: neurospheres. (A color version of this figure is available in the online journal.)
The purified Müller cells were cultured in stem cell-conditioned medium. At 5–7 days of culture, the cell spheres increased in both number and size, cells displayed good refraction and exhibited well-defined cell boundaries at the edge of cell spheres (Figure 1(d)). Thereafter, the cell spheres showed no significant increase in number and size. After seven days, the neurospheres were collected and dissociated into single stem cells which were cultured in serum-free dedifferentiation media to generate new clonal neurospheres (Figure 1(e)). Immunofluorescence staining showed that stem cells within the spheres were positive for retinal stem cell-specific markers Nestin (green fluorescence, 92.94 ± 6.48%) and Ki-67 (red fluorescence, 85.96 ± 6.04%) (Figure 1(f) and (h)), suggesting that retinal Müller cells dedifferentiate into retinal stem cells in the conditioned medium. Meanwhile, BrdUrd staining showed that most of the nuclei within the cell spheres were stained red (82.80 ± 6.65%), suggesting that the new cell spheres have the capacity for effective proliferation (Figure 1(g) and (h)). RT-PCR analysis showed that cell spheres in the culture expressed a battery of transcripts characteristic of stem cells such as Nestin and Ki-67, which were absent in the Müller cells (Figure 1(i)). Western blot analysis further confirmed the expression of Nestin and Ki-67 in the cell spheres but not in the Müller cells (Figure 1(j)). Taken together, these data suggest that Müller cells were dedifferentiated into retinal stem cells.
Apoptosis of retinal ganglion cells in rat chronic ocular hypertension glaucoma model
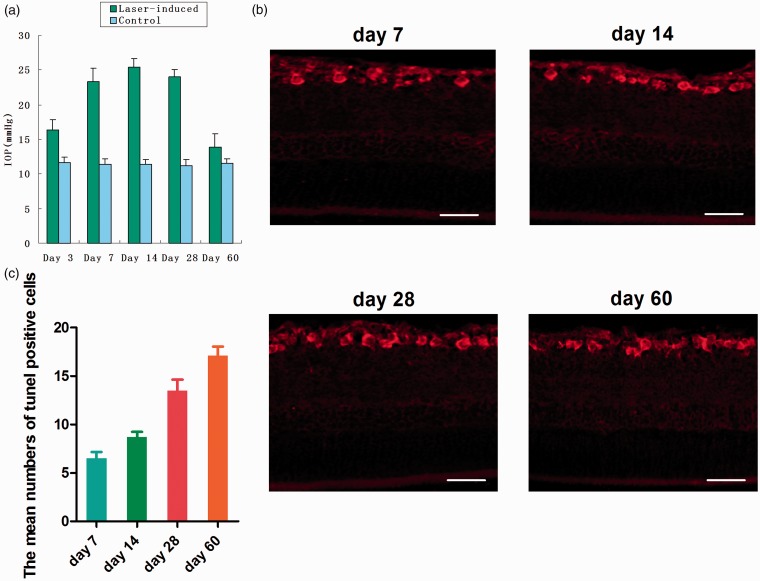
IOP was measured bilaterally under anesthesia at day 3, day 7, day 14, day 28, day 60 after laser treatment using a digital tonometer. The results showed that the mean IOP of glaucomatous eyes was elevated significantly compared with those of contralateral eyes from day 3 to day 28. IOP was gradually increased with the time and reached the maximum in 7–14 days. IOP began to decline about day 28 and reached normal level about two months later (Figure 2(a)).
Figure 2.
Apoptosis of retinal ganglion cells in rat chronic ocular hypertension glaucoma model. Ocular hypertension was induced using laser photocoagulation. The mean IOP of glaucomatous eyes was elevated significantly compared with those of contralateral eyes from day 3 to day 28. IOP was gradually increased with the time and reached the maximum in 7–14 days. IOP began to decline about day 28 and returned to normal level about two months later (a). TUNEL staining of retinal ganglion cells in rat chronic ocular hypertension glaucoma model. At day 60, IOP was decreased to the normal level, but the number of apoptotic cells was still increasing (b, c). Bar = 100 µm. (A color version of this figure is available in the online journal.)
To determine whether retinal ganglion cells undergo apoptosis in rat chronic ocular hypertension glaucoma model, we performed TUNEL staining and found apoptosis of the retinal ganglion cells in retinal ganglion cell layer (Figure 2(b)). The proportion of TUNEL positive cells were 6.5 ± 2.1,8.7 ± 1.7,13.5 ± 3.6,17.1 ± 3.0%, respectively. These data showed that the number of apoptotic nuclei was gradually increased with the continuous HIOP. At day 60, IOP was decreased to the normal level, but the number of apoptotic cells was still increasing (Figure 2(c)).
Lentivirus PGC-FU-Atoh7-GFP transfection and transplantation of retinal stem cells
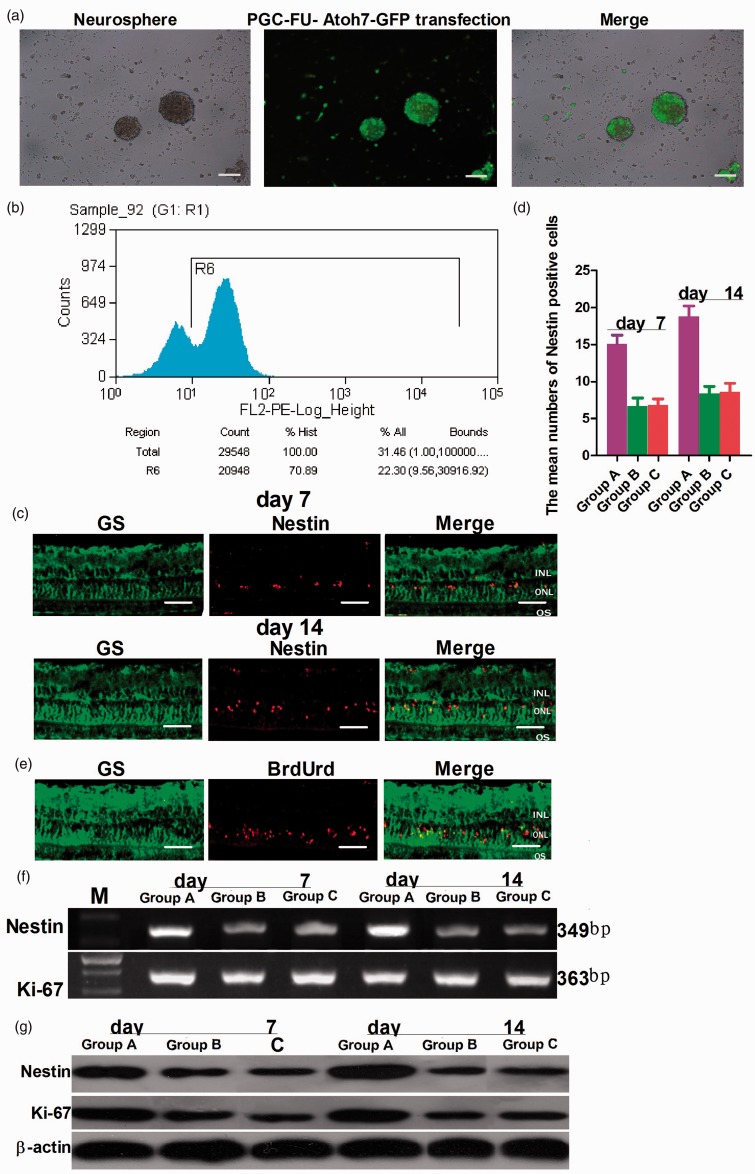
Next, stem cells dedifferentiated from Müller cells were transfected with lentivirus PGC-FU-Atoh7-GFP or empty vector PGC-FU-GFP. After 24 h, the number of GFP labeled cells increased and fluorescence intensity was enhanced and distributed homogeneously in the cytoplasm (Figure 3(a)). FACS analysis showed that the transfection efficiency was 70.89% (Figure 3(b)). At this time, the neurospheres of the three groups were separated into single cells by Accutase. The vitreous cavity of the rat glaucoma model was injected through the pars plana on the superotemporal conjunctiva. At days 7 and 14, immunohistochemical staining of retinal tissue sections showed that the retinal Müller cells were immunoreactive against GS (green fluorescence). Nestin positive cells (red fluorescence) were found in the inner nuclear layer (INL) in the right eyes (30 sections examined, n = 5 animals; group A: 151 cells; group B: 67 cells; group C: 68 cells) and the numbers of positive cells were gradually increased in INL at 14 days (30 sections examined, n = 5 animals; group A: 211 cells; group B: 93 cells; group C: 91 cells) (Figure 3(c) and (d)). However, Nestin positive cells were not detected in the contralateral eye (left eyes) (data not shown). Simultaneously, BrdUrd-positive cells were detected in INL at seven days (Figure 3(e)). These results indicated that retinal stem cells have been integrated into INL. At seven and 14 days, Western blot and RT-PCR analysis in group A and group B showed that retinal tissue in rat chronic ocular hypertension glaucoma model expressed protein and a battery of transcripts characteristic of stem cells such as Nestin and Ki-67, and the protein and mRNA levels of Nestin and Ki-67 at 14 days were more than at seven days. However, there was no difference in the contralateral eye at seven days and 14 days (Figure 3(f) and (g)).
Figure 3.
The transfection and transplantation of retinal stem cells. Stem cells dedifferentiated from Müller cells were transfected with lentivirus PGC-FU-Atoh7-GFP (a). Bar = 100 µm. FACS analysis showed that the transfection efficiency was 70.89% (b). The vitreous cavity of the rat was injected with the stem cells. Immunocytochemical staining of retinal tissue sections showed that the retinal Müller cells were immunoreactive against GS antibodies (green fluorescence). Nestin positive cells (red fluorescence) could be found in the INL in the right eyes (30 sections examined, n = 5 animals; group A: 151 cells; group B: 67 cells; group C: 68 cells). At seven days, the number of positive cells was gradually increased in INL at 14 days (30 sections examined, n = 5 animals; group A: 211 cells; group B: 93 cells; group C: 91 cells) (c, d). Bar = 100 µm. Simultaneously, BrdUrd-positive cells were detected in INL at seven days (e). Bar = 100 µm. Western blot and RT-PCR analysis showed that the amount of protein and mRNA of Nestin and Ki-67 at 14 days were more than at seven days. However, there was no difference in the contralateral eye at seven days and 14 days (f, g). f,g: lane M: DNA marker; lane A: group A: transfected with lentivirus PGC-FU-Atoh7-GFP; lane B: group B: transfected with empty vector PGC-FU-GFP; lane C: group C: no transfection. (A color version of this figure is available in the online journal.)
Differentiation of retinal stem cells in rat chronic ocular hypertension glaucoma model
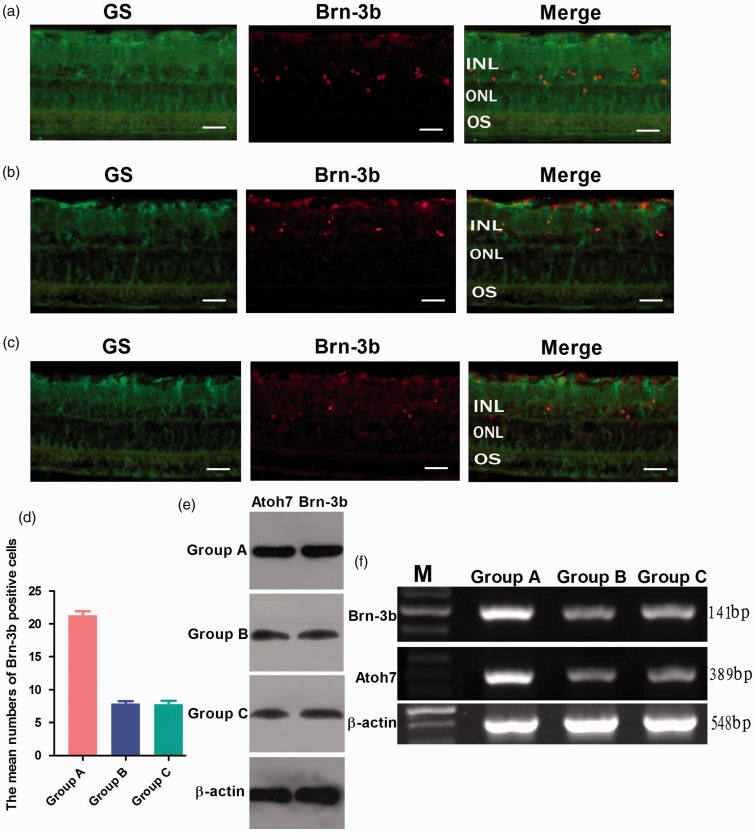
To determine whether Atoh7 promotes the differentiation of Müller cells-derived stem cells into ganglion cells in rat chronic ocular hypertension glaucoma model, immunohistochemical staining of retinal tissue sections was performed for the detection of cell-specific markers Brn-3b (ganglion cell) and GS (Müller cells) after 14 days of retinal stem cells transplantation. The results showed that the proportion of Brn-3b positive cells was 21.3 ± 2.0% in group A, but only 7.9 ± 1.1 and 7.8 ± 1.7% of positive cells expressed Brn-3b in group B and C (Figure 4(a) to (d)). Western blot and RT-PCR analysis showed that the expression of Atoh7 and Brn-3b at both mRNA and protein levels were significantly increased in group A. There were no significant differences between group B and group C (Figure 4(e) and (f)). These data indicate that Atoh7 promotes the differentiation of Müller cells-derived stem cells into ganglion cells in rat chronic ocular hypertension glaucoma model.
Figure 4.
Differentiation of retinal stem cells in rat chronic ocular hypertension glaucoma model. Immunocytochemical staining of retinal tissue sections showed that the proportion of Brn-3b positive cells was 21.3 ± 2.0% in group A, but only 7.9 ± 1.1 and 7.8 ± 1.7% of positive cells expressed Brn-3b in group B and C (a–d). Western blot and RT-PCR analysis showed that the expression of Atoh7 and Brn-3b at both mRNA and protein levels was significantly increased in group A. There were no significant differences between group B and group C (e, f). e,f: lane M: DNA marker; group A: transfected with lentivirus PGC-FU-Atoh7-GFP; group B: transfected with empty vector PGC-FU-GFP; group C: no transfection. Bar = 100 µm. (A color version of this figure is available in the online journal.)
Discussion
Glaucoma is one of the important irreversible blinding eye diseases. The blockade of retinal ganglion cell apoptosis, the reduction of intraocular pressure, and nourishment of the optic nerve are known to be somewhat effective in prolonging the life of ganglion cells and retarding disease progression for patients with early glaucoma or progressing glaucoma.18 Stem cell engineering has emerged as a promising approach for retinal regeneration therapy.19,20 By incorporating stem cells into the retina and inducing their proliferation and differentiation into target cells, it is possible to replenish retinal neurons and restore retinal function. Retinal Müller cells, the glial cells in the retina, retain proliferation potential and offer an abundant source for cell engineering.21,22 Extensive studies in recent years have shown that retinal Müller cells are potential retinal stem cells.23,24 In addition, Müller cells span the entire width of the retina and are widely distributed among ganglion cells. This increases the chance to better integrate the cells converted from Müller cells into the ganglion cell layer. Therefore, retinal Müller cells are the most promising source of stem cells in the treatment of glaucoma.
The directed differentiation of retinal stem cells is mainly co-regulated by the extracellular microenvironment factors and endogenous cytokines. bHLH (basic helix–loop–helix) family plays an important role in regulating retinal cell differentiation.25 Atoh7 is a member of bHLH family and its expression pattern is consistent with the spatiotemporal pattern of retinal ganglion cell differentiation. Atoh7 is a key regulatory factor essential for the formation of retinal ganglion cells in vertebrates.26,27 Ectopical expression of Atoh7 has been shown to increase the number of retinal ganglion cells differentiated from stem cells. Our previous study showed that Atoh7 promoted the differentiation of the stem cells dedifferentiated from Müller cells into large quantities of ganglion cells in vitro.13 To verify whether the regulatory mechanism is the same in vivo, we established rat chronic ocular hypertension glaucoma model in this study. The results showed that the normal value of IOP was between 10 and 12 mmHg in six-week-old male Sprague–Dawley rats. IOP began to rise at three days after laser treatment and gradually reached the maximum in 7–14 days (maximum: 27 mmHg). However, IOP began to decline from 28 days after laser treatment, and IOP returned to normal level about two months later. Simultaneously, apoptosis of the retinal ganglion cells was found in retinal ganglion cell layer after seven days of high IOP (the proportion of tunnel positive cells was 6.5 ± 2.1%). The number of apoptotic cells was gradually increased with the continuous HIOP. However, IOP was decreased to the normal level at day 60, but the number of apoptotic cells was still increasing. These data indicate that HIOP leads to retinal ganglion cell apoptosis.
To determine whether Atoh7 promotes the differentiation of Müller cells-derived stem cells into ganglion cells in rat chronic ocular hypertension glaucoma model, first we established SD rat glaucoma model by a modification of the laser photocoagulation method. Then, the stem cells dedifferentiated from Müller cells were transfected with lentivirus PGC-FU-Atoh7-GFP. After 24 h, the stem cells were transplanted into the vitreous cavity of the rat glaucoma model through the pars plana. At different time points, the retinal sections of glaucoma model eyes to examine immunoreactivity for stem cell-specific marker Nestin. The results showed that Nestin positive cells gradually migrated from the inner limiting membrane to INL layer. The number of Nestin positive cells was gradually increased in INL with the maintenance of hypertension IOP state. At day 14, most of Nestin positive cells were labeled in INL. Simultaneously, BrdUrd-positive cells were detected in INL. These data indicate that Nestin positive cells in INL are from migrating retinal stem cells.
Furthermore, Western blot and RT-PCR analysis showed that the expression of protein and a battery of transcripts characteristic of stem cells such as Nestin and Ki-67 in glaucoma model was gradually increased with the duration of migration. Simultaneously, the location of BrdUrd-labeled proliferative cells was consistent with Nestin positive cells. In addition, immunohistochemistry, Western blot, and RT-PCR analysis showed that the expression of Atoh7 and Brn-3 b at both mRNA and protein levels were significantly increased in group A, but there were no significant differences between group B and group C. These data indicate that Atoh7 promotes the differentiation of Müller cells-derived stem cells into ganglion cells in rat chronic ocular hypertension glaucoma model.
In conclusion, Atoh7 promotes the differentiation of Müller cells-derived retinal stem cells into retinal ganglion cells in a rat model of glaucoma, thus opening up a new avenue for gene therapy and optic nerve regeneration in glaucoma.
Acknowledgements
This study is supported by grants from National Scientific Foundation of China (81170844 and 81400400). We are grateful to Dr Yingqun Wang for his valuable advice and guidance; Dr Siqi Xiong and Qi Zeng for their helpful suggestion; Professor Hong Shen, Dr Jingyu He, and Dr Sai Zhang for technical assistance.
Authors’ contributions
WTS: Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; XYZ: Collection and assembly of data; XBX: corresponding author, conception and design, financial support, final approval of manuscript. All authors read and approved the final manuscript.
References
- 1.Fang JH, Wang XH, Xu ZR, Jiang FG. Neuroprotective effects of bis(7)-tacrine against glutamate-induced retinal ganglion cells damage. BMC Neurosci 2010; 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seki M, Lipton SA. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog Brain Res 2008; 173: 495–510. [DOI] [PubMed] [Google Scholar]
- 3.Tezel G, Yang X, Luo C, Kain AD, Powell DW, Kuehn MH, Kaplan HJ. Oxidative stress and the regulation of complement activation in human glaucoma. Invest Ophthalmol Vis Sci 2010; 51: 5071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coassin M, Lambiase A, Sposato V, Micera A, Bonini S, Aloe L. Retinal p75 and bax overexpression is associated with retinal ganglion cells apoptosis in a rat model of glaucoma. Graefes Arch Clin Exp Ophthalmol 2008; 246: 1743–9. [DOI] [PubMed] [Google Scholar]
- 5.Danesh-Meyer HV. Neuroprotection in glaucoma: recent and future directions. Curr Opin Ophthalmol 2011; 22: 78–86. [DOI] [PubMed] [Google Scholar]
- 6.Kanamori A, Naka M, Fukuda M, Nakamura M, Negi A. Tafluprost protects rat retinal ganglion cells from apoptosis in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol 2009; 247: 1353–60. [DOI] [PubMed] [Google Scholar]
- 7.Giannelli SG, Demontis GC, Pertile G, Rama P, Broccoli V. Adult human muller glia cells are a highly efficient source of rod photoreceptors. Stem Cells 2010; 29: 344–56. [DOI] [PubMed] [Google Scholar]
- 8.Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol 2006; 299: 283–302. [DOI] [PubMed] [Google Scholar]
- 9.Bull ND, Limb GA, Martin KR. Human Müller stem cell (MIO-M1) transplantation in a rat model of glaucoma: survival, differentiation, and integration. Invest Ophthalmol Vis Sci 2008; 49: 3449–56. [DOI] [PubMed] [Google Scholar]
- 10.Fischer AJ, Reh TA. Potential of Müller glia to become neurogenic retinal progenitor cells. Glia 2003; 43: 70–76. [DOI] [PubMed] [Google Scholar]
- 11.Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci USA 2004; 101: 13654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasov L, Glaser T. Pushing the envelope of retinal ganglion cell genesis: context dependent function of Math5 (Atoh7). Dev Biol 2012; 368: 214–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song WT, Zhang XY, Xia XB. Atoh7 promotes the differentiation of retinal stem cells derived from Müller cells into retinal ganglion cells by inhibiting Notch signaling. Stem Cell Res Ther 2013; 4: :49–:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiu K, Chang R, So KF. Laser-induced chronic ocular hypertension model on SD rats. J Vis Exp 2007; 4: 549–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 2006; 9: 268––75. [DOI] [PubMed] [Google Scholar]
- 16.Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science 2000; 287: 2032–6. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad I. Stem cells: new opportunities to treat eye diseases. Invest Ophthalmol Vis Sci 2001; 42: 2743–8. [PubMed] [Google Scholar]
- 18.Baron M. An overview of the Notch signalling pathway. Semin Cell Dev Biol 2003; 14: 113–9. [DOI] [PubMed] [Google Scholar]
- 19.Yu H, Vu TH, Cho KS, Guo C, Chen DF. Mobilizing endogenous stem cells for retinal repair. Transl Res 2014; 163: 387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Shamekh S, Goldberg JL. Retinal repair with induced pluripotent stem cells. Transl Res 2014; 163: 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helledie T, Nurcombe V, Cool SM. A simple and reliable electroporation method for human bone marrow mesenchymal stem cells. Stem Cells Dev 2008; 17: 837–48. [DOI] [PubMed] [Google Scholar]
- 22.Geoffroy CG, Raineteau O. A cre-lox approach for transient transgene expression in neural precursor cells and long-term tracking of their progeny in vitro and in vivo. BMC Dev Biol, 7, 2007, pp. 45–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Müller cell line. Invest Ophthalmol Vis Sci 1998; 39: 212–6. [PubMed] [Google Scholar]
- 24.Kubota A, Nishida K, Nakashima K, Tano Y. Conversion of mammalian Müller glia cells into a neuronal lineage by in vitro aggregate. Biochem Biophys Res Commun 2006; 351: 514–20. [DOI] [PubMed] [Google Scholar]
- 25.Brown NL, Patel S, Brzezinski J, Glaser T. Math5 is required for retinal ganglion cell and optic nerve formation. Development 2001; 128: 2497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for Math5 in the development of retinal ganglion cells. Genes Dev 2001; 15: 24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao J, Sun X, Wang Y, Xu G, Qian J. Atoh7 promotes retinal ganglion cell expression patterns in retinal progenitor cells. Mol Vis 2007; 13: 1066–72. [PMC free article] [PubMed] [Google Scholar]