Abstract
Prostate cancer is a frequently diagnosed cancer in males with high mortality in the world. As a heterogeneous tissue, the tumor mass contains a subpopulation that is called as cancer stem cells and displays stem-like properties such as self-renewal, epithelial–mesenchymal transition, metastasis, and drug resistance. Cancer stem cells have been identified in variant tumors and shown to be regulated by various molecules including microRNAs. MicroRNAs are a class of small non-coding RNAs, which can influence tumorigenesis via different mechanisms. In this review, we focus on the functions of microRNAs on regulating the stemness of prostate cancer stem cells with different mechanisms and propose the potential roles of microRNAs in prostate cancer therapy.
Keywords: miRNAs, PCSCs, EMT, metastasis, drug resistance
Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer and the third leading cause of cancer-related deaths among men in developed countries.1 According to American cancer statistics, approximately 233,000 new cases of PCa were diagnosed and approximately 29,480 PCa deaths occurred in the United States in 2014.2 Up to now, the molecular mechanisms underlying PCa progressions are still unclear. However, recent cancer stem cell hypothesis has provided a novel sight for the diagnosis and treatment of PCa.3
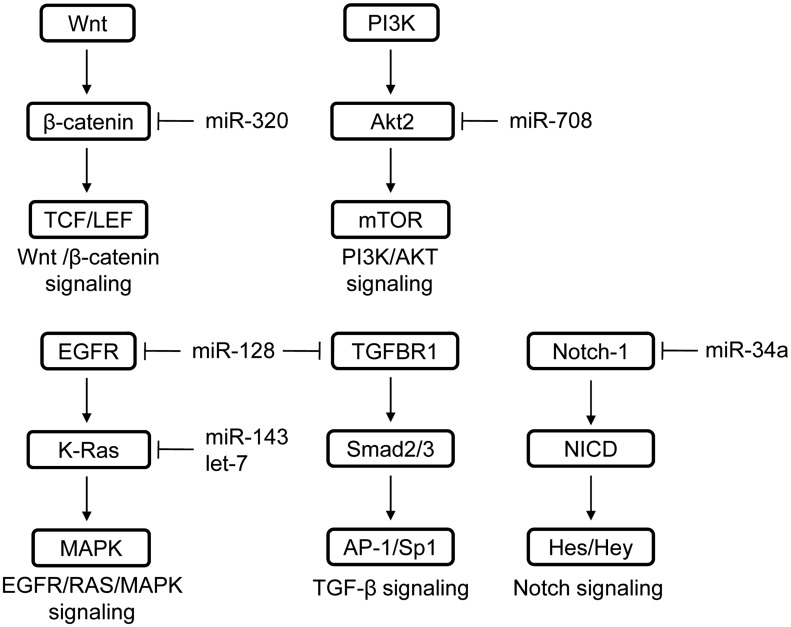
According to this hypothesis, cancer stem cells (CSCs) are a subset of cancer cell subpopulations in the tumor mass and are considered to be responsible for tumor initiation, resistance to anti-cancer therapies, recurrence, and metastasis.4 It was reported that CSCs exist in almost solid tumors including PCa.5 In order to further understand and explore mechanisms for the regulation of stemness of CSCs, especially prostate cancer stem cells (PCSCs), several regulatory factors were identified and among them microRNA (miRNA) is one of the critical factors.6 At present, miRNAs were found to regulate the stemness of PCSCs either directly by targeting stemness-related transcription factors and markers, or indirectly by targeting epithelial–mesenchymal transition (EMT), metastasis-related factors, and drug resistance-related factors. In addition, miRNAs were shown to be associated with regulation of several stemness-related pathways, such as TGF-β,7–9 Wnt/beta-catenin,10,11 and MAPK12 pathway (Figure 1). The aim of this review is to summarize the involvement of specific miRNAs in regulating the stemness of PCSCs (Table 1) and prospect the potential therapeutic application of these miRNAs for PCa.
Figure 1.
MiRNAs that have been reported to regulate the stemness-related pathways. Multiple signaling pathways, which are associated with the maintenance of stemness of PCSCs, have been identified to be regulated by miRNAs.
Table 1.
MiRNAs and related targets for regulation of PCSCs’ stemness. (A color version of this table is available in the online journal.)
| miRNA | Target |
|||
|---|---|---|---|---|
| PCSC-related surface marker | PCSC-related transcription factor | EMT & metastasis related gene | Drug resistance- related gene | |
| miR-34a | CD4424 | AR25 | N/A | SIRT1, Bcl283 |
| miR-708 | CD4426 | N/A | N/A | N/A |
| let-7 | N/A | c-Myc12 EZH231 | N/A | N/A |
| miR-143 | CD133, CD4432 | OCT4, c-Myc, KLF432 | N/A | N/A |
| miR-145 | CD133, CD4432 | OCT4, c-Myc, KLF432 | HEF152 Zeb253 | N/A |
| miR-128 | N/A | Bmi-1, Nanog37 | N/A | N/A |
| miR-100 | N/A | Argonaute 240 | N/A | N/A |
| miR-200b | N/A | N/A | Zeb1/2, Snail258 Slug59 | N/A |
| miR-200c | N/A | N/A | Zeb1/2, Slug60 | N/A |
| miR-205 | N/A | N/A | Zeb1/2, Slug60 PKCɛ61, ΔNp63α62 | N/A |
| miR-409-3p/-5p | N/A | N/A | STAG2, RSU167 | N/A |
| miR-21 | N/A | N/A | BTG269, TGFBR271 | N/A |
| miR-30d miR-181a miR-199a-5p | N/A | N/A | N/A | GRP7879 |
N/A: data not reported (not appreciable)
miRNAs directly regulate the stemness of PCSCs
CSCs are a dynamic and heterogeneous population,13 and can be isolated by specific biomarkers, including cell surface markers and intracellular transcription factors. Up to date, several different cell surface markers have been employed to identify PCSCs from prostate tumors, including CD44,14 CD133,15 integrin α2β1,16 ABCG2,17 and Sca-1.18,19 On the other hand, several well-known transcription factors that function in embryonic or pluripotency stem cells were also found to be involved in the maintenance of stemness in PCSCs.20–23 Interestingly, these surface markers and transcription factors have been shown to be regulated by specific miRNAs, which affect expression and function of specific stemness-related surface markers as well as transcription factors.
miRNAs regulate the stemness of PCSCs by targeting PCSC-related surface markers
MiR-34a
By comparing the miRNA profiling between PCSCs and non-PCSCs, Liu et al.24 have found that miR-34a is consistently under-expressed in six xenograft PCSC populations (three CD44+, one CD133+, one α2β1high, and one SP) as well as in CD44+ PCSC subpopulations from primary tumors, suggesting that miR-34a may play a key role in negatively regulating PCSC features. Functional experiments further demonstrated that miR-34a overexpression inhibits holoclone formation, clonogenic capacity, and sphere establishment in Du145, LAPC4, and PPC-1 PCa cells. In addition, miR-34a was found to be down-regulated in CD44+ PCa and restoration of miR-34a in CD44+ PCa also inhibited PCSC characteristics. To further explore the molecular mechanisms, they discovered that miR-34a inhibits PCSCs functions via suppressing CD44 expression and directly binding to the complimentary site in CD44 3′UTR. Collectively, these results suggest that miR-34a possesses tumor-inhibitory effects in PCa cells through negatively regulating stem cell properties in PCa cells. Besides CD44, miR-34a was also found to regulate self-renewal capacity of PCSCs via directly targeting AR and Notch-1 signaling, both of which are critically involved in growth and metastasis of PCa.25
MiR-708
Besides miR-34a, it was demonstrated that miR-708 is another PCSCs suppressive gene by targeting CD44 in PCa.26 MiR-708 is down-regulated in CD44+ PCa population, indicating that miR-708 is associated with the stemness of PCSCs. Overexpression of miR-708 in CD44+ PCa represses sphere formation and clonogenic potential, while inhibition of miR-708 in CD44– cells increased growth and sphere formation ability. MiR-708 also directly regulates Akt2, a core member of PI3K/Akt signaling pathway, which plays a key role in tumor progression and maintenance of cancer stem-like cell features.27
miRNAs regulate PCSCs-related transcriptional factors
Besides cell surface markers, many intracellular stem cell-related transcription factors are also used to identify PCSCs, such as KLF4,20 OCT4,21 Sox2,22 Nanog,23 and Bmi-1.28 miRNAs can regulate the stemness of CSCs also via targeting these key transcription factors and their downstream pathways.6
Let-7 family
Let-7 family had been demonstrated to possess strong CSC-suppressing functions in breast cancer,29 lung cancer,30 and PCa12 through miRNA profiling assays. Kong et al.31 reported that ectopic expression of let-7 suppresses stemness of PCSCs through inhibiting the expression of enhancer of Zeste homolog 2 which plays a key role in embryogenesis, normal stem cells, and CSCs. They also found that let-7 family expression is positively regulated by BR-DIM (metabolite 3,3′-diindolylmethan), which inhibits the growth of PCa cells. Moreover, it has been reported that let-7b overexpression inhibits clonal and sphere formation, via repressing c-Myc and K-Ras, both of which are oncogenic and self-renewal molecules.12 These results indicated a complex regulatory network between let-7 and CSCs-associated genes, and an essential role of let-7 in regulating the stemness of PCSCs.
MiR-143 and miR-145
Overexpression of miR-143 and miR-145 in PC3 cells was found to inhibit the expression of PCSC markers and stemness factors, such as CD133, CD44, OCT4, c-Myc, and KLF4, and to suppress tumor sphere formation as well as tumorigenesis.32 Moreover, the transcription of miR-145 is repressed by OCT4, which uncovers a double-negative feedback loop between OCT4 and miR-145.33 This double-negative feedback loop was also found to modulate cell differentiation, suggesting an important role of miR-145 in repressing stemness of PCSCs.
MiR-128
MiR-128 has been reported to inhibit the growth of glioblastoma34 and breast CSCs35,36 by directly targeting Bmi-1 (B lymphoma Mo-ML V insertion region 1 homolog), a component of the PRC2 polycomb repressor complex and a critical regulator of stem cell self-renewal and malignant transformation.28 Jin et al.37 demonstrated that overexpression of miR-128 has a similar effect on suppressing proliferation, invasion, clonogenic, and sphere-forming capacities in PCa. They further found that endogenous miR-128 levels in PCa are reversely correlated with their clonogenic and tumorigenic potential. These findings suggest that miR-128 shows inhibitory effects in PCa initiation. By mechanistic study, miR-128 regulates a cohort of oncogenic and stem cell-related genes in PCa cells, including Bmi-1, Nanog, TGFBR1, and EGFR, all of which are implicated in maintenance of CSCs’ stemness.37 In addition, several PCSC populations, including CD133+ and CD44+ show a reduced miR-128 expression level. Forced miRNA-128 expression in CD44+ PCSCs strongly suppresses PCSC properties, while down-regulation of miRNA-128 in CD44– PCa enhances the stemness. These data indicate that miR-128 can weaken the stemness of cancer stem-like cells.
MiR-320
MiR-320 has been reported to be down-regulated in multiple cancers, such as breast cancer,38 colon cancer,39 as well as PCa.10 In PCa cell line PC3 and DU145, overexpression of miR-320 directly inhibits β-catenin and significantly represses the expression of Wnt/β-catenin pathway regulatory factors (c-Myc, LFF1, CD44, Sox9, OCT4, cyclin D1) and stem cell markers (CD133, CD117, CXCR4, ABCG2), so as to suppress the stemness of PCSCs.10
MiR-100
It was found that miR-100 impairs stemness properties of PCa cells through directly targeting Argonaute 2, an oncogene that directly regulates expression of stemness factors, such as Oct4, Sox2, Nanog, KLF4, and c-Myc, following its binding to their regulatory regions.40 MiR-100 negatively regulates colony formation and spheroid formation of PCSCs and its expression is dramatically down-regulated in PCa especially in the bone metastasis patients.40,41 On the other hand, it was also reported that a higher miR-100 expression level is positively correlated with biochemical recurrence after radical prostatectomy.42 Therefore, these studies indicate a possible context-dependent role shift of miR-100 between a tumor suppressor and an oncogene.
Thus, examples above demonstrate that tumor suppressor miRNAs can impair PCSCs’ stemness by directly targeting stemness-related transcription factors as well as stemness-associated markers and in turn inhibit tumor progression.
miRNAs regulate PCSCs indirectly through targeting EMT- and metastasis-associated factors
Epithelial–mesenchymal transition (EMT) is a multistep change in which epithelial characteristics are lost and mesenchymal phenotypes are acquired.43 EMT endows cancer cells with malignant properties of metastasis, invasiveness as well as stemness.44–46 On the other hand, CSCs also have mesenchymal-like features.47 On the molecular mechanism, EMT and CSC development may share common signaling pathways, such as Wnt, Notch, and hedgehog (Hh) pathways.48 The EMT process is regulated by multiple transcription factors including N-cadherin, Snail1/2, and Zeb1/2, most of which are implicated in CSCs features and are regulated by miRNAs, suggesting that miRNAs can modulate the stemness of CSCs via regulating these EMT-associated factors. In PCa, there are multiple miRNAs reported to regulate EMT, such as miR-145, miR-200 family, miR-205, and so on.
MiR-145
MiR-145 has been reported to regulate EMT and CSCs in breast cancer,49 lung cancer,50 and renal cancer.51 In PCa, Guo et al. demonstrated that miR-145 suppresses EMT and invasion partially through repressing HEF1.52 Ren et al.53 also found that miR-145 directly suppresses Zeb2 expression and reversely Zeb2 inhibits transcription of miR-145. Hence, there is a negative feedback loop between Zeb2 and miR-145. Functional experiments showed that this feedback loop inhibits EMT and stem cell properties in PCa. Additionally, it has also been reported that p53 inhibits EMT and the stemness of PC3 and DU145 PCa cells by directly increasing the expression of miR-145 at the transcriptional level.54 All these data indicate that miR-145 plays an essential role in regulating the stemness of PCSCs and EMT.
MiR-200 family
All the four miR-200 family members (miR-200a/b/c and miR-141) have been reported to be associated with EMT.55 Among these, miR-200b is a critical regulator for EMT, CSC maintenance, and cancer chemosensitivity.56,57 In PDGF-D (platelet-derived growth factor-D) induced EMT phenotype in PC3 cells, miR-200b is down-regulated and restoration of miR-200b leads to a reversal of the EMT phenotype and enhanced expression of epithelial markers following down-regulation of Zeb1/2 and Snail2.58 Liu et al. further demonstrated that miR-200b along with miR-1 directly targets Slug and in turn inhibits tumor proliferation and delays tumorigenesis.59 Furthermore, they also found that Slug inhibits miR-200b expression by directly binding to the promoter of pri-miR-200b, showing that there is a mutually inhibitory feedback loop between miR-200b and Slug. Besides miR-200b, overexpression of another member miR-200c as well as miR-205 is also found to enhance E-cad expression and to reduce expression of Zeb1/2 and Slug, two key components closely associated with both mesenchymal phenotypes and stemness of PCSCs.60
MiR-205
Gandellini et al. have reported that miR-205 reverses EMT progression by negatively regulating expression of multiple genes, such as Zeb2 and PKCɛ, and in turn inhibits cancer stem-like properties in PCa.61 Ectopic overexpression of miR-205 inhibits prostasphere formation and decreases the proportion of CD44high/CD24low and CD133+ cancer stem-like cells in PCa cells. Moreover, they also found that HIF-1a (hypoxia-inducible factor-1a), a malignant transformation factor, directly represses miR-205 expression at a transcriptional level.61 In addition, miR-205 can also repress invasion through a miR-205-ΔNp63α auto-regulatory network, which is essential for the maintenance of the basement membrane in the prostate epithelium.62
MiR-409-3p and -5p
Besides the tumor-suppressor miRNAs described above, there are several important oncogenic miRNAs that enhance EMT and the stemness of PCSCs, including miR-409-3p and −5p.63 These two miRNAs are members of the DLK1-DIO3 (delta-like 1 homolog–deiodinase, iodothyronine 3) gene cluster which are associated with pluripotency levels in embryonic stem cells64 and cancer development.65,66 MiR-409-3p has been reported to be up-regulated in the serum of patients with high risks compared to those with low risks.63 Josson et al. further demonstrated that miR-409-3p and −5p are notably up-regulated in two aggressive, bone metastatic PCa models and play an important role in facilitating tumor growth, EMT, and bone metastasis through inhibiting multiple tumor suppressors67 such as STAG2 (stromal antigen 2) and RSU1 (ras suppressor protein 1). MiR-409-3p and -5p were also found to be involved in prostatic tumorigenesis via tumor–stromal interactions. Up-regulation of miR-409 in normal prostate fibroblasts confers a cancer-associated stroma-like phenotype and releases miR-409 into the tumor microenvironment to promote tumor induction and EMT.68
MiR-21
A well-known oncogenic miRNA, miR-21, has also been found to contribute to EMT by directly targeting BTG2 (basal protein B-cell translocation gene 2), which is implicated in PCa transformation and progression.69 Bao et al. reported that miR-21 is induced by hypoxia and HIF and involved in acquisition of EMT, maintenance of CSCs functions, and therapeutic resistance.70 They also demonstrated that miR-21 down-regulation represses prostasphere formations, expression of CSC markers CD44, and EpCAM as well as CSCs-related factors VEGF.70 In addition, AR was reported to activate the transcription of miR-21 and in turn inhibit TGF-β receptor II (TGFBR2) expression in PCa cells, so to escape the growth inhibition from the TGF-β/Smad 3 pathway.71 These results indicate that miR-21 plays an important role in enhancing PCSCs’ stemness by enhancing EMT.
Thus, besides repressing the expression of stemness factors directly, tumor suppressive miRNAs can also impair PCSCs’ stemness indirectly by reversing EMT and inhibiting metastasis. On the contrary, oncogenic miRNAs can also enhance PCSCs’ stemness indirectly by repressing the expression of these EMT-inhibiting factors.
miRNAs regulate PCSCs indirectly through targeting drug resistance-associated factors
As mentioned above, one feature of CSCs is therapeutic resistance.72,73 In PCa, after androgen deprivation and chemoradiotherapy, most patients eventually progress to castration resistance prostate cancers and metastasis.74 PCSCs have been reported to be tightly associated with drug resistance and to express related genes at a high level.75,76 Several signaling pathways regulating the self-renewal behavior of CSCs, including PI3K/Akt and Ras pathway, are identified to be associated with chemotherapy.77,78 Underlying mechanisms of CSCs-related therapy resistance are associated with DNA damage response, apoptosis resistance, autophage, etc.72 Thus, multiple miRNAs related to drug resistance, including miR-143 and miR-34a, are reported to regulate the stemness of PCSCs indirectly.
MiR-143
Xu et al. found that PC3 and DU145 transfected with miR-143 inhibits cell growth and enhances the sensitivity to docetaxel through suppressing K-Ras, a key component in EGFR/RAS/MAPK pathway, which is associated with PCSCs’ stemness.78
MiR-30d, miR-181a, and miR-199a-5p
Su et al. demonstrated that miR-30d, miR-181a, and miR-199a-5p cooperatively increases the sensitivity of several different human cancer cell lines including C42B (a PCa cell line) to an HDAC inhibitor TSA through suppressing a key signaling regulator GRP78,79 which is significantly associated with stemness maintenance, drug resistance, and apoptosis resistance in different types of cancers.80–82
MiR-34a
In addition to the direct regulatory function of miR-34a on repression of PCSCs’ stemness, Kojima et al. reported that miR-34a attenuates paclitaxel-resistance of castration resistance prostate cancers through directly targeting SIRT1 (silent mating type information regulation 2 homolog 1) and Bcl2, both of which play a crucial role in promoting tumorigenesis and developing drug resistance.83 Moreover, Corcoran et al. also demonstrated that miR-34a is essential in reducing chemoresistance to docetaxel in PCa patients.84
The above studies demonstrate that besides repressing the expression of stemness factors directly, tumor suppressive miRNAs can also impair PCSCs’ stemness indirectly by reducing chemoresistance and increasing their drug sensitivity.
Conclusion
Mounting evidences indicate that tumor suppressive miRNAs can repress the stemness of PCSCs both directly (via inhibiting the expression of stemness-related transcription factors and stemness-associated markers) and indirectly (via reversing EMT and restoring the drug sensitivity). In the future, identification of more miRNAs that specifically block the PCSCs’ stemness or stemness-associated pathways (Figure 1) directly or indirectly and elucidation of their acting mechanisms will still be helpful. We predict that specific stemness-related miRNAs will become not only very useful markers for the diagnosis and prognosis but also potential therapeutic molecules for PCa.
Acknowledgements
This study was supported by funds to Yu-Xiang Fang from the National Natural Science Foundation of China (81301857), Shanghai Education Committee Supporting Project for Youth Investigator in Colleges and Universities, Shanghai Jiao Tong University Foundation of Medicine-Engineering Science Project (YG2012MS47). This study was also supported by funds to Wei-Qiang Gao from the Chinese Ministry of Science and Technology (2012CB966800 and 2013CB945600), the National Natural Science Foundation of China (81130038 and 81372189), Science and Technology Commission of Shanghai Municipality (Pujiang program), Shanghai Education Committee Key Discipline and Specialty Foundation (J50208), Shanghai Health Bureau Key Discipline and Specialty Foundation and KC Wong foundation.
Author contributions
All authors participated in the design and interpretation of the studies; Y-XF and Y-LC wrote the manuscript, and WQG edited the manuscript. Y-XF and Y-LC contributed equally to this work.
References
- 1.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev 2010; 24: 1967–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 3.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008; 8: 755–68. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell 2012; 10: 717–28. [DOI] [PubMed] [Google Scholar]
- 5.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, Bonal DM, Charytonowicz E, Gladoun N, de la Iglesia-Vicente J, Petrylak DP, Benson MC, Silva JM, Cordon-Cardo C. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell 2012; 22: 373–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Tang DG. MicroRNA regulation of cancer stem cells. Cancer Res 2011; 71: 5950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal J, Sarkar FH, Majumdar AP. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFbetaR2) in colon cancer cells. Carcinogenesis 2012; 33: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Yu Y, Tsuyada A, Ren X, Wu X, Stubblefield K, Rankin-Gee EK, Wang SE. Transforming growth factor-beta regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene 2011; 30: 1470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu K, Ding J, Chen C, Sun W, Ning BF, Wen W, Huang L, Han T, Yang W, Wang C, Li Z, Wu MC, Feng GS, Xie WF, Wang HY. Hepatic transforming growth factor beta gives rise to tumor-initiating cells and promotes liver cancer development. Hepatology 2012; 56: 2255–67. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ, Lee KH, Yeh SD, Hong TM, Chen YL. MicroRNA-320 suppresses the stem cell-like characteristics of prostate cancer cells by downregulating the Wnt/beta-catenin signaling pathway. Carcinogenesis 2013; 34: 530–8. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Ruan B, You N, Huang Q, Liu W, Dang Z, Xu W, Zhou T, Ji R, Cao Y, Li X, Wang D, Tao K, Dou K. Downregulation of miR-200a induces EMT phenotypes and CSC-like signatures through targeting the beta-catenin pathway in hepatic oval cells. PLoS One 2013; 8: e79409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Kelnar K, Vlassov AV, Brown D, Wang J, Tang DG. Distinct microRNA expression profiles in prostate cancer stem/progenitor cells and tumor-suppressive functions of let-7. Cancer Res 2012; 72: 3393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 2009; 138: 822–9. [DOI] [PubMed] [Google Scholar]
- 14.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006; 25: 1696–708. [DOI] [PubMed] [Google Scholar]
- 15.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci 2004; 117: 3539–45. [DOI] [PubMed] [Google Scholar]
- 16.Rentala S, Yalavarthy PD, Mangamoori LN. Alpha1 and beta1 integrins enhance the homing and differentiation of cultured prostate cancer stem cells. Asian J Androl 2010; 12: 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001; 7: 1028–34. [DOI] [PubMed] [Google Scholar]
- 18.Mulholland DJ, Xin L, Morim A, Lawson D, Witte O, Wu H. Lin-Sca-1+CD49f high stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res 2009; 69: 8555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc Natl Acad Sci USA 2005; 102: 6942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moad M, Pal D, Hepburn AC, Williamson SC, Wilson L, Lako M, Armstrong L, Hayward SW, Franco OE, Cates JM, Fordham SE, Przyborski S, Carr-Wilkinson J, Robson CN, Heer R. A novel model of urinary tract differentiation, tissue regeneration, and disease: reprogramming human prostate and bladder cells into induced pluripotent stem cells. Eur Urol 2013; 64: 753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Y, Yang Y, Li W, Chen Q, Li J, Pan X, Zhou L, Liu C, Chen C, He J, Cao H, Yao H, Zheng L, Xu X, Xia Z, Ren J, Xiao L, Li L, Shen B, Zhou H, Wang YJ. Reciprocal regulation of Akt and Oct4 promotes the self-renewal and survival of embryonal carcinoma cells. Mol Cell 2012; 48: 627–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Pinilla SM, Sarrio D, Moreno-Bueno G, Rodriguez-Gil Y, Martinez MA, Hernandez L, Hardisson D, Reis-Filho JS, Palacios J. Sox2: a possible driver of the basal-like phenotype in sporadic breast cancer. Mod Pathol 2007; 20: 474–81. [DOI] [PubMed] [Google Scholar]
- 23.Noh KH, Kim BW, Song KH, Cho H, Lee YH, Kim JH, Chung JY, Kim JH, Hewitt SM, Seong SY, Mao CP, Wu TC, Kim TW. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J Clin Invest 2012; 122: 4077–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011; 17: 211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashat M, Azzouz L, Sarkar SH, Kong D, Li Y, Sarkar FH. Inactivation of AR and Notch-1 signaling by miR-34a attenuates prostate cancer aggressiveness. Am J Transl Res 2012; 4: 432–42. [PMC free article] [PubMed] [Google Scholar]
- 26.Saini S, Majid S, Shahryari V, Arora S, Yamamura S, Chang I, Zaman MS, Deng G, Tanaka Y, Dahiya R. miRNA-708 control of CD44(+) prostate cancer-initiating cells. Cancer Res 2012; 72: 3618–30. [DOI] [PubMed] [Google Scholar]
- 27.Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, Garcia-Echeverria C, Schultz PG, Reddy VA. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA 2009; 106: 268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell 2010; 7: 682–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007; 131: 1109–23. [DOI] [PubMed] [Google Scholar]
- 30.Hua S, Xiaotao X, Renhua G, Yongmei Y, Lianke L, Wen G, Yongqian S. Reduced miR-31 and let-7 maintain the balance between differentiation and quiescence in lung cancer stem-like side population cells. Biomed Pharmacother 2012; 66: 89–97. [DOI] [PubMed] [Google Scholar]
- 31.Kong D, Heath E, Chen W, Cher ML, Powell I, Heilbrun L, Li Y, Ali S, Sethi S, Hassan O, Hwang C, Gupta N, Chitale D, Sakr WA, Menon M, Sarkar FH. Loss of let-7 up-regulates EZH2 in prostate cancer consistent with the acquisition of cancer stem cell signatures that are attenuated by BR-DIM. PLoS One 2012; 7: e33729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S, Guo W, Tang Y, Ren D, Zou X, Peng X. miR-143 and miR-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncol Rep 2012; 28: 1831–7. [DOI] [PubMed] [Google Scholar]
- 33.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. microRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 2009; 137: 647–58. [DOI] [PubMed] [Google Scholar]
- 34.Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB, Chiocca EA, Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res 2008; 68: 9125–30. [DOI] [PubMed] [Google Scholar]
- 35.Masri S, Liu Z, Phung S, Wang E, Yuan YC, Chen S. The role of microRNA-128a in regulating TGFbeta signaling in letrozole-resistant breast cancer cells. Breast Cancer Res Treat 2010; 124: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y, Yu F, Jiao Y, Feng J, Tang W, Yao H, Gong C, Chen J, Su F, Zhang Y, Song E. Reduced miR-128 in breast tumor-initiating cells induces chemotherapeutic resistance via Bmi-1 and ABCC5. Clin Cancer Res 2011; 17: 7105–15. [DOI] [PubMed] [Google Scholar]
- 37.Jin M, Zhang T, Liu C, Badeaux MA, Liu B, Liu R, Jeter C, Chen X, Vlassov AV, Tang DG. miRNA-128 suppresses prostate cancer by inhibiting BMI-1 to inhibit tumor-initiating cells. Cancer Res 2014; 74: 4183–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bronisz A, Godlewski J, Wallace JA, Merchant AS, Nowicki MO, Mathsyaraja H, Srinivasan R, Trimboli AJ, Martin CK, Li F, Yu L, Fernandez SA, Pécot T, Rosol TJ, Cory S, Hallett M, Park M, Piper MG, Marsh CB, Yee LD, Jimenez RE, Nuovo G, Lawler SE, Chiocca EA, Leone G, Ostrowski MC. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol 2011; 14: 159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schepeler T, Reinert JT, Ostenfeld MS, Christensen LL, Silahtaroglu AN, Dyrskjot L, Wiuf C, Sorensen FJ, Kruhoffer M, Laurberg S, Kauppinen S, Orntoft TF, Andersen CL. Diagnostic and prognostic microRNAs in stage II colon cancer. Cancer Res 2008; 68: 6416–24. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Ren D, Guo W, Wang Z, Huang S, Du H, Song L, Peng X. Loss of miR-100 enhances migration, invasion, epithelial-mesenchymal transition and stemness properties in prostate cancer cells through targeting Argonaute 2. Int J Oncol 2014; 45: 362–72. [DOI] [PubMed] [Google Scholar]
- 41.Leite KR, Sousa-Canavez JM, Reis ST, Tomiyama AH, Camara-Lopes LH, Sañudo A, Antunes AA, Srougi M. Change in expression of miR-let7c, miR-100, and miR-218 from high grade localized prostate cancer to metastasis. Urol Oncol 2011; 29: 265–9. [DOI] [PubMed] [Google Scholar]
- 42.Leite KR, Tomiyama A, Reis ST, Sousa-Canavez JM, Sañudo A, Dall'Oglio MF, Camara-Lopes LH, Srougi M. MicroRNA-100 expression is independently related to biochemical recurrence of prostate cancer. J Urol 2011; 185: 1118–22. [DOI] [PubMed] [Google Scholar]
- 43.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119: 1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9: 265–73. [DOI] [PubMed] [Google Scholar]
- 45.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarrio D, Franklin CK, Mackay A, Reis-Filho JS, Isacke CM. Epithelial and mesenchymal subpopulations within normal basal breast cell lines exhibit distinct stem cell/progenitor properties. Stem Cells 2012; 30: 292–303. [DOI] [PubMed] [Google Scholar]
- 47.Leth-Larsen R, Terp MG, Christensen AG, Elias D, Kuhlwein T, Jensen ON, Petersen OW, Ditzel HJ. Functional heterogeneity within the CD44 high human breast cancer stem cell-like compartment reveals a gene signature predictive of distant metastasis. Mol Med 2012; 18: 1109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia 2010; 15: 235–52. [DOI] [PubMed] [Google Scholar]
- 49.Hu J, Guo H, Li H, Liu Y, Liu J, Chen L, Zhang J, Zhang N. MiR-145 regulates epithelial to mesenchymal transition of breast cancer cells by targeting Oct4. PLoS One 2012; 7: e45965. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Hu J, Qiu M, Jiang F, Zhang S, Yang X, Wang J, Xu L, Yin R. MiR-145 regulates cancer stem-like properties and epithelial-to-mesenchymal transition in lung adenocarcinoma-initiating cells. Tumour Biol 2014; 35: 8953–61. [DOI] [PubMed] [Google Scholar]
- 51.Lu R, Ji Z, Li X, Zhai Q, Zhao C, Jiang Z, Zhang S, Nie L, Yu Z. miR-145 functions as tumor suppressor and targets two oncogenes, ANGPT2 and NEDD9, in renal cell carcinoma. J Cancer Res Clin Oncol 2014; 140: 387–97. [DOI] [PubMed] [Google Scholar]
- 52.Guo W, Ren D, Chen X, Tu X, Huang S, Wang M, Song L, Zou X, Peng X. HEF1 promotes epithelial mesenchymal transition and bone invasion in prostate cancer under the regulation of microRNA-145. J Cell Biochem 2013; 114: 1606–15. [DOI] [PubMed] [Google Scholar]
- 53.Ren D, Wang M, Guo W, Huang S, Wang Z, Zhao X, Du H, Song L, Peng X. Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells. Cell Tissue Res 2014; 358: 763–78. [DOI] [PubMed] [Google Scholar]
- 54.Ren D, Wang M, Guo W, Zhao X, Tu X, Huang S, Zou X, Peng X. Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR145. Int J Oncol 2013; 42: 1473–81. [DOI] [PubMed] [Google Scholar]
- 55.Feng B, Wang R, Chen LB. Review of miR-200b and cancer chemosensitivity. Biomed Pharmacother 2012; 66: 397–402. [DOI] [PubMed] [Google Scholar]
- 56.Zhu W, Xu H, Zhu D, Zhi H, Wang T, Wang J, Jiang B, Shu Y, Liu P. miR-200bc/429 cluster modulates multidrug resistance of human cancer cell lines by targeting BCL2 and XIAP. Cancer Chemother Pharmacol 2012; 69: 723–731. [DOI] [PubMed] [Google Scholar]
- 57.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, Zur HA, Brunton VG, Morton J, Sansom O, Schuler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol 2009; 11: 1487–95. [DOI] [PubMed] [Google Scholar]
- 58.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells 2009; 27: 1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, Martin P, Kelly K. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 2013; 32: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puhr M, Hoefer J, Schafer G, Erb HH, Oh SJ, Klocker H, Heidegger I, Neuwirt H, Culig Z. Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am J Pathol 2012; 181: 2188–201. [DOI] [PubMed] [Google Scholar]
- 61.Gandellini P, Giannoni E, Casamichele A, Taddei ML, Callari M, Piovan C, Valdagni R, Pierotti MA, Zaffaroni N, Chiarugi P. miR-205 hinders the malignant interplay between prostate cancer cells and associated fibroblasts. Antioxid Redox Signal 2014; 20: 1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gandellini P, Profumo V, Casamichele A, Fenderico N, Borrelli S, Petrovich G, Santilli G, Callari M, Colecchia M, Pozzi S, De Cesare M, Folini M, Valdagni R, Mantovani R, Zaffaroni N. miR-205 regulates basement membrane deposition in human prostate: implications for cancer development. Cell Death Differ 2012; 19: 1750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen HC, Xie W, Yang M, Hsieh CL, Drouin S, Lee GS, Kantoff PW. Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancer. Prostate 2013; 73: 346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu L, Luo GZ, Yang W, Zhao X, Zheng Q, Lv Z, Li W, Wu HJ, Wang L, Wang XJ, Zhou Q. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J Biol Chem 2010; 285: 19483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luk JM, Burchard J, Zhang C, Liu AM, Wong KF, Shek FH, Lee NP, Fan ST, Poon RT, Ivanovska I, Philippar U, Cleary MA, Buser CA, Shaw PM, Lee CN, Tenen DG, Dai H, Mao M. DLK1-DIO3 genomic imprinted microRNA cluster at 14q32.2 defines a stemlike subtype of hepatocellular carcinoma associated with poor survival. J Biol Chem 2011; 286: 30706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benetatos L, Hatzimichael E, Londin E, Vartholomatos G, Loher P, Rigoutsos I, Briasoulis E. The microRNAs within the DLK1-DIO3 genomic region: involvement in disease pathogenesis. Cell Mol Life Sci 2013; 70: 795–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Josson S, Gururajan M, Hu P, Shao C, Chu GC, Zhau HE, Liu C, Lao K, Lu CL, Lu YT, Lichterman J, Nandana S, Li Q, Rogatko A, Berel D, Posadas EM, Fazli L, Sareen D, Chung LW. miR-409-3p/-5p Promotes Tumorigenesis, Epithelial-to-Mesenchymal Transition, and Bone Metastasis of Human Prostate Cancer. Clin Cancer Res 2014; 20: 4636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Josson S, Gururajan M, Sung SY, Hu P, Shao C, Zhau HE, Liu C, Lichterman J, Duan P, Li Q, Rogatko A, Posadas EM, Haga CL, Chung LW. Stromal fibroblast-derived miR-409 promotes epithelial-to-mesenchymal transition and prostate tumorigenesis. Oncogene, Epub ahead of print, 2014. DOI: 10.1038/onc.2014.212. [DOI] [PubMed] [Google Scholar]
- 69.Coppola V, Musumeci M, Patrizii M, Cannistraci A, Addario A, Maugeri-Sacca M, Biffoni M, Francescangeli F, Cordenonsi M, Piccolo S, Memeo L, Pagliuca A, Muto G, Zeuner A, De Maria R, Bonci D. BTG2 loss and miR-21 upregulation contribute to prostate cell transformation by inducing luminal markers expression and epithelial-mesenchymal transition. Oncogene 2013; 32: 1843–53. [DOI] [PubMed] [Google Scholar]
- 70.Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li Y, Banerjee S, Padhye S, Sarkar FH. Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF IL-6 and miRNAs that are attenuated by CDF. PLoS One 2012; 7: e43726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra S, Deng JJ, Gowda PS, Rao MK, Lin CL, Chen CL, Huang T, Sun LZ. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene 2014; 33: 4097–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cojoc M, Mabert K, Muders MH, Dubrovska A. A role for cancer stem cells in therapy resistance: cellular and molecular mechanisms. Semin Cancer Biol 2015; 31: 16–27. [DOI] [PubMed] [Google Scholar]
- 73.Liu T, Xu F, Du X, Lai D, Liu T, Zhao Y, Huang Q, Jiang L, Huang W, Cheng W, Liu Z. Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1. Mol Cell Biochem 2010; 340: 265–73. [DOI] [PubMed] [Google Scholar]
- 74.Millikan RE, Wen S, Pagliaro LC, Brown MA, Moomey B, Do KA, Logothetis CJ. Phase III trial of androgen ablation with or without three cycles of systemic chemotherapy for advanced prostate cancer. J Clin Oncol 2008; 26: 5936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCubrey JA, Abrams SL, Stadelman K, Chappell WH, Lahair M, Ferland RA, Steelman LS. Targeting signal transduction pathways to eliminate chemotherapeutic drug resistance and cancer stem cells. Adv Enzyme Regul 2010; 50: 285–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castillo V, Valenzuela R, Huidobro C, Contreras HR, Castellon EA. Functional characteristics of cancer stem cells and their role in drug resistance of prostate cancer. Int J Oncol 2014; 45: 985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 2009; 4: 226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, Li P, Zhang W, Wu H, Feng N, Wang Z, Hua L, Wang X. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem 2011; 350: 207–13. [DOI] [PubMed] [Google Scholar]
- 79.Su SF, Chang YW, Andreu-Vieyra C, Fang JY, Yang Z, Han B, Lee AS, Liang G. miR-30d, miR-181a and miR-199a-5p cooperatively suppress the endoplasmic reticulum chaperone and signaling regulator GRP78 in cancer. Oncogene 2013; 32: 4694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li B, Cheng XL, Yang YP, Li ZQ. GRP78 mediates radiation resistance of a stem cell-like subpopulation within the MCF-7 breast cancer cell line. Oncol Rep 2013; 30: 2119–26. [DOI] [PubMed] [Google Scholar]
- 81.Chiu CC, Lee LY, Li YC, Chen YJ, Lu YC, Li YL, Wang HM, Chang JT, Cheng AJ. Grp78 as a therapeutic target for refractory head-neck cancer with CD24(-)CD44(+) stemness phenotype. Cancer Gene Ther 2013; 20: 606–15. [DOI] [PubMed] [Google Scholar]
- 82.Wang N, Wang Z, Peng C, You J, Shen J, Han S, Chen J. Dietary compound isoliquiritigenin targets GRP78 to chemosensitize breast cancer stem cells via beta-catenin/ABCG2 signaling. Carcinogenesis 2014; 35: 2544–54. [DOI] [PubMed] [Google Scholar]
- 83.Kojima K, Fujita Y, Nozawa Y, Deguchi T, Ito M. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate 2010; 70: 1501–12. [DOI] [PubMed] [Google Scholar]
- 84.Corcoran C, Rani S, O'Driscoll L. miR-34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. Prostate 2014; 74: 1320–34. [DOI] [PubMed] [Google Scholar]