Abstract
In the past 50 years, disease burden has steadily shifted from infectious disease to cancer. Standard chemotherapy has long been the mainstay of cancer medical management, and despite vast efforts towards more targeted and personalized drug therapy, many cancers remain refractory to treatment, with high rates of relapse and poor prognosis. Recent dramatic immunotherapy clinical trials have demonstrated that engineering T-cells with chimeric antigen receptors (CARs) to target CD19 can lead to complete remission in relapsed or refractory B-cell malignancies, generating a great deal of enthusiasm in the field. Here we provide a comprehensive overview of the history of adoptive T-cell therapy, including CARs, in solid tumors as well as hematologic malignancies. CAR therapy has the potential to fundamentally transform cancer treatment with specific and even personalized targeting of tissue- and tumor-specific antigens. However, before CARs become standard first-line treatment modalities, critical issues regarding efficacy, combinatorial regimens, and mechanisms of treatment failure and toxicity will need to be addressed.
Keywords: Cancer, tumor, therapy, lymphocyte, medicine/oncology, immunology/molecular
Introduction
Engineering T-cells with tumor-targeting receptors can overcome immune tolerance to tumors and eradicate large established tumors. Chimeric antigen receptors (CARs) contain a targeting moiety, typically a single chain variable region from a monoclonal antibody (mAb), linked to a hinge region, a transmembrane domain, and an intracellular tyrosine-based activation motif comprised of either a region of CD3 T-cell receptor (TCR) complex, the CD3 ζ chain, or FcR receptor γ (Figure 1). The hinge region acts a “spacer” between the “chimeric” antigen-recognizing domain and the transmembrane domain, thus increasing conformational flexibility for antigen binding. Crucially, the chimeric pairing of an antigen receptor with the TCR intracellular signaling domain allows CD8+ cytotoxic T-cells to target cell surface makers with robust yet major histocompatibility complex (MHC)-independent activation. Second and third generation CAR T-cells include co-activator domains that further enhance the T-cell activation signal, thereby increasing proliferation and cytokine production. Remarkably, small doses of CAR T-cells are effective in lyzing a far larger tumor burden, and optimizing cell composition can further enhance CAR potency. Whereas CAR T-cell therapy was originally conceived as a bridge to transplantation in B-cell leukemias and lymphomas, clinical trials targeting CD19 have been effective therapies in their own right because the CD19 marker is highly sensitive and specific for B-cells. Importantly, since CAR T-cell therapy can be autologous as well as allogeneic, it can circumvent graft-versus-host disease, a longstanding concern with earlier adoptive T-cell therapies.1 By far the most common and dangerous side effect of CAR T-cell therapy is cytokine-release syndrome and its most severe manifestation, “cytokine storm”, in which massive T-cell activation triggers a cascade of released pro-inflammatory cytokines causing fever, flushing, and dyspnea. Severe cytokine storm can be potentially life threatening, however it can be effectively treated with the anti-interleukin-6 receptor antibody tocilizumab, along with hydration and supportive therapy.2 With advances in CAR design, CAR T-cells are poised to move beyond clinical trials and become standard treatment.2 However, this transition will require greater understanding of the mechanisms of failure in patients who do not respond to CAR, as well as characterization of CAR efficacy in the context of concomitant treatment, e.g. chemotherapy. Furthermore, CAR T-cells could potentially be combined with drugs that prevent tumors from co-opting immune checkpoints, notably the programmed death 1 (PD-1) pathway, in order to evade host immune defenses. In short, the ideal CAR T-cell therapy should be long lasting and durable and mechanisms of toxicity should be mitigated. The history of immunotherapy and adoptive T-cell therapy holds valuable lessons for optimizing CAR approach and design. In this review, we will highlight key developments in the history of immunotherapy and the specific implications they hold for CAR T-cell therapy in both hematological malignancies and solid tumors.
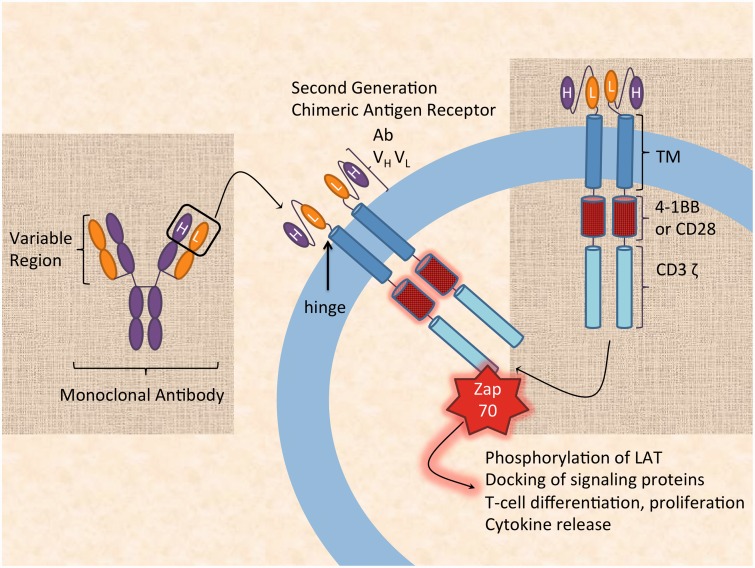
Figure 1.
Building a chimeric antigen receptor (CAR). The terminal variable heavy and light regions from a monoclonal antibody (Ab VH VL) are re-arranged into a single chain and attached to a transmembrane domain (TM), an activation domain (4-1BB or CD28), and the TCR intracellular signaling domain (CD3 ζ). A hinge region is included extracellularly to allow for increased conformational range of the antigen-binding domain. Upon CD3 ζ intracellular activation, Zap-70 is recruited to CD3 ζ and is positioned to phosphorylate the transmembrane protein linker of activated T-cells (LAT), resulting in T-cell activation, differentiation and proliferation, docking of signaling proteins, and cytokine release. H: heavy chain; L: light chain. (A color version of this figure is available in the online journal.)
History of non-specific immunotherapy
Cancer is among the leading causes of death worldwide, accounting for 8.2 million deaths in 2012.3 Within the next 20 years, cancer incidence is expected to rise from 14 million in 2012 to 22 million per year.3 Landmark findings in the 1950s and 1960s first demonstrated that tumors could be immunogenic.4,5 However, since tumors possess mechanisms by which they can evade the endogenous immune response, e.g. an immunosuppressive tumor microenvironment, directed immunotherapy has emerged as an attractive treatment strategy. The underlying premise of immunotherapy is simple: engineer or prime the immune system to detect the cancer, bypass its defenses, and destroy it effectively.
Cytokine priming
In 1891, in an early recorded example of cancer immunotherapy, William B Coley, a New York surgeon, injected beta-hemolytic pyogenic streptococcus into a patient with an inoperable osteosarcoma, causing the tumor to shrink.6 It is likely that the streptococcal injection would have up-regulated interleukin-12 (IL-12), a potent upregulator of interferon gamma (IFN-γ) in T-cells and natural killer (NK) cells, thereby priming the immune system against the tumor, albeit non-specifically. Almost a hundred years later, early modern-day immunotherapies used cytokines in order to prime the immune response against a tumor non-specifically. For instance, lymphokine-activated killer cells (LAKs) were peripheral blood leukocytes (PBLs) that were incubated ex vivo with IL-2. Soon, other PBL studies sought to further increase tumor lysis efficacy with far more complex cytokine cocktails. For instance, cytokine-induced killer cells (CIKs) were incubated with the standard anti-CD-3 antibodies—used to activate the TCR—and IL-2, in the presence of additional IL-1, IFN-γ, IL-7, IL-15, and CH-296 stimulation.7 Although non-specific to tumor cells, CIKs in particular were found to augment pre-existing treatment regimens for a number of metastatic malignancies, notably non-small cell lung carcinoma (NSCLC) and advanced gastric cancer, renal, hepatocellular, and nasopharyngeal carcinomas—with a remarkably decreased recurrence rate of hepatocellular carcinoma.7 In addition to IL-2 and interferon therapy, BCG vaccination against tuberculosis was also used as a means to prime the immune response against a tumor.8 However, these early CIK-based immunotherapies showed disappointing efficacy as stand-alone regimens. Low efficacy was not only caused by weak and non-specific targeting of tumor lesions, but also because cancers develop the ability to evade the immune response.
Checkpoint pathways and cancer immune evasion
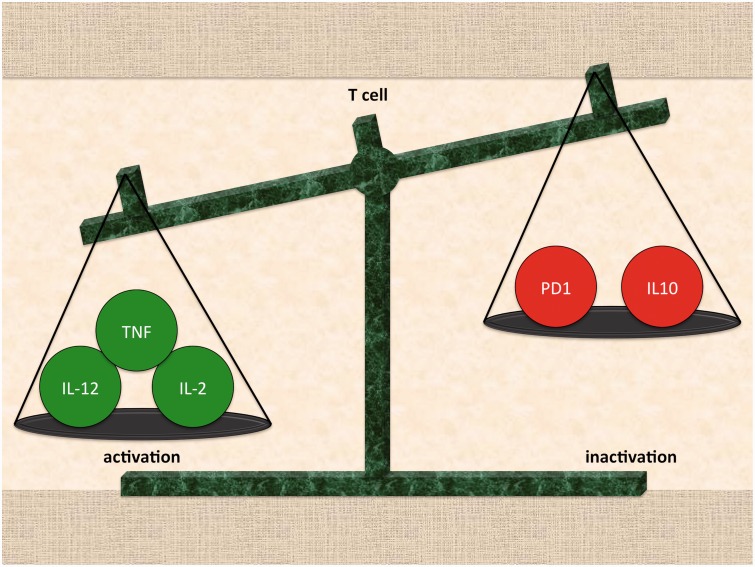
The inherent difficulty of specifically targeting tumors with an enhanced immune response is compounded by cancer's ability to evade the immune system by co-opting the pathways that promote self-tolerance. The body’s endogenous anti-inflammatory responses are designed to decrease damage to normal tissue caused in the event of a hyperactive immune response. The immune response to a foreign bacterial or viral invader or to a somatic cell that has undergone neoplastic transformation is a balance between pro-inflammatory factors, which lead to the necrosis or apoptosis of affected tissues, and protective anti-inflammatory mechanisms (Figure 2). Notably, immunosuppressive cytokines such as IL-10 and T-regulatory cells prevent excessive damage to unaffected cells.9,10 Additionally, immune checkpoint pathways that downregulate T-cell activation also maintain self-tolerance.11 By co-opting checkpoint pathways, tumor cells can develop mechanisms of immune resistance. Unsurprisingly, efforts to eradicate resistant cancers have centered on targeting specific immune checkpoint pathways. We will discuss the two checkpoint pathways of considerable interest for cancer therapy applications: PD-1 and CLTA-4.12
Figure 2.
T-cell activation is determined by the balance of competing signals. (A color version of this figure is available in the online journal.)
PD-1 blockade
The programmed-cell death protein 1 (PD-1) is a critical checkpoint to limit T-cell mediated immune responses. PD-1 is expressed on the surface of activated T-cells, but is also present on dendritic cells, B-cells, NK cells, and activated monocytes. PD-1 has two ligands, PD-L1 and PD-L2, which are members of the B7 family and mediate a protective response to prolonged inflammation. PD-L1 is expressed not only on macrophages and dendritic cells, but also on T-cells and B-cells whereas PD-L2 is expressed primarily in dendritic cells. PD-1 and its ligands negatively regulate T-cell proliferation and decrease IFN-γ secretion TNF-α, and IL-2 production.13 Unsurprisingly, neoplasms can co-opt the PD-1 checkpoint to escape detection by the adaptive immune system.14 PD-L1 is expressed in many solid tumors, conferring a proliferative advantage to tumor cells that evade the immune response. Indeed, PD-L1 expression in tumors has been linked with poor prognosis in breast cancer, gastric cancer, esophageal cancer, hepatocellular carcinoma, malignant melanoma, ovarian cancer, pancreatic cancer, renal cell carcinoma, and urothelial cancer.15 In NSCLC, it was discovered that tumor-infiltrating lymphocytes (TIL) upregulate PD-1 compared to circulating T-lymphocytes as means of overcoming increased tumor PD-L1 expression.16 Recent efforts at increasing the immunogenicity of cancer cells have focused on a strategy of blocking the PD-1 pathway.17 Suppression of tumor cell growth was observed in PD-1 deficient mice, and anti-PD-L1 antibody treatment of established tumors was associated with a decrease in tumor burden and increased survival in mice.18,19 In an expansive clinical trial in patients with advanced NSCLC, melanoma, and renal-cell cancer, anti-PD-L1 antibodies induced durable tumor regression, with an objective response rate of 6–17% and long-term stabilization of disease at rates of 12–41%.20 The parallel trial with an anti-PD-1 mAb, nivolumab, produced objective responses in ∼25% of patients with NSCLC, melanoma, or renal cell cancer.21 Currently, there are multiple phase III trials underway that target the PD-1/PD-L1 pathway for several cancers, including melanoma, renal cell carcinoma, and NSCLC in treatment naïve patients—with notably encouraging results for NSCLC reported.22 Additionally, nivolumab was associated with a substantial survival benefit in treatment-naive metastatic melanoma as compared to standard treatment.23
PD-1 checkpoint blockade in hematological malignancies has led to striking results in the clinic. The characteristic “owl-shaped” Reed-Sternberg cells in Hodgkin's lymphoma also exploit the PD-1 pathway to avoid immune detection. Classical Hodgkin's lymphoma typically presents with alterations in chromosome 9p24.24 The aberrant chromosome induces the JAK-STAT signaling pathway, which leads to a subsequent increase in PD-1 ligands, PD-L1 and PD-L2.24 In an ongoing clinical trial, nivolumab was shown to have a significant therapeutic effect and an acceptable safety profile in patients with Hodgkin's lymphoma. These patients were difficult to treat as they had relapsed following autologous stem-cell transplantation and/or standard medical management with brentuximab vedotin. The results were dramatic for such an intractable lymphoma: an objective response was reported in 87% of patients (20 out of 23 enrolled), and, at 24 weeks, the rate of progression-free survival was 86%.25 Since blockade of the PD-1/PD-L1 checkpoint pathway is effective in increasing tumor immunogenicity across solid tumors and hematological malignancies, checkpoint strategy could potentially be used with CAR therapy. Although the mechanism of CAR action is MHC-independent, it is possible that PD-1 blockade could impact CAR therapy beyond increased tumor immunogenicity. T-cell exhaustion is regulated by PD-1 in the setting of chronic infections, and blocking the PD-1: PD-L interaction rescues a subset of exhausted CD8 T-cells.26,27 These findings suggest that PD-1 blockade could help mitigate T-cell exhaustion in CAR therapy.
CTLA-4 blockade
Cytotoxic T-lymphocyte-associated protein 4, CTLA-4, comprises another checkpoint pathway that downregulates the immune response. When CD28 on the T-cell surface binds to B7, it activates the T-cell and promotes its survival, however when CTLA-4 binds to B7, it inhibits T-cell activation. Therefore, CTLA-4 blockade augments the ability of tumor-specific T-cells to target the tumor.28 CTLA-4 blockade can be combined with adoptive T-cell transfer or even with PD-1 blockade.29–31 For instance, concurrent blockade of both PD-1 with nivolumab and CTLA-4 with ipilimumab in patients with advanced melanoma resulted in an objective response rate of 40% overall, which increased to 53% at maximum drug doses. Of the patients who responded to high dose PD-1 and CTLA-4 blockade, all had a reduction in tumor mass of 80% or more,31 suggesting all-or-nothing susceptibility to the regimen.
Targeting cancers with adoptive T-cell immunotherapy
In adoptive T-cell therapy, autologous or allogeneic T-cells are first harvested and then either selected or genetically modified for enhanced immunologic function against a target. The T-cells are then expanded ex vivo prior to introduction into the patient. Over the course of the past several years, there has been a vast effort and renewed enthusiasm regarding adoptive T-cells in the context of CAR therapy. However, the history of adoptive T-cell therapy spans several decades and holds important lessons for current efforts to expand CAR applications to new cancers and to new delivery systems.
Tumor infiltrating lymphocytes
The development of tumor specific T-cells was pioneered in 1988 by Rosenberg’s group. The investigators selected TILs from patient melanoma tissue and expanded them ex vivo. These TILs were naturally activated by the tumor to have tumor-specific cytotoxic effects, and when expanded ex vivo and re-implanted, TILs were more selectively reactive against tumor cells when compared to LAK therapy with IL-2. However, a key limiting factor was the low efficacy of TIL-based therapy: only one out of 11 patients mounted a complete response.32 In 2000, Rosenberg sought to address low TIL efficacy by increasing the modified T-lymphocyte’s ability to engraft and clonally expand, thus granting long-term immunity against cancer cells. This was accomplished by preceding TIL treatment with a immune depleting regimen, resulting in a robust TIL effect.33 Subsequently, TIL therapy was shown to be effective in melanoma, with additional evidence that TILs can cross the blood–brain barrier and cause regression of brain metastases.34 Although the melanoma data constituted a very powerful model, TILs are not present in most types of cancer.35,36 And among the subset of cancers that produce TILs, e.g. melanoma, TILs are only present in about half of cases.35 Consequently, using TILs alone had limited potential as an expansive model for cancer therapy.36
On-target, off-tumor effects
Since some patients were particularly responsive to TIL therapy, determining the genetic sequence of those patients' TCR α and β chains, would allow for the generation of T-cells with high affinity TCRs.37 The first clinical trial utilizing this protocol targeted the melanoma antigen MART-1, with only two of 17 patients demonstrating tumor regression.38 A trial was then done targeting both MART-1 and GP100, two proteins expressed on both melanomas and normal melanocytes in the skin, eye, and ear. This trial showed far greater cancer regression: objective cancer regressions were seen in 30% and 19% of patients who received the human or mouse TCR, respectively.39 The price of improved cancer regression was a pronounced “on-target, off-tumor” effect, which consisted of a widespread erythematous skin rash in 29 of 36 patients, destruction of epidermal melanocytes, and diffuse infiltrates of CD8+ cells in skin, requiring treatment with local administration of steroids. Of great concern, 11 of 20 patients receiving the more reactive GP100 TCR treatment developed anterior uveitis, and evidence of hearing loss was present in 10 of 20 patients. The modified T-cells were able to specifically bind to their respective cellular targets, MART-1 and GP100, with high affinity. Unfortunately, the targets themselves were not specific to cancer cells and were also normally expressed off-tumor, hence the designation of “on-target, off-tumor”. As a result, modified T-cells targeted normal tissue as a serious side effect. Although CAR-modified T-cells in current hematological applications in the clinic are CD19-specific and target all B-cells as part of the regimen, the MART-1/GP100 trial is a potent reminder of the need to understand the nature of target antigen presence in normal cells. These results highlight the importance of targeting appropriate tumor-associated antigens (TAAs), especially if CAR-modified T-cell therapy is to be applied to solid tumors.
Tumor-specific antigens
Carcinoembryonic antigen (CEA) is a tumor-associated protein aberrantly highly expressed in tumor cells. First discovered in 1965, by Gold et al. who demonstrated humoral responses to CEA, CEA was the first identified cancer-specific antigen and has long been a target for immunotherapy, including DNA vaccine development and TCR therapy.40–42 More recently, in 2011, three patients with colorectal cancer were treated with adoptive T-cells genetically modified to express anti-CEA TCRs with high avidity, which refers to the significant accumulated strength of multiple single affinity interactions. All three patients had an observed reduction in CEA levels, and one patient had a partial response with a 49% reduction of lung and liver metastases and subsequent metastatic cancer regression in six months. However, all three patients developed a dose-limiting transient colitis, as the reengineered T-cells attacked the CEA-rich, highly differentiated epithelial cells in the upper third of the colonic crypts in another example of a “on-target, off-tumor” effect.40 This study highlighted the off-tumor risk involved even with very small numbers of highly avid modified T-cells.
Cancer-testis antigens (CTAs) are another promising potential target for adoptive T-cell therapy.43 This family of antigens is very specific to cancer cells, including bladder, lung and liver carcinomas, germ cells, and trophoblasts. For instance, New York Esophageal Squamous Cell Carcinoma 1 (NY-ESO-1), a member of the CTA family, is a potential cellular target that is expressed in synovial cell carcinoma (in 80% of cases) and also less frequently melanoma (in 25% of cases) as well as in normal adult human testis cells that lack MHC-1 expression. This would theoretically make this target a more specific and therefore safer alternative to other targets for adoptive TCR therapy, with a decreased risk of “on-target, off-tumor” effects. However, when TCR therapy against NY-ESO-1 was used in patients with synovial cell carcinoma and melanoma, the clinical results were mixed. One year after therapy, two of 11 patients with melanoma had complete tumor regression, and one patient with synovial cell carcinoma mounted a partial response.44 Fortunately, there was no toxicity associated with the transferred cells.
Off-target, off-tumor effects
Because CTAs are highly specific to cancer cells, they were expected to be a safer target for TCR therapy. However, highly specific CTA expression on target cells only decreases the chances of an on-target, off-tumor event. If the affinity-enhanced TCR cross-reacts with a distinct, albeit similar, antigen expressed in normal tissue, then an “off-target, off-tumor event” is possible. Off-target, off-tumor effects are very concerning because they progress rapidly, with potentially lethal consequences, and are notoriously hard to predict. For instance, melanoma-associated antigen 3 (MAGE-A3) is a CTA that is specifically expressed in more than 30% of common epithelial malignancies, including melanoma, breast, lung, esophageal, and head and neck cancers.45 In a TCR study targeting the MAGE-A3 antigen, two patients undergoing treatment experienced cardiogenic shock, resulting in death. Autopsy revealed severe myocardial damage and histopathological analysis revealed T-cell infiltration. Alarmingly, despite preclinical screening for cross-reactivity, MAGE-A3 was found to have a similar structure to a human cardiac protein, Titin, suggesting that the TCR-modified T-cell mistook a Titin-derived peptide on cardiomyocyte MHCs for MAGE-A3. In the follow-up, the TCR-modified T-cells reacted in vitro with beating human cardiomyocytes, thus confirming that Titin was the cross reactive protein.46 In short, in the MAGE-A3 TCR trial, an off-target, off-tumor event led to the fatal destruction of cardiac tissue in the two patients.47
Off-target, off-tumor toxicity is fast progressing and devastating. As evidenced by the MAGE-A3 trial, off-target, off-tumor effects are hard to completely rule out in preclinical studies due to variable protein sequence homology between animal models and humans. As a result, it is far more difficult to identify potential off-target, off-tumor effects than it is to determine on-target off-tumor effects. This is as relevant to CAR applications as it was for TCR therapy for solid tumors.
CAR therapy
Lessons learned: Addressing off-target off-tumor events for CAR therapy
In order for CAR therapy targeting CTAs or other TAAs to succeed in the clinic, the risk of off-target off-tumor effects needs to be addressed using various safety strategies. For example, prior to a clinical trial, in-vitro studies could use human genome data to synthesize and test candidate cross reactive proteins for reactivity. Additionally, future CAR safety studies could potentially deploy high-throughput proteomics to screen for cross-reactivity with the selected tumor-specific antigens, to test off-target off-tumor antigenic candidates, or even to select for the safest epitope. Another potentially advantageous safety strategy would be to include small molecule-inducible suicide switches in the modified T-cells.48 Finally, perhaps the best conceptual approach in terms of therapeutic safety would be to use mRNA-modified T-cells in CAR applications. The CAR mRNA modifying the T-cell would be short-lived and would degrade a short time after infusion, allowing for enhanced dosage control and a safer therapy.
CAR therapy is MHC-independent
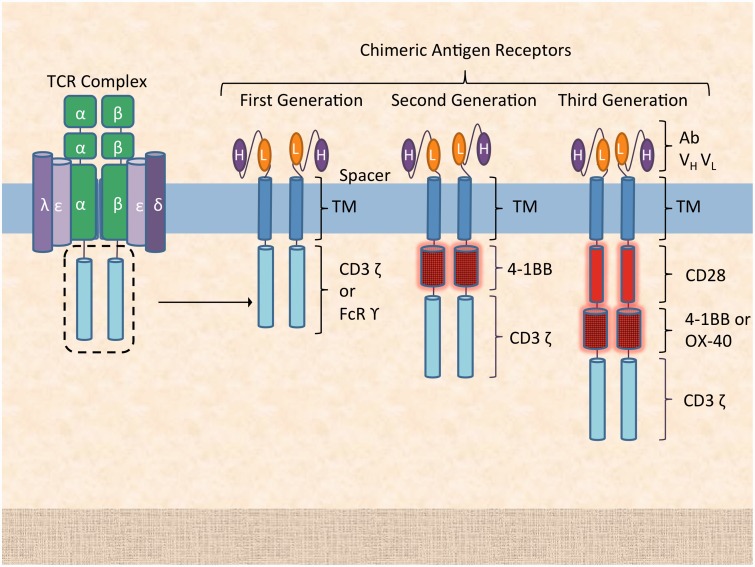
As previously discussed, CARs consist of an antigen-derived binding motif, linked with a hinge to transmembrane and intracellular signaling domains, the latter derived from the ζ chain of CD3 or the FcR receptor γ chain. In other words, CAR T-cells do not require MHC-mediated recognition of the target antigen, allowing for a far greater range of potential cellular targets. Furthermore, modified CAR T-cells have increased power relative to TCR-based therapies, as they are immune to down-regulation of MHC expression in neoplastic cells. The correlate is that, by bypassing the MHC, CAR T-cells can only target cell surface markers. It is important to note that CAR affinity for the target epitope is a key determinant of CAR therapy efficacy. A murine antibody single chain variable fragment (Fv) is articulated to the transmembrane domain via a hinge region, which allows for greater flexibility of the antigen-receptor configuration. Since CAR T-cells are not MHC-restricted, their interaction with antigen presenting cells (APCs) is limited, and they do not receive a co-stimulatory signal from an APC. Indeed, whereas first generation CAR design consists of the CAR fused to the intracellular portion of the TCR domain, second generation CAR design includes one co-stimulatory domain, e.g. CD28 or 4-1BB, and third generation CARs include two or more co-stimulatory signaling domains, e.g. CD28 and 4-1BB (Figure 3).
Figure 3.
Chimeric antigen receptor generations. First generation CARs include a single-chain variable region from a monoclonal antibody paired with an intracellular signaling domain, the CD3 ζ chain from the CD3 TCR or FcR γ. Second and third generation CARs include an additional one or two co-stimulating domains (e.g. CD28, 4-1BB, OX-40) that increase signal strength and persistence, with increased proliferation and cytokine production. TM: transmembrane domain; H: heavy chain; L: light chain. (A color version of this figure is available in the online journal.)
CAR T-cell therapy and unprecedented success in B-cell malignancies
The first successful CAR therapies were directed against hematologic cancers, notably B-cell malignancies. B-cell ablation is considered safe for patients in the context of the prompt recovery of other blood counts. For that reason, the CD19 marker, highly specific to B-cells including those transformed to lymphoma and leukemia, was chosen as target for CAR T-cell therapy. The first CAR targeting CD19 was dramatically effective in a patient with B-cell lymphoma, with eradication of B-lineage cells for a prolonged period of time, with concomitant marked lymphoma regression.49
In order to further strengthen CAR T-cell activation, a co-stimulatory domain from CD137 (4-1BB) was added in a subsequent trial for advanced B-cell chronic lymphocytic leukemia (CLL), with two out of three patients undergoing complete remission. CAR-modified T-cell lines successfully expanded ex vivo more than 1000-fold, engrafted in the bone marrow and could establish CAR memory for at least six months.50 The combination of a CAR with a co-stimulatory signal led to a robust response: each CAR-expressing T-cell was estimated to lyze at least 1000 CLL cells.50 Consequently, tumor lysis syndrome was reported as common serious adverse event in both CLL and B-cell acute lymphoid leukemia (AML) trials.51,52 The lesson was that, during CAR therapy, patients need to be closely monitored for signs of tumor lysis syndrome since CAR T-cells ablate large amounts of target cells at levels vastly superior to those of past immunotherapies.
CAR T-cell therapy has succeeded where conventional therapies have failed, and CAR T-cell therapy has led to remission in patients with refractory disease. In a landmark clinical trial, autologous T-cells transduced with a CD19-directed CAR led to a 90% remission rate (27/30 cases) for patients with relapsed or refractory acute lymphoblastic leukemia (ALL), including 15 patients for whom stem cell transplantation had previously failed.2 The six-month event-free survival rate was 67%; overall survival rate was 78%. All patients experienced cytokine-release syndrome, with severe cytokine-release syndrome observed in 27% of patients. Severe cases of cytokine-release syndrome were associated with a higher tumor burden and were treated effectively with tocilizumab, the anti-IL-6 receptor antibody. The results of this trial were without precedent and demonstrated that CAR T-cells can overcome mechanisms of B-cell neoplasm resistance in subsets of patients with abysmally low prognosis. Indeed, 90% remission was a quasi-reversal of the odds for these patients, and firmly made the case that CAR therapy, if done correctly, can completely upend longstanding cancer treatment paradigms, providing hope for millions of patients with dire prognosis.
CAR therapy in solid tumors
Although CAR T-cell therapy was first attempted against a solid tumor, CAR therapy for solid tumors has seen limited progress due to safety concerns. In a 2008 neuroblastoma trial, Epstein-Barr virus (EBV)-specific T-cells were engineered to also co-express a CAR targeted at diasialoganglioside GD2, an antigen expressed by neuroblastoma cells.53 Half the patients (4/8) had evidence of tumor regression. Intriguingly, the virus-specific CAR T-cells survived longer than T-cells with CAR alone. Additionally, on-target, off-tumor toxicity is an especially critical safety concern for CAR therapy, even more so than for TCR therapy because CAR T-cell destruction of cells is not MHC-mediated, leading to a stronger and faster response during activation. For instance, a CAR therapy derived from a mAb against carboxyanhydrase-IX (CAIX), a marker of clear cell renal cell carcinoma, also destroyed normal bile duct epithelial cells, which happened to also express CAIX.53 It was a striking example of an on-target, off-tumor effect in a CAR therapy, and a potent reminder that future CAR strategies will need to further guarantee targeting specificity.53
Overcoming mechanisms of CAR failure
The power of CAR therapy resides in the ability to choose any surface marker as a target, and to hit that target with high efficacy. Nonetheless, not all patients respond completely to CAR therapy and the mechanisms of failure will need to be defined in non-responding patients. For instance, the chimeric murine mAb Fv presents the risk of immunogenicity, potentially resulting in CAR neutralization. Tumor antigen loss and the development of antigen variants could lead to tumor resistance to CAR therapy. Another challenge is the development of treatment-related toxicity, cytokine-release syndrome, which has been linked to massive macrophage and IL-6 activation. Although IL-6 blockade has been successful in managing the most severe manifestations of cytokine-release syndrome, regulating the level or dose of CAR expression would allow for greater control over toxicity as well.
CAR therapy in tumor relapse surveillance
CAR T-cell therapy targeting CD19 risks selecting for neoplastic clones that express low or no CD19.52 Beyond absence of CD19, dynamic or cyclical CD19 expression at a single cell or subclone level would constitute another potential mechanism of resistance, and would require constitutive CAR T-cell presence over a period of 3–6 months in order to provide immune surveillance and prevent relapse. Indeed, otherwise, transient down-regulation of CD19 expression during CAR T-cell therapy would conceal the B-cell malignancy from cytotoxic T-cells, increasing the chances of subsequent tumor recurrence.
Retroviral and lentiviral transduction are currently the most commonly used procedure employed to create CAR T-cells. Using an integrated transgene allows for long-term expression of CAR-modified T-cells, which engraft and can provide constitutive surveillance in hematologic malignancy applications. However, with long-term and constitutive expression of CAR T-cells, there is an increased chance of an immune reaction developing against the CAR provirus, decreasing the efficacy of the CAR constructs.54 There is always a risk of insertional mutagenesis with viral integration, even in this well-defined and more differentiated T-cell population.
Conversely, the modified T-cells could induce autoimmunity and an exaggerated immune response, leading to the unpredictable destruction of healthy tissue in “on-target, off-tumor” and “off-target, off-tumor” effects, and cytokine storm.55 The use of small molecule-inducible suicide genes, especially the inducible caspase-9 safety switch, has shown promise as a safety mechanism for the rapid elimination of T-cells in graft versus host disease.48 This mechanism could be useful in CAR T-cell therapy as well, however by the time potentially lethal off-tumor events are detected, it might be already too late to trigger the suicide gene, due to the fast kinetics of CAR-mechanism.56
Simultaneous multiple CAR targets
Targeting multiple markers simultaneously with a combination of different CAR constructs could also potentially prevent selection of resistant clones and decrease tumor recurrence. Since the proportion of CD19- B-cells would be very small relative to the total number of CD19+ cells in a CD19+ B-cell lymphoma or leukemia, we do not anticipate that adding additional CAR targets would have a significant effect on the severity of cytokine-release syndrome, given that the bulk of the side effects would be caused regardless by the CD19 CAR T-cell regimen alone.
Combining several different CARs to treat malignancies is analogous to drug combination therapy in cancer, where improved survival can be achieved without increased toxicity.57 For instance, in treating hematological malignancies, often an oncolytic drug cocktail is greater than the sum of its parts. The combination therapy will succeed more often compared to a single drug or compared to even sequential administration of the individual drugs in the cocktail. The principle that denies neoplasms the opportunity to develop drug-resistant clones in traditional standard-of-care medical management should also be applicable to CAR-modified T-cell therapy.
Using mRNA for CAR therapy
CAR T-cells modified with mRNA express a short-lived CAR mRNA that naturally degrades within several days, without the risk associated with long-term engraftment. Clinical delivery of mRNA CARs would likely be injected in regular doses, and in the event of an adverse off-tumor event, the next injection could be withheld to avert destruction of healthy tissue. Using mRNA-based CARs decreases the risk of the persistence of autoreactive T-cells, whereas other methods might leave a small population of toxic autoimmune reactive cells. To illustrate, anti-ErbB2 and anti-CEA CAR T-cells were created through electroporation with mRNA, with a CAR expression half-life of two days, and complete disappearance of the CARs after nine days.58 mRNA has been also used to create CAR T-cells targeting mesothelin. Mesothelin is a TAA that is over-expressed in the majority of malignant pleural mesotheliomas (MPM), as well as pancreatic, ovarian, and select lung cancers but is also expressed in low levels on normal peritoneal, pleural, and pericardial cells.59 The first human study of mesothelin-directed mRNA CARs in patients with metastatic pancreatic cancer presented evidence of antitumor activity. Despite the transient nature of the transplanted T-cells, the mRNA CAR T-cells were functional and did not require pretreatment lymphodepletion. There was no overt evidence of off-tumor toxicities for this trial, where in previous studies “off-tumor” peritonitis was the dose-limiting toxicity.60 Elsewhere, mRNA CARs directed against HER2/Neu have been promising in a xenograft model targeting ovarian cancer, showing greater inhibition of tumor growth compared to herceptin, the HER2/Neu targeting standard of care.61
Given the heterogeneity of soft tissue tumors and solid non-hematologic tumors, there is an opportunity to personalize CAR therapy. Indeed, using mRNA technology, multiple CARs could target multiple tumor-specific targets. It would be feasible to obtain longitudinal samples from a patient, determine which of the pre-screened biomarkers are present and utilize this information in order to develop personalized CAR therapy.62 Combining multiple CARs with multiple targets addresses the issue of a heterogeneous solid tumor, and would give the tumor less time to develop mechanisms of resistance, however this strategy does not address potential off-tumor effects. Off-tumor effects could be addressed with the use of dual targeting proteins. Recombinant proteins with the capacity for dual targeting can be used to re-direct an effector cell to a tumor cell with a much greater specificity, avoiding potentially dangerous off-tumor effects.
NK cells for CAR therapy
Future clinical CAR therapies might also involve NK cells instead of T-cells for certain applications. There are several advantages to using NK cells instead of T-cells for CAR therapy. Chiefly, NK cells have a limited lifespan in patients, addressing concerns about persistent CAR-associated side effects, and eliminating the need for including an inducible suicide gene on the construct. Furthermore, CAR-modified NK cells are not restricted in their killing to a CAR specific mechanism: they can also deploy endogenous MHC-independent cytotoxic activity. For instance, if TAA-specific antibodies bind to the target cell, the NK cell can recognize the Fc fragment of the target-bound antibody, thereby triggering antibody-dependent cell-mediated cytotoxicity (ADCC). Thus, NK cells could recognize the same TAA twice, or two separate TAAs, via distinct CAR and ADCC pathways. This specific feature of NK cells would enable the combination of two NK-dependent targeted therapies: CAR-expressing NK cells and a TAA-specific mAb.
The exciting future of CAR therapy
Remarkably, small doses of CAR T-cells are effective and potency can be further enhanced by enhancing T-cell capacity to self-renew.50,51 The only exception is therapy utilizing mRNA-transduced CAR T-cells, which require the infusion of large numbers of mRNA CAR T-cells at regular intervals due to the short-lived nature of mRNA-mediated CAR expression. A key advantage of mRNA technology is avoiding chromosomal instability and possible cancerous transformation of lentiviral derived CAR T-cells.63
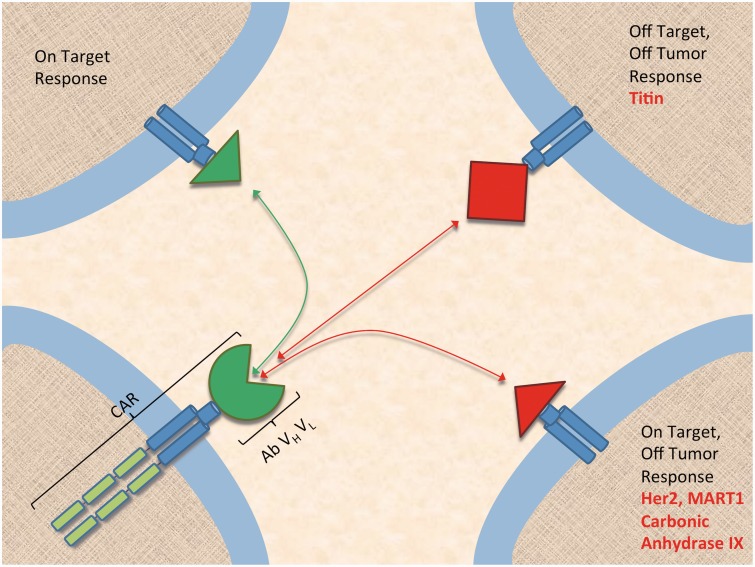
Chemotherapy and hematopoietic stem cell transplantation are often poorly tolerated in patients, and especially in the case of transplantation, adverse events constitute a large share of patient mortality. However, for many patients stem cell transplantation becomes the only option to prolong survival in those with difficult to treat disease. In comparison, CD19-directed CAR-modified T-cells have achieved 90% remission in B-cell ALL in refractory and relapsed patients, including many for whom stem cell transplantation had failed, reaching a level of efficacy that is unprecedented in immunotherapy. As a result, CAR therapy has the potential not only to supplant the current standard of care in hematology/oncology, but also to expand to solid tumors, providing hope for the millions of cancer patients with poorly tolerated treatment regimens and dire prognosis. However, future expansive CAR applications to targets other than CD19 will have to learn from immunotherapy’s long history of adverse outcomes and non-specific targeting. In short, building CARs directed at tumor-specific antigens will require careful consideration of off-tumor effects, be they on-target or off-target (Figure 4). For this reason, epitope specificity appears to be more important than CARs of similar affinity targeting distinct epitopes. Off-tumor responses are dangerous and difficult to predict and are the leading cause of morbidity and mortality related to adoptive cell therapy. Efforts to make CAR therapy safer include using a suicide gene, substituting NK cells for T-cells, and delivery of CAR via multiple injections of mRNA that can be discontinued safely.
Figure 4.
Risk of off-tumor effects in chimeric antigen receptor therapy. CAR: chimeric antigen receptor. (A color version of this figure is available in the online journal.)
TRUCKs
The tumor microenvironment can be suppressive, as evidenced by regulatory T-cells (T regs), myeloid-derived suppressor cells, and soluble inhibitors of TGFβ-mediated resistance mechanisms. Since tumor cells can co-opt PD1:PD-L1 and CTLA-4 checkpoint pathways and reduce tumor immunogenicity, an adjuvant approach would be to block checkpoint pathways concurrent with CAR therapy.20 This would be especially relevant in cases where checkpoint blockade has been already been shown to be successful as a monotherapy, e.g. nivolumab in Hodgkin's Lymphoma.20 Finally, in cases where the cancer-specific antigen targeted by CAR is not fully sensitive, there are T-cells redirected for universal cytokine-mediated killing (TRUCKs). TRUCKs are activated by CAR antigen binding on the target, thus releasing IL-12 in the vicinity of the targeted cell. The result is collateral damage beyond the CAR-recognized target cancer cell, as IL-12 recruits an overwhelming innate immune response to the neighboring neoplastic cells that are not recognized by the CAR.
CAR therapy is upending the longstanding paradigms of cancer research, with some dramatic successes in B-cell malignancies. A key concern will be to determine the durability of CAR’s effect and the malignancies best adapted to CAR targeting. It is possible that a fourth generation CAR will be needed to address the issue of T-cell exhaustion and ensure surveillance in the critical months following initial therapy. New evidence suggests that CAR T memory stem cells could potentially survive and remain active for up to 12 years following infusion.64 In other words, a surveillance CAR could likely outlast residual tumor cells and help prevent recurrence. Indeed, CAR therapy’s ability to induce lasting remission will be the ultimate test of this very exciting development in immunotherapy.
Acknowledgments
This work was supported by the New York Stem Cell Science Program (NYSTEM) NG 11G-25. The content is solely the responsibility of the authors and does not necessarily represent the official views of the New York Stem Cell Science Program.
Author contributions
All authors participated in the writing and review of this mini-review.
Declaration of conflicting interest
Dr Yupo Ma is a Founder of iCell Gene Therapeutics LLC.
References
- 1.Korngold R, Sprent J. Graft-versus-host disease in experimental allogeneic bone marrow transplantation. Proc Soc Exp Biol Med Soc Exp Biol Med 1991; 197: 12–8. [DOI] [PubMed] [Google Scholar]
- 2.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart BW, Wild C. International Agency for Research on Cancer, World Health Organization. World cancer report 2014. Lyon: WHO Press, 2014. xiv, 630 p.
- 4.Gross L. Intradermal immunization of C3H mice against a sarcoma that originated in an animal of the same line. Cancer Res 1943; 3: 326–33. [Google Scholar]
- 5.Foley EJ. Antigenic properties of methylcholanthrene-induced tumors in mice of the strain of origin. Cancer Res 1953; 13: 835–7. [PubMed] [Google Scholar]
- 6.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthopaed J 2006; 26: 154–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Zang Y-W, Gu X-D, Xiang J-B, Chen Z-Y. Clinical application of adoptive T cell therapy in solid tumors. Med Sci Monit 2014; 20: 953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larson CL, Baker RE, Ushijima RN, Baker MB, Gillespie C. Immunotherapy of Friend disease in mice employing viable BCG vaccine. Proc Soc Exp Biol Med Soc Exp Biol Med 1972; 140: 700–2. [DOI] [PubMed] [Google Scholar]
- 9.Murphy K. Janeway’s immunobiology, 8th ed New York, NY: Garland Science, 2011. [Google Scholar]
- 10.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010; 10: 170–81. [DOI] [PubMed] [Google Scholar]
- 11.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 2010; 37: 430–9. [DOI] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ, Sharpel AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvey RD. Immunologic and clinical effects of targeting PD-1 in lung cancer. Clin Pharmacol Ther 2014; 96: 214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, Mackensen A. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer 2006; 119: 317–27. [DOI] [PubMed] [Google Scholar]
- 17.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol 2005; 17: 133–44. [DOI] [PubMed] [Google Scholar]
- 19.Hirano F, Kaneko K, Tamura H, Dong HD, Wang SD, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu GF, Tamada K, Chen LP. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 2005; 65: 1089–96. [PubMed] [Google Scholar]
- 20.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366: 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharon E, Streicher H, Goncalves P, Chen HX. Immune checkpoints in cancer clinical trials. Chin J Cancer 2014; 33: 434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbe C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA. Nivolumab in previously untreated melanoma without BRAF mutation. New Engl J Med 2015; 372: 320–30. [DOI] [PubMed] [Google Scholar]
- 24.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, Chapuy B, Takeyama K, Neuberg D, Golub TR, Kutok JL, Shipp MA. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 2010; 116: 3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blackburn SD, Crawford A, Shin H, Polley A, Freeman GJ, Wherry EJ. Tissue-specific differences in PD-1 and PD-L1 expression during chronic viral infection: implications for CD8 T-cell exhaustion. J Virol 2010; 84: 2078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A 2008; 105: 15016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371: 2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahvi DA, Meyers JV, Tatar AJ, Contreras A, Suresh M, Leverson GE, Sen S, Cho CS. Ctla-4 blockade plus adoptive T-cell transfer promotes optimal melanoma immunity in mice. J Immunother 2015; 38: 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, Dhodapkar KM. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 2015; 194: 950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma – a preliminary-report. N Engl J Med 1988; 319: 1676–80. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 2009; 21: 233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, Sherry RM. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res 2010; 16: 4892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 2003; 26: 332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med 2011; 236: 567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dembic Z, Haas W, Weiss S, McCubrey J, Kiefer H, Vonboehmer H, Steinmetz M. Transfer of specificity by murine alpha-t-cell and beta-t-cell receptor genes. Nature 1986; 320: 232–8. [DOI] [PubMed] [Google Scholar]
- 38.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314: 126–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, Lee C-CR, Restifo NP, Schwarz SL, Cogdill AP, Bishop RJ, Kim H, Brewer CC, Rudy SF, VanWaes C, Davis JL, Mathur A, Ripley RT, Nathan DA, Laurencot CM, Rosenberg SA. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009; 114: 535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan D-AN, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, Hughes MS, Kammula US, Phan GQ, Lim RM, Wank SA, Restifo NP, Robbins PF, Laurencot CM, Rosenberg SA. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011; 19: 620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hance KW, Zeytin HE, Greiner JW. Mouse models expressing human carcinoembryonic antigen (CEA) as a transgene: evaluation of CEA-based cancer vaccines. Mutat Res Fundam Mol Mech Mutagen 2005; 576: 132–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haupt K, Roggendorf M, Mann K. The potential of DNA vaccination against tumor-associated antigens for antitumor therapy. Exp Biol Med 2002; 227: 227–37. [DOI] [PubMed] [Google Scholar]
- 43.Simpson AJG, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 2005; 5: 615–25. [DOI] [PubMed] [Google Scholar]
- 44.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee C-CR, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011; 29: 917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sang M, Lian Y, Zhou X, Shan B. MAGE-A family: attractive targets for cancer immunotherapy. Vaccine 2011; 29: 8496–500. [DOI] [PubMed] [Google Scholar]
- 46.Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013; 122: 863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK. Identification of a titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 2013;5. [DOI] [PMC free article] [PubMed]
- 48.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011; 365: 1673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan D-AN, Lanier BJ, Morgan RA, Rosenberg SA. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010; 116: 4099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011;3. [DOI] [PMC free article] [PubMed]
- 51.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamers CHJ, Sleijfer S, Vulto AG, Kruit WHJ, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006; 24: e20–2. [DOI] [PubMed] [Google Scholar]
- 54.Lamers C, van Elzakker P, van Steenbergen S, Oosterwijk J, Sleijfer S, Debets R, Gratama J. Immune responses to transgene and retroviral vector in patients treated with ex vivo engineered T-cells. Immunology 2012; 137: 688–688. [DOI] [PubMed] [Google Scholar]
- 55.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010; 18: 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Stasi A, Tey S-K, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011; 365: 1673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, Chiarion-Sileni V, Drucis K, Krajsova I, Hauschild A, Lorigan P, Wolter P, Long GV, Flaherty K, Nathan P, Ribas A, Martin AM, Sun P, Crist W, Legos J, Rubin SD, Little SM, Schadendorf D. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372: 30–9. [DOI] [PubMed] [Google Scholar]
- 58.Birkholz K, Hombach A, Krug C, Reuter S, Kershaw M, Kaempgen E, Schuler G, Abken H, Schaft N, Doerrie J. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4(+) and CD8(+) T cells for use in the adoptive immunotherapy of cancer. Gene Ther 2009; 16: 596–604. [DOI] [PubMed] [Google Scholar]
- 59.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, Kalos M, June CH. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce antitumor activity in solid malignancies. Cancer Immunol Res 2014; 2: 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, Pastan I. Phase I study of SS1P a recombinant anti-mesothelin immunotoxin given as a bolus IV infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007; 13: 5144–9. [DOI] [PubMed] [Google Scholar]
- 61.Yoon SH, Lee JM, Cho HI, Kim EK, Kim HS, Park MY, Kim TG. Adoptive immunotherapy using human peripheral blood lymphocytes transferred with RNA encoding Her-2/neu-specific chimeric immune receptor in ovarian cancer xenograft model. Cancer Gene Ther 2009; 16: 489–97. [DOI] [PubMed] [Google Scholar]
- 62.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366: 883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCormack MP, Rabbitts TH. Activation of the T-cell oncogene LMO2 after gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 2004; 350: 913–22. [DOI] [PubMed] [Google Scholar]
- 64.Biasco L, Scala S, Basso Ricci L, Dionisio F, Baricordi C, Calabria A, Giannelli S, Cieri N, Barzaghi F, Pajno R, Al-Mousa H, Scarselli A, Cancrini C, Bordignon C, Roncarolo MG, Montini E, Bonini C, Aiuti A. In vivo tracking of T cells in humans unveils decade-long survival and activity of genetically modified T memory stem cells. Sci Transl Med 2015; 7: 273ra13–273ra13. [DOI] [PubMed] [Google Scholar]